SUMMARY
Dendritic arborization patterns are consistent anatomical correlates of genetic disorders such as Down syndrome (DS) and autism spectrum disorders (ASD). In a screen for abnormal dendrite development, we identified Minibrain(MNB)/DYRK1a, a kinase implicated in DS and ASD, as a regulator of the microtubule cytoskeleton. We show that MNB is necessary to establish the length and cytoskeletal composition of terminal dendrites by controlling microtubule growth. Altering MNB levels disrupts dendrite morphology and perturbs neuronal electrophysiological activity, resulting in larval mechanosensation defects. Using in vivo and in vitro approaches, we uncover a molecular pathway whereby direct phosphorylation of β-tubulin by MNB inhibits tubulin polymerization, a function that is conserved for mammalian DYRK1a. Our results demonstrate that phospho-regulation of microtubule dynamics by MNB/DYRK1a is critical for dendritic patterning and neuronal function, revealing a previously unidentified mode of post-translational microtubule regulation in neurons and uncovering a conserved pathway for a DS- and ASD-associated kinase.
INTRODUCTION
Neurons typically develop discrete dendritic and axonal compartments, which enable them to functionally integrate into neuronal circuitry (Stiess and Bradke, 2011; Witte and Bradke, 2008). Individual types of neurons develop dramatically different morphologies of mature dendrites, and the arborization pattern of a dendritic field is a critical determinant of neuronal function (Kaufmann and Moser, 2000). Dendrite morphological defects are one of the strongest anatomical correlates of intellectual disability, and hence an understanding of the intracellular pathways that lead to proper dendrite development is critical for human health (Kaufmann and Moser, 2000). Because dendritic development and function are tightly linked to the underlying dynamics of the cellular cytoskeleton (Witte and Bradke, 2008; Hoogenraad and Bradke, 2009), an understanding of how the neuron establishes such dynamics will provide direct insight into the normal developmental pathways that may be perturbed in certain disorders, such as DS.
The microtubule (MT) cytoskeletal network is organized into stable and dynamic arrays that provide structural support, serve as tracks for molecular motors, and function as signaling platforms during neuronal development and plasticity (Dent and Baas, 2014; Hoogenraad and Bradke, 2009). Dynamic MTs, in particular, help to drive the extension of a neurite, dendrite (or dendritic spine), or axon, and when MT growth into a branch or growth cone is inhibited, overall extension is stunted (Grabham et al., 2007; Hu et al., 2008; Jaworski et al., 2008; Myers and Baas, 2007; Ori-McKenney et al., 2012; Witte and Bradke, 2008). Although many molecules contribute to the organization of the MT cytoskeleton into specific arrays either in the axons or the dendrites, little is known about the pathways that regulate MT dynamics through post-translational modifications to establish a dendritic pattern. In addition, the influence of MT organization on dendrite morphogenesis and overall neuronal function is still being explored. The Drosophila dendritic arborization (da) neurons of the larval peripheral nervous system display complex dendrite morphologies and can be separated into four distinct classes with branch complexity and arbor size increasing with class number (I–IV) (Grueber et al., 2003b). Each of these classes of da neurons also performs a unique and independent function within the peripheral nervous system (Kim et al., 2012; Tracey et al., 2003; Xiang et al., 2010; Yan et al., 2013). They therefore provide an excellent system for studying how the differential regulation of the MT cytoskeleton contributes to the development of unique dendrite morphologies, and subsequently, unique functions.
Utilizing this system, we performed a screen for cytoplasmic kinases involved in the production of the distinct dendritic arborization pattern of class III da neurons. Kinases are of particular interest because they are often key regulators of biological processes including dendrite morphogenesis (Emoto et al., 2004; Ultanir et al., 2012), and they are attractive potential drug targets (Bishop et al., 1998). We identified MNB kinase as a regulator of the MT cytoskeleton during dendrite morphogenesis. MNB is a dual-specificity tyrosine regulated kinase (DYRK), and is 82% identical to its human homologue, DYRKla (Lochhead et al., 2005; Shindoh et al., 1996). MNB was originally identified in Drosophila, where hypomorph mutants produce a smaller brain and exhibit defects in visual and olfactory behavior (Fischbach and Heisenberg, 1981; Tejedor et al., 1995). Located on chromosome 21, DYRK1a in trisomy produces learning and memory defects associated with DS pathology (Altafaj et al., 2001; Guimera et al., 1996; Shindoh et al., 1996; Smith et al., 1997; Song et al., 1996), and is one of the genes most strongly associated with ASD (O’Roak et al., 2014; Willsey and State, 2015). However, its mode of action and downstream molecular pathways are not well defined.
In this study, we identify a direct role for MNB in the regulation of MT growth dynamics both in vivo and in vitro, uncover the first evidence for phospho-regulation of MTs in neurons, and reveal how the contributions of the MNB pathway to dendrite branch structure and cytoskeleton composition are essential for neuronal morphology and function. MNB binds directly to the acidic C-terminal tails (CTT) of tubulin through a conserved N-terminal basic patch, and inhibits MT polymerization by phosphorylating β-tubulin at a conserved serine residue. Interestingly, MNB is expressed in the class III, but not the class IV da neuron subtype, and we find that its phospho-regulation of MT growth contributes to the development of terminal branches that are a specific morphological feature characteristic of the class III da neurons. As a consequence, altering the levels of MNB severely perturbs the electrophysiological functioning of these neurons, resulting in defects in larval mechanosensation. The MNB pathway therefore contributes to proper neuronal function by regulating the cytoskeletal composition of dendrites to establish and maintain the dendritic arbor. Our study reveals a surprising mechanism of post-translational MT regulation during neuronal development and uncovers a previously unidentified molecular pathway for an important disease-related kinase, providing insight into the neuronal pathologies of DS and ASD.
RESULTS
Drosophila melanogaster class III and class IV dendritic arborization neurons develop distinct terminal branch morphologies with unique cytoskeletal compositions
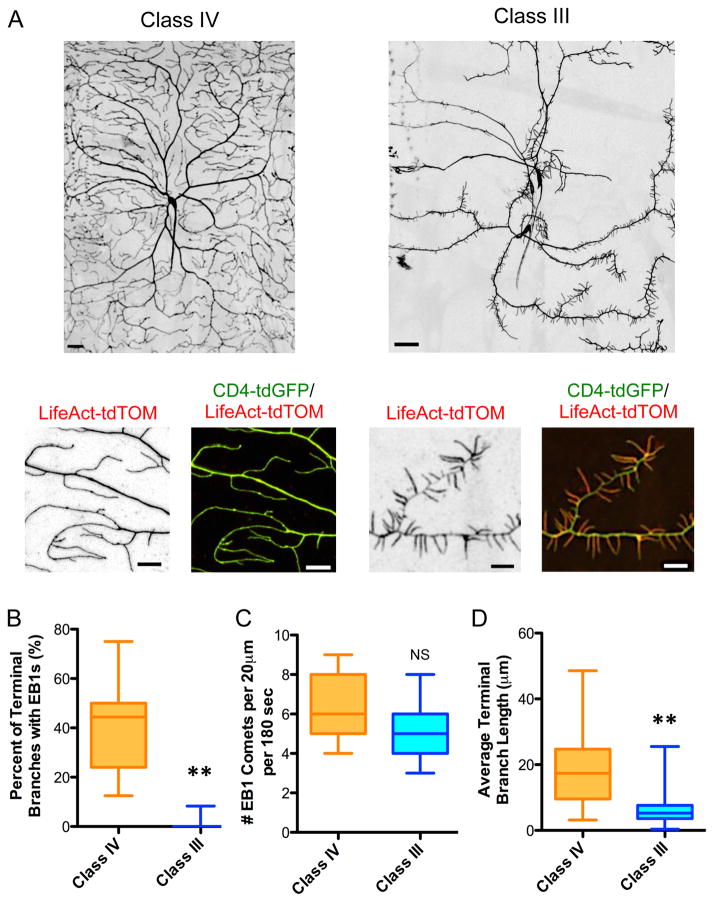
Class IV and class III da neurons develop dramatically different arborization patterns: while class IV da neurons contain many subsets of branches that cover the entire larval body wall, class III da neurons extend primary dendrites which sprout short, spikey terminal branches with limited coverage (Fig. 1A). We found that while the filamentous actin (F-actin) cytoskeleton filled the entire dendritic arbor of class IV da neurons, F-actin was enriched in the terminal dendrite branches of class III da neurons (Fig. 1A), consistent with previous reports (Nagel et al., 2012; Tsubouchi et al., 2012). Conversely, neither stable nor dynamic MTs are present in the terminal branches of class III da neurons (Ye et al., 2011), unlike those of class IV da neurons, though the number of dynamic MTs was similar in the primary branches of both neurons (Fig. 1B–C). For this study, we define MT dynamics as MT assembly that we visualize using EB1-GFP, which marks the growing plus end of the MT. While class IV terminal branches contain both actin and dynamic MTs, class III terminal branches contain predominantly actin, and appear to exclude dynamic MTs. This may account for the ~3-fold difference in average terminal branch length between class III and class IV da neurons (6.1 μm vs. 18.5 μm, Fig. 1D). These results indicate that differential regulation of the MT and actin cytoskeletons may form the basis of the distinct dendrite morphologies of class III and class IV da neurons.
