Abstract
Echinoderm population collapses, driven by disease outbreaks and climatic events, may be important drivers of population dynamics, ecological shifts and biodiversity. The northeast Pacific recently experienced a mass mortality of sea stars. In Howe Sound, British Columbia, the sunflower star Pycnopodia helianthoides—a previously abundant predator of bottom-dwelling invertebrates—began to show signs of a wasting syndrome in early September 2013, and dense aggregations disappeared from many sites in a matter of weeks. Here, we assess changes in subtidal community composition by comparing the abundance of fish, invertebrates and macroalgae at 20 sites in Howe Sound before and after the 2013 sea star mortality to evaluate evidence for a trophic cascade. We observed changes in the abundance of several species after the sea star mortality, most notably a four-fold increase in the number of green sea urchins, Strongylocentrotus droebachiensis, and a significant decline in kelp cover, which are together consistent with a trophic cascade. Qualitative data on the abundance of sunflower stars and green urchins from a citizen science database show that the patterns of echinoderm abundance detected at our study sites reflected wider local trends. The trophic cascade evident at the scale of Howe Sound was observed at half of the study sites. It remains unclear whether the urchin response was triggered directly, via a reduction in urchin mortality, or indirectly, via a shift in urchin distribution into areas previously occupied by the predatory sea stars. Understanding the ecological implications of sudden and extreme population declines may further elucidate the role of echinoderms in temperate seas, and provide insight into the resilience of marine ecosystems to biological disturbances.
Keywords: Marine diseases, Starfish, Community shifts, Mass mortality, Environmental change, Sea star wasting syndrome, Echinoderm population
Introduction
Echinoderms can be subject to dramatic population fluctuations (Uthicke, Schaffelke & Byrne, 2009). Rapid declines are often driven by disease or extreme climatic events. For example, the spread of mass mortality of the black sea urchin, Diadema antillarum, in the 1980s suggests that it was most likely caused by a pathogen (Lessios, Robertson & Cubit, 1984). The event impacted an estimated 3.5 million km2 of the Caribbean region, causing up to 99% urchin mortality at some sites (Lessios, 1988). While the precipitous decline of Diadema was a unique occurrence, other echinoderm mass mortality events occur repeatedly. On the Atlantic coast of North America, an amoeboid parasite causes episodic mortality events in green sea urchins, Strongylocentrotus droebachiensis (Jones & Scheibling, 1985), which are linked to hurricanes and are predicted to increase in frequency with climate change (Scheibling & Lauzon-Guay, 2010). Similarly, recurring events of wasting disease involving asteroids (sea stars), echinoids (sea urchins) and holothurians (sea cucumbers) in the Channel Islands, California, are associated with climate regime shifts and extreme weather events (Engle, 1994; Eckert, Engle & Kushner, 2000).
Because sea stars and sea urchins play key ecological roles in many marine ecosystems, echinoderm population collapses can be important drivers of biodiversity, population dynamics and ecological shifts. In fact, the term ‘keystone predator’ was originally coined for the purple star, Pisaster ochraceus, after experiments showed that its absence led to significant decreases in intertidal biodiversity (Paine, 1966). Many other echinoderm species have since been shown to influence community composition through predation or herbivory. These effects are apparent on coral reefs following echinoderm population booms (e.g., coral cover declines owing to eruptive crown-of-thorns star, Acanthaster planci Sano et al., 1984) or busts (e.g., the transition from coral- to algae-dominated reefs following the D. antillarum mortality event Carpenter, 1990). On temperate rocky reefs, fluctuations in the abundance of herbivorous urchins can also result in major community shifts, from kelp forests to urchin barrens and back again (Estes & Duggins, 1995; Steneck et al., 2003).
The northeast Pacific region has recently experienced a protracted mass mortality of sea stars that might rival the magnitude of the Diadema die-off of the 1980s (Johnson, 2016). The event was first noticed on the Olympic coast of Washington in June 2013 (Hewson et al., 2014). In affected sea stars, the signs progress from a loss of turgor pressure, to lesions and ruptures of the body wall and autotomization of arms, and ultimately, disintegration and death (Fig. 1). The wasting syndrome has continued through 2014 and 2015, and has so far affected some 20 species from Alaska to Southern California (Stockstad, 2014). A virus may be involved (Hewson et al., 2014), but the precise causes and contributing factors remain poorly understood. Moreover, little is known so far of the extent and ecological consequences of this sea star mortality event at any location.
Figure 1. Progression of sea star wasting disease.
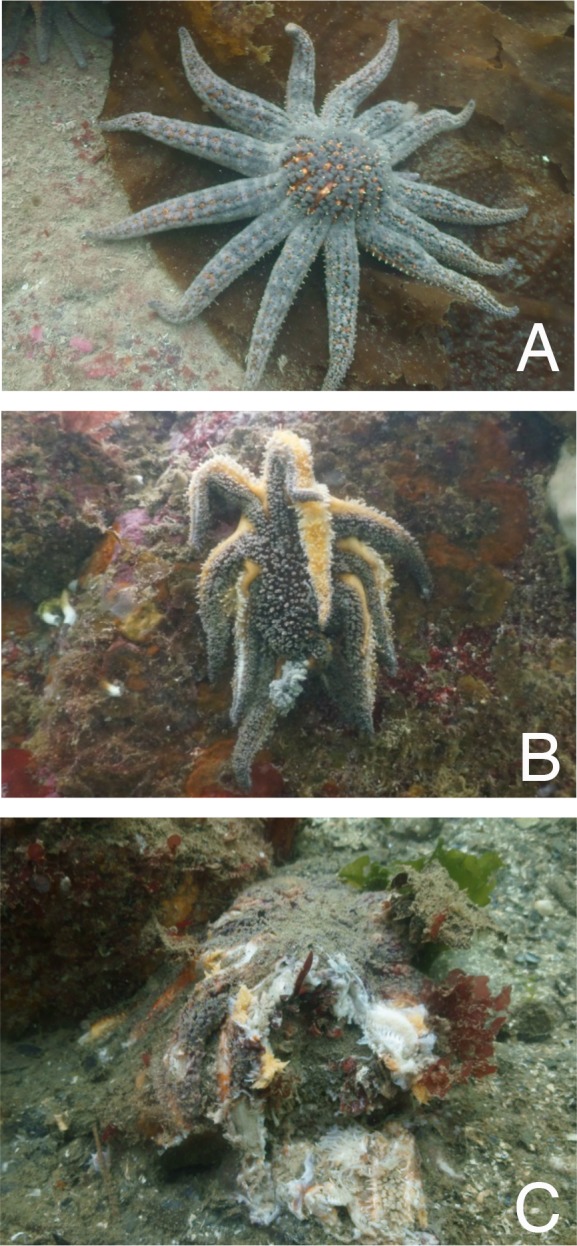
(A) A healthy-looking specimen of P. helianthoides moves across the kelp, Agarum fimbriatum. (B) Afflicted sea stars exhibit a loss of turgor pressure and body wall ruptures, followed by (C) limb autotomization, disintegration and death. Photos by Donna Gibbs.
