Abstract
Metabolic stress sensors like AMP-activated protein kinase (AMPK) are known to confer stress adaptation and promote longevity in lower organisms. This study demonstrates that activating the metabolic stress sensor AMP-activated protein kinase (AMPK) in endothelial cells helps maintain normal cellular function by promoting mitochondrial biogenesis and stress adaptation. To better define the mechanisms whereby AMPK promotes endothelial stress resistance, we used 5-aminoimidazole-4-carboxamide riboside (AICAR) to chronically activate AMPK and observed stimulation of mitochondrial biogenesis in wild type mouse endothelium, but not in endothelium from endothelial nitric oxide synthase knockout (eNOS-null) mice. Interestingly, AICAR-enhanced mitochondrial biogenesis was blocked by pretreatment with the mammalian target of rapamycin complex 1 (mTORC1) inhibitor, rapamycin. Further, AICAR stimulated mTORC1 as determined by phosphorylation of its known downstream effectors in wild type, but not eNOS-null, endothelial cells. Together these data indicate that eNOS is needed to couple AMPK activation to mTORC1 and thus promote mitochondrial biogenesis and stress adaptation in the endothelium. These data suggest a novel mechanism for mTORC1 activation that is significant for investigations in vascular dysfunction.
Keywords: endothelial dysfunction, AICAR, mitochondrial biogenesis, eNOS, nitric oxide, mTORC1, AMPK, rapamycin
1: Introduction
Mitochondrial biogenesis is induced during times of physiologic stress and most commonly serves to improve efficiency of cellular energy while also preserving the integrity of the cell. Though a complex process, this response has been repeatedly associated with the activation of the heterotrimeric protein AMP-activated protein kinase (AMPK), which is most often activated by high intracellular AMP/ATP ratios (1). In skeletal muscle, AMPK is required for mitochondrial biogenesis in response to chronic energy deprivation (2). Similarly, mice lacking skeletal muscle AMPK exhibit impaired mitochondrial biogenesis after caloric restriction, and AMPK activation alone is sufficient to improve running endurance, likely through mitochondrial biogenesis (3). However, in tissues that do not rely on mitochondria as the primary source of ATP, the activation and significance of mitochondrial biogenesis is largely unclear.
Endothelial cells, for example, rely on mitochondrial dynamics to maintain vascular homeostasis (4,5) despite being primarily glycolytic (6). The endothelium, in part, also controls events in adjacent tissues via sensing environmental cues and contributing to signals that help tissues respond to stress (e.g. nutrient deprivation, hypoxia). Indeed endothelial cells contribute to such diverse events as the maintenance of hematopoietic stem cells (7) and osteogenesis (8). In the vasculature, endothelial dysfunction is often associated with a decrease in the bioavailabilty of nitric oxide (NO) and an increase in reactive oxygen species (ROS) production, leading to impaired control of vascular tone. Indeed, mice lacking endothelial nitric oxide synthase (eNOS) demonstrate endothelial dysfunction, increased ROS, and become spontaneously hypertensive (9).
Chronic AMPK activation with the AMP analog 5-aminoimidazole-4-carboxamide riboside (AICAR) limited the endothelial dysfunction associated with angiotensin II-induced hypertension in wild type mice (10,11). One study showed that this response was dependent on PGC1α (11), a master regulator of mitochondrial biogenesis, suggesting that induction of mitochondrial biogenesis itself is protective in the endothelium. However the precise mechanisms correlating AMPK to mitochondrial biogenesis and vascular stress adaptation are not well defined. In this paper we investigate endothelial AMPK activation and define a novel pathway that leads to mitochondrial biogenesis in the endothelium.
2: Materials and Methods
2.1: Materials
AICAR was obtained from Toronto Research Chemicals (Toronto, Canada). Anti-actin antibody was from Sigma (St. Louis, MO, USA). Anti-phospho-AMPK, anti-AMPK, anti-phospho-ACC, anti-ACC, anti-phospho-4EBP1, anti-4EBP1, anti-phospho-eNOS, anti-phospho-p70 S6K, and anti-p70 S6K were obtained from Cell Signaling Inc. (Danvers, MA, USA). Anti-cyt c, anti-PECAM-1 and anti-ICAM-2 antibodies were from BD Pharmingen (San Diego, CA, USA). Anti-eNOS antibody was obtained from BD Transduction (San Diego, CA, USA). Anti-SOD-2 antibody was obtained from Upstate Biotechnology (Lake Placid, NY). MitoTracker Green FM was obtained from Invitrogen Corporation (Carlsbad, CA, USA). Other chemicals were obtained from Sigma.
2.2: Cell culture
Primary cultures of HAECs were obtained from Lonza Group Ltd. (Switzerland) and used in experiments during passages 2–5. Cultures were maintained in EBM-2 media with supplements (Lonza). Primary cultures of rat aortic smooth muscle cells (RASMCs) were obtained from VEC Technologies (Rensselaer, NY, USA) and used in experiments during passages 2–5. Cultures were maintained in DMEM (Cellgro, Manassas, VA, USA) that contained 10% fetal bovine serum (FBS), 500 IU/ml penicillin, and 500 IU/ml streptomycin.
2.3: cGMP assay
NO release from HAECs was measured by the bioassay procedure first described by Ishii et al. (35) with the modifications described previously (36).