Figure 1. Drosophila melanogaster class IV and class III dendritic arborization neurons develop distinct terminal branch morphologies with different cytoskeletal compositions.
(A) Dendrite morphologies of class IV and class III wild type neurons, visualized by UAS-CD4-tdGFP expressed by ppk-Gal4 and 1912-Gal4 for class IV and class III, respectively (scale bars are 30 μm). Enlarged regions illustrate the differences in terminal branch length and actin composition between class IV and class III terminal branches (scale bars are 10 μm). UAS-CD4-tdGFP and UAS-LifeAct-tdTOM were expressed in class IV and class III da neurons by ppk-Gal4 and 1912-Gal4, respectively. (B) Quantification of the percent of terminal branches that contain dynamic MTs, as evidenced by EB1 entry into a terminal branch, for class III da neurons vs. class IV da neurons (P < 0.0001; n = 7 for class IV and 23 neurons for class III da neurons). UAS-EB1-GFP was expressed in class IV and class III da neurons by ppk-Gal4 and Gal4-1003.3, respectively. (C) Quantification of the number of EB1 comets that grow along the primary branches of class IV and class III da neurons (P = 0.10; n = 11 neurons for class IV and 6 neurons for class III da neurons). (D) Quantification of terminal branch length in class III and IV da neurons (P < 0.0001; n = 3 neurons per genotype). All graphs are box plots with min to max whiskers, which include all datapoints.
Minibrain kinase drives class III da neuron terminal dendrite morphology
Based on the above observations, we performed a screen for cytoplasmic kinases that could regulate the cytoskeleton to produce the class III short, actin-rich terminal branches as opposed to the class IV long, MT-filled terminal branches, and identified MNB/DYRK1a kinase, which is localized throughout the cytoplasm of class III, but not class IV da neurons (Fig. S1A–C). We analyzed two different MNB mutants, mnb3 and mnb1, as well as mnb-RNAi, which abolishes mnb expression (Fig. S2A). Mnb3 and mnb1 are mutant alleles that have been previously hypothesized to affect MNB protein levels and/or function (Tejedor et al., 1995). In order to understand the effects of these mutants on protein levels, we generated a MNB antibody to detect total levels of MNB protein in whole larval lysate (Fig. S2B), and found that MNB levels were decreased ~10% and ~50% in the mnb1 and mnb3 mutants, respectively (Fig. S2C). These results are consistent with the previous literature on mnb3, but the slight decrease in MNB levels in the mnb1 mutants is surprisingly different from the original report of a ~60% decrease (Tejedor et al., 1995), though similar to a recent study showing a ~25% reduction by immunohistochemistry (Chen et al., 2014). In order to understand the true nature of the mnb1 mutant, we expressed and purified recombinant full-length MNB kinase protein containing the alanine to threonine (A191T) mutation found in the mnb1 fly (Fig. S2D). Examination of the tyrosine phosphorylation state of the purified protein suggested that, in contrast to wild type MNB, the A191T mutation likely prevents autophosphorylation of tyrosines in the mutant protein (Fig. S2E). Because the activation mechanism of DYRK family kinases requires autophosphorylation on a tyrosine within the activation loop, it is likely that the A191T mutation prevents kinase activation (Lochhead et al., 2005). Another MNB mutation located in the same region of the kinase domain (K193M) was previously shown to produce a kinase inactive protein (Lochhead et al., 2005).
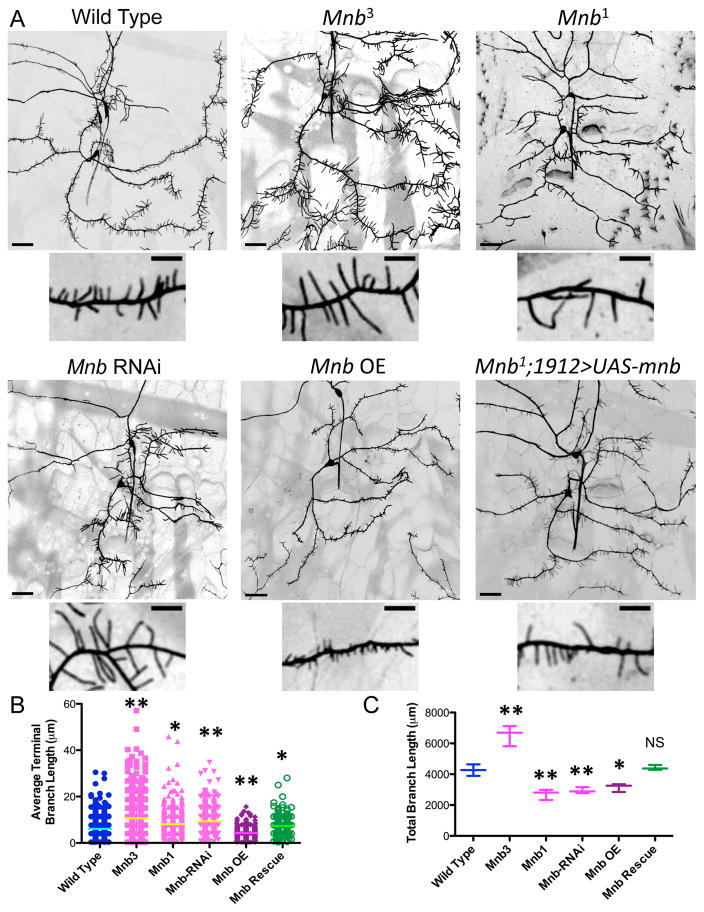
The hypomorph, Mnb3 produced longer terminal branches, without compromising the outgrowth of the dendritic arbor (Fig. 2A–B and S2F), leading to an overall increase in total branch length (Fig. 2C). The kinase dead mutant, Mnb1, and mnb RNAi also resulted in significantly longer terminal branches (Fig. 2A–B), but reduced the overall outgrowth of the primary dendrites and the number of terminal branches (Fig. S2F–G), leading to a decrease in total branch length (Fig. 2C). We were able to rescue most aspects of the mnb1 phenotype by driving expression of UAS-mnb with 1912-Gal4 (Fig. 2A–C and S2F–G). Overexpression of MNB resulted in shorter terminal branches and a significant reduction in total branch length (Fig. 2A–C). Expressing one extra copy of MNB using a duplication line also produced shorter terminal branches, but did not affect the overall outgrowth of the neuron (Fig. S3A). These results are consistent with previous observations of decreased dendritic spine length in trisomic DS mouse models (Tejedor and Hammerle, 2011). Mnb RNAi did not perturb the overall morphology of class I or class IV neurons, but overexpression of MNB reduced the complexity of the dendritic arbors of both classes (Fig. S3B). Together, these results reveal that MNB contributes to overall neuronal outgrowth and maintenance of terminal branch length, as well as highlight the impact of MNB gene dosage on dendrite development.
Figure 2. MNB/DYRK1a kinase mutants perturb the dendritic architecture of class III da neurons.
(A) Dendrite morphologies of class III wild type neurons compared with those of class III mnb3 and mnb1 mutant da neurons, 1912-Gal4>UAS-mnb-RNAi neurons, 1912-Gal4>UAS-mnb overexpression (OE) neurons, and mnb1 mutant neurons rescued with 1912-Gal4>UAS-MNB. Neurons were visualized using UAS-CD4-tdGFP expressed by 1912-Gal4 (scale bars are 30 μm). Enlarged pictures show the difference in terminal branch length between each genotype (scale bars are 10 μm). (B) Quantification of the average terminal branch length for each genotype (one star indicates P < 0.05 and two stars indicate P < 0.005; n = 3 neurons per genotype). Graph is a scatterplot of all datapoints with the line indicating the mean. (C) Quantification of the total neuronal branch length for each genotype (one star indicates P < 0.05 and two stars indicate P < 0.005; for mnb rescue, P = 0.56; n = 3 neurons per genotype). Graph is a box plot with min to max whiskers, which include all datapoints.
