Abstract
Tissues derived from induced pluripotent stem cells (iPSCs) are a promising source of cells for building various regenerative medicine therapies; from simply transplanting cells to reseeding decellularized organs to reconstructing multicellular tissues. Although reprogramming strategies for producing iPSCs have improved, the clinical use of iPSCs is limited by the presence of unique human leukocyte antigen (HLA) genes, the main immunologic barrier to transplantation. In order to overcome the immunological hurdles associated with allogeneic tissues and organs, the generation of patient-histocompatible iPSCs (autologous or HLA-matched cells) provides an attractive platform for personalized medicine. However, concerns have been raised as to the fitness, safety and immunogenicity of iPSC derivatives because of variable differentiation potential of different lines and the identification of genetic and epigenetic aberrations that can occur during the reprogramming process. In addition, significant cost and regulatory barriers may deter commercialization of patient specific therapies in the short-term. Nonetheless, recent studies provide some evidence of immunological benefit for using autologous iPSCs. Yet, more studies are needed to evaluate the immunogenicity of various autologous and allogeneic human iPSC-derived cell types as well as test various methods to abrogate rejection. Here, we present perspectives of using allogeneic vs autologous iPSCs for transplantation therapies and the advantages and disadvantages of each related to differentiation potential, immunogenicity, genetic stability and tumorigenicity. We also review the current literature on the immunogenicity of syngeneic iPSCs and discuss evidence that questions the feasibility of HLA-matched iPSC banks. Finally, we will discuss emerging methods of abrogating or reducing host immune responses to PSC derivatives.
Keywords: reprogramming, induced pluripotent stem cells, transplantation, HLA antigens, epigenetics, co-stimulatory blockade, immunogenicity, regenerative medicine, pluripotent stem cells, alloimmunity, autoimmunity
INTRODUCTION
Understanding the immune responses to transplanted human pluripotent stem cells (PSCs) and PSC-derived tissues, and how immune responses can be mitigated or prevented, is of critical importance for the future clinical application of this technology. Both categories of PSCs--embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)--offer an unprecedented opportunity to create replacement cells and tissues for regenerative medicine therapies. However, the advent of human iPSCs uniquely allows for the generation of autologous cell therapies that, at least in theory, should not elicit immunologic rejection when transplanted back into the original donor [1–3]. This concept has the potential to transform the field of transplantation, which today is beset with the clinical reality of scarce donor tissue availability and the prospect for recipients to endure a lifetime of immunosuppressive drugs with associated side effects. Hopefully, one day this could be eclipsed by abundant personalized therapies with low potential for rejection and graft failure. However, to achieve this goal of patient-specific iPSC-based therapies biological, logistical, regulatory and financial barriers will need to be overcome [4, 5]. The biological concerns associated with the generation of iPSCs for use in regenerative medicine, such as the reprogramming methods used, input cell source used for generating iPSCs, as well as the teratoma-forming potential of the differentiated cell populations--all of which potentially may elicit an unintended immune response--pose a threat to the integrity and survival of the graft. However, we believe these issues will be surmountable in time and, further, the fact that iPSCs are derived ex vivo offers opportunities to modulate the immunogenicity and functionality of iPSC-derived tissues, as has been demonstrated by recent studies [6]. This review will comment on critical available evidence related to the immunogenicity of allogeneic and autologous PSCs and potential methods that could be utilized to circumvent alloimmunity.
Background: Derivation of human iPSCs
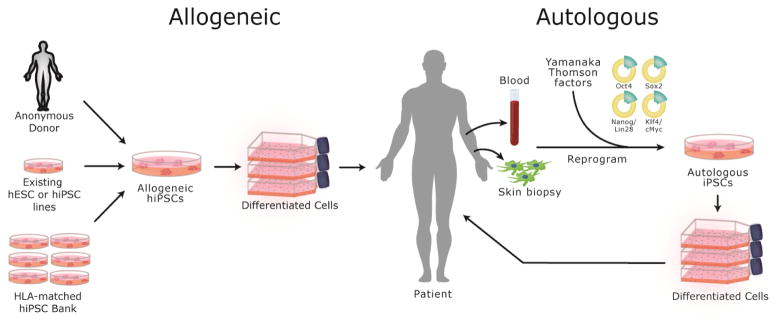
Two similar but distinct types of human PSCs are available for use in future clinical regenerative medicine strategies. Whereas ESCs are derived from the inner cell mass of donated embryos, iPSCs are generated directly by genetically “reprogramming” terminally differentiated somatic cells into a pluripotent state via forced expression of pluripotency-associated factors. Reprogramming is a clonal process i.e. one input cell is reprogrammed into a clonal iPSC line. The resultant iPSCs very closely resemble ESCs in phenotype and function. Reprogramming technology was discovered through early work from Sir John Gurdon [7] and then built upon by the laboratories of Shinya Yamanaka [3, 8] and James Thomson [2]. Gurdon and Yamanaka were awarded the Nobel Prize for their contributions in 2012. Both categories of PSCs are capable of nearly unlimited, undifferentiated proliferation in vitro and are considered pluripotent by retaining the ability to form the numerous adult cell types derived from the three embryonic germ layers [9, 10]. However, iPSCs have a unique added benefit of containing a near replica of the genetic material of the individual cell of origin, thus offering the potential to create patient-specific therapies which may be tolerated as “self” by the patient’s immune system (Figure 1).
Figure 1.
Both allogeneic and autologous sources of human pluripotent stem cells (hPSCs) are potentially available for therapeutic use. Allogeneic hPSCs could be sourced from anonymous living or cadaver donors, existing cGMP grade lines, or established banks of HLA-defined cGMP hiPSC lines. Alternatively, customized patient-specific hiPSC lines can be derived by reprogramming somatic cells from the patient, such as peripheral blood cells or skin fibroblasts. Advantages and disadvantages of each approach and current challenges are discussed in the text.
iPSC technology circumvents the main ethical objection associated with the use of hESCs by using terminally differentiated somatic cells rather than discarded embryos as the input cell source. Further, it allows for the development of patient-specific cell therapies and potentially moves medicine away from allogeneic transplants and the specter of immunologic rejection into autologous transplants and the promise of donor-specific tolerance. However, currently available reprogramming technologies will need to be refined prior to widespread clinical application. Initial reports of successful reprogramming used integrating retroviral vectors [2, 3]. Because of concerns related to potential unintended effects on differentiation, teratoma formation and genetic stability, researchers strove to develop reprogramming methods using non-integrating approaches to obtain safer iPSCs. These non-integrative approaches include episomal plasmid DNA [11], piggyBac transposon [12], Sendai virus [13], adenovirus [14], mRNA [15] minicircle vectors [16], as well as protein transduction and small molecules (reviewed in [17] and [18]). One common disadvantage to many of these strategies is the decreased reprogramming efficiency compared to that achieved with lentiviral/retroviral approaches. Nonetheless, some methods such as Sendai virus show increased reprogramming efficiency and many of these methods are effective in practice.
A recent review by Schlaeger et al. [19] examined several non-integrative reprogramming methods including Sendai virus (SeV) [13], episomal plasmid DNA [20] and mRNA [15]. They summarized the pros and cons associated with these methods, as they compare to integrating vector approaches. In addition to directly testing the above-mentioned methods they further polled the field of human cell reprogramming laboratories through an online survey and compiled results from >1400 human somatic reprogramming experiments from around the world. They conclude that while the Sendai virus was efficient and reliable it presently cannot be applied to the clinical setting due to lack of clinical grade SeV. RNA, protein transduction and small molecule based approaches are very promising but need further optimization due to technical challenges. They concluded that the best approach for reprogramming cells that can be applied to a clinical setting at this point in time is the episomal plasmid DNA method due to its reliability and integration-free mechanism.