2.4: Isolation of mouse lung endothelial cells (MLECs)
WT and eNOS-null mice (C5BL/6J background) were purchased from Jackson Laboratory and housed in the Animal Facility at University of Massachusetts Medical School. MLECs were prepared by double immunoselection with anti-PECAM-1 and anti-ICAM-2 antibodies. Briefly, mouse lungs were isolated and minced to paste, and dissolved with 0.1% collagenase. After incubation with tilting for 1 h at 37°C, the lung samples were filtered through 100 µm and 40 µm cell strainers. The pellet was obtained by centrifugation and resuspended in fresh MLEC media (38% DMEM low glucose medium and 38% Ham F12 Nutrient Mixture, supplemented with 20% FBS, 0.1 mg/ml endothelial mitogen, 2 mM L-glutamine, 0.1 mg/ml heparin, and 500 IU/ml penicillin-streptomycin) and plated into gelatin-coated flasks. Endothelial cells were then selected twice with PECAM-1 and ICAM-2 antibody coated dynabeads (Invitrogen). 2.5:
2.5: Western blot
Cells were treated as indicated. Cells were then washed twice with cold PBS and lysed with cold lysis buffer (Cell Signaling) as described (12). The supernatant was resuspended in equal volume of 2× Laemmli sample buffer (Cell Signaling) and resolved by SDS-PAGE followed by immunoblotting with antibodies as indicated. The protein bands were visualized using ECL reagent (GE Healthcare, Piscataway NJ) using FluroChem HD2 system (Alpha Tech, Burlington MA).
2.6: Analysis of AMPK activation
In order to confirm the activation of AMPK after AICAR treatment, cells were processed (as described above in Section 2.5) for Western blotting and probed for AMPK, phospho-AMPK, and phospho-ACC. Samples that showed a sufficient increase in AMPK and ACC phosphorylation after AICAR treatment were used for further experiments.
2.7: Adenoviral constructs
The adenoviral vector expressing bovine eNOS was a gift from Dr. Richard C. Venema (Medical College of Georgia, Augusta, Georgia). Cells were typically infected at a multiplicity of infection (MOI) of 10 to 50 with a control adenovirus at the same MOI.
2.8: Analysis of mitochondrial mass
Mitochondrial mass was estimated by fluorescence of MitoTracker Green FM, a mitochondrial specific dye, independent of the mitochondrial membrane potential (13). MLECs were treated with AICAR (1 mM) in 2% fetal bovine serum EBM-2 media for 24 hours and then washed once with serum free media, followed by 20 min incubation with 200 nM MitoTracker Green FM, respectively, in serum free media at 37°C and 5% CO2. Cells were then washed twice to eliminate unincorporated dye. Cells were then scraped and transferred into black 96-well plate with clear bottom and read in fluorescence plate reader (excitation 490 nm, emission 516 nm for MitoTracker Green FM).
2.9: Endothelial ROS production
Endothelial ROS production was assessed by measuring 2’, 7’-dichlorofluorescin diacetate fluorescence (DCF). Cells were washed and preloaded with 10 µM DCF (in HBSS containing 1 ml/mg glucose) for 30 min. Media was replaced with new HBSS buffer to remove extracellular DCF. MLECs were treated with 200 µM H2O2 for 10 min. Cells were then quickly washed, scraped in ice-cold PBS, and dispersed by repeated pipetting. Aliquots were added to black 96-well plates. DCF fluorescence was assessed with a fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA) with excitation at 485 nm and emission at 535 nm.
2.10: Cell survival assays
MLECs in 12-well plates were treated with vehicle or AICAR for 24 h in growth media. Cells were then kept in Krebs-Hepes buffer for 30 min and treated with 500 µM H2O2 for 2 hours. Cell viability was assessed by the CellTiter96Aqueous One Solution Cell Proliferation Assay (Promega, San Luis Obispo, CA, USA) following the manufacturer’s instructions.
2.11: Vascular ring relaxation
To study NO-dependent vascular relaxation, mice aged 8–12 weeks were randomly treated with vehicle (200 µl PBS) or AICAR (200 mg/kg) via intraperitoneal (i.p.) injections for 24h. Mice were euthanized and thoracic aortae were isolated, cleaned of connective tissue, and cut into 2 mm segments. The vessel segments were mounted on stainless-steel holders in organ baths (Danish Myo Technology, Denmark) containing Physiological Saline Solution (PSS; 119 mM NaCl, 4.69 mM KCl, 1.17 mM MgSO4, 1.18 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, 0.03 mM EDTA and 5.5 mM Glucose) aerated with 95% O2 / 5% CO2 for isometric force recording. Preparations were allowed to equilibrate for 30 min under constant passive force of (~7 mN) and synchronized with KCl and phenylephrine. After treatment with vehicle or 1 µM 20-Hete, segments were instantly contracted with phenylephrine and then relaxation was analyzed in response to acetylcholine (ACh) or sodium nitroprusside (SNP) using PowerLab software (AD Instruments).
2.12: Statistics
All immunoblots are representative of 4–6 independent experiments. Numerical data are presented as mean ± SE. Overall differences between groups were analyzed using one-way ANOVA or two-way ANOVA and tested with Tukey-Kramer Multiple-Comparison Test for determining differences between the means when more than two groups were compared. An independent t-test was used when only two groups were compared. In all tests, significance was accepted at p < 0.05.