Minibrain regulates microtubule dynamics within the class III da neurons
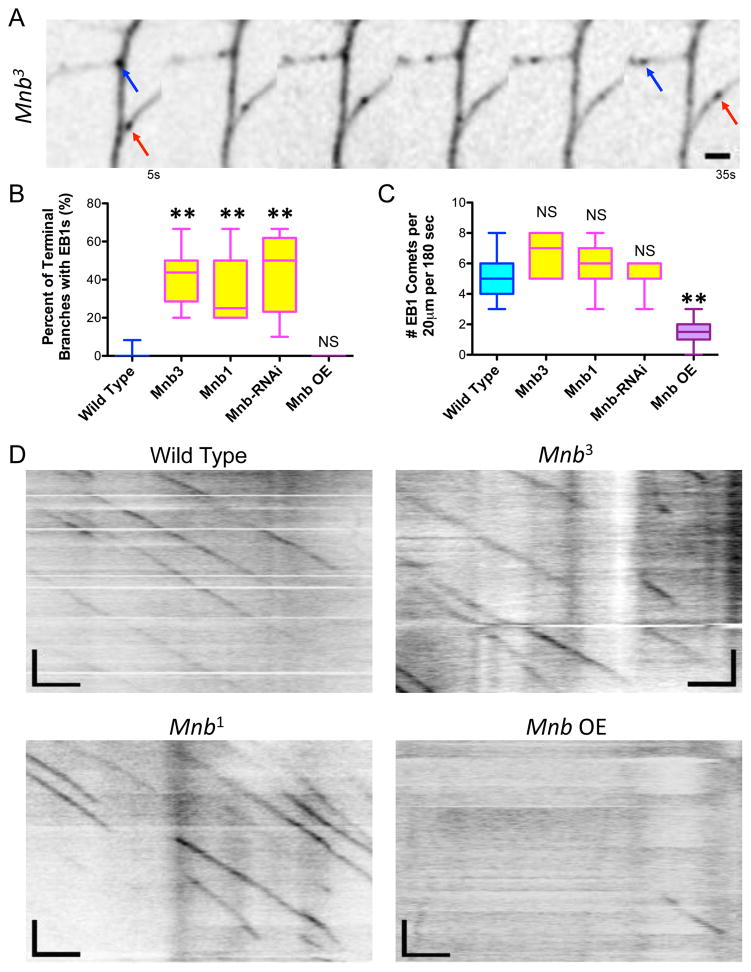
In order to understand how MNB contributes to the development of the dendritic arbor, we analyzed its effects on the MT cytoskeleton. Based on our previous observations that MT growth into a short terminal branch correlates with branch extension and stability (Ori-McKenney et al., 2012), we wanted to investigate if dynamic MTs contribute to the extension and stabilization of the longer terminal branches in the MNB mutant class III da neurons. We analyzed MT dynamics in the mnb mutant and RNAi backgrounds and found that a much higher percentage of terminal branches contained MTs, as evidenced by EB1-GFP comet growth into the branches of mnb mutant neurons, compared with wild type neurons (Fig. 3A–B: 42.5 ± 15%, 33.6 ± 18.2%, and 44.0 ± 12.8% for mnb3, mnb1 and mnb RNAi, respectively, compared with 0.4 ± 1.7 % for wild type; means ± s.d.). Actin was still localized within the terminal branches of the mnb RNAi neurons, though not as prominently in the distal branch tips, indicating that the presence of dynamic MTs in these branches could affect the actin cytoskeleton (Fig. S4A). Interestingly, the number of dynamic MTs growing within the primary branches of class III da neurons was unaffected in the mnb mutant and RNAi neurons (Fig. 3C–D). However, upon mnb overexpression, the number of dynamic MTs within the primary branches was dramatically reduced (Fig. 3C–D: 1.6 ± 0.8 EB1 comets for mnb overexpression compared with 5.1 ± 1.4 comets for wild type neurons; means ± s.d.), indicating that excessive levels of MNB leads to a suppression of MT dynamics. MT polarity was similar, but MT growth rate was significantly slower in mnb OE neurons compared with wild type and mnb1 neurons (Fig. S4B–C). These results reveal a role for MNB in controlling MT dynamics, and thus neuronal morphology, in class III da neurons, because knockdown of MNB results in MTs entering terminal branches from which they are normally excluded, leading to longer terminal branches, and overexpression of MNB results in fewer dynamic MTs overall, leading to stunted arborization.
Figure 3. MNB regulates microtubule dynamics both within the terminal and primary branches of class III da neurons in vivo.
(A) Movie montage depicts the movement of EB1-GFP comets within the terminal branches of a mnb1 mutant neuron (scale bars are 2 μm). Blue and red arrows indicate the starting and ending positions of two comets growing into a particular terminal branch. (B) Quantification of the percent of terminal branches that contain dynamic MTs, as evidenced by EB1 entry into a terminal branch, for each genotype (two stars indicate P < 0.0001; for mnb overexpression, P = 0.52; n = 23, 7, 7, 7, and 10 neurons for wild type, mnb3, mnb1, mnb RNAi, and mnb OE, respectively). (C) Quantification of the number of EB1 comets in the primary branches of class III da neurons for each genotype (two stars indicate P < 0.0001; for mnb3, P = 0.66; for mnb1, P = 0.18; for mnb RNAi, P = 0.86; n = 23, 6, 12, 7, and 22 neurons for wild type, mnb3, mnb1, mnb RNAi, and mnb OE, respectively). All graphs are box plots with min to max whiskers, which include all datapoints. (D) Kymographs showing the number of EB1-GFP comets growing along the primary branches of wild type, mnb3, mnb1, and mnb OE neurons (scale bars are 2.5 μm (x-axis) and 30 s (y-axis)). UAS-EB1-GFP was expressed in class III da neurons by Gal4-1003.3.
Minibrain binds directly to microtubules through a conserved basic patch in its N-terminus
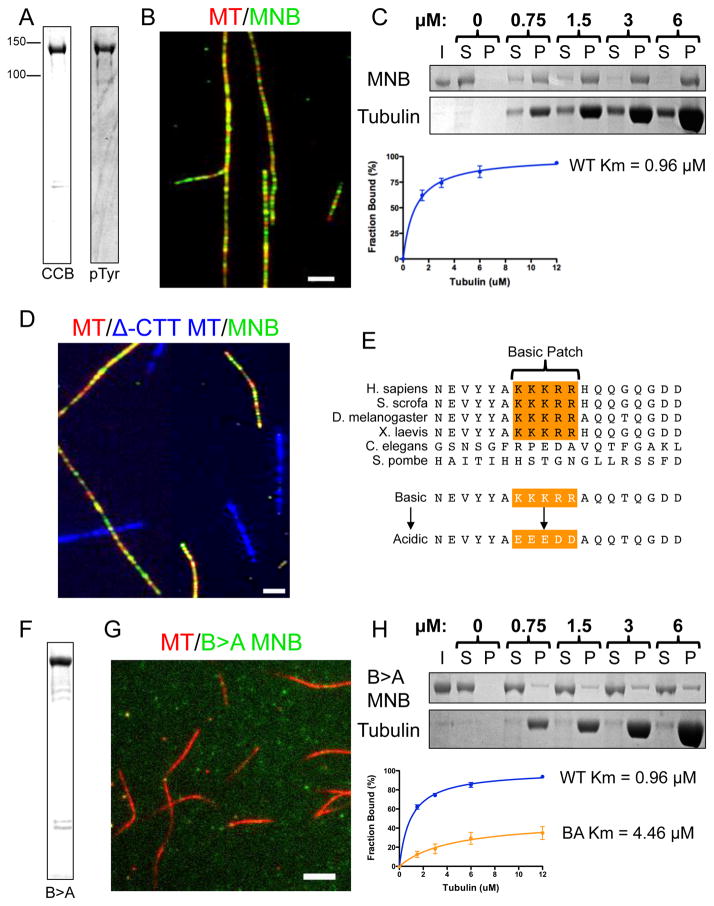
We next set out to determine if MNB could directly regulate the MT cytoskeleton, or if its effects on MT dynamics in vivo are exerted indirectly. In order to answer this question, we turned to an in vitro reconstitution system. We purified recombinant full-length, active GFP-MNB kinase (Fig. 4A). There are three major isoforms of MNB in Drosophila that are enriched at various developmental stages (Chen et al., 2014). We chose to express MNB isoform E, because it is the predominant isoform in the larval body wall, which is where the da neurons are located (Chen et al., 2014). Using total internal reflection (TIRF) microscopy, we imaged purified GFP-MNB and fluorescently labeled taxol-stabilized porcine MTs, and found that GFP-MNB bound along the lattice of these MTs (Fig. 4B). Thus, purified GFP-MNB interacts directly with MTs in vitro. MT co-sedimentation assays with increasing concentrations of taxol-stabilized MTs revealed that GFP-MNB bound MTs with an apparent Km of 0.96 μM (Fig. 4C).
Figure 4. Drosophila MNB binds microtubules through a conserved basic cluster in vitro.
(A) Coomassie Brilliant Blue (CBB) stained SDS-Polyacrylamide gel electrophoresis (SDS-PAGE) of purified recombinant GFP-tagged MNB kinase (left), and an immunoblot of MNB protein detected by an antibody against phosphotyrosine (right). (B) TIRF-M image showing GFP-MNB (50nM, green) binds along the lattice of taxol-stabilized MTs (red) in vitro (scale bar is 2.5 μm). (C) CBB stained SDS-PAGE shows the binding behavior of GFP-MNB in the presence of increasing concentrations of taxol-stabilized MTs. Results from five separate experiments were plotted and fit to a Michaelis-Menten equation (right) producing a Km of 0.96 ± 0.10 μM (mean ± s.d.). (D) GFP-MNB (green) binds normal taxol-stabilized MTs (red), but does not bind subtilisin-treated MTs that lack the CTTs (blue). Scale bar is 2.5 μm. (E) Sequence alignment reveals that Drosophila MNB-E contains a basic cluster (orange, residues 118–122) that is highly conserved. We mutated each of these basic residues to acidic residues to produce a Basic-to-Acidic mutant (B>A) MNB. (F) CBB stained SDS-PAGE of purified recombinant GFP-B>A-MNB kinase. (G) TIRF-M imaging of GFP-B>A-MNB (50nM, green) reveals only weak binding to taxol-stabilized MTs (red) in vitro (scale bar is 3 μm). (H) CBB stained SDS-PAGE gels shows the binding behavior of recombinant GFP-B>A-MNB in the presence of increasing concentrations of taxol-stabilized MTs. Results of three separate co-sedimentation experiments are plotted and fit to the Michaelis-Menten equation (right) producing a Km of 4.46 ± 2.62 μM (mean ± s.d.), which is significantly different from that of wild type MNB (P = 0.019).
