Highlights
-
•
Scant research has examined neural correlates of development of working memory filtering.
-
•
Working memory filtering was examined in adults and adolescents using fMRI.
-
•
Age-independent neural recruitment for load and filtering was as expected.
-
•
Adolescents differed from adults in neural recruitment to load in non-frontal regions.
-
•
Filter-preparatory activity in the basal ganglia supported filtering in adults only.
Keywords: Working memory, Working memory filtering, Basal ganglia, Adolescence
Abstract
While most measures of working memory (WM) performance have been shown to plateau by mid-adolescence and developmental changes in fronto-parietal regions supporting WM encoding and maintenance have been well characterized, little is known about developmental variation in WM filtering. We investigated the possibility that the neural underpinnings of filtering in WM reach maturity later in life than WM function without filtering. Using a cued WM filtering task (McNab and Klingberg, 2008), we investigated neural activity during WM filtering in a sample of 64 adults and adolescents. Regardless of age, increases in WM activity with load were concentrated in the expected fronto-parietal network. For adults, but not adolescents, recruitment of the basal ganglia during presentation of a filtering cue was associated with neural and behavioral indices of successful filtering, suggesting that WM filtering and related basal ganglia function may still be maturing throughout adolescence and into adulthood.
1. Introduction
Working memory (WM) is the ability to maintain representations of recently experienced or recalled information over a short period of time (Curtis and D’Esposito, 2003). The capacity of working memory in humans is limited (Cowan, 2001, Miller, 1956), and individual differences in capacity are correlated with a variety of cognitive and social outcomes including school performance (Dumontheil and Klingberg, 2012, Finn et al., 2014, Gathercole et al., 2003). Working memory encoding and maintenance without distractors is reliant on the middle frontal gyrus (MFG) and superior parietal cortex, specifically, the intraparietal sulcus (IPS; Todd and Marois, 2004). Recent research has shown that WM filtering ability – the ability to filter extraneous or distracting information from WM during encoding – is strongly associated with overall WM capacity and accuracy (Vogel et al., 2005). Evidence from neuroimaging suggests that the basal ganglia (BG) play an important role in filtering out extraneous information (McNab and Klingberg, 2008). Although a variety of studies have tracked the development of WM and associated neural systems from childhood to early adulthood (Asato et al., 2010, Curtis and D’Esposito, 2003, Lenroot and Giedd, 2006, Sowell et al., 1999, Sowell et al., 2004), developmental variation in patterns of neural function that support WM filtering, specifically, remain largely unexplored.
Contemporary neurocognitive theories of WM function suggest that, during encoding and maintenance, activity in the MFG reflects a top-down control process that serves to maintain representations of visual stimuli which are processed in the IPS (Curtis and D’Esposito, 2003). Research on WM in humans and other primates has consistently shown activity in the MFG (Goldman-Rakic, 1996) and IPS (Hartley and Speer, 2000, Nelson et al., 2000, Thomas et al., 1999) during tasks where working memory load is manipulated. The MFG and IPS appear to make distinct contributions to WM. Activity in the MFG, but not IPS, is implicated in top-down control over representations in WM (Feredoes et al., 2011, Sakai et al., 2002). Activity in the IPS appears to reflect actual WM storage or maintenance; this idea is supported by several ERP studies which have found that contralateral delay signal over parietal scalp scales with WM load but plateaus when load exceeds the capacity of the subject (McCollough et al., 2007, Vogel and Machizawa, 2004, Vogel et al., 2005). Functional magnetic resonance imaging (fMRI) studies have localized this ‘contralateral delay’ signal to the IPS, such that blood oxygen level dependent (BOLD) signal in the IPS is associated with the number of items being maintained in WM (Todd and Marois, 2004; see also: Xu and Chun, 2006).
Recent research additionally suggests a crucial role for the BG in filtering information into WM. Evidence for this hypothesis comes from several sources. Computational models of WM that model the BG as responsible for selectively updating information in WM reproduce core human WM abilities including acquiring new filtering rules, selective rapid encoding of new information, and robust maintenance (Frank et al., 2001, Hazy et al., 2006). Importantly, general hypotheses about patterns of neural activity during WM tasks based on these models have been tested successfully in empirical studies (Braver and Bongiolatti, 2002, Frank, 2005, Frank et al., 2004, Koechlin et al., 2000, Koechlin et al., 2003; for review, see Hazy et al., 2006). However, none of these studies attempted to isolate the BG's role in filtering specifically. McNab and Klingberg (2008) addressed this problem directly with a novel task in which subjects were prepared for a delayed match to sample task via a cue, which indicated the presence or absence of distractors in the upcoming encoding period. Neural activity during cues which indicated the upcoming presence of distractors compared to cues which did not was thought to reflect activity in filtering-related structures absent confounding activity from encoding or maintenance processes (McNab and Klingberg, 2008, Sakai and Passingham, 2006). In this study the authors demonstrated that activity in the BG was increased for distractor cues relative to non-distractor cues in their adult sample. This increased filter preparatory activity in the BG was associated with reduced encoding of distractors as measured by reduced IPS activity during maintenance as well as better task performance. Additional studies of the BG's role in filtering have shown that individuals with lesions in the BG are more likely to attend to irrelevant information in WM tasks (Baier et al., 2010, Voytek and Knight, 2010).
1.1. Development of WM during childhood
The greatest increases in WM capacity occur before mid-adolescence, with gains continuing more gradually into young adulthood (Cowan et al., 2006, Gathercole, 1999, Kwon et al., 2002, Sander et al., 2011). The fronto-parietal WM network follows a similar developmental course, with frontal and parietal gray matter volume peaking between 10 and 14 years of age and decreasing into the twenties (Dempster, 1992, Lenroot and Giedd, 2006). These structural changes have been associated with changes in WM ability (Darki and Klingberg, 2014, Kharitonova et al., 2013). Additionally, WM related activity in the prefrontal and parietal cortices increases with increasing age during childhood and throughout adolescence (Casey et al., 1995, Klingberg et al., 2002, Kwon et al., 2002, Thomas et al., 1999, Thomason et al., 2008). As children develop they are better able to recruit brain regions associated with WM encoding and maintenance (e.g. MFG, IPS) and their performance on WM tasks improves in parallel to the structural and functional development of these structures.
