ABSTRACT
Many methylotrophic taxa harbor two distinct methanol dehydrogenase (MDH) systems for oxidizing methanol to formaldehyde: the well-studied calcium-dependent MxaFI type and the more recently discovered lanthanide-containing XoxF type. MxaFI has traditionally been accepted as the major functional MDH in bacteria that contain both enzymes. However, in this study, we present evidence that, in a type I methanotroph, Methylomicrobium buryatense, XoxF is likely the primary functional MDH in the environment. The addition of lanthanides increases xoxF expression and greatly reduces mxa expression, even under conditions in which calcium concentrations are almost 100-fold higher than lanthanide concentrations. Mutations in genes encoding the MDH enzymes validate our finding that XoxF is the major functional MDH, as XoxF mutants grow more poorly than MxaFI mutants under unfavorable culturing conditions. In addition, mutant and transcriptional analyses demonstrate that the lanthanide-dependent MDH switch operating in methanotrophs is mediated in part by the orphan response regulator MxaB, whose gene transcription is itself lanthanide responsive.
IMPORTANCE Aerobic methanotrophs, bacteria that oxidize methane for carbon and energy, require a methanol dehydrogenase enzyme to convert methanol into formaldehyde. The calcium-dependent enzyme MxaFI has been thought to primarily carry out methanol oxidation in methanotrophs. Recently, it was discovered that XoxF, a lanthanide-containing enzyme present in most methanotrophs, can also oxidize methanol. In a methanotroph with both MxaFI and XoxF, we demonstrate that lanthanides transcriptionally control genes encoding the two methanol dehydrogenases, in part by controlling expression of the response regulator MxaB. Lanthanides are abundant in the Earth's crust, and we demonstrate that micromolar amounts of lanthanides are sufficient to suppress MxaFI expression. Thus, we present evidence that XoxF acts as the predominant methanol dehydrogenase in a methanotroph.
INTRODUCTION
An increasing surplus in the global methane budget exists due to human activity. The industrial use of microorganisms to convert methane into useful chemicals or biofuels represents one way to mitigate atmospheric methane (1, 2). Methanotrophs, or methane-oxidizing bacteria, utilize methane as their carbon and energy source and are prime candidates for the industrial bioconversion of methane (1). Renewed interest in the industrial use of methanotrophs has come about partially because of the discovery of rapidly growing strains and new tools for genetic manipulation, allowing for fast-paced metabolic engineering (3, 4). The success of metabolic engineering strategies in these methanotrophs depends upon a strong foundation of knowledge concerning the metabolic pathways that methanotrophs employ, both in the laboratory and in their natural environments, and an understanding of how various branches of metabolic pathways are regulated.
The majority of methanotrophs have two systems for oxidizing methane to methanol: the particulate methane monooxygenase (pMMO) is a copper-dependent enzyme, and the soluble methane monooxygenase (sMMO) is an iron-dependent enzyme with a broader substrate specificity (5, 6). The transcriptional expression of these two methane oxidation systems is regulated by the presence of copper. Expression of the genes encoding pMMO is increased in the presence of copper, and expression of the genes encoding sMMO is decreased under these conditions (7, 8).
The methanol generated from methane oxidation is further oxidized to formaldehyde by a methanol dehydrogenase (MDH) enzyme. In methanotrophs, as well as in nonmethanotrophic methylotrophs, the oxidation of methanol to formaldehyde was traditionally thought to be catalyzed primarily by a calcium-dependent MDH, MxaFI. A gene similar to the one encoding MxaF, named xoxF, has been identified in many methylotrophs (6), and it has been recently demonstrated that xoxF encodes an alternative MDH, named XoxF (9–15). Moreover, it has become evident that xoxF genes are more widespread in methylotrophs than mxaFI genes (6, 16). An additional function for XoxF, in controlling mxa gene expression in the model methylotroph Methylobacterium extorquens AM1, has also been demonstrated (17).
The traditional MxaFI-type MDH is much more well studied than XoxF. MxaFI is a pyrroloquinoline quinone (PQQ)-linked MDH and is located in the periplasm (18). In methylotrophs, the mxa operon typically consists of genes encoding the large and small subunits of MDH (mxaF and mxaI, respectively), the cytochrome c electron acceptor (mxaG), proteins for calcium insertion (mxaACKL), and other genes of unknown function that are required for a functional MDH (6, 19). Transcription of this operon in M. extorquens AM1 is controlled by at least two two-component systems, MxcQE and MxbDM, as well as by an orphan response regulator, MxaB (20–23).
XoxF enzymes have been isolated as active MDH enzymes in multiple methylotrophs when the cultivation medium was supplemented with lanthanides (13-15, 24). Growth in a medium supplemented with lanthanides has been demonstrated to increase the MDH activity of the methylotrophs Methylobacterium radiotolerans, Bradyrhizobium sp., and M. extorquens AM1, when XoxF acts as the dominant MDH (13–15). Lanthanides have been copurified with the PQQ-linked XoxF enzyme, indicating that the active XoxF enzyme contains alternative metals, in contrast to the calcium-dependent MxaFI MDH (13, 24). Lanthanides are stronger Lewis acids than calcium and are proposed to act as a strong Lewis acid in the XoxF enzyme, allowing the active carbons in PQQ to become stronger electrophiles and remove electrons from methanol (24). The discovery of XoxF functionality in the presence of lanthanides has allowed for the cultivation of a novel acidophilic methanotroph, Methylacidiphilum fumariolicum SolV (24), as well as for growth of methylotrophic bacteria cultured from the phyllosphere (25). Lanthanides belong to a group of elements known as the rare earth elements, but they are relatively abundant in the Earth's crust despite the group's name (25). Some rare earth elements are present at concentrations similar to those of typical industrial metals (26, 27).
More recently, it has been shown that lanthanides transcriptionally regulate the expression of genes encoding the two alternative MDH enzymes in multiple methylotrophic organisms (25, 28). In the type II methanotroph Methylosinus trichosporium OB3b, mxaF gene expression was reduced in the presence of cerium, while xoxF expression was increased (28). These cerium-dependent effects were attenuated by the presence of copper, which led the authors to hypothesize that the MxaFI MDH likely formed a supercomplex with pMMO in the periplasm.
