Abstract
Pituitary tumor transforming gene (PTTG) is a novel oncogene that is expressed at higher level in most of the tumors. PTTG overexpression correlates with lymph node infiltration and a higher degree of tumor recurrence in breast cancer. However, the cellular functions and precise signals elicited by PTTG in breast cancer are not fully understood. Here, we established a breast cancer cell line which stably overexpressed PTTG. In vitro experiments showed that overexpression of PTTG in MCF-7 cells was associated with enhanced cell migration and invasion as well as EMT. Our results also demonstrated that PTTG overexpression correlated with elevated EMMPRIN level, which mediated the enhanced cell migration, invasion and EMT. Moreover, our findings suggested that PTTG enhances metastatic potential of breast cancer cells by inducing EMMPRIN through activating FAK/Akt/mTOR pathway. Our findings may lead to a better understanding of the biological effect of PTTG and provide mechanistic insights for developing potential therapeutic strategies for inhibiting the invasion and metastasis of breast cancer.
Keywords: PTTG, breast cancer, metastasis, EMMPRIN, FAK
Introduction
Breast cancer, accounting for 21% of all new cancer diagnoses in females, is the third most frequent cancer worldwide and one of the most frequent causes of cancer mortality in females in developed countries [1,2]. Survival rates have been steadily extending over the past 5 decades, primarily due to early detection, precise resection using wide margins, and systematic adjuvant therapy. However, recurrence and metastasis remains the leading cause of breast cancer-related mortality [3,4].
Numerous studies have showed that oncogene plays an important role on the metastatic behavior of tumor. Pituitary tumor-transforming gene (PTTG), first successfully cloned from a rat pituitary tumor by Pei etc [5], has been reported to regulates chromosomal segregation under normal physiological conditions [6,7]. PTTG protein is expressed at higher than normal levels in several tumors, including those of the pituitary [8], thyroid [9], colon [10], lung [11], uterine [12], liver [13], brain [14], ovary [15,16], testis [15], renal cell carcinoma [17] and breast [18], as well as in hematopoietic neoplasms [19]. An early study has showed that overexpression of PTTG is capable of stimulating cell proliferation in HEK293 and inducing cellular transformation in vitro using NIH3T3 and HEK293 cells as well as promotes tumor development in nude mice [20]. Kakar et al. later reported that silencing PTTG gene using siRNA in the lung cancer cell line H1299 showed inhibited cell proliferation and reduced xenograft tumor formation in nude mice [21]. PTTG has also been showed to function in a variety of cellular activities, such as mitosis [22,23], cell cycle progression [7], DNA repair [24] and secretion and expression of basic fibroblast growth factor (bFGF) [25] and vascular endothelial growth factor (VEGF) [26]. Furthermore, increase in expression levels of PTTG have been found to correlate with increased tumor invasiveness in human pituitary tumors with hormone overproduction [26], and with the degree of malignancy, pathogenesis and/or progression of colorectal and thyroid tumors [10,27]. Based on clinical, in vitro and in vivo evidence, PTTG has been identified as one of eight signature genes that is associated with tumor metastasis and is up regulated in human primary solid tumors [27,28]. In terms of breast cancer, PTTG level has been found to correlate with tumor size, histologic grade, the presence of lymph node metastasis, and tumor node metastasis (TNM) stage [29]. However, the contribution of PTTG to these aspects of neoplasia remains unclear.
Extracellular matrix metalloprotease inducer (EMMPRIN/basigin), also known as CD147 [30,31], is a transmembrane glycosylated member of the immunoglobulin superfamily molecules made up of a single transmembrane domain required for counter receptor binding activity and a short cytoplasmic domain known to interact with Cav-1 [32,33]. EMMPRIN is expressed at the surface of human tumor cells or is released by these cells through microvesicle shedding, then increasing tumor invasion by inducing matrix metalloproteinases (MMPs) synthesis of the surrounding stromal cells [34]. It has been found that EMNMPRIN is overexpressed in breast cancer, hepatoma, esophageal squamous cell carcinoma, colorectal cancer and ovarian cancer [35-37] and also serves as prognostic marker in some tumor entities including prostate cancer, colorectal cancer, bladder cancer and breast cancer [38-40]. Given the important functional role of EMMPRIN in the metastasis, it has the potential to be a target for novel therapeutic agent. The present study is aimed to investigate the role of PTTG in metastatic behavior of breast cancer cells and characterized PTTG-elicited signal transduction. Human breast cancer cell line MCF-7 was used to evaluate the effect of PTTG overexpression on cell proliferation, migration and invasion. Our results showed that PTTG gene is strongly associated with breast cancer metastasis. Moreover, our findings suggested that PTTG enhances metastatic potential of breast cancer cells by inducing EMMPRIN through activating FAK/Akt/mTOR pathway.
Materials and methods
Cell lines and cultures
Human breast cancer MCF-7 cell lines, obtained from the Shanghai Institutes for Biological Science (Shanghai, China), were cultured in RPMI-1640 medium (Gibco, MD, USA) supplemented with 10% fetal bovine serum (Gibco, MD, USA ) in a 5% CO2-humidified incubator.
Generation of plasmid constructs and establishment of PTTG-ovexpression cell lines
To generate PTTG overexpression vectors, CDS region of PTTG-coding sequences were obtained by RT-PCR and cloned into pEGFP-N1 vector (Takara Biomedical Technology Co., Ltd., Beijing, China). The resulting plasmid was named pEGFP-N1-PTTG. MCF-7 cells were transfected with pEGFP-N1-PTTG vector to induce excessive PTTG expression or pEGFP-N1 vector to generate stable clones expressing PTTG constitutively as control. The resulting cell lines were named MCF-7/pEGFP-N1-PTTG and MCF-7/pEGFP-N1-Vector, respectively. Two days after transfection, G418 solution was added to cells for selection of stable clones (MCF-7/pEGFP-N1-PTTG and MCF-7/pEGFP-N1-Vector cells). Stable clones were selected and maintained in medium containing G418.
Quantitative RT-PCR analysis (qRT-PCR)
Total RNA was extract from cells using RNA simple Total RNA Kit (TIANGEN Co., Beijing, China) and 3 µg of RNA was converted into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR was carried out in the MX3000p PCR system (Stratagene, Europe). Reaction was performed using KAPASYBR Green fast PCR master mix PCR kit. The primers used for PTTG, EMMPRIN, Slug, Snail and Twist detection were synthesized based on published sequence [41-43]. Data were analyzed using the comparative ΔCt method (ABPrism software, Applied Biosystems, Foster City, CA) using GAPDH as an internal normalization control.
Western blot analysis
Western blot analysis was performed using a standard protocol. The cell lysate (30-50 μg) samples were mixed with 6×sample buffer, boiled for 5 minutes, electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel and thereafter transferred to PVDF membranes. The membrane was then blocked in PBS containing 5% bovine serum albumin (BSA) for 1 hour at room temperature. The membranes were incubated with specific primary antibodies in Tris-buffered saline at 4°C overnight. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (Beyotime Institute of Biotechnology, Shanghai, China). ECL detection reagent (7Sea Biotech., Shanghai, China) was used for blot detection according to the manufacturer’s instructions.
Wound healing assay
Each well of 24-well tissue culture plate was seeded with cells to a final density of 100,000 cells per well and these cells were maintained at 37°C and 5% CO2 for 24 hours to permit cell adhesion and the formation of a confluent monolayer. These confluent monolayers were then scored with a sterile pipette tip to leave a scratch of approximately 0.4-0.5 mm in width. Cell surface was then washed with serum-free culture medium for three times to remove dislodged cells. Wound closure was monitored by collecting digitized images at 0, 12, and 24 hours after the scratch was performed. Digitized images were captured with an inverted microscope (MOTIC CHINA GROUP CO., Xiamen, China) and digital camera (Nikon, Tokyo, Japan). The digitized images were then analyzed using Image-J software.
