Abstract
To assess the role of κ opioid receptors in astrocyte development, the effect of κ agonists on the growth of astroglia derived from 1–2 day-old mouse cerebra was examined in vitro. κ-Opioid receptor expression was assessed immunocytochemically (using KA8 and KOR1 antibodies), as well as functionally by examining the effect of κ receptor activation on intracellular calcium ([Ca2+]i) homeostasis and DNA synthesis. On days 6–7, as many as 50% of the astrocytes displayed κ receptor (KA8) immunoreactivity or exhibited increases in [Ca 2+]i in response to κ agonist treatment (U69,593 or U50,488H). Exposure to U69,593 (100 nM) for 72 h caused a significant reduction in number and proportion of glial fibrillary acidic protein-immunoreactive astrocytes incorporating bromodeoxyuridine (BrdU) that could be prevented by co-administering the κ antagonist, nor-binaltorphimine (300 nM). In contrast, on day 14, only 5 or 14%, respectively, of the astrocytes were κ opioid receptor (KA8) immunoreactive or displayed functional increases in [Ca2+]i. Furthermore, U69,593 (100 nM) treatment failed to inhibit BrdU incorporation at 9 days in vitro. Experimental manipulations showed that κ receptor activation increases astroglial [Ca 2+]i both through influx via L-type channels and through mobilization of intracellular stores (which is an important Ca2+ signaling pathway in cell division). Collectively, these results indicate that a subpopulation of developing astrocytes express κ opioid receptors in vitro, and suggest that the activation of κ receptors mobilizes [Ca 2+]i and inhibits cell proliferation. Moreover, the proportion of astrocytes expressing κ receptors was greatest during a period of rapid cell growth suggesting that they are preferentially expressed by proliferating astrocytes.
Keywords: Opioid receptors, Cell division, Central nervous system maturation, Neuroglia, Drug abuse, Endogenous opioid system, U 69593, U 50488H, nor-Binaltorphimine, Fura-2, Intracellular calcium, Nifedipine, Thapsigargin
1. Introduction
Endogenous opioid peptides and receptors are present during development and can modify nervous system maturation [23,27,72]. During development, endogenous opioid neuropeptides typically act by inhibiting the genesis of neurons and glia [23,27,65,79]. Pathophysiological changes in the endogenous opioid system may contribute to sudden infant death syndrome, autism or self-destructive behavior [19]. Furthermore, opiate drugs with abuse liability, such as heroin or morphine, also inhibit neural development presumably by disrupting the endogenous opioid system [23,27]. Children born to opioid addicted mothers display neurobehavioral deficits which are directly or indirectly caused by prenatal or perinatal exposure to opiates [12,30].
The endogenous opioid system consists of multiple types of opioid receptors (e.g., μ, δ, and κ) as well as a variety of endogenous opioid peptides (i.e., proopiomelanocortin, proenkephalin, and prodynorphin). μ, δ, And κ opioid receptors serve many distinct functions in the adult. These receptors have been characterized pharmacologically and more recently at the molecular level [16,44,53,62]. Despite the importance of the endogenous opioid system in development, no single opioid receptor type has been exclusively linked to growth [27]. Moreover, in addition to the traditional μ, δ and κ opioid receptor types [34,35], there are putative opioid receptor types which are present during development and which may affect growth [5,78]. It is uncertain whether these immature receptors may represent novel opioid receptor types or variants of the traditional opioid receptor types or subtypes. Thus, although multiple opioid receptor types exist, the role of the individual types in growth has not been adequately assessed.
Recent findings suggest that astrocytes are important targets of opioid action. Astrocytes express opioid binding sites [38] and opioids affect astroglial function [38,76], including glycogen metabolism [48], cyclic AMP formation [14] and Ca2+ homeostasis [15,28,67]. During development, not only do astrocytes themselves express endogenous opioid peptides [25,43,60,61,63], but opioids and/or opiate drugs inhibit the proliferation of flat, polyhedral (type 1) astrocytes [26,64–66].
Despite numerous effects of opioids on astroglial function, little is known about the particular opioid receptor type(s) or the cellular mechanisms by which opioids act. Previous studies from our laboratory suggested that κ opioid receptors are expressed by many developing astrocytes in vitro[14,15,67], and that κ receptors can affect astrocyte differentiation through a Ca 2+-dependent mechanism [67]. The onset of κ opioid receptor expression occurs early during development (by mid-gestation in rodents) [34,35,54]. Moreover, dynorphins--a class of endogenous opioid peptides which have high affinity for κ opioid receptors --also are expressed in the brain prenatally [34,35]. In addition to dynorphins, several of the intermediate/partially-processed peptide products of proenkephalin are also expressed during early development. Recent findings suggest that proenkephalin-derived peptides can also selectively activate κ opioid receptors, in addition to their action at δ sites [33] (review [34,35]). This collective evidence prompted us to further assess the potential role of this receptor type in astrocyte development. Our results indicate that κ opioid receptors are expressed by a subpopulation of astrocytes during development in vitro and that the activation of κ receptors inhibits cell division.
2. Materials and Methods
2.1. Materials
nor-Binaltorphimine was obtained from RBI (Research Biochemicals, Inc., Natick, MA)[68]. (−)-Naloxone was a gift from Dr. Joel G. Whitney, E.I. DuPont (Wilmington, DE). trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl] benzeneacetamide methanesulfonate (U50,488H) and [(5α,7α,8β) -(−)-N-Methyl-N-[7-(1-pyrrolidinyl) -1-oxaspiro(4,5)dec-8-yl] bezeneacetamide (U69,593)[32] were obtained through Dr. Paul Hillary (NIDA). Nifedipine and thapsigargin were both obtained from Sigma (St. Louis, MO) and were used as previously described [28].
2.2. Cell culture
Dissociated, mixed-glial cultures were obtained from 1–4 day-old (date of birth = day 0) ICR mouse (Harlen Sprague Dawley, Indianapolis, IN) cerebra as previously described (Stiene-Martin and Hauser, 1990). Briefly, cultures were maintained in growth media containing Dulbecco’s modified Eagle’s Media (DMEM) (Gibco, Grand Island, NY) with 0.5% glucose, 0.06% Na2HCO3, and 10% fetal bovine serum (FBS) (JRH Biosciences, Lenexa, KS). For assessment of DNA synthesis and anti-κ opioid receptor immunocytochemistry experiments, 16 mm glass coverslips coated with poly-L-lysine (40.1 kDa; Sigma, St. Louis, MO) were placed into 22 mm wells and seeded with 1 ml of cell suspension (approximately 3.5 x 105cells/well). To measure [Ca2+]i, cells were seeded onto poly-L-lysine coated MatTek dishes (MatTek Co., Natick, MA). All cultures were incubated at 34–35 °C in 5% CO2/95% air and high humidity. Media was change 24 h after plating and subsequently replaced every 2–3 days thereafter. Cultures were maintained for 1 to 28 days in vitro. To characterize astroglia in these cultures, glial fibrillary acidic protein (GFAP) immunocytochemistry was performed as described below.