3: Results
3.1: AICAR promotes endothelial cell stress adaptation
AMPK-mediated stress adaptation has been associated with improved cell viability and their ability to reduce excess intracellular ROS (10,11). To examine the role of AMPK in endothelial stress adaptation, we treated human aortic endothelial cells (HAECs) with H2O2 to mimic oxidative stress. As shown in Figure 1A, H2O2 induced significant endothelial cell death and this effect was attenuated by AMPK activation. Consistent with these findings, H2O2 significantly increased intracellular ROS levels in HAECs, which were attenuated with AICAR treatment (Fig. 1B). Given that one target of AMPK in the endothelium is endothelial nitric oxide synthase (eNOS) (14,15), we probed the activation of eNOS. Treatment with calcimycin (A23187), a calcium ionophore that constitutively stimulates calmodulin-dependent production of NO, demonstrated that AICAR increases both basal and stimulated NO bioactivity in HAECs (Fig. 1C). AMPK activation by AICAR also increased the phosphorylation of eNOS at its activation site Ser-1177 (Fig. 1D).
Figure 1. AICAR promotes stress adaptation in HAEC.
(A) Confluent HAECs were treated with vehicle (PBS) or 1 mM AICAR for 24 h. Cells were then kept in Krebs-Hepes buffer for 30 min and exposed to 500 µM H2O2 for 2h and MTS assay was performed. (B) Confluent HAECs were treated with vehicle or 1 mM AICAR for 24 h. Cells were then kept in Krebs-Hepes buffer for 30 min and exposed to 200 µM H2O2 for 10 min. Endothelial ROS production was assessed by DCF assay. (C) Confluent HAECs were treated with vehicle or 1mM AICAR for 30 min in the presence or absence of calcimycin (A23187). Cell culture medium was then transferred to confluent rat aortic smooth muscle cells (RASMCs) for 3 min and removed. RASMCs were lysed and cGMP was measured in the cell lysates using an enzyme immunoassay. (D) Confluent HAECs were treated with AICAR (1mM) for 0–4 h. Cells were lysed and subjected to immunoblotting for phospho-Ser-1177 eNOS and total eNOS. *, P < 0.05.
3.2: AMPK-mediated prevention of endothelial dysfunction requires eNOS
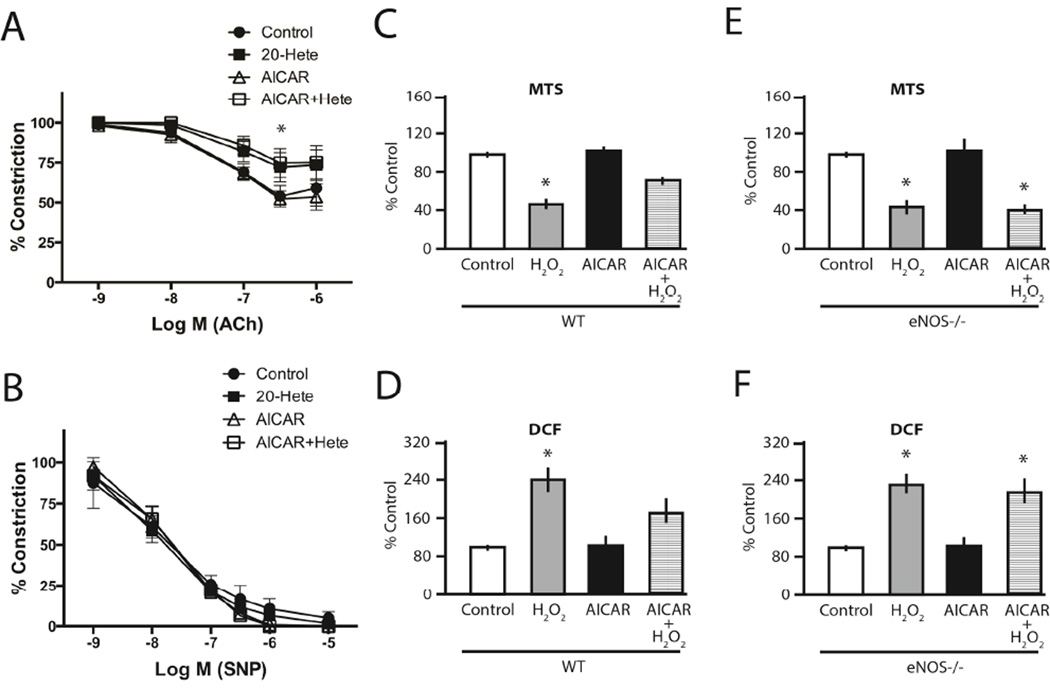
We have previously demonstrated that chronic AMPK activation with AICAR prevents endothelial dysfunction instigated by angiotensin II (11), LPS (11), and 20-hydroxy-eicosatetraenoic acid (20-HETE) (10). In eNOS-null mice, endothelium-dependent relaxation is mediated predominantly by prostacyclin and, as expected, 20-HETE, a potent mediator of vascular injury, produced endothelial dysfunction in eNOS-null aorta (Fig. 2A). However, in contrast to our previous findings in WT aorta (10), chronic AICAR treatment was not able to rescue 20-HETE-induced endothelial dysfunction in eNOS-null aorta (Fig. 2A). Importantly, these observations were not due to smooth muscle cell dysfunction as nitroprusside-mediated relaxation was unaffected in both WT (10) and eNOS-null aorta (Fig. 2B). Similarly, in contrast to WT MLECs, eNOS-null MLECs exhibited no benefit of AMPK activation with regards to cell survival (Fig. 2C and 2E) and intracellular ROS in response to H2O2 (Fig. 2D and 2F). These results suggest that eNOS is needed in order for AMPK to preserve endothelial function under stress conditions.