Invasion assay
24-well Transwells coated with Matrigel (8-μm pore size; BD Biosciences, San Jose, California) were used for cell invasion assays. Equal numbers (1 × 105) of wells were plated onto separate well. Cells were starved overnight in serum-free medium, trypsinized and washed three times in DMEM containing 1% FBS. A total of 1×105 cells were then resuspended in 500 μl DMEM containing 1% FBS and added to the upper chamber, while MEM with 10% FBS was added to the lower chamber as chemoattractant. For the control, medium containing 1% FBS was added to the lower chamber. After 24 hours of incubation, the Matrigel and the cells remaining in the upper chamber were removed by cotton swabs. The cells on the lower surface of the membrane were fixed in formaldehyde and stained with hematoxylin staining solution. The cells in at least five random microscopic fields (magnification, ×200) were counted and photographed.
Immunoflorescence staining and confocal image
Cells were grown on glass coverslip until 80% confluent, and then fixed with 4% formaldehyde solution. Transfected and untransfected cells were then incubated with rabbit monoclonal anti-β-catenin antibody (Cell Signaling Technology, Inc., Beverly, MA, USA, 1:100) for detection of specific protein, respectively. Next, the cells were incubated with Cy3-labeled goat anti-rabbit antibody (Beyotime Institute of Biotechnology, Shanghai, China, 1:100) at room temperature for 1 hour in the dark, followed by incubation with DAPI (Biosharp Biotech., Hefei, China, 1:1000) for 5 minutes before washed three times with PBS to remove excessive staining solution. The cells were then subjected to laser scanning confocal microscope (Olympus FV1000S-SIM/IX81, Tokyo, Japan).
Transfection of EMMPRIN shRNA
ShRNA targeting EMMPRIN were synthesized according to previously published sequences [42] with nonspecific shRNA which has no target in the human transcriptome was used as a negative control. Briefly, 1 day before transfection cells were plated at 5 × 103 cells/well in 96-well culture plate and allowed to reach 30% confluence after 24 hours of incubation. The transfection mixture containing MMP-2 targeted shRNA and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was incubated for 20 minutes at room temperature before applied to the cells. The cells were then incubated for 6 hours at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, cells were washed with PBS and maintained in culture medium for 48 hours before expression of EMMPRIN was assessed with western blot.
Statistical analysis
Data presented in this study are means ± standard error of the mean.Statistical analysis of differences between groups was performed using analysis of variance (ANOVA) followed by Dunnett’s t test. The statistical significance of the differences between mean values was defined as P < 0.05.
Results
Overexpression of PTTG in MCF-7 cells
To overexpressing PTTG in MCF-7 cells, an EGFP-containing vector pEGFP-N1 was used in study. After transfection, more than 75% cells in each group were GFP-positive, indicating a high transfection efficiency (Figure 1A). To confirm whether the established MCF-7/pEGFP-N1-PTTG cell line could stably express high level of PTTG, the expression levels of PTTG mRNA and protein were determined by qRT-PCR and western blot analysis, respectively. Our results showed that MCF-7/pEGFP-N1-PTTG cells could stably express significantly higher level of PTTG than MCF-7/pEGFP-N1 cells or parental MCF-7 cells (Figure 1B and 1C).
Figure 1.
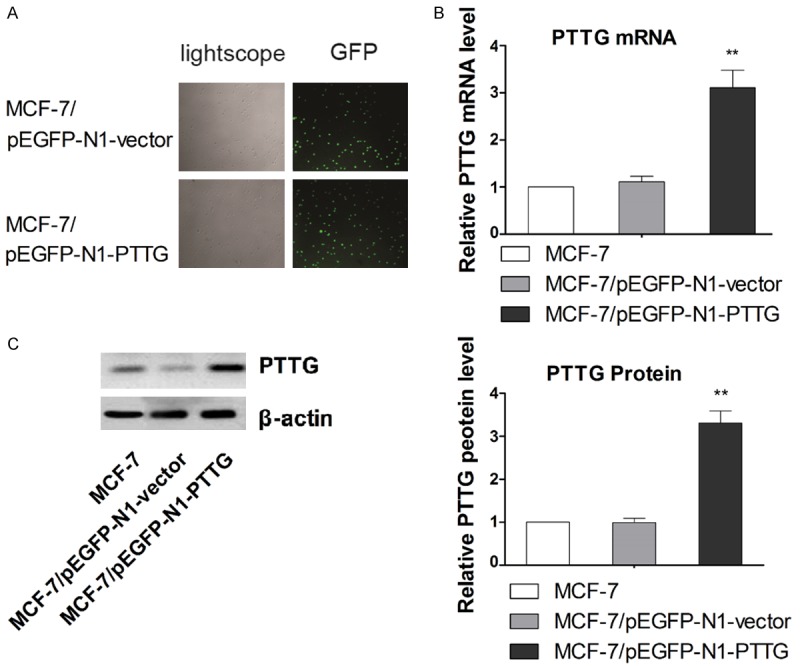
Transfection of PTTG overexpressing plasmid and confirmation of overexpressing PTTG in MCF-7 cells. A. The transfection efficiency was evaluated by fluorescence microscopy. Representative transfection results are shown. B. The expression of PTTG mRNA was measured with qRT-PCR. C. The expression of PTTG protein was detected with western blots. The average signal intensity was standardized to β-actin and data are shown as mean ± SD of triplicate experiments. ** P < 0.01 vs. control. The representative western blot is shown. Data are shown as mean ± SD of triplicate experiments. **P < 0.01 vs. MCF-7.
Overexpression of PTTG leads to increase in MCF-7 cells migration and invasion
To determine whether alteration on PTTG expression led to changed in cell migration and invasion, would scratch and Transwell assay were performed, respectively. PTTG overexpression clones (MCF-7/pEGFP-N1-PTTG) showed significant increase in cell migration rate compared to vector control and parental MCF-7 cells at all time point (Figure 2A). In vitro invasion activity was determined by the number of cells that migrated across the membrane after 24-hour incubation. As shown in Figure 2B, a significant increase in cell invasion was observed in PTTG-overexpressed MCF-7 cells. These beforementioned results suggested that PTTG overexpression significantly enhanced cell migration and invasion in vitro in MCF-7 cells.
Figure 2.
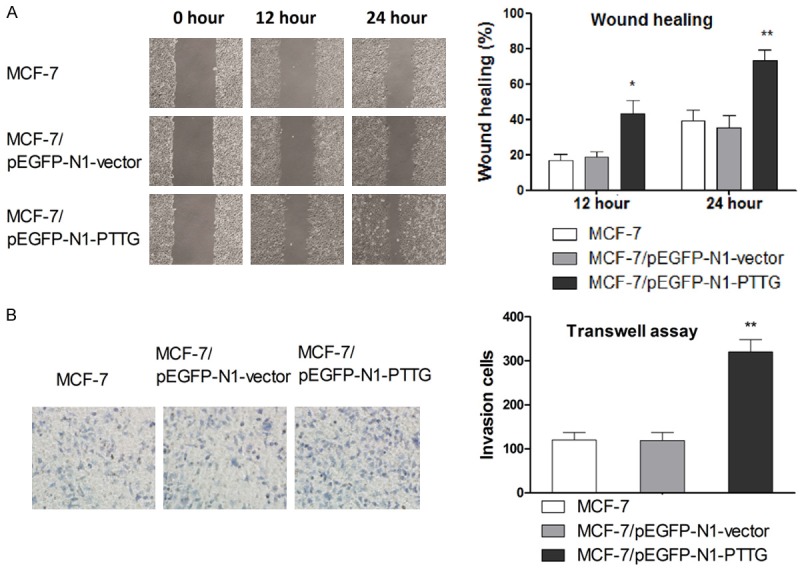
Increase PTTG expression enhances MCF-7 cell migration and invasion in vitro. A. Cell migration after transfection was detected by wound healing assay. Representative wound closure results are shown. B. Cell invasion after transfection was determined by Transwell assay. Representative figures of cell invasion are shown. Data are shown as mean ± SD of triplicate experiments. *P < 0.05 vs. MCF-7, ** P < 0.01 vs. MCF-7.
