Abstract
INTRODUCTION
Most pituitary macroadenomas (PMA) are soft and suckable allowing transsphenoidal resection. A small percentage of PMA are firm, which significantly alters the time, technical difficulty, and effectiveness of transsphenoidal surgery. No current imaging technology can reliably assess PMA viscoelastic consistency in preparation for surgery. Magnetic Resonance Elastography (MRE) is a MRI-based technique that measures the propagation of mechanically induced shear waves through tissue to calculate stiffness. We prospectively evaluated MRE in 10 patients undergoing transsphenoidal resection of PMA to determine feasibility and potential usefulness.
METHODS
10 patients with PMA > 2.0 cm in maximum diameter were prospectively imaged with MRE prior to transsphenoidal surgery. Mean patient age was 59.5 ± 16.2 (22-78) years. Five were female and five male. MRE was performed with a modified single-shot spin-echo echo-planar-imaging pulse sequence on a 3T MRI. MRE values were independently calculated. The surgeon, blinded to the MRE results, graded tumor consistency at surgery as soft, intermediate, or firm. Chi-squared test compared surgical grading and MRE stiffness values.
RESULTS
MRE was accomplished in all patients with excellent resolution. By surgical categorization, six tumors were soft and four intermediate. The mean MRE value for soft tumors was 1.38 ± 0.36 (1.08−1.87) kPa, while for intermediate tumors it was 1.94 ± 0.26 (1.72−2.32) kPa (p= 0.020).
CONCLUSION
Determination of PMA stiffness is feasible with MRE. There was a statistically significant difference in MRE values between soft and intermediate PMAs. Further study in a larger series is ongoing to determine whether MRE will prove useful in preoperative planning for PMA.
Keywords: magnetic resonance elastography, MRE, pituitary macroadenoma, consistency, stiffness
INTRODUCTION
Most pituitary macroadenomas (PMA) are currently resected through transsphenoidal approaches, which work well if tumors are soft and suckable. However, approximately 10% of PMA are firm which alters surgical technique and may impact surgical outcome and approach [1,2]. While various magnetic resonance imaging (MRI) based methods have been investigated to determine the consistency of pituitary macroadenomas (PMA), none of the methods have proven capable of characterizing tumor stiffness [3-6]. These methods, including measurement of standard T1 and T2 signal and features of contrast enhancement and diffusion, are based on static imaging characteristics and cannot directly measure stiffness.
Magnetic Resonance Elastography (MRE) is a dynamic MRI-based technique that measures the propagation of mechanically induced shear waves through a tissue of interest to calculate the stiffness, offering a method to evaluate tissue stiffness [7-10] in vivo. In essence, MRE “palpates” by imaging and determines a property called the shear modulus, which is a measure of the underlying viscoelastic properties of the tissue. MRE of various brain tumors has been reported [11,12]. The purpose of this study was to evaluate the feasibility and potential usefulness of MRE in patients undergoing transsphenoidal resection of PMA.
METHODS
After institutional review board approval and written consent, 10 patients with a PMA > 2.0 cm in maximum diameter were prospectively evaluated by MRE prior to transsphenoidal surgery from September 2013 to January 2015 (Table 1). The mean patient age was 59.5 + 16.2 (22-78) years. Five were female and five were male. The mean tumor size in maximal diameter was 3.5 ± 0.9 (2.5 − 5.2) cm.
Table 1.