Figure 2. AMPK-mediated prevention of endothelial dysfunction requires eNOS.
(A) eNOS-null mice were i.p. injected with either vehicle (200 µl PBS) or AICAR (200 mg/kg) 24 hours prior to tissue harvest. After thoracic aortic ring preparation, the vessel segments were treated with vehicle or 1 µM 20-Hete and then contracted with a submaximal concentration of phenylephrine immediately. When the contraction reached a plateau phase, a response curve of relaxation to acetylcholine (10-9 to 10-5 M) was then determined. (B) Similarly, thoracic aortic rings were pre-constricted with phenylephrine before measuring the relaxation curve in response to increasing concentration of sodium nitroprusside (10-9 to 10-5 M). *, P < 0.05 vs. control. (C, E) Confluent MLECs from WT (C) and eNOS-null (E) mice were treated with vehicle or 0.2 mM AICAR for 24 h. Cells were then kept in Krebs-Hepes buffer for 30 min and exposed to 500 µM H2O2 for 2h and MTS assay was performed. (D, F) Confluent MLECs from WT (D) and eNOS-null (F) mice were treated with vehicle or 0.2 mM AICAR for 24 h. Cells were then kept in Krebs-Hepes buffer for 30 min and exposed to 200 µM H2O2 for 10 min. Endothelial ROS production was assessed by DCF assay. *, P < 0.05 vs. control.
3.3: Endothelial AMPK-induced mitochondrial biogenesis requires eNOS
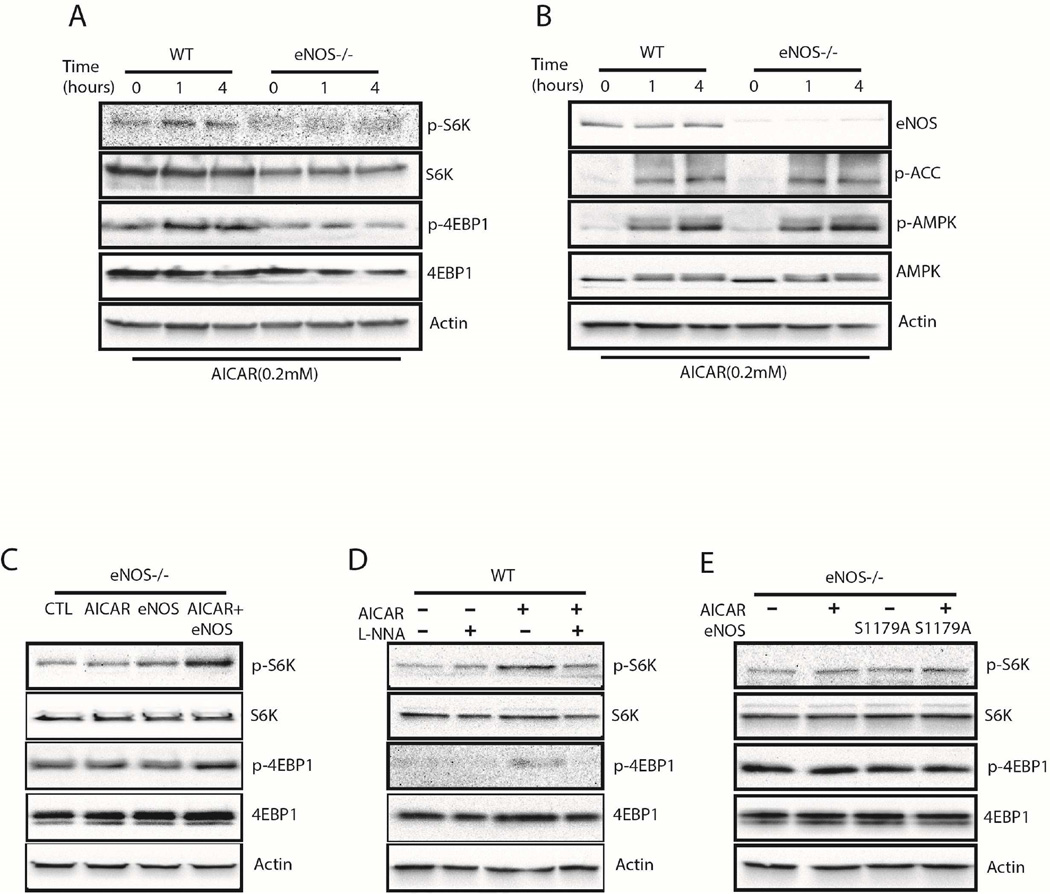
Mitochondrial biogenesis is one element of cellular adaptation to stress and energy deprivation (16). Since AMPK has been implicated in the regulation of mitochondrial biogenesis (2), we examined the effects of AICAR treatment on MLECs isolated from WT and eNOS-null mice. In response to AICAR, WT MLECs showed evidence of enhanced mitochondrial biogenesis by increased mitochondrial protein expression (cytochrome C (cyt C) and superoxide dismutase 2 (SOD2); Fig. 3A) and mitochondrial mass (Fig. 3B). These endothelial responses to AICAR were specific for AMPK, as they were inhibited by dominant-negative AMPK (Fig. 3C and D).