2.3. κ-Opioid receptor immunocytochemistry
Media was removed and cultures were fixed for 20 min with 1 ml of paraformaldehyde at 4 °C. Kappa opioid receptor immunoreactivity was determined using monoclonal antibody KA8 diluted in 0.1% BSA (1:400 dilution) [37]. Cells were incubated in primary antibody for at least 24 hr at 4 °C. To determine specificity of staining, some cells were incubated in 0.1% BSA without primary antibody. The immunocytochemical procedure was continued at room temperature using biotinylated donkey anti-mouse-IgG secondary antibodies and an ABC reagent (avidin conjugated to peroxidase; Vector Laboratories) for 1 hr. The reaction was visualized by using 2.5% nickel ammonium sulfate, 0.35% DAB, and 0.012% H2O2in 0.1 M sodium acetate (pH 6.0). Affinity -purified rabbit anti-KOR1 antibodies [1] were also used as described above to assess the presence of κ opioid receptors in astrocytes.
2.4. Effect of κ Opioids on [Ca 2+]i
The concentration of free intracellular calcium ([Ca2+]i) was measured by ratiometric fluorescence imaging of the Ca2+ sensitive dye, fura -2/AM. Cells were loaded (45 min at 34 –35 °C ) with 4 μM or 10 μM fura-2/AM (Molecular Probes, Eugene, OR) in growth media that included 10 or 25 mM Hepes buffer (pH 7.2) and 2% DMSO in 5% CO2/95% air. Following incubation, cells were washed 3 times with growth media containing 10 or 25 mM Hepes buffer, and allowed to incubate for 30 min to permit complete hydrolysis of the fura-2/AM. Ratiometric [Ca2+]i measurements were acquired at 26–28 °C using an inverted Nikon microscope with an oil immersion, fluoro 40x (N.A. 1.3 objective), a Dage 72 CCD camera (Michigan City, IN), and a Hamamatsu C2400 intensifier (Hamamatsu Photonics K.K., Hamamatsu-City, Japan). MCID MI or M4 imaging systems (Imaging Research Inc., St. Catharines, Ontario) with fura-2 ratiometric software were used to acquire and process the images. [Ca2+]i was determined from the ratio of fluorescence emission at 340 and 380 nm excitation wavelengths [21] as previously described [28,67]. A computer-controlled, optical filter wheel (Lambda-10, Axon/Sutter Instruments, Novato, CA) was used to change excitation filters.
To assess the effect of opioids on [Ca2+]i, repeated measures were made on the same cells at 1–20 sec. intervals. [Ca2+]i determinations were made while the exposed to varied concentrations of the κ agonists U69,593 [32] or U50,488H; with initial measurements made to established basal [Ca2+]i. Three-fold greater concentrations of the κ antagonist nor-binaltorphimine, or the μ, δ, and κ antagonist naloxone, were used to block κ agonist effects. To manipulate [Ca 2+]i, 6 day-old cultures were pretreated for 10–30 min. with nifedipine (1 μM) (which blocks L-type Ca2+ channels), thapsigargin (100 nM) which depletes inositol 1,3,4-trisphosphate-(IP3) sensitive Ca2+ stores, or media without extracellular Ca2+ (0 [Ca 2+]o + 1 mM EGTA) as previously described [28]. Measurements for individual cells were taken from the cytoplasm, but not over the nucleus. Following Ca2+ imaging, some cultures were fixed, processed for GFAP immunocytochemistry, air-dried and coverslipped to verify that the imaged cells were type 1 astrocytes. In addition, the proportion of flat, polyhedral cells displaying a positive response to κ agonist treatments was determined at various days in vitro. A positive response was defined as at least a 50% increase in the 340:380 ratio within 20 sec. of agonist treatment. Each culture consisted of an independent sample of cells derived from separate animals.
2.5. Effect of κ opioids on DNA synthesis and astrocyte numbers
To assess the effect of opioids on astrocyte DNA synthesis, cultures received nutrient media alone (control), or were continuously exposed to varied concentrations of the κ agonists U69,593 or U50,488H with or without 3-fold greater concentrations of the κ antagonist nor-binaltorphimine for 72 h before harvesting at 6 or 9 days in vitro. Cultures were treated with either U69,593 (1 or 100 nM), U69,593 plus nor-binaltorphimine (3 or 300 nM), or nor-binaltorphimine alone (300 nM).
The effect of opioids on astrocyte DNA synthesis was analyzed using combined glial fibrillary acidic protein (GFAP) and 5-bromodeoxyuridine (BrdU) immunocytochemistry. Six hours before opioid-treated cultures were fixed, BrdU (50 μM final concentration; Sigma) was added to the media. Cells were fixed in Zamboni’s fixative[80] containing 3% paraformaldehyde at 4 °C and incubated with primary monoclonal antibodies (1:1000 dilution) against BrdU (Chemicon, Temecula, CA) for 48 hr at 4 °C. BrdU was detected using donkey anti-mouse IgG secondary antibodies and a biotin-avidin-peroxidase detection system as directed (Vectastain ABC kit, Vector laboratories, Burlingame, CA). A black BrdU reaction product was visualized by using 2.5% nickel ammonium sulfate, 0.35% diaminobenzidine (DAB), and 0.012% H2O2in 0.1 M sodium acetate (pH 6.0). GFAP immunocytochemistry was then performed using primary, anti-GFAP polyclonal antibodies (Chemicon; 1:1500 dilution). Secondary goat anti-rabbit IgG antibodies conjugated to an avidin-biotin-peroxidase complex were then used to detect GFAP as directed (ABC kit, Vector Laboratories). A brown GFAP reaction product was visualized using 0.06% DAB, 0.02% hydrogen peroxide in 50 mM Tris buffer, pH 7.6. The labeling index is reported as the number of GFAP immunoreactive flat, polyhedral (type 1) astrocytes containing a BrdU immunoreactive nucleus (labeled cell) divided by the total number of astrocytes. The number of astrocytes per field were counted using a Leitz microscope at 40x. About 500–800 astrocytes were sampled in each of six cultures; each culture consisted of independent samples of cells pooled from separate animals.
2.6. Statistics
The effects of opioids on DNA synthesis and cell numbers were assessed using analysis of variance (ANOVA) with post-hoc comparisons using Newman -Keuls test (Statistica, StatSoft, Tulsa, OK). Mean [Ca2+]i levels were determined using repeated measures ANOVA with post-hoc Newman -Keuls analysis to [Ca2+]i levels in individual cells before and after opioid treatment (Statistica, StatSoft, Tulsa, OK). The effect of days in vitro on κ-receptor expression among astrocytes was assessed using Student’s t-test. Data are reported as the mean ±SEM.
3. Results
After 3days in vitro, 95–97% of the flat, polyhedral cells in our mixed-glial cultures were GFAP immunoreactive. These flat, polyhedral cells do not express A2B5 antigenicity and are morphologically similar to “type 1” astrocytes [22,26](Stiene-Martin, unpublished) described by other investigators [50], and are referred to as type 1 astrocytes in the remainder of the present study. We have previously found that opioids affect the development of type 1 astrocytes, but not A2B5 immunoreactive process-bearing (type 2) astrocytes [22,26].