In this study, we examined the role of XoxF in a type I methanotroph, Methylomicrobium buryatense 5GB1C. Type I methanotrophs are particularly attractive for industrial use, as they assimilate formaldehyde into biomass using a highly efficient variant of the ribulose monophosphate pathway (5, 29–31). Our results suggest that XoxF is not an accessory MDH but is the preferred methanol oxidation system in M. buryatense 5GB1C. We show that lanthanides increase xoxF expression and decrease mxa expression, even in the presence of excess calcium. Results from knockout mutations in the MDH genes indicate that each system is dispensable under specific environmental conditions, i.e., the presence versus the absence of lanthanides. Overall, XoxF likely dominates environmental MDH activity, as micromolar concentrations of lanthanides are sufficient to completely block mxa expression. We demonstrate that the lanthanide-mediated MDH switch is regulated in part by the response regulator MxaB.
MATERIALS AND METHODS
Strains and growth conditions.
M. buryatense 5GB1C and its derivatives were grown in modified nitrate mineral salts medium (NMS2) as previously described (3). For growth without copper, the 500× high-purity trace elements solution was modified to contain 1.0 g of Na2-EDTA/liter, 2.0 g of FeSO4·7H2O/liter, 0.8 g of ZnSO4·7H2O/liter, 0.03 g of MnCl2·4H2O/liter, 0.03 g of H3BO3/liter, 0.2 g of CoCl2·6H2O/liter, 0.02 g of NiCl2·6H2O/liter, and 0.05 g of Na2MoO4·2H2O/liter. Supplements were added as follows: 2.5% (weight/volume) sucrose, 100 μg of hygromycin/ml, 50 μg of kanamycin/ml, 30 μg of zeocin/ml, 30 μM lanthanum chloride (unless a different concentration is specified) (Sigma-Aldrich), and 30 μM cerium chloride (Sigma-Aldrich). All culturing glassware for experiments performed without lanthanides or copper was acid washed overnight in 1 M hydrochloric acid before use. All strains used in this study are listed in Table S1 in the supplemental material.
Genetic manipulations.
All gene knockout constructs were composed of assembled PCR products that were electroporated into M. buryatense 5GB1C, as described previously (4). Briefly, a construct containing the zeocin resistance gene flanked by two FLP recombination target (FRT) sites was assembled in the middle of ∼800 bp of the flanking region for each target gene deletion. The zeocin resistance gene was amplified from the zeocin resistance-sacB cassette described by Yan et al. (4). The resulting constructs were electroporated into M. buryatense 5GB1C. The knockout mutants were selected for by growth on media containing zeocin, which selects for colonies having undergone homologous recombination in the desired region, and confirmed by sequencing. The confirmed knockout strains were unmarked by electroporation of a plasmid, pFC25, containing the flippase gene (flp) to induce a site-specific recombination between the two FRT sites, thereby deleting the zeocin resistance gene (4). The final knockout strains contained unmarked gene deletions, with a single FRT site remaining, and were cured of pFC25. Primers for knockout assembly are listed in Table S2 in the supplemental material.
Strain FC31 containing PmxaF-xylE was obtained by conjugation of a pCM433-based suicide plasmid, pFC30, harboring the PmxaF-xylE construct between genes METBUDRAFT_2794 and METBUDRAFT_2795, into M. buryatense 5GB1C (4, 32) (see Table S1 in the supplemental material). Sucrose counterselection was used to unmark the strain (3). The PmxaF portion contained 300 bp of sequence upstream of the mxaF open reading frame. This sequence contained almost the entire intergenic region between the mxaF and mxaB open reading frames, except for the 18 bp immediately upstream of the mxaB open reading frame. The xylE gene was amplified from pCM130 (33). Conjugation was performed with Escherichia coli S17-1 λpir acting as the donor strain, as previously described (3). Construction of the plasmid was performed using Gibson assembly (34). Primers for Gibson assembly are listed in Table S2 in the supplemental material.
Construction of the complementation strains is described in the Supplemental Materials and Methods.
Catechol 2,3-dioxygenase activity assay.
Strain FC31, harboring PmxaF-xylE, was grown to stationary phase in media with different concentrations of supplemented lanthanum, as indicated below (see Fig. 1C). Whole-cell quantitative catechol 2,3-dioxygenase (XylE) activity assays were performed using a protocol adapted from Ali and Murrell (35). Cells were centrifuged, and cell pellets were resuspended to an optical density at 600 nm (OD600) of 0.5 in 50 mM Tris-HCl (pH 7.5). A catechol solution in 50 mM Tris-HCl (pH 7.5) was added to the cells to a final concentration of 1 mM (100 μl of total volume). Cells were assayed in a 96-well plate for catechol 2,3-dioxygenase activity by monitoring the absorbance at 375 nm to assay for the production of 2-hydroxymuconate semialdehyde. XylE activity was expressed in milliunits per minute per milligram of protein. Whole-cell XylE assays were performed successfully in other bacterial strains (36, 37).
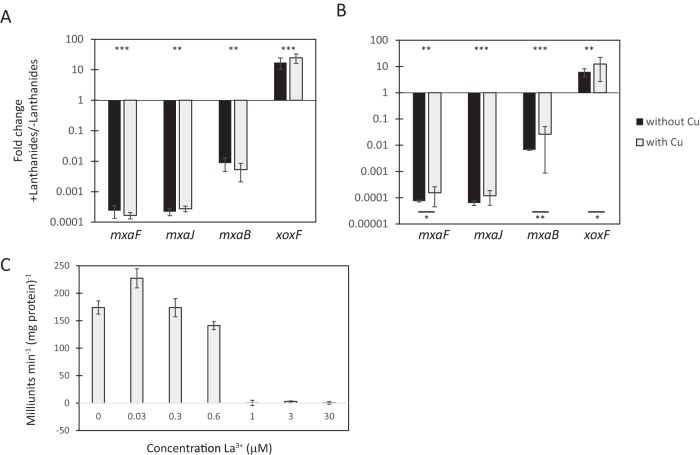
FIG 1.