To ascertain how MNB interacts with MTs, we treated taxol-stabilized MTs with the protease, subtilisin, to remove the CTT domains of both alpha and beta tubulin (McKenney et al., 2014). Strikingly, this treatment completely abolished GFP-MNB binding to MTs (Fig. 4D). Thus, purified GFP-MNB binds directly to MTs through interactions with the acidic CTTs of tubulin. We located a basic patch of residues (a.a. 118–122: KKKRR) that was highly conserved in all Drosophila isoforms and in metazoans (Fig. 4E and Fig. S5A). Interestingly, this basic patch lies within the previously described, bi-partite nuclear localization signal for MNB (a.a. 105–139, see discussion)(Becker et al., 1998). In order to test if this basic patch plays a role in the MNB-MT interaction, we mutated five basic residues to acidic residues (KKKRR → EEEDD) (Fig. 4E), and purified the full-length mutant MNB protein which we termed B>A for ‘basic to acidic’ (Fig. 4F). We found that, at comparable protein concentrations, the B>A mutations strongly diminished MT binding by MNB in both the TIRF assay (Fig. 4G) and the MT co-sedimentation assay (Fig. 4H), increasing the Km nearly fivefold to 4.46 μM. We found that the Drosophila MNB1 A191T mutation, which produces a kinase dead version of MNB (Fig. S2D–E), did not affect MT binding (Fig. S5B). Thus, MNB kinase directly interacts with the CTTs of tubulin through a highly conserved basic motif, independent of its kinase activity.
Minibrain inhibits microtubule polymerization by phosphorylating β-tubulin at serine 172
Because our in vivo results suggested that MNB affects dynamic MTs, we examined the possibility that MNB could directly affect MT polymerization in vitro. The polymerization of purified tubulin in solution can be monitored as a change in turbidity (Borisy et al., 1972). Studies of mammalian tubulin polymerization typically perform such assays at physiological temperature (37ºC)(Szyk et al., 2011). However, initial trials suggested that our recombinant Drosophila GFP-MNB protein is unstable at this temperature, and we thus performed our assays at 25ºC. We observed that our purified porcine tubulin polymerized more slowly at 25ºC than at 37ºC, as expected (Fig. S6A).
While 25 μM tubulin alone incubated with 1 mM GTP and 1 mM ATP polymerized into MTs over time, the addition of 500 nM GFP-MNB dramatically inhibited tubulin polymerization over the course of the assay (Fig. 5A). Strikingly, the addition of GFP-MNB without ATP did not affect MT polymerization, strongly suggesting the kinase activity of MNB is required to suppress MT growth. GFP-MNB also significantly reduced tubulin polymerization in the presence of the slowly hydrolyzable GTP analogue, GMP-CPP, a potent MT nucleator (Fig S6B). Importantly, both the kinase dead mutant MNB1, and the MT-binding deficient mutant B>A MNB proteins displayed dramatically reduced abilities to inhibit tubulin polymerization (Fig. 5B). These results reveal that MNB requires both its ability to interact with tubulin and its kinase activity to directly inhibit tubulin polymerization.
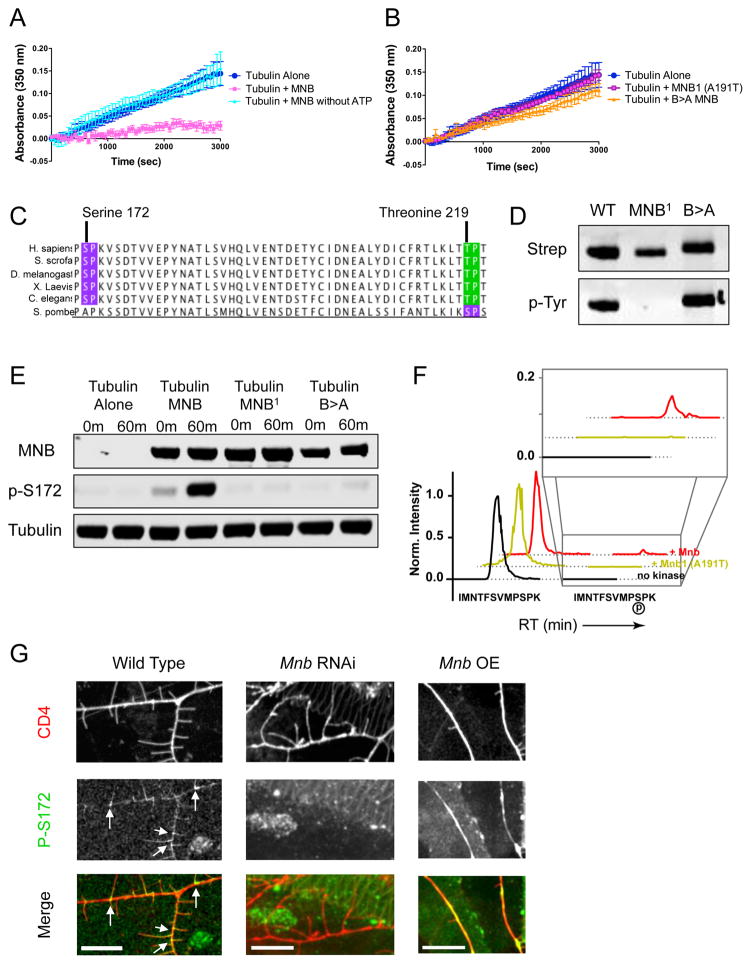
Figure 5. Mnb inhibits microtubule polymerization in vitro by phosphorylating β-tubulin at serine-172.
(A) Tubulin polymerization determined via turbidity (at 350nm) in the absence of MNB (dark blue), in the presence of MNB (pink), and in the presence of MNB without ATP (light blue). (B) Tubulin polymerization determined via turbidity in the presence of either the MNB1 (A191T) mutant MNB (pink/purple) or the B>A mutant MNB (orange) (P = 0.0007 and 0.004 for MNB1 and B>A MNB vs. WT MNB). All turbidity assays were conducted at 25ºC in BRB80 buffer with 25 μM tubulin, 5 μM GFP-MNB, 1 mM GTP and 1 mM ATP, unless otherwise noted. Means ± s.d. are plotted from at least n = 3 experiments per condition. (C) Sequence analysis of β-tubulin shows two potential MNB phosphorylation sites: serine 172 (purple) and threonine 219 (green), both of which are highly conserved. (D) Immunoblots of total MNB protein and autophosphorylated MNB protein detected by antibodies against the strep-tag and phosphotyrosine, respectively, show that wild type and B>A MNB recombinant proteins are active kinases, while MNB1 is not. (E) Immunoblots of in vitro kinase assays with samples taken at 0 min and 60 min after incubation of 500 nM tubulin and 1 mM ATP alone, or with 500 nM wild type, MNB1 or B>A MNB at 25ºC. MNB proteins were detected by an anti-strep antibody, β-tubulin phosphorylated at serine-172 was detected by a phospho-specific antibody, and total tubulin was detected by an anti-alpha-tubulin antibody. (F) Extracted ion chromatogram from MS1 filtering of LC-MS/MS data shows the unmodified peptide, IMNTFSVMPSPK, containing Ser172 (earlier retention time peak) and the phosphorylated Ser172 peptide (later retention time and inset). The MS1 intensity is normalized to the peak of the unmodified peptide identified in each sample. The phosphorylated Ser172 peptide appears in the presence of wild type MNB protein, but does not appear in the presence of the kinase dead mutant, MNB1, or in the absence of the kinase. See also Dataset S1. (G) Immunohistochemical staining of wild type, mnb RNAi and mnb OE larval fillets with anti-phospho-S172 and anti-GFP (against the membrane marker, CD4). Phospho-S172 is normally present at the base of terminal branches in wild type class III neurons (white arrows), but expression decreases in mnb RNAi neurons and increases in mnb overexpressing neurons. Scale bars are 10 μm.
Our results with purified components strongly suggested that tubulin might be a direct substrate for the kinase activity of MNB. Sequence analysis of α- and β-tubulin genes revealed two potential MNB consensus target sites within β-tubulin: serine 172 (S172) and threonine 219, which are highly conserved across metazoan β-tubulin isoforms (Fig. 5C and S6C). We performed a kinase assay with porcine tubulin and wild type GFP-MNB, the kinase dead MNB1 mutant, or the kinase active, but MT-binding deficient B>A MNB mutant (Fig. 5D). Using a phospho-specific antibody, we found GFP-MNB directly phosphorylated purified β-tubulin at S172, but did not phosphorylate threonines on either tubulin, suggesting that S172 in β-tubulin is the major target site for MNB (Fig. 5E and S6D). Neither the MNB1 nor the B>A mutant was able to phosphorylate β-tubulin at S172 in our assay (Fig. 5E), demonstrating that tubulin phosphorylation by MNB requires both kinase activity and tubulin binding. Analysis of these samples by mass spectrometry revealed that S172 is the only residue within the tubulin dimer that was phosphorylated by MNB (Fig. 5F and Dataset S1). S172 is located near the exchangeable nucleotide-binding site of β-tubulin (Fig. S6E)(Hesse et al., 1985). These findings suggest that the addition of a phosphate group to S172 may interfere with nucleotide binding and/or exchange, which is necessary for tubulin assembly into MTs (Fourest-Lieuvin et al., 2006; Yu et al., 2015).