3.1. Findings during the first week in vitro
3.1.1. κ-Opioid receptor immunocytochemistry
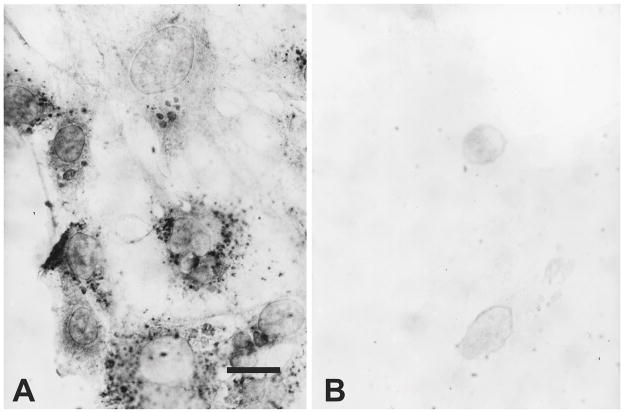
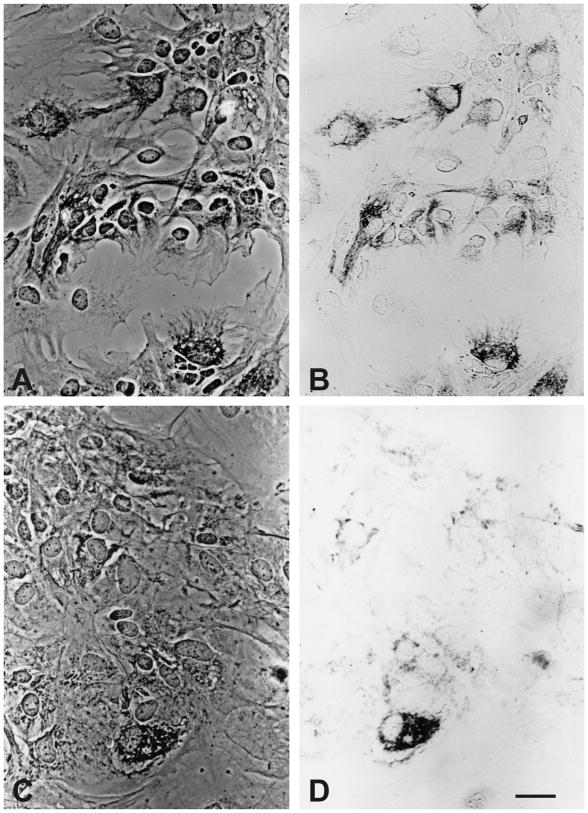
Using KA8 antibodies, many type 1 astrocytes expressed κ-opioid receptor immunoreactivity at 7 days in vitro (Fig. 1). Punctate patterns of KA8 immunoreactivity associated with the cytoplasm and/or plasma membrane were most evident, although a more diffuse immunoreactive pattern was also evident in some cells (Fig. 1A). The apparent cytoplasmic immunoproducts may be associated with sites of receptor synthesis at the endoplasmic reticulum or with internalized receptors within endosomal compartments. The unambiguous localization of κ-immunoproducts at the subcellular level requires confocal and/or electron microscopic localization. Both the level of immunoreactivity (including the number of punctate immunoreactive sites) varied among individual type 1 astrocytes. Often entire clusters of adjacent astrocytes either lacked or possessed immunoreactivity. The pattern of KA8 and KOR1 immunoreactivity were generally similar with some exceptions; the diffuse immunostaining pattern was less evident and the punctate sites were less discrete with KOR1 antibodies (see Fig. 5). Both KA8 and KOR1 antibodies are believed to preferentially recognize postsynaptic κ receptors [1,37,39,57].
Fig. 1.
Brightfield photomicrographs showing KA8 (κ receptor) immunoreactivity in astrocyte -enriched cultures at 7 days in vitro. κ-Opioid receptor immunoreactivity was typically punctate with some diffuse staining and associated with the cytoplasm/plasma membrane of a subset of type 1 astrocytes (A). Controls; KA8 primary antibody was omitted from the reaction (B). Scale bar = 10 μm.
Fig. 5.
Phase-contrast and brightfield photomicrographs showing KOR1 (κ receptor) immunoreactivity in astrocyte-enriched cultures at 7 and 26 days in vitro. At 7 days in vitro, κ opioid receptor immunoreactivity was associated with large numbers of type 1 astroglia (A & B). Alternatively, at 26 days in vitro, fewer cells displayed κ opioid receptor immunoreactivity and these were often clustered (non-uniformly) within the culture (not shown) (C & D). Scale bar = 20 μm.
3.1.2. Effect of κ Opioids on [Ca 2+]i
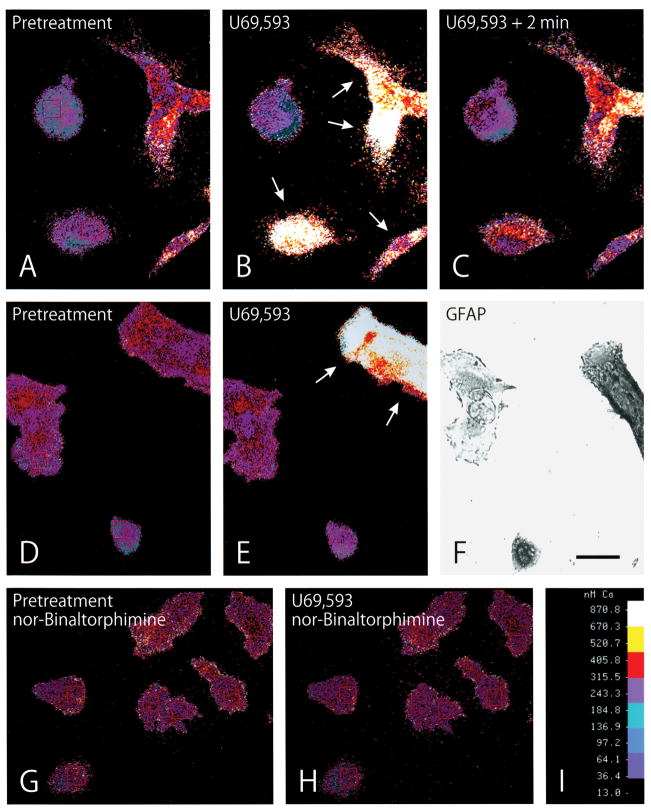
Treatment with the κ opioid receptor agonists U69,593 or U50,488H caused concentration -dependent increases in free [Ca2+]i in a subpopulation of GFAP -immunoreactive, type 1 astrocytes (Figs. 2; 3). κ-Agonist-induced increases in [Ca2+]i were prevented by pretreatment with the κ antagonist nor-binaltorphimine (Figs. 2H; 3H) or naloxone (Fig. 3B,D). When changes in [Ca2+]i were compared among individual astrocytes, it was apparent that the response to opioids was not uniform; many astrocytes did not respond to high concentrations (10 μM) of κ receptor agonists (Figs. 2; 3). Nevertheless, sufficient numbers of astrocytes responded to U69,593 treatment (1 μM) to significantly elevate mean [Ca2+]i within the entire population by about 40% (Fig. 3H).
Fig. 2.