PTTG promotes EMT process in MCF-7 cells
EMT is crucial step in cancer metastasis; we examined the effect of SOX2 overexpression on EMT process to further evaluate the role of PTTG in the metastasis of breast cancer. Since EMT is marked by nuclear translocation of β-catenin, we stained cells with β-catenin antibody and observed the fluorescent signals. As shown in Figure 3A, there was a significant increase of the β-catenin in the cytoplasm and nucleus relative to cell membrane in PTTG overexpressing MCF-7 cells. The epithelial marker E-cadherin and mesenchymal markers N-cadherin, vimentin, fibronectin and α-SMA were also examined by western blots to further demonstrate the role of PTTG in promoting EMT. As shown in Figure 3B, PTTG overexpression led to remarkable downregulation of E-cadherin and upregulation of N-cadherin, vimentin, fibronectin and α-SMA compared with parental cell line. Transcriptional repressors Snail, Slug and Twist are known to regulate the expression of mesenchymal and epithelial markers and hence play a crucial role in EMT [44]. Therefore, in order to completely understand the mechanism of PTTG’s action, the effect of PTTG overexpression in these regulatory molecules were also examined. As shown in Figure 3C and 3D, in PTTG overexpressing MCF-7 cells, the expression of these transcription repressors was inhibited at both mRNA and protein levels. Taken together, these results indicated that overexpression of PTTG promoted the transition from epithelial to mesenchymal phenotype.
Figure 3.
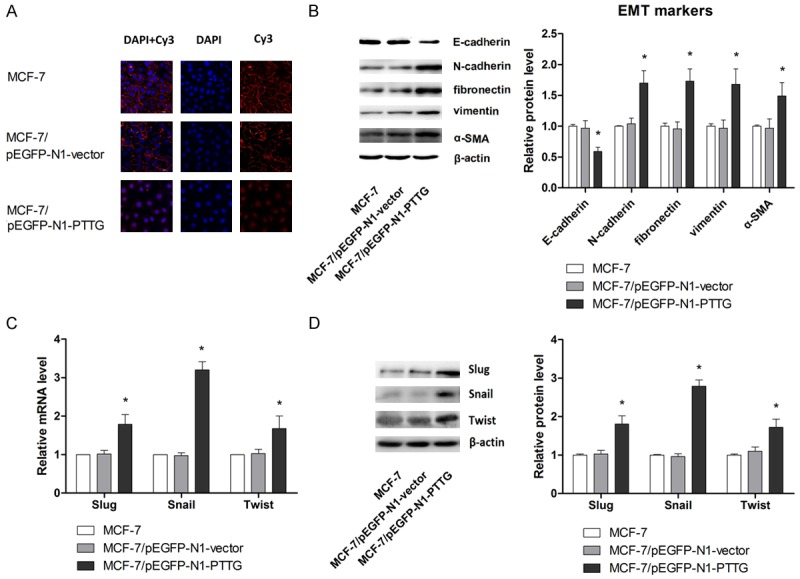
PTTG overexpression promotes EMT process. A. Effect of PTTG overexpression on cell morphology was evaluated by fluorescent microscopy. B. Expression of epithelial cell markers and mesenchymal cell markers (E-cadherin, N-cadherin, vimentin, fibronectin and α-SMA) were examined by western blot. The representative western blot is shown. C. mRNA expression of Slug, Snail and Twist were determined by qRT-PCR. D. Protein expression of Slug, Snail and Twist were determined by western blot. The representative western blot is shown. The values are expressed as the mean ± SD from three independent experiments. Data are shown as mean ± SD of triplicate experiments. *P < 0.05 vs. MCF-7.
Altered PTTG expression affects metastatic potential by regulating EMMPRIN in MCF-7 cells
EMMPRIN is a factor functioning in tumor metastasis including breast cancer, so the expression of EMMPRIN was examined in this study to further determine the mechanism by which overexpression PTTG affects the migration and invasion. mRNA and protein level of EMMPRIN in MCF-7/pEGFP-N1-PTTG, MCF-7/pEGFP-N1-vector and parental MCF-7 cell lines were determined by qRT-PCR and western blot analysis, respectively. Results showed that PTTG overexpression in MCF-7 cells was associated correlated with higher EMMPRIN expression compared with controls (Figure 4A and 4B). These above results indicated that PTTG expression was associated with EMMPRIN expression. To investigate whether PTTG overexpression promotes cell migration and invasion via regulating EMMPRIN, EMMPRIN gene was silenced in MCF-7/pEGFP-N1-PTTG using EMMPRIN-targeting shRNA. Results from western blot analysis confirmed that EMMPRIN expression was significantly suppressed compared with cells transfected with control shRNA or MCF-7/pEGFP-N1-PTTG cells (Figure 5A). Next, the effect of EMMPRIN knockdown on cell migration and invasion was examined. As shown in (Figure 5B), cell migration rate of MCF-7/pEGFP-N1-PTTG cells was significantly reduced after transfected with EMMPRIN-targeted shRNA, compared with MCF-7/pEGFP-N1-PTTG cells transfected with control shRNA (P < 0.05 at 12 hours and P < 0.01 at 24 hours), whereas no significant difference was found in the migration rate of control shRNA transfected and parental MCF-7/pEGFP-N1-PTTG cells. Cell invasion assay also demonstrated that suppression of EMMPRIN expression in MCF-7/pEGFP-N1-PTTG cells resulted in a significant decrease in cell invasion compared with control shRNA transfected and parental MCF-7/pEGFP-N1-PTTG cells.
Figure 4.
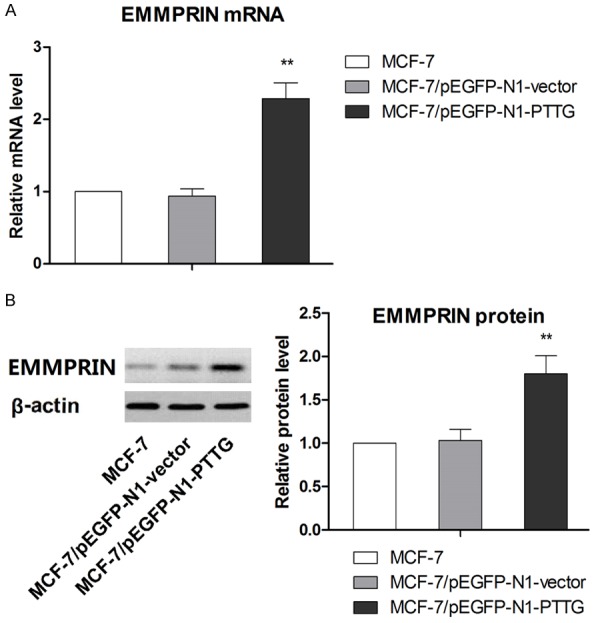
PTTG overexpression induced increase in EMMPRIN expression. A. The expression of EMMPRIN mRNA was measured with qRT-PCR. B. The expression of EMMPRIN protein was detected with western blots. The representative western blot is shown. Data are shown as mean ± SD of triplicate experiments. **P < 0.01 vs. MCF-7.
