Abstract
Biodegradable tissue engineering scaffolds have great potential for delivering cells/therapeutics and supporting tissue formation. Polyesters, the most extensively investigated biodegradable synthetic polymers, are not ideally suited for diverse tissue engineering applications due to limitations associated with their hydrophobicity. This review discusses the design and applications of amphiphilic block copolymer scaffolds integrating hydrophilic poly(ethylene glycol) (PEG) blocks with hydrophobic polyesters. Specifically, we highlight how the addition of PEG results in striking changes to the physical properties (swelling, degradation, mechanical, handling) and biological performance (protein & cell adhesion) of the degradable synthetic scaffolds in vitro. We then perform a critical review of how these in vitro characteristics translate to the performance of biodegradable amphiphilic block copolymer-based scaffolds in the repair of a variety of tissues in vivo including bone, cartilage, skin, and spinal cord/nerve. We conclude the review with recommendations for future optimizations in amphiphilic block copolymer design and the need for better-controlled in vivo studies to reveal the true benefits of the amphiphilic synthetic tissue scaffolds.
Keywords: poly(ethylene glycol) (PEG), tissue engineering scaffolds, amphiphilic polymers, biodegradable, block copolymers
1. INTRODUTION
The field of tissue engineering often employs biodegradable scaffolds that deliver cells/therapeutics, template tissue formation, and ultimately regenerate the tissue of interest.1,2 An ideal scaffold should be one that can recapitulate the key features (mechanical / biological) of the tissue of interest while possessing handling characteristics that facilitate cell/therapeutic loading and surgical delivery. The scaffold should then induce healing by supporting the growth and differentiation of pre-seeded cells or surrounding endogenous stem/progenitor cells. Finally, the scaffold should degrade at a rate that is synchronous with tissue healing and eventually become completely replaced by the tissue. These complex requirements can potentially be met through the rational design of synthetic polymers and composite materials.
Synthetic polymers are widely applied as tissue engineering scaffolds because they can be reproducibly manufactured and are amenable to chemical modification. Biodegradable polymers presently used in Food and Drug Administration (FDA)-approved devices are attractive building blocks for synthetic tissue scaffolds because of their established biocompatibility and simplified regulatory approval process. The most widely used biodegradable polymers are the hydrophobic polyesters poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), and poly(ε-caprolactone) (PCL), which have been used in surgical products such as sutures or resorbable orthopedic fixation devices.3 These polymers, however, were not originally designed to guide tissue regeneration. Modification of polymers used in FDA-approved implants to improve their mechanical properties, degradation behavior, and bioactivity is a promising strategy to develop translational tissue engineering scaffolds.4
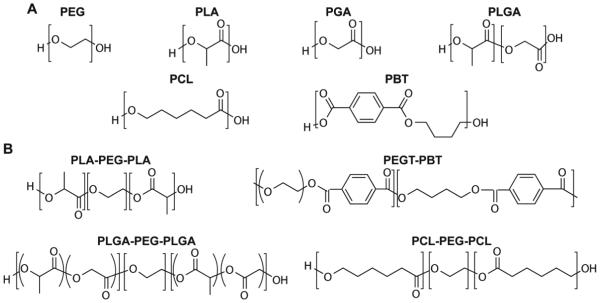
Amphiphilic molecules are a universal modality in living systems, from cell membranes (phospholipids and glycolipids) to amphiphilic proteins and their supramolecular assemblies. For example, the extracellular matrix (ECM) of all tissues is composed of proteins with hydrophilic and hydrophobic amino acid residues that govern protein folding, bioactivity, and protein-protein interactions. The binding and release of growth factors to/from hydrophilic or hydrophobic pockets of the ECM proteins mediates their activity and localization which is critical for tissue maintenance and regeneration.5 Protein and peptide engineering approaches have been used to create amphiphilic proteins or lipid-peptide amphiphiles that mimic the self-folding/assembling and soluble factor-binding capabilities of the ECM.6,7 An alternative approach to develop biomimetic tissue scaffolds is to use synthetic amphiphilic block copolymers. Such amphiphilic polymers often combine biodegradable hydrophobic polyester blocks with the biocompatible hydrophilic polyether poly(ethylene glycol) (PEG, Figure 1).
Figure 1.
Chemical structures of the repeating units of (A) PEG, PLA, PGA, PLGA, PCL, PBT and (B) common types of amphiphilic block copolymers. The repeating units of these blocks vary widely from 100 to 2000. PEG: Poly(ethylene glycol); PLA: Poly(lactic acid); PGA: poly(glycolic acid); PLGA: poly(lactic-co-glycolic acid); PCL: poly(ε-caprolactone); PBT: poly(butylene terephthalate).
The hydrophilic and low-fouling nature of PEG has resulted in its clinical applications in pharmaceutical formulation, such as for increasing the circulation time of protein therapeutics.8 This increased circulation time is a result of PEG’s resistance to non-specific protein adhesion/denaturing through the entropic penalty of releasing and displacing bound water from the hydrophilic PEG surface.9 Although there are reports of anti-PEG antibodies produced following the administration of PEGylated proteins,10 PEG is widely considered as bioinert, minimally immunogenic and safe for in vivo applications.11 The non-degradable, non-cell-adhesive, and water-soluble nature of unmodified PEG, however, has impeded its broader biomedical uses beyond drug delivery. Meanwhile, as PEG fragments shorter than 30-50 kDa are readily cleared through the kidneys, the incorporation of PEG segments into degradable polymers has been explored for modulating the physical and biological properties of biomaterials without compromising their biocompatibility.8,12 Indeed, PEG has been copolymerized with commonly studied water-stable and degradable hydrophobic blocks, such as PLA, PLGA, or PCL. These amphiphilic polymers have been processed into nanoparticles that encapsulate hydrophobic drugs or proteins and extend their circulation time.13–16 Alternatively, amphiphilic polymers can form membranes or gels for use as degradable anti-adhesion tissue barriers for surgical applications.17–21 By decreasing the weight percentage of PEG to permit some degree of protein adsorption, adding bioactive fillers, or by chemically modifying the PEG surface, PEG-based amphiphilic polymers can also be engineered into bioactive/cell-adhesive scaffolds for tissue engineering.
While a number of reviews have been published on block copolymers including PEG-based amphiphilic block copolymers,15,22–24 most focus on the use of amphiphilic polymers for drug delivery applications. PEG-based amphiphilic block copolymers for tissue engineering applications were reviewed by Tessmar & Göpferich in 2007.25 This review will discuss the application of biodegradable amphiphilic block copolymers as structural scaffolds for tissue engineering with an emphasis on more recent developments since 2007. More specifically, we will focus on how hydrophilic PEG was used to tune the physical properties, protein interactions, cell interactions, and in vivo performance of synthetic biodegradable tissue scaffolds.
2. TYPICAL BIODEGRADABLE BLOCKS OF AMPHIPHILIC BLOCK COPOLYMERS
The types of biodegradable polymers, block copolymers, and methods of synthesis have been reviewed previously.15,23,24,26 Here we will briefly review some of the common hydrophobic blocks used in amphiphilic degradable biomaterial scaffolds. The choice of a hydrophobic polymer block to be combined with PEG in the design of amphiphilic tissue engineering scaffolds is typically based on their biocompatibility, processing characteristics, mechanical properties, and degradation profiles. The most commonly used hydrophobic polymer blocks are the biodegradable PLA, PGA, PLGA, and PCL (Figure 1A). The degradation of these polymer blocks results in acidic degradation products lactic acid, glycolic acid, and caproic acid, respectively. Although these degradation products can be cleared by the body, local accumulation of these acidic degradation products is known to be immunogenic and lead to bone resorption in the case of bone tissue engineering applications.27,28 Poly(butylene terephthalate) (PBT, Figure 1A), barely hydrolytically degradable , has also been used as a component in biodegradable amphiphilic block copolymers as will be described later in this review. The thermal transitions, mechanical properties and general degradability of these hydrophobic polymers are summarized in Table 1.
Table 1.
