Abstract
Vocal imitation involves incorporating instructive auditory information into relevant motor circuits through processes that are poorly understood. In zebra finches, we find that exposure to a tutor’s song drives spiking activity within premotor neurons in the juvenile but that inhibition suppresses such responses upon learning in adulthood. We measure inhibitory currents evoked by the tutor song throughout development while simultaneously quantifying each bird’s learning trajectory. Surprisingly, we find that the maturation of synaptic inhibition onto premotor neurons is correlated with learning but not age. We used synthetic tutoring to demonstrate that inhibition is selective for specific song elements that have already been learned and not those still in refinement. Our results suggest that structured inhibition is playing a crucial role during song acquisition, enabling a piece-by-piece mastery of complex tasks.
Humans (1) and several other animal species (2–4) learn motor sequences by imitation. In the observer, a sensory percept must inform relevant motor circuits involved in the generation of the target behavior, but little is known about neural mechanisms underlying this process. We address this issue in the zebra finch, which acquires its courtship song by listening to (5) and imitating (6–9) a tutor. The forebrain nucleus HVC acts as an important sensorimotor interface because it receives direct connections from higher-order auditory centers (10–12) and generates commands essential for song production (13–15). In the juvenile zebra finch, tutor song exposure influences structural plasticity within HVC (16), a process that is thought to be crucial for song imitation (17). The tutor song has also been shown to drive network activity within HVC (18), but the responses of individual HVC premotor neurons during observational learning had not yet been explored.
We performed intracellular recordings in identified HVC neurons projecting to the robust nucleus of the arcopallium (RA) of 10 awake juvenile zebra finches during exposure to their tutor song. In 13 out of 29 of these HVC premotor neurons, tutor song playback caused spiking activity (Fig. 1, A to C). In those neurons, the timing of the evoked spikes was often highly precise across trials (see Methods for details), demonstrating that exposure to the tutor song is sufficient to drive patterned spiking activity within HVC and may serve an instructive role for the developing HVC premotor circuit. We also observed reliably timed tutor-evoked spiking in RA (18 neurons in 3 birds) (fig. S1) that was presumably driven by HVC. In contrast, tutor song playback did not evoke a suprathreshold response in HVC premotor neurons of awake adult zebra finches (Fig. 1D, 0 of 24 cells in 7 birds) and had only a minimal impact on subthreshold activity in those neurons (Fig. 1, D to F).
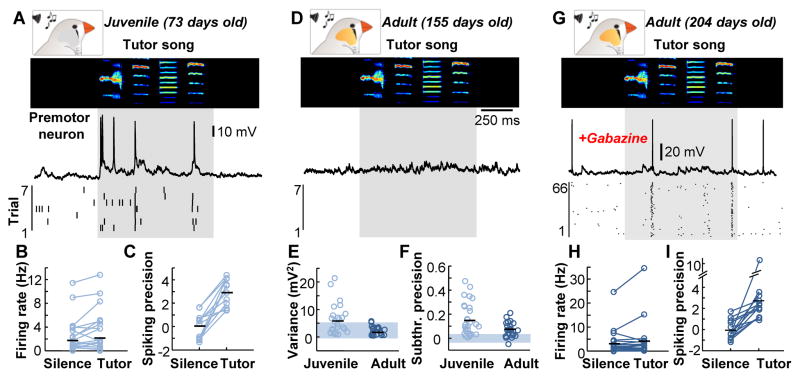
Figure 1. Responses of HVC premotor neurons to the tutor song are suppressed during development.