THE IMPORTANCE OF CELL SOURCE FOR iPSCs
Starting Material
Cell lines derived from fetal fibroblasts, neonatal foreskin fibroblasts, and adult dermal fibroblasts were the first human cells to be reprogrammed [2, 3]. In the following years a wide variety of cell types have been documented to be amenable to reprogramming, for example, adipose cells [21] and epithelial cells collected from the urine [22]. Deriving iPSCs from various blood cell sources, including mobilized CD34+ cells [23] and peripheral blood T cells [24], has emerged as an attractive methodology due to routine clinical collection of patient blood samples, compared to the more invasive and labor-intensive approach of performing skin punch biopsies followed by tissue processing and cell culture associated with establishing primary fibroblast cell lines. Minimally manipulated primary cells, such as blood-derived cells, have the advantage of being less likely to exhibit stochastically acquired mutations prior to reprogramming. Additionally, the availability of cryopreserved specimens from living and deceased donors [25] makes blood-derived cells a leading choice for reprogramming as iPSC therapies move towards the clinic.
The choice of input cell type for therapeutic reprogramming should be evaluated based on convenience of obtaining samples, as mentioned above, but additional factors should also be considered. Donor cell age is an important factor, as advanced age of the somatic cell donor has been associated with decreasing reprogramming efficiency [26]; however, Lapasset et al. [27] showed no differences in efficiencies between aged and young donor input cells. Furthermore, the cells from an older individual will have a greater likelihood to have accumulated mutations over time (e.g. UV-induced DNA damage in skin fibroblasts), which may detrimentally affect the cell/tissue of origin and the derivative cell/tissue. Overall, the age and possibility of accumulated genetic changes in the donor could have a significant and unavoidable influence on the success of the therapy. In the autologous setting, these risks may be acceptable i.e. in cases where personalized therapies are required. On the other hand, in allogeneic settings, such as in HLA tissue banking strategies, the choice of cell source and donor age should be carefully considered, as the safety requirements for clinical application would likely be more stringent.
An intriguing example of the importance of input cell type involves the use of T cells for generating iPSCs, as described by Brown et al. [24].During the process of T cell receptor (TCR) rearrangement, germline DNA is excised prior to the formation of a functional TCR. This phenomenon can be exploited to generate monoclonal T cells by selecting a T cell clone with known peptide/MHC specificity (e.g. HIV or Melanoma [28, 29]) from a patient’s blood sample, reprogramming the cell and then redifferentiating the iPSCs into a new population of monoclonal T cells which maintain the parent cell’s HLA background as well as clonal specificity. Besides being a method to generate large quantities of therapeutic T cells, the resultant “rejuvenated” cells have longer telomeres and a more central memory phenotype [28]. Moreover, this approach of generating clonal T cell populations avoids the alpha and beta TCR chain exogenous/endogenous mispairing encountered with traditional TCR cloning adoptive transfer therapies which can elicit autoimmune-related side effects [24, 30].
Genome-wide gene expression profiling [31, 32] and DNA methylation pattern analyses [33] have detected incomplete reprogramming in iPSCs, which can result in aberrant differentiation and immunogenicity of iPSC-derived cells. Therefore these approaches should be incorporated into the evaluation of a particular iPSC line whenever possible. In addition, whether or not an iPSC line exhibits incomplete reprogramming should be correlated with lineage-specific differentiation potential to determine its clinical utility and its propensity for teratoma formation after transplantation of lineage-enriched or purified populations [34]. For example, while an “epigenetic memory” (to be discussed further, below) can be considered incomplete reprogramming, in certain instances it may be beneficial by biasing the cells toward differentiation to a desired lineage. Identifying gene expression profiling differences among iPSC lines and whether they are functionally relevant illustrates the importance of evaluating multiple lines from a given individual and will aid in determining the cell lines with the highest likelihood of success for future potential therapeutic applications.
Epigenetic Memory
Recent studies have suggested significant molecular and functional differences between iPSCs that are derived from distinct cell types and sources, demonstrating a so-called “epigenetic memory” [35]. In order for the complete reprogramming of a somatic cell to a pluripotent state the epigenetic modifications (e.g. DNA methylation, histone acetylation, etc.) must be erased or reset to what is observed in an embryonic state. Pluripotency is typically assessed by gene expression and teratoma formation; however, these assays do not assess the persistent epigenetic and molecular signatures, which may have negative (or positive) effects on the functional differentiation potentials of these cell lines. The reprogramming process may introduce new aberrations and/or leave intact epigenetic marks of the original input somatic cell, which could then be inherited by the iPSCs.
For example, Daley and colleagues described the derivation of iPSC lines from neonatal keratinocytes and umbilical cord blood cells from the same donor, which met all criteria for pluripotency, and used these two lines to interrogate the epigenetic status of human iPSCs [36]. They found that iPSCs made from keratinocytes expressed keratin-14 at 9.4 fold higher levels than iPSCs made from cord blood, indicating a much higher differentiation potential for these cells into the skin cell lineage. In the reciprocal direction, cord blood cells showed a greater propensity toward hematopoiesis. Further, they could show that these cells maintained epigenetic signatures that are intrinsic to the cell of origin even after extended in vitro culture. This could be viewed on one hand as a disadvantage or on the other hand as beneficial. Meaning, one could manipulate the outcome of the differentiation by knowing the epigenetic proclivities of a particular cell line, which would be particularly advantageous in situations where directed differentiation remains a challenge. Other groups have demonstrated that the retention of an input cell source’s epigenetic signature can be transient, as the marks tended to disappear upon further passaging, suggesting that complete reprogramming is a gradual process [37, 38]. Thus, epigenetic memory effects may be more of a concern, or benefit, for early passage cell lines, while at the same time it still reflects the technical limitations of current reprogramming strategies.
Various groups have shown that the epigenetic status of iPSCs may prove to be an important consideration for development of PSC therapies [33, 39, 40]. For example, an iPSC line derived from the hematopoietic lineage, at least in early passages, may retain epigenetic signatures of the starting input cell source and thus bias the differentiated cell products towards other mesodermal lineages and against more disparate ectodermal lineages [41]. Additionally, some cell types may be more amenable to reprogramming than others based on their own gene transcriptional profile [42] and there are data indicating that reprogramming more developmentally immature cell types such as hematopoietic stem cells is more efficient [43] and may provide a more “blank-slate” epigenome compared to reprogramming more terminally differentiated cells [44]. Perhaps utilizing a “naive” input cell source such as stem or progenitor cells will allow for more appropriate differentiation through manipulation of the epigenetic signatures that occur during early development and throughout the specification process [45]. Additionally, using these cell types may have a further advantage for reprogramming because of the avoidance of issues related to accumulated mutations or other chromosomal damage seen in older cells [44]. Future research directions in this area may include determining whether epigenetic memory can contribute to immunogenicity or tolerance of iPSC-derived tissues, in which case the selection of cell type or passage number could be a crucial factor in development of cell therapies.
Finally, epigenetic memory may affect the immunogenicity of iPSCs and thus choosing an input cell type that is weakly immunogenic may be advantageous if it can confer its weak immunogenicity onto differentiated daughter cells. For example, Wang et al. tested whether the low immunogenicity of Sertoli cells (SC) was maintained in SC-derived iPSCs, either freshly isolated or after extensive in vitro passaging, as well as in the in vivo setting [46]. They demonstrated that SC-derived iPSCs resulted in a high frequency of teratoma formation when injected into syngeneic B6 mice, as compared to MEF-iPSCs and ES cells; suggesting that SC-derived iPSCs elict a blunted immune response thereby allowing for more efficient teratoma formation. Although they discovered that initially low passage SC-iPSCs were less immunogenic in vivo than MEF-iPSCs, the observed reduced immunogenicity was lost following extended culturing. Similar to the finding described by Polo et al., [38] these immune-privileged cells still exhibited reduced immunogenicity when compared to MEF-iPSCs [46]. This is likely due to epigenetic memory from the donor cell impacting the immunogenicity of the derived iPSCs. Given this interesting observation the underlying mechanisms should be addressed in further research. It will also be valuable to study the immunogenicity of SC-iPSC-derived differentiated cell types, rather than just undifferentiated SC-iPSCs themselves. It may also be interesting to compare human and mouse SCs for their ability to convey reduced immunogenicity to SC-iPSC-derived cells.