Representative physical properties and degradation rates of hydrophobic polymers used in biodegradable amphiphilic polymers.
| Polymer | Tg | Tm | Tensile Modulus |
Degradation | References |
|---|---|---|---|---|---|
| PLLA | 60-65 ºC | 175 ºC | 2.7 GPa | 2-5 years | 28,29 |
| PDLLA | 55-60 ºC | n/a | 1.9 GPa | ~ 1 year | 3,30 |
| PGA | 35-40 ºC | 225 ºC | 12.8 GPa | 6 – 12 months | 3,30 |
| PLGA | 45-55 ºC | n/a | 1-2 GPa | 1 – 6 months | 3,31 |
| PCL | −60 ºC | 60 ºC | 400 MPa | > 2 years | 32,33 |
| PBT | 30-50 ºC | 220 ºC | 2.8 GPa | n/a | 34,35 |
PLLA: Poly(L-lactic acid); PDLLA: Poly (D,L-lactic acid); PGA: poly(glycolic acid); PLGA: poly(lactic-co-glycolic acid); PCL: poly(ε-caprolactone); PBT: poly(butylene terephthalate); Tg: glass transition temperature; Tm: melting temperature.
2.1 Poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PGLA)
PLA can be polymerized from chiral lactide building blocks in an enantiomerically pure L form (PLLA) or a racemic D/L form (PDLLA).36 PLLA is semi-crystalline and PDLLA is amorphous, resulting in vastly different mechanical properties and degradation rates, as summarized in Table 1. PLLA and PDLLA are thermoplastics that can be fabricated into scaffolds with a variety of architectures including dense and nanoporous films by solvent-casting and electrospinning, respectively, as well as dense filaments and macroporous 3-D scaffolds by extrusion and fused deposition modeling techniques. Relatively high melting temperatures are required for thermoplastic processing of PLLA (> 175 ºC).
PLA degrades by hydrolysis of the ester bonds into lactic acid, which is a natural metabolic byproduct and may be cleared by the body as carbon dioxide and water.3 The accumulation of lactic acid released from degrading PLA and the resulting pH drop in the vicinity of the implant can cause an inflammatory immune response and bone resorption.30,31 The semi-crystalline PLLA takes over 2 years to disintegrate, and full degradation of PLLA crystallites can take over 5 years,31 while the amorphous PDLLA degrades in ~1 yr.3
PGA is a highly crystalline polymer, resulting in its insolubility in most organic solvents and high stiffness (12.8 GPa tensile modulus, Table 1).30 PGA degrades faster than PLA, and was used as the first biodegradable synthetic suture material (Dexon®).3 However its use has been relatively limited in tissue engineering due to its low solubility for polymer purification/processing, limited high-temperature processing options (with a high melting point of 225 ºC, it is typically processed by injection molding/extrusion), and high stiffness.
Copolymers of PLA and PGA, called PLGA, overcome the processing difficulties (solubility) and excessive stiffness of PGA. The degradation rate of PLGA can be tuned by varying the ratio of PLA to PGA from 1-2 months (50/50 ratio) to 6 months (85/15 ratio).32 The tunable degradation rates, ease of processing and established medical uses of PLA and PLGA have made them attractive choices for integration with PEG to form amphiphilic di-block,17,33–37 tri-block,38–42 or multi-block copolymers.43–47
2.2. Poly (ε-caprolactone) (PCL)
The medical uses of semi-crystalline PCL have generally been restricted to slow degrading drug delivery devices and sutures, but more recently its application has been extended to tissue engineering applications, as reviewed by Woodruff and Hutmacher.32 The thermoplastic nature and relatively low melting point (60 °C) of PCL enables its facile processing into a variety of scaffold architectures. Since PCL is more hydrophobic than PLA,37 it has a slower hydrolytic degradation rate (Table 1). Amphiphilic di-block, tri-block, and multi-block copolymers have been synthesized by copolymerizing PCL with hydrophilic PEG43,51,52 or poly(hydroxyethyl methacrylate) (pHEMA).53–56
2.3 Poly(butylene terephthalate) (PBT)
Poly(butylene terephthalate) (PBT) is a hard, semi-crystalline material34 far more resistant to hydrolytic degradation than PLA or PCL-based materials (Table 1). PBT has been traditionally used for automotive, electrical, and plumbing applications,34 but more recently also copolymerized with PEG for biomedical applications.35 Commercialized as PolyActive®, poly(ethylene glycol terephthalate)-PBT (PEGT-PBT)-based devices have been FDA approved as bone cement restrictors38 and tympanic membrane reconstruction materials.39 The mechanical properties, biological performance, and degradation behavior can be tuned by varying the length and ratio of PEG and PBT blocks. However, as the PBT blocks do not fully degrade in vivo,40 the crystalline segments of partially degraded PBT could elicit undesired immune responses.41
3. PHYSICAL PROPERTIES
The incorporation of hydrophilic blocks into biodegradable hydrophobic polymers results in substantial changes in a number of surface and bulk physical properties. Here we will discuss how the incorporation of PEG can influence the surface wettability and swelling behavior, degradation, and mechanical properties of degradable polymers that are key to their biomedical applications. The ideal weight percentage of incorporated PEG for a particular application varies and should be optimized case-by-case. This is because the incorporation of PEG alters all of these properties while some might be more critical than others for a specific end application. Furthermore, unique properties such as hydration-induced stiffening, shape memory, and improved blending with minerals can be introduced by the addition of PEG. These properties are attractive for tissue engineering applications and will also be discussed.
3.1. Surface Hydrophilicity and Aqueous Swelling Behavior
It is difficult to load aqueous suspensions of cells or growth factors on hydrophobic scaffolds, which tend to float and suffer from hydrophobicity-driven collapse in aqueous media. In addition, the hydrophobic surfaces are responsible for non-specific absorption of proteins and denaturing their natural conformations. Thus, one of the most obvious benefits of amphiphilic degradable polymers is their improved surface aqueous wettability.
Multi-block copolymer films of PEG and PLLA exhibit water contact angles ranging from 71º to 21º with increased PEG content.42 Similarly, Wurth et al. recently showed that the incorporation of oligo(ethylene glycol) side chains into PCL can reduce the water contact angle of films from ~90º to ~35º.43,44 Dramatic increases in surface aqueous wettability have also been observed for electrospun amphiphilic scaffolds. Electrospun hydrophobic PLA and PLGA scaffolds have been characterized with water contact angles as high as 120º.45,46 Electrospun tri-block PDLLA-PEG-PDLLA containing 19 wt.% PEG resulted in a water contact angle of 71º compared to 118º for unmodified PDLLA.45 Equilibrating the scaffolds in water, which allowed the hydrophilic PEG block to preferentially segregate to the fiber surface,45,47 further reduced the water contact angle to 53º. The surface hydrophilicity of the scaffolds can be tuned by adjusting the PEG weight percentage. For example, the water contact angle on amphiphilic electrospun di-block PEG-PDLLA scaffolds was tuned from 45º (14 wt.% PEG) to <10º (33 wt.% PEG).47
By adjusting the content of hydrophilic blocks, the aqueous swelling behavior of amphiphilic scaffolds can also be tuned. In the case of hydrogels, for example those composed of PEG-PCL as described by Park et al., the swelling ratio positively correlated with their PEG content.48 Park et al. found that the more swelled amphiphilic hydrogels supported greater proliferation of rabbit chondrocytes, presumably due to increased nutrient transport and cellular penetration/migration.48 The swelling of amphiphilic scaffolds can also have a positive impact on scaffold performance in vivo. Radder et al. found that increasing PEG content and subsequent swelling of PEGT-PBT correlated with increased bone contact and calcification of the polymer plugs when implanted into goat femoral defects.49
3.2. Degradation
As previously described, aliphatic polyesters such as PLA and PCL have long degradation times (2+ years) that are incompatible with the rate of tissue regeneration. Prolonged presence of scaffolds can impede tissue ingrowth and remodeling, and elicit undesired immune responses.27,28,50 Aliphatic polyesters mostly undergo degradation by bulk erosion,3 with a few exceptions.51,52 During bulk erosion, the mass of the scaffold remains unchanged at the onset of degradation since the degraded chains are unable to diffuse out of the bulk hydrophobic material. Once the chain scission proceeds to a critical point where the smaller polymer chains are able to diffuse away, the mass of the scaffold rapidly drops. As the degradation is driven by hydrolysis, it is expected that the incorporation of hydrophilic PEG blocks would accelerate degradation by increasing water up-take and accelerating the release of hydrophilic degradation products.