Few studies have examined the development of WM filtering specifically. Spronk et al. (2012) found that 12–16 year olds show reduced WM filtering capability relative to adults. WM performance without filtering was also impaired in adolescents in this study, suggesting a possible confounding effect of simple WM performance. Other research has suggested that age-related discrepancies in filtering performance may occur only when WM capacity is taxed (Cowan et al., 2010). Alternately, the development of filtering ability may drive increases in WM capacity in middle childhood and early adolescence, as several authors have linked filtering ability and related brain activity to WM capacity in adults (McNab and Klingberg, 2008, Vogel et al., 2005). Simple WM encoding and maintenance performance appears to stabilize by late adolescence, but performance on more complex WM tasks, such as those involving distraction or manipulation of stimuli in memory, have shown performance gains during this period (Bunge and Wright, 2007, Crone et al., 2006, Schleepen and Jonkman, 2010). Relatedly, the BG appear to mature into young adulthood (Asato et al., 2010, Lenroot and Giedd, 2006, Østby et al., 2009, Sowell et al., 1999) suggesting that WM filtering may continue to develop throughout adolescence even after simple maintenance and encoding processes have reached adult levels. Examination of filtering function in later adolescence may help disentangle changes in WM filtering function from developmental changes in WM encoding and maintenance.
No studies to date have examined the functional neurodevelopment of WM filtering in late adolescence. The present study addresses these gaps in the literature by adapting an established fMRI paradigm (McNab and Klingberg, 2008) for use in a sample of older adolescents, around 16 years in age on average, and adults. Given that WM maintenance is likely to be well developed by late adolescence, but WM filtering may continue to mature, we hypothesized that adolescents would perform worse on filter trials than adults and that they would recruit the BG less robustly than adults in response to cues indicating the presence of distractors.
2. Methods
2.1. Sample
A sample of 64 adolescents and adults aged 13–36 participated. Of these, three adolescents and one adult participant whose total accuracy were less than 2.5 SD below the mean (<39% correct) were excluded as behavioral outliers. Of the remaining participants, Forty-three were adolescents age 13–18 (M = 16.74, SD = 1.22, 70% female) and 17 were adults age 19–36 (M = 24.44, SD = 5.18, 53% female). Exclusion criteria included psychiatric medication use with the exception of stimulant medications for ADHD (discontinued 24 h before the scan), metal orthodontics unsuitable for MRI, claustrophobia incompatible with entering the MRI machine, active substance use disorder, major developmental or genetic disorders, and non-English speaking. All procedures were approved by the institutional review board for the protection of human subjects at Boston Children's Hospital.
47 subjects (43 adolescents and 4 adults) were recruited as part of a previous study. These participants took part in a two-session lab visit that included assessments of IQ using the 2-subtest short form of the Wechsler Abbreviated Scale of Intelligence (WASI), violence exposure, mental health, and emotional reactivity. Of these approximately 1/3 of the subjects reported experiencing some form of violence during childhood1. Data from this study are described elsewhere (McLaughlin et al., 2015). The remaining 13 adults were recruited from the Boston and Cambridge area using flyers and word of mouth. These individuals participated in a one-session lab visit that included MRI and fMRI only. To estimate IQ in this group, we administered the matrix reasoning subscale of the WASI; t-scores on this subscale were used for all participants as an index of IQ.
Matrix reasoning data were missing for 13 subjects due to experimenter error. In the entire sample, matrix reasoning ability was significantly higher in adults (M = 59.8, SD = 6.81) than adolescents (M = 49.4, SD = 11.26, t(45) = 2.772, p = .008). When including this estimate of IQ as a covariate modified our results, we report this difference.
2.2. Task design
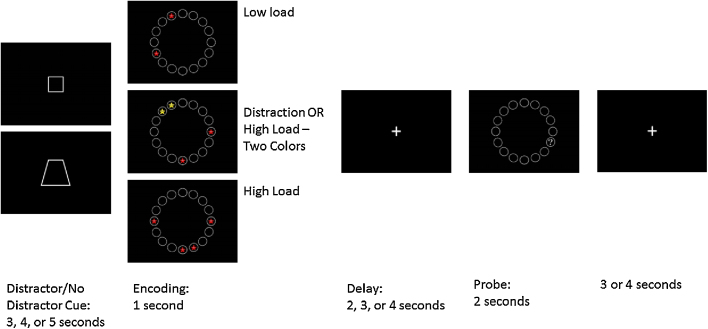
A delayed match to sample WM task with and without distractors was administered during fMRI scanning (see Fig. 1). This task was modeled after an existing filtering task with some modifications (McNab and Klingberg, 2008). Participants first viewed a cue, either a square or a trapezoid. This cue lasted 3–5 s and indicated if there would be distractors present on the subsequent trial. The specific shape indicating distractors was counterbalanced across subjects. Following the cue, an encoding screen was presented for 1 s. During encoding participants viewed an array of 16 circles presented in a circle around a centrally located fixation cross with red or yellow stars with eyes in 2 or 4 of the circles. Participants were told to remember the location of the stars. There were four conditions: low load where participants saw 2 red stars, high load where participants saw 4 red stars, distraction trials where participants saw 4 stars (two red and two yellow) and were cued to ignore the yellow stars, and high load-two color trials where participants saw 2 red and 2 yellow stars but were cued to remember the locations of all stars. A delay period lasting 2, 3, or 4 s followed encoding. During the delay period, participants saw a fixation cross hair and needed to maintain in memory the location of the stars viewed during encoding. Following delay, participants viewed the same 16 circle array for 2 s. One circle had a question mark in it, and participants indicated with a button press if that question mark was in the same location as one of the to-be-remembered stars. The next trial began 2, 3, or 4 s after the probe ended. Participants completed scanning in four functional runs lasting 9 min each. Each run contained 10 trials of each condition, for a total of 160 trials per subject.
Fig. 1.
Schematic diagram of task showing Cue, Encoding, Delay, and Probe Phase. Trials with red and yellow stars were modeled as high load-two color or distraction trials depending on the presence of a distractor cue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Equal numbers of all durations for cue, delay, and ITI were present across each condition and run (each cue and delay duration was used in 33% of trials, and each ITI duration in 50% of trials). Red stars in the encoding phase were placed in a pseudorandom fashion such that they were distributed evenly across available spaces. In distraction and high load-two color trials, yellow stars regularly occurred in one of 4 patterns, which were counterbalanced by subject between high-load two color and distraction conditions. A recognition test given immediately post-scan showed no sensitivity to the presence of these patterns, indicating that pattern learning was not a significant factor in performance. In 56% of trials, the probe was presented in a target location (match trials). On trials with a distractor present, the probe was presented in the location of a distractor for 31% of trials. For trials where the probe was presented in a previously empty circle (non-match trials), the probe was located 1 space away from a filled circle in 90% of trials.
Prior to scanning, participants were given instructions on how to complete the task and the meaning of the distraction cue. Participants practiced the task and were quizzed as to the meaning of the distraction cues prior to completing it in the scanner. To ensure that all participants had equally good knowledge of the meaning of the ‘distractor’ cue and the ‘non-distractor’ cue, all participants were quizzed on the meaning of different cues directly prior to imaging the task. They were allowed to continue once they correctly identified if the square or trapezoid indicated that there would be a distractor present on a trial at least 2 times in a row.