Computer-generated pseudocolor images showing the effect of the κ opioid receptor agonist U69,593 on free intracellular calcium ([Ca2+]i) in astrocytes at 6 days in vitro. A–C: The same cells before (A) and after (B and C) κ agonist treatment. Treatment with U69,593 (100 nM) markedly increased in [Ca2+]i in a subset of astrocytes (arrows) 10 sec following exposure (B). Continued exposure to U69,593 (100 nM) for 2 min caused sustained elevations in [Ca2+]i (C) compared to pretreatment levels. D–F: The same cells before (D) and after U69,593 (1 μM) treatment (E). The subset of cells responding to κ agonist treatment ( arrows in E) were subsequently shown to be glial fibrillary acidic protein (GFAP) immunoreactive—confirming their identity as astrocytes (F). G–H: Pretreatment (55 min) with the κ antagonist nor-binaltorphimine (30 μM) (G) prevented U69,593 (1 μM) induced increases in [Ca2+]i (H). [Ca 2+]i scale (I). Scale bar in F = 25 μM.
Fig. 3.
Heterogeneous effects of κ-opioid receptor agonist treatment on free intracellular calcium ([Ca2+]i) show that a subset of astroglia respond to κ opioids at 6 days in vitro. Either U69,593 (A) or U50,488H (C), which are related κ agonists, caused concentration -dependent increases in [Ca2+]i within subpopulations of astrocytes. Naloxone pretreatment (10 min) completely prevented U69,593 (B) or U50,488H (D) induced increases in [Ca2+]i. Neither nifedipine pretreatment (E), nor the removal of extracellular Ca2+ (F) prevented κ-agonist-induced increases in [Ca2+]i in a subset of astrocytes, suggesting that within this subpopulation, [Ca2+]i increases did not result from influx through L-type Ca2+-channels or influx across the plasma membrane, respectively. Alternatively, thapsigargin pretreatment (G) did not prevent κ agonist induced increases in [Ca 2+]i within a subset of astrocytes, suggesting that within this subpopulation, [Ca2+]i increases did not result from Ca 2+mobilization from IP3-sensitive intracellular stores. Data in panels A through G show the responses of individual astrocytes; responsive (solid line) and non-responsive (dashed line). Arrows show when drugs were added; data are representative results of sampling 432 astrocytes in 8 separate experiments. H: Effect of κ agonists on mean [Ca 2+]i levels across all cells ( κ and non -κ-expressing astrocytes) at 6 days in vitro. U69,593 (1 μM) treatment significantly increased mean [Ca2+]i levels in astrocytes compared to pretreatment levels (a) (*P<0.05 vs. pretreatment [Ca2+]i at time 0). Nifedipine (1 μM) attenuated κ opioid-induced increases in [Ca2+]i levels at 2 min ( #P<0.05 vs. nifedipine-treatment), but not 6 or 10 min (H). [Ca2+]i increases were prevented by nor-binaltorphimine (nor-BNI) (3 μM) (Data in H are repeated measurements of 7–14 astrocytes per treatment and representative of 3 experiments).
Nifedipine pretreatment (1 μM for 20 min) significantly reduced U69,593-induced increases in mean [Ca2+]i in astrocyte populations at 2 min (but not at 6 or 10 min), suggesting that κ agonists can increase [Ca2+]i by coupling to L -type Ca2+ channels in a subset of astrocytes (Fig. 3H). Moreover, some astrocytes still responded to U69,593 with increases in [Ca2+]i following thapsigargin pretreatment (100 nM for 30 min). This further suggested that [Ca2+]i increases in some individual astrocytes did not result from Ca2+ mobilization from IP 3-sensitive Ca2+ stores (Fig. 3G).
Alternatively, nifedipine pretreatment did not prevent κ opioid agonist -induced increases in [Ca2+]i within some individual astrocytes (Fig. 3E) or in mean [Ca 2+]i levels in astrocyte populations at 6 or 10 min (Fig. 3H). This suggested that [Ca2+]i increases within a subset of astrocytes did not result from influx through L-type Ca2+ channel s. Furthermore, κ agonist -induced increases in [Ca2+]i occurred in the absence of extracellular Ca 2+ (0 [Ca 2+]o and 1 mM EGTA) also suggesting that the increased [Ca2+]i did not originate from Ca 2+ influx across the plasma membrane (Fig. 3F). Collectively, these findings suggest that κ agonists can mobilize Ca 2+ from internal stores in a subset of astrocytes.
3.1.3. Effect of κ opioids on DNA synthesis and astrocyte numbers
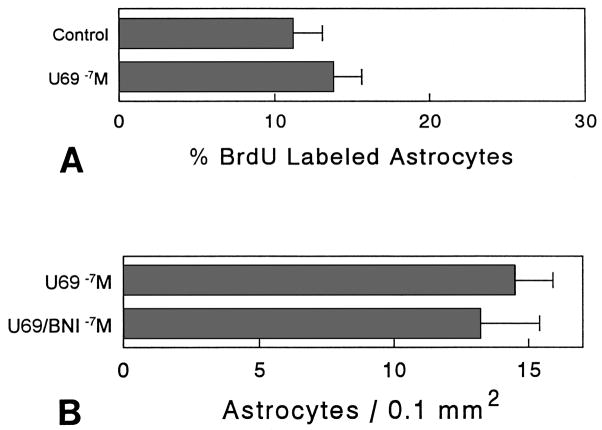
Continuous exposure to U69,593 (100 nM) for 72 h caused a significant decrease (35.4%) in BrdU incorporation in type 1 astrocytes compared to control cultures at 6 days in vitro (P< 0.05). The inhibitory effect of U69,593 on BrdU incorporation was prevented by concurrent treatment with 300 nM nor-binaltorphimine (Fig. 4A). Lower concentrations of U69,593 (1 nM) did not affect BrdU incorporation in astrocytes. Treatment with U69,593 (100 nM) significantly decreased (56.5%) the number of type 1 astrocytes, compared to cultures treated with U69,593 (100 nM) plus nor-binaltorphimine (300 nM) (P< 0.05) (Fig. 4B).
Fig. 4.
Effect of κ agonist and/or antagonists on the percentage of type 1 astrocytes incorporating bromodeoxyuridine (BrdU) and astrocyte numbers at 6 days in vitro. Continuous exposure to 100 nM U69,593 (U69 −7M) significantly reduced the proportion of BrdU-labeled astrocytes in treated cultures compared to controls, cultures treated with nor-binaltorphimine (300 nM) plus U69,593 (U69/BNI −7M), or cultures treated with nor-binaltorphimine (300 nM) alone (BNI −7M). Treatment with 1 nM U69,593 did not affect BrdU incorporation (U69 −9M) (A). Continuous exposure to U69,593 (100 nM) caused a significant reduction in the number of astrocytes in treated cultures compared to cultures concurrently treated with U69,593 and nor-binaltorphimine (300 nM). Mean ± SEM of 6 cultures; each culture consisted of independent samples of cells from separate mice (*P< 0.05 vs. control or BNI-treated cultures) (B).
3.2. Temporal Differences in κ opioid receptor expression
3.2.1. κ-Opioid receptor immunocytochemistry
The immunocytochemical results revealed temporal differences in the number of type 1 astrocytes containing κ-opioid receptor immunoreactivity. At 6–7 days in vitro, about 50% of the astrocytes sampled expressed κ receptor (KA8) immunoreactivity. By 14 days in vitro, significantly fewer (5%) of the type 1 astrocytes expressed KA8 immunoreactivity (Table 1). A reduction in the proportion of KOR1 immunoreactive astrocytes was also evident after the first week in vitro (Fig. 5) (the percentages of KOR1 positive astrocytes were not quantified). The astrocytes expressing κ receptors were not uniformly distributed within the cultures. Often, κ-expressing astrocytes were segregated into isolated clusters surrounded by cells lacking the receptor (not shown).