Despite this exciting progress, further research will be required to elucidate the molecular mechanisms involved in the induction of pluripotency and there is still an active debate in the field as to the long-term stability of reprogrammed cells. Accumulated genomic mutations that are not expressed in the cell of origin could be expressed in the derived iPSCs and/or in derivative differentiated cells. The extent to which these somatic cell mutations might influence the function, tumorigenicity, genetic stability and immunogenicity of therapeutic cell populations is not known. These issues, which have the potential to affect both the safety and efficacy of iPSC-derived cell types, must be adequately addressed prior to widespread clinical use.
BENEFITS OF iPSCs FOR CELLULAR THERAPIES
PSCs are intended to provide the cellular pillar for regenerative and personalized medicine. PSC-derived replacement cells or tissues have many theoretical advantages over allogeneic cadaver donor sources of tissues. Key attributes are that they are scalable and can potentially provide an unlimited amount of tissue that is available at any time, uniform in its condition and properties, and free of the risk of transmitted donor-derived diseases. By virtue of their scalability, PSCs allow for growth of a large number of differentiated cells, even if the differentiation process is not very efficient. The scalability, availability and consistent quality of human PSCs address critical existing supply problems in the field of organ and tissue transplantation. For example, applications include beta cell transplants for treating diabetes where there are an insufficient supply of pancreata or islets for the millions of patients with diabetes [47, 48] and hematopoietic stem cell (HSC) transplants, which are still hampered by low cell numbers which fail to meet therapeutic dose thresholds even when a suitable donor can be found [49].
Whether autologous (patient-specific) iPSCs or allogeneic PSCs (either ESCs or iPSCs) prove to be the best cell type may depend on the disease application, the differentiated cell type, transplant site or other aspects of the therapy. As such, is it worthwhile to review the proposed clinical advantages and disadvantages of patient-specific iPSCs vs. allogeneic iPSCs (Table 1). If autologous to the prospective recipient, then transplants, in theory, would require minimal or no immunosuppression, and therefore the recipient would be significantly less likely to experience immunological rejection and immunosuppression-related complications such as opportunistic infections, cancer, diabetes, bone marrow suppression, and kidney failure, among others. For applications where autoimmunity is not an issue, autologous iPSC-derived cells may offer significant advantage, whereas in the presence of autoimmunity it is not clear to what extent autologous iPSC-derived cells would be destroyed in the absence of immunological protection. On the other hand, in applications where the cellular product may function in an immunoprotective environment (e.g. micro or macro-encapsulated cells, immune-privileged transplant sites), then allogeneic cells may be equally effective as autologous cells, and could circumvent some cost and regulatory barriers.
Table 1.
Advantages and disadvantages of hPSCs from varying sources. Total anticipated costs of a therapy are difficult to estimate because for different applications there would be different indirect costs, some of which would include immunosuppressive drugs, encapsulation devices and/or other cellular and matrix components.
| Syngeneic iPSCs | Allogeneic iPSCs | Allogeneic ESCs | |
|---|---|---|---|
| Adaptive Immune Response | No (?) | Yes | Yes |
| Susceptible to autoimmunity | Possible (?) | Unknown | Unknown |
| Immunosuppression or Immunoprotection Needed | No (?) | Yes | Yes |
| Epigenetic Memory | Maybe | Maybe | No |
| Differentiation Capacity | May be influenced by input cell | May be influenced by input cell | All tissue types |
| Tissue Source | Varies | Varies | Embryonic |
| Genetic Disease - Gene Correction? | Yes | Yes | Yes |
| Manufacturing | Months (custom made) | Ready to use | Ready to use |
| Expected cost to produce cells | High | Low to moderate | Low |
IMMUNOGENICITY OF AUTOLOGOUS PSCs
A major obstacle hindering the development of alternatives for repairing or replacing tissues or organs is immunogenicity. In addition to being a scalable and abundant resource from which to generate replacement tissues, iPSCs have the important attribute of enabling differentiation of patient-specific autologous tissues potentially devoid of many of the immunogenic properties that hinder modern allogeneic organ transplants. This key quality of iPSCs may reduce or eliminate alloimmunity (i.e. transplant rejection) thereby obviating or reducing the need for immunosuppressive drugs to maintain function of the graft [50]. However, whether recipients would display donor-specific unresponsiveness to autologous iPSC-based therapies has been called into question by several studies, although subsequent research has allayed some of the concerns. Indeed, changes on the epigenomic and protein expression level that can arise from reprogramming, gene editing and culturing could potentially generate immunogenic antigens [51, 52].
A 2011 study by Zhao et al. was the first to address the question of immunogenicity of autologous iPSCs. This study concluded that iPSC-derived tissues may indeed be immunogenic [53] in that they observed the lack of teratoma formation after inoculation of autologous undifferentiated mouse iPSCs into C57BL/6 mice, which they attributed to an immunological, rejection-like response. Through comparative gene expression studies they attributed the rejection of the cells and absence of teratomas to the abnormal expression of two genes, Hormad1 and Zg16. This finding was alarming and seemed to call into question a fundamental utility of this novel cell source. Even though these findings were described in the mouse, and only focused on the immunogenicity of undifferentiated iPSCs or iPSCs in the process of differentiating in vivo, they nonetheless suggested that autologous iPSC self-tolerance should not be assumed. Careful experimentation, ideally with human cells, is needed to conclusively determine whether iPSC-derived tissues will indeed be tolerated by the autologous host immune system or if these differentiated tissues will elicit an immune response detrimental to engraftment and/or graft function.
Two groups in 2013, both using mouse models but including more clinically relevant terminally differentiated iPSC-derived tissues, challenged the results from Zhao et al. and showed iPSCs and iPSC-derived tissues survived in syngeneic hosts. Araki et al. [54] used skin grafts and bone marrow from chimeric PSC-derived mice, showing negligible immunogenicity. An important caveat is that they did see immune cell infiltration in supplementary experiments with impure iPSC-derived cardiomyoctyes. They did not, however, conclusively determine whether these infiltrating T cells were cytotoxic or regulatory in nature. Guha et al. [55] looked at in vitro PSC-differentiated endothelial cells, hepatocytes and neuronal mouse cell types in their study and did not find evidence of immune rejection. De Alemeida et al., also in the murine model, performed a more in depth investigation of the specific immune responses to both teratomas and iPSC-derived endothelial cells [56]. The authors showed markedly different immune responses on the clonal and functional level in the different tissue types, the latter being infiltrated by tolerogenic T cells and macrophages and the former by cytotoxic T cells. This more detailed investigation of the graft infiltrating cells and their associated cytokines and cytolytic proteases offers strong evidence that autologous iPSC-derived tissues will be tolerated in a clinical setting.
It may be difficult to make direct comparisons between the Zhao et al. report and the others, due to the many technical differences in the reprogramming methods, cell lineage, cellular differentiation states and transplantation sites used. That said, the approach used by Zhao et al. is not directly applicable to proposed clinical uses of PSCs, since the aim of any cellular therapy would be to minimize any transfer of undifferentiated PSCs and only implant purified cell populations.
The results from these papers, as well as from Morizane et al. [57], also suggest that the method of somatic cell reprogramming may have impacted the immunogenicity of the differentiated cells. Working in a non-human primate system, Morizane et al. demonstrated that viral integration of “first-generation” reprogramming factors can elicit an immune response while non-viral, non-integrating (“second-generation”) episomal strategies exhibit greatly reduced immunogenicity. Integrating vectors in general are capable of producing immunogenic polypeptides, potentially instigating an immune response against the genetically engineered cells [58, 59]. In contrast, non-integrating vectors are designed specifically to be devoid of immunogenic viral factors and the vector DNA is lost from the cell lines within relatively few passages of the initial transfection [19]. Indeed, the Zhao et al. study used integrative reprogramming methods, and in retrospect, it is possible that immunological responses to neoantigens produced through reprogramming could have accounted for the reduced teratoma formation from the inoculated cells in this study. Thus, data are accumulating now showing iPSCs generated using “second-generation” vectors yield differentiated products which are tolerated in a syngeneic host, though much of the data to date are related to non-human iPSCs derivatives.