2.3. Image acquisition
Scanning was performed on a 3T Siemens Tim Trio scanner at the Harvard Center for Brain Science using a 32-channel head coil. Anatomical scans (T1-weighted multi-echo MPRAGE volumes) were acquired for co-registration with fMRI (TR = 2530 ms, TE = 1640–7040 ms, flip angle = 7°, FOV = 220 mm, 176 slices, in-plane voxel size = 1 mm3). To reduce motion-related artifacts a navigator echo was used prior to scan acquisition, which compares slices to this echo online and permits up to 20% of slices be re-acquired.
BOLD signal during functional runs was acquired using a gradient-echo T2*-weighted EPI sequence. Thirty-two 3 mm thick slices were acquired parallel to the AC-PC line (TR = 2 s, TE = 30 ms, flip angle = 90°, bandwidth = 2240 Hz/Px, echo spacing = 0.51 ms, FOV = 216 mm, matrix size = 64 × 64). 270 Volumes were acquired for each of four functional runs. Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. An online prospective motion correction algorithm (PACE) was used to reduce the effect of motion artifacts.
2.4. Image processing
Pre-processing and analysis of fMRI data was performed in Nipype, a platform that implements analysis tools from multiple software packages using the Python programming language (Gorgolewski et al., 2011). fMRI pre-processing included a 4-dimensional spatial realignment and slice-time correction (Sladky et al., 2011) followed by spatial smoothing (6 mm FWHM) implemented in FSL. Data were inspected for artifacts using the RapidART library in Nipype; single point outlier regressors were generated for any volume in which scan to scan motion of any center point of a cuboid drawn around the brain exceeded 1.5 mm or in which over-all image intensity was more than 3 standard deviations from the mean. 6 Rigid-body motion regressors were included as nuisance covariates in person-level models. Person and group-level models were estimated in FSL. A component-based anatomical noise correction method was used to reduce noise associated with physiological fluctuations (Behzadi et al., 2007). Following estimation of person-level models, the resulting contrast images were normalized into standard anatomical space, and anatomical co-registration of the functional data with each participant's T1-weighted image was performed using Advanced Normalization Tools (ANTs) software (Avants et al., 2011).
To identify regions of interest based on structurally defined boundaries, each participants T1 weighted images were automatically segmented and parcellated using FreeSurfer (Destrieux et al., 2010, Fischl et al., 2002). FreeSurfer morphometric procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths (Han et al., 2006). In addition, these procedures have been successfully used in studies of children as young as age four (Ghosh et al., 2010).
2.5. Statistical analysis
2.5.1. Behavioral performance
Behavioral task response was indexed using discrimination sensitivity (d′) (Stanislaw and Todorov, 1999). Reaction time (RT) was not considered a measure of performance on this task because of the long delay between encoding and probe. This is consistent with previous studies showing weak associations between performance and RT using the delayed match to sample task (Sheridan et al., 2010). Behavioral response was analyzed using a 4 × 2 within-subjects ANOVA (trial type × age group). Within the ANOVA model of task performance we defined contrasts to test for effects of load, distraction, and stimulus color (high load vs. high load-two color) on d′.
2.5.2. fMRI analysis (whole brain)
Regressors were created by convolving a boxcar function of phase duration and amplitude one with the standard hemodynamic response function (HRF) for each phase of the task: cue, encoding + delay, and probe. Previous work has demonstrated that use of the standard HRF is appropriate when comparing adults to children as young as 7 (Kang et al., 2003, Moses et al., 2014, Richter and Richter, 2003). Cue was modeled separately for “distractor instruction” (i.e., cues that indicated participants were in a distraction trial) and “no distractor instruction” (i.e., cues that indicated all other trials) trials. The encoding + delay period was modeled as a single regressor, and all four encoding + delay conditions were modeled separately (low load single color, low load with distractors, high load single color, high load two colors). Probe was also modeled separately for each of these four conditions. Using FSL, a general linear model (GLM) was constructed to estimate the association between variation in BOLD signal and task demands across time for each subject, prior to normalization. Individual-level estimates of BOLD activity were submitted to group-level random effects models that contrasted activity across conditions. We defined contrasts to examine the effect of load (high load > low load) at encoding + delay, the effect of distraction (distraction > low load) at encoding + delay, and the effect of the distractor instruction (distractor instruction > no distractor instruction) at cue. We corrected for multiple comparisons to p < .05 using cluster-level correction in FSL; our primary threshold was z > 2.3. We examined differences in BOLD response during contrasts of interest as a function of age group (adults vs adolescents). For each trial phase, accurate and inaccurate trials were modeled separately; only accurate trials were used to examine neural activity at the whole brain level.
2.5.3. fMRI analysis (region of interest)
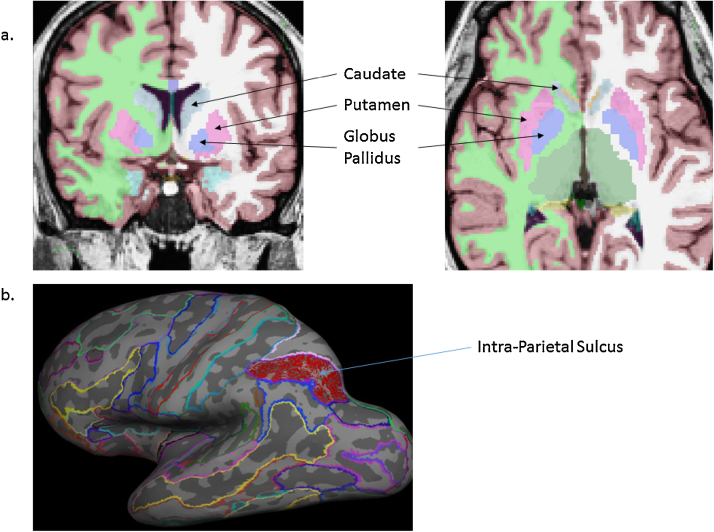
We examined 8 a priori regions of interest (ROIs), defined structurally, in the BG and IPS. The “Intra-Parietal and Parietal Transverse Sulcus” area from FreeSurfer's 2009 atlas (Destrieux et al., 2010) was used to define the IPS and ROIs were defined for the putamen, caudate, and globus pallidus from the subcortical atlas (Fischl et al., 2002) (see Fig. 2 for a visual depiction of all ROIs). These ROIs were defined in each participant's native space using FreeSurfer and the average parameter estimate from the contrast of interest in the functional data was extracted for each participant within the structurally defined region. The ROI analysis was used to specifically test hypotheses engendered by models of failures in filtering and filtering preparatory activity. To capture potential errors due to encoding of distractors, both accurate and inaccurate trials were included in the ROI analysis. Following methods in previous work (McNab and Klingberg, 2008), we tested regression models in which distractor-related maintenance activity in the IPS (representing the encoding of distractors) was predicted by age group (adolescents vs. adults), filter preparatory activity in the BG, and the interaction between age group and BG filter preparatory activity. Significant age × preparatory activity interactions were followed up by examining these associations separately within each group (adults, adolescents).