NMR studies suggest that chain cleavage in biodegradable amphiphilic polymers occurs in a similar rate at the hydrophilic-hydrophobic block linkage as within the hydrophobic chains.53,54 However, the cleaved hydrophilic blocks are the first to elute from the polymer due to better aqueous solubility, while the slow-eluting hydrophobic blocks tend to remain trapped within the bulk material.37,45,55,56 This results in a rapid initial mass loss of the amphiphilic scaffold as the hydrophilic blocks are eluted, followed by a much slower mass loss as the hydrophobic chains are cleaved into more soluble units.57 Di-block PCL-PEG and tri-block PCL-PEG-PCL polymers containing ~25% molar ratio of PEG lost ~3% and ~7% of mass over 15 months in vitro, respectively.56 While both contained similar molar contents of PEG, the tri-block PCL-PEG-PCL had a longer PEG block (8000 Da vs. 5000 Da in the di-block copolymer), which led to greater mass loss upon its cleavage and release from the scaffold. The degradation rate of these amphiphilic polymers during the same period is accelerated compared to unmodified PCL (~0% mass loss), though the increases in degradation rate are modest. Subcutaneous implantation of PCL and PEG-PCL pellets showed similar trends where the PEG-PCL disintegrated faster than PCL.58 However, in this study the initial molecular weights for PCL and PEG-PCL were different, so the degradation outcomes are difficult to interpret. Overall, data from different studies support that the incorporation of a hydrophilic block to hydrophobic degradable polymers accelerates the scaffold mass reduction during degradation due to the release of faster eluting hydrophilic blocks.59,60
The incorporation of PEG blocks to poly(glycerol sebacate) (PGS), a material known to degrade by the mode of surface erosion,61 also accelerated its degradation. Whereas unmodified PGS lost only ~9% mass after a 21-day incubation in aqueous buffer, the rate of degradation of crosslinked PGS-PEG increased with increasing PEG contents (20%, 40%, 60%), with the amphiphilic polymer incorporating 60% PEG losing over 80% of its mass upon incubation for the same period of time. The authors hypothesized that the increased degradation rate is a result of the increased water uptake by the PEG-modified PGS. However, a confounding factor in this study is the presence of un-crosslinked pre-polymer that can readily elute from the material. This un-crosslinked fraction increased with PEG content, from ~10% in unmodified PGS to ~45% of un-crosslinked pre-polymer in the PEG-PGS containing 60% PEG. Thus, the observed degradation may partially be a result of un-crosslinked PEG-containing pre-polymer being rapidly eluted from the scaffold.
Conventional strategies can be applied to tuning the degradation rate of amphiphilic polymers such as using combinations of PCL and PLA, where PCL-PLA-PEG degraded faster than PCL alone.37 PLGA also allows for control over degradation rate by controlling the feed ratio of lactide to glycolide. However, ideal control over degradation would be the design of “smart” materials that degrade in response to the demands of the regenerating tissue in vivo, potentially by incorporation of cleavable sites in the polymer that are specific to cellular/enzymatic activities of the tissue environment.62,63 This strategy has been elegantly implemented in PEG-based hydrogels,64 and may be extended to the environmentally responsive degradation control of amphiphilic scaffolds. For instance, matrix metalloproteinase (MMP) cleavable oligopeptides may be covalently integrated in between hydrophilic and hydrophobic polymer blocks to accelerate the scaffold degradation upon implantation in an in vivo tissue environment rich in MMP activities.
3.3. Mechanical Properties
Amphiphilic block copolymers may be designed with unique mechanical properties that enable convenient surgical handling (i.e. elasticity for press-fitting in a confined defect), improve resistance to fracture, or better emulate the mechanical properties of the desired tissue of interest. PEG blocks can act as soft segments (Tg < room temperature) that exert a plasticizing effect on hard hydrophobic polymers (Tg > room temperature). This plasticizing effect was manifested in blends of PEG and PLA, where the strain at break increased from ~20% to ~550% with the addition of 10 wt.% PEG.65 Higher contents of PEG increased the strain at break while compromising elastic modulus and ultimate stress. Similar observations have been described for amphiphilic block copolymers including PEGT-PBT,66 PEG-PLA,67 and PEG-PGS.61 Reduced brittleness and increased extensibility can facilitate the press-fitting of scaffolds into confined bone defects or enable soft tissue engineering applications (e.g. skin, cardiac, or vocal fold). The elastic modulus of polymers has also been shown to be important for guiding stem cell differentiation,68 thus the ability to tune the modulus of amphiphilic polymer scaffolds is beneficial for their tissue engineering applications.
3.4. Novel Handling Characteristics
3.4.1 Hydration-Induced Stiffening and Self-Fixation
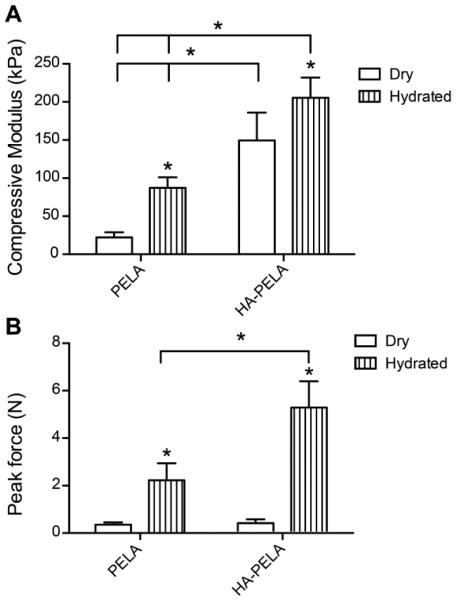
A unique behavior of some amphiphilic polymers is their moduli increases upon hydration.45,69–71 For example, the compressive modulus of rapid prototyped PDLLA-PEG-PDLLA (PELA) and hydroxyapatite (HA)-PELA composites increases 1.34 and 4-fold, respectively, following hydration in water at 37 ºC for 24 h (Fig. 2A). One hypothesis explaining this phenomena is that hydration-induced phase segregation in the amphiphilic network results in a change in load transfer within the polymer.70,72 Another competing hypothesis is that the swelling of the hydrophilic (PEG) domains stiffens the polymer.71 For tissue engineering applications, hydration-induced stiffening may be exploited to engineer amphiphilic scaffolds with self-fixation behaviors.69 For example, the force required to pull-out a rapid prototyped PELA scaffold from a simulated confined defect increased by 6-fold after hydration.69 When the osteoconductive mineral HA was incorporated into the scaffold, the pull-out force increased by 15-fold upon hydration (Fig. 2B). Self-fixation behavior of a synthetic tissue scaffold could potentially reduce the reliance on stabilization/fixation devices required to secure the scaffold in place73 and minimize bone resorption resulting from the loosening of the implant.74
Figure 2.
Hydration-induced modulus increase and self-fixation of rapid prototyped PELA and HA-PELA scaffolds. (A) Compressive modulus of dry and hydrated (24 h in water at 37 ºC) PELA and HA-PELA scaffolds. * p < 0.05 (ANOVA with Tukey post-hoc). (B) Peak force required to dislodge scaffolds from a simulated confined defect either dry (placed in defect) or following hydration (allowed to swell in defect for 2 h, water 37 ºC). Adapted with permission from ref 69. Copyright 2014 Mary Ann Liebert.
3.4.2 Thermal-Responsive Shape Memory Properties
Thermal responsive shape memory polymers (SMPs) can be programed into a temporary shape and then be triggered by a temperature trigger to recover to a pre-programmed permanent shape.75,76 Thermoplastic SMPs typically contain two polymer phases, one phase with a high glass transition temperature (Tg) or melting temperature (Tm) that acts as physical cross-links or hard segments that maintain the permanent shape of the polymer network, and another switching phase with a lower Tg or Tm that allows for programing of the temporary shape of the network. The polymer is deformed above the transition temperature of the switching phase into a temporary shape and then cooled below the transition temperature to fix the temporary shape. Shape recovery to the pre-programed permanent shape, fixed by the hard segments, occurs when the polymer is heated back above the transition temperature of the switching phase.