Patient characteristics
| Age | Sex | Tumor Size |
Knosp Grade (R/L) |
Secreting | Pathology | Resection | Overall Surgical Consistency |
MRE | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | 4.3 × 5.2 × 5.2 |
4/4 | No | Null cell | ST | Soft | 1.08 |
| 2 | 78 | M | 3.3 × 2.7 × 2.6 |
4/3 | No | LH, FSH | ST | Soft | 1.81 |
| 3 | 67 | F | 3.0 × 3.5 × 2.6 |
4/3 | No | Null cell | ST | Soft | 1.08 |
| 4 | 70 | M | 3.3 × 2.9 × 2.8 |
4/3 | No | Null cell | ST | Intermediate | 1.72 |
| 5 | 60 | F | 2.2 × 2.3 × 2.1 |
4/3 | No | Null cell | NT | Intermediate | 1.87 |
| 6 | 58 | M | 2.3 × 2.4 × 2.5 |
3/3 | Yes | GH | ST | Soft | 1.86 |
| 7 | 22 | F | 4.8 × 4.6 × 3.0 |
4/4 | Yes | GH | ST | Intermediate | 2.32 |
| 8 | 78 | F | 2.4 × 2.6 × 1.7 |
3/3 | No | LH, FSH | ST | Intermediate | 1.84 |
| 9 | 55 | M | 3.0 × 2.4 × 1.9 |
3/3 | No | Null cell | NT | Soft | 1.13 |
| 10 | 54 | M | 4.1 × 2.5 × 2.4 |
3/3 | No | FSH | ST | Soft | 1.30 |
R= Right; L= Left; LH= luteinizing hormone; FSH= follicle stimulating hormone; GH= growth hormone; ST= subtotal; NT= near total
MRE was performed with a modified single-shot spin-echo echo-planar-imaging pulse sequence on a 3T MRI system (Signa Excite, GE Healthcare, Waukesha, WI) as previously reported[13]. Shear waves were introduced intracranially with a soft, pillow-like pneumatic driver positioned under the subject’s head. The resulting displacement field was acquired with: TR/TE 3600/62 ms, FOV = 24 cm, 3 x parallel imaging acceleration, matrix = 72×72, 48 contiguous 3 mm thick axial slices and 8 phase offsets sampled over one period of the 60 Hz motion. The matrix was reformatted to 80×80 in order to have 3 mm isotropic resolution and the entire volume was acquired in just under 7 minutes. Post processing of the wave data includes a) registration of the MRE data to a standard anatomical atlas, b) calculating the curl of the first temporal harmonic of the acquired displacement field to reduce the effects of longitudinal waves and c) masking of the results from regional boundaries to minimize edge effects. The stiffness in each region of interest (ROI) was reported as the median value in the elastogram (stiffness map) within that region.
MRE values were calculated prospectively by radiologists unaware of the surgical findings. Tumor size was noted in centimeters (cm) of the largest dimension.
The participating experienced pituitary surgeon (JVG) was blinded to the results of the MRE and reported tumor consistency at the time of surgery in detailed surgical notes. Surgeon impression of tumor consistency at resection was considered the reference standard. Tumor stiffness was graded on the following scale: soft: primarily removed with suction; intermediate: parts easily removed with suction but other portions difficult to remove with suction requiring mechanical techniques such a sharp dissection; hard: unable to remove with suction requiring sharp dissection.
A chi-squared test was performed with JMP Version 10.0 (SAS, Cary, NC) to compare surgical grading and MRE stiffness values. P-values <0.05 were considered significant.
RESULTS
All patients were first approached transsphenoidally. Two patients (Table 1, patients 3 and 7) underwent subsequent craniotomy, the first on postoperative day one for residual hemorrhagic tumor causing worsened vision and the second 3 months after the index surgery for residual tumor in the lateral cavernous sinus and petrous apex with continued elevation of IGF-1 and GH. Given the patients young age, aggressive cavernous tumor resection was undertaken to reduce her total residual disease.
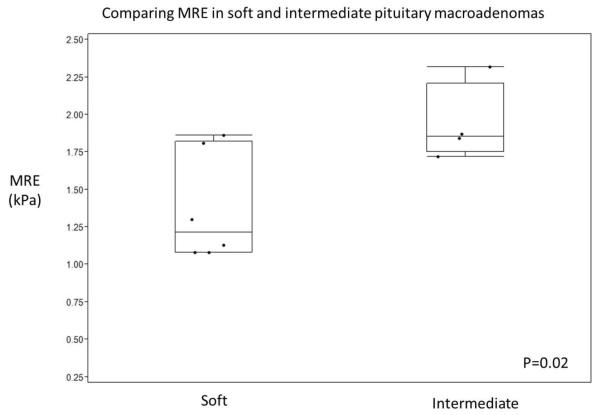
By surgical categorization, six tumors were soft (Figure 1) and four tumors intermediate (Figures 2 and 3) (Table 1). The mean MRE value for soft tumors was 1.38 + 0.36 (1.08 − 1.86) kPa, while for intermediate tumors it was 1.94 + 0.26 (1.72 − 2.32) kPa. The difference between the two groups was significant (p = 0.020) (Figure 4).