Fig. 2.
Anatomical ROIs for the caudate, putamen, globus pallidus (a) and intra-parietal sulcus (b) as identified by the aseg and aparc-2009 atlases within FreeSurfer.
3. Results
3.1. Behavioral results
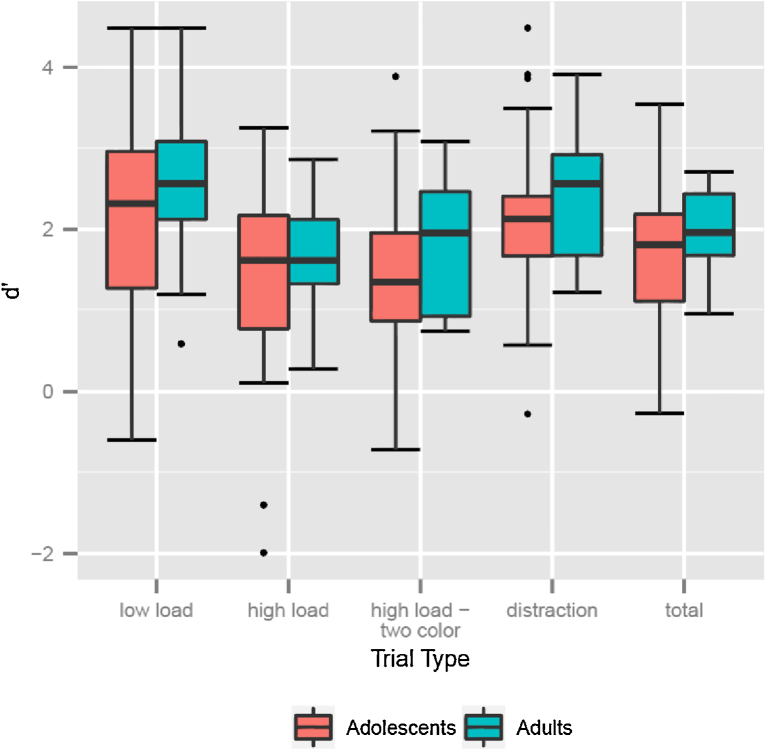
Performance (d′) on the task, including all trial types, indicated that both adolescents and adults were able to perform the task well (see Table 1, Fig. 3). A 4 × 2 (trial type × age group) ANOVA showed a significant effect of trial type on performance (F(3,56) = 21.781, p < 001, ηp2 = .274). Contrasts demonstrated that d′ varied with load–scores were higher on low load trials relative to high load trials (F(1,58) = 63.414, p < .001, ηp2 = .522). However, performance did not vary between distraction and low load (F(1,58) = 285, p = .596, ηp2 = .002) or between high load and high load-two color trials (F(1,58) = 037, p = .849, ηp2 = .023). No significant main effect of age was found, nor was there any interaction of age and trial type. Performance trends were similar for response time and accuracy, with low load and distraction trials showing higher accuracy and faster response times than high load and high load—two color trials.
Table 1.
d′, response time, and accuracy by trial type.
| Low load |
High load |
High load–two colors |
Distraction |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| d′ | Adol. | 2.15 | 1.15 | 1.47 | 1.12 | 1.37 | 0.96 | 2.12 | 1.00 | 1.69 | 0.86 |
| Adults | 2.56 | 0.93 | 1.70 | 0.66 | 1.84 | 0.80 | 2.44 | 0.82 | 1.99 | 0.54 | |
| Total | 2.26 | 1.10 | 1.54 | 1.01 | 1.50 | 0.94 | 2.21 | 0.96 | 1.78 | 0.79 | |
| Response time (s) | Adol. | 1.06 | 0.17 | 1.16 | 0.19 | 1.15 | 0.19 | 1.05 | 0.18 | 1.10 | 0.17 |
| Adults | 1.11 | 0.22 | 1.24 | 0.24 | 1.19 | 0.23 | 1.09 | 0.21 | 1.16 | 0.22 | |
| Total | 1.08 | 0.18 | 1.18 | 0.21 | 1.16 | 0.20 | 1.06 | 0.19 | 1.12 | 0.18 | |
| Accuracy (% correct) | Adol. | 0.81 | 0.13 | 0.74 | 0.14 | 0.73 | 0.13 | 0.82 | 0.12 | 0.78 | 0.12 |
| Adults | 0.87 | 0.09 | 0.79 | 0.09 | 0.79 | 0.12 | 0.86 | 0.08 | 0.83 | 0.07 | |
| Total | 0.83 | 0.12 | 0.76 | 0.13 | 0.75 | 0.13 | 0.83 | 0.11 | 0.79 | 0.11 | |
Fig. 3.
d′ by trial type and age group.
3.2. fMRI data (whole brain)
3.2.1. Main effects of task on BOLD response
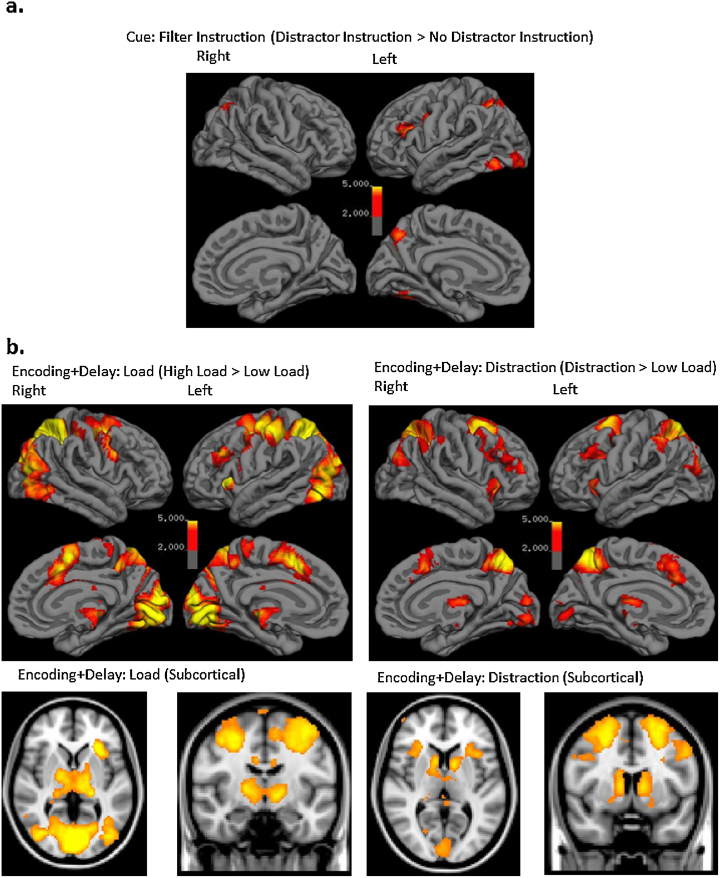
Across all participants, BOLD signal was greater in the bilateral IPS, bilateral occipital cortex, left MFG, and left fusiform gyrus for cue trials with a distraction instruction than trials with a no distractor instruction (see Table 2 and Fig. 4 for all main effects of task).