To investigate if β-tubulin is phosphorylated at S172 in vivo, we performed immunohistochemistry on larvae using the phospho-specific antibody against S172. We found that the phospho-S172 β-tubulin antibody stained the base of terminal branches in wild type class III da neurons, but this reactivity was largely abolished in mnb RNAi neurons (Fig. 5G). Upon mnb overexpression, however, the levels of phospho-S172 β-tubulin increased throughout the primary branches (Fig. 5G). These data indicate that MNB phosphorylates β-tubulin within the class III neurons, consistent with its mechanism of action in vitro.
The mechanisms of microtubule binding and tubulin phosphorylation are conserved in mammalian DYRK1a
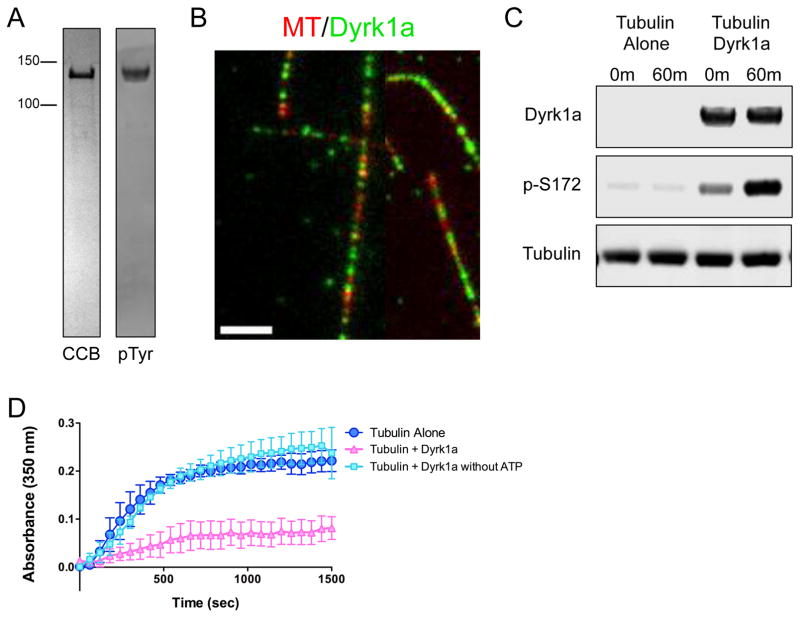
Given the sequence conservation between MNB and DYRKla, we wanted to directly test whether the MT binding and tubulin phosphorylation activities are conserved in mammalian DYRKla. We therefore purified recombinant full-length, active mouse GFP-DYRKla kinase (Fig. 6A). Using TIRF microscopy, we imaged purified GFP-DYRKla and fluorescently labeled taxol-stabilized MTs, and found that GFP-DYRKla bound along the lattice of these MTs (Fig. 6B), similar to GFP-MNB. We also performed a kinase assay and found that GFP-DYRK1a directly phosphorylates purified porcine β-tubulin at S172 (Fig. 6C). Finally, turbidity assays revealed that the addition of GFP-DYRKla dramatically inhibited tubulin polymerization, in an ATP-dependent manner (Fig. 6D). Together, these results indicate that the mechanisms of MT binding and tubulin phosphorylation are conserved between Drosophila MNB and mammalian DYRKla.
Figure 6. Mammalian DYRK1a exhibits a conserved mechanism of action on tubulin.
(A) CBB stained SDS-PAGE of purified recombinant GFP-tagged DYRK1a kinase (left), and an immunoblot of DYRK1a protein detected by an antibody against phosphotyrosine (right) to show that the recombinant GFP-DYRK1a kinase is active and able to autophosphorylate its own tyrosine residues. (B) TIRF-M reveals GFP-DYRK1a (green) binds along the lattice of taxol-stabilized MTs (red) in vitro (scale bar is 2.5 μm). (C) Immunoblots of in vitro kinase assays with samples taken at 0 min and 60 min after incubation of 500 nM tubulin and 1 mM ATP alone, or with 500 nM GFP-DYRK1a at 25ºC. DYRK1a protein was detected by an anti-strep antibody, β-tubulin phosphorylated at serine-172 was detected by a phospho-specific antibody, and total tubulin was detected by an anti-alpha-tubulin antibody. (D) Tubulin polymerization determined via turbidity (at 350nm) in the absence of DYRK1a (dark blue), in the presence of DYRK1a (pink), and in the presence of DYRK1a without ATP (light blue). All turbidity assays were conducted at 37ºC in BRB80 buffer with 25 μM tubulin, 5 μM GFP-DYRK1a, 1 mM GTP and 1 mM ATP, unless otherwise noted. Means ± s.d. are plotted from at least n = 3 experiments per condition.
Mnb mutants impair the mechanosensory response of class III da sensory neurons by altering the dendritic architecture and perturbing the localization of NompC
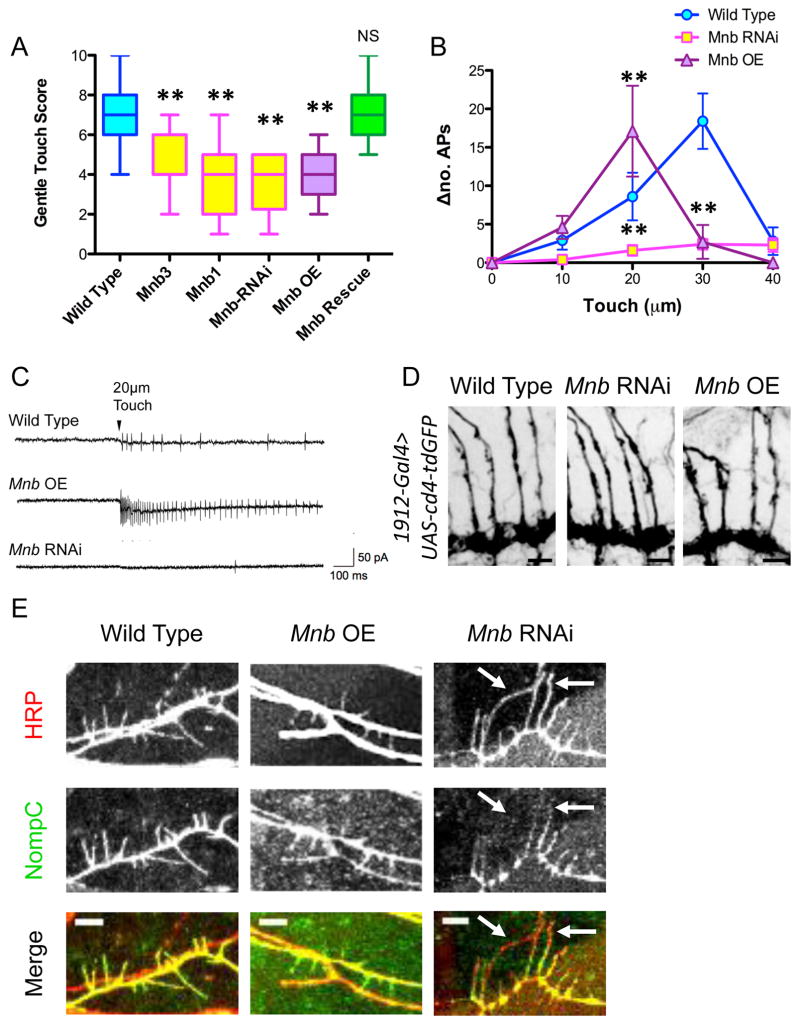
Class III da neurons are mechanosensitive neurons that respond to gentle touch (Bagley et al., 2014; Tsubouchi et al., 2012; Yan et al., 2013). Using a previously described gentle touch assay (Kernan et al., 1994), we observed significant reductions in touch response scores in mnb1, mnb3, mnb RNAi, and mnb overexpression larvae (Fig. 7A). In addition, rescuing the dendritic arbor phenotype of mnb1 by driving UAS-mnb in class III da neurons using the 1912-Gal4 also rescued the gentle touch response (Fig. 7A). To determine whether mnb RNAi or mnb overexpression affects mechanosensation of class III da neurons as opposed to subsequent signaling in the CNS, we performed extracellular recordings from class III da neurons while delivering a touch stimulus of varying intensity. Wild type class III da neurons responded with a progressively higher frequency of action potentials (APs) as the touch stimulus intensity increased up to 30 μm (Fig. 7B). This response was dramatically reduced in class III da neurons expressing mnb RNAi at all stimulus intensities; however, mnb overexpression neurons exhibited a much higher frequency of APs at 20 μm, then substantially fewer APs at higher intensities (Fig. 7B–C). These results are consistent with the defects shown in our gentle touch assay; however, while mnb RNAi nearly eliminates the mechanosensitive response of the class III da neurons, mnb overexpression causes an initial increase in sensitivity of these neurons, which then fail to fire at higher intensities, possibly resulting from excessive depolarization or channel desensitization/inactivation.