Many of the affected sea stars were predatory species, raising the possibility of trophic cascades associated with their disappearance and marked community restructuring. In Howe Sound, southern Strait of Georgia, British Columbia, the sunflower star Pycnopodia helianthoides showed signs of advanced wasting in early September 2013. Dense aggregations disappeared from many sites in a matter of weeks (J Schultz, pers. obs., 2013). This species is one of the world’s largest predatory sea stars and it consumes a variety of prey, including echinoderms, gastropods and crustaceans (Herrlinger, 1983; Shivji et al., 1983). In areas that lack other predators such as sea otters Enhydra lutris, such as in Howe Sound, sunflower stars can become the dominant predator of urchins (Duggins, 1983). By altering the abundance and/or distribution of sea urchins, which in turn can have a conspicuous impact on the abundance of kelp, sunflower stars can influence the formation and persistence of urchin barrens, i.e., areas devoid of kelp because of the grazing activity of urchins (Duggins, 1981). Indeed, most well-substantiated examples of tri-trophic cascades in rocky subtidal ecosystems involve urchins as prey and major herbivore (Pinnegar et al., 2000). We therefore expected that Pycnopodia prey, in particular urchins, would increase in abundance following the disappearance of their major predator, leading to reductions in kelp cover.
Here, we evaluate the extent of mortality of P. helianthoides in Howe Sound and test whether changes in the benthic community following the rapid decline of this predatory sea star are consistent with the hypothesis of a trophic cascade. We compare rocky reef community composition before and after the mass mortality using quantitative data derived from subtidal transects and qualitative information gathered by citizen scientists. In doing so, we provide empirical evidence that a trophic cascade quickly followed what might be one of the largest wildlife die-off events ever recorded (Johnson, 2016).
Materials and Methods
Subtidal surveys
We compared sunflower star abundance and benthic community composition before (2009/2010) and after (2014) the 2013 wasting event using scuba-based surveys of 20 sites in Howe Sound, British Columbia (BC), Canada (Fig. 2). Surveys before the wasting event were conducted as part of a study of rockfish (Sebastes spp) habitat (Cloutier, 2011). We repeated these surveys after the wasting event using the same method, at the same GPS locations, depths (within 2 m) and time of year (within 14 days). Ten sites were surveyed in early summer (June–July) and 10 sites in late summer (August–October). In all surveys, we recorded the abundance of 18 taxa (species or species groups) of common benthic fishes and invertebrates (Table 1).
Figure 2. Rocky reef survey sites in Howe Sound, British Columbia.
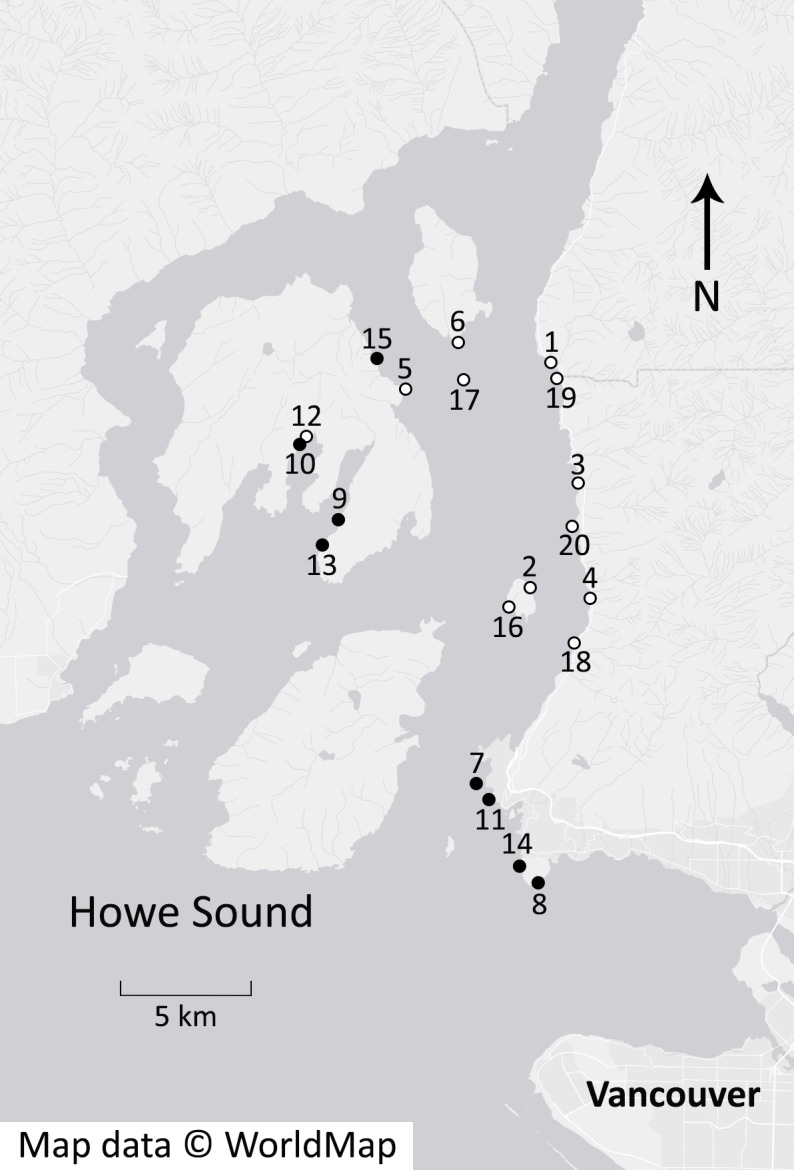
Benthic community composition was assessed at each of the 20 sites once in 2009 or 2010 and again in 2014. A mass mortality of sea stars occurred in the summer and fall of 2013 in this area. A site-level trophic cascade following the mortality was detectable at some sites (solid circles) but not others (open circles). (Map data © 2015 WorldMap).
Table 1. Taxa recorded during subtidal surveys in Howe Sound, British Columbia.
Mean density and standard deviation per 15 m2 are given for each taxon as recorded before and after the sea star mortality event.