Table 2.
Main effects of task on BOLD response.
| Contrast | Cluster label | x | y | z | Voxels | t Value |
|---|---|---|---|---|---|---|
| Cue: distractor instruction > no distractor instruction | L IPS | −30 | −62 | 40 | 2715 | 5.61 |
| R IPS | 34 | −62 | 46 | 1541 | 4.84 | |
| L Fusiform gyrus | −34 | −64 | −12 | 1133 | 4.25 | |
| Bilateral occipital cortex | −36 | −68 | −22 | * | 3.15 | |
| L MFG | −36 | 2 | 32 | 1020 | 4.55 | |
| Encoding + delay: load (high load > low load) | Bilateral IPS | −22 | −64 | 42 | 48,239 | 6.82 |
| Bilateral occipital cortex | 6 | −90 | 12 | * | 6.5 | |
| Bilateral middle frontal gyrus | −24 | 2 | 56 | * | 5.08 | |
| Thalamus | 16 | −24 | 12 | * | 5.05 | |
| Bilateral anterior cingulate† | −6 | 2 | 30 | * | 4.1 | |
| L Pallidum† | −18 | −10 | 2 | * | 3.82 | |
| Encoding + delay: distraction (distraction > low load) | Bilateral IPS | 12 | −68 | 62 | 13,415 | 5.87 |
| Bilateral SFG | −24 | 0 | 54 | 8340 | 5.58 | |
| Bilateral caudate† | −10 | 6 | 6 | 4332 | 4.72 | |
| Bilateral IFG† | 32 | 26 | −2 | * | 4.46 | |
| Bilateral occipital cortex | 14 | −92 | −12 | 1985 | 4.03 | |
| Probe: Load | Anterior cingulate | −6 | 20 | 32 | 1840 | 4.14 |
| Probe: Distraction | (no significant results) | |||||
Contiguous with above region.
Not significant when cluster-level correction was applied with a primary threshold of p = .001 (all reported results were cluster-level corrected at p = .05–see Woo et al. (2014)).
Fig. 4.
Main effects of task in the cue (a) and encoding + delay (b) phases.
We observed a main effect of load (high load > low load) on BOLD response during encoding and delay bilaterally in the IPS and MFG, as well as in the thalamus and occipital cortex. We observed a main effect of filtering during encoding and delay (distraction > low load) in a network of regions including the bilateral IPS, superior frontal gyrus (SFG), caudate, inferior frontal gyrus (IFG), and occipital cortex.
3.2.2. Interaction of task contrasts with age
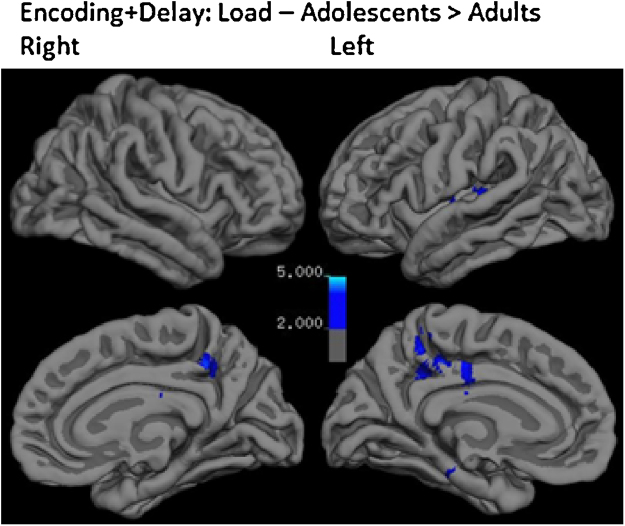
No significant interaction of BOLD response with age was found in the cue phase. During encoding + delay, adolescents showed greater BOLD response with increased load than adults bilaterally in the precuneus as well as in the left temporoparietal junction and cerebellar vermis (See Table 3, Fig. 5). Adults did not show greater load-related recruitment as compared with adolescents. There were no significant differences in neural activation between adults and adolescents during encoding + delay in the distraction > low load contrast at the whole-brain level. In order to allow comparison with McNab and Klingberg (2008), we examined this contrast for BG activity prior to correction for multiple comparisons and found activity in the right putamen, but this result did not survive correction for multiple comparisons.
Table 3.
Interaction of Age with task-related activity.
| Contrast | Cluster Label | X | Y | z | Voxels | t Value |
|---|---|---|---|---|---|---|
| Cue: distractor instruction | (No significant differences) | |||||
| Encoding + delay: load adolescents > adults | Precuneus (bilateral)† | 4 | −48 | 46 | 1126 | 4.92 |
| L Temporoparietal Junction† | −44 | −22 | 8 | 614 | 3.68 | |
| L Cerebellar Vermis† | −22 | −38 | −20 | 560 | 3.90 | |
| Encoding + delay: distraction | (No significant differences) | |||||
| Probe: Load | (No significant differences) | |||||
| Probe: distraction adults > adolescents | Bilateral IPS† | 2 | −72 | 54 | 4286 | 4.28 |
| Bilateral Occipital Cortex† | −4 | −94 | 26 | * | 4.05 | |
Contiguous with above region.
Not significant when cluster-level correction was applied with a primary threshold of p = .001 (all reported results were cluster-level corrected at p = .05—see Woo et al. (2014)).
Fig. 5.
Interaction of Age on load-related activity during encoding + delay.