(A), Example intracellular recording from an HVC premotor neuron in an awake juvenile zebra finch during tutor song presentation (sonogram frequency: 0.5–7.5 kHz). Below, a spike raster plot showing seven repetitions from the same neuron. The shaded region indicates the time of tutor song exposure with an additional 50 milliseconds after the end of the song. (B and C) The rate [silence: 1.7±2.7 Hz; tutor: 2.1±3.3 Hz, P = 0.114, repeated measures ANOVA] (B) and precision [silence: 0.0±1.1; tutor: 3.0±1.2; P = 0.007, repeated measures ANOVA] (C) of HVC premotor neuron firing in juvenile zebra finches. (D) Example HVC premotor neuron recording in an awake adult bird during tutor song presentation with spike raster below. (E) Membrane potential variance of HVC premotor neurons was greater in juveniles (5.8±5.3 mV2) than in adults (2.3±1.6 mV2) (P = 0.004, Wilcoxon rank sum test) (shaded region = 95% confidence interval). (F) Subthreshold precision in HVC premotor neurons was greater in juveniles (0.16±0.14) than in adults (0.08±0.06) (P = 0.025, Wilcoxon rank sum test). (G) Tutor song responses from an HVC premotor neuron in an awake adult bird after local gabazine infusion (0.01mM). (H) The firing rate during silence (3.6±5.8 Hz) and tutor song presentation (4.6±8.2 Hz, n = 14 neurons, P < 0.001, repeated measures ANOVA,) after local gabazine (0.01 mM-0.1 mM) infusion. (I) Spiking precision was greater during the tutor song presentation (2.7±2.5) than during silence (−0.1±0.9) (P = 0.016, repeated measures ANOVA).
To directly investigate sensory-evoked synaptic events in HVC premotor neurons in awake zebra finches, we used in vivo whole-cell voltage clamp recordings (fig. S2). Using a fluorescent retrograde tracer injected into RA, we targeted HVC premotor neurons with 2-photon microscopy guidance. In both juveniles (25 cells in 7 birds) and adults (15 cells in 5 birds), excitatory events could be evoked by the tutor song (fig. S3), suggesting that the observed lack of spiking is not explained by a decrease in the strength of sensory afferents from auditory projections to HVC in the adult bird. Our results seemed inconsistent with previous findings in which HVC projection neuron spiking could be reliably driven by song playback in urethane-anesthetized adult zebra finches (19). Because urethane has been shown to act as an antagonist for GABAergic transmission (20), we reasoned that synaptic inhibition might suppress tutor song-evoked excitation in HVC of awake adult zebra finches. To test that hypothesis, we locally infused a GABAA antagonist (gabazine) and recorded HVC premotor neurons during tutor song exposure in adults (Fig. 1G). Once local inhibition was attenuated, HVC premotor neurons exhibited tutor song-evoked patterned spiking responses (Fig. 1, G to I), similar to that seen in juvenile zebra finches (Fig. 1, A to C). This finding indicates that inhibition can effectively silence sensory inputs onto HVC premotor neurons in the adult zebra finch.
Local circuit interneurons are likely to be the sole source of inhibition in HVC (21), and they exhibit song selective auditory responses in the awake adult zebra finch (22, 23). Thus, the lack of a tutor-song response in HVC premotor neurons in adulthood may be due to a stronger recruitment of the inhibitory network. To address this idea, we performed juxtacellular recordings of HVC interneurons during tutor song presentation in both juvenile (34 cells in 10 birds) and adult (15 cells in 4 birds) zebra finches (Fig. 2, A to D). Across all ages tested, interneurons increased their spiking activity to tutor song playback compared with a silent baseline period (fig. S4), and we observed that the tutor song could evoke precise spiking activity in some cases (Fig. 2B). When considering all data across both juvenile and adult zebra finches, however, we noticed no consistent age-related difference in the regularity of interneuron firing across trials (Fig. 2C). Because the song learning process has been shown to be highly variable across individuals (6) (fig. S5), we reexamined our data based on the similarity of recent song recordings from each bird to the tutor song. We found that interneuron firing precision in response to the tutor song tended to correlate with the extent of acoustic similarity to that song (Fig. 2D).