Figure 7. Mnb mutant class III da neurons exhibit a reduced mechanosensitive response due to altered morphology and abnormal localization of NompC.
(A) Quantification of the larval behavioral response to gentle touch reveals defects in the mnb mutant and RNAi animals, as well as in the mnb overexpressing animals (two stars indicate P < 0.0001; for mnb1 rescued with 1912-Gal4>UAS-mnb, P = 1.00; n = 24, 19, 19, 20, 19, and 20 larvae for wild type, mnb3, mnb1, mnb RNAi, and mnb OE, and mnb rescue, respectively). Graph is a box plot with min to max whiskers, which include all datapoints. (B) Extracellular electrophysiological recordings from class III da neurons expressing mnb-RNAi and mnb OE indicate an alteration in the number of action potentials (APs) fired per second for a mechanical stimulus of a particular intensity compared with baseline (Δno. APs) (two stars indicate P < 0.005; n = 8, 9, and 7 larvae for wild type, mnb RNAi, and mnb OE, respectively). (C) Representative recording traces from wild type, mnb OE, and mnb-RNAi neurons for a stimulus of 20-μm intensity. (D) Immunohistochemical staining of larval fillets expressing UAS-cd4-tdGFP specifically in class III da neurons (driven by 1912-Gal4) with antibodies against GFP reveals that the axon terminals of wild type, mnb RNAi, and mnb OE class III da neurons appear similar. Scale bars are 30 μm. (E) Immunohistochemical staining of wild type, mnb OE and mnb RNAi larval fillets with anti-nompC and anti-HRP reveals altered patterns of NompC localization. NompC fills the terminal branches of wild type and mnb OE neurons. In mnb RNAi neurons, NompC is apparent in the proximal regions of the terminal branches, but is not localized throughout the entire terminal branch (white arrows). Scale bars are 5 μm.
To understand the basis of this defective response to gentle touch, we examined the axon terminals of mnb RNAi and mnb overexpression class III da neurons, but found that the axon terminals appeared similar between all genotypes (Fig. 7D). We then analyzed the localization pattern of the mechanosensitive channel, NompC (Tsubouchi et al., 2012; Yan et al., 2013), and found that NompC localized to the terminal branches of both wild type and mnb overexpression neurons, but its pattern was altered in mnb RNAi neurons (Fig. 7E and S7). NompC was either entirely absent from the longer terminal branches, or only appeared in the proximal regions of the branches of mnb RNAi neurons (Fig. 7E and S7), which is similar to the pattern of F-actin in the mnb RNAi neurons (Fig. S4A). Therefore, the impaired gentle touch response in mnb RNAi and overexpression neurons is likely caused by altered intracellular cytoskeletal dynamics leading to improper trafficking of NompC, perturbed dendrite morphology, or both.
DISCUSSION
Our results uncover a direct role for MNB in the regulation of MT growth both in vivo and in vitro, revealing the mechanism by which the MNB pathway controls the cytoskeletal composition and branch structure of the dendritic arbor for proper neuronal function. Additionally, we have uncovered a previously unidentified mode of post-translational MT regulation during neuronal development.
Surprisingly, MNB is localized throughout the cytoplasm of the class III da neurons, not just within terminal branches, indicating that its kinase activity may be spatially regulated to ensure MT growth is not inhibited throughout the entire neuron. We identified a conserved N-terminal basic patch in MNB/DYRK1a that is responsible for binding to the CTT of MTs. This patch lies within the putative nuclear localization signal (NLS) for MNB/DYRK1a (Becker et al., 1998), which could serve as an important regulator of MNB/DYRK1a expression and activity. Several other key organizers of the MT cytoskeleton, such as TPX2, XCTK2, NuMA, and GM130, also localize to the interphase nucleus (Walczak et al., 1997; Wei et al., 2015; Wiese et al., 2001; Wittmann et al., 1998; Wittmann et al., 2000). Strikingly, the binding of importin α or β to the NLS of these proteins prevents either their association with MTs, or their action upon MTs (Ems-McClung et al., 2004; Schatz et al., 2003; Wei et al., 2015; Wiese et al., 2001). We speculate a similar mechanism may regulate MNB/DYRK1a localization and MT association both before and after neuronal differentiation.
MNB directly inhibits MT polymerization by phosphorylating β-tubulin at S172, a mechanism that is conserved in mammalian DYRK1a. Phosphorylation of β-tubulin at S172 by CDK1 has been shown to suppress MT dynamics during the cell cycle (Fourest-Lieuvin et al., 2006). We expand this work and provide evidence that phospho-regulation of MT dynamics is important for MT-driven morphogenesis in postmitotic cells. Based on previously characterized roles for MNB/DYRK1a during the cell cycle, there may be a broader utility for this action. MNB/DYRK1a is expressed throughout the cell cycle in Drosophila and mammalian neural progenitors, and numerous studies have reported that both loss and gain of MNB/DYRK1a function impair proliferation (Tejedor and Hammerle, 2011). Furthermore, Pom1p, the MNB/DYRK1a homologue in S. pombe, regulates mitotic entry based on cell length (Martin and Berthelot-Grosjean, 2009). Therefore, the purpose of β-tubulin phosphorylation to inhibit MT growth by MNB/DYRK1a may involve not only dendrite morphogenesis, but also the control of MT dynamics during the cell cycle.
Finally, our results suggest that the cytoskeletal composition of the dendrite branches critically drives both cellular morphology and functional output. Altering MNB levels perturbs the MT dynamics of the dendrite branches, which results in either hypersensitivity, due to abnormal dendrite morphology, or hypo-sensitivity, due to the improper trafficking of NompC, of these neurons to mechanosensitive stimuli. In the latter case, in mnb RNAi neurons, the presence of MTs within the terminal branches may disrupt the distribution of the actin cytoskeleton, leading to an altered localization of NOMPC and a decrease in neuronal firing. These results indicate that the organization of the cytoskeleton can significantly affect intracellular dynamics and contribute to proper functioning of a neuron on multiple levels. MNB/DYRK1a kinase may have additional signaling pathways that regulate dendrite morphogenesis and function, and it will be interesting to investigate the roles of its other substrates in future studies.
In summary, our study provides the first evidence that MNB/DYRK1a kinase can directly control MT growth through phosphorylation of β-tubulin, and that this action has a profound influence not only on how a dendritic arbor forms, but also on how a neuron functions. Not only do our results reveal a previously unidentified mode of post-translational MT regulation in neurons, but they also uncover a conserved molecular pathway for a DS and ASD critical kinase, providing insight into the nature of the neuronal pathologies of these disorders.
EXPERIMENTAL PROCEDURES
Fly Stocks
We used Gal4 driver lines ppk-Gal4, 1912-Gal4, and Gal41003.3 (Grueber et al., 2003a; Yan et al., 2013) to drive the expression of UAS-EB1-GFP (Zheng et al., 2008) to visualize the growing plus ends of MTs in class IV and III da neurons, of the membrane marker, UAS-cd4-tdGFP (Han et al., 2011) to visualize dendrite morphology, and of UAS-LifeAct-tdTOM (Han et al., 2011) to visualize actin localization in wild type and mutant backgrounds. The mnb3, mnb1, and UAS-mnb fly stocks were provided by Ulrike Heberlein and Kiernan Harvey. The UAS-mnb-RNAi lines (Stocks 36657 (II; RRID:BDSC_36657) and 31386 (III; RRID:BDSC_31386)) and the mnb Duplication line (Dp(1;3)DC337, Stock 30441, RRID:BDSC_30441) were obtained from the Bloomington Drosophila Stock Center (Department of Biology, Indiana University, Bloomington, IN).
Genetic Screen
We screened 60 fly lines harboring mutant cytoplasmic kinases for their ability to alter class III terminal branches from short, spikey protrusions to longer, class IV-like terminal branches. We only focused on kinases that were expressed within the peripheral nervous system during larval development, based on expression data found in FlyBase. Fifty-seven of the fly lines were mutant alleles, while 3 were UAS-RNAi. Homozygous and hemizygous mutants were analyzed, but for those mutants whose homozygosity caused lethality before third larval instar, we analyzed the heterozygote and a UAS-RNAi line driven by the class III specific promoter, 1912-Gal4.
Live Imaging and Analysis
Whole, live third instar larvae were mounted in 90% glycerol under coverslips sealed with grease, and imaged using a Leica SP5 laser scanning confocal microscope. Live imaging and analysis of EB1-GFP were performed as previously described (Ori-McKenney et al., 2012; Zheng et al., 2008). Please refer to Supplemental Experimental Procedures for detailed methods.