Lanthanides regulate mxa and xoxF genes divergently at the transcriptional level. Real-time qRT-PCR was performed on RNA harvested from M. buryatense 5GB1C cells grown in the presence or absence of supplemental 30 μM lanthanum (A) or 30 μM cerium (B), with and without copper. The values shown represent the fold change in mxa and xoxF gene expression from wild-type M. buryatense 5GB1C cells grown with lanthanides compared to wild-type M. buryatense 5GB1C cells grown without lanthanides. All CT values were normalized to 16S rRNA. Multiple-way analysis of variance (ANOVA) was performed to determine significance of changes in gene expression levels (***, P < 0.001; **, P < 0.01; *, P < 0.05). Asterisks above the x axis indicate significance between with- and without-lanthanide conditions. Asterisks below the x axis indicate significance between the two data points connected by the bar. (C) Whole-cell catechol 2,3-dioxygenase activity from the mxaF promoter reporter strain FC31 (METBUDRAFT_2794::PmxaF-xylE) grown in the indicated concentrations of supplemental lanthanum. Data represent means from three replicates ± standard deviations.
RNA isolation.
Cells were grown to early stationary phase for harvesting RNA. A 1:10 volume of stop solution (5% buffer-saturated phenol in ethanol) was added to cells prior to harvesting the cells by centrifugation. Cells were resuspended in RNA extraction buffer (1:3 ratio of 5% cetrimonium bromide in 2.5 M NaCl to 0.1 M phosphate buffer [pH 5.8]) and lysed by bead beating with 0.1-mm zirconia-silica beads (Biospec Products) in 50% phenol–chloroform–isoamyl alcohol (at a 25:24:1 ratio), 0.5% sodium dodecyl sulfate, and 0.5% N-lauroylsarcosine sodium salt. After centrifugation, the aqueous layer was harvested and mixed with an equal volume of chloroform-isoamyl alcohol (24:1 ratio). The aqueous layer was harvested again, and RNA was precipitated with 150 mM sodium acetate, 1.5 mM MgCl2, and 50% isopropanol (all final concentrations) overnight at −80°C.
The precipitated RNA was harvested by centrifugation and treated with DNase I (Life Technologies) before purification with the RNeasy minikit and RNase-free DNase (Qiagen). We ensured that the harvested RNA was DNA free by using iScript reverse transcription supermix (Bio-Rad) with and without reverse transcriptase.
Real-time qRT-PCR assays.
cDNA was generated using 100 to 500 ng of isolated RNA as the template with the SensiFast cDNA synthesis kit (Bioline). PCRs consisted of the following: 400 μM primers, SensiFast SYBR No-Rox kit (Bioline), cDNA, and double-distilled H2O up to 10 μl of volume. The PCR mixtures were placed into LightCycler capillaries (Roche Diagnostics), and reactions were run using a LightCycler 2.0 (Roche Diagnostics). Threshold cycle (CT) values were determined using LightCycler software, version 3.5 (Roche), and all gene expression values were normalized to 16S rRNA CT values. All primers used for real-time quantitative reverse transcription-PCR (qRT-PCR) are listed in Table S2 in the supplemental material.
RESULTS
Lanthanides mediate the MDH switch in M. buryatense 5GB1C.
We first determined whether lanthanides act as a switch between the two MDH enzymes, XoxF and MxaFI, in a type I methanotroph. We employed M. buryatense strain 5GB1C as our model type I methanotroph, as it is genetically tractable, has a rapid doubling time, and is an industrially promising methanotroph (3, 4). In M. buryatense 5GB1C, only one xoxF gene is present in the genome, and XoxF and MxaF share 49% identity at the amino acid level. We tested whether two lanthanides, lanthanum and cerium, regulate the expression of the mxa operon (consisting of genes mxaF to mxaL) and the xoxF gene. Real-time qRT-PCR was performed to measure relative transcript abundances using RNA isolated from M. buryatense 5GB1C cultures grown in the presence or absence of supplemental lanthanides. As shown in Fig. 1A and B, the transcription of two genes in the mxa operon, mxaF and mxaJ, was significantly reduced (3,700- to 15,000-fold decrease) when grown in normal growth medium containing calcium in the presence of cerium or lanthanum. Conversely, transcription of the xoxF gene was induced 6- to 25-fold in the presence of lanthanides (Fig. 1A and B). Together, these results suggest that lanthanides control a transcriptional MDH switch, with the MxaFI MDH operating in the absence of lanthanides and XoxF acting as the predominant MDH when lanthanides are present.
The effect of lanthanides on mxa gene expression was almost completely attenuated in the presence of copper in M. trichosporium OB3b, a type II methanotroph (28). To determine if a similar phenomenon occurred in M. buryatense 5GB1C, the real-time qRT-PCR experiments were performed on cells grown in the absence of copper, a condition which has been shown to induce sMMO activity (4). The absence of copper, and therefore the presence of sMMO expression, did not significantly modify mxa or xoxF expression in experiments with lanthanum (Fig. 1A). Copper slightly attenuated the effect of cerium on mxaF expression and slightly enhanced the influence of cerium on xoxF expression (Fig. 1B), but these copper-mediated effects were small compared to the effect of adding cerium, in contrast to the results reported for a type II methanotroph (28). Our results suggest that in M. buryatense 5GB1C, the choice between the two MDH enzymes is independent of the presence of copper. Since no significant effects were observed with copper when studying the lanthanum-dependent changes in mxa operon and xoxF expression, only lanthanum was used in subsequent experiments.
The decrease of 3 to 4 orders of magnitude in mxa expression by lanthanides in the presence of calcium suggests that XoxF may be the dominant MDH in environmental settings, where lanthanides are relatively abundant (26, 27, 38). To test this hypothesis in a laboratory setting, an xylE reporter gene was fused to the promoter of mxaF to create an M. buryatense 5GB1C strain with PmxaF-xylE integrated into the chromosome at a region known to be transcriptionally silent (4). The resulting PmxaF reporter strain was grown in media supplemented with different concentrations of lanthanides, and catechol 2,3-dioxygenase (XylE) activity was assayed. As little as 1 μM supplemented lanthanum was sufficient to completely repress PmxaF, indicating that minute amounts of lanthanides in the environment would favor the utilization of XoxF over MxaFI (Fig. 1C).