3.3. Filter preparatory activity
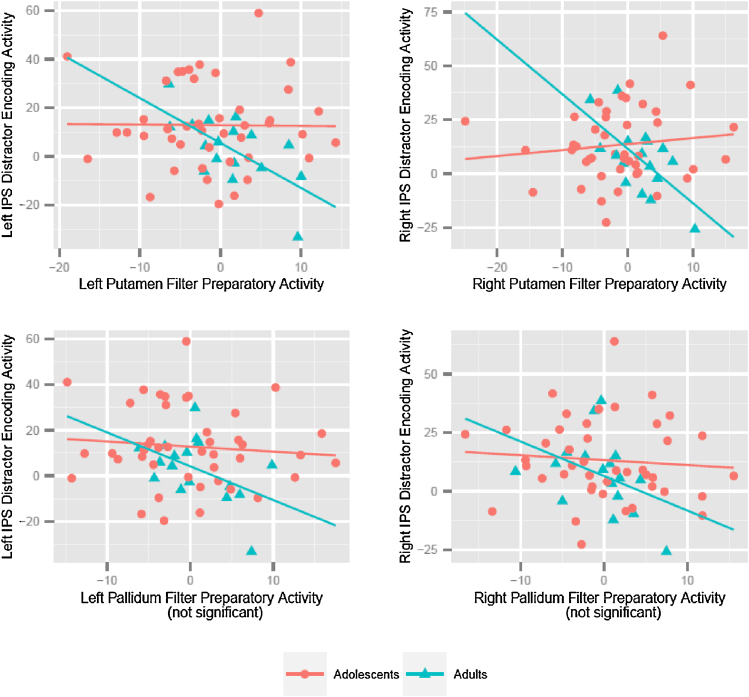
In our ROI data, there was no main effect of filter preparatory activity (distractor instruction > no distractor instruction during cue) in the caudate, putamen, or globus pallidus on distractor-related activity in the ipsilateral IPS (distraction > low load during encoding + delay) (all p values >.22). To examine the effect of age, we first tested a model wherein IPS preparatory activity was predicted by the interaction of BG preparatory activity and the centered square of age and found a quadratic interaction of age with putamen preparatory activity in the right hemisphere (β = −.363, t(54) = −2.007, p = .05)2. This quadratic interaction was driven by the strength of the effect in the adult sample. In group analysis we found an interaction between age group and preparatory activity for the left (β = −.284, t(56) = −2.072, p = .043) and right (β = −.386, t(56) = −2.639, p = .011) putamen, indicating an effect of age group on the relationship between filter preparation and distractor related activity3.
In adults, we observed the expected negative relationship between filter preparatory activity in the putamen and distractor maintenance in the IPS in both the left (β = −.656, t(15) = −3.37, p = .004) and right (β = −.643, t(15) = −3.256, p = .005) hemispheres (see Fig. 6); these relationships were maintained in a skipped correlation model where extreme data points were removed (Wilcox, 2004). In adolescents these associations were not significant in the left (β = −.01, t(41) = −.069, p = .945) or right (β = −.002, t(41) = −.013, p = .99) hemisphere. In order to allow direct comparison to results from McNab and Klingberg (2008), who identified this negative relationship for filter preparatory activity in the pallidum, we also tested the relationship between filter preparatory activity in the pallidum and distractor maintenance in the IPS in adults only. This relationship was in the expected direction, but only marginally significant for both the left (β = −.469, t(15) = −2.059, p = .057) and right (β = −.401, t(15) = −1.696, p = .11) hemispheres.
Fig. 6.
Plot of IPS Distractor Encoding (distraction > low load contrast; encoding + delay) with putamen and pallidum filter preparatory activity (distractor instruction > no distractor instruction; cue) by age and hemisphere.
3.4. Relationship of brain activity to task performance
Our measure of IPS inefficiency correlated negatively with performance (d′) in the distraction condition of the task in both the left (β = −.3, t(57) = −2.904, p = .005) and right (β = −.322, t(57) = −3.19, p = .002) hemispheres while controlling for performance in the low load condition. There was no main effect of filter preparatory activity in the putamen on distraction performance while controlling for low load performance, however an interaction of preparatory activity in the putamen with age group was significant in the left (β = .753, t(55) = 2.467, p = .017) and right (β = .964, t(55) = 2.65, p = .011)4 hemispheres. This interaction was driven by an effect of putamen preparatory activity on distraction performance (again, controlling for low load performance) in adults only in the left (β = .525, t(14) = 2.332, p = .035) and right (β = .629, t(15) = 3.064, p = .008) putamen. This effect was not significant in adolescents (p > .14).
4. Discussion
This study examined neural activity related to WM filtering in a sample of older adolescents and adults. Our primary hypothesis was that adolescents would show worse performance in WM filtering trials relative to adults and exhibit a different pattern of neural activity in WM filtering related networks. Unexpectedly, adolescents and adults performed equally well in filtering trials of the WM task. However, the predicted negative relationship between filter preparatory activity in the BG and activity related to distractor encoding in the IPS was present in adults but not adolescents. Relatedly, filter preparatory activity was not correlated with distraction trial performance in adolescents but it was for adults. These findings suggest that adolescents did not recruit their BG in response to filter instructions so as to support later filtering, whereas adults did. The finding that adolescents do not recruit the BG in support of filtering in the same manner as adults provides evidence that filtering-related neurodevelopment is ongoing in late adolescence which is consistent with our initial hypothesis.
Across age, main effects of WM activity on BOLD response generally conformed to existing theoretical models and empirical data of neural recruitment during WM function. Increased WM load was associated with activity in the MFG, IPS, and globus pallidus during encoding + delay, consistent with prior work highlighting these areas’ role in encoding and maintenance of items in WM (Hartley and Speer, 2000, Nelson et al., 2000, Postle and D’Esposito, 1999, Thomas et al., 1999). BOLD response during encoding + delay in the distraction condition, relative to same load no-distractor trials, included the MFG and IPS as well as a strong cluster of BG activity centered in the caudate nucleus. Increased BG activity during encoding in the presence of distractors is consistent with theoretical models of WM filtering wherein the BG serve to selectively filter stimuli into WM (Hazy et al., 2006).
Compared to adults, adolescents showed greater activity with increasing WM load in a network of regions involved in processing visual stimuli during encoding + delay. These included the precuneus, temporoparietal junction, and cerebellar vermis. This increase in activity for adolescents relative to adults may indicate inefficient processing, more effort or time devoted to visual processing of stimuli, or differences in strategy. However, there were no corresponding differences in MFG or IPS activity. This equivalence is consistent with previous evidence suggesting that systems devoted to WM maintenance are reasonably well developed by late adolescence (Darki and Klingberg, 2014, Dempster, 1992, Kharitonova et al., 2013, Lenroot and Giedd, 2006).
At the group level, adults and adolescents exhibited no differences in neural recruitment in support of WM filtering during encoding or cue. However, as described above, recruitment of the BG when preparing to filter was associated with a neural index of successful filtering as well as behavioral performance on filtering trials in adults but not in adolescents. This difference suggests that adolescents may use different cognitive strategies to support WM in the presence of distractors. These findings parallel those in other work wherein children switch from a reactive (responding to task demands as they arise) to proactive (preparing ahead of time to meet task demands) cognitive control style as they develop (Chatham et al., 2009).