Another potential mechanism of unexpected immunogenicity of autologous iPSCs is the development of immunogenic proteins due to spontaneous gene mutations. Mutations arising in somatic cells in culture prior to reprogramming or in the reprogrammed iPSCs during subsequent culture could theoretically contribute to iPSC immunogenicity. Provided such mutations do not involve the HLA genes directly, any antigen that develops would be considered a minor histocompatibility antigen. For the affected protein to be immunogenic, the mutation would need to be located within a peptide fragment that would be presented by specific donor or recipient HLA molecules, and the mutated peptide would need to be capable of activating an antigen-specific T cell [60]. While the risk of an immunogenic mutation developing is real, this risk is tempered by the strict requirements to achieve immunogenicity.
Very recently, the laboratory of Yang Zu that produced the 2011 Zhao et al. [53] paper described an elegant strategy to test immunogenicity of hPSC derivatives in an autologous setting. They evaluated hiPSC-derived retinal pigmented epithelium (RPE) and smooth muscle cell (SMC) syngeneic transplants in humanized mice where the iPSC cells were derived from the same tissues that reconstituted the immune system within the mice (see Humanized Mouse Models section below for descriptions of these and other mice) [61]. Interestingly, they observed differential immune responses depending on the cell type and transplantation location, with rejection of autologous iPSC-SMC grafted into skeletal muscle and acceptance of both autologous and allogeneic iPSC-RPE injected into the immune-privileged subretinal space. The exact mechanisms for the differential immune responses were not explored; instead the authors used immune cell infiltration as an indication of rejection and a lack of infiltration as evidence of tolerance. Deeper level examination of infiltrating lymphocytes is warranted, as previous studies have shown that infiltration alone can be correlated with tolerance e.g. if the infiltrating cells are Foxp3+ CD4+ T cells instead of cytotoxic CD8+ cells [62]. Additionally, Zhao et al. demonstrate that aberrant expression of hormad1 and Zg16 are associated with immune cell infiltration, which is a similar finding to their previous report showing immune responses to syngeneic mouse iPSCs. The recent paper by Zhao et al. highlights the need for a greater understanding of how different cell types transplanted to different sites influence host immune responses, as well as the role that terminal cell differentiation protocols play in introducing immunogenic gene expression patterns in the cells. In the aforementioned reports it is important to note that immunogenicity associated with aberrant gene expression may be a surmountable obstacle particular to specific differentiation protocols/methodologies. In other words, immune rejection may be a natural process resulting from flawed differentiation, rather than from a response to inherent properties of iPSCs or their differentiated products. The utility of humanized mouse models for studying these critical issues will be discussed in more detail below.
Many of the hiPSCs currently in use for pre-clinical experimentation, as well as for the first and only iPSC trial in progress [63–66] utilize “second-generation” episomal reprogramming methodologies. Nonetheless, given the aforementioned conflicting data, further work is needed to verify whether autologous human iPSC-derived tissues would be susceptible to destructive adaptive or innate immunity in immunocompetent hosts. Studies to date on this topic have been limited. Two reports utilizing autologous neuronal cell types derived from iPSCs in the non-human primate model showed enduring engraftment and tolerance of the iPSC-derived cells without the requirement for immunosuppression [67, 68]. These results are quite promising, giving evidence to the one of the fundamental promises of iPSC technology--that autologous tissues can be used for regenerative medicine applications without the need for immunosuppression. However, the use of cells introduced into the immune-privileged site of the brain in these reports, still leaves unanswered as to how a functional immune system would recognize more immunogenic tissue transplants in less immunologically privileged sites such as skin derived from patient-specific iPSCs. Further work in non-human primates will no doubt shed light on this question. However, due to the expense and lack of widespread availability of non-human primates, we believe that future preclinical investigations into the immunogenicity of human PSCs will be buttressed by use of humanized mouse models, as demonstrated by the very recent Zhao et al. paper above and discussed further in the next section below. Moreover, we feel it is critical in early human clinical trials to study immune responses of autologous and allogeneic PSC-derivatives.
Lastly, it is important to mention that for disease applications such as Type 1 diabetes, there may also be risks of destruction of autologous iPSC-derived beta cells. No studies to date have studied this possibility however. Pancreas transplants performed in the 1980s between identical twins without immunosuppression that resulted in recurrent insulitis in the graft [69, 70] would predict that human autologous iPSC-derived beta cells would ultimately be susceptible to recurrent autoimmune destruction in the recipient. In this setting then it is likely that some means of immunoprotection would be required for both allogeneic and autologous iPSC-derived beta cells. On the other hand, non-autoimmune forms of diabetes, e.g. Type 2 diabetes, could be targeted by autologous stem cell treatments.
HUMANIZED MOUSE MODELS
Humanized mouse models offer a tractable in vivo system to experimentally test therapeutic interventions in the context of a functional human immune system. These model systems have the potential for comprehensive analysis of a patient-specific immune system repertoire and its interaction with autologous and allogeneic iPSC-derived tissues, thereby offering valuable insight into questions of PSC immunogenicity and tolerance required for clinical translation of these technologies.
Humanized mouse models applicable to PSC studies fall into three basic categories, each based on the concept of engrafting human hematopoietic and lymphoid tissues into an immunocompromised mouse. The first type, often referred to as the “Hu-PBL” model, is created by injecting human peripheral blood leukocytes intravenously into the mouse, with and without radiation conditioning [71]. The animals are useful for studies where a quick determination of immunogenicity is needed, as they are prone to developing Graft vs Host Disease (GVHD) within 4–8 weeks of cell injection. The second type of model is known as the “Hu-HSC model” [72]. This model is created by intravenous injection of HSCs which then engraft and differentiate into the various hematopoietic cell types within the mouse. These animals allow for longer-term experiments and are especially useful for studies of HSC engraftment, including studies involving PSC-derived HSCs/hematopoietic progenitor cells (HPCs) [73, 74]. Hu-HSC animals have a longer experimental window prior to the onset of GVHD, but are suboptimal for studies that seek to understand how T cells, in particular, encounter PSC-derived tissues, owing to the suboptimal T cell development seen in these models.
The third type of humanized mouse model circumvents the above-mentioned limitations, and is most relevant to PSC studies. This model is called the “BLT” mouse [75, 76], and more recent iterations of this model are referred to as the “Humanized Immune Mouse (HIM)” or the “Personalized Immune (PI)” mouse [77]. The common technique used in this type of humanized mouse model is the I.V. injection of human HSCs coupled with the incorporation of a human thymus and liver fragment surgically implanted under the mouse kidney capsule which facilitates T cell development in an HLA-restricted manner. The resultant mice harbor a human immune repertoire with clinically relevant distribution frequencies, crucially containing de novo generated T cells. As T cells are a critical component of the adaptive response in tissue transplantation, these animals are well suited for PSC transplantation studies.
Further iterations of the “HIM”/“PI” models are currently being developed by our lab and others to enhance the human cytokine profile within the animals [78, 79] as well as explore the use of more developmentally mature thymic tissue and PSC-derived thymic epithelial cells (TECs) instead of patient tissue explants [80, 81]. The use of PSC-derived TECs or TEC progenitors is an intriguing future direction for research which could solve the issue of passenger-lymphocyte related GVHD in the mice, as well as creating patient-specific thymic niches to study T cell development and function.
