Abstract
Klotho functions as a tumor suppressor predominantly expressed in renal tubular cells, the origin of clear cell renal cell carcinoma (ccRCC). Altered expression and/or activity of growth factor receptor have been implicated in ccRCC development. Although Klotho suppresses a tumor progression through growth factor receptor signaling including insulin-like growth factor-1 receptor (IGF-1R), the role of Klotho acting on IGF-1R in ccRCC and its clinical relevance remains obscure. Here, we show that Klotho is favorable prognostic factor for ccRCC and exerts tumor suppressive role for ccRCC through inhibiting IGF-1R signaling. Our data shows the following key findings. First, in tumor tissues, the level of Klotho and IGF-1R expression are low or high, respectively, compared to that of adjacent non-neoplastic parenchyma. Second, the Klotho expression is clearly low in higher grade of ccRCC and is closely associated with clinical outcomes in tumor progression. Third, Klotho suppresses IGF-1-stimulated cell proliferation and migration by inhibiting PI3K/Akt pathway. These results provide compelling evidence supporting that Klotho acting on IGF-1R signaling functions as tumor suppressor in ccRCC and suggest that Klotho is a potential carcinostatis substance for ccRCC.
Keywords: Clear cell renal cell carcinoma, IGF-1 receptor, Klotho, Migration, Prognosis
INTRODUCTION
The klotho gene was identified as an aging suppressor gene in mice, the mutation of which causes multiple premature-aging phenotypes and the overexpression of which extended life span [1,2]. Aging is the primary risk factor for major human pathologies including cancer. Though klotho-deficient (kl/kl) mice manifested multiple aging-like features, cancer has not been identified in kl/kl mice that may be due to short lifespan. Even though it has been suggested that Klotho may have an anti-cancer effect, the link between Klotho and cancer had remained ill-defined until 2007. A homozygous missense mutation in human KLOTHO gene causing severe tumoral calcinosis with severe defects in mineral homeostasis is the first evidence linking Klotho and cancer [3]. Recent studies demonstrated that expression of KLOTHO gene is suppressed by hypermethylation of DNA at the CpG island in the promoter and the first exon in various cancer cells, including cervical, colorectal, gastric and breast cancers [4,5,6,7] suggesting that Klotho expression may be closely correlated with tumorigenesis. In addition, multiple studies have reported that Klotho suppresses cancer proliferation and metastasis by inhibiting growth factor signaling such as IGF-1 and TGF-β [8,9].
Clear cell renal cell carcinoma (ccRCC) is the most common type of renal cancer with increasing incidence [10]. Altered expression and/or activity of growth factor receptor are a primary event in ccRCC development [11,12,13]. Augmented IGF-1 receptor (IGF-1R) signaling has been implicated in proliferation and metastasis in diverse cancers including ccRCC [11]. Clinically, IGF-1R expression has been associated with development of highly invasive metastatic ccRCC with adverse prognosis [12,13]. Klotho inhibits IGF-1 signaling in mice [2] and suppresses IGF-1-mediated breast cancer progression [8]. Although Klotho is predominantly expressed in distal convoluted and proximal tubules which are a common origin of ccRCC, little is known about the biological role of Klotho acting on IGF-1R signaling involved in renal malignances and its clinical relevance.
Here, we examined the expression levels of Klotho and IGF-1R associated with the clinico-pathological parameters of ccRCC and molecular mechanism of Klotho suppression of ccRCC progression by IGF-1R. The results indicate that Klotho expression is a favorable prognostic factor of ccRCC and Klotho ameliorates tumor progression via inhibiting IGF-1R suggesting that Klotho regulation acting on IGF-1R is an attractive target for therapeutic intervention for ccRCC.