| Taxon | Species or genera included in taxon | Mean density (SD) | |
|---|---|---|---|
| Before | After | ||
| Invertebrates | |||
| Sunflower star | Pycnopodia helianthoides Brandt, 1835 | 6.4 (11.4) | 0.9 (3.3) |
| Green urchin | Strongylocentrotus droebachiensis O. F. Müller, 1776 | 18.3 (41.0) | 77.2 (157.4) |
| Red urchin | Strongylocentrotus franciscanus Aggasiz, 1863 | 0.4 (0.9) | 0.3 (0.6) |
| White urchin | Strongylocentrotus pallidus G. O. Sars, 1871 | 1.1 (2.0) | 0.3 (0.4) |
| California cucumber | Parastichopus californicus Linnaeus, 1758 | 6.1 (9.0) | 13.1 (8.9) |
| Dungeness crab | Metacarcinus magister Dana, 1852 | 0.1 (0.2) | 0.0 |
| Red rock crab | Cancer productus Randall, 1839 | 0.1 (0.3) | 0.4 (0.7) |
| Spot prawn | Pandalus platyceros Brandt, 1851 | 22.1 (89.1) | 0.3 (0.8) |
| Squat lobster | Munida quadrispina Benedict, 1902 | 4.0 (9.0) | 0.3 (0.6) |
| Miscellaneous crabs | Primarily anomurans, including lithode and hermit crabs; several brachyuran genera including Cancer, Pugettia, Scyra, and Oregonia | 21.7 (35.0) | 16.3 (23.5) |
| Miscellaneous shrimps | Primarily Pandalus danae Stimpson, 1857, but also other members of the genus Pandalus, as well as the genera Lebbeus, Eualus, Heptocarpus and possibly others | 37.0 (38.6) | 15.8 (11.2) |
| Giant Pacific octopus | Enteroctopus dofleini Wülker, 1910 | 0.1 (0.2) | 0.0 |
| Cup corals | Balanophyllia elegans Verrill, 1864, Caryophyllia alaskensis Vaughan, 1941 | 6.7 (15.8) | 22.1 (19.0) |
| Benthic fishes | |||
| Grunt sculpin | Rhamphocottus richardsonii Günther, 1974 | 0.1 (0.2) | 0.1 (0.2) |
| Longfin sculpin | Jordania zonope Starks, 1895 | 0.2 (0.4) | 2.7 (4.1) |
| Sailfin sculpin | Nautichthys oculofasciatus Girard, 1858 | 0.1 (0.2) | 0.0 |
| Scalyhead sculpin | Artedius harringtoni Starks, 1896 | 0.8 (1.6) | 1.8 (2.3) |
| Miscellaneous sculpins | Cottid genera including Artedius, Orthanopias, Oligocottus, Radulinus, Chitonotus and possibly others. | 5.5 (4.6) | 0.7 (1.2) |
At each site we surveyed four transects (25 m long by 4 m wide) at depths between 8 and 15 m (chart datum). We quantified fish and invertebrate abundance by counting all individuals of the target taxa occurring fully or partly within 0.25 m2 quadrats placed at 15 random positions along each transect. We also estimated visually the percent cover of kelp (mainly the genera Agarum, Costaria, Laminaria and Saccharina) within the same quadrats.
Citizen-contributed (REEF) surveys
To verify that the patterns of echinoderm abundance detected at our 20 study sites reflected local trends accurately, we compiled qualitative data on the abundance of sunflower star and green urchin in Howe Sound and adjacent Indian Arm, east of Vancouver, from the Reef Environmental Education Foundation (REEF; REEF, 2014) citizen science database. Through REEF, scuba divers are trained in species identification and collect data on abundance of species sighted during recreational dives. Divers assign an abundance score from 1–4 to each species they can positively identify: score 1 = a single individual, 2 = 2–10 individuals, 3 = 11–100 individuals and 4 = >100 individuals. Species with no abundance score were assumed to be absent, which we deemed to be a fair assumption given that our target taxa were easy to identify.
We extracted the abundance scores of sunflower stars and green sea urchins for all REEF surveys submitted between January 1, 2010 and November 1, 2014 in Washington and BC. To depict trends in abundance over time, we plotted 60-day running averages of the abundance scores for both species. Missing values were filled in using linear interpolation.
Data analyses
We used linear mixed-effects models in the R statistical platform (nlme package; Pinheiro et al., 2015) to compare sunflower star abundance, green urchin abundance and kelp cover before and after the sea star mortality. We obtained sunflower and green urchin abundance for each transect by summing the number of sunflower stars and, separately, green urchins across all quadrats and log-transforming the values prior to analysis. Kelp cover was averaged across all quadrats within each transect. In all cases, we included ‘site’ as a random effect, and verified the assumptions of normally distributed residuals, homoscedasticity and the absence of leverage by visually examining quantile, residual vs. fitted and Cook’s distance diagnostic plots, respectively.
To depict graphically site-level changes in the abundance of sunflower stars, green sea urchins and kelp, we plotted the relative difference in abundance for each group at each site. Relative abundance was calculated as the abundance after the mortality event minus the abundance prior to it divided by the mean abundance for both time periods. Abundance was calculated as the total count of each species at each site for sunflower stars and green urchins, and as the average percent cover at each site for algae.
To compare overall benthic community composition before and after the sea star mortality, we ran a permutation-based, non-parametric analysis of similarity (ANOSIM; Clarke, 1993) using PRIMER (v. 1.0.3; Clarke & Gorley, 2006). Abundance matrices (species by site) were compiled for each period (i.e., pre- and post-mortality), in which abundance was estimated as the total count of each taxon across transects and/or quadrats at each site. The raw data were square-root-transformed to reduce the influence of very abundant or very rare species. Bray-Curtis similarity coefficients were computed between pairs of sites (Clarke & Warwick, 2001). The ANOSIM procedure was carried out on the similarity matrix. ANOSIM generates an R statistic, which varies between 0 (samples are as similar across groups as they are within group) and 1 (all samples within groups are more similar to each other than to any sample across groups) and is tested for difference from zero with a permutation test (in this study, N = 999 permutations). The differences in benthic assemblages were visualized in a non-metric, multidimensional scaling (MDS) plot in which samples that are more similar in community composition appear closer together than more dissimilar samples. Stress values of <0.1 suggest that distances among samples in an MDS plot accurately reflect the extent of community differences (Clarke & Warwick, 2001). Finally, we conducted an analysis of similarity percentages (SIMPER) to identify the main taxa responsible for any differences observed between pre- and post-mortality assemblages. We considered a taxon to be important to community differences if its individual contribution was 11% or more, which is twice the expected value if dissimilarity contributions were evenly distributed among all taxa in the analysis (i.e.,100 percent divided by 18 taxa, multiplied by 2). The SIMPER analysis also includes an indication of evenness, expressed as a consistency ratio (CR). CR is the average dissimilarity contribution of a taxon divided by the standard deviation in dissimilarity values of that taxon, for each time period. CR values greater than one suggest that the taxon contributed to dissimilarity between time periods equally across all sites (Terlizzi et al., 2005).
Results
Sea star mortality
At our monitored sites, the abundance of sunflower stars declined by 89% ± 29% (mean ± SD), from an average of 0.42 (±0.76) sunflower stars per m2 before the mortality event to 0.06 (±0.22) individuals per m2 after it (LME: t = 4.62, df = 139, p < 0.0001; Fig. 3). Three sites had no sunflower stars in 2009/2010, and were not included in the percent decline calculation. All 17 sites with sunflower stars in 2009/2010 had fewer sunflower stars in 2014.
Figure 3. Mortality of sea stars, and subsequent change in urchin abundance and kelp cover after sea star mortality.