Our study had a number of limitations that should be addressed in future research. First, the study utilized a cross-sectional design to examine a developmental question. A longitudinal design would better control for individual differences in the sample. Second, it is possible that the task, particularly the low load and distraction conditions, was too easy. Over-all WM load in the task was reduced relative to McNab and Klingberg (2008) in order to adapt the task to a younger sample. A more difficult low load condition might have revealed greater performance differences between low load and distraction trials, providing a behavioral index of how distracted participants were by the yellow stars. Third, complete data on IQ (both a complete estimate of IQ and IQ data on all participants) may have helped clarify our findings. However, the lack of performance differences between groups suggest that a gross difference in cognitive ability, as indexed by estimated IQ was not a major factor in our findings. Additionally, IQ is not an ideal control for ability differences in the sample as WM capacity may be related to IQ (Fukuda et al., 2010). We observed that some of our significant effects were attenuated when IQ was included as covariate. This may be because of the known correlation between IQ and WM capacity or because including estimated IQ in our models reduced our sample size dramatically. Fourth, our adult and adolescent sample sizes were unequal. A larger sample of adults would have provided greater sensitivity. This limitation might explain the absence of some effects observed in McNab and Klingberg (2008) in our adult sample, such as the lack of a main effect of filtering on BG activity (this is supported by the marginal activity found in the right putamen when comparing adults to adolescents in the distraction > no-distraction contrast during encoding + delay). It is unlikely that unequal sample sizes explain our group differences. If anything, the smaller adult sample would undermine our observation of group differences because it limits the reliability of our estimations in the adult sample. Finally, future research should examine WM filtering beginning at earlier developmental stages. Although investigating brain development in older adolescents is important to capture later development in filtering related structures, differences in neural recruitment between older adolescents and adults in this study were subtle. Studies with a wider age range may help isolate age differences related to maturity in filtering and encoding related structures.
Overall, our findings support the conclusion that brain networks supporting WM filtering are still maturing in later adolescence. For instance, predicted BG-IPS correlations indicating preparatory filtering activity were not observed in adolescents, but were observed in adults. In contrast, while we observed differences between adolescents and adults in recruitment of visual attention and processing areas for encoding of high vs. low load, recruitment of WM specific regions (MFG, IPS) was similar for adults and adolescents.
Although we observed differences in neural recruitment between adolescents and adults, we did not observe differences in behavioral performance. This lack of behavioral difference suggests that observed neural differences reflect differences in maturity or strategy but not gross failure to perform the task. If replicated, these findings could complicate models of WM development in which the ability to filter extraneous information from WM is a core component of WM capacity (Vogel et al., 2005). The observation that WM filtering and WM capacity may be dissociated developmentally makes the argument that they are intrinsically linked less strong. Finally, immaturity of WM filtering in adolescence could have wide-ranging implications for adolescent cognition and development. Such a deficit might have implications for adolescents’ ability to focus on long-term goals in the presence of distraction and suggests that adolescents might benefit from additional support in maintaining focus in clinical and educational contexts.
Conflict of interest statement
The authors have no financial disclosures or conflicts of interest to report.
Acknowledgements
This research was funded by the National Institute of Mental Health (K01-MH092625 to McLaughlin and K01-MH092555 to Sheridan). The authors would like to thank Mr. Marcus Way for his contributions in task programming and data collection.
Footnotes
Because some of the children in the previous study's sample had been exposed to violence (e.g., maltreatment, community violence) a composite violence exposure score was calculated based on measures of exposure to home and neighborhood violence using the Screen for Adolescent Violence Exposure (Hastings and Kelley, 1997) and the Childhood Trauma Questionnaire (Bernstein et al., 1997). Violence scores for the additional adult sample who were not part of the original study were imputed from the mean score of the control group from the original study which assumes that adults were not exposed to traumatic violence. We performed all analyses with and without this variable as a control and found that it did not account for any finding presented in this paper.
This relationship became marginal (p = .057) when violence exposure was not included as a covariate, and thus it is included here.
Including IQ as a regressor in these models reduced the number of adult participants to 7 and the number of adolescents to 42. The interaction of age and filter preparatory activity was no longer significant when IQ was modelled as a covariate.
Interaction of right putamen activity with age was marginally related to distraction performance (p = .06) when controlling for IQ and low load performance.
References
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. http://doi.org/10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. http://doi.org/10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B., Karnath H.-O., Dieterich M., Birklein F., Heinze C., Müller N.G. Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J. Neurosci. 2010;30(29):9788–9792. doi: 10.1523/JNEUROSCI.1513-10.2010. http://doi.org/10.1523/JNEUROSCI. 1513-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. http://doi.org/10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. http://doi.org/10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Bongiolatti S.R. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage. 2002;15(3):523–536. doi: 10.1006/nimg.2001.1019. http://doi.org/10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. http://doi.org/10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cohen J.D., Jezzard P., Turner R., Noll D.C., Trainor R.J. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. http://doi.org/10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Frank M.J., Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. U.S.A. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. http://doi.org/10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav. Brain Sci. 2001;24(1):87–114. doi: 10.1017/s0140525x01003922. Discussion 114–185. [DOI] [PubMed] [Google Scholar]
- Cowan N., Morey C.C., AuBuchon A.M., Zwilling C.E., Gilchrist A.L. Seven-year-olds allocate attention like adults unless working memory is overloaded. Dev. Sci. 2010;13(1):120–133. doi: 10.1111/j.1467-7687.2009.00864.x. http://doi.org/10.1111/j.1467-7687.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N., Naveh-Benjamin M., Kilb A., Saults J.S. Life-span development of visual working memory: when is feature binding difficult? Dev. Psychol. 2006;42(6):1089–1102. doi: 10.1037/0012-1649.42.6.1089. http://doi.org/10.1037/0012-1649.42.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U.S.A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. http://doi.org/10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C.E., D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Darki F., Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb. Cortex. 2014 doi: 10.1093/cercor/bht352. http://doi.org/10.1093/cercor/bht352, bht352. [DOI] [PubMed] [Google Scholar]