METHODS
Ethics approval and tissue samples
This study has been approved by the Institutional Ethics Committee of Yonsei University Wonju College of Medicine and has followed the principles outlined in the Declaration of Helsinki. After institutional approval, human kidney tissue samples from patients with ccRCC were obtained (YWMR-12-4-048). Pathologic reports and clinical records were reviewed. The tissue samples were collected from patients diagnosed with ccRCC who had undergone surgery at the Yonsei University Wonju Severance Christian Hospital. Collecting formalin-fixed paraffin embedded (FFPE) and fresh tissues for immunohistochemistry (IHC) and immunoblotting were described previously [14].
Cell culture and materials
Cell culture of Caki1 ccRCC cell-line was previously described [14]. Unless otherwise noted, all chemicals and reagents were obtained from Sigma-Aldrich (St Louis, MO, USA). Recombinant human Klotho protein was purchased from R&D Systems (Minneapolis, MN, USA).
Immunohistochemistry and western blot
Procedures and analysis of IHC and immunoblotting of tissue specimens were previously described [14]. Primary antibodies against Klotho (KM2076 and ab69208), IGF-1R and β-actin (Abcam, Cambridge, MA, USA), p-IGF-1R, p-Akt, Akt, p-Erk1/2 and Erk1/2 (Cell Signaling, Beverly, MA, USA) and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for immunoblotting and IHC.
Colony formation and wound healing assay
Cells were cultured with IGF-1 and/or Klotho. Culture medium was changed every three days and colonies were visualized with 1% methylene blue. Colony formation and wound-healing assays were performed as described previously [14].
Statistical analysis
Data analysis was performed with the Prism software (version 6, GraphPad Software, San Diego, CA). For statistical comparisons, a two-tailed unpaired Student's t-test, χ2-test and ANOVA were used to compare the categorical and continuous variables. p-values less than 0.05 and 0.01 were considered significant and represented by single- or double-asterisk, respectively. All data were presented as mean±SEM, unless stated otherwise.
RESULTS
Expression levels of Klotho and IGF-1R in ccRCC
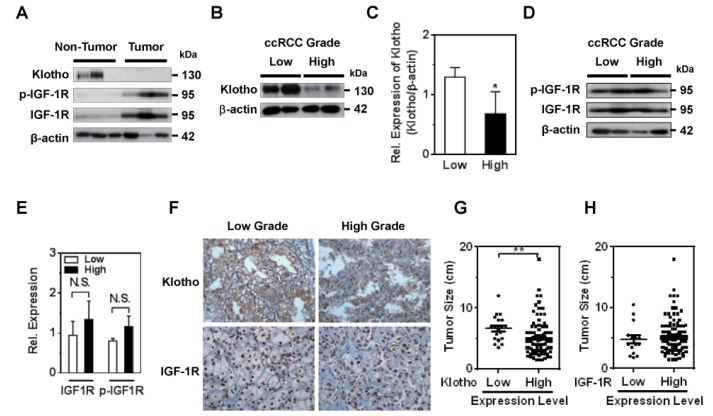
ccRCC is the most common carcinoma originated from the renal tubular epithelium in which Klotho is highly expressed. IGF-1R expression is augmented in diverse human malignancies including ccRCC [11,13] and Klotho blunts IGF-1R signaling [2]. Those observations prompt us to examine the correlation of Klotho and IGF-1R expression and clinical significance in ccRCC. To clarify whether expression level of Klotho and IGF-1R is associated with ccRCC development and Klotho inhibition of IGF-1R contributes to tumor progression, ccRCC with pair tissues of carcinoma and adjacent non-neoplastic parenchyma were analyzed using immunoblotting (Fig. 1A). Whereas Klotho expression level was decreased in tumor tissues compared to that of adjacent non-tumor tissues, expression and phosphorylation of IGF-1R were elevated in tumor tissues suggesting that inverse correlation of Klotho and IGF-1R expression may contribute to ccRCC development.
Fig. 1. Expression of Klotho and IGF-1R and clinical outcome.