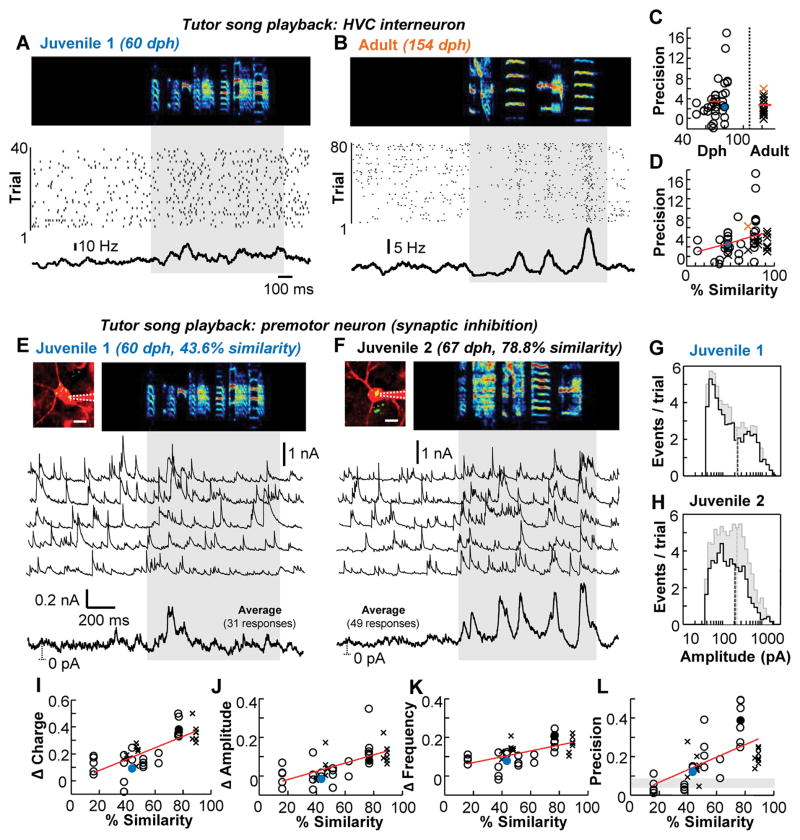
Figure 2. Tutor song-evoked inhibition strengthens and sharpens with improved song performance.
(A and B) Awake spiking activity of example HVC interneurons recorded in a juvenile (A) and an adult (B) bird during silence and tutor song presentation. (C) Across the population, the precision of HVC interneuron firing did not differ between juveniles (3.8±4.0Hz) and adults (2.9±1.7 Hz) (P = 0.51, Wilcoxon rank sum test). (D) Spiking precision of HVC interneurons depending on performance (P = 0.056, linear mixed-effect model). The filled circles/colored crosses represent data shown in the examples to the left. (E and F) Awake voltage-clamp recordings of inhibitory currents onto two HVC premotor neurons (images at top, scale bar: 10 μm) in response to a tutor song. For each cell, five single sweeps are presented as well as an average. The dotted line represents the distance from baseline (0 pA). (G and H) Amplitude histograms of detected inhibitory events during silence (black) and tutor song (gray) for juveniles 1 (G) and 2 (H). Mean of the amplitude distribution is indicted as a dotted vertical line. (I to L), Changes in tutor-song evoked inhibition onto HVC premotor neurons as a function of performance. Increasing similarity to the tutor song is associated with an increase (P < 0.01, linear mixed-effect model) in the inhibitory charge (I), the amplitude (J) and frequency (K) of inhibitory events, and the precision of inhibition across trials (L) (shaded region = 95% confidence interval). The filled circles represent data shown in the examples above.