SMP-based tissue engineering scaffolds have been used to study the effects of stress/strain on cellular behavior77, as “smart” biomedical devices (e.g. self-tightening suture),75 and for minimally invasive delivery of scaffolds to tissue defect sites.78 Amphiphilic polymer networks composed of soft hydrophilic blocks functioning as the switching phase and hydrophobic blocks functioning as hard segments can exhibit shape memory properties when properly designed.59,79–81 High molecular weight (>200 kDa) PCL-PEG-PCL block copolymers can be deformed >800% at room temperature and recover to their original shape after they are heated to 70 ºC for 1 min.81 Cross-linked foams of PCL-PEG can be compressed and recover to their original shape at body temperature, albeit with reduced dimensional stability at room temperature (Figure 3).79 Photo-polymerized PLGA-PEG based materials also exhibit shape memory behavior and are less brittle than photopolymerized PLGA-dimethacrylates.80 We recently reported high molecular weight (> 120 kDa) PLA-PEG-PLA thermoplastics exhibiting shape memory properties around physiological temperatures. The amphiphilic PLA-PEG-PLA can be blended with HA and rapid prototyped into macroporous composite scaffolds. The shape memory behavior was maintained in these composites, exhibiting stable temporary shape fixing at room temperature and nearly complete shape recovery at 50 ºC, albeit the shape recovery slowed when >10 wt% HA was incorporated.82 These macroporous scaffolds could potentially be delivered minimally-invasively to a bone defect site to facilitate scaffold-guided bone regeneration.
Figure 3.
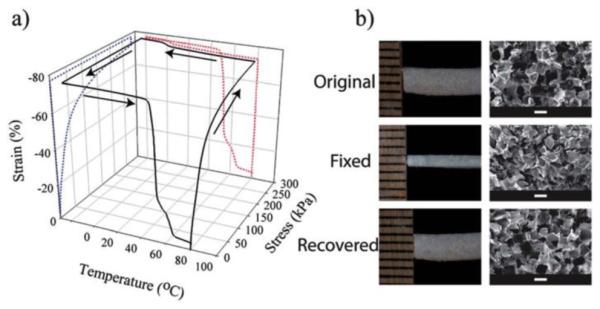
Shape memory behavior of cross-linked PCL-PEG foam scaffolds. (a) Cyclic shape memory testing of PCL-PEG foams showing strain fixing at 0 ºC and recovery at 60-80 ºC. (b) Optical images (left) and scanning electron micrographs (right) of the original porous scaffolds, those with a fixed extended shape (50% strain), and following recovery at 80 ºC. Reproduced with permission from ref 79. (a) Copyright 2013 Royal Society of Chemistry.
3.4. Blending with Calcium Phosphates
Calcium phosphate minerals such as HA, the main mineral component in bone, provide biological cues for the growth and differentiation of bone progenitor cells. Thus, composites of calcium phosphate minerals and biodegradable polymers that combine the bioactivity of HA with the favorable processing and handling properties of synthetic polymers are sought after for bone tissue engineering applications.83 A challenge with this approach is that most biodegradable polymers are relatively hydrophobic and do not blend well with the hydrophilic HA.84,85 This hydrophobic/hydrophilic mismatch complicates the fabrication of uniform composites and results in inferior mechanical properties (e.g. brittleness) and inconsistent bioactivity.45,86,87 Various approaches have been used to improve the polymer/HA interfacial adhesion, including the addition of surfactants, the surface modification of HA,87 and the use of amphiphilic polymers instead of the hydrophobic biodegradable polymers.
PEG88 and pHEMA89 hydrogels are known to blend well with HA, forming highly elastic and tough composite materials that are suitable for press-fitting into bone defects. Osteoconductive pHEMA-HA scaffolds expedited the healing of critical-size bone defects in rats when implanted alone or pre-loaded with low-doses of osteogenic recombinant growth factors.90,91 However, the pHEMA-HA composites were not biodegradable and needed to be cross-linked to achieve aqueous stability. Composites of amphiphilic polymers and bone minerals have the potential to combine the favorable blending characteristics and bioactivity of pHEMA-HA composites while introducing biodegradation and flexible thermoplastic material processing.
PCL-PEG92–95 and PLA-PEG-based amphiphilic polymers45,69,96–98 have been blended with HA and fabricated into tissue engineering scaffolds. Unfortunately, the effect of the addition of PEG blocks to these polymer/HA composites was not extensively investigated. In the case of electrospun composites, the use of PLA-PEG-PLA instead of PLA improved HA dispersion and composite fiber uniformity.45 The amphiphilic polymer/HA (25 wt.% HA) composites were tougher (~2 MPa for PLA-PEG-PLA/HA vs. 0.5 MPa PLA/HA, ultimate tensile stress) and more elastic (~200% vs. ~40% failure strain) than PLA/HA. This may be a result of the improved interfacial adhesion between HA and the amphiphilic polymer.
The impact of HA on the aqueous wettability and degradation behavior of amphiphilic scaffolds can depend on the scaffold morphology and hydration history. For dense solvent cast PCL-PEG-PCL membranes, the addition of >5 wt.% HA resulted in increased hydrophilicity (decreased water contact angle)93 whereas in the form of an electrospun mesh, the water contact angle increased with the addition of HA.92 Equilibration in water and subsequent freeze drying reduced the water contact angle of electrospun PLA-PEG-PLA/HA composites to ~0º, potentially due to the structural rearrangement of the amphiphilic composite to expose the hydrophilic PEG to the surface.45 The in vitro degradation rate of the PCL-PEG-PCL dense films in PBS was somewhat accelerated with the addition of HA, with ~8% increased mass loss over 10 weeks.93 The in vitro degradation rate of the electrospun PLA-PEG-PLA/HA scaffolds in PBS, on the other hand, was barely affected by HA incorporation.45 Overall, the addition of HA may further increase the hydrophilicity of amphiphilic tissue engineering scaffolds, especially with adequate equilibration in water, but has limited impact on in vitro degradation rate of the amphiphilic scaffold.
4. PROTEIN AND CELL INTERACTIONS
The interactions of a tissue engineering scaffold with adhered proteins and cells are critical for the outcome of scaffold-guided tissue regeneration in vivo. Hydrophobic polymer blocks tend to favor protein adhesion while hydrophilic blocks are relatively low-fouling. Preserving protein bioactivity and controlling the binding of specific proteins and/or cells requires a delicate hydrophobic-hydrophilic balance.99,100 In 2013, Bhushan & Schricker reviewed the control of protein and cell interactions by block copolymers in general.101 Here we will describe how scaffolds composed of biodegradable amphiphilic polymers are uniquely suited for encouraging/discouraging protein and cell adhesion.
4.1. Role of Amphiphilic Polymers in Protein and Cell Adhesion
When a tissue engineering scaffold is placed into the culture media or implanted into the body, modification of scaffold surface properties occurs due to rapid protein adsorption.102 Hydrophobic surfaces could change the native conformation of adhered proteins and compromise their bioactivity.102,103 Amphiphilic polymers have been used to modulate the wettability of tissue engineering scaffolds and in turn protein adhesion. Altankov et al. grafted PEG to hydrophobic polysulfone films and found the highest numbers of fibroblasts adhered to the surfaces grafted with moderate levels of PEG.104 Tziampazis et al. found that increasing the PEG concentration in tyrosine-derived polycarbonate films resulted in lower overall fibronectin absorption but the bioactivity of the adhered fibronectin was increased at a PEG content of 6 wt.%.105 Increasing PEG content beyond 6 wt.% resulted in reduced fibronectin absorption and surface bioactivity. PEG-PLA films with low contents of PEG (~5 wt.%) exhibited reduced cell spreading and adhesion but supported more robust osteogenic differentiation of bone marrow stromal cells (MSCs).106,107 The authors hypothesized that the improved differentiation was due to increased bioactivity of cell-adhesion proteins on the materials.107 Cardiomyogenic differentiation of embryonic stem cells was also shown to be improved on electrospun PCL-based membranes containing 4% PEG.108 However, increasing the PEG content to 8% resulted in decreased cell adhesion and cardiomyogenic protein expression.