(A) Western blot analysis of expression of Klotho, p-IGF-1R and IGF-1R in tumor and adjacent non-tumor tissues of ccRCC. (B~E) Representative immunoblotting of Klotho (B), and IGF-1R and p-IGF-1R (D) in low and high nuclear grades of ccRCC. β-actin was used for loading control. (C and E) Densitometry from panel B and D, respectively (Rel.=relative). *p<0.05 versus low. N.S., not significant (F) IHC staining of Klotho and IGF-1R in low and high nuclear grade ccRCC. (G and H) Association between tumor size and expression levels of Klotho and IGF-1R, respectively. **p<0.01 versus high nuclear grade.
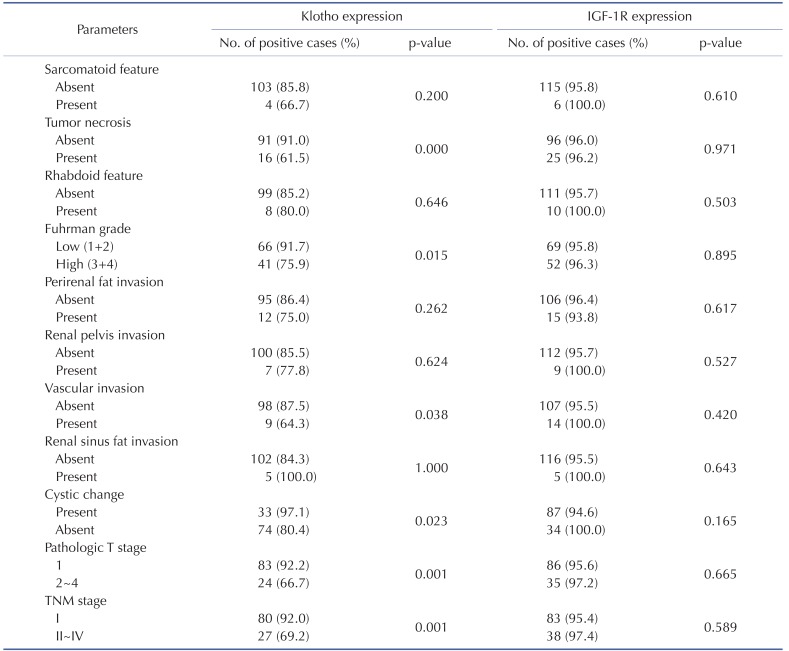
Next, the levels of Klotho and IGF-1R expression in tumor tissue with clinical outcomes were examined using IHC and immunoblotting from FFPE (n=126) and fresh pair samples (n=17), respectively. While the expression level of Klotho expression was clearly low in higher nuclear grade of ccRCC tissues compared to that of lower grade, IGF-1R expression level was not significant between tumor grades (Fig. 1B~E). Consistent with immunoblotting assay, the IHC staining confirmed that Klotho expression was clearly low in higher nuclear grade of ccRCC and IGF-1R expression was not significantly different between tumor grades (Fig. 1F, Table 1 and 2). These results support the notion that the expression level of Klotho was closely correlated with ccRCC progression.
Table 1. Correlation of Klotho and IGF-1R expression and clinico-pathological parameters in ccRCC.
χ2 test.