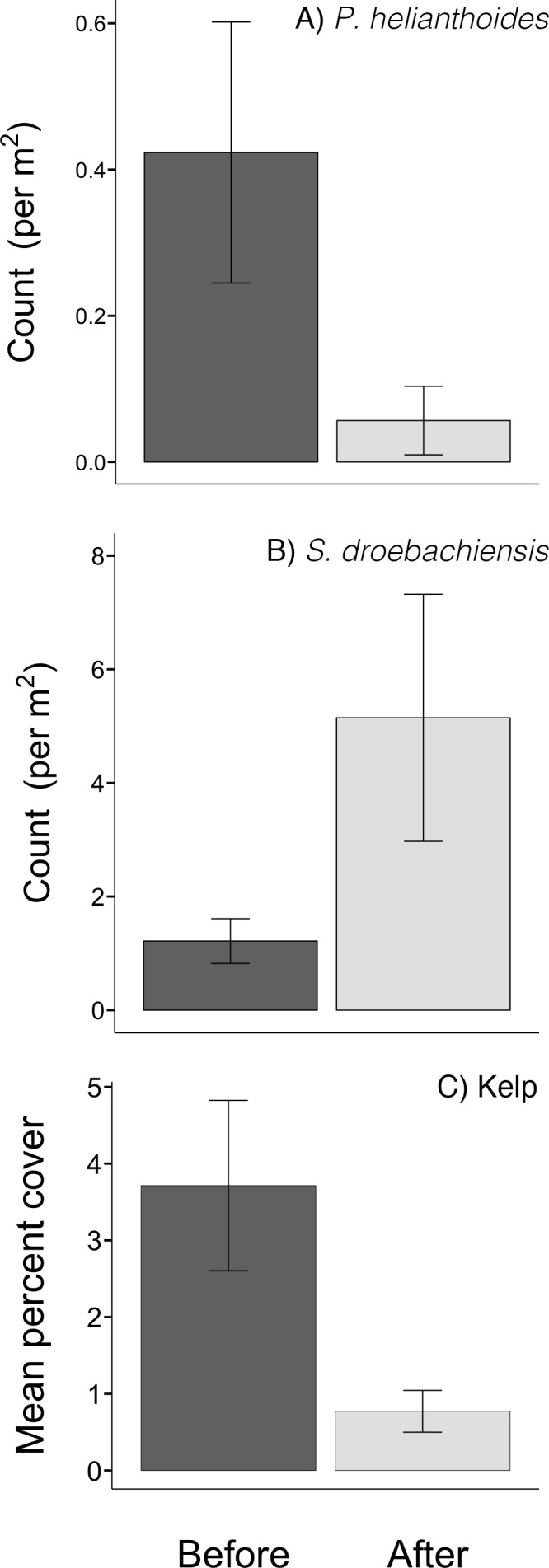
Mean abundance (per m2) of (A) sunflower stars and (B) green sea urchins, and (C) percent cover of kelp on rocky reefs in Howe Sound, British Columbia, on 80 transects before and after the mass mortality of sea stars in 2013. Error bars represent standard error. The dominant kelp was the sea colander kelp, Agarum fimbriatum.
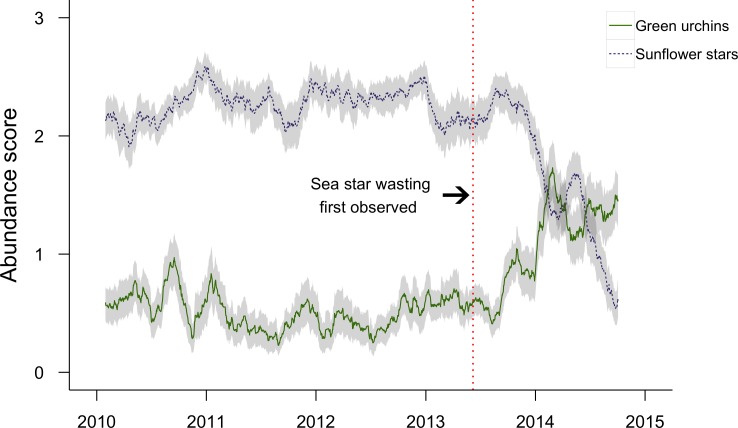
The REEF data included 1,568 surveys carried out at 28 sites broadly distributed across BC and Washington between 2010 and 2014. Although sunflower stars were sighted on 98% of surveys in the years before the mortality event and on 89% of surveys in the years afterward, a marked decline was evident in their abundance score trajectory (Fig. 4). At this larger geographic scale, sunflower stars started declining in approximately the third week of September, some 15 weeks after the first report of sea star wasting in the region.
Figure 4. Sunflower star and green sea urchin abundance trajectories.
Sixty-day running average abundance scores for green sea urchins (Strongylocentrotus droebachiensis; green solid line) and sunflower stars (Pycnopodia helianthoides; purple dashed line) recorded in REEF surveys from January 2010 to November 2014 in Washington and British Columbia (n = 1568 surveys). Grey bands indicate 95% confidence intervals of the running average. The vertical red dotted line indicates the date of the first recorded observation of sea star wasting syndrome (7 June 2013), which was on the Olympic coast of WA.
We were unable to detect a geographic pattern in the spread of the sea stars’ mortality in our study area because of the speed at which the sea star wasting progressed. It was first observed in Howe Sound (at Whytecliff Park; , ) on 2 September, 2013, and we then noted it at all of our study sites the following month.
Benthic community composition
There was a significant shift in overall community composition following sea star mortality in Howe Sound (ANOSIM: R = 0.326, p = 0.001; Fig. 5), and many species changed in abundance from one period to the next (Table 1). The community shift was largely driven by an increase in abundance of green urchins (Table 2). Green urchin abundance quadrupled after the near-disappearance of sunflower stars (LME: t = − 3.10, df = 139, p = 0.0023; Fig. 3). This trend is supported by the REEF surveys, although these qualitative data suggest that green urchin numbers began increasing in the first week of September, two to three weeks before the detectable onset of sea star decline (Fig. 4). There was also an increase in the abundance of cup corals, while the numbers of small shrimps and crabs decreased (Table 2). Cumulatively, these four taxa accounted for nearly two-thirds (62%) of the dissimilarity in benthic community composition before and after the sea star mortality, and their contributions were consistent across sites (CRs > 1; Table 2). Despite their marked decline, sunflower stars did not contribute disproportionately to the dissimilarity between time periods (SIMPER; individual contribution to dissimilarity = 7.15%). Overall, within-year similarity was higher after than before sea stars died (SIMPER; average inter-site similarity before = 46.28%, after = 58.11%; Fig. 5), suggesting that communities became more homogeneous following the sea star mortality.
Figure 5. Rocky reef species assemblages before and after sea star mortality.