Immunohistochemistry
Immunohistochemical staining was performed as reported previously (Bagley et al., 2014). The primary antibodies used were chicken anti-GFP (GFP-1020, Aves Labs, RRID:AB_10000240), mouse anti-NOMPC-NT (N-terminal epitope, a gift from J. Howard, Max Planck Institute, Dresden, Germany), goat anti-HRP-Cy5 (123-605-021, Jackson ImmunoResearch, RRID:AB_2338967) and rabbit anti-phospho-serine 172 (ab76286, Abcam, RRID:AB_1523210). For the phospho-S172 antibody staining, it was essential that larval muscle be cleared entirely from the fillet in order to get staining of the neurons, because this antibody prominently stains the muscles. The affinity purified rabbit antibody against MNB was generated against amino acids 106–145, a region that is conserved between all MNB isoforms (Yenzym Antibodies, LLC). To validate the MNB antibody that we produced, we determined its specificity using western blot and immunohistochemistry analysis. We blotted larval lysate and saw specific bands at the known molecular weights for four MNB isoforms: isoforms E (~90kDa), F/G (Both ~60kDa), and H (~100kDa), and one band that is either a nonspecific band or an unidentified MNB isoform (Fig. S2B). We then used the MNB antibody to stain larval fillets, and the antibody recognized class I and class III neurons (Fig. S1). Upon MNB knockdown with RNAi specifically in class III neurons, our MNB antibody still stained class I neurons, but no longer recognized class III neurons, further validating its specificity (Fig. S2A). Finally, these results were reproduced on different larval preps on different days using different aliquots of antibody. Secondary antibodies consisted of appropriate fluorescence-conjugated anti-donkey IgG (Jackson ImmunoResearch). Slides were imaged on a Leica SP5 confocal microscope using an oil immersion 40x objective.
Behavioral Assays and Electrophysiology
Larval gentle touch assays and electrophysiological experiments were performed as previously described (Kernan et al., 1994; Yan et al., 2013). Please refer to Supplemental Experimental Procedures for detailed methods.
Protein Expression and Purification
Tubulin was purified from porcine brain by the method of (Castoldi and Popov, 2003). The Drosophila MNB-E cDNA was codon-optimized and synthesized by GenScript Corporation. The cDNA of mouse DYRK1a was acquired from GE Dharmacon MGC Collection (accession # BC034550). Both MNB-E and DYRK1a were cloned in frame into pFastBacHTA vector containing an N-terminal strepII-superfolder GFP (sfGFP) cassette (McKenney et al., 2014). For baculovirus expression, the Bac-to-Bac protocol (Invitrogen) was followed. SF9 cells were grown in shaker flasks to ~2x106/mL and infected at a ratio of 10mL virus to 250 mL cells. The infection was allowed to proceed for 48 hr for MNB or 60 hr for DYRK1a before cells were harvested and frozen in LN2. Cell pellets were resuspended in lysis buffer (50mM Tris-HCl, pH 8.0, 150mM K-acetate, 2mM Mg-acetate, 1mM EDTA, 10% glycerol, 0.1mM ATP) with protease inhibitor cocktail (Roche). Cells were lysed by addition of 1% Triton X-100 for 10 min on ice. The lysate was clarified by centrifugation (250,000g for 10 min). The lysate was incubated with 5mL Streptactin agarose (GE healthcare) for 30 min, and the beads were washed with lysis buffer containing an additional 100mM KCl extensively to remove unbound proteins. The MNB or DYRK1a protein was eluted with 3mM desthiobiotin in lysis buffer. The protein was concentrated on Amicon concentrators and flash frozen in LN2. Protein concentration was determined by measuring the absorbance of the sfGFP tag at 488nm and calculated using the molar extinction coefficient of sfGFP (83300 M−1 cm−1). The resulting preparations were analyzed by SDS-PAGE and by immunoblot using antibodies against strep tag II (ab76949, Abcam, RRID:AB_1524455) and phospho-tyrosine (05–321, clone 4G10, Millipore, RRID:AB_309678), to ensure the kinase was active. Immunoblots were visualized using an Odyssey LiCor system.
Total Internal Reflection (TIRF) Microscopy
TIRF experiments were performed as previously described (McKenney et al., 2014). A mixture of native tubulin, biotin-tubulin, and fluorescent-tubulin purified from porcine brain (~10:1:1 ratio) was assembled in BRB80 buffer (80mM PIPES, 1mM MgCl2, 1mM EGTA, pH 6.8 with KOH) with 1mM GTP for 15 min at 37ºC, then polymerized MTs were stabilized with 20 μM taxol. MTs were pelleted over a 25% sucrose cushion in BRB80 buffer to remove unpolymerized tubulin. For subtilisin removal of the C-terminal tubulin tails, the assembled MTs were digested with 200 μg/mL subtilisin for 1 hr at 37ºC. The digestion was terminated by addition of 1mM PMSF and the digested MTs were centrifuged over a 25% sucrose cushion in BRB80 to remove the subtilisin protease.
Flow chambers containing immobilized MTs were assembled as described (McKenney et al., 2014). Imaging was performed on a Nikon Eclipse TE200-E microscope equipped with an Andor iXon EM CCD camera, a 100X, 1.49 NA objective, three laser lines (491, 568, 647 nm) and Micro-Manager software (Edelstein et al., 2010). All assays were performed in assay buffer (30mM Hepes, pH 7.4, 50nM K-acetate, 2mM Mg-acetate, 1mM EGTA, 10% glycerol), supplemented with 0.1mg/mL biotin-BSA, 0.5% Pluronic F-168, and 0.2mg/mL κ–casein. A final concentration of 50 nM GFP-MNB wild type and mutant proteins or 50 nM GFP-DYRK1a was used in all assays.
Co-sedimentation Assays
Co-sedimentation assays were performed as previously described (Ori-McKenney et al., 2010). Please refer to Supplemental Experimental Procedures for detailed methods.
Turbidity Assays
Turbidity experiments were performed with 25 μM tubulin and 5 μM GFP-MNB wild type or mutant proteins (Fig. 5 and S6) or GFP-DYRK1a (Fig. 6). Purified proteins were mixed with tubulin in the presence or absence of 1mM ATP and incubated in BRB80 buffer (supplemented with 25% glycerol) on ice for 10 min, then 1mM GTP was added and the entire solution was injected into either a 25ºC or 37ºC pre-heated cuvette. Absorbance at 350 nm was monitored at 1 min intervals for 25–50 min. When turbidity assays were conducted with GMP-CPP, every step was the same except we used 10 μM Tubulin, 2 μM GFP-MNB, and 1mM GMP-CPP instead of 1mM GTP immediately before transferring the sample to the cuvette.
Kinase Assays and Mass Spectrometry
Phosphorylation reactions were performed as previously described (Fourest-Lieuvin et al., 2006) with the following modifications: 500nM porcine brain tubulin was incubated for 60 min at 25ºC with 1mM ATP either in the presence or absence of 500nM GFP-MNB or GFP-DYRK1a proteins in BRB80 buffer (supplemented with 1mM DTT and 1mM PMSF). Please refer to Supplemental Experimental Procedures for detailed methods.
For mass spectrometry, 25 μM of porcine brain tubulin was incubated for 60 min at 25ºC with 1mM ATP either in the presence or absence of 1 μM GFP-MNB (wild type or kinase dead) proteins in 50mM Tris-HCl buffer (150mM K-acetate, 2mM Mg-acetate, 10% glycerol, pH 8.0) in low retention tubes. The reaction was quenched by adding EDTA (pH 8.0) to a final concentration of 20mM, then solid urea was added to a final concentration of 1 M. Next, 0.5 μg of trypsin was added to each sample and the samples were incubated overnight. The trypsinization was quenched with 10% trifluoroacetic acid to a final concentration of 0.5%, and the samples were spun at 15,000 x g for 10 min to pellet any precipitate. The supernatant of each sample was desalted using Pierce C18 Spin Tips and eluted in 50% acetronitrile/0.1% formic acid into another low retention tube, then dried using a SpeedVac.
An estimated 4.3 pmol of peptides were resuspended to a concentration of 100 nM in 0.1% formic acid, 2% acetonitrile. Approximately, 200 fmol of each sample was injected onto a Dionex Ultimate 3000 RSLCnano UHPLC with a 75 μm x 15 cm Acclaim Pepmap C18 column. This column was coupled directly in-line with a Q-Exactive Plus hybrid Orbitrap (Thermo Fisher) mass spectrometer. Reverse-phase separation was performed in buffer A (0.1% formic acid in water) and buffer B (0.08% formic acid in 80% acetonitrile) at a flow rate of 300 nL/min. A non-linear gradient extended from 3% to 40% buffer B over 29 min, followed by a column wash to 99% buffer B over 13 min, and then re-equilibration at 3% buffer B for a total run time of 60 min.
Data-dependent acquisition was performed using X-Calibur version 3.0.63 software (Thermo) across an m/z range of 350–1500 with an isolation window of 1.7 m/z for ions selected for MS/MS with a dynamic exclusion window of 20 s after acquisition. The top twelve most intense ions in each MS survey scan (MS1) were chosen for fragmentation and sequencing. Peptides were fragmented in a higher-energy collisional dissociation (HCD) cell at a normalized collision energy (NCE) of 27.
Raw data from unbiased MS experiments were processed using Protein Prospector (University of California San Francisco) from peaklists generated by ProteoWizard MSConvert (Chambers et al., 2012). All spectra were searched using the full porcine SwissProt database (downloaded March 5, 2015 from www.uniprot.org/downloads). Search parameters included fixed modification of cysteine carbamidomethylation, variable modifications of methionine oxidation and phosphorylation of serine/threonine, up to two missed tryptic cleavages, parent mass tolerance 6 ppm, and fragment mass tolerance 6 ppm in the first biological replicate and 20 ppm in the second replicate. The full list of identified peptides based on Protein Prospector search is shown in Dataset S1.