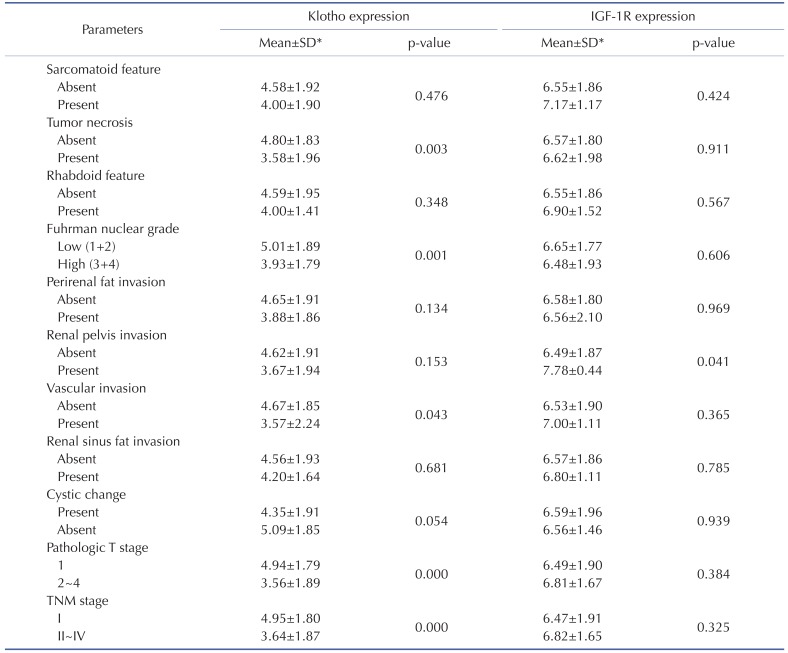
Table 2. Correlation of mean staining score of Klotho and IGF-1R expression and clinico-pathological parameters of ccRCC.
Student's t-test. *SD, standard deviation.
Tumor expression level of Klotho is associated with clinico-pathological parameters of ccRCC
Several clinico-pathological parameters including TNM staging, Fuhrman nuclear grades, and pathologic T stage are used to predict the ccRCC prognosis [15]. TNM staging is the most reliable prognostic factor and Fuhrman nuclear grade is an independent prognostic factor based on nuclear and nucleoli morphology. To determine if Klotho and IGF-1R have any prognostic value, correlation analysis between mean staining score of Klotho and IGF-1R and clinico-pathological parameters was examined. The level of Klotho expression in tumor tissues was significantly lower in the higher TNM stages, Fuhrman nuclear grades, and pathologic T stage (Table 1 and 2). Additionally, Klotho level was significantly higher in the groups of existing tumor necrosis, vascular invasion and absent cystic changes (Table 1 and 2) suggesting that Klotho is the favorable prognostic factor of ccRCC. On the other hand, the expression level of IGF-1R was not statistically significant with clinico-pathological parameters among patients with ccRCC. More importantly, the expression level of Klotho, not IGF-1R, in tumor tissues was significantly correlated with tumor size (Fig. 1G and H). These results suggest that Klotho is more favorable prognostic factor of ccRCC.
Klotho suppresses IGF-1-stimulated ccRCC cell proliferation and migration
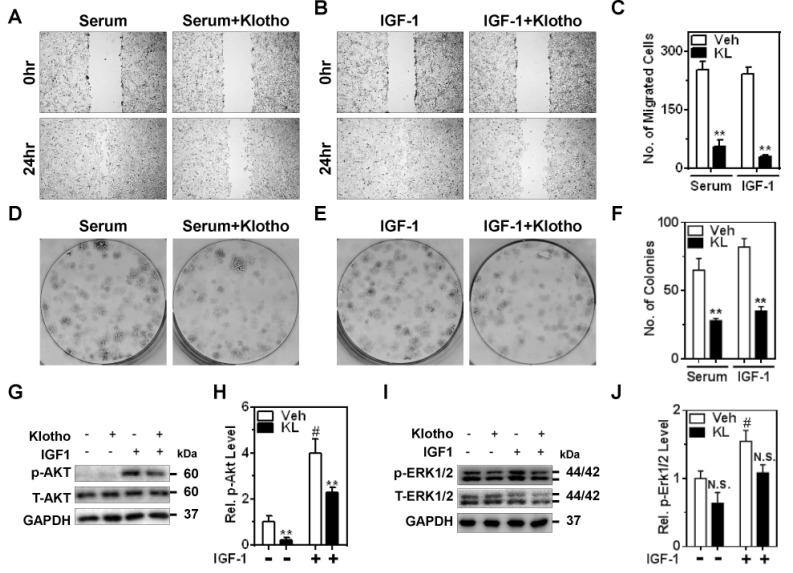
Augmented expression and/or activity of growth factor receptors are primary feature of cancer. Exaggerated IGF-1R signaling has been implicated in invasive metastasis and proliferation of ccRCC [11,16]. Next, whether Klotho inhibits ccRCC progression stimulated by serum growth factors including IGF-1 was examined using ccRCC cell line, Caki1. Serum- and IGF-1-stimulated ccRCC cell migration was analyzed via wound-healing assay in the presence of mitomycin C to exclude the effect of cell proliferation. Consistent with previous reports, serum and IGF-1 stimulated migration of Caki1, ability of which was blunted by incubation of purified Klotho protein (Fig. 2A~C). Additionally, serum and IGF-1 facilitated proliferation of Caki1 and Klotho prevented colony formation induced by serum and IGF-1 (Fig. 2D~F). These data suggest that Klotho ameliorates cell motility and proliferation stimulated by serum growth factors such as IGF-1.