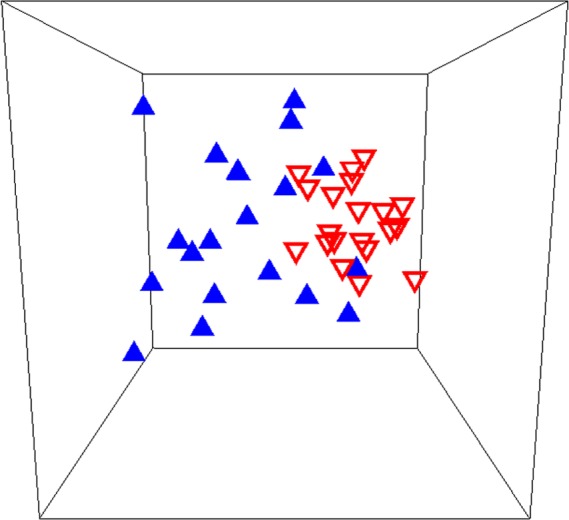
Multidimensional scaling plot of benthic community composition on rocky reefs before (blue triangles) and after (inverted red triangles) the 2013 sea star mass mortality event in Howe Sound, British Columbia. The analysis included 18 fish and invertebrate taxa at 20 sites, surveyed both in 2009/2010 and 2014. The associated stress value (0.13) suggests some distortion in the multivariate representation of the data.
Table 2. Differences in pre- and post-mortality benthic assemblages.
The four taxa that contributed disproportionately to dissimilarity in benthic community composition on rocky reefs before and after the 2013 sea star mass mortality, as well as the focal sea star, Pycnopodia helianthoides. Mean densities (# per 30 m2 ± 1 SD), consistency ratios, and individual and cumulative contributions (in %) to differences between years are shown. The consistency ratio is calculated as a species’ average dissimilarity contribution divided by the standard deviation of dissimilarity values. A consistency ratio > 1 indicates an even contribution to community dissimilarity across sites. The analysis was conducted on square-root-transformed data (see Methods) but untransformed densities are presented here.
| Taxon | Mean density (SD) | Consistency ratio | Individual contribution | Cumulative contribution | |
|---|---|---|---|---|---|
| Before | After | (%) | (%) | ||
| Strongylocentrotus droebachiensis | 18.3 (41.0) | 77.2 (157.5) | 1.09 | 18.91 | 18.91 |
| Cup corals | 6.7 (15.8) | 22.2 (19.1) | 1.41 | 13.04 | 31.95 |
| Misc. shrimps | 37.0 (38.7) | 15.9 (11.2) | 1.3 | 11.29 | 43.23 |
| Misc. crabs | 21.7 (35.0) | 16.3 (23.5) | 1.05 | 11.15 | 54.38 |
| Pycnopodia helianthoides | 6.4 (11.4) | 0.9 (3.3) | 1.18 | 7.15 | 69.05 |
In addition to shifts in benthic animal community composition, there was also a change in the abundance of kelp. Kelp cover decreased from 4% (±10%) in 2009/2010 to <1% (±2%) in 2014 (LME: t = 2.669, df = 139, p = 0.0085; Fig. 3). In all years, the kelp at our sites was almost exclusively the sea colander kelp, Agarum fimbriatum, but also included Saccharina latissima.
At the regional scale, the changes in abundance of sunflower stars (decline), green urchins (increase) and kelp (decline) were consistent with a trophic cascade (Fig. 3). At the site level, the patterns were more variable (Fig. 6). Eleven of the 17 sites that had some P. helianthoides before the sea star mortality showed increases in green urchin abundance concomitant with declines in sea star abundance (Fig. 6). Eight of these 17 sites showed declines in kelp cover concomitant with increases in green urchin abundance (Fig. 6). A clear alternation of population trajectories from predators to herbivores to kelp was clear at eight of the 16 sites (Fig. 6).
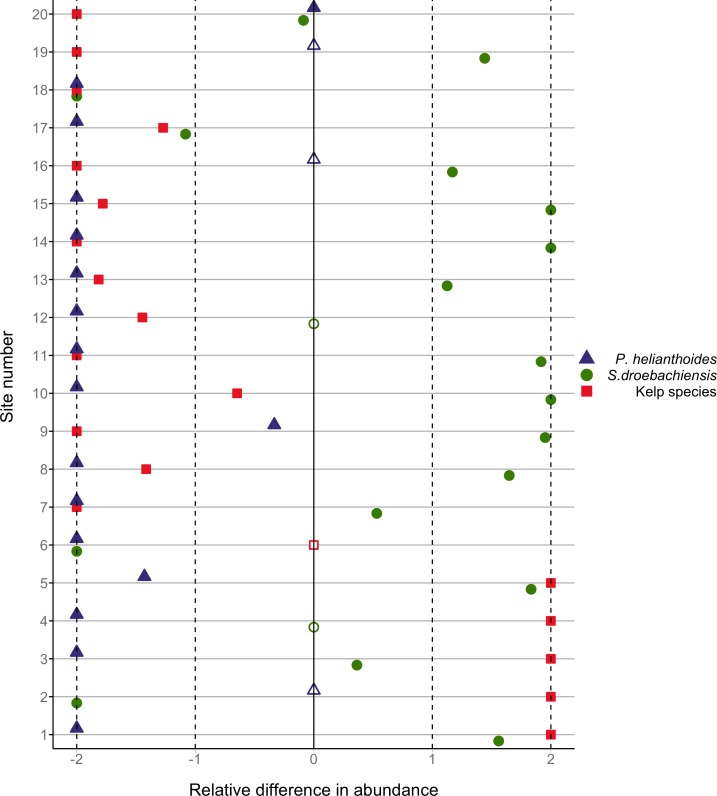
Figure 6. Relative difference in abundance of sea stars, urchins and kelp by site.
The relative difference in total count of sunflower stars (blue triangles) and green urchins (green circles), and the relative difference in the mean percent cover of algae (red squares) before and after the sea star mass mortality. Open symbols indicate sites where population density was zero both before and after the mass mortality. Relative difference was calculated as the change in abundance divided by the mean abundance of both time periods. A relative difference of −2 indicates the population declined to zero. Sites are numbered chronologically according to the order in which they were surveyed, from June to August, 2014. The geographic location of these sites is shown in Fig. 2.
Discussion
The wasting disease that affected echinoderms in the northeast Pacific in 2013/2014 heavily impacted populations of sunflower stars, the sea stars that formerly dominated subtidal communities. We found a noticeable shift in benthic community structure following the sea star decline. Community changes were largely driven by changes in the abundance of green sea urchins, cup corals, shrimps and crabs. The temporal coincidence of the alternating trajectories of abundance of sea stars, urchins and kelp, as well as the overlapping distributions and documented trophic linkages among these three taxa, meet the diagnostic criteria of a tri-trophic cascade (Grubs et al., 2016), triggered by the mass mortality of predatory sunflower stars.
Sunflower star densities declined by almost 90%, on average, at our sites in Howe Sound, BC. Such a decline in sea stars rivals the largest magnitudes reported for disease-induced echinoderm mass mortalities (e.g., 70% of Strongylocentrotus droebachiensis in Nova Scotia Scheibling & Stephenson, 1984; 95% of S. franciscanusin California Pearse et al., 1977; 97% of Diadema antillarum across the Caribbean Lessios, 1988). The percent change in biomass of P. helianthoides must be even greater than the change in relative abundance because the sea stars we observed following the mortality event were almost exclusively juveniles (<6 cm diameter). The very large individuals (>50 cm diameter) present before the mortality event likely played a larger role in structuring benthic communities than the juveniles present after the event. The steep decline in sunflower star numbers, occurring some 15 weeks after the first official sighting of sea star wasting, was clearly evident in the qualitative density scores generated by citizen science (REEF) surveys, which covered a broader geographic area. The time-series of REEF data suggests that sunflower star population levels were somewhat variable, perhaps reflecting variation in the sites surveyed by divers, but largely stable between the first snapshot in 2009/2010 and the onset of the wasting event in 2013. More generally, the benthic species composition of the Strait of Georgia region has remained remarkably stable in recent decades, even in the face of climate regime shifts (Marliave et al., 2011). It therefore seems unlikely that the sea star population declines, and concomitant changes in benthic community composition, could be ascribed to a different, unreported disturbance occurring prior to 2013.