TABLE 1.
Effect of days in vitro on the percentage of type 1 astrocytes expressing κ opioid receptor (KA8) immunoreactivity.
| Days in Vitro | Appearance of cultures | Astrocytes expressing κ receptors (%) | Astrocyte density (Cells/0.1 mm2) |
|---|---|---|---|
|
| |||
| 6–7 | Subconfluent | 52.2 ± 3.0 | 6.8 ± 1.0 |
| 14 | Confluent | 13.6 ± 4.0* | 25.6 ± 3.1* |
Mean ±SEM of 7 cultures; each culture consisted of independent samples of cells from separate mice.
P< 0.01 vs. cultures at 6 –7 days in vitro.
3.2.2. Effect of κ Opioids on [Ca 2+]i
The proportion of astrocytes exhibiting increased [Ca2+]i in response to κ agonist treatment depended on the days spent in culture (Fig 6). Approximately 40% of the astrocytes sampled between 4 and 7 days in vitro responded to U69,593 (100 nM). However, at 1 –3 days in vitro or at 14 days in vitro, less than 10% of the astrocytes sampled displayed increases in [Ca2+]i in response to U69,593 treatment.
Fig. 6.
Effect of days in vitro on the percentage of flat, polyhedral cells in astrocyte -enriched cultures showing increases in intracellular calcium ([Ca2+]i) following 100 nM U69,593 treatment. The greatest proportion of cells responded on days 4 through 7. Data are the mean ±SEM of about 150 cells sampled from 4 to 10 cultures per day (percentage responding vs. days in vitro, P< 0.05 ANOVA).
3.2.3. Effect of κ opioids on DNA synthesis and astrocyte numbers
In contrast to our observations at 6 days in vitro, continuous exposure to U69,593 (100 nM) for 72 h did not affect BrdU incorporation or type 1 astrocyte numbers at 9 days in vitro(Fig. 7). Importantly, the cells in our cultures remained dividing at 9 days in vitro and were not confluent until about day 12.
Fig. 7.
Effect of κ agonist and/or antagonists on the percentage of type 1 astrocytes incorporating bromodeoxyuridine (BrdU) and astrocyte numbers at 9 days in vitro. Continuous treatment with 100 nM U69,593 did not affect BrdU incorporation in astrocytes compared to astrocytes in control cultures (A), and did not affect astrocyte numbers compared to cultures concurrently treated with U69,593 and 300 nM nor-binaltorphimine (U69/BNI −7M) (B). Mean ±SEM of 6 cultures; each culture consisted of independent samples of cells from separate mice.
4. Discussion
Our results show that two phenotypically distinct populations of astrocytes can be identified based on whether or not they express κ opioid receptors. In type 1 astrocyte cultures in which a large percentage of cells expressed κ receptors, κ agonists increased [Ca2+]i and inhibited astrocyte proliferation. In addition, age-related differences in the proportion of κ to non -κ-expressing cells were striking. A large proportion of astrocytes expressed κ-opioid receptors during the first week in vitro, but proportionately fewer cells expressed κ receptors thereafter. The changing nature of κ receptor expression may result from: (i) a change in the phenotype of individual astrocytes, (ii) a slower rate of growth of κ compared to non-κ receptor expressing subpopulations of astrocytes, (iii) the selective loss of cells with κ receptors, or (iv) the absence of specific factors in vitro that may normally promote opioid receptor expression. Interleukin-1β regulates μ receptor expression in astrocytes [56], and κ receptors may be similarly regulated. The extent to which the in vitro findings accurately reflect events occurring in vivo is uncertain. Further studies are needed to determine the role of κ opioid receptors in astrocyte growth in vivo, and to determine whether the dynamic changes in κ receptor expression by astrocytes might contribute to critical periods of opioid sensitivity during neural development.
Endogenous opioids are not uniformly synthesized by cells within the brain or even within particular brain regions. Osborne et. al. [47] proposed that local differences in opioid production contribute to maturational gradients in the cerebellum by finely coordinating regional differences in the developmental interactions among two neuronal types. Interestingly, astrocytes express proenkephalin, but not prodynorphin, in vitro and in vivo [25,31,43,73]. Moreover, astrocytes are able to process proenkephalin precursors into smaller, biologically active peptides [31,43] and this processing is developmentally regulated [61]. Opioids have been proposed to regulate astrocyte development through paracrine/autocrine mechanisms [25], and the possibility that astrocyte-derived proenkephalin peptides could activate κ opioid receptors on astrocytes themselves infers a potentially interesting opioid signaling pathway [33]. Of course, neuronally-produced κ opioids are likely to be important in astrocyte function. Based on the above evidence, it is interesting to speculate that locally produced κ opioids might limit the number of κ receptor -expressing astrocytes within a brain region; thereby decreasing the proportion of κ-to non -κ-receptor expressing astrocytes.
That type 1 astrocytes express κ opioid receptors is supported by other studies. [Ca2+]i activation by selective ligands has been suggested as functional evidence for the presence of specific receptors in astroglia [18,59]. Physiologically relevant concentrations of selective κ agonists cause functional changes in [Ca2+]i in type 1 astrocytes [15,67]. Although μ or δ opioid receptor activation also elevates [Ca2+]i in a few cells (Hauser, unpublished), κ opioid agonists elevate [Ca2+]i in far greater numbers of astrocytes prior to 7 days in vitro[67]. Other investigators have identified κ opioid receptor mRNA using ribonuclease protection and/or reverse transcriptase PCR assays [55,69]. Although some experimenters have failed to identify opioid receptor expression by astroglia [58,71], an explanation for dissimilar findings might be the apparent plasticity of opioid receptor expression by astroglia evident in the present study.
Both KA8 and KOR1 antibodies most often yielded punctate patterns of immunoreactivity associated with the cytoplasm/plasma membrane of astrocytes. This pattern is consistent with the pattern of KA8 immunoreactivity in cultured embryonic chick neurons [36]. KA8 monoclonal antibodies selectively recognize an epitope of the κ opioid receptor associated with opioid binding sites. KA8 antigenicity is largely postsynaptic in chick (2-day-old) and human (30 to 35-day-old) neurons and has also been demonstrated in glia [36,37,39,57]. KOR1 antibodies were generated against specific epitopes of the cloned κ opioid receptor [1], and also recognize postsynaptic sites [1], although KOR1 antigenicity has not associated with glia in adult rats --except in the hypothalamo-hypophyseal system. The postsynaptic distribution suggests that both antibodies recognize the κ2 receptor subtype, which is in general agreement with the pharmacological agents used in the present study. U50,488H reportedly is a κ1 and κ2 agonist; whereas U69,593 is a preferential κ1 agonist but can activate κ2 subtypes at high concentrations [70,77]. A potential role for the naloxone benzoylhydrazone-sensitive κ3 receptor subtype in astrocyte function is uncertain [7,77].