Figure 3. AICAR-induced AMPK specifically regulates mitochondrial biogenesis in endothelium.
(A) Confluent MLECs from WT mice were treated with various doses (0–1mM) of AICAR for 24 h. Cells were lysed and subjected to immunoblotting for cyt C, SOD2, and control actin. (B) Confluent WT MLECs were treated with 0.2 mM of AICAR for 24 h. MitoTracker Green FM fluorescence assay was performed as indicated. (C, D) Confluent HAECs were incubated with control, lacZ, or DN-AMPK (dominant negative AMPK) adenoviruses for 24h. Cells were then treated with vehicle or 1 mM AICAR for 24h. Immunoblotting (C) and MitoTracker Green FM fluorescence assay (D) were performed as indicated. *, P < 0.05 vs. control.
MLECs from eNOS-null mice showed no such mitochondrial response to AMPK activation, however this response could be rescued in eNOS-null MLECs after the reintroduction of wild-type eNOS (Fig. 4A and B). We next examined the requirement of eNOS catalytic activity in AMPK-mediated stress adaptation using the eNOS catalytic activity inhibitor L-nitro-arginine (L-NNA) and observed attenuation of AMPK-induced mitochondrial biogenesis (Fig. 4C and D). Furthermore, the introduction of the activation-insensitive mutant eNOS (S1179A; 15) into eNOS-null MLECs failed to rescue AMPK-mediated mitochondrial biogenesis (Fig. 4E and F). Thus, eNOS and eNOS-derived NO are necessary for AMPK-induced mitochondrial biogenesis in the stressed endothelium.
Figure 4. eNOS is required for AMPK-mediated mitochondrial biogenesis.
(A, B) Confluent MLECs from WT and eNOS-null mice were treated with vehicle or 0.2 mM AICAR for 24h. An additional well of eNOS-null cells was preincubated with eNOS adenoviruses for 24h before treatment with 0.2 mM AICAR for 24 h. Immunoblotting (A) and MitoTracker Green FM fluorescence assay (B) were performed as indicated. (C, D) Confluent MLECs from WT mice were treated with 0.2 mM AICAR for 24 h in the presence or absence of 1mM L-NNA. Immunoblotting (C) and MitoTracker Green FM fluorescence assay (D) were performed as indicated. (E, F) Confluent MLECs from eNOS-null mice were preincubated for 24h with either control or mutant eNOS (S1179A) adenovirus before treatment with vehicle or 0.2 mM AICAR for 24 h. Immunoblotting (E) and MitoTracker Green FM fluorescence assay (F) were performed as indicated. *, P < 0.05 vs. control.
3.4: AMPK-mediated mitochondrial biogenesis involves eNOS/mTORC1 signaling
The mammalian target of rapamycin complex 1 (mTORC1) is a central regulator of cell growth, metabolism, and survival, as well as mitochondrial oxidative function (18,19). Unlike in other cell types (20,21), we found AICAR induced endothelial mTORC1 activation as determined by increased phosphorylation of its targets, p70 ribosomal S6 kinase (S6K) and eukaryotic translation initiation factor 4E binding protein 1 (4EBP1; Fig. 5A). We studied the role of endothelial mTORC1 in AMPK-induced mitochondrial biogenesis and found that mTORC1 inhibition with rapamycin blunted AMPK-mediated mitochondrial biogenesis (Figs. 5B and C) and abrogated AICAR-induced resistance to H2O2 –mediated injury (Fig. 5D).
Figure 5. AICAR-induced mitochondrial biogenesis and oxidative stress adaptation involves mTORC1.
(A) Confluent HAECs were treated with vehicle or 1 mM AICAR over 0–24 h. Cells were lysed and subjected to immunoblotting as indicated. (B, C) Confluent HAECs were treated with vehicle or 1 mM AICAR for 24h in the presence or absence of the mTORC1 inhibitor, rapamycin. Immunoblotting (B) and MitoTracker Green FM fluorescence assay (C) were performed as indicated. (D) Confluent HAECs were treated with vehicle or 1 mM AICAR for 24 h in the presence or absence of rapamycin. Cells were then subjected to H2O2 and the cell viability was assessed by MTS assay. *, P < 0.05 vs. control.