Statistical Analysis
All statistical tests were performed with two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank Susan Younger for help with fly work, and members of the Jan lab for useful discussion and critical reading of the manuscript. The authors also thank the DRGC for providing mnb cDNA constructs, the Bloomington Drosophila Stock Center for providing fly stocks, and Kieran Harvey and Ulrike Heberlein for providing the mnb1 and UAS-mnb, and mnb3 fly stocks, respectively. This work was supported by NIH grant 2R37NS040929 to Y-NJ, NIH grant GM38499 to RDV, the Jane Coffin Childs Postdoctoral Fellowship and NIH grant 1K99HD080981 to KMOM, and NIH grant 1K99NS089428 to RJM. Y-NJ and LYJ and RDV are Investigators of the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTIONS
KMOM conceived of the project. KMOM and RJM designed and performed the in vitro experiments. TL performed the electrophysiological recordings from larval da neurons. SM performed the larval behavioral assays. HHH and AW performed the mass spectrometry analysis. KMOM, RJM, Y-NJ, RDV, and LYJ wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altafaj X, Dierssen M, Baamonde C, Marti E, Visa J, Guimera J, Oset M, Gonzalez JR, Florez J, Fillat C, et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down’s syndrome. Human molecular genetics. 2001;10:1915–1923. doi: 10.1093/hmg/10.18.1915. [DOI] [PubMed] [Google Scholar]
- Bagley JA, Yan Z, Zhang W, Wildonger J, Jan LY, Jan YN. Double-bromo and extraterminal (BET) domain proteins regulate dendrite morphology and mechanosensory function. Genes & development. 2014;28:1940–1956. doi: 10.1101/gad.239962.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Weber Y, Wetzel K, Eirmbter K, Tejedor FJ, Joost HG. Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. The Journal of biological chemistry. 1998;273:25893–25902. doi: 10.1074/jbc.273.40.25893. [DOI] [PubMed] [Google Scholar]
- Bishop AC, Shah K, Liu Y, Witucki L, Kung C, Shokat KM. Design of allele-specific inhibitors to probe protein kinase signaling. Current biology : CB. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- Borisy GG, Olmsted JB, Klugman RA. In vitro aggregation of cytoplasmic microtubule subunits. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:2890–2894. doi: 10.1073/pnas.69.10.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein expression and purification. 2003;32:83–88. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Bregere C, Paluch J, Lu JF, Dickman DK, Chang KT. Activity-dependent facilitation of Synaptojanin and synaptic vesicle recycling by the Minibrain kinase. Nature communications. 2014;5:4246. doi: 10.1038/ncomms5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Baas PW. Microtubules in neurons as information carriers. Journal of neurochemistry. 2014;129:235–239. doi: 10.1111/jnc.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. In: Ausubel Frederick M, et al., editors. Current protocols in molecular biology. Unit 14. Chapter 14. 2010. p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Ems-McClung SC, Zheng Y, Walczak CE. Importin alpha/beta and Ran-GTP regulate XCTK2 microtubule binding through a bipartite nuclear localization signal. Molecular biology of the cell. 2004;15:46–57. doi: 10.1091/mbc.E03-07-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach KF, Heisenberg M. Structural brain mutant of Drosophila melanogaster with reduced cell number in the medulla cortex and with normal optomotor yaw response. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:1105–1109. doi: 10.1073/pnas.78.2.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourest-Lieuvin A, Peris L, Gache V, Garcia-Saez I, Juillan-Binard C, Lantez V, Job D. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Molecular biology of the cell. 2006;17:1041–1050. doi: 10.1091/mbc.E05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003a;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Current biology : CB. 2003b;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Guimera J, Casas C, Pucharcos C, Solans A, Domenech A, Planas AM, Ashley J, Lovett M, Estivill X, Pritchard MA. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Human molecular genetics. 1996;5:1305–1310. doi: 10.1093/hmg/5.9.1305. [DOI] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Bradke F. Control of neuronal polarity and plasticity--a renaissance for microtubules? Trends in cell biology. 2009;19:669–676. doi: 10.1016/j.tcb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Hoogenraad CC, Akhmanova A. Microtubule plus-end tracking proteins in differentiated mammalian cells. The international journal of biochemistry & cell biology. 2008;40:619–637. doi: 10.1016/j.biocel.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead PA, Sibbet G, Morrice N, Cleghon V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–936. doi: 10.1016/j.cell.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–856. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science. 2014;345:337–341. doi: 10.1126/science.1254198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. The Journal of cell biology. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J, Delandre C, Zhang Y, Forstner F, Moore AW, Tavosanis G. Fascin controls neuronal class-specific dendrite arbor morphology. Development. 2012;139:2999–3009. doi: 10.1242/dev.077800. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Stessman HA, Boyle EA, Witherspoon KT, Martin B, Lee C, Vives L, Baker C, Hiatt JB, Nickerson DA, et al. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nature communications. 2014;5:5595. doi: 10.1038/ncomms6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney KM, Xu J, Gross SP, Vallee RB. A cytoplasmic dynein tail mutation impairs motor processivity. Nature cell biology. 2010;12:1228–1234. doi: 10.1038/ncb2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz CA, Santarella R, Hoenger A, Karsenti E, Mattaj IW, Gruss OJ, Carazo-Salas RE. Importin alpha-regulated nucleation of microtubules by TPX2. The EMBO journal. 2003;22:2060–2070. doi: 10.1093/emboj/cdg195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindoh N, Kudoh J, Maeda H, Yamaki A, Minoshima S, Shimizu Y, Shimizu N. Cloning of a human homolog of the Drosophila minibrain/rat Dyrk gene from “the Down syndrome critical region” of chromosome 21. Biochemical and biophysical research communications. 1996;225:92–99. doi: 10.1006/bbrc.1996.1135. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Stevens ME, Sudanagunta SP, Bronson RT, Makhinson M, Watabe AM, O’Dell TJ, Fung J, Weier HU, Cheng JF, et al. Functional screening of 2 Mb of human chromosome 21q22.2 in transgenic mice implicates minibrain in learning defects associated with Down syndrome. Nature genetics. 1997;16:28–36. doi: 10.1038/ng0597-28. [DOI] [PubMed] [Google Scholar]
- Song WJ, Sternberg LR, Kasten-Sportes C, Keuren ML, Chung SH, Slack AC, Miller DE, Glover TW, Chiang PW, Lou L, et al. Isolation of human and murine homologues of the Drosophila minibrain gene: human homologue maps to 21q22.2 in the Down syndrome “critical region”. Genomics. 1996;38:331–339. doi: 10.1006/geno.1996.0636. [DOI] [PubMed] [Google Scholar]
- Stiess M, Bradke F. Neuronal polarization: the cytoskeleton leads the way. Developmental neurobiology. 2011;71:430–444. doi: 10.1002/dneu.20849. [DOI] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Piszczek G, Roll-Mecak A. Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nature structural & molecular biology. 2011;18:1250–1258. doi: 10.1038/nsmb.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F, Zhu XR, Kaltenbach E, Ackermann A, Baumann A, Canal I, Heisenberg M, Fischbach KF, Pongs O. minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron. 1995;14:287–301. doi: 10.1016/0896-6273(95)90286-4. [DOI] [PubMed] [Google Scholar]
- Tejedor FJ, Hammerle B. MNB/DYRK1A as a multiple regulator of neuronal development. The FEBS journal. 2011;278:223–235. doi: 10.1111/j.1742-4658.2010.07954.x. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Current biology : CB. 2012;22:2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 Uncovers their roles in dendrite arborization and spine development. Neuron. 2012;73:1127–1142. doi: 10.1016/j.neuron.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Verma S, Mitchison TJ. XCTK2: a kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevis egg extracts. The Journal of cell biology. 1997;136:859–870. doi: 10.1083/jcb.136.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JH, Zhang ZC, Wynn RM, Seemann J. GM130 Regulates Golgi-Derived Spindle Assembly by Activating TPX2 and Capturing Microtubules. Cell. 2015;162:287–299. doi: 10.1016/j.cell.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. doi: 10.1126/science.1057661. [DOI] [PubMed] [Google Scholar]
- Willsey AJ, State MW. Autism spectrum disorders: from genes to neurobiology. Current opinion in neurobiology. 2015;30:92–99. doi: 10.1016/j.conb.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Current opinion in neurobiology. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Wittmann T, Boleti H, Antony C, Karsenti E, Vernos I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. The Journal of cell biology. 1998;143:673–685. doi: 10.1083/jcb.143.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Wilm M, Karsenti E, Vernos I. TPX2, A novel xenopus MAP involved in spindle pole organization. The Journal of cell biology. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Kim JH, Yang L, McLachlan I, Younger S, Jan LY, Jan YN. Differential regulation of dendritic and axonal development by the novel Kruppel-like factor Dar1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3309–3319. doi: 10.1523/JNEUROSCI.6307-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Garnham CP, Roll-Mecak A. Writing and Reading the Tubulin Code. The Journal of biological chemistry. 2015;290:17163–17172. doi: 10.1074/jbc.R115.637447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nature cell biology. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.