MDH enzyme mutants display lanthanum-dependent growth defects.
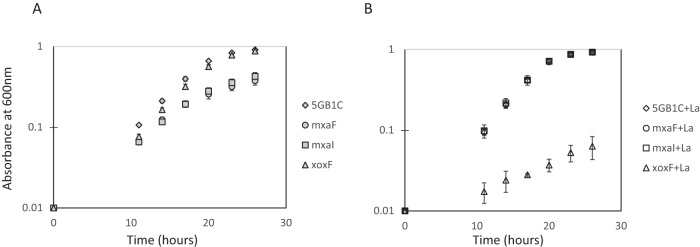
The differential regulation of the mxa operon and xoxF by lanthanides informed our construction of mutations in genes encoding the two MDH enzymes. A knockout mutant of xoxF was obtained by selecting for zeocin-resistant colonies in the absence of lanthanides. Conversely, knockout mutants in mxaF and mxaI, genes that encode the large and small subunits of the Mxa MDH, respectively, were selected on zeocin-containing media in the presence of lanthanum. The final versions of these mutants were unmarked, as described in Materials and Methods. Growth of these mutants was tested in the presence or absence of supplemental lanthanum. The ΔxoxF mutant displayed a wild-type growth rate in the absence of lanthanum, when the MxaFI enzyme was maximally expressed. However, we observed a marked growth defect (about a 70% decrease in growth rate) when ΔxoxF was grown in the presence of lanthanum, a condition under which mxa expression was significantly reduced (Fig. 2; Table 1). Likewise, the ΔmxaF and ΔmxaI mutants grew as well as wild-type M. buryatense 5GB1C did in the presence of lanthanum, when XoxF was maximally expressed (Fig. 2B) but displayed about a 20% decreased growth rate in the absence of supplemental lanthanum, when XoxF expression decreased by an order of magnitude (Fig. 2A; Table 1). Complementation of all three MDH mutants restored growth rates to wild-type levels, regardless of the presence or absence of lanthanum in the cultivation medium (see Table S3 in the supplemental material). These growth rate results are in line with the real-time qRT-PCR results presented above and further support the hypothesis that the MxaFI enzyme is required for MDH activity in the absence of lanthanides and that XoxF is required for MDH activity in the presence of lanthanides.
FIG 2.
Analysis of MDH (mxaF, mxaI, and xoxF) mutants. Growth curves for MDH mutant strains and wild-type M. buryatense 5GB1C grown without (A) and with (B) supplemental 30 μM lanthanum (La). Data represent means from three replicates ± standard deviations.
TABLE 1.
Doubling times of MDH mutants
| Strain | Doubling timea (h) |
|
|---|---|---|
| Without La3+ | With La3+ | |
| WT 5GB1Cb | 3.17 ± 0.13 | 2.84 ± 0.15 |
| ΔmxaF mutant | 3.87 ± 0.45 | 2.88 ± 0.24 |
| ΔmxaI mutant | 3.87 ± 0.16 | 2.86 ± 0.12 |
| ΔxoxF mutant | 2.93 ± 0.07 | 10.3 ± 6.6 |
Doubling times represent the means for three technical replicates with standard deviations. Doubling times were calculated from three time points during the exponential phase of growth.
WT, wild-type Methylomicrobium buryatense.
MxaB partially mediates the lanthanide switch.
The mxa operon expression is known to be activated by at least two two-component systems, MxcQE and MxbDM, and an orphan response regulator, MxaB, in M. extorquens AM1 (20–23). The M. buryatense genome lacks homologs of genes encoding MxcQE or MxbDM, but a homolog of the mxaB gene is located upstream of the mxa operon and is divergently transcribed (39). Real-time qRT-PCR was performed to determine whether mxaB itself was regulated by lanthanides. Similar to gene expression results with mxaF and mxaJ, mxaB expression was significantly reduced in the presence of lanthanides (38- to 189-fold decrease) (Fig. 1A and B). This decrease in transcript abundance was independent of the presence of copper when lanthanum was present but was slightly attenuated by copper when cerium was added to the cultivation medium (Fig. 1A and B).
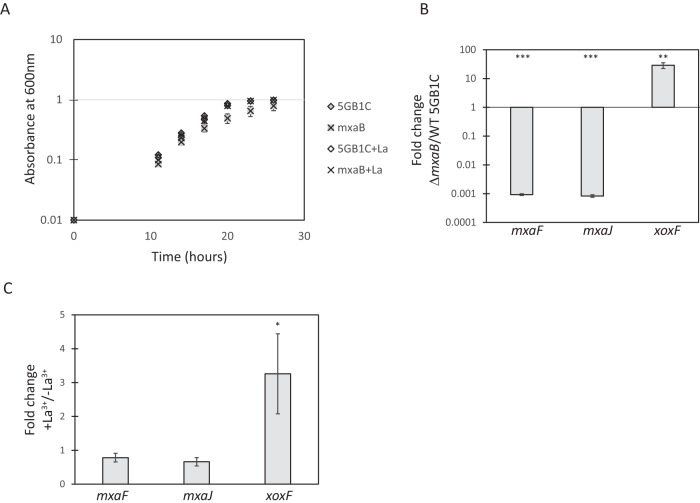
We next investigated whether MxaB was involved in regulating mxa operon transcription in M. buryatense 5GB1C by creating a deletion mutation in mxaB. The ΔmxaB mutant grew at a similar rate to wild-type M. buryatense 5GB1C in the presence of lanthanum, but a slight growth defect was observed in the absence of lanthanum (Fig. 3A; Table 2). This phenotype is similar to the phenotypes of the ΔmxaF and ΔmxaI strains, indicating that MxaB may be required for obtaining a functional MxaFI enzyme. Real-time qRT-PCR was performed to determine MDH gene expression levels in the ΔmxaB mutant. Compared to wild-type M. buryatense 5GB1C, when the ΔmxaB mutant was grown in the absence of lanthanum, mxaF and mxaJ expression levels were reduced by 1,000- and 1,200-fold, respectively (Fig. 3B). Accordingly, ΔmxaB cells harvested during growth with or without lanthanum had similar levels of mxaF and mxaJ expression (Fig. 3C). Together, these results indicate that mxaB expression is regulated by lanthanum and that its gene product activates mxa expression.