The most striking change we observed in community composition was a marked increase in the abundance of green urchins. Overall, green urchins were nearly four times more numerous following the sea star mortality event than before. However, the mechanism of this population increase remains unclear. One possibility is that a recruitment pulse of green urchins coincided with sea star wasting disease, which would have generated a large urchin cohort even in the presence of sunflower stars. Another possibility is that urchin recruits—whether part of a normal or a large cohort—were able to survive better in the absence of abundant sea star predators (Duggins, 1981). The size (3–5 cm diameter) of the majority of urchins present a year following the sea star mortality makes this explanation perhaps unlikely. Green urchins of this size on the east coast of North America are at least three years of age, and possibly more than a decade old (Russel, Ebert & Petraitis, 1998; Vadas et al., 2000). If these growth rates are similar on the Pacific coast, then most of the urchins we saw could have settled several years before the sea star mortality event. However, urchin growth rates can be highly variable (Vadas et al., 2000), depending on food supply and temperature (Thompson, 1983; Meidel & Scheibling, 1999; Pearce et al., 2005), Urchin growth rates have not yet been estimated in BC. A third possible explanation is that the observed increase in urchin abundance resulted from a shift in urchin behaviour following the sea star mortality event. The impact of ‘intimidation’ on predator–prey interactions can be as important as direct consumption (Lima & Dill, 1990; Preisser, Bolnick & Benard, 2005). Under risk of predation, prey individuals alter a suite of behaviours, including habitat choice, foraging range, and time under cover (Werner et al., 1983; Peacor & Werner, 2001; Trussell, Ewanchuk & Bertness, 2003; Schmitz, Krivan & Ovadia, 2004). The effect of sunflower stars on urchin behaviour is well documented. In field experiments in Alaska, both green and purple (S. purpuratus) urchins moved away after P. helianthoides arms were placed in the centre of urchin aggregations (Duggins, 1981), and urchin distribution shifted rapidly when sea star abundance was experimentally increased (Duggins, 1983). Fear-released urchins could therefore respond by moving from refuges, perhaps in very shallow or deep habitats or in sheltered crevices inaccessible to sea stars (and divers), to more open substrates, making them easier to see and count.
The data from REEF surveys support a behavioural rather than a consumptive mechanism for the increase in urchin numbers. Whereas one would expect a delayed increase in urchin numbers following a release from predation (Wangersky & Cunningham, 1957), green urchin numbers began to increase at approximately the same time as the decline in sunflower stars was evident (Fig. 4). The observed change in green urchin abundance may therefore be due, at least in part, to green urchins modifying their distribution in response to the decline of sunflower stars.
Another conspicuous change we observed was a ∼80% reduction in kelp cover (Fig. 3), pointing to a potential trophic cascade triggered by the sea star mortality event. There are many documented examples of urchin abundance directly influencing the abundance of algae (e.g., Fletcher, 1987; Carpenter, 1990; Estes & Duggins, 1995; McClanahan et al., 1996; Palacin et al., 1998; Scheibling, Hennigar & Balch, 1999; Villouta et al., 2001). As urchin numbers rise, either due to a large recruitment event (Hart & Scheibling, 1988) or the absence of a predator (Watson & Estes, 2011), kelp is rapidly depleted. The alternating directions of population trends of sea stars, urchins and kelp observed here are consistent with the hypothesis of a trophic cascade triggered by the sea star disease. The tri-trophic cascade was clearly evident at the larger scale of Howe Sound (Fig. 3), but detectable at only half of the sites, with a few additional sites showing only part of the cascade (Fig. 6). It is notable that the sites surveyed earliest (i.e., sites 1–5 on Figs. 2 and 6) showed an increase in kelp cover, perhaps because not enough time had passed for changes to take place. At other sites where the trophic cascade was not detectable, it is possible that urchins moved elsewhere in search of better food sources (e.g., at sites 17 and 18 on Fig. 6), or that the presence of juvenile sea stars (i.e., site 20 on Fig. 6) resulted in different trophic interactions.
In contrast to green urchins, the abundance of many prey species did not increase in the near-absence of sunflower star predators. For example, there was no change in the abundance of red urchins (S. franciscanus) and white urchins (S. pallidus). Neither species is common in Howe Sound, and little is known about the ecology of S. pallidus. However, S. franciscanus may generally be less susceptible to sea star predation than other urchin species because they grow too large to be consumed (Duggins, 1981). Moreover, although crustaceans constitute a significant portion of the diet of sunflower stars (Shivji et al., 1983; Estes & Duggins, 1995; Lambert, 2000), shrimps and crabs declined following the sea star mortality. Several of the crustaceans we monitored use kelp for both food and habitat. The spot prawn, Pandalus platyceros, for instance, specifically uses sea colander kelp as nursery habitat (Marliave & Roth, 1995). The decline of some crustacean taxa could result from the reduced kelp cover and therefore be a fourth step in the cascade documented here.
Another fourth link in the ecological cascade triggered by sea star mortality might involve cup corals. Their increase in abundance was surprising as cnidarians are not normally consumed by P. helianthoides (Shivji et al., 1983; Herrlinger, 1983). However, cup corals are known to fare poorly in areas dominated by macroalgae (Fadlallah, 1983). Contact with algae causes coral polyp retraction, which in turn allows overgrowth by filamentous and coralline algae (Coyer et al., 1993). Increases in density of cup corals can be swift (<1 year), and of the magnitude observed here (3–4 times), after algae disappear (Coyer et al., 1993). Of course, the reduced abundance of kelp and of sea stars may also have allowed for a less obstructed view of the substrate by the observers. As a number of taxa were not monitored in this study, there were likely other changes following the sea star mortality event that we did not detect.
In conclusion, our study contributes to understanding the ecological consequences of the northeast Pacific sea star mass mortality. The most notable change was a marked increase in the number of green sea urchins, which might have already had trickle-down effects on other levels of the ecosystem by the time we detected it. It is unclear whether the changes observed will persist as long-term consequences of the near-disappearance of sea stars. Nonetheless, further monitoring will help elucidate the resilience of this ecosystem in the face of acute biological disturbances. Although such a sudden and drastic decline in sea star populations is alarming, it provides a large-scale natural experiment that may advance our understanding of subtidal trophic cascades and invertebrate population dynamics.