To date, the use of BLT/HIM/PI mice in PSC immunogenicity studies has been limited [6], compared to their successful use in other fields, e.g. HIV [82, 83], and we believe this is a fruitful avenue for future research. Rong et al. [6] demonstrated the utility of the HIM model for studying alloreactivity of hESC-derived cell types, as well as induction of tolerance of those cell types upon genetic introduction of costimulatory blockade molecules (discussed below in Modulation of PSCs). Future research should include examination of how human iPSC-derived cells interact with a syngeneic immune repertoire within these humanized mice, similar to the recent paper by Zhao et al. [61]. Determining the specific T cell subsets involved in the immune response to autologous and allogeneic iPSC-derived tissue will be critical. Additionally, as Zhao et al. demonstrate, the differentiation protocol used to create the transplanted iPSC-derived cells may inadvertently introduce aberrant gene expression patterns into the cells, resulting in immunogenicity. Humanized mouse models may fill a crucial niche in translating PSC therapies to the clinic by allowing for careful experimental observation of the interaction between PSC-derived tissues and the syngeneic human immune system, which cannot be studied using traditional mouse or non-human primate models.
STRATEGIES TO REDUCE IMMUNOGENICITY OF HUMAN PSCs
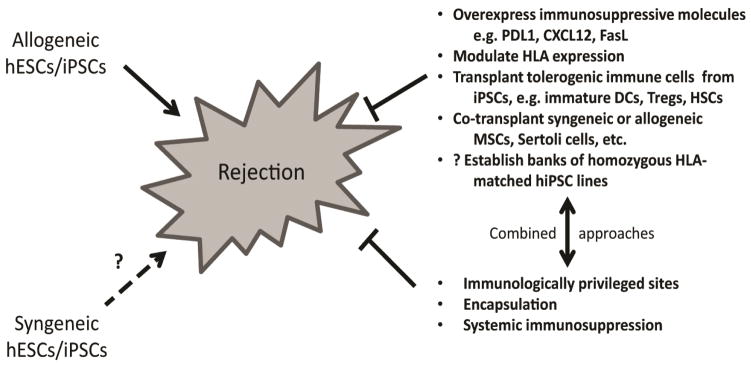
A variety of strategies for down-regulating immune responses to allogeneic hPSC-derived therapeutic cell populations are being evaluated. Figure 2 provides an overview of these approaches and the following sections will review work reported to date in several thematic areas.
Figure 2.
Potential Strategies to Reduce Immunogenicity of Allogeneic Human Pluripotent Stem Cell (PSC)-Derived Therapeutic Cellular Grafts. Allogeneic hPSCs would most likely elicit rejection and be susceptible to destruction via adaptive immunity without modification, immunosuppression or encapsulation. Whether autologous hiPSCs would be tolerated by the immune system of an immunocompetent donor/recipient is unclear presently and may be dependent on the method of reprogramming, the cell line, the cell type, transplant site and the purity of the transplanted cells. A number of strategies, alone and in combination, are being tested to abrogate or down-regulate immune responses.
Direct Immunomodulation of PSCs
With advancements in the efficiency of gene editing technologies, modification of PSCs and their progeny can now be readily achieved. Most reports of PSC gene editing to date have been in the context of either correcting gene mutations to reverse the pathological cellular phenotype, inserting new mutations to endogenous genes to study effects on function or insert gene expression reporters [84, 85]. Relatively few studies exist to date where the genetic manipulation, either done with or without gene editing achieved an immunomodulatory effect on the cells or altered the host immune system in some way. There are a few examples, however. Rong et al. used an efficient bacterial artificial chromosome (BAC)-based strategy to knock-in PDL1 and CTLA4-Ig into hESCs lines, yielding progeny cells capable of eliciting co-stimulatory blockade and thus tolerance in an allogeneic humanized mouse system [6]. The results illustrate encouraging applications of recent advances in our understanding of co-stimulatory pathways. Moreover, the use of genetic engineering to confer ectopic expression of an immunosuppressive or tolerogenic molecule for preventing rejection of an iPSC graft has several advantages and opens up many possibilities for modifying host responses to allogeneic PSCs. One potential disadvantage of this approach is that the genetic modification designed to allow cells to “escape the immune system” could simultaneously render the recipient more vulnerable to teratoma growth if residual pluripotent stem cells are present.
An additional example involves the use of T cell reprogramming mentioned above. By selecting an input T cell clone with known TCR gene rearrangement and peptide/MHC specificity, reprogramming to iPSCs and differentiation back to T cells one can generate a monoclonal T cell population with TCR specificity matching the input cell clone. This shares a similar goal to traditional approaches of modifying mature patient T cells via transduction of a cloned TCR [30], but with certain potential advantages. By selecting for a naturally occurring TCR rearrangement, and in effect locking it into the progeny cells, this method sidesteps some of the problems reported with the traditional methods, namely mispairing of ectopically expressed and endogenous TCR chains which can result in autoreactivity and immunogenicity [86, 87].
Another example is of the disruption of the CCR5 gene in hematopoietic stem cells (HSCs) using gene editing approaches, recapitulating the HIV controller phenotype of the “Berlin patient” when the modified HSCs were transferred to immunodeficient mice [88].
Gene Editing and Techniques
In recent years, efficient gene modification using zinc finger nucleases ushered in a wave of increasingly efficient approaches to genome engineering within human cells, including PSCs [89, 90]. The use of zinc finger nucleases, transcription activator-like effector nucleases (TALENs) and, most recently, the clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas RNA-guided DNA endonucleases have opened a new era of targeted gene editing with fewer off-target effects and much greater efficiency than traditional approaches such as homologous recombination [91]. Hou et al. demonstrated efficient targeting of endogenous genes in iPSCs using the CRISPR system, effectively demonstrating the utility of this approach for a wide variety of potential modifications in PSCs [92]. The relative ease of use, and ability to target multiple loci in one experiment, make CRISPR an attractive approach for experimental and potentially therapeutic genetic engineering.
Improvements in traditional homologous recombination strategies are also being effectively used to genetically engineer PSCs. Methods of enhancing homologous recombination efficiency in human cells have been designed such as by using viral gene-free helper-dependent adenoviral vectors (HDAdVs) to deliver large pieces of DNA into the cell [93]. The ZFN, TALEN, CRISPR and HDAdV methods have already been used to correct several genetic diseases in patient-derived iPSCs [94–96], unfortunately an in-depth analysis of the genomic, epigenomic, and immunogenicity changes incurred through these approaches is lacking. A 2012 study by Liu et al. is perhaps one of the more complete analyses of gene-corrected iPSCs to date. Using the HDAdV system and analyzing copy number variations (CNV), single nucleotide polymorphisms (SNP), RNA expression profiles and methylation patterns, they determined that their gene-corrected iPSCs maintained the genomic and epigenomic integrity of the parental line [96]. It should be noted that to date, there have been few in depth studies performed to verify a lack of immunogenicity with the above-mentioned editing methods themselves [97]. In summary, the development of efficient gene editing methods enables the correction of gene defects in autologous iPSCs, yet it is important for the “gene-corrected cells” to be tested for potential immunogenicity. In addition, genome editing opens up exciting opportunities for modifying the immunogenicity of PSCs.
IMMUNOLOGICALLY PRIVILEGED TISSUES AND SITES
Another important factor to consider in using patient-specific iPSCs is that certain cell types may be more or less immunogenic than others (e.g. keratinocytes vs. Sertoli cells, SMCs vs RPE). Although it is well known that native tissues possess different capacities to stimulate the immune system, it is not well understood what underlies these differences. One possibility is the relative expression levels of MHC Class I on parenchymal cells while other possibilities include differences in the relative abundance of antigen presenting cells or density of endothelial cells in the grafted tissue. Thus, separate derivations of pure populations of differentiated parenchymal cells and antigen presenting cells from iPSCs and subsequent experimental manipulation could provide a platform for dissecting the mechanisms behind this poorly understood phenomenon.