We further confirmed the role of eNOS in AMPK-mediated mTORC1 activation using WT and eNOS-null MLECs. We observed AICAR-induced mTORC1 activation in WT but not in eNOS-null MLECs (Fig. 6A), despite robust AMPK activation and phosphorylation of its downstream target, acetyl-CoA carboxylase (ACC; Fig. 6B). In parallel with its rescue of AMPK-mediated mitochondrial biogenesis, reintroduction of wild-type eNOS into eNOS-null MLECs was able to rescue AICAR-induced mTORC1 activation (Fig. 6C). Correspondingly, pharmacological inhibition of eNOS enzymatic activity with L-NNA hindered AICAR-induced mTORC1 activation (Fig. 6D). These observations were linked to eNOS phosphorylation and activation by AMPK, as eNOS-null MLECs transfected with eNOS harboring the mutated AMPK phosphorylation site (S1179A eNOS) was not able to rescue AMPK-induced mTORC1 activation (Fig. 6E).
Figure 6. eNOS is an upstream regulator of mTORC1 in AICAR-induced AMPK activation.
(A, B) Confluent MLECs from WT and eNOS-null mice were treated with vehicle or 0.2 mM AICAR for 0–4h. Cell lysates were subjected to immunoblotting as indicated. (C) Confluent MLECs from eNOS-null mice were treated with vehicle or 0.2 mM AICAR for 1 h. An additional two wells of eNOS-null cells were pre-incubated with either control or wild-type eNOS adenovirus for 24h before the 1h treatment with 0.2 mM AICAR. Cells were lysed and subjected to immunoblotting as indicated. (D) Confluent MLECs from WT mice were treated with 0.2 mM AICAR for 1h with or without 30 min pretreatment with 1 mM L-NNA. Cells were lysed and subjected to immunoblotting as indicated. (E) Confluent MLECs from eNOS-null mice were treated with vehicle or 0.2 mM AICAR. Additional wells of eNOS deficient cells were preincubated with either control or mutant eNOS (S1179A) adenovirus, before treatment with 0.2 mM AICAR for 1 h. Immunoblotting was performed as indicated.
4: Conclusions
The principal finding of this work is that AMPK-mediated endothelial mitochondrial biogenesis depends upon the coordinated activation of eNOS and mTORC1. We found that endothelial cells lacking eNOS were unable to upregulate mitochondrial biogenesis in response to AICAR-induced AMPK activation. The mitochondrial biogenesis response was important for endothelial cell survival, and eNOS-null cells derived no protection against stress with chronic AMPK activation. Importantly, AMPK signaling to other canonical targets (i.e. acetyl-CoA carboxylase) was not dependent upon eNOS. Interestingly, we also found that eNOS was important for endothelial cell signaling from AMPK to mTORC1, as both eNOS gene deletion and eNOS inhibition prevented both AICAR-induced mTORC1 activation and mitochondrial biogenesis. We could rescue the defect in mTORC1 activation in eNOS-null cells by reintroduction of catalytically active eNOS. Further, inhibition of mTORC1 with rapamycin prevented induction of mitochondrial biogenesis after AMPK activation, thus indicating that a pathway involving AMPK, eNOS, and mTORC1 coordination is involved in endothelial mitochondrial biogenesis and vascular stress adaptation.
While mitochondrial dysfunction has been linked to endothelial dysfunction and subsequent vascular disease states, particularly in the aging population (22), mitochondrial biogenesis is typically a hallmark of adaptation to stress and often coincides with cell survival and improved signaling (4). In the primarily glycolytic endothelium, mitochondria appear to serve as essential signaling organelles rather than simple mediators of oxidative phosphorylation. Indeed, glycolysis generates roughly 85% of endothelial ATP production, despite the high availability of circulating oxygen from blood (6). The high expression of eNOS increases levels of nitric oxide (NO), which is known to have an inhibitory effect on oxidative phosphorylation (23), further rendering the mitochondria as environmental stress sensors rather than energy powerhouses (24). Indeed, we found that eNOS-null aorta exhibit a higher degree of dysfunction in response to environmental stressors than wild-type aorta. Likely this dysfunction stems from inadequate NO signaling which therefore impair mitochondrial dynamics (25).
Stimuli promoting mitochondrial biogenesis confer endothelial protection from stress (11), and AMPK has been repeatedly associated with induction of mitochondrial biogenesis (2,16,26). We observed that endothelial AMPK-induced mitochondrial biogenesis was dependent upon mTORC1, a serine/threonine kinase that is highly conserved amongst eukaryotes. This central regulator of cell growth is nutrient-sensitive, and typically becomes activated only with the dual positive input of nutrient availability and growth factor stimulation (21). The coordination of growth stimulation with nutrient availability is logical, as proliferative signals in the absence of adequate growth substrates would not be productive. Thus, it is curious our study found a link between AMPK, a signal typically associated with nutrient deprivation (27), and mTORC1 activation. In multiple other cell types AMPK activation has been associated with mTORC1 inhibition and further linked to protection from apoptosis (18,28–32). This apparent discrepancy might be reconciled by considering the requisite role of the endothelium in promoting angiogenesis in response to reduced blood flow and hypoxia. Unlike other cells that are growth inhibited by hypoxia, endothelial cells migrate towards hypoxic tissue via a VEGF gradient and actively divide under these conditions (33). In addition to angiogenesis, the endothelium serves to signal surrounding tissues to respond to stress (e.g. nutrient deprivation, hypo-/hypervolemia, increased ROS) and therefore must survive these conditions to do so. Thus, it stands to reason that endothelial cells require coordinated activation of both nutrient stress (AMPK) and growth signals (mTORC1).