Our study suggests that κ receptor activation can increase [Ca 2+]i by stimulating either of two separate pathways. This agrees with findings in other developing cell types that show κ-opioids can increase [Ca2+]i via influx through L -type Ca2+ channels and/or via [Ca 2+]i release from inositol trisphosphate-sensitive intracellular stores [2–4,15,40]. Earlier studies showed that κ agonists can increase [Ca2+]i in astrocytes through Ca 2+ influx via L -type channels [15], but did not provide evidence for Ca2+ mobilization from intracellular stores. Although our findings show that κ agonists can mobilize Ca2+, we have not attempted to show whether κ opioid -induced Ca2+ influx and intracellular Ca2+ release occurs in the same cell or separate subpopulations of cells. As mentioned, the response to κ opioid agonists among astroglia was not uniform; the κ expressing subpopulation might be further subdivided into separate subsets in which κ receptors are coupled to L-type channels and/or Ca2+ mobilization. Interestingly, κ receptors can couple to multiple G proteins in the same cell type suggesting that both pathways could coexist within individual astrocytes [49]. Lastly, that there are pharmacologically distinct opioid receptor-expressing astrocyte subpopulations is perhaps not surprising, since there is considerable diversity among astrocytes in the expression of non-opioid neurotransmitter receptors [18,59].
[Ca2+]i release from intracellular stores is likely to affect cell division. Evidence suggests that opioid-induced alterations in phospholipase C-dependent production of IP3[20,40] --and subsequent release of Ca2+ from intracellular stores [3,28] --is important in regulating DNA synthesis. Both Ca2+ and/or IP 3modulate cell division, and there is a requirement for Ca 2+for entry into and exit from mitosis[6,42,52]. Moreover, cells cannot advance through the G 1/S phase of the cell division cycle in the absence of Ca2+ [42,52]. We have previously shown that morphine inhibits DNA synthesis and causes cellular hypertrophy by activating μ opioid receptors and mobilizing [Ca2+]i in astroglia [28,67]. The antiproliferative effect of morphine can be mimicked by experimentally increasing [Ca2+]i, or prevented by pretreating cells with dantrolene which blocks Ca2+-dependent Ca2+ release; however, morphine’s action is not prevented by blocking L-type Ca2+ channels [28]. Therefore, based on these earlier studies [28], present findings suggest that κ agonists could also inhibit DNA synthesis by mobilizing [Ca2+]i. Moreover, κ receptor activation affects the replication of other cell types suggesting the effects are not limited to astrocytes [4,20]. Lastly, although a Ca2+- dependent pathway may inhibit DNA synthesis, opioids may additionally affect developing cells through alternative mechanisms. For example, Mangoura and Dawson have shown that μ agonists affect neuronal differentiation by activating protein kinase C-ε via a Ca2+-independent mechanism [41]. Böttger and Spruce have found that proenkephalin protein can be directly targeted to the cell nucleus and is associated with growth arrest and differentiation [9].
Findings that both μ and κ opioid receptor activation can similarly inhibit DNA synthesis in astrocytes (perhaps through a common Ca2+-dependent mechanism) have important implications for the role of particular opioid receptor types in growth. Collectively, our evidence strongly supports the hypothesis that no single class of opioid receptor is exclusively involved in cellular growth--instead the activation of μ and κ (and perhaps δ) receptor types can affect growth depending on the subpopulation and age of the astrocytes studied [66]. These findings differ from the view that a single opioid receptor type preferentially mediates growth (e.g., the putative ζ receptor) [78]. A unified hypothesis regarding the endogenous opioid system and cellular development needs to consider both the role of “traditional” μ, δ and κ receptors and the role of immature, non -traditional putative opioid receptor variants. Although many opioid functions are segregated among particular opioid receptor types [53,62], there is emerging evidence that different opioid receptor types can have identical/overlapping functions. This appears to result from promiscuity between individual opioid receptor types and intracellular effectors [5,10,11,16,17,29,49,74], especially during development [24]. What might be the purpose for μ and κ receptors having a similar antiproliferative function in astrocytes? There is evidence that multiple opioid signals can simultaneously converge on individual neurons [13,75] (or glia; Stiene-Martin and Hauser, personal observations); i.e., a single cell can have more than one opioid receptor type. The resultant intracellular “input signature” as defined by Evans [16] is integrative and may convey substantial information depending on which signaling pathways are coupled to particular opioid receptor types. Varying the particular opioid receptor types and effectors during development may be a mechanism by which individual cells simultaneously “choose” among different extracellular signals and how those signals are translated into a unique “intracellular logic” [16,24]. Also, the evidence that an opioid receptor type can couple to different intracellular effectors suggests that the type of G protein coupling may be more important than the identity of the opioid receptor type itself in determining the intracellular response and subsequent developmental outcome (see [8,46]).
During development, astroglia affect the genesis of neurons [45,51,59]. Thus, the impact of opiate drugs on gliogenesis may contribute to the neurobehavioral defects in the offspring of opiate-dependent mothers [27,28,64,67]. Findings that κ opioid receptor activation mobilizes intracellular Ca2+ and inhibits proliferation in cultured astroglia suggest a basic mechanism by which opioids might affect neural development. Furthermore, the transient nature of κ opioid receptor expression by astrocytes, suggests that the timing of κ opioid exposure is essential in determining developmental outcome.
Acknowledgments
The authors wish to thank Carol Turbek, Rong Zhou, Chrystal C. Godleske, and S. Eric Ryan for technical assistance. Supported by DA 06204 and Equipment Grants from NIDA, and by the Univ. Kentucky Medical Center.