Since HVC interneurons densely interconnect with HVC premotor neurons (24, 25), they are well-poised to directly inhibit HVC premotor neurons during tutor song presentation. Using 2-photon targeted voltage-clamp recordings in awake birds, we found that inhibitory currents onto HVC premotor neurons were often reliably evoked by tutor song playback (Fig. 2, E to H and fig. S6). Consistent with our results concerning HVC interneuron firing, we found no evidence for an age-related change in the regularity or strength of tutor song-evoked inhibition onto HVC premotor neurons (fig. S7). Additionally, we found no age-related change in the amplitude or frequency of spontaneous inhibitory events (fig. S8) and a developmental decrease in the amount of current needed to hold premotor neurons at 0 mV, which could reflect a downregulation of tonic inhibition. However, in both juvenile and adult zebra finches we found that inhibitory charge, event frequency and amplitude, and the regularity of inhibition across trials were significantly correlated with song imitation accuracy (Fig. 2, E to L and fig. S6). These results demonstrate that the maturation of sensory-evoked inhibition in HVC matches the bird’s learning progress rather than his developmental stage.
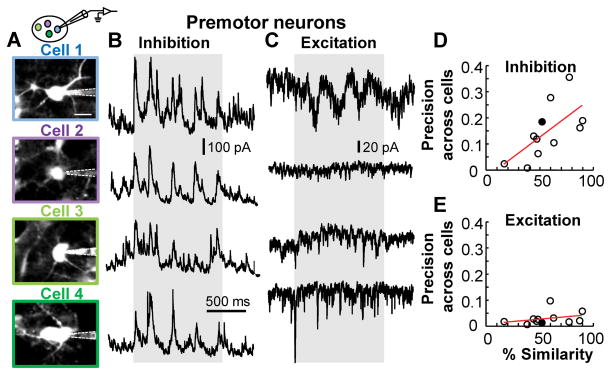
What function might this inhibition serve? We hypothesized that precisely timed inhibition in HVC could selectively target portions of the song that have been adequately learned, suppressing the effect of sensory inputs on premotor neurons during those times, thus preventing further plasticity in motor output. We tested two predictions stemming from this idea. One prediction is that all premotor neurons should receive the inhibitory signal synchronously, which would allow for robust suppression of sensory inputs on the entire premotor system. A second prediction is that the global inhibitory signal should vary in strength as a function of how well each segment of the song has been learned, with stronger inhibition associated with better learned segments. To test the first prediction, we considered 11 cases in which multiple HVC premotor neurons (mean: 3.5±1.3 neurons/bird) were recorded in the same bird (Fig. 3A). We found that tutor song-evoked inhibition was often highly correlated across neurons (Fig. 3B), and this effect was monotonically related to the degree of learning (Fig. 3D). The excitatory current profiles, however, seemed to be unique for each neuron (Fig. 3C), and did not significantly change with song learning (Fig. 3E). Our results are consistent with a global entrainment of a motor circuit by an inhibitory network whose coherent song responsiveness may effectively suppress certain segments of the tutor song in a learning-dependent manner.
Figure 3. Learning is associated with synchronous network inhibition.
(A) Recording schematic and 2-photon images of four recorded HVC premotor neurons in the same zebra finch. (B and C) Average inhibitory (B) and excitatory (C) current traces of premotor neurons shown in (A) during tutor song presentation. (D) Regularity of tutor song-evoked inhibitory currents across different neurons within one bird significantly increases with learning (P < 0.05, Pearson linear correlation). The filled circles represent data shown in the examples to the left. (E) Regularity of tutor song-evoked excitatory currents across different neurons within one bird does not significantly change depending on performance (P = 0.35, Pearson linear correlation).