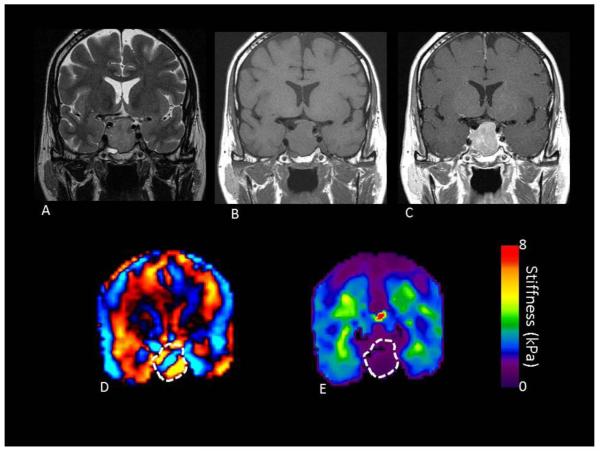
FIGURE 1.
A 56 year old male with a macroadenoma measuring 3.0 × 2.4 × 1.9 cm on preoperative MRI T2 (A), T1 without (B) and with contrast (C). Preoperative MRE showed the entire tumor to be soft (wave image D and elastogram E) with a MRE value of 1.13 kPa. At the time of surgery, the tumor was soft and suckable.
FIGURE 2.
A 61 year old female with a macroadenoma measuring 2.3 × 2.1 × 2.2 cm on preoperative MRI T2 (A), T1 without (B) and with contrast (C). Preoperative MRE showed the entire tumor to be of intermediate stiffness (wave image D and elastogram E) with an overall value of 1.86 kPa. At the time of surgery, the tumor was not suckable and required curettage for resection, although a near total resection was achieved.
FIGURE 3.
A 22 year old female with a macroadenoma measuring 4.8 × 4.6 × 3.0 cm on preoperative MRI T2 (A), T1 without (B) and with contrast (C). Preoperative MRE showed the anterior portion of the tumor (wave image D and elastogram E) to be soft while the lateral cavernous sinus portion was more firm. Overall, the tumor had an MRE value of 2.32 kPa. At the time of surgery, initially the tumor was soft and suckable, but as the resection proceeded posterolaterally, the tumor became more firm and unable to be removed with suction. This portion of the tumor was left behind due to the consistency as seen in the postoperative T1 with contrast MRI (F).
FIGURE 4.
Box plot comparing the MRE values and surgical grading of pituitary macroadenoma and showing there was a statistically significant difference between soft and intermediate tumors.
DISCUSSION
Clinically, there is a need to evaluate tumor PMA tumor stiffness/firmness as improved preoperative prediction would lead to better counseling of the risks and benefits to the patient. Further, while most PMAs are suckable tumors, a small proportion are not and these cases take a substantial amount of additional operative time and further operative techniques than suckable tumors. The surprisingly firm tumor can make for a difficult operative schedule when a case that typically consumes a couple of hours takes longer to achieve an inferior operative result with higher risk. Several studies have evaluated a variety of MRI methods to find correlation between imagining characteristics and operative tumor consistency. Results have been variable, rendering them less useful to surgeons (Table 2).
Table 2.
Summary of MRI Studies and Pituitary Tumor Consistency
| Study | N | MRI Modality | N Firm (%) | Correlation |
|---|---|---|---|---|
| Yamamoto et al 2014 | 29 | CE-BSSGES | 5 (17) | Yes |
| Alimohamadi et al 2014 | 30 | DW | 6 (20) | No |
| Bahyleyan et al 2006 | 80 | T2W | 12 (15) | No |
| Mahmoud et al 2010 | 24 | DW | 4 (17) | No |
| Pierallini et al 2006 | 22 | DW | 4 (18) | Yes |
| Thomas et al 2014 | 31 | DW | 6 (19) | Yes |
CE-BSSES= contrast-enhanced balanced steady-state gradient echo sequence; DW= diffusion weighted; T2W= T2 weighted
Yamamoto et al[6] evaluated contrast-enhanced balanced steady-state gradient echo sequence to categorize tumors as hard or soft, hypothesizing that tumors with intratumoral hyperintense dots labeled “mosaic” would be soft and tumors without these dots, labeled “solid” would be hard. There were 5 tumors classified as hard at surgery and all 5 were labeled as solid tumors by balanced steady-state gradient echo sequence. No tumors classified as mosaic were found to be hard at the time of surgery. They calculated balanced steady-state gradient echo sequence had 100% sensitivity and 88% specificity. On histologic analysis, solid tumors also had higher collagen content than their mosaic counterparts.