- Dempster F.N. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev. Rev. 1992;12(1):45–75. http://doi.org/10.1016/0273-2297(92)90003-K. [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. http://doi.org/10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb. Cortex (New York, N.Y.: 1991) 2012;22(5):1078–1085. doi: 10.1093/cercor/bhr175. http://doi.org/10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Feredoes E., Heinen K., Weiskopf N., Ruff C., Driver J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci. U.S.A. 2011;108(42):17510–17515. doi: 10.1073/pnas.1106439108. http://doi.org/10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A.S., Kraft M.A., West M.R., Leonard J.A., Bish C.E., Martin R.E. Cognitive skills, student achievement tests, and schools. Psychol. Sci. 2014;25(3):736–744. doi: 10.1177/0956797613516008. http://doi.org/10.1177/0956797613516008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frank M.J. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005;17(1):51–72. doi: 10.1162/0898929052880093. http://doi.org/10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Loughry B., O’Reilly R.C. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn., Affective Behav. Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Seeberger L.C., O’Reilly R.C. By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. http://doi.org/10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Vogel E., Mayr U., Awh E. Quantity, not quality: the relationship between fluid intelligence and working memory capacity. Psychon. Bull. Rev. 2010;17(5):673–679. doi: 10.3758/17.5.673. http://doi.org/10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E. Cognitive approaches to the development of short-term memory. Trends Cogn. Sci. 1999;3(11):410–419. doi: 10.1016/s1364-6613(99)01388-1. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Brown L., Pickering S.J. Working memory assessments at school entry as longitudinal predictors of National Curriculum attainment levels. Educ. Child Psychol. 2003;20(3):109–122. [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4-to-11 years of age. NeuroImage. 2010;53(1):85–93. doi: 10.1016/j.neuroimage.2010.05.075. http://doi.org/10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. U.S.A. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinformatics. 2011;5:13. doi: 10.3389/fninf.2011.00013. http://doi.org/10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. http://doi.org/10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hartley A.A., Speer N.K. Locating and fractionating working memory using functional neuroimaging: storage, maintenance, and executive functions. Microsc. Res. Tech. 2000;51(1):45–53. doi: 10.1002/1097-0029(20001001)51:1<45::AID-JEMT5>3.0.CO;2-O. http://doi.org/10.1002/1097-0029(20001001)51:1<45::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hastings T.L., Kelley M.L. Development and validation of the screen for adolescent violence exposure (SAVE) J. Abnorm. Child Psychol. 1997;25(6):511–520. doi: 10.1023/a:1022641916705. http://doi.org/10.1023/A: 1022641916705. [DOI] [PubMed] [Google Scholar]
- Hazy T.E., Frank M.J., O’Reilly R.C. Banishing the homunculus: making working memory work. Neuroscience. 2006;139(1):105–118. doi: 10.1016/j.neuroscience.2005.04.067. http://doi.org/10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kharitonova M., Martin R.E., Gabrieli J.D.E., Sheridan M.A. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev. Cogn. Neurosci. 2013;6:61–71. doi: 10.1016/j.dcn.2013.07.002. http://doi.org/10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. http://doi.org/10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Corrado G., Pietrini P., Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc. Natl. Acad. Sci. U.S.A. 2000;97(13):7651–7656. doi: 10.1073/pnas.130177397. http://doi.org/10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E., Ody C., Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. http://doi.org/10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U.S.A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. http://doi.org/10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. http://doi.org/10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McCollough A.W., Machizawa M.G., Vogel E.K. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43(1):77–94. doi: 10.1016/s0010-9452(08)70447-7. (A Journal Devoted to the Study of the Nervous System and Behavior) [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. http://doi.org/10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F., Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. http://doi.org/10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miller G.A. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol. Rev. 1956;63(1):81–97. [PubMed] [Google Scholar]
- Moses P., Hernandez L.M., Orient E. Age-related differences in cerebral blood flow underlie the BOLD fMRI signal in childhood. Front. Psychol. 2014;5:300. doi: 10.3389/fpsyg.2014.00300. http://doi.org/10.3389/fpsyg.2014.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Monk C.S., Lin J., Carver L.J., Thomas K.M., Truwit C.L. Functional neuroanatomy of spatial working memory in children. Dev. Psychol. 2000;36(1):109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. http://doi.org/10.1523/JNEUROSCI. 1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle B.R., D’Esposito M. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Brain Res. Cogn. Brain Res. 1999;8(2):107–115. doi: 10.1016/s0926-6410(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Richter W., Richter M. The shape of the fMRI BOLD response in children and adults changes systematically with age. NeuroImage. 2003;20(2):1122–1131. doi: 10.1016/S1053-8119(03)00347-1. http://doi.org/10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- Sakai K., Passingham R.E. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J. Neurosci. 2006;26(4):1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. http://doi.org/10.1523/JNEUROSCI. 3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Rowe J.B., Passingham R.E. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat. Neurosci. 2002;5(5):479–484. doi: 10.1038/nn846. http://doi.org/10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sander M.C., Werkle-Bergner M., Lindenberger U. Contralateral delay activity reveals life-span age differences in top-down modulation of working memory contents. Cereb. Cortex. 2011;21(12):2809–2819. doi: 10.1093/cercor/bhr076. http://doi.org/10.1093/cercor/bhr076. [DOI] [PubMed] [Google Scholar]
- Schleepen T.M.J., Jonkman L.M. The development of non-spatial working memory capacity during childhood and adolescence and the role of interference control: an N-back task study. Dev. Neuropsychol. 2010;35(1):37–56. doi: 10.1080/87565640903325733. http://doi.org/10.1080/87565640903325733. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., Hinshaw S., D’Esposito M. Stimulant medication and prefrontal functional connectivity during working memory in ADHD: a preliminary report. J. Atten. Disord. 2010;14(1):69–78. doi: 10.1177/1087054709347444. http://doi.org/10.1177/1087054709347444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Friston K.J., Tröstl J., Cunnington R., Moser E., Windischberger C. Slice-timing effects and their correction in functional MRI. NeuroImage. 2011;58(2):588–594. doi: 10.1016/j.neuroimage.2011.06.078. http://doi.org/10.1016/j.neuroimage.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. http://doi.org/10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. http://doi.org/10.1523/JNEUROSCI. 1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk M., Vogel E.K., Jonkman L.M. Electrophysiological evidence for immature processing capacity and filtering in visuospatial working memory in adolescents. PLoS ONE. 2012;7(8):e42262. doi: 10.1371/journal.pone.0042262. http://doi.org/10.1371/journal.pone.0042262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. http://doi.org/10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., King S.W., Franzen P.L., Welsh T.F., Berkowitz A.L., Noll D.C. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10(3):327–338. doi: 10.1006/nimg.1999.0466. http://doi.org/10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D.E. Development of spatial and verbal working memory capacity in the human brain. J. Cogn. Neurosci. 2008;21(2):316–332. doi: 10.1162/jocn.2008.21028. http://doi.org/10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J.J., Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. doi: 10.1038/nature02466. http://doi.org/10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Vogel E.K., Machizawa M.G. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. doi: 10.1038/nature02447. http://doi.org/10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel E.K., McCollough A.W., Machizawa M.G. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. doi: 10.1038/nature04171. http://doi.org/10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Voytek B., Knight R.T. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc. Natl. Acad. Sci. U.S.A. 2010;107(42):18167–18172. doi: 10.1073/pnas.1007277107. http://doi.org/10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R. Inferences based on a skipped correlation coefficient. J. Appl. Stat. 2004;31(2):131–143. http://doi.org/10.1080/0266476032000148821. [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. http://doi.org/10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Chun M.M. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–95. doi: 10.1038/nature04262. http://doi.org/10.1038/nature04262. [DOI] [PubMed] [Google Scholar]