FIG 3.
Lanthanide switch is mediated in part by MxaB. (A) Growth curves for the ΔmxaB mutant and for wild-type M. buryatense 5GB1C grown without and with supplemental 30 μM lanthanum (La). (B) Real-time qRT-PCR was performed on RNA harvested from the ΔmxaB mutant and wild-type M. buryatense 5GB1C cells grown in the absence of lanthanum. Results shown represent the fold change in gene expression in the ΔmxaB mutant compared to wild-type M. buryatense 5GB1C grown without lanthanum. (C) Real-time qRT-PCR was performed on RNA harvested from the ΔmxaB mutant grown with and without supplemental 30 μM lanthanum. Results shown represent the fold change in gene expression in ΔmxaB cells grown with lanthanum compared to gene expression in ΔmxaB grown without lanthanum. Unpaired t tests were used to determine significance (***, P < 0.001; **, P < 0.01; *, P < 0.05) between gene expression levels in panels B and C. Data represent means from three replicates ± standard deviations.
TABLE 2.
Doubling times of ΔmxaB and ΔxoxFS mutants
| Strain | Doubling timea (h) |
|
|---|---|---|
| Without La3+ | With La3+ | |
| WT 5GB1Cb | 2.85 ± 0.18 | 2.79 ± 0.02 |
| ΔmxaB mutant | 3.07 ± 0.19 | 2.71 ± 0.08 |
| ΔxoxFS mutant | 2.71 ± 0.13 | 2.77 ± 0.03 |
Doubling times represent the means for three technical replicates with standard deviations. Doubling times were calculated from three time points during the exponential phase of growth.
WT, wild-type Methylomicrobium buryatense.
In M. extorquens AM1, the two-component system MxbDM acts to increase mxa expression while also decreasing xoxF expression (17). We found that MxaB functions similarly in M. buryatense 5GB1C by activating transcription of mxa genes and decreasing xoxF transcription. In the ΔmxaB strain, xoxF expression was enhanced 29-fold compared to the wild-type M. buryatense 5GB1C strain when both strains were grown in the absence of lanthanum (Fig. 3B). Transcription of the xoxF gene still exhibited a significant lanthanum-dependent increase in the ΔmxaB strain. However, this effect was attenuated in the ΔmxaB strain (3-fold increase) compared to the wild-type strain (∼20-fold increase) (Fig. 1A and 3C).
Complementation of the ΔmxaB mutant restored the growth rate to wild-type levels, regardless of the presence or absence of lanthanum in the cultivation medium (see Table S3 in the supplemental material). The complemented strain also restored wild-type lanthanide-mediated regulation of mxaF, mxaJ, and xoxF gene transcription (see Fig. S1 in the supplemental material).
Suppressors of the ΔxoxF mutant phenotype increase mxa expression in the presence of lanthanum.
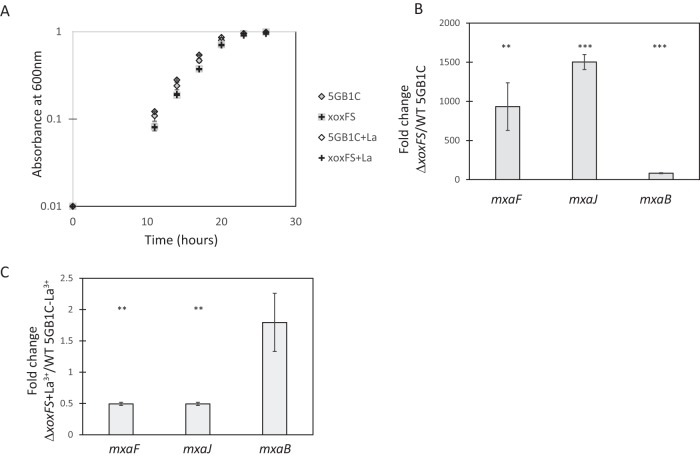
To determine whether additional components might be present that function in the lanthanide-mediated MDH switch, spontaneous mutants that suppressed the ΔxoxF mutant growth phenotype were isolated, as follows. We noted that, when grown for prolonged periods of time with lanthanum, many ΔxoxF cultures exhibited improved growth. Single colonies were isolated from these cultures and retested for growth in media supplemented with lanthanum. These isolates were grown in the absence of lanthanum before transfer to media supplemented with lanthanum to ensure that adaptation was not responsible for the improved growth. Six of eight of these isolates maintained robust growth in the presence of lanthanum, and one isolate was selected for further study. The ΔxoxF suppressor strain, named ΔxoxFS, displayed a wild-type growth rate regardless of the presence or absence of lanthanum (Fig. 4A; Table 2). This is in contrast with the results described above for the original ΔxoxF strain, which displayed a marked growth defect in the presence of lanthanum (Fig. 2B; Table 1).
FIG 4.
Suppressor mutation in ΔxoxFS mutant permits mxa expression in the presence of lanthanum (La). (A) Growth curves for ΔxoxFS strain and wild-type M. buryatense 5GB1C grown without and with supplemental 30 μM lanthanum. (B) Real-time qRT-PCR was performed on RNA harvested from the ΔxoxFS suppressor strain and wild-type M. buryatense 5GB1C grown in the presence of 30 μM supplemental lanthanum. Results represent the fold change in mxa gene expression in the ΔxoxFS strain compared to wild-type M. buryatense 5GB1C grown with lanthanum. (C) Real-time qRT-PCR was performed on RNA harvested from the ΔxoxFS suppressor strain grown in the presence of 30 μM supplemental lanthanum and wild-type M. buryatense 5GB1C grown without lanthanum. Results represent the fold change in mxa gene expression in ΔxoxFS grown with lanthanum compared to wild-type M. buryatense 5GB1C grown without lanthanum. Unpaired t tests were used to determine significance (***, P < 0.001) between gene expression levels in panels B and C. Data represent means from three replicates ± standard deviations.