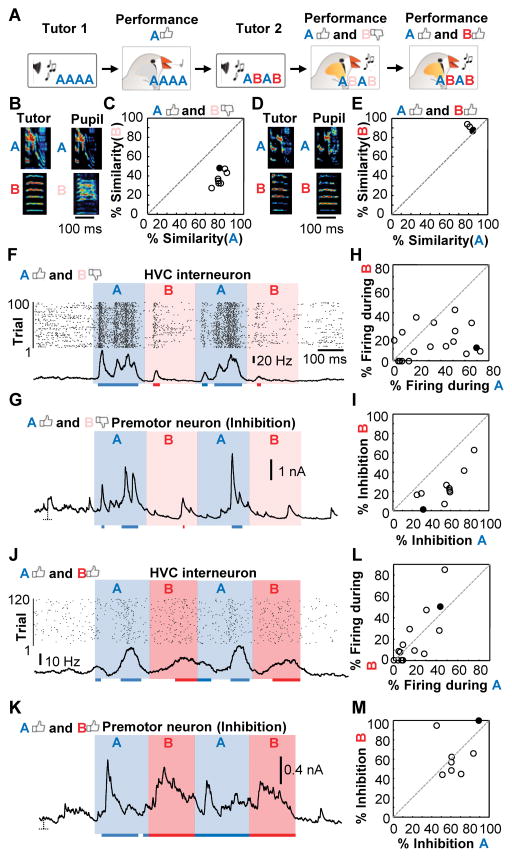
Zebra finches often learn individual song elements in series, focusing on specific passages at certain times (7). To test whether the second prediction of our model - that local inhibition in HVC is accurately targeting the syllables of the tutor song that the zebra finch had learned - we used a previously established method for controlling the learning process of individual song syllables (7, 26). Zebra finches were first trained using a synthetic tutor that produced four concatenated copies of a single syllable ‘A’ (Fig. 4A). Once the song ‘AAAA’ was learned, the tutoring paradigm was altered to introduce an additional syllable ‘B’. Juvenile finches can eventually copy the new song ‘ABAB’ (Fig. 4A), which leads to two distinct learning phases: an early learning phase in which ‘A’ is performed well and ‘B’ is performed poorly (Fig. 4, B and C) followed by a later learning phase in which both ‘A’ and ‘B’ are performed well (Fig. 4, D and E). We can exploit this artificially induced learning trajectory in order to address whether inhibition can be specifically directed towards portions of the song that have already been learned. In the early learning phase, when juveniles could perform syllable ‘A’ well and not ‘B’, both interneuron firing (Fig. 4F) as well as inhibitory currents onto HVC premotor neurons (Fig. 4G) preferentially targeted the learned syllable (Fig. 4, H and I). Zebra finches at the later learning stage, which produced a good copy of both ‘A’ and ‘B’, showed equivalent interneuron firing and synaptic inhibition across both syllable types (Fig. 4, J to M). In contrast, excitatory currents did not change their relative timing across learning conditions (fig. S9).
Figure 4. Inhibition accurately targets learned portions of the tutor song.
(A) A schematic detailing the training procedure. (B), Example syllables produced by a juvenile during an early learning phase in which syllable A is copied with 82.5% similarity and syllable B is copied with 48.8% similarity. (C) Syllable performance for 8 individuals in their early learning stage singing a good copy of A (77.9±4.7% similarity) and a poor copy of B (42.2±7.6% similarity). (D) Example syllables produced by a juvenile during a late learning phase in which both syllable A and B are copied well (85.7% and 88.9% similarity, respectively). (E) Syllable performance for 4 individual adults in their final learning stage singing a good copy of A (84.6±1.9% similarity) and B (89.7±2.2% similarity). (F and G) HVC interneuron activity (F) and inhibitory currents onto an HVC premotor neuron (G) during ABAB presentation recorded in two juvenile zebra finches performing a good copy of A (similarity: 75.6% and 84.6%, respectively) and a poor copy of B (similarity: 36.0% and 44.7%). The red and blue horizontal lines represent periods in which either the interneuron firing rate or HVC premotor neuron inhibition exceeds a 95% confidence interval. (H and I) Percentage of time that interneuron firing rates (n = 18 cells) (H) or the HVC premotor inhibition (n = 10 cells) (I) exceeded the 95% confidence interval threshold across a population of 4 and 5 birds respectively that copied syllable A well and B poorly. (J and K) HVC interneuron activity (J) and HVC premotor neuron inhibition (K) during ABAB presentation recorded in a juvenile zebra finch performing a good copy of syllables A (similarity: 85.7% and 79.8%) and B (similarity: 88.9% and 94.9%). (L and M) Percentage of time that interneuron firing rates (L) (n = 14 cells) or the HVC premotor inhibitory current amplitudes (M) (n = 8 cells) exceeded the 95% confidence interval threshold in two birds respectively that copied syllables A and B well.