Certain anatomical regions of the body appear to be relatively protective of allografts, at least in experimental rodent models. Although for a given therapeutic application, it is often not possible to control either cell type or route of administration, it is worthwhile to consider options in cases where there is flexibility. Immunologically privileged sites in rodents have been described and include the anterior chamber of the eye [98, 99], thymus [100, 101], pedicled skin graft [102] and intra-abdominal testes [103, 104], among others. It may be wise to allocate resources for the study of autologous iPSC transplantation of certain tissues that are weakly immunogenic and/or are being transplanted into immune-privileged anatomical locations. For example, vocal fold tissue [62], mesenchymal stem cells [105], neurons, and liver are generally considered less immunogenic than tissues such as skin, lung and small bowel. To this point, two early PSC clinical trials have taken advantage of transplanting weakly immunogenic tissues into the “immune-privileged” sites of the spinal cord [106–108] and the retina [65]. In determining the utility of PSC-derived cellular therapies it may be advantageous to continue to focus early translational studies on these types of tissues--and to develop and characterize additional PSC-derived tissues for inherent immunogenicity--until progress has been made on answering questions regarding patient-specific iPSC immunogenicity and cost-effective scale up. Nonetheless, transplanting cell types having lower immunogenicity or modified immunogenicity, or in tandem with support cells that modulate immunogenicity, into more immunologically privileged sites may be advantageous approaches.
HLA TYPED iPSC BANKS
In light of the anticipated high costs of developing large-scale personalized iPSC therapies [5], an alternative approach of banking multiple donors with known HLA status has been proposed. The idea is that, in the near-term while the expenses of personalized therapies would likely be formidable, one could take advantage of iPSC technology by proactively seeking out rare individuals that are homozygous for different HLA alleles that would potentially HLA-match a large segment of the population of recipients in need of a transplant. From these HLA homozygous donors one would create a “bank” of lines all homozygous for different HLA haplotypes (i.e. so-called haplobanks). It has been proposed that a bank of <100 cell lines would allow for matching to a majority of the population [109–113]. Some of the pros and cons of this approach have been reviewed elsewhere [114]. For example, it has been estimated that 50 donors could provide matched tissue for over 90% of the Japanese population, albeit after screening from a population of 24,000 donors [112] or a bank of 150 selected HLA typed volunteers could match 93% of the UK population [109]. The utilization of “super donors”, those individuals with type O negative blood and homozygous HLA alleles, may be a particularly effective approach, though finding such individuals will require extensive screening of the population [115].
Although PSCs and their differentiated products were once considered to be less immunogenic than adult tissues [116], recent research has demonstrated robust immune responses to allogeneic PSC-derived tissues [55], illustrating the need for careful consideration of appropriate HLA matching of PSC-derived tissues. Due to the degree of screening required to cover different blood types, HLA haplotypes, and ethnicities, as well as other considerations, the HLA matched cell line banking approach rapidly becomes less feasible than it seemed at first glance. Indeed, the reported estimates of required bank size are based on populations that are considerably more homogeneous than the US population and therefore may underestimate the necessary number of cell lines required in the bank. For instance, Gourraud et al. estimated that due to the diversity of ethnicities in the US population one would need to screen between 26,000 European Americans and 110,000 African Americans to generate enough cell lines and that 100 cell lines would exclude 22% of European Americans, 37% of Asians, 48% of Hispanics and 55% of African Americans [110]. Zimmermann et al. also point out the fact that the limited number of HLA-compatible loci and HLA typing resolution complicate the establishment of a bank of cell lines that would be reliably non-immunogenic to the majority of patients [117].
Experience in human solid organ transplantation predicts significant challenges to the HLA haplobank strategy. A fundamental transplant immunology concept is that minor antigen mismatches can indeed elicit rejection in HLA matched transplants. For example, it is well known that kidney transplants between HLA identical siblings still typically require at least two drug immunosuppressive therapy to prevent rejection. In this discussion it is also worth considering that only 8% of deceased donor kidney transplants in the US are zero mismatched (HLA-matched) despite mandatory sharing. Furthermore, a straightforward analysis reveals that it is not feasible to cover all HLA haplotypes in a proposed bank. If one considers the 10 most common HLA-A, B, DR haplotypes in Caucasians, then the top 10 haplotypes represent only 24% of the most common Caucasian haplotypes representing only ~6% of all HLA genotypes. The likelihood of finding a Caucasian who is homozygous for the 10th most common HLA haplotype is 1 in 10,000 individuals. Finding a match to the remainder of the Caucasian population (76%) with more unusual HLA haplotypes would require screening exponentially more individuals, not to mention other ethnicities or mixed ethnicity individuals. Another point worth mentioning in this context is that HLA-DPB1, a recognized target for rejection in organ and hematopoietic stem cell transplants, is not in linkage disequilibrium with the other loci comprising the HLA complex. As a consequence, HLA haplotype frequencies (which are calculated using only HLA loci in linkage disequilibrium) underestimate the number of potential donors and iPSC bank size required to cover a reasonable portion of the population. While not a precise analysis, this does speak to the challenges of creating a cell line bank that would cover most of the world population.
The very nature of designing tissue banks for a majority of a given country’s population will potentially aggravate one of the issues that plague current clinical trial design and implementation; the lack of participants from traditionally underrepresented groups within the population i.e. women, ethnic minorities and people from economically disadvantaged backgrounds [118]. Thus, further discussion is warranted within the stem cell and healthcare communities to determine the most cost-effective short and long-term plans to translate PSCs to the clinic, and to do so in a way that benefits as many members of society as possible. Further research to clarify the immunogenicity and associated potential need for immunosuppressive drugs in the context of autologous iPSC transplantation will help guide the decision making process.
TOLEROGENIC IMMUNE CELLS FROM INDUCED PSCs
Another salient experimental approach designed to temper immune responses to therapeutic iPSC-derived cells is to generate specific cells having tolerogenic or immunomodulatory properties. As protocols for differentiating hPSCs into defined cell types become more refined and able to generate highly-enriched/nearly pure cell populations, it becomes feasible to test the effects of such cells on host innate and adaptive immune responses in a rigorous manner, and further, it becomes possible to derive immunomodulatory cells from patient iPSCs. Mesenchymal stromal or stem cells (MSCs) have been derived from hPSCs [119, 120] and have been shown to dampen innate immunity of transplanted islets (reviewed in [121]). The Zavazava group demonstrated that iPSC-derived hematopoietic progenitor cells are capable of inducing anergy in T cells in an in vitro setting [122]. These are two examples but other possibilities exist as well. If regulatory T cells and immature dendritic cells could be efficiently differentiated from hiPSCs or hESCs, then one could explore whether these cell populations could enhance graft survival of allogeneic or autologous hPSC-derived cell transplants. The field is likely to see further work in this area in the coming years as differentiation protocols continue to evolve.
ENCAPSULATION
In some therapeutic applications of PSC derivatives (i.e. PSC-derived beta cells) it would be permitted for the therapeutic cell population to be isolated from host tissues using macro- or microencapsulation techniques. The principle goal of encapsulation is to immunoisolate transplanted cells by preventing access by T and B cells and immunoglobulins. A full discussion of the many macroencapsulation devices in development and the various approaches to microencapsulation are beyond the scope of this review – the reader is instead referred to recent reviews such as by Tomei, Villa and Ricordi [123]. Numerous challenges remain in achieving optimal immunoisolation by encapsulation including overcoming foreign body reaction, hypoxia induced celI death, bioincompatibility, biofouling, loss of capsule integrity, lack of retrievability, and volume/packing density issues, among others. It is notable, however, that macroencapsulation using a proprietary, optimized Theracyte®-like device has shown promise in preventing rejection of human pancreatic progenitor cells and enriched endocrine cell populations in rodent studies and is currently in early clinical trials [124].
CONCLUDING REMARKS
Human PSCs are now widely considered the future foundation of regenerative medicine. As this science continues to mature, stem cell researchers will increasingly rely upon the knowledge base and expertise developed by the tissue transplantation field over the past 50 years. Understanding the innate and adaptive host immune responses to various cell and tissue types derived from either allogeneic hESCs/iPSCs, or autologous hiPSCs will be critical to successful clinical translation. Equally important is to understand how different routes of administration/transplant sites, and co-transplantation with other biomaterials may affect immunogenicity. Improved humanized murine models are needed for studying human-specific immune responses, but mouse models and non-human primate models will also play a role. Studies refining our understanding of the epigenetic effects of various reprogramming methods are also needed. Gene editing technology used in conjunction with autologous iPSCs undoubtedly opens up numerous possibilities for personalized cell replacement therapies for rare diseases, yet the nature of the immune responses to these “gene corrected” cells has not be defined. Devising safe and effective methods to cell-autonomously or indirectly down regulate host immune responses will be essential to optimizing the clinical potential of stem cell therapies. It will be exciting to see the rapid advances over the next decade as research is carried out at the nexus of transplant immunology and stem cell biology.