Fig. 2. Klotho suppresses serum growth factors and IGF-1-stimulated cell migration and proliferation of ccRCC via PI3K/Akt pathway.
(A and B) Caki1 cell migration was examined using wound-healing assay with the presence of mitomycin C (0.1 µg/ml). Serum (10% FBS) and IGF-1 (100 nM)-induced cell migration was inhibited by pretreatment of Klotho (KL; 1 nM) in Caki1. (C) Number (No.) of migrated cells in the wound area in panel A and B. **p<0.01 versus vehicle (Veh). (D and E) Effect of Klotho on serum and IGF-1-induced colony formation in Caki1 cells. (F) Quantitative analysis of colony formation assay from three independent experiments in panel D and E. **p<0.01 versus Veh (G and I) Representative immunoblotting of p-Akt and p-Erk1/2 by IGF-1R stimulation and Klotho effect. GAPDH was used for loading control. (H and J) Quantitative analysis from panel G and I, respectively (Rel.= elative). **p<0.01 versus Veh. #p<0.01 versus no IGF-1. N.S., not significant. Note; in panel J, p-values for Veh vs KL are 0.13 and 0.08 in control (no IGF-1) and IGF-1-treated groups, respectively.
Klotho downregulates IGF-1R signaling
IGF-1R governs diverse signaling cascades including a phosphoinositie-3-kinase (PI3K)/Akt and Erk1/2 pathways. Next, the effect of Klotho on IGF-1R signaling in ccRCC was examined. Major downstream effector of IGF-1R is a PI3K/Akt pathway, which is highly activated and genetically mutated in ccRCC [16,17]. While IGF-1 activated Akt in Caki1 cells, Klotho blunted basal and IGF-1-stimulated Akt activation (Fig. 2G and H). Although IGF-1 activated Erk1/2 in Caki1 cells, only minor effects were noted on Klotho inhibition of Erk1/2 activation by IGF-1R (Fig. 2I and J). These results suggest that Klotho might have a suppressive role for ccRCC through inhibiting PI3K/Akt-dependent pathway of IGF-1R.
DISCUSSION
Previous studies have shown that Klotho exhibits tumor suppressive function in various cancers [3,4,5,6,7,8,9,18] except hepato cellular carcinoma [19]. Klotho is predominantly expressed in the kidney tubular epithelium which is the origin of ccRCC. Augmented expression and/or activity of growth factor receptor have aggravated ccRCC progression [16]. Altered IGF-1R expression is a hallmark of most cancer including ccRCC, the expression and activity of which have been augmented [5,18]. The role of Klotho acting on IGF-1R regulating ccRCC progression and its clinico-pathological relevance has been ill-defined. Here, we ask the question whether Klotho exerts tumor suppressive role for ccRCC through inhibiting IGF-1R signaling and its clinical significance. Our data shows the following key findings. First, in tumor tissues, the level of Klotho and IGF-1R expression are low or high, respectively, compared to that of adjacent non-neoplastic parenchyma. Second, the level of Klotho expression is clearly low in higher grade of ccRCC and is closely associated with clinical outcomes in tumor progression. Third, Klotho suppresses IGF-1-stimulated cell proliferation and migration by inhibiting PI3K/Akt pathway. These results provide compelling evidence supporting the notion that Klotho acting on IGF-1R signaling functions as tumor suppressor in ccRCC.