Supplemental Information
Acknowledgments
We thank the Vancouver Aquarium Marine Science Centre for all aspects of this study, and in particular the Howe Sound Research team: Jeff Marliave, Donna Gibbs, Laura Borden, and Boaz Hung. Thank you to volunteer divers Roya Esragh, Marielle Wilson, Brian Caron, Justin Lisaingo, Crystal Kulstar, and Alex Clegg. Comments by Jeff Marliave, Alejandro Frid, Jane Williamson and one anonymous reviewer greatly improved the manuscript.
Funding Statement
Jessica Schultz received funding through the Vancouver Aquarium Howe Sound Research Program. Ryan N. Cloutier received funding through the Canadian Healthy Oceans Network. Isabelle M. Côté received funding through a Discovery grant of the Natural Sciences and Engineering Research Council of Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jessica A. Schultz conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Ryan N. Cloutier performed the experiments, wrote the paper, reviewed drafts of the paper.
Isabelle M. Côté conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Data S1 and the code has been supplied as a Supplemental Information 1.
References
- Carpenter (1990).Carpenter RC. Mass mortality of Diadema antillarum. Marine Biology. 1990;104:67–77. doi: 10.1007/BF01313159. [DOI] [Google Scholar]
- Clarke (1993).Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecology. 1993;18:117–143. [Google Scholar]
- Clarke & Gorley (2006).Clarke KR, Gorley RN. PRIMER version 6: user manual/tutorial. Plymouth: Primer-E Limited; 2006. [Google Scholar]
- Clarke & Warwick (2001).Clarke KR, Warwick RM. PRIMER version 5: user manual/tutorial. Plymouth: Primer-E Limited; 2001. [Google Scholar]
- Cloutier (2011).Cloutier RN. MSc Thesis. 2011. Direct and indirect effects of marine protection: Rockfish Conservation Areas as a case study. [Google Scholar]
- Coyer et al. (1993).Coyer JA, Ambrose RF, Engle JM, Carroll JC. Interactions between corals and algae on a temperate zone rocky reef: mediation by sea urchins. Journal of Experimental Marine Biology and Ecology. 1993;167(1):21–37. doi: 10.1016/0022-0981(93)90181-M. [DOI] [Google Scholar]
- Duggins (1981).Duggins DO. Interspecific facilitation in a guild of benthic marine herbivores. Oecologia. 1981;48:157–163. doi: 10.1007/BF00347958. [DOI] [PubMed] [Google Scholar]
- Duggins (1983).Duggins DO. Starfish predation and the creation of mosaic patterns in a kelp-dominated community. Ecology. 1983;64:1610–1619. doi: 10.2307/1937514. [DOI] [Google Scholar]
- Eckert, Engle & Kushner (2000).Eckert GL, Engle JM, Kushner DJ. Sea star disease and population declines at the Channel Islands. Proceedings of the fifth California Islands symposium; 2000. pp. 390–393. [Google Scholar]
- Engle (1994).Engle JM. Perspectives on the structure and dynamics of nearshore marine assemblages of the California Channel Islands. Proceedings of the fourth California Islands symposium: update on the status of resources; 1994. pp. 13–26. [Google Scholar]
- Estes & Duggins (1995).Estes JA, Duggins DO. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs. 1995;65(1):75–100. doi: 10.2307/2937159. [DOI] [Google Scholar]
- Fadlallah (1983).Fadlallah YH. Population dynamics and life history of a solitary coral, Balanophyllia elegans, from central California. Oecologia. 1983;58(2):200–207. doi: 10.1007/BF00399217. [DOI] [PubMed] [Google Scholar]
- Fletcher (1987).Fletcher WJ. Interactions among subtidal Australian sea urchins, gastropods and algae: effects of experimental removals. Ecological Monographs. 1987;57(1):89–109. doi: 10.2307/1942640. [DOI] [Google Scholar]
- Grubs et al. (2016).Grubs RD, Carlson JK, Romine JG, Curtis TH, McElroy WD, McCandless CT, Cotton CF, Musick JA. Critical assessment and ramifications of a purported marine trophic cascade. Scientific Reports. 2016;6:20970. doi: 10.1038/srep20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart & Scheibling (1988).Hart MW, Scheibling RE. Heat waves, baby booms, and the destruction of kelp beds by sea urchins. Marine Biology. 1988;99:167–176. doi: 10.1007/BF00391978. [DOI] [Google Scholar]
- Herrlinger (1983).Herrlinger TJ. M.A. Thesis. 1983. The diet and predator–prey relationships of the sea star Pycnopodia helianthoides (Brandt) from a central California kelp forest. [Google Scholar]
- Hewson et al. (2014).Hewson I, Button JB, Gudenkauf BM, Miner B, Newton AL, Gaydos JK, Wynne J, Groves CL, Hendler G, Murray M, Fradkin S, Breitbart M, Fahsbender E, Lafferty KD, Kilpatrick AM, Miner CM, Raimondi P, Lahner L, Friedman CS, Daniels S, Haulena M, Marliave J, Burge CA, Eisenlord ME, Harvell CD. Densovirus associated with sea-star wasting disease and mass mortality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17278–17283. doi: 10.1073/pnas.1416625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson (2016).Johnson L. Sea star wasting disease among worst wildlife die-offs say scientists. CBC News. 2016 Available at http://www.cbc.ca/news/canada/british-columbia/sea-star-wasting-die-off-1.3414607 . [Google Scholar]
- Lambert (2000).Lambert P. Sea stars of British Columbia, Southeast Alaska, and Puget Sound. Vancouver: Royal British Columbia Museum, UBC Press; 2000. [Google Scholar]
- Lessios (1988).Lessios HA. Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annual Review of Ecology and Systematics. 1988;19:371–393. doi: 10.1146/annurev.es.19.110188.002103. [DOI] [Google Scholar]
- Lessios, Robertson & Cubit (1984).Lessios HA, Robertson DR, Cubit JD. Spread of Diadema mass mortality through the Caribbean. Science. 1984;226:335–337. doi: 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- Lima & Dill (1990).Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology. 1990;68:619–640. doi: 10.1139/z90-092. [DOI] [Google Scholar]
- Marliave et al. (2011).Marliave JB, Gibbs CJ, Gibbs DM, Lamb AO, Young SJF. Biodiversity stability of shallow marine benthos in Strait of Georgia, British Columbia, Canada through climate regimes, overfishing and ocean acidification. In: Grillo O, Verona G, editors. Biodiversity loss in a changing planet. Rijeka: InTech; 2011. pp. 49–74. [Google Scholar]
- Marliave & Roth (1995).Marliave JB, Roth M. Agarum kelp beds as nursery habitat of spot prawns, Pandalus platyceros Brandt, 1851 (Decapoda, Caridea) Crustaceana. 1995;68:27–37. doi: 10.1163/156854095X01132. [DOI] [Google Scholar]
- McClanahan et al. (1996).McClanahan TR, Kamukuru AT, Muthiga NA, Gilagabher Yebio M, Obura D. Effect of sea urchin reductions on algae, coral and fish populations. Conservation Biology. 1996;10(1):136–154. doi: 10.1046/j.1523-1739.1996.10010136.x. [DOI] [Google Scholar]