Acknowledgments
The authors would like to acknowledge that this work was in part supported by grants from the Juvenile Diabetes Research Foundation and the American Society of Transplant Surgeons (to J.S.O.) and by NIH grant R01-AI066219 to W.B. M.E.B was supported under NIH awards UL1TR000427 and TL1TR000429. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy, Nature reviews. Molecular cell biology. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.McKernan R, Watt FM. What is the point of large-scale collections of human induced pluripotent stem cells? Nature biotechnology. 2013;31:875–877. doi: 10.1038/nbt.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott C. The business of exploiting induced pluripotent stem cells, Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2011;366:2323–2328. doi: 10.1098/rstb.2011.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, Yi H, Goldrath A, Yang YG, Xu Y, Fu X. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell stem cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Journal of embryology and experimental morphology. 1962;10:622–640. [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 15.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nature methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higuchi A, Ling QD, Kumar SS, Munusamy MA, Alarfaj AA, Chang Y, Kao SH, Lin KC, Wang HC, Umezawa A. Generation of pluripotent stem cells without the use of genetic material. Lab Invest. 2015;95:26–42. doi: 10.1038/labinvest.2014.132. [DOI] [PubMed] [Google Scholar]
- 18.Augustyniak J, Zychowicz M, Podobinska M, Barta T, Buzanska L. Reprogramming of somatic cells: possible methods to derive safe, clinical-grade human induced pluripotent stem cells. Acta Neurobiol Exp (Wars) 2014;74:373–382. doi: 10.55782/ane-2014-2000. [DOI] [PubMed] [Google Scholar]
- 19.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ. A comparison of non-integrating reprogramming methods. Nature biotechnology. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nature methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 21.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, Bao X, Tse HF, Grillari J, Grillari-Voglauer R, Pei D, Esteban MA. Generation of human induced pluripotent stem cells from urine samples. Nature protocols. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 23.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Ng K, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, Zitur LJ, Learish RD, Nuwaysir EF. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PloS one. 2010;5:e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajesh D, Dickerson SJ, Yu J, Brown ME, Thomson JA, Seay NJ. Human lymphoblastoid B-cell lines reprogrammed to EBV-free induced pluripotent stem cells. Blood. 2011;118:1797–1800. doi: 10.1182/blood-2011-01-332064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Ait-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes & development. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, Takayama N, Yamada D, Nishimura K, Ohtaka M, Watanabe N, Takahashi S, Iwamoto A, Koseki H, Nakanishi M, Eto K, Nakauchi H. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell stem cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell stem cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell stem cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PloS one. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, Miller J, Schlaeger T, Daley GQ, Feinberg AP. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature genetics. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyanagi-Aoi M, Ohnuki M, Takahashi K, Okita K, Noma H, Sawamura Y, Teramoto I, Narita M, Sato Y, Ichisaka T, Amano N, Watanabe A, Morizane A, Yamada Y, Sato T, Takahashi J, Yamanaka S. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20569–20574. doi: 10.1073/pnas.1319061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Lee JB, Shapovalova Z, Fiebig-Comyn A, Mitchell RR, Laronde S, Szabo E, Benoit YD, Bhatia M. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nature communications. 2014;5:5605. doi: 10.1038/ncomms6605. [DOI] [PubMed] [Google Scholar]
- 36.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature biotechnology. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature biotechnology. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature cell biology. 2011;13:541–549. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell stem cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Firas J, Liu X, Polo JM. Epigenetic memory in somatic cell nuclear transfer and induced pluripotency: evidence and implications. Differentiation; research in biological diversity. 2014;88:29–32. doi: 10.1016/j.diff.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PloS one. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature genetics. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94:451–454. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacoby M, Gohrbandt S, Clausse V, Brons NH, Muller CP. Interindividual variability and co-regulation of DNA methylation differ among blood cell populations. Epigenetics : official journal of the DNA Methylation Society. 2012;7:1421–1434. doi: 10.4161/epi.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Qin J, Zhao RC, Zenke M. Reduced immunogenicity of induced pluripotent stem cells derived from Sertoli cells. PloS one. 2014;9:e106110. doi: 10.1371/journal.pone.0106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kandaswamy R, Skeans MA, Gustafson SK, Carrico RJ, Tyler KH, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: pancreas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(Suppl 2):1–20. doi: 10.1111/ajt.13196. [DOI] [PubMed] [Google Scholar]
- 48.I.D. Federation. International Diabetes Federation 2014. 2014. IDF Federation Atlas Sixth Edition Update. [Google Scholar]
- 49.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124:3201–3211. doi: 10.1182/blood-2014-07-589176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyd AS, Rodrigues NP, Lui KO, Fu X, Xu Y. Concise review: Immune recognition of induced pluripotent stem cells. Stem cells. 2012;30:797–803. doi: 10.1002/stem.1066. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz S, Diep D, Gore A, Panopoulos AD, Montserrat N, Plongthongkum N, Kumar S, Fung HL, Giorgetti A, Bilic J, Batchelder EM, Zaehres H, Kan NG, Scholer HR, Mercola M, Zhang K, Izpisua Belmonte JC. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16196–16201. doi: 10.1073/pnas.1202352109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene therapy. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 53.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 54.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 55.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell stem cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 56.de Almeida PE, Meyer EH, Kooreman NG, Diecke S, Dey D, Sanchez-Freire V, Hu S, Ebert A, Odegaard J, Mordwinkin NM, Brouwer TP, Lo D, Montoro DT, Longaker MT, Negrin RS, Wu JC. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nature communications. 2014;5:3903. doi: 10.1038/ncomms4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem cell reports. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo E, Akatsuka Y, Nawa A, Kuzushima K, Tsujimura K, Tanimoto M, Kodera Y, Morishima Y, Kuzuya K, Takahashi T. Retroviral vector backbone immunogenicity: identification of cytotoxic T-cell epitopes in retroviral vector-packaging sequences. Gene therapy. 2005;12:252–258. doi: 10.1038/sj.gt.3302406. [DOI] [PubMed] [Google Scholar]
- 59.Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 60.Warren EH. Minor histocompatibility antigens in allogeneic hematopoietic cell transplantation. Current Opinion in Organ Transplant. 2006;11:31–36. [Google Scholar]
- 61.Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, Rong Z, Kim J, He J, Wang M, Clegg DO, Yang YG, Zhang K, Friedlander M, Xu Y. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell stem cell. 2015;17:353–359. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling C, Li Q, Brown ME, Kishimoto Y, Toya Y, Devine EE, Choi KO, Nishimoto K, Norman IG, Tsegyal T, Jiang JJ, Burlingham WJ, Gunasekaran S, Smith LM, Frey BL, Welham NV. Bioengineered vocal fold mucosa for voice restoration. Science translational medicine. 2015;7:314ra187. doi: 10.1126/scitranslmed.aab4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem cell reports. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cyranoski D. Stem cells cruise to clinic. Nature. 2013;494:413. doi: 10.1038/494413a. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 66.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neuroscience letters. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 67.Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang SC. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell reports. 2013;3:646–650. doi: 10.1016/j.celrep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, Schumacher JM, Spealman RD, Isacson O. Successful Function of Autologous iPSC-Derived Dopamine Neurons following Transplantation in a Non-Human Primate Model of Parkinson’s Disease. Cell stem cell. 2015 doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–144. [PubMed] [Google Scholar]