References
- 1.Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee J-H, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R. The kappa-opioid receptor is primarily postsynaptic: Combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barg J, Belcheva MM, Rowinski J, Coscia CJ. kappa-opioid agonist modulation of [3H]thymidine incorporation into DNA: Evidence for the involvement of pertussis toxin-sensitive G protein-coupled phosphoinositide turnover. J Neurochem. 1993;60:1505–1511. doi: 10.1111/j.1471-4159.1993.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barg J, Belcheva MM, Zimlichman R, Levy R, Saya D, Mchale RJ, Johnson FE, Coscia CJ, Vogel Z. Opioids inhibit endothelin-mediated DNA synthesis, phosphoinositide turnover, and Ca2+ mobilization in rat C6 glioma cells. J Neurosci. 1994;14:5858–5864. doi: 10.1523/JNEUROSCI.14-10-05858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barg J, Nah S-Y, Levy R, Saya D, Vogel Z. Modulation of thymidine incorporation by kappa-opioid ligands in rat spinal cord-dorsal root ganglion co-cultures. Brain Res. 1993;629:109–114. doi: 10.1016/0006-8993(93)90488-9. [DOI] [PubMed] [Google Scholar]
- 5.Barg J, Simantov R. Transient expression of opioid receptors in defined regions of developing brain: Are embryonic receptors selective. J Neurochem. 1991;57:1978–1984. doi: 10.1111/j.1471-4159.1991.tb06412.x. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 7.Berzetei-Gurske IP, White A, Polgar W, DeCosta BR, Pasternak GW, Toll L. The in vitro pharmacological characterization of naloxone benzoylhydrazone. Eur J Pharmacol. 1995;277:257–263. doi: 10.1016/0014-2999(95)00088-3. [DOI] [PubMed] [Google Scholar]
- 8.Bourne HR, Stryer L. The target sets the tempo. Nature. 1992;358:541–543. doi: 10.1038/358541a0. [DOI] [PubMed] [Google Scholar]
- 9.Böttger A, Spruce BA. Proenkephalin is a nuclear protein responsive to growth arrest and differentiation signals. J Cell Biol. 1995;130:1251–1262. doi: 10.1083/jcb.130.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- 11.Cruciani RA, Dvorkin B, Morris SA, Crain SM, Makman MH. Direct coupling of opioid receptors to both stimulatory and inhibitory guanine nucleotide-binding proteins in F-11 neuroblastoma-sensory neuron hybrid cells. Proc Natl Acad Sci USA. 1993;90:3019–3023. doi: 10.1073/pnas.90.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doberczak TM, Kandall SR, Wilets I. Neonatal opiate abstinence syndrome in term and preterm infants. J Pediatr. 1991;118:933–937. doi: 10.1016/s0022-3476(05)82214-0. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, North RA. Both μ and δ opiate receptors exist on the same neuron. Science. 1981:923–924. doi: 10.1126/science.6272393. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson PS, Hansson E, Rönnbäck L. δ and kappa opiate receptors in primary astroglial cultures. Part II: Receptor sets in cultures from various brain regions and interactions with β-receptor activated cyclic AMP. Neurochem Res. 1992;17:545–551. doi: 10.1007/BF00968781. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson PS, Nilsson M, Wagberg M, Hansson E, Rönnbäck L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neurosci. 1993;54:401–407. doi: 10.1016/0306-4522(93)90261-d. [DOI] [PubMed] [Google Scholar]
- 16.Evans CJ. Diversity among the opioid receptors. In: Korenman SG, Barchas JD, editors. Biological Basis of Substance Abuse. Oxford University Press; New York: 1993. pp. 31–48. [Google Scholar]
- 17.Fields A, Gafni M, Oron Y, Sarne Y. Multiple effects of opiates on intracellular calcium levels and on calcium uptake in three neuronal cell lines. Brain Res. 1995;687:94–102. doi: 10.1016/0006-8993(95)00475-6. [DOI] [PubMed] [Google Scholar]
- 18.Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- 19.Gillberg C. Endogenous opioids and opiate antagonists in autism: Brief review of empirical findings and implications for clinicians. Dev Med Child Neurol. 1995;37:239–245. doi: 10.1111/j.1469-8749.1995.tb11998.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorodinsky A, Barg J, Belcheva MM, Levy R, Mchale RJ, Vogel Z, Coscia CJ. Dynorphins modulate DNA synthesis in fetal brain cell aggregates. J Neurochem. 1995;65:1481–1486. doi: 10.1046/j.1471-4159.1995.65041481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985;2603440:3440–3450. [PubMed] [Google Scholar]
- 22.Gurwell JA, Hauser KF. Morphine does not affect astrocyte survival in developing primary mixed-glial cultures. Dev Brain Res. 1993;76:293–298. doi: 10.1016/0165-3806(93)90222-v. [DOI] [PubMed] [Google Scholar]
- 23.Hammer RP., Jr . Effects of opioids on the developing brain. In: Hammer RP Jr, editor. The Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 1–21. [Google Scholar]
- 24.Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1996 in press. [PubMed] [Google Scholar]
- 25.Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser KF, Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- 27.Hauser KF, Stiene-Martin A. Opiates and the regulation of nervous system development: Evidence from in vitro studies. In: Hammer RP Jr, editor. Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 23–61. [Google Scholar]
- 28.Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996 doi: 10.1016/0006-8993(96)00103-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin W, Lee NM, Loh HH, Thayer SA. Opioid-induced inhibition of voltage-gated calcium channels parallels expression of omega-conotoxin-sensitive channel subtype during differentiation of NG108-15 cells. Brain Res. 1993;607:17–22. doi: 10.1016/0006-8993(93)91484-a. [DOI] [PubMed] [Google Scholar]
- 30.Kaltenbach KA, Finnegan LP. Prenatal narcotic exposure: perinatal and developmental effects. Neurotoxicology. 1989;10:597–604. [PubMed] [Google Scholar]
- 31.Klein RS, Fricker LD. Heterogeneous expression of carboxypeptidase E and proenkephalin mRNAs by cultured astrocytes. Brain Res. 1992;569:300–310. doi: 10.1016/0006-8993(92)90643-n. [DOI] [PubMed] [Google Scholar]
- 32.Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF. [3H]U-69,593 a highly selective ligand for the kappa opioid receptor. Eur J Pharmacol. 1985;109:281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- 33.Leng G, Bicknell RJ, Brown D, Bowden C, Chapman C, Russell JA. Stimulus-induced depletion of pro-enkephalins, oxytocin and vasopressin and pro-enkephalin interaction with posterior pituitary hormone release in vitro. Neuroendocrinology. 1994;60:559–566. doi: 10.1159/000126797. [DOI] [PubMed] [Google Scholar]
- 34.Leslie FM, Loughlin SE. Development of multiple opioid receptors. In: Miller MW, editor. Development of the Nervous System: Effects of Alcohol and Opiates. Wiley-Liss; New York: 1992. [Google Scholar]
- 35.Leslie FM, Loughlin SE. Ontogeny and plasticity of opioid systems. In: Hammer RP Jr, editor. The Neurobiology of Opiates. CRC Press; Boca Raton, FL: 1993. pp. 85–123. [Google Scholar]
- 36.Maderspach K, Németh K. Immunocytochemical visualization of kappa-opioid receptors on chick embryonic neurons differentiating in vitro. Neuroscience. 1993;57:459–465. doi: 10.1016/0306-4522(93)90078-t. [DOI] [PubMed] [Google Scholar]
- 37.Maderspach K, Németh K, Simon J, Benyhe S, Szücs M, Wollemann M. A monoclonal antibody recognizing kappa-but not μ-and δ-opioid receptors. J Neurochem. 1991;56:1897–1904. doi: 10.1111/j.1471-4159.1991.tb03446.x. [DOI] [PubMed] [Google Scholar]
- 38.Maderspach K, Solomonia R. Glial and neuronal opioid receptors: apparent positive cooperativity observed in intact cultured cells. Brain Res. 1988;441:41–47. doi: 10.1016/0006-8993(88)91381-9. [DOI] [PubMed] [Google Scholar]
- 39.Maderspach K, Takács J, Niewiadomska G, Csillag A. Postsynaptic and extrasynaptic localization of kappa-opioid receptor in selected brain areas of young rat and chick using an anti-receptor monoclonal antibody. J Neurocytol. 1995;24:478–486. doi: 10.1007/BF01181608. [DOI] [PubMed] [Google Scholar]