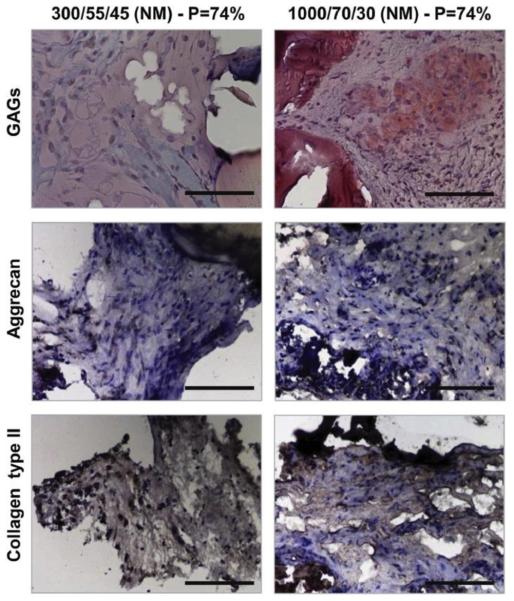
Incorporation of high PEG contents (e.g. ~ 20 wt.%) was shown to significantly reduce protein and cell adhesion.69,107,109 Surface segregation of hydrophilic low-fouling PEG to the surface of the amphiphilic scaffold in an aqueous environment is likely responsible for the anti-fouling behavior.45,110 Biodegradable amphiphilic low-adhesion membranes, such as those composed of PLA-PEG, have been exploited to prevent post-surgical tissue adhesions.17–21,69 Amphiphilic low-adhesion scaffolds could also be beneficial for cartilage tissue engineering because they may help preserve the native chondrocyte phenotype (e.g. rounded rather than spread-out cellular morphology) and encourage cartilage matrix deposition (e.g. type II collagen, sulfated proteoglycans), possibly due to the prevention of integrin-mediated cellular adhesion and spreading.111–113 Mahmood et al. grew human articular chondrocytes on PEGT-PBT surfaces with varying PEG molecular weight and weight percentages.111 They found that the chondrocytes maintained a more rounded morphology and expressed more Type II collagen, both features of native chondrocytes, on the high molecular weight PEG materials. More recently, Hendriks et al. grew articular chondrocytes on rapid prototyped 3-D PEGT-PBT scaffolds.113 In agreement with the prior study, the scaffolds with higher molecular weight PEG supported a more typical rounded chondrocyte morphology, more uniform cell distribution, and greater glycosaminoglycan (GAG) content following in vitro culture.113 When the scaffolds where pre-seeded with bovine articular chondrocytes and implanted subcutaneously in mice, the 70 wt.% PEG containing scaffolds resulted in qualitatively more staining for the typical cartilage matrix components GAG, aggrecan, and Type II collagen (Figure 4).
Figure 4.
The effect of scaffold PEG content (hydrophilicity) on cartilage formation in chondrocyte-seeded rapid prototyped PEGT-PBT scaffolds following 4 week subcutaneous implantation in nude mice. The scaffolds containing 70 wt.% PEG (1000/70/30) with 74% porosity (P=74%) resulted in greater staining for sulfated glucosaminoglycans (Safranin O), aggrecan, and type II collagen than scaffolds containing 55 wt.% PEG (300/55/45) with 74% porosity. Scale bar = 100 µm. Reproduced with permission from ref 113. Copyright 2013 Elsevier.
4.2. Enabling Controlled Protein and Cell adhesion
Amphiphilic scaffolds can be designed to encourage cell adhesion through covalent modification with targeted binding motifs.46,47,114 Grafahrend et al. modified an electrospun PLGA scaffold with star-shaped PEG and demonstrated the reduced adhesion of proteins and human dermal fibroblasts.46 Modifying the fiber surface with the integrin-binding peptide RGD, but not a scrambled peptide control, restored fibroblast adhesion. No cells adhered if the fibers were modified with a scrambled peptide, showing that the cell adhesion was specific to the controlled scaffold modification (Figure 5). These proof-of-concept studies showed that PEG modified surfaces can be tailored to enable cell adhesion and potentially cellular response to the scaffold. This could allow for the development of scaffolds that spatially control cell adhesion or stem cell differentiation.
Figure 5.
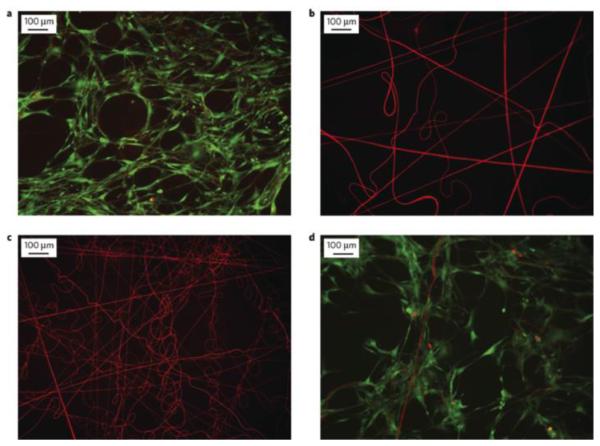
Control of cell adhesion on electrospun PLGA/star-shaped PEG modified scaffolds. (a) Live (green)/Dead(red) staining of human dermal fibroblasts after 1 week culture on unmodified PLGA, (b) PEG-modified PLGA, (c) PEG-modified PLGA with covalently attached scrambled cell adhesion peptide (GRGES), (d) PEG-modified PLGA with covalently attached cell adhesion peptide (GRGDS). Reproduced with permission from ref 46. Copyright Macmillan Publishers 2011.
The addition of bioactive components to the amphiphilic polymer scaffold can also offset the anti-adhesive effect of PEG. We recently showed that by incorporating osteoconductive HA, electrospun PLA-PEG-PLA/HA composite scaffolds containing ~19 wt.% PEG induced higher levels of osteogenic gene expression in MSCs than electrospun HA/PLA, with similar levels of cell attachment.45 The HA in this electrospun scaffold served to increase cell adhesion even with the anti-adhesive high PEG content. Restoration of cell adhesion was also observed when HA was incorporated into rapid prototyped PLA-PEG-PLA scaffolds.69 The anti-adhesion effect of PEG can also be offset by incorporating cell-adhesive blocks such as poly(L-lysine).110 PEG-PLA/L-lysine films exhibited better osteoblast attachment and proliferation than PEG-PLA or unmodified PLA.
4.3. Controlled Release of Protein and Small Molecule Therapeutics
Amphiphilic block copolymers have been extensively studied for drug delivery applications.15,115 However, they are more typically used as nanoparticles rather than tissue engineering scaffolds. One of the advantages of amphiphilic carriers is that they can be exploited for the delivery of hydrophobic, hydrophilic and amphiphilic cargos. General approaches to deliver protein factors from tissue engineering scaffolds were reviewed by Tessmar and Göpferich in 2007.116 Here we will highlight how amphiphilic tissue engineering scaffolds have been exploited for more effective therapeutic deliveries of recombinant protein growth factors, hydrophobic steroids, hydrophilic antibiotics, as well as amphiphilic lipid factors.
Recombinant human bone morphogenetic protein-2 (rhBMP-2) is a potent osteoinductive growth factor that is FDA-approved for treating certain bone non-unions and spinal fusion.117 Localized and sustained delivery of a suitable dose of rhBMP-2 using an appropriate carrier is essential to prevent negative side effects from a burst release of the growth factor. Miyamato et al. first described the use of amphiphilic PLA-PEG as a carrier for BMP.118 They found that the PLA-PEG, when blended with semi-purified BMP and implanted into the dorsal muscle of mice, supported more extensive ectopic bone formation than PLA homopolymers. The authors suggested that this improved bone induction could be due to reduced acidic degradation products from the lower fraction of PLA and/or the increased degradation rate of the PLA-PEG copolymer compared to the PLA homopolymer. In this early work, the molecular weight of PLA-PEG employed was only 850 Da, which was a viscous liquid rather than a hard material suitable for bone grafting or tissue engineering. The same group went on to optimize the PLA-PEG molecular weight and PLA:PEG ratio to make solid materials that induce bone formation with rhBMP-2.119 They found that a low molecular weight (9,500 Da) polymer with 32% PEG induced the greatest quantity of ectopic bone formation. They attributed this to the high degree of swelling and fast degradation rate of the polymer. Further work tuned the degradation profile of PEG-PLA by incorporating poly(dioxanone) (DX) blocks.120 The PLA-DX-PEG implants pre-loaded with 1 µg to 10 µg rhBMP-2 produced the same amount of ectopic bone in mice as rhBMP-2 loaded collagen implants.121 When 20 µg of rhBMP-2 was used, PLA-PEG-DX supported more robust bone formation than collagen. In order to translate this to structural bone scaffolds, porous HA or beta-tricalcium phosphate blocks were coated with PLA-PEG or PLA-DX-PEG polymers preloaded with rhBMP-2.96,97
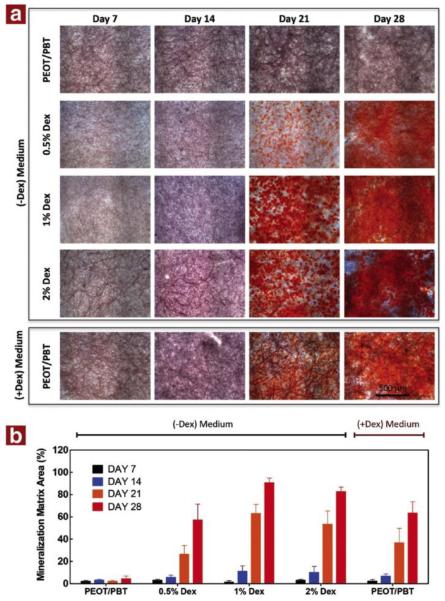
Moroni et al. described the potential of electrospun PEGT-PBT-based scaffolds for sustained drug release.122 The authors incorporated dyes of different molecular weight into the electrospinning solution. Both methylene blue (MW= 319.19 g/mol) and pyrogallol red (400.36 g/mol) exhibited an initial burst release of 20% and 35% of the initial dye loaded over 1 – 2 h, respectively, in PBS. This was followed by a limited release over a month, totaling ~30% methylene blue released and ~45% pyrogallol red released. Electrospun PEGT-PBT scaffolds were also used for the sustained release of the hydrophobic drug dexamethasone during in vitro cell culture.123 The dexamethasone was found mainly sequestered to beads within the electrospun fibers, and the scaffolds enabled sustained release of 60% of the drug over 28 days. The dexamethasone-containing scaffolds induced more robust osteogenic differentiation of human MSCs than when the same dose of dexamethasone was supplemented directly into the cell culture media. The increased differentiation of the human MSCs is evidenced by the increased Alizarin Red S staining of mineralized matrix on the scaffolds with pre-incorporated dexamethasone (Figure 6). The delivery of hydrophilic drugs, such as the antibiotic cefazolin, was also improved by adding PLA-PEG to electrospun PLGA scaffolds, achieving a more sustained release of antibiotic and significantly higher antimicrobial activity than unmodified PLGA.124
Figure 6.