Figure 5.
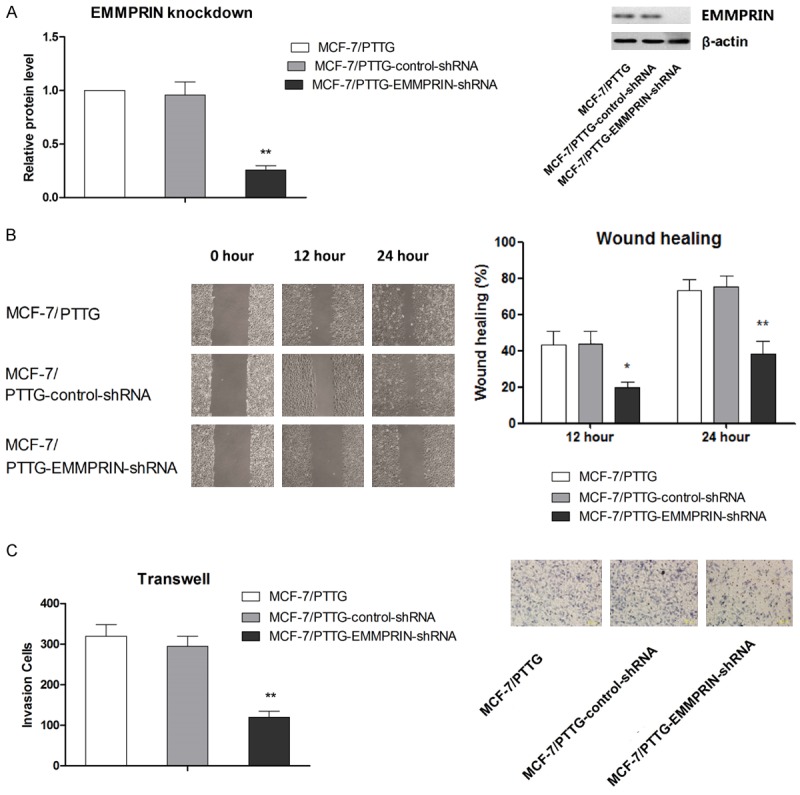
PTTG promotes cell migration and invasion by increasing EMMPRIN expression. A. Suppressed EMMPRIN expression in MCF-7/pEGFP-N1-PTTG by shRNA was confirmed by western blot. Scramble shRNA was used as control. The representative western blot is shown. B. The effect of EMMPRIN knockdown on cell migration in MCF-7/pEGFP-N1-PTTG was evaluated by wound healing. C. The effect of MMP-2 knockdown on cell invasion in MCF-7/pEGFP-N1-PTTG was evaluated by Transwell assay. Representative figures of cell invasion are shown. Data are shown as mean ± SD of triplicate experiments. **P < 0.01 vs. MCF-7/pEGFP-N1-PTTG.
To further demonstrate the involvement of EMMPRIN modulation by PTTG in the PTTF-induced enhancement in metastatic potential of MCF-7 cells, levels of EMT markers and transcriptional repressors were also examined. As shown in Figure 6A, expression levels of the epithelial marker E-cadherin were significantly upregulated in MCF-7 transfected with EMMPRIN-targeting shRNA compared with control shRNA transfected and parental MCF-7/pEGFP-N1-PTTG cells (P < 0.05). In contrast, expression levels of the mesenchymal markers N-cadherin, vimentin, fibronectin and α-SMA were all significantly downregulated in MCF-7 transfected with EMMPRIN-targeting shRNA compared with control shRNA transfected and parental MCF-7/pEGFP-N1-PTTG cells (P < 0.05). In terms of the expression levels of transcriptional repressors Slug, Snail and Twist, knockdown of EMMPRIN was associated with significantly suppressed expression of Slug, Snail and Twist. Taken together, these above results indicated the involvement of EMMPRIN modulation in the promoting effect of PTTG on metastatic potential in MCF-7 cells.
Figure 6.
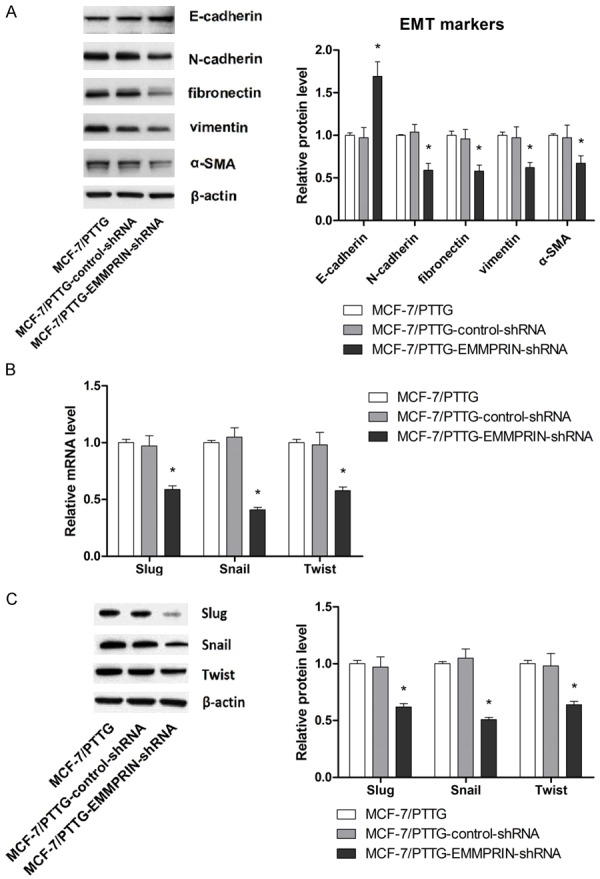
PTTG promotes EMT by increasing EMMPRIN expression. A. The effect of EMMPRIN knockdown on expression of EMT markers was evaluated western blot. The representative western blot is shown. B. The effect of EMMPRIN knockdown on mRNA expression of Slug, Snail and Twist was evaluated by qRT-PCR. C. The effect of EMMPRIN knockdown on expression of transcriptional repressore Slug, Snail and Twist was evaluated western blot. The representative western blot is shown. Data are shown as mean ± SD of triplicate experiments. *P < 0.05 vs. MCF-7/PTTG (MCF-7 cells overexpressing PTTG).
PTTG regulates EMMPRIN expression through the FAK/Akt/mTOR signaling pathway
It has been reported that Akt/mTOR pathway is associated with tumor progression and Akt/mTOR-mediated cell signals induce the expression of MMPs including MMP-2 [45]. It has also been noted that PTTG overexpression was associated with activation of FAK [46]. Given the inducing effect of EMMPRIN on MMPs expressions, we examine the effect of PTTG on activation of FAK/Akt/mTOR signaling pathway to explore whether this signaling pathway functions as an essential kinase in EMMPRIN expression induced by EMMPRIN overexpression. As shown in Figure 7A, PTTG overexpression was strongly associated with activation of FAK/Akt/mTOR signaling compared with controls. To determine whether PTTG regulates EMMPRIN expression in an FAK/Akt/mTOR-dependent manner, selective inhibitors specific to FAK (Y15), Akt (LY294002) and mTOR (rapamycin) were used. As shown in Figure 7B, any of the above three inhibitors effectively abolished PTTG-induced elevated expression of EMMPRIN, indicating that PTTG-induced EMMPRIN expression was strongly associated with FAK/Akt/mTOR activation.