Recent data has implicated the inhibitor of mTORC1, rapamycin, in endothelial dysfunction, further linking mTORC1 activation to endothelial adaptation to stress (34). Our data further indicate that mTORC1 is a downstream target of eNOS, likely via eNOS-derived NO as catalytically incompetent forms of eNOS did not rescue either AMPK-mediated mTORC1 activation or mitochondrial biogenesis in eNOS-null cells. Indeed rapamycin has been used to prevent neovascularization of tumors by targeting mTORC1-dependent VEGF production (35), and there have been reports that link AMPK activation to eNOS through investigations with VEGF (36), AICAR (14) and metformin (15,37).
Interestingly, one study found that inhibition of mTORC1 paradoxically upregulates mTORC2 activity and promotes endothelial survival and angiogenesis (38). Further, inhibition of mTORC2 activity prevents angiogenic sprouting in response to VEGF. This points toward paradoxical signaling between the two mTOR complexes during stress adaptation, which coincides with the fact that we did not find differences in Akt (a known target of mTORC2) signaling after treatment with AICAR (data not shown) but still saw improvement in cell survival during oxidative stress and increased mTORC1 activity. How this paradoxical mTOR balance coincides with AMPK activation and mitochondrial biogenesis in the endothelium remains to be thoroughly elucidated. One study has already shown that mitochondrial oxidative function and mitochondrial biogenesis require coordination of PGC1a and mTOR (19), and AICAR is known to increase protein expression levels of this mitochondrial biogenesis “master-regulator” PGC1a (39–41). Though it was not the focus of this particular study, it would be interesting to investigate further how PGC1a could fit into our proposed paradigm.
Other studies have implicated AMPK in ROS-induced autophagy, a crucial process for cell survival via reallocation of nutrients that coordinates with mitochondrial biogenesis, although it was hypothesized to operate through mTORC1 inhibition rather than activation (42). Indeed we observed attenuated ROS levels in endothelial cells after AMPK activation, and this correlated with improved cell survival during stress. It is possible that our observations in eNOS-null aorta and ECs (i.e. decreased stress adaptation and decreased mitochondrial biogenesis) are due to the failure of AMPK-mediated mechanisms to trigger autophagy in response to stress. Indeed, impaired autophagy and mitophagy has been implicated in several vascular pathologies (5,43,44). Likely the observed mitochondrial biogenesis from AICAR-induced AMPK activation is a component of cellular nutrient reorganization, and that process is impaired in the absence of eNOS and contributes to increased oxidative stress and decreased cell survival.
In summary, our studies have demonstrated that AICAR induces mitochondrial biogenesis and stress adaptation via an AMPK/eNOS/mTORC1 pathway in endothelial cells. The link between eNOS and mTORC1 is novel, and may be one reason why the eNOS-derived NO signal is an integral component of normal endothelial function.
Highlights.
endothelial AMPK activation leads to mitochondrial biogenesis and protection
eNOS activation is required for AMPK stimulated mitochondrial biogenesis
eNOS couples AMPK to mTORC1 activation in the endothelial stress response
Acknowledgments
We thank Dr. Richard C. Venema for his generous donation of eNOS adenoviruses. This work was supported by grants R01HL092122, and R01HL098407 from the NHLBI (to J.F.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
C.L., M.R., S.C., S.K. and J.F.K. designed research. C.L. and M.R. performed research. C.L., M.R., S.C., S.K. and J.F.K. analyzed the data. M.R., C.L., S.C., S.K. and J.F.K. wrote the manuscript.
Conflict of interest
None declared.
References
- 1.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 2.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter Ea, Kiens B, Wojtaszewski JFP. Can Exercise Mimetics Substitute for Exercise? Cell Metab [Internet] 2008;8(2):96–98. doi: 10.1016/j.cmet.2008.07.004. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1550413108002118. [DOI] [PubMed] [Google Scholar]
- 4.Kluge Ma, Fetterman JL, Vita Ja. Mitochondria and Endothelial Function. Circ Res [Internet] 2013;112(8):1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. Available from: http://circres.ahajournals.org/cgi/doi/10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimasaki Y, Pan N, Messina LM, Li C, Chen K, Liu L, et al. Uncoupling protein 2 impacts endothelial phenotype via p53-mediated control of mitochondrial dynamics. Circ Res [Internet] 2013;113(7):891–901. doi: 10.1161/CIRCRESAHA.113.301319. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3869454&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154(3):651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Kopp HG, Hooper aT, Avecilla ST, Rafii S. Functional heterogeneity of the bone marrow vascular niche. Ann N Y Acad Sci [Internet] 2009;1176:47–54. doi: 10.1111/j.1749-6632.2009.04964.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19796232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature [Internet]. Nature Publishing Group. 2014;507(7492):376–380. doi: 10.1038/nature13146. Available from: http://www.nature.com/doifinder/10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz Ma, Bevan Ja, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 10.Ward N, Chen K, Li C, Croft K, Keaney JF. Chronic activation of AMP-activated protein kinase prevents 20-hydroxyeicosatetraenoic acid-induced endothelial dysfunction. Clin Exp Pharmacol Physiol. 2011;38(5):328–333. doi: 10.1111/j.1440-1681.2011.05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118(13):1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SR, Chen K, Keaney JF. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277(8):6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 13.