A few studies have evaluated diffusion-weight MRI to determine PMA consistency, with particular attention to apparent diffusion coefficient (ADC) values. Alimohamadi et al [14] found that an ADC value of 0.600-0.740 × 10-3 mm2/s was associated with more residual tumor after transsphenoidal resection. However, ADC values were not predictive of surgical consistency. Mahmoud et al also concluded ADC values did not correlate with operative consistency. In contrast, both Pieralliniet et al [4] and Thomas et al [15] reported a correlation between PMA consistency and ADC values.
Bahuleyan et al [16] evaluated T2 weighted MRI to predict consistency with the hypothesis that firm tumors would appear homogenously hypointense on T2W MRI. They found no correlation between the appearance of macroadenomas and T2W MRI appearance. Several of the above studies have concluded the same [6,14,15].
Clearly, given the number of studies investigating tumoral stiffness, this would imply a clinical need likely driven by the surgical dichotomy of this tumor, where firm tumors pose a different surgical case. In contrast to these studies using standard MRI imaging characteristics, MRE is a technology intended to measure stiffness based on the underlying tissue biomechanics. As the pituitary is enclosed deep within the boney sella, it was unknown whether the MRE shear waves would propagate to the central skull region. It is possible that boney thinning or destruction that occurs with PMA aids in obtaining MRE measurements. In our first 10 PMA, MRE showed that there was a statistically significant difference in tumors that were determined to be soft or intermediate at surgery. No hard tumors were present in this series. However, during the recruitment period of the study a stiff sellar lesion was imaged (Figure 5). Preoperatively this mass had imaging findings consistent with a PMA. The MRE demonstrated that the lesion was very stiff and the resection was difficult. The final pathology determined the mass represented a metastasis from adenoid cystic carcinoma. There were two tumors that had higher MRE measurement within the range of the intermediate tumors, yet they were determined to be soft at surgery. One of these had both a soft and stiffer fibrous portion but was classified as soft at surgery. The anterior resected portion of the PMA was found to be soft on MRE (Figure 3). The other had a significant cystic portion which can be misrepresented by MRE as firm. This study has limitations, mainly, the small population size. However, this was primarily a feasibility study and not focused on patient accrual for in-depth analysis.
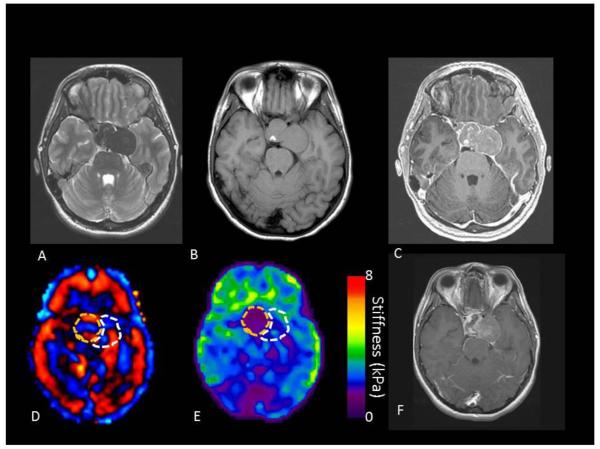
FIGURE 5.
A 73 year old female with metastatic adenoid cystic carcinoma. On preoperative MRI T2 (A), T1 without (B) and with contrast (C). The tumor was presumed to be an adenoma preoperatively but was found to be stiff at on MRE (wave image D and elastogram E) and at resection.
Given the small study population and that one patient had residual tumor hemorrhage causing worsened vision that led to craniotomy with the goal of improving vision, our study has a hemorrhage rate of 10%. However, this study represents a small portion of our institutional pituitary practice and our incidence of significant postoperative hemorrhage is less than 1%.
CONCLUSION
We found that it is feasible to obtain MRE measurements in PMA. While the series is small, there was a statistically significant difference in MRE values between soft and intermediate PMA. Further study in a larger series will help determine whether MRE will prove useful in preoperative planning for PMA.