We postulated that the ΔxoxFS variant was able to grow in the presence of lanthanum because the switch to reduce mxa expression under these conditions was nonfunctional in this strain. Indeed, mxaF and mxaJ expression levels were increased 900-fold and 1,500-fold, respectively, in the ΔxoxFS strain compared to wild-type M. buryatense 5GB1C when grown with lanthanum (Fig. 4B). This resulted in a comparable level of mxaF and mxaJ transcripts when the ΔxoxFS strain grown with lanthanum was compared to wild-type M. buryatense 5GB1C grown in the absence of lanthanum (Fig. 4C). We found that mxaB expression in the ΔxoxFS strain followed a similar pattern to that of the mxaF and mxaJ genes (Fig. 4B and C). The MxaB regulator present in the ΔxoxFS strain grown with lanthanum may be increasing mxaF to mxaL operon expression under these conditions. Together, the above results indicate that the lanthanide-mediated MDH switch is broken in the ΔxoxFS strain, permitting mxa expression in the presence of lanthanides.
We sought to identify the causal mutation in ΔxoxFS that allowed for lanthanide-independent expression of the mxa genes. The obvious candidates were (i) mutations in the shared promoter region of mxaB and mxaF that rendered the promoters unresponsive to lanthanide or (ii) mutations in the mxaB gene that allowed its product to be constitutively active. However, both of these regions had wild-type sequences in the ΔxoxFS strain (data not shown). These results suggest that there are additional control elements acting in the regulatory pathway upstream of MxaB and that these regulators function in a lanthanum-responsive manner to control mxa and xoxF transcription.
DISCUSSION
In this study, we demonstrated the existence of a lanthanide-mediated switch controlling the transcriptional expression of genes encoding two MDHs, the xoxF gene and the mxa operon, in a type I methanotroph. Similar results have been shown previously in the type II methanotroph M. trichosporium OB3b (28). However, in contrast to the results with M. trichosporium OB3b, copper does not significantly affect this switch, and lanthanides are the dominant factor determining which MDH is expressed, even in the presence of excess calcium. It has been suggested that, in M. trichosporium OB3b, XoxF can only serve as the MDH under conditions in which sMMO is expressed and that growth with pMMO would require MxaFI (28). However, in the type I methanotroph M. buryatense 5GB1C, it is clear that either MDH can support growth when either pMMO or sMMO is expressed. If an MDH forms a supercomplex with the pMMO, as has been suggested (28, 40), both MxaFI and XoxF must be able to form such a complex.
In the presence of 95 μM calcium in the cultivation medium, as little as 1 μM supplemental lanthanum was sufficient to completely abolish mxa gene transcription (Fig. 1C), showing that even with a major imbalance in the two metals, XoxF is the preferred MDH. Many methanotrophs, including M. buryatense isolates, have been isolated from sediment samples. In freshwater sediment samples collected from standard sediment collections, lanthanum concentrations have been reported to be around 42 μg lanthanum per g sediment (38). Lanthanum is largely insoluble under most environmental conditions; however, the sedimentary lanthanum concentrations found are approximately 300 times the concentration of lanthanum shown to inhibit PmxaF transcription (0.14 μg lanthanum per g cultivation medium) (Fig. 1C). Therefore, the lanthanide concentrations found in the environment are likely sufficient to repress mxa transcription, and XoxF is likely the major functional MDH in M. buryatense in the environment. Metaproteomic analyses of environmental samples from the phyllosphere have shown that XoxF is expressed in naturally occurring methylotrophs that also encode mxaFI in their genomes (41, 42).
The growth defect of the ΔxoxF mutant cultured in the presence of lanthanum was much more pronounced than the growth defects exhibited by the mxa mutants (ΔmxaF, ΔmxaI, and ΔmxaB) cultured in the absence of supplemental lanthanum (Tables 1 and 2). From preliminary RNA sequencing results, we still observed a moderate number of xoxF transcript reads in cells grown without lanthanum (D. A. C. Beck, F. Chu, A. Gilman, and M. E. Lidstrom, unpublished results). However, we know that the mxa operon expression is completely suppressed in the presence of 1 μM lanthanum (Fig. 1C). Therefore, it is likely that XoxF is still being produced, albeit at significantly reduced levels, in the absence of supplemental lanthanum. Moreover, the ΔmxaB mutant had even more xoxF transcripts than wild-type cells in the absence of lanthanum (Fig. 3B) and, correspondingly, had a higher growth rate than the ΔmxaF or ΔmxaI mutants (Tables 1 and 2). In this study, all glassware was acid washed prior to any experiments performed without lanthanides, and all medium components were of the highest purity available. However, lanthanides are a component of some types of glass, and there may have been minute amounts present from this source or from contaminants in the media that allowed some XoxF function and therefore some growth in the Δmxa mutants (27). Alternatively, it is possible that another metal present, such as Ca2+, was able to partially support XoxF activity in the absence of lanthanum. Our results demonstrated that the ΔxoxF mutant had a more severe growth defect when grown in the presence of lanthanum than Δmxa mutants when grown in the absence of lanthanum, and these results further support the conclusion that XoxF is the major functional MDH in M. buryatense.
The lanthanide MDH switch is mediated in part by the orphan response regulator MxaB, which has been shown to increase expression of the mxa operon in other methylotrophs (21, 22). No histidine kinase has been discovered to be responsible for MxaB phosphorylation, but we hypothesize the presence of a lanthanide-responsive histidine kinase that regulates MxaB activity. Lanthanides can be transported into the periplasm of Gram-negative cells to activate a regulatory cascade, as an engineered periplasmic histidine kinase has been shown to be responsive to lanthanides in Escherichia coli (43).
We hypothesize that additional regulatory genes are involved in the lanthanide-mediated MDH switch. In the ΔmxaB mutant, the xoxF gene is still partially induced by lanthanum, indicating that there is an additional factor controlling its transcription. Moreover, the ΔxoxFS mutant, which bears a broken lanthanide MDH switch, does not harbor mutations in the mxa operon promoter or the MxaB coding region. We postulate that the responsible mutation lies in a gene that is upstream of MxaB in the lanthanide-regulatory cascade. In the ΔxoxFS mutant, we demonstrated that mxaB expression in the presence of lanthanum was similar to that of wild-type cells grown in the absence of lanthanum (Fig. 4C). Therefore, the regulatory cascade was broken upstream of mxaB expression in the ΔxoxFS mutant. We propose the existence of a lanthanum-binding regulatory protein that likely activates MxaB and other factors in the absence of lanthanum, and we propose that MxaB in turn activates the mxa operon and represses xoxF transcription.