- 40.Mangoura D, Dawson G. Chronic opioid treatment attenuates carbachol-mediated polyphosphoinositide hydrolysis in chick embryo neuronal cultures. Brain Res. 1991;548:273–278. doi: 10.1016/0006-8993(91)91132-k. [DOI] [PubMed] [Google Scholar]
- 41.Mangoura D, Dawson G. Opioid peptides activate phospholipase D and protein kinase C-ε in chicken embryo neuron cultures. Proc Natl Acad Sci USA. 1993;90:2915–2919. doi: 10.1073/pnas.90.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Means AR. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 1994;347:1–4. doi: 10.1016/0014-5793(94)00492-7. [DOI] [PubMed] [Google Scholar]
- 43.Melner MH, Low KG, Allen RG, Nielson CP, Young SL, Saneto RP. The regulation of proenkephalin expression in a distinct population of glial cells. EMBO J. 1990;9:791–796. doi: 10.1002/j.1460-2075.1990.tb08175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami M, Satoh M. Molecular biology of the opioid receptors: Structures, functions and distributions. Neurosci Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- 45.Muller CM. Glial cells and activity-dependent central nervous system plasticity. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford; New York: 1995. pp. 805–814. [Google Scholar]
- 46.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 47.Osborne JG, Kindy MS, Spruce BA, Hauser KF. Ontogeny of proenkephalin mRNA and enkephalin peptide expression in the cerebellar cortex of the rat: Spatial and temporal patterns of expression follow maturational gradients in the external granular layer and in Purkinje cells. Dev Brain Res. 1993;76:1–12. doi: 10.1016/0165-3806(93)90117-s. [DOI] [PubMed] [Google Scholar]
- 48.Pearce B, Cambray-Deakin M, Murphy S. Astrocyte opioid receptors: activation modifies the noradrenaline-evoked increase in 2-[14C]deoxyglucose incorporation into glycogen. Neurosci Lett. 1985;55:157–160. doi: 10.1016/0304-3940(85)90012-6. [DOI] [PubMed] [Google Scholar]
- 49.Prather PL, McGinn TM, Claude PA, Liu-Chen LY, Loh HH, Law PY. Properties of a kappa-opioid receptor expressed in CHO cells: Interaction with multiple G-proteins is not specific for any individual Gαsubunit and is similar to that of other opioid receptors. Mol Brain Res. 1995;29:336–346. doi: 10.1016/0169-328x(94)00264-f. [DOI] [PubMed] [Google Scholar]
- 50.Raff MC, Abney RE, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface ganagliosides and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rakic P. Radial glial cells: scaffolding for brain construction. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford; New York: 1995. pp. 746–762. [Google Scholar]
- 52.Reddy GP. Cell cycle: regulatory events in G1-->S transition of mammalian cells. J Cell Biochem. 1994;54:379–386. doi: 10.1002/jcb.240540404. [DOI] [PubMed] [Google Scholar]
- 53.Reisine T. Opiate receptors. Neuropharmacology. 1995;34:463–472. doi: 10.1016/0028-3908(95)00025-2. [DOI] [PubMed] [Google Scholar]
- 54.Rius RA, Barg J, Bem WT, Coscia CJ, Loh YP. The prenatal developmental profile of expression of opioid peptides and receptors in the mouse brain. Dev Brain Res. 1991;58:237–241. doi: 10.1016/0165-3806(91)90010-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express μ, δ and kappa opioid receptor mRNA. Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- 56.Ruzicka BB, Thompson RC, Watson SJ, Akil H. Interleukin-1β-mediated regulation of μ-opioid receptor mRNA in primary astrocyte-enriched cultures. J Neurochem. 1996;66:425–428. doi: 10.1046/j.1471-4159.1996.66010425.x. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt P, Schöder H, Maderspach K, Staak M. Immunohistochemical localization of kappa opioid receptors in the human frontal cortex. Brain Res. 1995;654:223–233. doi: 10.1016/0006-8993(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz JP, Nishiyama N, Wilson D, Taniwaki T. Receptor-mediated regulation of neuropeptide gene expression in astrocytes. Glia. 1994;11:185–190. doi: 10.1002/glia.440110212. [DOI] [PubMed] [Google Scholar]
- 59.Shao Y, McCarthy KD. Plasticity of astrocytes. Glia. 1994;11:147–155. doi: 10.1002/glia.440110209. [DOI] [PubMed] [Google Scholar]
- 60.Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989;245:415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- 61.Shinoda H, Marini AM, Schwartz JP. Developmental expression of the proenkephalin and prosomatostatin genes in cultured cortical and cerebellar astrocytes. Dev Brain Res. 1992;67:205–210. doi: 10.1016/0165-3806(92)90220-q. [DOI] [PubMed] [Google Scholar]
- 62.Simon EJ, Hiller JM. Opioid peptides and opioid receptors. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry. Raven Press; New York: 1994. pp. 321–339. [Google Scholar]
- 63.Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: Evidence for a direct effect of opiates on neural maturation. Dev Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: Suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- 66.Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stiene-Martin A, Mattson MP, Hauser KF. Opiates selectively increase intracellular calcium in developing type-1 astrocytes: role of calcium in morphine-induced morphologic differentiation. Dev Brain Res. 1993;76:189–196. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- 68.Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- 69.Thorlin T, Eriksson PS, Nilsson M, Hansson E, Rönnbäck L. Co-localized opioid and glutamate receptors on astroglial cells -Possible regulators of synaptic transmission. Soc Neurosci Abstr. 1994;20:1731. [Google Scholar]
- 70.Tiberi M, Magnan J. Quantitative analysis of multiple kappa-opioid receptors by selective and nonselective ligand binding in guinea pig spinal cord: Resolution of high and low affinity states of the kappa2 receptors by a computerized model -fitting technique. Mol Pharmacol. 1990;37:694–703. [PubMed] [Google Scholar]
- 71.Vaysse PJ-J, Zukin RS, Fields KL, Kessler JA. Characterization of opioid receptors in cultured neurons. J Neurochem. 1990;55:624–631. doi: 10.1111/j.1471-4159.1990.tb04179.x. [DOI] [PubMed] [Google Scholar]
- 72.Vernadakis A, Sakellaridis N, Geladopoulos T, Mangoura D. Function of opioidsearly in embryogenesis. Ann N Y Acad Sci. 1990;579:109–122. doi: 10.1111/j.1749-6632.1990.tb48354.x. [DOI] [PubMed] [Google Scholar]
- 73.Vilijn MH, Vaysse PJ-J, Zukin RS, Kessler JA. Expression of preproenkephalin mRNA in cultured astrocytes and neurons. Proc Natl Acad Sci (USA) 1988;85:6551–6555. doi: 10.1073/pnas.85.17.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Gintzler AR. Bimodal opioid regulation of cyclic AMP formation: Implications for positive and negative coupling of opiate receptors to adenylyl cyclase. J Neurochem. 1994;63:1726–1730. doi: 10.1046/j.1471-4159.1994.63051726.x. [DOI] [PubMed] [Google Scholar]
- 75.Werz MA, Macdonald RL. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu-and delta -opiate receptors. Nature. 1982;299:730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- 76.Wilkin GP, Marriott DR, Pearce B. Peptide receptors on astrocytes. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford; New York: 1995. pp. 367–376. [Google Scholar]
- 77.Wollemann M, Benyhe S, Simon J. The kappa-opioid receptor: evidence for the different subtypes. Life Sci. 1993;52:599–611. doi: 10.1016/0024-3205(93)90451-8. [DOI] [PubMed] [Google Scholar]
- 78.Zagon IS, Goodman SR, McLaughlin PJ. Zeta (ζ), the opioid growth factor receptor: Identification and characterization of binding subunits. Brain Res. 1993;605:50–56. doi: 10.1016/0006-8993(93)91355-v. [DOI] [PubMed] [Google Scholar]
- 79.Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991;542:318–323. doi: 10.1016/0006-8993(91)91585-o. [DOI] [PubMed] [Google Scholar]
- 80.Zamboni L, De Martino C. Buffered picric acid formaldehyde: a new rapid fixative for electron microscopy. J Cell Biol. 1967;35:148A. [Google Scholar]