Alizarin Red S (calcium deposition) staining of PEG/PBT scaffolds with dexamethasone incorporated in the scaffold versus added to the medium. Human MSCs where cultured on the scaffolds for 7 to 28 days prior to staining. (a) Images of the Alizarin Red S staining over time as a function of dexamethasone content. Scsale bar = 500 µm. (b) Image quantification of mineralized matrix area showing elevated mineralized area for the PEO/PBT scaffolds containing 1% dexamethasone compared to control (dexamethasone added to medium). Reprinted with permission from ref. 123. Copyright 2014 Elsevier.
Amphiphilic bioactive lipids can also interact favorably with amphiphilic scaffolds and be released in a sustained manner with retained bioactivity. One example of this is the encapsulation/release of the pro-angiogenic phospholipid, sphingosine 1-phosphate (S1P) by amphiphilic scaffolds, as we recently demonstrated.125 Angiogensis is critical for wound healing and S1P has been shown to enhance healing in models of diabetes126 and ischemic limb injury,127 among others. However, hydrophobic biodegradable polymers lack tunable control of S1P delivery. Using electrospun membranes of PLA-PEG-PLA and alkylated PLA-PEG-PLA block copolymers, we demonstrated controlled release of S1P from the amphiphilic scaffolds.125 We found that PLA-PEG-PLA and PLA-PEG-PLA modified with 14-carbon alkylated side chains supported release of S1P over 7 days. This sustained release of S1P resulted in an increased length of vessel-like tubes formed by human umbilical vein endothelial cells in a Matrigel tube formation assay. There was also a noticeable shift of surrounding vessels toward the electrospun S1P bearing scaffolds in an ex ovo CAM assay, supporting the bioactivity of the encapsulated and locally released S1P from the scaffold.
5. IN VIVO APPLICATIONS
The unique mechanical and biological characteristics of amphiphilic scaffolds have already been exploited for tissue engineering applications in animal studies and clinical trials. These in vivo applications include the scaffold-guided repair of bone, cartilage, skin, and nerve tissue. The critical overview of these studies will focus on whether the animal studies are likely to translate to clinical uses and how animal study designs may be improved to maximize successful clinical translation.
5.1. Bone
5.1.1. PEGT-PBT
Amphiphilic block copolymer PEGT-PBT has been actively explored for bone grafting/tissue engineering applications in vivo. Early studies examined the bone-bonding ability of PEGT-PBT with PEG contents ranging from 30% to 70%.49 The highest degree of cortical bone integration and scaffold calcification was observed for the formulation with 70% PEG, while no calcification was found in compositions containing less than 55% PEG. This outcome may be due to the differential swelling behaviors of the polymers, where greater swelling of the higher PEG-content scaffolds in vivo may result in closer contact with the surrounding bone and greater absorption of precursor ions from the serum and tissue environment. The importance of in situ scaffold swelling within the bone defect was demonstrated by implanting pre-swelled PEGT-PBT into goat femoral defects, this resulted in fibrous encapsulation rather than osteointegration.128 In a human trail, PEGT-PBT (70% PEG) foam blocks were implanted into iliac crest defects and the bone healing was compared to untreated control defects.129 Nine months after implantation, the PEGT-PBT foams resulted in limited inflammation but were encapsulated by fibrous tissue. The PEGT-PBT foams failed to calcify even after one year, and the un-treated control defects resulted in greater bone formation than the PEGT-PBT filled defects. The authors pointed out that the difference in study design might be responsible for the difference between the human trial and prior animal trial outcomes. The animal studies did not use critical size defects, leaving the defect possibly healed even without intervention. In addition, the animal studies did not include empty defect controls. It is surprising that a human trial was carried out without an adequately designed/controlled animal study.
PEGT-PBT materials have also been used for metacarpophalangeal joint reconstruction in mini pigs.130 The authors found significant osteolysis and inflammation surrounding the PEGT-PBT implant up to 52 weeks post op. The inflammation was resolved after 3 years when the PEGT-PBT was mostly degraded, but sclerotic bone with large cysts was detected. The authors suggested that this adverse reaction may have been due to the cyclic loading stresses present in the joint that could prevent bone bonding and accelerate polymer degradation. Un-degraded crystalline PBT segments were also a potential cause for osteolysis.41
The performance of PEGT-PBT in bone tissue engineering applications may also be improved by blending more hydrophobic PEGT-PBT copolymer (< 50 wt.% PEG) with osteoconductive calcium phosphate minerals. The composites were fabricated into scaffolds with controlled porosity using fabrication techniques such as rapid prototyping and electrospinning, as previously described.131–133 The efficacy of these scaffolds for guiding bone regeneration would need to be tested in a clinically relevant surgical model with a critical-size bone defects along with appropriate controls (e.g. empty defect and gold standard controls).
5.1.2. PLA/PLGA-PEG or PCL-PEG
Amphiphilic block copolymers based on PLA and PCL have also been used for bone tissue engineering in vivo. Yoneda et al. examined the performance of porous beta-tricalcium phosphate cylinders coated with PLA-DX-PEG in augmenting the repair of critical-size rabbit femoral defects.97 They compared the healing with the implant alone or pre-loaded with 50 µg of rhBMP-2 to an empty defect. Negligible new bone formation was observed with the implant alone, but new bone formation and functional restoration of torsional strength were observed after 24 weeks with the addition of rhBMP-2. Importantly, the scaffold was completely resorbed by 24 weeks and the anatomy of the femur was restored. Similar results in a rabbit critical size radius defect were observed for porous HA implants coated with PLA-PEG and loaded with rhBMP-2.96 Interestingly, the rhBMP-2 dose used in the PLA-DX-PEG coated implant (50 µg) in the rabbit femoral defect was 10 times higher than the effective dose in the PLA-PEG coated implant (5 µg) in the rabbit radius defect, even though both defects were similar in size (1.5 cm). This difference was suggested to possibly be due to the more sustained release profile of rhBMP-2 from PLA-PEG (3 weeks) than from PLA-DX-PEG (2 weeks).96 Neither study compared the results to an uncoated calcium phosphate implant pre-loaded with the same dose of rhBMP-2.