Figure 7.
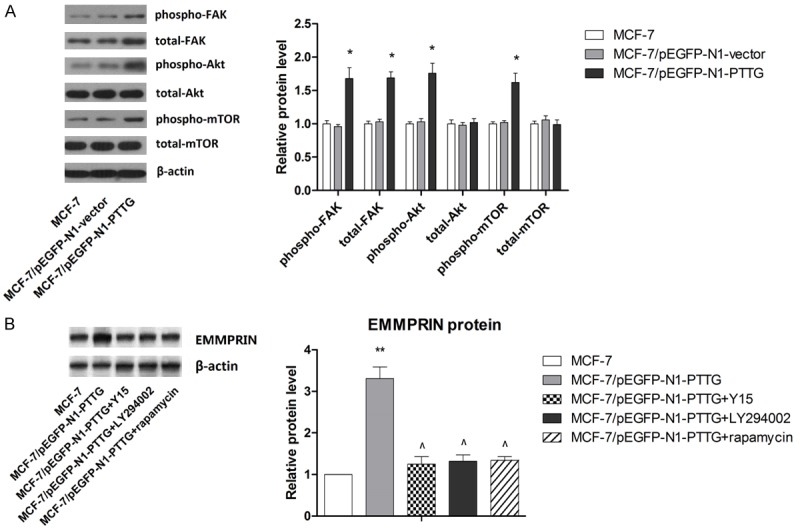
PTTG induces EMMPRIN expression in MCF-7 cells through FAK/Akt/mTOR pathway. A. Activation of FAK/Akt/mTOR pathway in MCF-7/pEGFP-N1-PTTG was detected by western blot. The representative western blot is shown. B. Inhibitors of FAK/Akt/mTOR signaling pathway significantly abolished the inducing effect of PTTG on EMMPRIN expression. The representative western blot is shown. *P < 0.05 vs. MCF-7, ** P < 0.01 vs. MCF-7, ^P < 0.05 vs. MCF-7/pEGFP-N1-PTTG.
FAK/Akt/mTOR signaling is critical for the PTTG-induced metastatic potential of MCF-7 cells
To further support our beforementioned findings, we examined the role of FAK/Akt/mTOR signaling in PTTG-induced metastatic potential of MCF-7 cells. As shown in Figure 8A, FAK inhibitor, Akt inhibitor as well as mTOR inhibitor significantly abolished the promoting effect of PTTG overexpression on cell migration, confirming that the inhibitory effect of PTTG on cell migration was mediated, at least partly, through FAK/Akt/mTOR signaling. Similar effects were observed when cell invasion were examined, as shown in Figure 8B. Moreover, the inducing effect of PTTG on EMT in MCF-7 cells were also abrogated by FAK inhibitor, Akt inhibitor or mTOR inhibtor, as indicated by the changes in the expression levels of two key EMT markers, E-cadherin and N-cadherin (Figure 8C) as well as transcriptional repressors Slug, Snail, and Twist (Figure 8D). Combined with the beforementioned results, our results suggested that PTTG activated FAK/Akt/mTOR signaling, which in turn led to upregulation on EMMPRIN levels, resulting in promotion on metastatic potential in breast cancer cell line MCF-7.
Figure 8.
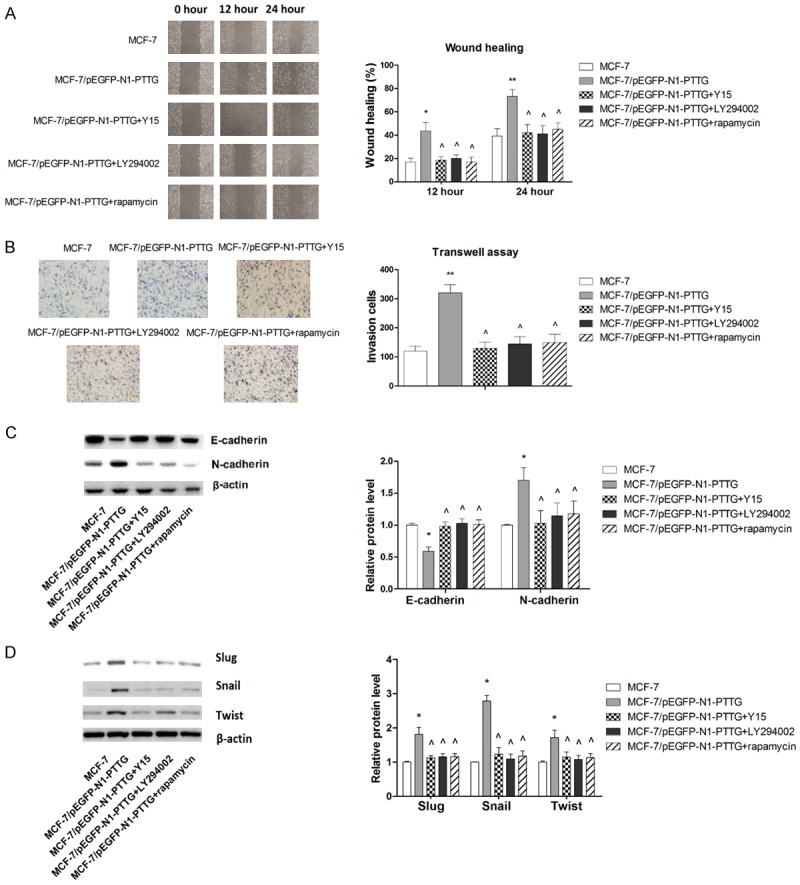
PTTG enhances metastatic potential of MCF-7 cells through FAK/Akt/mTOR pathway. A. Inhibitors of FAK/Akt/mTOR signaling pathway significantly abolished the inducing effect of PTTG on cell migration. Representative wound closure results are shown. B. Inhibitors of FAK/Akt/mTOR signaling pathway significantly abolished the inducing effect of PTTG on cell invasion. Representative figures of cell invasion are shown. C. Inhibitors of FAK/Akt/mTOR signaling pathway significantly abolished the changes in EMT marker expressions induced by PTTG. The representative western blot is shown. D. Inhibitors of FAK/Akt/mTOR signaling pathway significantly abolished the changes in transcriptional repressors expressions induced by PTTG. *P < 0.05 vs. MCF-7, ** P < 0.01 vs. MCF-7, ^P < 0.05 vs. MCF-7/pEGFP-N1-PTTG.
Discussions
PTTG, also known as securin, has been showed to play a vital role in tumor metastasis [47]. PTTG was first cloned from rat pituitary tumor [5] and the homologue of PTTG was subsequently cloned from human origin [48,49]. Predominantly located in the cytoplasm [23], PTTG can be translocated to nucleus under the facilitation by either interaction with PTTG binding factor (PBF) [50] or interaction with the mitogen-activating protein (MAP) kinase cascade [51]. Numerous studies have shown that PTTG can induce tumorigenesis and tumor progression in many aspects by demonstrating the carcinogenic properties of PTTG and the close association with most tumorigenesis while in the absence of the auxiliary genes. In the context of breast cancer, PTTG expression was found to be maximal in invasive ductal carcinoma, metastatic breast carcinoma, and breast carcinoma cell cultures in one cohort of 90 breast tumors [52]. Moreover, detection of PTTG mRNA in 72 breast carcinoma samples also demonstrated a correlation between its expression and lymph node involvement as well as recurrence over a 5-year period of follow-up [18]. Although these above findings suggested a role of PTTG in the metastasis of breast cancer, no experimental proof has been provided and the underlying mechanism by which PTTG affects the metastatic behavior of breast cancer has never been investigated. In present study, our results showed that PTTG gene is strongly associated with breast cancer metastasis. Moreover, our findings suggested that PTTG enhances metastatic potential of breast cancer cells by inducing EMMPRIN through activating FAK/Akt/mTOR pathway.