- Meidel & Scheibling (1999).Meidel SK, Scheibling RE. Effects of food type and ration on reproductive maturation and growth of the sea urchin Strongylocentrotus droebachiensis. Marine Biology. 1999;134:155–166. doi: 10.1007/s002270050534. [DOI] [Google Scholar]
- Paine (1966).Paine RT. Food web complexity and species diversity. The American Naturalist. 1966;100:65–75. doi: 10.1086/282400. [DOI] [Google Scholar]
- Palacin et al. (1998).Palacin C, Giribet G, Carner S, Dantart L, Turon X. Low densities of sea urchins influence the structure of algal assemblages in the western Mediterranean. Journal of Sea Research. 1998;39:281–290. doi: 10.1016/S1385-1101(97)00061-0. [DOI] [Google Scholar]
- Peacor & Werner (2001).Peacor SD, Werner EE. The contribution of trait-mediated indirect effects to the net effects of a predator. Proceedings of the National Academy of Sciences of Sciences of the United States of America. 2001;98:3904–3908. doi: 10.1073/pnas.071061998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce et al. (2005).Pearce CM, Williams SW, Yuan F, Castell JD, Robinson SMC. Effect of temperature on somatic growth and survivorship of early post-settled green sea urchins, Strongylocentrotus droebachiensis (Müller) Aquaculture Research. 2005;36:600–609. doi: 10.1111/j.1365-2109.2005.01264.x. [DOI] [Google Scholar]
- Pearse et al. (1977).Pearse JS, Costa DP, Yellin MB, Agegian CR. Localized mass mortality of red sea urchin, Strongylocentrotus franciscanus near Santa Cruz, California. Fishery Bulletin. 1977;75(3):645–648. [Google Scholar]
- Pinheiro et al. (2015).Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Development Team . nlme: Linear and Nonlinear Mixed Effects Models. 2015. R-project. org/package=nlme. R package version. [Google Scholar]
- Pinnegar et al. (2000).Pinnegar JK, Polunin NVC, Francour P, Badalamenti F, Chemello R, Harmelin-Vivien M-L, Hereu B, Milazzo M, Zabala M, D’Anna G, Piptone C. Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environmental Conservation. 2000;27(2):179–200. doi: 10.1017/S0376892900000205. [DOI] [Google Scholar]
- Preisser, Bolnick & Benard (2005).Preisser E, Bolnick D, Benard M. The high cost of fear: behavioral effects dominate predator–prey interactions. Ecology. 2005;86:501–509. doi: 10.1890/04-0719. [DOI] [Google Scholar]
- REEF (2014).REEF . Reef environmental education foundation. World Wide Web electronic publication; 2014. Available at www.reef.org (accessed 19 November 2014) [Google Scholar]
- Russel, Ebert & Petraitis (1998).Russel MP, Ebert TA, Petraitis PS. Field estimates of growth and mortality of the green sea urchin, Strongylocentrotus droebachiensis. Ophelia. 1998;48:137–153. doi: 10.1080/00785236.1998.10428681. [DOI] [Google Scholar]
- Scheibling, Hennigar & Balch (1999).Scheibling RE, Hennigar AW, Balch T. Destructive grazing, epiphytism, and disease: the dynamics of sea urchin-kelp interactions in Nova Scotia. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:2300–2314. doi: 10.1139/f99-163. [DOI] [Google Scholar]
- Scheibling & Lauzon-Guay (2010).Scheibling RE, Lauzon-Guay J-S. Killer storms: North Atlantic hurricanes and disease outbreaks in sea urchins. Limnology and Oceanography. 2010;55:2331–2338. [Google Scholar]
- Scheibling & Stephenson (1984).Scheibling RE, Stephenson RL. Mass mortality of Strongylocentrotus droebachiensis (Echinodermata: Echinoidea) off Nova Scotia, Canada. Marine Biology. 1984;78:153–164. doi: 10.1007/BF00394695. [DOI] [Google Scholar]
- Schmitz, Krivan & Ovadia (2004).Schmitz OJ, Krivan V, Ovadia O. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecology Letters. 2004;7:153–163. doi: 10.1111/j.1461-0248.2003.00560.x. [DOI] [Google Scholar]
- Shivji et al. (1983).Shivji M, Parker D, Hartwick B, Smith MJ, Sloan NA. Feeding and distribution study of the sunflower sea star Pycnopodia helianthoides (Brandt, 1835) Pacific Science. 1983;37:133–140. [Google Scholar]
- Steneck et al. (2003).Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation. 2003;29(4):436–459. [Google Scholar]
- Stockstad (2014).Stockstad E. Death of the stars. Science (New York) 2014;344:464–467. doi: 10.1126/science.344.6183.464. [DOI] [PubMed] [Google Scholar]
- Thompson (1983).Thompson RJ. The relationship between food ration and reproductive effort in the green sea urchin, Strongylocentrotus droebachiensis. Oecologia. 1983;56:50–57. doi: 10.1007/BF00378216. [DOI] [PubMed] [Google Scholar]
- Terlizzi et al., (2005).Terlizzi A, Benedetti-Cecchi L, Bevilacqua S, Fraschetti S, Guidetti P, Anderson MJ. Multivariate and univariate asymmetrical analyses in environmental impact assessment: a case study of Mediterranean subtidal sessile assemblages. Marine Ecology Progress Series. 2005;289:27–42. [Google Scholar]
- Trussell, Ewanchuk & Bertness (2003).Trussell GC, Ewanchuk PJ, Bertness MD. Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology. 2003;96:2049–2055. [Google Scholar]
- Uthicke, Schaffelke & Byrne (2009).Uthicke S, Schaffelke B, Byrne M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in Echinoderms. Ecological Monographs. 2009;79:3–24. [Google Scholar]
- Vadas et al. (2000).Vadas RL, Sr, Beal B, Dowling T, Fegley JC. Experimental field tests of natural algal diets on gonad index and quality in the green sea urchin, Strongylocentrotus droebachiensis: a case for rapid summer production in post-spawned animals. Aquaculture. 2000;182:115–135. doi: 10.1016/S0044-8486(99)00254-9. [DOI] [Google Scholar]
- Villouta et al. (2001).Villouta E, Chadderton WL, Pugsley CW, Hay CH. Effects of sea urchin (Evechinus chloroticus) grazing in Dusky Sound, Fiordland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 2001;35(5):1007–1024. doi: 10.1080/00288330.2001.9517060. [DOI] [Google Scholar]
- Wangersky & Cunningham (1957).Wangersky PJ, Cunningham WJ. Time lag in prey-predator population models. Ecology. 1957;38:136–139. doi: 10.2307/1932137. [DOI] [Google Scholar]
- Watson & Estes (2011).Watson J, Estes JA. Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecological Monographs. 2011;81:215–239. doi: 10.1890/10-0262.1. [DOI] [Google Scholar]
- Werner et al. (1983).Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. An experimental test of the effects of predation risk on habitat use in fish. Ecology. 1983;64:1540–1548. doi: 10.2307/1937508. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Data S1 and the code has been supplied as a Supplemental Information 1.