Acknowledgments
FINANCIAL MATERIAL & SUPPORT: This research received funding from the National Institutes of Health, R01 grant EB001981.
Footnotes
CONFLICT(S) OF INTEREST TO DECLARE: Richard Ehman and John Huston and the Mayo Clinic have intellectual property rights and a potential financial interest in some of the technology used in this study.
INSTITUTIONAL REVIEW BOARD APPROVAL: Yes
WORKS CITED
- 1.Snow RB, Lavyne MH, Lee BC, Morgello S, Patterson RH., Jr. Craniotomy versus transsphenoidal excision of large pituitary tumors: the usefulness of magnetic resonance imaging in guiding the operative approach. Neurosurgery. 1986;19(1):59–64. doi: 10.1227/00006123-198607000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Zada G, Du R, Laws ER., Jr. Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg. 2011;114(2):286–300. doi: 10.3171/2010.8.JNS10520. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud OM, Tominaga A, Amatya VJ, Ohtaki M, Sugiyama K, Sakoguchi T, Kinoshita Y, Takeshima Y, Abe N, Akiyama Y, El-Ghoriany AI, Abd Alla AK, El-Sharkawy MA, Arita K, Kurisu K, Yamasaki F. Role of PROPELLER diffusion-weighted imaging and apparent diffusion coefficient in the evaluation of pituitary adenomas. Eur J Radiol. 2011;80(2):412–417. doi: 10.1016/j.ejrad.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Pierallini A, Caramia F, Falcone C, Tinelli E, Paonessa A, Ciddio AB, Fiorelli M, Bianco F, Natalizi S, Ferrante L, Bozzao L. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging--initial experience. Radiology. 2006;239(1):223–231. doi: 10.1148/radiol.2383042204. [DOI] [PubMed] [Google Scholar]
- 5.Snow RB, Johnson CE, Morgello S, Lavyne MH, Patterson RH., Jr. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26(5):801–803. doi: 10.1097/00006123-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto J, Kakeda S, Shimajiri S, Takahashi M, Watanabe K, Kai Y, Moriya J, Korogi Y, Nishizawa S. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. AJNR Am J Neuroradiol. 2014;35(2):297–303. doi: 10.3174/ajnr.A3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21(7):755–764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 8.Kruse SA, Rose GH, Glaser KJ, Manduca A, Felmlee JP, Jack CR, Jr., Ehman RL. Magnetic resonance elastography of the brain. Neuroimage. 2008;39(1):231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sack I, Beierbach B, Hamhaber U, Klatt D, Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed. 2008;21(3):265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- 10.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23(5):497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MC, Huston J, Glaser KJ, Manduca A, Meyer FB, Lanzino G, Morris JM, Felmlee JP, Ehman RL. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013;118(3):643–648. doi: 10.3171/2012.9.Jns12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Lin Y, Han JC, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 2007;48(3):327–330. doi: 10.1080/02841850701199967. [DOI] [PubMed] [Google Scholar]
- 13.Murphy MC, Huston J, 3rd, Jack CR, Jr., Glaser KJ, Senjem ML, Chen J, Manduca A, Felmlee JP, Ehman RL. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8(12):e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alimohamadi M, Sanjari R, Mortazavi A, Shirani M, Moradi Tabriz H, Hadizadeh Kharazi H, Amirjamshidi A. Predictive value of diffusion-weighted MRI for tumor consistency and resection rate of nonfunctional pituitary macroadenomas. Acta Neurochir (Wien) 2014;156(12):2245–2252. doi: 10.1007/s00701-014-2259-6. discussion 2252. [DOI] [PubMed] [Google Scholar]
- 15.Thomas TCVGBTSN. Evaluation of Consistency of Pituitary Macroadenoma Using Diffusion-weighted Imaging in Correlation with Surgical Findings. Neurosurgery Quaterly. 2014;24(2):131–135. [Google Scholar]
- 16.Bahuleyan B, Raghuram L, Rajshekhar V, Chacko AG. To assess the ability of MRI to predict consistency of pituitary macroadenomas. Br J Neurosurg. 2006;20(5):324–326. doi: 10.1080/02688690601000717. [DOI] [PubMed] [Google Scholar]