In this study, we demonstrated that the activity of a motor circuit can be directly driven by sensory afferents during song learning. Specifically, exposure to the tutor song can elicit precise spiking in HVC premotor neurons of the juvenile bird (fig. S10A). This result is reminiscent of the ‘mirroring’ previously observed in mammalian motor systems (27) as well as in other songbird species (28) in which motor neurons respond to actions that are observed in others, but the temporal similarity between the tutor song-evoked firing patterns and singing-related activity in individual HVC neurons of the juvenile remains unknown. Importantly, a previous experiment (17) demonstrated the necessity of tutor song-dependent HVC dynamics during vocal learning. In that study, juvenile zebra finches were unable to imitate the tutor when HVC activity was optogenetically scrambled during the presentation of the tutor song. These results are consistent with the idea that precise sensory-driven activity may have a pivotal role in establishing song-related premotor sequences. We next demonstrated a loss of auditory responsiveness in HVC of the adult bird, but we were able to use GABA antagonists to unmask tutor song-evoked spiking, highlighting the role of inhibition on the suppression of these responses. Auditory-evoked responses were suppressed in all adults, even those that poorly copied the tutor song, indicating that the phasic inhibitory currents that are central to this study are not the only factor mediating this phenomenon. We did not find an age-related increase in tonic inhibition, which could have explained these results. Future studies could investigate the role of other developmentally regulated factors, such as intrinsic neuronal properties or chloride reversal potential, that could contribute to the further suppression of sensory inputs during development.
We further demonstrate that inhibition during song learning can precisely target specific portions of the song that have already been mastered (fig. S10B). As a result, certain components of the HVC sequence representing unlearned aspects of the song may be left ‘exposed’ to the influence of the incoming auditory stream. These neurons may then fire in a patterned way in response to the tutor song until an appropriate behavior is established. After the song has been learned completely, inhibition can shield HVC premotor neurons from the impact of the tutor song (fig. S10C). We do not yet understand the mechanisms that compare the current song performance to the tutor song and then transform this information into a change in inhibition throughout learning. This process is likely to be primarily mediated through an increase in the regularity of inhibitory neuron firing across trials, which is driven from inputs from higher-order auditory centers (10–12).
In sensory systems, inhibitory network maturation can result in the closure of critical periods (29). However, this procedure is strongly dependent on the developmental stage of the animal, while the inhibitory network changes observed in HVC are correlated not with age but with song performance (fig. S10C). Additionally, because the extent of tutor imitation is variable across birds and even within the span of a single bird’s song, the maturation of HVC inhibition proceeds in a self-directed, nonuniform manner. This stands in stark contrast to sensory systems, where inhibitory maturation primarily relies on external factors, such as visual experience (30–32). Despite these differences, our findings offer the opportunity to potentially enable latent afferent streams to engage with motor circuits through the manipulation of local inhibition. Using this approach, we may help to extend (29) or reopen critical periods (33) in order to rebuild or refine skilled behaviors throughout life.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (Grant R01NS075044), the New York Stem Cell Foundation, and the Deutsche Forschungsgemeinschaft (Grant VA 742/1–2). We thank Robert Froemke, Jesse Goldberg, Gaby Maimon, Daniel Okobi, Bence Ölveczky, and Richard Tsien for comments on earlier versions of this manuscript and Kalman Katlowitz, Kishore Kuchibhotla and Josh Merel for assistance with statistics and analysis. We also thank Ofer Tchernichovski for valuable discussions and for providing the birds used in this study.
Footnotes
Supplement contains additional data.
The authors declare no competing financial interests.