In conclusion, we demonstrate that M. buryatense 5GB1C has a lanthanide-mediated switch regulating the choice between the two MDH enzymes, MxaFI and XoxF. This switch is mediated in part by the response regulator MxaB. Finally, the gene expression and MDH mutant growth rate analyses presented in this work lead us to postulate that XoxF is the major operational MDH in this type I methanotroph.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Chistoserdova for helpful discussions and critical reading of the manuscript. We thank M. Hendershott, D. A. C. Beck, A. Gilman, and A. W. Puri for useful discussions. We thank T. C. Franklin for help with statistical analyses.
This work was supported by a grant from DOEARPA-E (DE-AR0000350) to Mary E. Lidstrom.
The funding agency had no role in experimental design, data collection or interpretation, or the decision to submit this work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00959-15.
REFERENCES
- 1.Kalyuzhnaya MG, Puri AW, Lidstrom ME. 2015. Metabolic engineering in methanotrophic bacteria. Metab Eng 29:142–152. doi: 10.1016/j.ymben.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Haynes CA, Gonzalez R. 2014. Rethinking biological activation of methane and conversion to liquid fuels. Nat Chem Biol 10:331–339. doi: 10.1038/nchembio.1509. [DOI] [PubMed] [Google Scholar]
- 3.Puri AW, Owen S, Chu F, Chavkin T, Beck DA, Kalyuzhnaya MG, Lidstrom ME. 2015. Genetic tools for the industrially promising methanotroph Methylomicrobium buryatense. Appl Environ Microbiol 81:1775–1781. doi: 10.1128/AEM.03795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan X, Chu F, Puri AW, Fu Y, Lidstrom ME. 22 January 2016. Electroporation-based genetic manipulation in type I methanotrophs. Appl Environ Microbiol doi: 10.1128/AEM.03724-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol Rev 34:496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 6.Chistoserdova L. 2011. Modularity of methylotrophy, revisited. Environ Microbiol 13:2603–2622. doi: 10.1111/j.1462-2920.2011.02464.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi DW, Kunz RC, Boyd ES, Semrau JD, Antholine WE, Han JI, Zahn JA, Boyd JM, de la Mora AM, DiSpirito AA. 2003. The membrane-associated methane monooxygenase (pMMO) and pMMO-NADH:quinone oxidoreductase complex from Methylococcus capsulatus Bath. J Bacteriol 185:5755–5764. doi: 10.1128/JB.185.19.5755-5764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen AK, Gerdes K, Murrell JC. 1997. Copper-dependent reciprocal transcriptional regulation of methane monooxygenase genes in Methylococcus capsulatus and Methylosinus trichosporium. Mol Microbiol 25:399–409. doi: 10.1046/j.1365-2958.1997.4801846.x. [DOI] [PubMed] [Google Scholar]
- 9.Chistoserdova L, Lidstrom ME. 1997. Molecular and mutational analysis of a DNA region separating two methylotrophy gene clusters in Methylobacterium extorquens AM1. Microbiology 143:1729–1736. [DOI] [PubMed] [Google Scholar]
- 10.Chistoserdova L. 1996. Metabolism of formaldehyde in M. extorquens AM1: molecular genetic analysis and mutant characterization. In Lidstrom M, Tabita FR (ed), Microbial growth on C1 compounds. Kluwer Academic Publishers, Dordrecht, the Netherlands. [Google Scholar]
- 11.Schmidt S, Christen P, Kiefer P, Vorholt JA. 2010. Functional investigation of methanol dehydrogenase-like protein XoxF in Methylobacterium extorquens AM1. Microbiology 156:2575–2586. doi: 10.1099/mic.0.038570-0. [DOI] [PubMed] [Google Scholar]
- 12.Harms N, Ras J, Koning S, Reijnders WNM, Stouthamer AH, van Spanning RJM. 1996. Genetics of C1 metabolism regulation in Paracoccus denitrificans; microbial growth on C1 compounds. Kluwer Academic Publishers, Dordrecht, the Netherlands. [Google Scholar]
- 13.Nakagawa T, Mitsui R, Tani A, Sasa K, Tashiro S, Iwama T, Hayakawa T, Kawai K. 2012. A catalytic role of XoxF1 as La3+-dependent methanol dehydrogenase in Methylobacterium extorquens strain AM1. PLoS One 7:e50480. doi: 10.1371/journal.pone.0050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitriyanto NA, Fushimi M, Matsunaga M, Pertiwiningrum A, Iwama T, Kawai K. 2011. Molecular structure and gene analysis of Ce3+-induced methanol dehydrogenase of Bradyrhizobium sp. MAFF211645. J Biosci Bioeng 111:613–617. doi: 10.1016/j.jbiosc.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Hibi Y, Asai K, Arafuka H, Hamajima M, Iwama T, Kawai K. 2011. Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J Biosci Bioeng 111:547–549. doi: 10.1016/j.jbiosc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Keltjens JT, Pol A, Reimann J, Op den Camp HJ. 2014. PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98:6163–6183. doi: 10.1007/s00253-014-5766-8. [DOI] [PubMed] [Google Scholar]
- 17.Skovran E, Palmer AD, Rountree AM, Good NM, Lidstrom ME. 2011. XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. J Bacteriol 193:6032–6038. doi: 10.1128/JB.05367-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony C. 2004. The quinoprotein dehydrogenases for methanol and glucose. Arch Biochem Biophys 428:2–9. doi: 10.1016/j.abb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Chistoserdova L, Chen SW, Lapidus A, Lidstrom ME. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J Bacteriol 185:2980–2987. doi: 10.1128/JB.185.10.2980-2987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer AL, Morris CJ, Lidstrom ME. 1997. Molecular analysis of mxbD and mxbM, a putative sensor-regulator pair required for oxidation of methanol in Methylobacterium extorquens AM1. Microbiology 143 (Part 5):1737–1744. [DOI] [PubMed] [Google Scholar]
- 21.Springer AL, Auman AJ, Lidstrom ME. 1998. Sequence and characterization of mxaB, a response regulator involved in regulation of methanol oxidation, and of mxaW, a methanol-regulated gene in Methylobacterium extorquens AM1. FEMS Microbiol Lett 160:119–124. doi: 10.1111/j.1574-6968.1998.tb12900.x. [DOI] [PubMed] [Google Scholar]
- 22.Morris CJ, Lidstrom ME. 1992. Cloning of a methanol-inducible moxF promoter and its analysis in moxB mutants of Methylobacterium extorquens AM1rif. J Bacteriol 174:4444–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu HH, Janka JJ, Viebahn M, Hanson RS. 1995. Nucleotide sequence of the mxcQ and mxcE genes, required for methanol dehydrogenase synthesis in Methylobacterium organophilum XX: a two-component regulatory system. Microbiology 141:2543–2551. [DOI] [PubMed] [Google Scholar]
- 24.Pol A, Barends TR, Dietl A, Khadem AF, Eygensteyn J, Jetten MS, Op den Camp HJ. 2014. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 16:255–264. doi: 10.1111/1462-2920.12249. [DOI] [PubMed] [Google Scholar]
- 25.Skovran E, Martinez-Gomez NC. 2015. Microbiology: just add lanthanides. Science 348:862–863. [DOI] [PubMed] [Google Scholar]
- 26.Haxel GB, Hedrick JB, Orris GJ. 2002. Rare earth elements: critical resources for high technology. U.S. Geological Survey, Washington, DC. http://pubs.usgs.gov/fs/2002/fs087-02/.