EMMPRIN is highly expressed on the surface of various malignant tumor cells where its main function is that of a cellular adhesion molecule that induces the secretion of matrix metalloproteinases (MMP; mainly MMP-1, MMP-2 and MMP-9), thus promoting invasion and metastasis [53,54]. In addition, EMMPRIN has been found to promote EMT, another crucial step in metastasis [55]. As breast cancer is concerned, up-regulated tumor expression of EMMPRIN was found to be a significant predictor of reduced disease progression-free survival rate and a shorter overall survival [56], and EMMPRIN-positive rate in patients with breast cancer correlated with clinical stage, number of metastatic lymph nodes, and tumor size [35], suggesting that EMMPRIN has the potential to be the target for novel therapeutic agent. In fact, Quemener et al. has showed that EMMPRIN upregulates the urokinase-type plasminogen activator system and promotes tumor cell invasion [57]. Consistent with these previous studies, evidence from present study also showed that the promoting effect of PTTG on metastatic potential of MCF-7 cells was mediated, at least partly, by regulating EMMPRIN expression, supporting the role of EMMPRIN as novel target for suppressing breast cancer metastasis. It has been found that EMMPRIN was involved in the chemoresistance and angiogenesis of breast cancer [58,59]. Additionally, EMMPRIN has been found to play an anti-apoptotic role in both solid tumor cells and hematological malignant cells [42,53,60]. Meanwhile, the role of EMMPRIN in autophagic cell death has also been reported [61,62]. Therefore, by upregulating the expression of EMMPRIN, PTTG might function in multiple aspects to promote tumor progression and development.
Results from epidemiological studies and animal studies have showed that PI3K could induce activation of MMP-2, MMP-9 and urokinase-type plasminogen activator (uPA), resulting in ECM degradation [63]. Given the fact that EMMPRIN could induce MMP-2 and MMP-9 as well as upregulate uPA, we asked the question whether PTTG upregulated the expression of EMMPRIN via activating Akt/mTOR signaling. In fact, our results showed that overexpressing PTTG in MCF-7 cells did cause activation of Akt/mTOR signaling. Moreover, by using Akt inhibitor LY294002 and mTOR inhibitor rapamycin, we were able to demonstrate that PTTG upregulated EMMPRIN by activating Akt/mTOR signaling. A recent study found that EMMPRIN was transcriptional regulated by Slug [55], which has been found to act as downstream molecule of Akt/mTOR signaling in tumor cells [64]. Agreeing with these results, our data also demonstrated that PTTG increased the expression level of Slug in MCF-7, providing further mechanistic evidence for the mediating role of Akt/mTOR on the regulatory effect of PTTG on EMMPRIN expression.
FAK is a nonreceptor tyrosine kinase that regulates signal transduction at sites of integrin-mediated cell adhesions, where clustering of integrins facilitates the autophosphorylation of FAK at Tyr397 through undefined mechanisms [65]. In fact, activating FAK ensures successive signaling events, as this protein binds to the Src-homology 2 domain (SH2) domain of PI3K, thereby transporting the catalytic subunit of PI3K to the membrane, where it catalyzes the phosphorylation of Akt [66]. In present study, we found that PTTG induced activation of FAK, leading to subsequent activation of Akt/mTOR signaling and elevated EMMPRIN expression. Therefore, the data in present study established that PTTG regulated EMMPRIN expression via FAK/Akt/mTOR signaling. However, it has also been found that EMMPRIN acts as upstream signaling and regulated the activation of FAK in human melanoma cells [67]. A recently study in hepatocellular carcinoma cells also showed that a chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway [68]. Combining these above results with ours, we wondered whether EMMPRIN and FAK were involved in a regulatory loop. Actually, a regulatory loop involving miR-21, SP1 and c-Myc has been reported to modulates EMMPRIN expression in breast cancer invasion and metastasis, suggesting the role of EMMPRIN in a complex network rather than linear signaling pathway in tumor progression [69]. Nevertheless, whether a similar network signaling, which might involve a feedback mechanism, is responsible for the regulatory effect of PTTG on EMMPRIN needs to be evidenced by further studies.
In summary, our findings in present study indicate that PTTG upregulation in MCF-7 cells enhanced cell metastatic potential by increasing EMMPRIN expression. Meanwhile, our findings suggested that PTTG enhances metastatic potential of breast cancer cells by inducing EMMPRIN through activating FAK/Akt/mTOR pathway.
Though more studies are needed, this study suggests that PTTG may be a potential molecular target for developing novel gene targeted therapy against breast cancer.
Acknowledgements
This work was supported by National Natural Science Foundation (No. 31470570, 81473384 and 81274126), Chongqing Natural Science Foundation (No. cstc2014jcyjA80013), Chongqing Creative Programe for Graduate Students (CYS15155) and Science Foundation of Chongqing Education Commission (No. kj1400534).
Disclosure of conflict of interest
None.