Several observations argue that low expression of Klotho in tumor tissue is associated with a poor prognosis [8,18,19]. Although Klotho is known as a favorable prognostic factor in breast and renal cancers [8,18], oncogenic function of Klotho facilitating tumor migration and invasion is also suggested in hepatocellular carcinoma [19]. While pathologic poor prognostic factors in ccRCC include the presence of tumor necrosis and vascular invasion [20], cystic change is a favorable prognostic factor in predicting survival of RCC [21]. Our results support previous reports that Klotho is a favorable prognostic factor of ccRCC and extend that Klotho has an additional clinical outcomes such as a presence of cystic change and an absence of tumor necrosis and vascular invasion. Additionally, Klotho expression was decreased in tumor tissues compared to that of adjacent normal tissues suggesting that Klotho expression is closely related to ccRCC development.
IGF-1R signaling has been augmented in ccRCC with poor prognosis [11]. Consistently, IGF-1R expression was much higher in tumor tissues compared to that of adjacent normal parenchyma supporting that overexpression of IGF-1R is related with tumorigenesis of ccRCC. However, IGF-1R expression was weakly associated with clinico-patholgical paramenters of ccRCC in the present study. It is conceivable that IGF-1R expression may be saturable in tumor tissues of ccRCC. These data suggest that Klotho expression is more important prognostic factor for ccRCC than IGF-1R expression.
Altered expression and/or activity of IGF-1R are critical for ccRCC development and progression. Augmented expression of IGF-1R is a typical feature of most types of cancer including ccRCC [11,22]. Genetic alterations invlove in initiation and progression of ccRCC. The IGF-1R gene has been transcriptionally downregulated by variety of tumor suppressors including von-Hippel Lindau (VHL) gene, somatic mutation of which occurs in most case of ccRCC [11,17,22]. E3 ligase VHL protein functions as tumor suppressor via inhibiting hypoxia-inducible factor-1α (HIF-1α) whose regulation by VHL is crucial for ccRCC tumorigenesis. Additonally, components of PI3K/Akt pathway, major downstream of IGF-1R, have been frequently mutated in ccRCC [17]. Multiple studies demonstrated that crosstalk between the VHL/HIF-1 and PI3K/Akt pathways is critical for ccRCC progression supporting that altered activity of IGF-1R may contribute to ccRCC tumorigenesis. In addtion, Erk1/2 is downstream target of IGF-1R that regulates cell growth and prolifeartion, activation of which promotes tumor progressoin including RCC [23]. Klotho potentiates activation of Erk1/2 by FGF23 [24] but inhibits its activation by IGF-1R [8]. Although activation of Erk1/2 has been implicated in RCC progression [23], only minor effect of Klotho on Erk1/2 activation were noted in ccRCC suggesting that PI3K/Akt pathway may be major target of IGF-1R involving ccRCC.
Indeed, exaggerated IGF-1R signaling has been aggravated ccRCC progression notoriously resistant to chemo- and radiotherapies [11,17]. Genetic or pharmacological targeting IGF-1R signaling inhibition may provide new insights to overcome resistance of conventional chemo- and/or radiotherapy in ccRCC. Thus, Klotho inhibition targeting IGF-1R signaling in ccRCC offers clues for treatment stragegies of ccRCC.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT&Future Planning (NRF-2012R1A1A1004233) and the Ministry of Education (NRF-2013R1A1A2060764, and NRF-2015R1D1A1A01060454) and a research grant from Yonsei University Wonju College of Medicine (2010-7-0310 to S.-K.C).