- 27.Van Gosen BS, Verplanck PL, Long KR, Gambogi J, Seal RR II. 2014. The rare-earth elements: vital to modern technologies and lifestyles. U.S. Geological Survey, Washington, DC. http://pubs.usgs.gov/fs/2014/3078/.
- 28.Farhan Ul Haque M, Kalidass B, Bandow N, Turpin EA, DiSpirito AA, Semrau JD. 2015. Cerium regulates expression of alternative methanol dehydrogenases in Methylosinus trichosporium OB3b. Appl Environ Microbiol 81:7546–7552. doi: 10.1128/AEM.02542-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalyuzhnaya MG, Yang S, Rozova ON, Smalley NE, Clubb J, Lamb A, Gowda GA, Raftery D, Fu Y, Bringel F, Vuilleumier S, Beck DA, Trotsenko YA, Khmelenina VN, Lidstrom ME. 2013. Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat Commun 4:2785. doi: 10.1038/ncomms3785. [DOI] [PubMed] [Google Scholar]
- 30.Kemp MB, Quayle JR. 1966. Microbial growth on C1 compounds: incorporation of C1 units into allulose phosphate by extracts of Pseudomonas methanica. Biochem J 99:41–48. doi: 10.1042/bj0990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp MB, Quayle JR. 1967. Microbial growth on C1 compounds: uptake of [14C]formaldehyde and [14C]formate by methane-grown Pseudomonas methanica and determination of the hexose labelling pattern after brief incubation with [14C]methanol. Biochem J 102:94–102. doi: 10.1042/bj1020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx CJ. 2008. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes 1:1. doi: 10.1186/1756-0500-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx CJ, Lidstrom ME. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065–2075. doi: 10.1099/00221287-147-8-2065. [DOI] [PubMed] [Google Scholar]
- 34.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 35.Ali H, Murrell JC. 2009. Development and validation of promoter-probe vectors for the study of methane monooxygenase gene expression in Methylococcus capsulatus Bath. Microbiology 155:761–771. doi: 10.1099/mic.0.021816-0. [DOI] [PubMed] [Google Scholar]
- 36.Curcic R, Dhandayuthapani S, Deretic V. 1994. Gene expression in mycobacteria: transcriptional fusions based on xylE and analysis of the promoter region of the response regulator mtrA from Mycobacterium tuberculosis. Mol Microbiol 13:1057–1064. doi: 10.1111/j.1365-2958.1994.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 37.Olson JW, Maier RJ. 2002. Molecular hydrogen as an energy source for Helicobacter pylori. Science 298:1788–1790. doi: 10.1126/science.1077123. [DOI] [PubMed] [Google Scholar]
- 38.Bentlin FRS, Pozebon D. 2010. Direct determination of lanthanides in environmental samples using ultrasonic nebulization and ICP OES. J Braz Chem Soc 21:627–634. doi: 10.1590/S0103-50532010000400007. [DOI] [Google Scholar]
- 39.Khmelenina VN, Beck DA, Munk C, Davenport K, Daligault H, Erkkila T, Goodwin L, Gu W, Lo CC, Scholz M, Teshima H, Xu Y, Chain P, Bringel F, Vuilleumier S, Dispirito A, Dunfield P, Jetten MS, Klotz MG, Knief C, Murrell JC, Op den Camp HJ, Sakai Y, Semrau J, Svenning M, Stein LY, Trotsenko YA, Kalyuzhnaya MG. 2013. Draft genome sequence of Methylomicrobium buryatense strain 5G, a haloalkaline-tolerant methanotrophic bacterium. Genome Announc 1:e00053-13. doi: 10.1128/genomeA.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culpepper MA, Rosenzweig AC. 2014. Structure and protein-protein interactions of methanol dehydrogenase from Methylococcus capsulatus (Bath). Biochemistry 53:6211–6219. doi: 10.1021/bi500850j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knief C, Delmotte N, Chaffron S, Stark M, Innerebner G, Wassmann R, von Mering C, Vorholt JA. 2012. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J 6:1378–1390. doi: 10.1038/ismej.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA. 2009. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H, Deng X, Bosscher M, Ji Q, Jensen MP, He C. 2013. Engineering bacterial two-component system PmrA/PmrB to sense lanthanide ions. J Am Chem Soc 135:2037–2039. doi: 10.1021/ja312032c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.