References
- 1.Tao M, Ma D, Li Y, Zhou C, Li Y, Zhang Y, Duan W, Xu X, Wang R, Wu L, Liu H. Clinical significance of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011;129:247–254. doi: 10.1007/s10549-011-1512-4. [DOI] [PubMed] [Google Scholar]
- 2.Lian L, Li W, Li ZY, Mao YX, Zhang YT, Zhao YM, Chen K, Duan WM, Tao M. Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs. Oncol Lett. 2013;5:675–680. doi: 10.3892/ol.2012.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shou LM, Zhang QY, Li W, Xie X, Chen K, Lian L, Li ZY, Gong FR, Dai KS, Mao YX, Tao M. Cantharidin and norcantharidin inhibit the ability of MCF-7 cells to adhere to platelets via protein kinase C pathway-dependent downregulation of alpha2 integrin. Oncol Rep. 2013;30:1059–1066. doi: 10.3892/or.2013.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K, Shou LM, Lin F, Duan WM, Wu MY, Xie X, Xie YF, Li W, Tao M. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs. 2014;25:652–662. doi: 10.1097/CAD.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 5.Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- 6.Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- 7.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 8.Yu R, Melmed S. Oncogene activation in pituitary tumors. Brain Pathol. 2001;11:328–341. doi: 10.1111/j.1750-3639.2001.tb00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaney AP, Nelson V, Fernando M, Horwitz G. Transforming events in thyroid tumorigenesis and their association with follicular lesions. J Clin Endocrinol Metab. 2001;86:5025–5032. doi: 10.1210/jcem.86.10.7886. [DOI] [PubMed] [Google Scholar]
- 10.Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716–719. doi: 10.1016/S0140-6736(99)10238-1. [DOI] [PubMed] [Google Scholar]
- 11.Honda S, Hayashi M, Kobayashi Y, Ishikawa Y, Nakagawa K, Tsuchiya E. A role for the pituitary tumor-transforming gene in the genesis and progression of non-small cell lung carcinomas. Anticancer Res. 2003;23:3775–3782. [PubMed] [Google Scholar]
- 12.Tsai SJ, Lin SJ, Cheng YM, Chen HM, Wing LY. Expression and functional analysis of pituitary tumor transforming gene-1 [corrected] in uterine leiomyomas. J Clin Endocrinol Metab. 2005;90:3715–3723. doi: 10.1210/jc.2004-2303. [DOI] [PubMed] [Google Scholar]
- 13.Cho-Rok J, Yoo J, Jang YJ, Kim S, Chu IS, Yeom YI, Choi JY, Im DS. Adenovirus-mediated transfer of siRNA against PTTG1 inhibits liver cancer cell growth in vitro and in vivo. Hepatology. 2006;43:1042–1052. doi: 10.1002/hep.21137. [DOI] [PubMed] [Google Scholar]
- 14.Chamaon K, Kirches E, Kanakis D, Braeuninger S, Dietzmann K, Mawrin C. Regulation of the pituitary tumor transforming gene by insulin-like-growth factor-I and insulin differs between malignant and non-neoplastic astrocytes. Biochem Biophys Res Commun. 2005;331:86–92. doi: 10.1016/j.bbrc.2005.03.124. [DOI] [PubMed] [Google Scholar]
- 15.Puri R, Tousson A, Chen L, Kakar SS. Molecular cloning of pituitary tumor transforming gene 1 from ovarian tumors and its expression in tumors. Cancer Lett. 2001;163:131–139. doi: 10.1016/s0304-3835(00)00688-1. [DOI] [PubMed] [Google Scholar]
- 16.El-Naggar SM, Malik MT, Kakar SS. Small interfering RNA against PTTG: a novel therapy for ovarian cancer. Int J Oncol. 2007;31:137–143. [PubMed] [Google Scholar]
- 17.Tang MH, Tan J, Cui FL. [Expression of pituitary tumor transforming gene in human renal clear cell carcinoma and its significance] . Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1712–1714. [PubMed] [Google Scholar]
- 18.Solbach C, Roller M, Fellbaum C, Nicoletti M, Kaufmann M. PTTG mRNA expression in primary breast cancer: a prognostic marker for lymph node invasion and tumor recurrence. Breast. 2004;13:80–81. doi: 10.1016/j.breast.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Saez C, Pereda T, Borrero JJ, Espina A, Romero F, Tortolero M, Pintor-Toro JA, Segura DI, Japon MA. Expression of hpttg proto-oncogene in lymphoid neoplasias. Oncogene. 2002;21:8173–8177. doi: 10.1038/sj.onc.1205954. [DOI] [PubMed] [Google Scholar]
- 20.Hamid T, Malik MT, Kakar SS. Ectopic expression of PTTG1/securin promotes tumorigenesis in human embryonic kidney cells. Mol Cancer. 2005;4:3. doi: 10.1186/1476-4598-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakar SS, Malik MT. Suppression of lung cancer with siRNA targeting PTTG. Int J Oncol. 2006;29:387–395. [PubMed] [Google Scholar]
- 22.Ramos-Morales F, Dominguez A, Romero F, Luna R, Multon MC, Pintor-Toro JA, Tortolero M. Cell cycle regulated expression and phosphorylation of hpttg proto-oncogene product. Oncogene. 2000;19:403–409. doi: 10.1038/sj.onc.1203320. [DOI] [PubMed] [Google Scholar]
- 23.Yu R, Ren SG, Horwitz GA, Wang Z, Melmed S. Pituitary tumor transforming gene (PTTG) regulates placental JEG-3 cell division and survival: evidence from live cell imaging. Mol Endocrinol. 2000;14:1137–1146. doi: 10.1210/mend.14.8.0501. [DOI] [PubMed] [Google Scholar]
- 24.Romero F, Multon MC, Ramos-Morales F, Dominguez A, Bernal JA, Pintor-Toro JA, Tortolero M. Human securin, hPTTG, is associated with Ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res. 2001;29:1300–1307. doi: 10.1093/nar/29.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Heaney AP, Yu R, Horwitz GA, Melmed S. Human pituitary tumor-transforming gene induces angiogenesis. J Clin Endocrinol Metab. 2001;86:867–874. doi: 10.1210/jcem.86.2.7184. [DOI] [PubMed] [Google Scholar]
- 26.Hunter JA, Skelly RH, Aylwin SJ, Geddes JF, Evanson J, Besser GM, Monson JP, Burrin JM. The relationship between pituitary tumour transforming gene (PTTG) expression and in vitro hormone and vascular endothelial growth factor (VEGF) secretion from human pituitary adenomas. Eur J Endocrinol. 2003;148:203–211. doi: 10.1530/eje.0.1480203. [DOI] [PubMed] [Google Scholar]
- 27.Boelaert K, McCabe CJ, Tannahill LA, Gittoes NJ, Holder RL, Watkinson JC, Bradwell AR, Sheppard MC, Franklyn JA. Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J Clin Endocrinol Metab. 2003;88:2341–2347. doi: 10.1210/jc.2002-021113. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 29.Chen CC, Chang TW, Chen FM, Hou MF, Hung SY, Chong IW, Lee SC, Zhou TH, Lin SR. Combination of multiple mRNA markers (PTTG1, Survivin, UbcH10 and TK1) in the diagnosis of Taiwanese patients with breast cancer by membrane array. Oncology. 2006;70:438–446. doi: 10.1159/000098557. [DOI] [PubMed] [Google Scholar]
- 30.Gabison EE, Mourah S, Steinfels E, Yan L, Hoang-Xuan T, Watsky MA, De Wever B, Calvo F, Mauviel A, Menashi S. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: role in epithelio-stromal interactions and matrix metalloproteinase induction. Am J Pathol. 2005;166:209–219. doi: 10.1016/S0002-9440(10)62245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 32.Yu XL, Hu T, Du JM, Ding JP, Yang XM, Zhang J, Yang B, Shen X, Zhang Z, Zhong WD, Wen N, Jiang H, Zhu P, Chen ZN. Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem. 2008;283:18056–18065. doi: 10.1074/jbc.M802694200. [DOI] [PubMed] [Google Scholar]
- 33.Luo J, Teplyakov A, Obmolova G, Malia T, Wu SJ, Beil E, Baker A, Swencki-Underwood B, Zhao Y, Sprenkle J, Dixon K, Sweet R, Gilliland GL. Structure of the EMMPRIN Nterminal domain 1: dimerization via betastrand swapping. Proteins. 2009;77:1009–1014. doi: 10.1002/prot.22577. [DOI] [PubMed] [Google Scholar]