Cell-based bone tissue engineering approaches have also been examined with PLA-PEG. Ren et al. compared the performance of MSC-seeded PLA-PEG vs. PLGA salt-leached foam scaffolds in repairing rabbit mandibular defects.134 They found that the MSC-seeded PLGA scaffold resulted in greater bone formation than the MSC-seeded PLA-PEG scaffold, but the conclusion was based on semi-quantitative bone area measurement without statistical analysis. This result could be due to better exogenous cell seeding efficiency to PLGA than to PLA-PEG, as cell-free PLGA and PLA-PEG scaffolds supported comparable degrees of new bone formation. Covalent surface modification of PLGA-PEG with aspartic acid (Asp) was shown to improve cell attachment and the resulting PLGA-PEG-Asp scaffolds supported better cell attachment than unmodified PLGA.135 Bone formation was induced in a rat subcutaneous implantation model by the covalent attachment of a 24-amino acid BMP-2-based peptide to the PEG-PLGA-Asp. However, the performance of PLGA-PEG-Asp scaffold has not been examined in critical size bone defects.
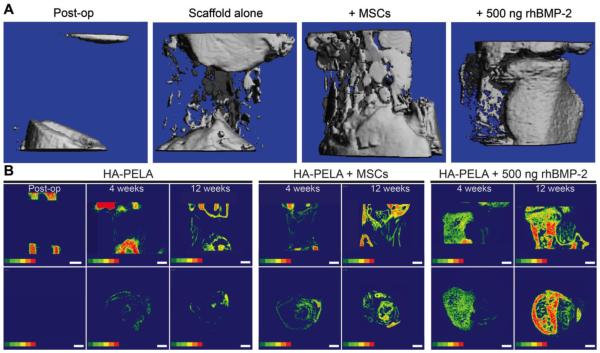
We have recently compared cell-based and growth-factor-based approaches for bone tissue engineering using spiral-wrapped electrospun PLA-PEG-PLA/HA in critical-size femoral defects in rats.136 We examined whether the previously reported in vitro bioactivity (promotion of osteogenic gene expression in MSCs)45 of these amphiphilic scaffolds translates to templated bone formation by exogenously seeded rat MSCs or endogenous cells recruited with a low dose (500 ng) of rhBMP-2. Pre-seeding the scaffolds with MSCs resulted in laminated bone formation that appeared templated by the spiral-wrapped scaffold. The scaffold treated with the rhBMP-2 but without exogenous cells resulted in the most robust bone formation although the new bone did not appear to be as templated by the scaffold (Figure 7). Overall the electrospun amphiphilic scaffolds were effective in guiding bone formation by either exogenous MSCs or endogenous stem/progenitor cells recruited by a low dose of rhBMP-2. It is important to note that the torsional strength of the femurs was not restored by 12 weeks in this study. Further optimization of the scaffold design including tuning the scaffold degradation rate to better match that of the bone formation, increasing exogenous cell survival, and synergistically delivering pro-angiogenic and osteogenic growth factors may further expedite the functional healing of the defect.
Figure 7.
Templated bone formation by PLA-PEG-PLA/HA (HA-PELA) scaffolds in critical-size rat femoral defects. (A) Microcomputed tomography (µ-CT) reconstructions of rat femoral defects treated with spiral-wrapped electrospun HA-PELA scaffolds immediately post-op or after 12 weeks. Treatment groups are the HA-PELA scaffold alone, HA-PELA with rat MSCs, or HA-PELA with 500 ng of rhBMP-2. (B) Two-dimensional bone mineral density color maps of the scaffold-filled defect over time (red representing higher mineral density). Longitudinal (top) and axial (bottom) midslices of the defect treated with HA−PELA immediately post-op, and HA−PELA, HA−PELA + MSCs, and HA−PELA + 500 ng rhBMP-2 at 4 and 12 weeks post-op. Bone formation that matches the spiral template of the scaffold is visible in the HA-PELA + MSCs treatment group. Scale bars = 1 mm. Adapted with permission from ref. 136. Copyright 2015 American Chemical Society.
Amphiphilic polymer solutions can undergo a sol-to-gel transition whereby a solution of the polymer undergoes a phase change at elevated temperature and gels. Such materials have been explored for minimally invasive repair of bone defects. PCL-PEG solutions mixed with MSCs supported the formation of bone-like mineral when subcutaneously injected in rats.137 Bone formation further increased when dexamethasone was added to the gel. Additives such as decellularized bone mineral,138,139 collagen,94 and hydroxyapatite140 have been blended with the PCL-PEG solutions to improve bioactivity. Fu et al. examined PEG-PCL-PEG/HA/collagen sol-gel scaffolds in rabbit cranial defects.94 At 20 weeks post-op, the defects treated with the sol-gel scaffolds were filled with new bone. No quantification of the bone volume or quality was performed but semi-quantitative analysis of the histological sections showed significantly higher bone coverage in the treated group than untreated controls. However, the defects were not critical size and untreated controls also healed. Similar results were observed by Ni et al. for PEG-PCL-PEG/decellularized bone sol-gel scaffolds in repairing rabbit calvarial defects.139 This study also demonstrated the benefit of the bioactive acellular bone component, as the PEG-PCL-PEG alone resulted in lower bone regeneration than the composite. However, this study also did not use a critical-size defect and did not compare the healing to an empty defect control. Quantification of the new bone area between these studies revealed that the group treated with PEG-PCL-PEG (~35% new bone after 20 weeks) performed worse than the untreated control (~70% new bone after 20 weeks). In a similar in vivo study also by Fu et al., electrospun PEG-PCL-PEG/hydroxyapatite nanofibrous scaffolds were implanted into non-critical-size rabbit cranial defects.95 The electrospun scaffold supported new bone formation after 20 weeks (82.6% defect fill) that was comparable to the PEG-PCL-PEG/collagen/HA sol-gel scaffold (82.3% defect fill). The empty control defects in the two studies healed to different extents, however, with the empty control filling 56.9% in the electrospun scaffold study and 71.6% in the sol-gel study. This discrepancy, coupled with a lack of quantitative micro-computed tomography quantification of bone quality (e.g. bone volume, bone mineral density), makes it difficult to interpret the efficacy and superiority of these scaffolds (electrospun vs. sol-gel).
Overall amphiphilic polymers have shown promise as bone tissue engineering scaffolds. Additional research is required to define the optimal hydrophilic/hydrophobic balance for bone healing and the optimal bioactive additives. Furthermore, in vivo studies employing appropriate critical-size defect models with relevant controls and quantitative outcome measures are needed to make meaningful comparisons between the various scaffolds and to reveal their benefits compared to conventional degradable scaffolds.
5.2. Cartilage
Articular cartilage is composed of chondrocytes sparsely distributed in a dense ECM containing primarily type II collagen and glycosaminoglycans.141 Repairing cartilage lesions is particularly difficult due to its avascular nature and low cellularity.142 A wide variety of scaffolds have been tested for cartilage repair in humans, with some approved for clinical uses.143 However, the efficacy of current cartilage regeneration approaches is controversial due to the formation of mechanically inferior fibrocartilage instead of the desired hyaline cartilage, and the limited long-term follow up data. The development of better-performing scaffolds for cartilage repair remains an active field of tissue engineering research.144
As discussed earlier, low-fouling materials may be beneficial for preserving the native phenotype of chondrocytes. Early studies showed some neocartilage formation by combining chondrocytes with high molecular weight PEG (100 kDa) and subcutaneously injecting the mixture into nude mice.145 However, PEG alone does not have adequate mechanical integrity in a physiological environment and high molecular weight PEG cannot be readily cleared by the body. Therefore, amphiphilic PEG-based materials that are biodegradable and mechanically stable have been explored for cartilage tissue engineering. Hendriks et al. used rapid prototyping to fabricate porous PEGT-PBT scaffolds.113 They found that scaffolds with 70% PEG, when pre-seeded with chondrocytes, supported the formation of more cartilage-like tissue than scaffolds with 55% PEG, when implanted subcutaneously in nude mice. Similarly, PEG-PCL hydrogels with 70% PEG supported better growth of encapsulated chondrocytes than hydrogels with 50% or 30% PEG.48 The chondrocyte-loaded PEG-PCL hydrogels were implanted into nude mice and supported the formation of neotissues that stained positive with Safranin O and for type II collagen. However, both the PEGT-PBT and PEG-PCL studies would need to be validated in a true cartilage defect model in order to determine the ideal PEG weight fraction for supporting chondrogenesis in vivo. Prior work by Jansen et al. indeed showed that an acellular 70% PEGT-PBT supported more robust repair of a full thickness osteochondral defect in rabbits 12 weeks post-implantation compared to a 55% PEGT-PBT scaffold.146 However, based on histological scoring, adequate healing was not accomplished by 12 weeks due to the absence of hyaline cartilage and presence of osteophytes. Furthermore, scaffold degradation elicited inflammatory responses (e.g. foreign body giant cells) that have contributed to an overall unfavorable healing outcome.