Footnotes
Author contributions: J.H.K., K.H.H. and S.L. performed experiments. J.H.J., H.C.C. and M.E. collected and analyzed ccRCC tissues samples. K.S.P., I.D.K., M.E. and S.K.C. supervised and coordinated the study. J.H.K. and K.H.H., M.E. and S.K.C. wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Jeong DJ, Kim J, Lee S, Park JH, Chang B, Jung SI, Yi L, Han Y, Yang Y, Kim KI, Lim JS, Yang I, Jeon S, Bae DH, Kim CJ, Lee MS. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinek T, Shulman M, Israeli S, Bose S, Avraham A, Zundelevich A, Evron E, Gal-Yam EN, Kaufman B, Wolf I. Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Breast Cancer Res Treat. 2012;133:649–657. doi: 10.1007/s10549-011-1824-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Wang X, Wang X, Jie P, Lu H, Zhang S, Lin X, Lam EK, Cui Y, Yu J, Jin H. Klotho is silenced through promoter hypermethylation in gastric cancer. Am J Cancer Res. 2011;1:111–119. [PMC free article] [PubMed] [Google Scholar]
- 7.Pan J, Zhong J, Gan LH, Chen SJ, Jin HC, Wang X, Wang LJ. Klotho, an anti-senescence related gene, is frequently inactivated through promoter hypermethylation in colorectal cancer. Tumour Biol. 2011;32:729–735. doi: 10.1007/s13277-011-0174-5. [DOI] [PubMed] [Google Scholar]
- 8.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 9.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 11.He X, Wang J, Messing EM, Wu G. Regulation of receptor for activated C kinase 1 protein by the von Hippel-Lindau tumor suppressor in IGF-I-induced renal carcinoma cell invasiveness. Oncogene. 2011;30:535–547. doi: 10.1038/onc.2010.427. [DOI] [PubMed] [Google Scholar]
- 12.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardillo TM, Trisal P, Arrojo R, Goldenberg DM, Chang CH. Targeting both IGF-1R and mTOR synergistically inhibits growth of renal cell carcinoma in vitro. BMC Cancer. 2013;13:170. doi: 10.1186/1471-2407-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 15.Lkhagvadorj S, Oh SS, Lee MR, Jung JH, Chung HC, Cha SK, Eom M. Insulin receptor expression in clear cell renal cell carcinoma and its relation to prognosis. Yonsei Med J. 2014;55:861–870. doi: 10.3349/ymj.2014.55.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(Suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, Ding Z. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42:343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H, Lv T, An H, Liu L, He H, Zhang H, Liu J, Xu J, Lin Z. Klotho suppresses tumor progression via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell carcinoma. Cancer Sci. 2013;104:663–671. doi: 10.1111/cas.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Liu H, Liu J, Zhu Y, Xu L, He H, Zhang H, Wang S, Wu Q, Liu W, Liu Y, Pan D, Ren S, Xu J, Gu J. Klotho endows hepatoma cells with resistance to anoikis via VEGFR2/PAK1 activation in hepatocellular carcinoma. PLoS One. 2013;8:e58413. doi: 10.1371/journal.pone.0058413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC European Association of Urology Guideline Group. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Park HS, Jung EJ, Myung JK, Moon KC. The prognostic implications of cystic change in clear cell renal cell carcinoma. Korean J Pathol. 2010;44:149–154. [Google Scholar]
- 22.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31:2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 23.Sharma U, Pal D, Prasad R. A novel role of alkaline phosphatase in the ERK1/2 dephosphorylation in renal cell carcinoma cell lines: a new plausible therapeutic target. Biochimie. 2014;107:406–409. doi: 10.1016/j.biochi.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]