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen a, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow Va, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278(34):31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 15.Davis BJ, Xie Z, Viollet B, Zou M. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. 2006 Feb;55 doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 16.Jornayvaz F, Shulman G. Regulation of mitochondrial biogenesis. Essays Biochem [Internet] 2010;47:69–84. doi: 10.1042/bse0470069. Available from: http://essays.biochemistry.org/bsessays/047/bse0470069.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106(52):22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450(7170):736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Dromparis P, Michelakis ED. Mitochondria in Vascular Health and Disease [Internet] Annual Review of Physiology. 2013:95–126. doi: 10.1146/annurev-physiol-030212-183804. Available from: http://www.annualreviews.org/doi/abs/10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- 23.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci [Internet] 2006;119(14):2855–2862. doi: 10.1242/jcs.03062. Available from: http://jcs.biologists.org/cgi/doi/10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 24.Widlansky ME, Gutterman DD. Regulation of Endothelial Function by Mitochondrial Reactive Oxygen Species. Antioxid Redox Signal [Internet] 2011;15(6):1517–1530. doi: 10.1089/ars.2010.3642. Available from: http://www.liebertonline.com/doi/abs/10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erusalimsky JD, Moncada S. Nitric Oxide and Mitochondrial Signaling: From Physiology to Pathophysiology. Arterioscler Thromb Vasc Biol [Internet] 2007;27(12):2524–2531. doi: 10.1161/ATVBAHA.107.151167. Available from: http://atvb.ahajournals.org/cgi/doi/10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Curry BJ, Brown PL, Zemel MB. Leucine Modulates Mitochondrial Biogenesis and SIRT1-AMPK Signaling in C2C12 Myotubes. J Nutr Metab [Internet] 2014;2014:1–11. doi: 10.1155/2014/239750. Available from: http://www.hindawi.com/journals/jnme/2014/239750/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93(4) doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 28.Hardie DG. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W Bin, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, et al. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem. 2010;285(52):40461–40471. doi: 10.1074/jbc.M110.164046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1 / AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116(20):1–3. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest. 2010;90(5):762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafique E, Choy WC, Liu Y, Feng J, Cordeiro B, Lyra A, et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging (Albany NY) [Internet] 2013;5(7):515–530. doi: 10.18632/aging.100569. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3765580&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Reineke DC, Müller-Schweinitzer E, Winkler B, Kunz D, Konerding Ma, Grussenmeyer T, et al. Rapamycin impairs endothelial cell function in human internal thoracic arteries. Eur J Med Res [Internet]. European Journal of Medical Research. 2015;20(1):59. doi: 10.1186/s40001-015-0150-4. Available from: http://www.eurjmedres.com/content/20/1/59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Bufalo D. Antiangiogenic Potential of the Mammalian Target of Rapamycin Inhibitor Temsirolimus. Cancer Res [Internet] 2006;66(11):5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 36.Reihill Ja, Ewart MA, Hardie DG, Salt IP. Biochem Biophys Res Commun [Internet] 4. Vol. 354. Elsevier Inc.; 2007. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production; pp. 1084–1088. Available from: http://dx.doi.org/10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou M-H, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, Schlattner U, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279(42):43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 38.Farhan Ma, Carmine-Simmen K, Lewis JD, Moore RB, Murray AG. Endothelial Cell mTOR Complex-2 Regulates Sprouting Angiogenesis. PLoS One [Internet] 2015;10(8):e0135245. doi: 10.1371/journal.pone.0135245. Available from: http://dx.plos.org/10.1371/journal.pone.0135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irrcher I, Adhihetty PJ, Sheehan T, Joseph A-M, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. [[cited 2016 Feb 26]];Am J Physiol Cell Physiol [Internet] 2003 Jun 1;284(6):C1669–C1677. doi: 10.1152/ajpcell.00409.2002. Available from: http://ajpcell.physiology.org/content/284/6/C1669. [DOI] [PubMed] [Google Scholar]
- 40.Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-Activated Protein Kinase-Regulated Activation of the PGC-103B1; Promoter in Skeletal Muscle Cells. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kukidome D. Activation of AMP-Activated Protein Kinase Reduces Hyperglycemia-Induced Mitochondrial Reactive Oxygen Species Production and Promotes Mitochondrial Biogenesis in Human Umbilical Vein Endothelial Cells. Diabetes. 2006;55(1):120–127. [PubMed] [Google Scholar]
- 42.Wang Q, Liang B, Shirwany Na, Zou M-H. 2-Deoxy-D-Glucose Treatment of Endothelial Cells Induces Autophagy by Reactive Oxygen Species-Mediated Activation of the AMP-Activated Protein Kinase. PLoS One [Internet] 2011;6(2):e17234. doi: 10.1371/journal.pone.0017234. Available from: http://dx.plos.org/10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124(4):444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryter SW, Lee S-J, Smith a, Choi aMK. Autophagy in Vascular Disease. Proc Am Thorac Soc [Internet] 2010;7(1):40–47. doi: 10.1513/pats.200909-100JS. Available from: http://pats.atsjournals.org/cgi/doi/10.1513/pats.200909-100JS. [DOI] [PMC free article] [PubMed] [Google Scholar]