References and Notes
- 1.Blandin Y, Lhuisset L, Proteau L. Cognitive processes underlying observational learning of motor skills. Q J Exp Psychol-A. 1999 Nov;52:957. [Google Scholar]
- 2.Fiorito G, Scotto P. Observational-Learning in Octopus-Vulgaris. Science. 1992 Apr 24;256:545. doi: 10.1126/science.256.5056.545. [DOI] [PubMed] [Google Scholar]
- 3.Whiten A. Imitation of the sequential structure of actions by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998 Sep;112:270. doi: 10.1037/0735-7036.112.3.270. [DOI] [PubMed] [Google Scholar]
- 4.Kenward B, Rutz C, Weir AAS, Kacelnik A. Development of tool use in New Caledonian crows: inherited action patterns and social influences. Animal Behaviour. 2006 Dec;72:1329. [Google Scholar]
- 5.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965 Dec;22:770. [PubMed] [Google Scholar]
- 6.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001 Mar 30;291:2564. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 7.Ravbar P, Lipkind D, Parra LC, Tchernichovski O. Vocal exploration is locally regulated during song learning. J Neurosci. 2012 Mar 7;32:3422. doi: 10.1523/JNEUROSCI.3740-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price PH. Developmental Determinants of Structure in Zebra Finch Song. J Comp Physiol Psych. 1979;93:260. [Google Scholar]
- 9.Immelman K. Song development in the zebra finch and other estrilidid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge Umiversity Press; Cambridge: 1969. [Google Scholar]
- 10.Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982 Jun 1;207:344. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 11.Bauer EE, et al. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008 Feb 6;28:1509. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akutagawa E, Konishi M. New brain pathways found in the vocal control system of a songbird. J Comp Neurol. 2010 Aug 1;518:3086. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- 13.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994 Nov;14:6924. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008 Nov 13;456:189. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008 May 2;320:630. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- 16.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010 Feb 18;463:948. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts TF, Gobes SM, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci. 2012 Oct;15:1454. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005 Feb 5;62:231. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- 19.Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci. 2000 Jul 15;20:5420. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accorsi-Mendonca D, Leao RM, Aguiar JF, Varanda WA, Machado BH. Urethane inhibits the GABAergic neurotransmission in the nucleus of the solitary tract of rat brain stem slices. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292:R396. doi: 10.1152/ajpregu.00776.2005. [DOI] [PubMed] [Google Scholar]
- 21.Scotto-Lomassese S, Rochefort C, Nshdejan A, Scharff C. HVC interneurons are not renewed in adult male zebra finches. Eur J Neurosci. 2007 Mar;25:1663. doi: 10.1111/j.1460-9568.2007.05418.x. [DOI] [PubMed] [Google Scholar]
- 22.Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol. 2003 Mar;89:1688. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- 23.Raksin JN, Glaze CM, Smith S, Schmidt MF. Linear and nonlinear auditory response properties of interneurons in a high-order avian vocal motor nucleus during wakefulness. J Neurophysiol. 2012 Apr;107:2185. doi: 10.1152/jn.01003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosche G, Vallentin D, Long MA. Interplay of inhibition and excitation shapes a premotor neural sequence. J Neurosci. 2015 Jan 21;35:1217. doi: 10.1523/JNEUROSCI.4346-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005 Feb 23;25:1952. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipkind D, Tchernichovski O. Quantification of developmental birdsong learning from the subsyllabic scale to cultural evolution. Proc Natl Acad Sci U S A. 2011 Sep 13;108(Suppl 3):15572. doi: 10.1073/pnas.1012941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 28.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008 Jan 17;451:305. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 29.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 30.Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002 Sep 15;22:8084. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YT, Ma WP, Pan CJ, Zhang LI, Tao HW. Broadening of cortical inhibition mediates developmental sharpening of orientation selectivity. J Neurosci. 2012 Mar 21;32:3981. doi: 10.1523/JNEUROSCI.5514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pecka M, Han Y, Sader E, Mrsic-Flogel TD. Experience-dependent specialization of receptive field surround for selective coding of natural scenes. Neuron. 2014 Oct 22;84:457. doi: 10.1016/j.neuron.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010 Feb 26;327:1145. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.