Abstract
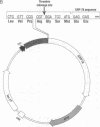
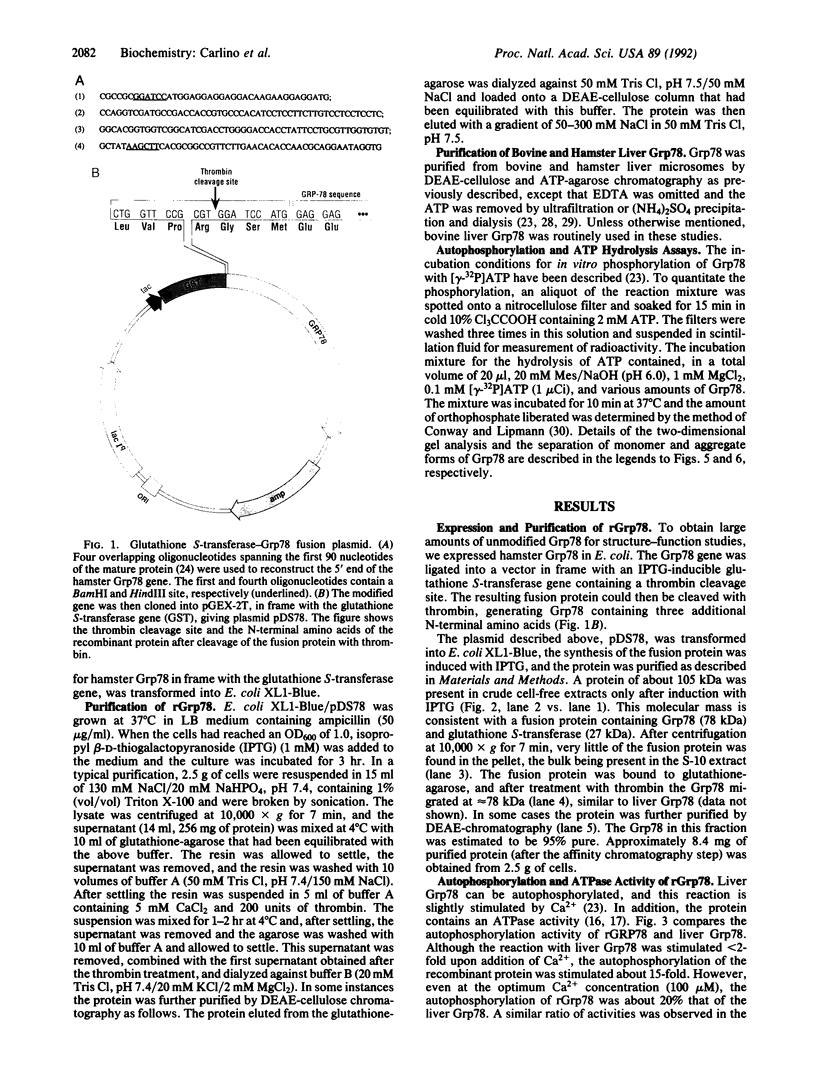
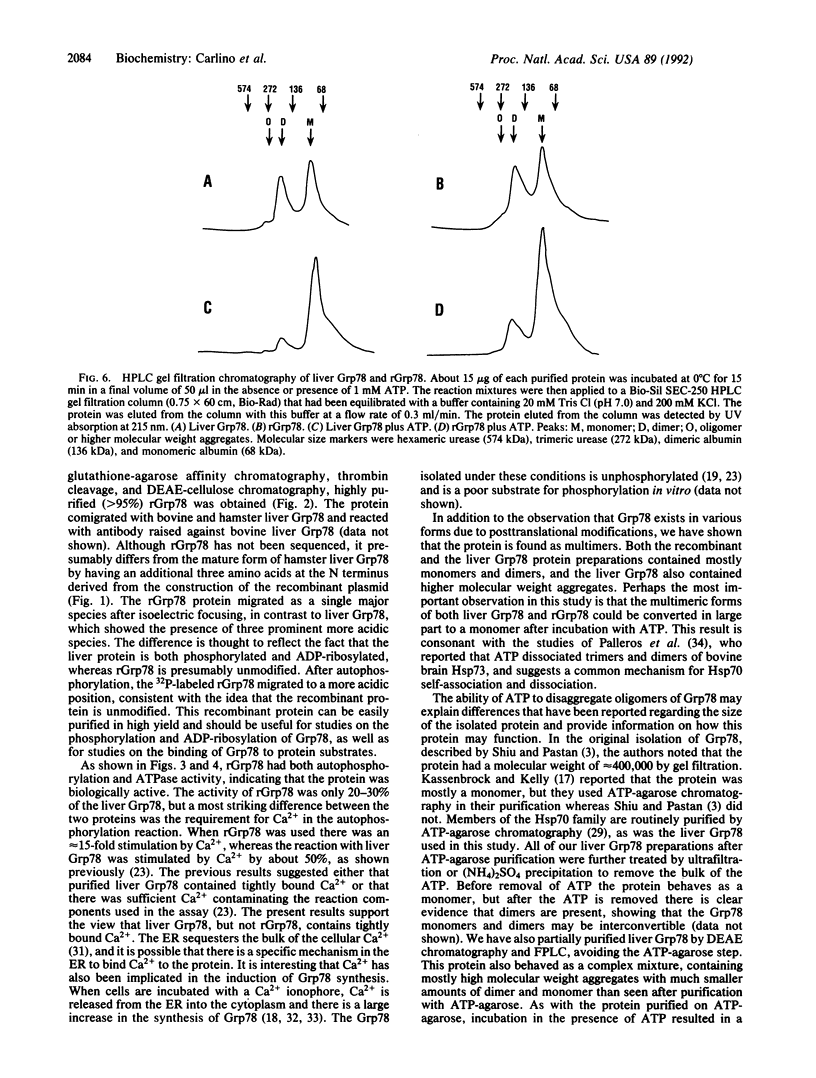
The hamster gene encoding the 78-kDa glucose-regulated protein (Grp78) was expressed in Escherichia coli as a fusion protein with glutathione S-transferase. After induction with isopropyl beta-D-thiogalactopyranoside, the recombinant Grp78 was purified to homogeneity by affinity column chromatography of the fusion protein followed by thrombin cleavage. The purified recombinant protein was compared with liver Grp78 for its ability to interact with ATP. Like liver Grp78, the recombinant protein contained a weak ATPase activity and a Ca(2+)-stimulated autophosphorylation activity. However, unlike liver Grp78, in which the autophosphorylation reaction is stimulated less than 50% by CaCl2, the reaction with the recombinant Grp78 was stimulated about 15-fold in the presence of Ca2+. Although the liver protein showed at least four isoforms after two-dimensional gel electrophoresis, the recombinant Grp78 had one major species corresponding to the most basic form seen in liver. Both the liver Grp78 and the recombinant protein existed primarily as monomers and dimers. A small amount of oligomers was also present in the liver Grp78. When either protein was incubated with ATP, there was a conversion of the higher molecular weight species to the monomeric form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L., Lazarides E. ADP-ribosylation of the Mr 83,000 stress-inducible and glucose-regulated protein in avian and mammalian cells: modulation by heat shock and glucose starvation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4664–4668. doi: 10.1073/pnas.80.15.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–12805. [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M., Ting J., Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988 Oct;8(10):4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenbrock C. K., Kelly R. B. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J. 1989 May;8(5):1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y., Normington K., Press E., Slaughter C., Sambrook J., Gething M. J. Identification of immunoglobulin heavy chain binding protein as glucose-regulated protein 78 on the basis of amino acid sequence, immunological cross-reactivity, and functional activity. J Cell Sci Suppl. 1989;11:115–137. doi: 10.1242/jcs.1989.supplement_11.10. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Ledford B. E. ADP-ribosylation of the 78-kDa glucose-regulated protein during nutritional stress. Eur J Biochem. 1989 Dec 8;186(1-2):205–211. doi: 10.1111/j.1432-1033.1989.tb15196.x. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Ledford B. E. Reversible ADP-ribosylation of the 78 kDa glucose-regulated protein. FEBS Lett. 1990 Dec 10;276(1-2):29–33. doi: 10.1016/0014-5793(90)80499-9. [DOI] [PubMed] [Google Scholar]
- Leustek T., Toledo H., Brot N., Weissbach H. Calcium-dependent autophosphorylation of the glucose-regulated protein, Grp78. Arch Biochem Biophys. 1991 Sep;289(2):256–261. doi: 10.1016/0003-9861(91)90469-y. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Jaworski C., Yamada K. M. Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):791–795. doi: 10.1073/pnas.76.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Schmid S. L., Braell W. A., Rothman J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pastan I. H. Properties and purification of a glucose-regulated protein from chick embryo fibroblasts. Biochim Biophys Acta. 1979 Jan 25;576(1):141–150. doi: 10.1016/0005-2795(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Ting J., Wooden S. K., Kriz R., Kelleher K., Kaufman R. J., Lee A. S. The nucleotide sequence encoding the hamster 78-kDa glucose-regulated protein (GRP78) and its conservation between hamster and rat. Gene. 1987;55(1):147–152. doi: 10.1016/0378-1119(87)90258-7. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Wu F. S., Park Y. C., Roufa D., Martonosi A. Selective stimulation of the synthesis of an 80,000-dalton protein by calcium ionophores. J Biol Chem. 1981 Jun 10;256(11):5309–5312. [PubMed] [Google Scholar]