- 70.Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Suppl 1):85–87. doi: 10.2337/diab.38.1.s85. [DOI] [PubMed] [Google Scholar]
- 71.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, Laning J, Fodor W, Foreman O, Burzenski L, Chase TH, Gott B, Rossini AA, Bortell R, Shultz LD, Greiner DL. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McIntosh BE, Brown ME, Duffin BM, Maufort JP, Vereide DT, Slukvin, Thomson JA. Nonirradiated NOD,B6.SCID Il2rgamma(−/−)Kit(W41/W41) (NBSGW) Mice Support Multilineage Engraftment of Human Hematopoietic Cells. Stem cell reports. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian X, Hexum MK, Penchev VR, Taylor RJ, Shultz LD, Kaufman DS. Bioluminescent imaging demonstrates that transplanted human embryonic stem cell-derived CD34(+) cells preferentially develop into endothelial cells. Stem cells. 2009;27:2675–2685. doi: 10.1002/stem.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slukvin Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122:4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature medicine. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 76.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 77.Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, Greenberg E, Spitzer TR, Savage DG, Tahara H, Choi G, Yang YG, Sykes M. A model for personalized in vivo analysis of human immune responsiveness. Science translational medicine. 2012;4:125ra130. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. Development and function of human innate immune cells in a humanized mouse model. Nature biotechnology. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parent AV, Russ HA, Khan IS, LaFlam TN, Metzger TC, Anderson MS, Hebrok M. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell stem cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell stem cell. 2013;13:230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP, Eisenbarth S, Eynon E, Flavell RA, Guzman CA, Huntington ND, Kremsdorf D, Manns MP, Manz MG, Mention JJ, Ott M, Rathinam C, Rice CM, Rongvaux A, Stevens S, Spits H, Strick-Marchand H, Takizawa H, van Lent AU, Wang C, Weijer K, Willinger T, Ziegler P. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell host & microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, Phillips K, Scott D, Peterson KE, Chan CK, Dittmer U, Dudek T, Allen TM, Weissman IL, Hasenkrug KJ. BLT-humanized C57BL/6 Rag2−/−gammac−/−CD47−/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122:4013–4020. doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vasileva EA, Shuvalov OU, Garabadgiu AV, Melino G, Barlev NA. Genome-editing tools for stem cell biology. Cell Death Dis. 2015;6:e1831. doi: 10.1038/cddis.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech. 2013;6:896–904. doi: 10.1242/dmm.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nature medicine. 2010;16:565–570. 561–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 87.van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, van Rood JJ, Falkenburg JH, Heemskerk MH. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, Cannon PM. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nature biotechnology. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moehle EA, Rock JM, Lee YL, Jouvenot Y, DeKelver RC, Gregory PD, Urnov FD, Holmes MC. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3055–3060. doi: 10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Zhang WY, Hu S, Lan F, Lee AS, Huber B, Lisowski L, Liang P, Huang M, de Almeida PE, Won JH, Sun N, Robbins RC, Kay MA, Urnov FD, Wu JC. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circulation research. 2012;111:1494–1503. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in biotechnology. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, Thomson JA. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aizawa E, Hirabayashi Y, Iwanaga Y, Suzuki K, Sakurai K, Shimoji M, Aiba K, Wada T, Tooi N, Kawase E, Suemori H, Nakatsuji N, Mitani K. Efficient and accurate homologous recombination in hESCs and hiPSCs using helper-dependent adenoviral vectors. Mol Ther. 2012;20:424–431. doi: 10.1038/mt.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun N, Zhao H. Seamless correction of the sickle cell disease mutation of the HBB gene in human induced pluripotent stem cells using TALENs. Biotechnology and bioengineering. 2014;111:1048–1053. doi: 10.1002/bit.25018. [DOI] [PubMed] [Google Scholar]
- 95.Ramalingam S, Annaluru N, Kandavelou K, Chandrasegaran S. TALEN-mediated generation and genetic correction of disease-specific human induced pluripotent stem cells. Current gene therapy. 2014;14:461–472. doi: 10.2174/1566523214666140918101725. [DOI] [PubMed] [Google Scholar]
- 96.Liu GH, Suzuki K, Qu J, Sancho-Martinez I, Yi F, Li M, Kumar S, Nivet E, Kim J, Soligalla RD, Dubova I, Goebl A, Plongthongkum N, Fung HL, Zhang K, Loring JF, Laurent LC, Izpisua Belmonte JC. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell stem cell. 2011;8:688–694. doi: 10.1016/j.stem.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nature medicine. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 99.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 100.Odorico JS, Posselt AM, Naji A, Markmann JF, Barker CF. Promotion of rat cardiac allograft survival by intrathymic inoculation of donor splenocytes. Transplantation. 1993;55:1104–1107. doi: 10.1097/00007890-199305000-00032. [DOI] [PubMed] [Google Scholar]
- 101.Barker CF, Posselt AM, Odorico JS, Markmann JF, Naji A. Intrathymic transplantation as a model for tolerance. Adv Nephrol Necker Hosp. 1993;22:387–400. [PubMed] [Google Scholar]
- 102.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selawry HP, Whittington K. Extended allograft survival of islets grafted into intra-abdominally placed testis. Diabetes. 1984;33:405–406. doi: 10.2337/diab.33.4.405. [DOI] [PubMed] [Google Scholar]
- 104.Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983;36:423–431. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- 105.van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatric research. 2012;71:474–481. doi: 10.1038/pr.2011.64. [DOI] [PubMed] [Google Scholar]
- 106.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alper J. Geron gets green light for human trial of ES cell-derived product. Nature biotechnology. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- 108.Geron C. World’s first clinical trial of human embryonic stem cell therapy cleared. Regenerative medicine. 2009;4:161. [PubMed] [Google Scholar]
- 109.Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell stem cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 110.Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem cells. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- 111.Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nature biotechnology. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 113.Turner M, Leslie S, Martin NG, Peschanski M, Rao M, Taylor CJ, Trounson A, Turner D, Yamanaka S, Wilmut I. Toward the development of a global induced pluripotent stem cell library. Cell stem cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Bravery CA. Do human leukocyte antigen-typed cellular therapeutics based on induced pluripotent stem cells make commercial sense? Stem cells and development. 2015;24:1–10. doi: 10.1089/scd.2014.0136. [DOI] [PubMed] [Google Scholar]
- 115.Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat Rev Immunol. 2010;10:868–875. doi: 10.1038/nri2878. [DOI] [PubMed] [Google Scholar]
- 116.Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 117.Zimmermann A, Preynat-Seauve O, Tiercy JM, Krause KH, Villard J. Haplotype-based banking of human pluripotent stem cells for transplantation: potential and limitations. Stem cells and development. 2012;21:2364–2373. doi: 10.1089/scd.2012.0088. [DOI] [PubMed] [Google Scholar]
- 118.Chalela P, Suarez L, Munoz E, Gallion KJ, Pollock BH, Weitman SD, Karnad A, Ramirez AG. Promoting Factors and Barriers to Participation in Early Phase Clinical Trials: Patients Perspectives. J Community Med Health Educ. 2014;4:1000281. doi: 10.4172/2161-0711.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hematti P. Human embryonic stem cell-derived mesenchymal progenitors: an overview. Methods in molecular biology. 2011;690:163–174. doi: 10.1007/978-1-60761-962-8_11. [DOI] [PubMed] [Google Scholar]
- 120.Hematti P. Human embryonic stem cell-derived mesenchymal stromal cells. Transfusion. 2011;51(Suppl 4):138S–144S. doi: 10.1111/j.1537-2995.2011.03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando) 2013;27:21–29. doi: 10.1016/j.trre.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Kim EM, Manzar G, Zavazava N. Human iPS cell-derived hematopoietic progenitor cells induce T-cell anergy in in vitro-generated alloreactive CD8(+) T cells. Blood. 2013;121:5167–5175. doi: 10.1182/blood-2012-11-467753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomei AA, Villa C, Ricordi C. Development of an encapsulated stem cell-based therapy for diabetes. Expert opinion on biological therapy. 2015;15:1321–1336. doi: 10.1517/14712598.2015.1055242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem cells translational medicine. 2015;4:1214–1222. doi: 10.5966/sctm.2015-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]