- 34.Ranuncolo SM, Armanasco E, Cresta C, Bal De Kier Joffe E, Puricelli L. Plasma MMP-9 (92 kDa-MMP) activity is useful in the follow-up and in the assessment of prognosis in breast cancer patients. Int J Cancer. 2003;106:745–751. doi: 10.1002/ijc.11288. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Cui L, Zhang Y, Chen L, Wang Y, Fan Y, Lei T, Gu F, Lang R, Pringle GA, Zhang X, Chen Z, Fu L. Expression of HAb18G is associated with tumor progression and prognosis of breast carcinoma. Breast Cancer Res Treat. 2010;124:677–688. doi: 10.1007/s10549-010-0790-6. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Wu J, Song F, Tang J, Wang SJ, Yu XL, Chen ZN, Jiang JL. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin beta1 to modulate malignant properties of hepatoma cells. J Biol Chem. 2012;287:4759–4772. doi: 10.1074/jbc.M111.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu S, Li Y, Mi L, Zhang Y, Zhang L, Gong L, Han X, Yao L, Lan M, Chen Z, Zhang W. Clinical impact of HAb18G/CD147 expression in esophageal squamous cell carcinoma. Dig Dis Sci. 2011;56:3569–3576. doi: 10.1007/s10620-011-1812-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhong WD, Liang YX, Lin SX, Li L, He HC, Bi XC, Han ZD, Dai QS, Ye YK, Chen QB, Wang YS, Zeng GH, Zhu G, Zhang Z, Chen ZN, Wu CL. Expression of CD147 is associated with prostate cancer progression. Int J Cancer. 2012;130:300–308. doi: 10.1002/ijc.25982. [DOI] [PubMed] [Google Scholar]
- 39.Afonso J, Longatto-Filho A, Baltazar F, Sousa N, Costa FE, Morais A, Amaro T, Lopes C, Santos LL. CD147 overexpression allows an accurate discrimination of bladder cancer patients’ prognosis. Eur J Surg Oncol. 2011;37:811–817. doi: 10.1016/j.ejso.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Xin T, Jiang QY, Huang DY, Shen WX, Li L, Lv YJ, Jin YH, Song XW, Teng C. CD147, MMP9 expression and clinical significance of basal-like breast cancer. Med Oncol. 2013;30:366. doi: 10.1007/s12032-012-0366-x. [DOI] [PubMed] [Google Scholar]
- 41.Xia YH, Li M, Fu DD, Xu SL, Li ZG, Liu D, Tian ZW. Effects of PTTG down-regulation on proliferation and metastasis of the SCL-1 cutaneous squamous cell carcinoma cell line. Asian Pac J Cancer Prev. 2013;14:6245–6248. doi: 10.7314/apjcp.2013.14.11.6245. [DOI] [PubMed] [Google Scholar]
- 42.Gao H, Jiang Q, Han Y, Peng J, Wang C. shRNA-mediated EMMPRIN silencing inhibits human leukemic monocyte lymphoma U937 cell proliferation and increases chemosensitivity to adriamycin. Cell Biochem Biophys. 2015;71:827–835. doi: 10.1007/s12013-014-0270-4. [DOI] [PubMed] [Google Scholar]
- 43.Xie J, Gao H, Jianjun P, Yantao H, Xuehong C, Qixiao J, Chunbo W. Hispidulin prevents hypoxia-induced epithelial-mesenchymal transition in human colon carcinoma cells. Am J Cancer Res. 2015;5:15. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thakur R, Trivedi R, Rastogi N, Singh M, Mishra DP. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah PP, Fong MY, Kakar SS. PTTG induces EMT through integrin alphaVbeta3-focal adhesion kinase signaling in lung cancer cells. Oncogene. 2012;31:3124–3135. doi: 10.1038/onc.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab. 2002;87:4238–4244. doi: 10.1210/jc.2002-020309. [DOI] [PubMed] [Google Scholar]
- 48.Kakar SS, Jennes L. Molecular cloning and characterization of the tumor transforming gene (TUTR1): a novel gene in human tumorigenesis. Cytogenet Cell Genet. 1999;84:211–216. doi: 10.1159/000015261. [DOI] [PubMed] [Google Scholar]
- 49.Kakar SS. Molecular cloning, genomic organization, and identification of the promoter for the human pituitary tumor transforming gene (PTTG) Gene. 1999;240:317–324. doi: 10.1016/s0378-1119(99)00446-1. [DOI] [PubMed] [Google Scholar]
- 50.Chien W, Pei L. A novel binding factor facilitates nuclear translocation and transcriptional activation function of the pituitary tumor-transforming gene product. J Biol Chem. 2000;275:19422–19427. doi: 10.1074/jbc.M910105199. [DOI] [PubMed] [Google Scholar]
- 51.Pei L. Activation of mitogen-activated protein kinase cascade regulates pituitary tumortransforming gene transactivation function. J Biol Chem. 2000;275:31191–31198. doi: 10.1074/jbc.M002451200. [DOI] [PubMed] [Google Scholar]
- 52.Ogbagabriel S, Fernando M, Waldman FM, Bose S, Heaney AP. Securin is overexpressed in breast cancer. Mod Pathol. 2005;18:985–990. doi: 10.1038/modpathol.3800382. [DOI] [PubMed] [Google Scholar]
- 53.Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, Chen ZS, Kanekura T. RNA interference targeting the CD147 induces apoptosis of multidrug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276:189–195. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. [PubMed] [Google Scholar]
- 55.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–4427. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 56.Nagashima S, Sakurai K, Suzuki S, Hara Y, Maeda T, Hirano T, Enomoto K, Amano S, Koshinaga T. [CD147 expression in non-invasive and invasive breast carcinoma] . Gan To Kagaku Ryoho. 2014;41:1267–1269. [PubMed] [Google Scholar]
- 57.Quemener C, Gabison EE, Naimi B, Lescaille G, Bougatef F, Podgorniak MP, Labarchede G, Lebbe C, Calvo F, Menashi S, Mourah S. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007;67:9–15. doi: 10.1158/0008-5472.CAN-06-2448. [DOI] [PubMed] [Google Scholar]
- 58.Zhou S, Liao L, Chen C, Zeng W, Liu S, Su J, Zhao S, Chen M, Kuang Y, Chen X, Li J. CD147 mediates chemoresistance in breast cancer via ABCG2 by affecting its cellular localization and dimerization. Cancer Lett. 2013;337:285–292. doi: 10.1016/j.canlet.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 59.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 60.Thammasit P, Sangboonruang S, Suwanpairoj S, Khamaikawin W, Intasai N, Kasinrerk W, Tayapiwatana C, Tragoolpua K. Intracellular Acidosis Promotes Mitochondrial Apoptosis Pathway: Role of EMMPRIN Down-regulation via Specific Single-chain Fv Intrabody. J Cancer. 2015;6:276–286. doi: 10.7150/jca.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing X, Xiao Q, Wang W, Gou X, Wang Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011;102:1287–1292. doi: 10.1111/j.1349-7006.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gou X, Ru Q, Zhang H, Chen Y, Li L, Yang H, Xing J, Chen Z. HAb18G/CD147 inhibits starvation-induced autophagy in human hepatoma cell SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer Sci. 2009;100:837–843. doi: 10.1111/j.1349-7006.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ispanovic E, Haas TL. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol Cell Physiol. 2006;291:C579–588. doi: 10.1152/ajpcell.00300.2005. [DOI] [PubMed] [Google Scholar]
- 64.Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari A, Lacour JP, Ballotti R, Deckert M, Tartare-Deckert S. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One. 2012;7:e40378. doi: 10.1371/journal.pone.0040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res. 2010;70:1645–1655. doi: 10.1158/0008-5472.CAN-09-2447. [DOI] [PubMed] [Google Scholar]
- 67.Delyon J, Khayati F, Djaafri I, Podgorniak MP, Sadoux A, Setterblad N, Boutalbi Z, Maouche K, Maskos U, Menashi S, Lebbe C, Mourah S. EMMPRIN regulates beta1 integrin-mediated adhesion through Kindlin-3 in human melanoma cells. Exp Dermatol. 2015;24:443–448. doi: 10.1111/exd.12693. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Yuan L, Yang XM, Wei D, Wang B, Sun XX, Feng F, Nan G, Chen ZN, Bian H. A chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway. Clin Exp Metastasis. 2015;32:39–53. doi: 10.1007/s10585-014-9689-7. [DOI] [PubMed] [Google Scholar]
- 69.Kong LM, Liao CG, Zhang Y, Xu J, Li Y, Huang W, Zhang Y, Bian H, Chen ZN. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014;74:3764–3778. doi: 10.1158/0008-5472.CAN-13-3555. [DOI] [PubMed] [Google Scholar]