Amphiphilic scaffolds combined with growth factors have also been explored for the repair of osteochondral or full-thickness cartilage defects in vivo. Tamai et al. coated porous hydroxyapatite blocks with a mixture of PLA-PEG and rhBMP-2 and implanted them into full-thickness osteochondral defects in rabbits.147 After 6 weeks, they found that these scaffolds supported the formation of some cartilage-like tissue that stained positive for proteoglycans and type-II collagen, albeit not as strongly or uniformly as native hyaline cartilage. They did not examine the tissue integration at longer time points and no mechanical testing was performed. They also used skeletally immature rabbits that have a high innate healing potential. Such a model does not recapitulate the clinical pathology of osteoarthritis (OA) in humans, particularly the late-stage OA that results in full-thickness cartilage defects. This study, nevertheless, demonstrated the potential utility of sustained rhBMP-2 release from PLA-PEG for cartilage repair. This strategy could potentially be extended for the delivery of other pro-chondrogenic growth factors such as transforming growth factor-beta.148
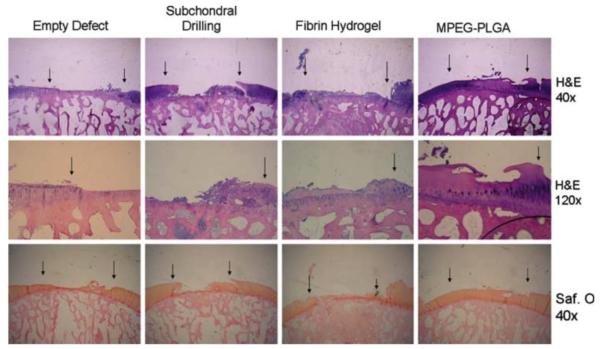
Methoxy-PEG(MPEG)-PLGA scaffolds have been used to support autologous chondrocyte implantation in goats149 and rabbits,143 and received a CE mark for this application (AseedTM, Coloplast N/S). Lind et al. used fibrin glue to help seed MPEG-PLGA scaffolds with autologous goat chondrocytes and implant the constructs into full-thickness cartilage defects (6 mm in diameter) in the goat’s femoral chondyle.149 Of note, to recapitulate a challenging clinical scenario, the defects were created 4 weeks prior to scaffold treatment and in weight-bearing areas of the femoral condyle. The authors also compared the scaffold performance to an un-treated control, a defect receiving microfracture intervention (current standard of care), and a defect receiving the cell-seeded fibrin alone. After 4 months, the best histological score was obtained in the defects treated with the cell-seeded MPEG-PLGA scaffold. However, the regenerated cartilage exhibited weak proteoglycan staining with the cartilage layer incongruent to the surrounding cartilage (Figure 8). The performance of the scaffold without cell seeding or without fibrin glue was not evaluated. Follow-up studies aimed to optimize the chondrocyte culture protocol150 and seeding density.143 Using a rabbit osteochondral defect model, however, no difference in healing outcome was observed with increasing cell seeding density (0 to 2.0 × 107 cells/cm3) on the MPEG-PLGA without fibrin.143 Interestingly, the O’Driscoll histological scores for all treatment groups in the rabbit model were lower than those in the goat model. Given relatively high endogenous healing potential of rabbit cartilage,151 this finding may suggest a positive role of fibrin in healing, which was absent from the rabbit study.
Figure 8.
Repair of articular cartilage defects in goats with MPEG-PLGA scaffolds. Hematoxylin & Eosin (H&E) and Safranin O (Saf. O) staining of 6-mm full thickness defects in goat femoral condyles receiving no treatment (empty defect), subchondral drilling, fibrin hydrogel with autologous chondrocytes, or MPEG-PLGA with fibrin and autologous chondrocytes. Arrows indicate the interface between repair tissue and the native cartilage. The best qualitative healing result based on histology was achieved in the MPEG-PLGA group. Reprinted with permission from ref. 149. Copyright 2008 Springer.
Amphiphilic scaffolds, with/without growth factors and pre-seeded/encapsulated cells, can potentially facilitate the repair of cartilage defects. Ideal scaffold formations and therapeutic loading regimens, however, are yet to be identified to encourage the regeneration of functional hyaline cartilage. Furthermore, it is critical to test the scaffold performance in large animal models that recapitulate/approximate true clinical pathology and physiological mechanical loading in humans, as reviewed by Chu et al.152
5.3. Skin
The elastomeric properties of amphiphilic copolymers coupled with their ability to encapsulate and locally release protein therapeutics make them well suited for soft tissue regeneration. PEGT-PBT membranes were initially developed and applied clinically for tympanic membrane reconstruction.39,153–155 Subsequent studies aimed to apply bi-layer PLLA/PEGT-PBT membranes for skin regeneration.156 Beumer et al. found that dense PEGT-PBT films supported the proliferation of keratinocytes and fibroblasts, with the 40 wt.% PEG film supporting most comparable proliferation to TCPS.156 Large PLLA/PEGT-PBT bi-layer membranes (approximating 25% of body surface area) were implanted subcutaneously in rats for 1 year.40 Foreign body giant cell reaction to the implants was observed with greater numbers of cells at the PLLA side rather than the PEGT-PBT side. The authors also observed vascular and fibrous tissue ingrowth into the implants, which is desired for skin regeneration. In a preclinical study, PEGT-PBT membranes with or without pre-seeded fibroblasts were used to repair full-thickness skin wounds in miniature pigs.157 The scaffolds prevented wound contraction compared to untreated controls and resulted in collagen deposition in the wound site. Pre-seeding the scaffolds with fibroblasts improved the alignment of newly formed collagen. Polymer fragmentation and uptake by foreign body giant cells was apparent after 2 months. A subsequent small-scale (7 patient) human trial for scar tissue repair found no improvement in the repair outcome with the use of PEGT-PBT scaffolds followed by split-thickness skin grafting compared to the use of split-thickness skin grafts alone.158 The PEGT-PBT scaffolds did not incorporate with surrounding tissue in 2 out of 7 patients, and a separate 2 patients withdrew from the study. In the other patients, wound contraction, scar tissue formation, and/or foreign body reactions were observed. This study exemplifies potential discrepancies between even large animal preclinical studies and human clinical outcomes. The slow degradation rate and foreign body response to scaffold degradation may have contributed to the unfavorable graft performance.
Controlled release of epidermal growth factor (EGF) can aid in the healing of diabetic ulcers, a common comorbidity in patients suffering from diabetes mellitus. Choi, Leong, and Yoo covalently conjugated rhEGF to the surface of electrospun PCL/PEG-PCL scaffolds.159 Keratinocytes grown on the rhEGF-modified PCL/PEG-PCL fibers better maintained the expression of keratinocyte-specific genes keratin 1 and loricrin than those grown on unmodified scaffolds with rhEGF supplemented in solution. Scaffold performance in vivo was tested in burn wounds in diabetic mice. The covalently modified scaffold accelerated the rate of wound closure for the first 7 days post-op. However, by day 14 post-op there was no significant difference in wound closure between the covalently modified scaffold and unmodified control.
5.4. Spinal Cord / Nerve
Spinal cord or peripheral nerve injury can cause significant functional impairment and morbidity.160,161 Amphiphilic degradable polymers have also been explored for the scaffold-assisted repair of these injuries. Maquet et al. used porous PDLLA/PDLLA-PEG scaffolds to repair spinal cord defects.162 The addition of the PDLLA-PEG component increased the hydrophilicity of the scaffold, which in turn improved the handling characteristics (e.g. the hydrophobic PDLLA foams float in cell culture while the amphiphilic scaffolds do not), and increased the drug release rate and scaffold degradation rate. The authors loaded the foams with acidic fibroblast growth factor and coated them with laminin to further improve their bioactivity. When implanted into spinal cord defects in rats, the scaffolds integrated with the surrounding spinal tissue after 15 days and Schwann cell growth into and around the foams was observed after 30 days. However, a foreign body giant cell reaction to some scaffolds was reported, and the scaffolds were not monitored beyond 30 days to examine the immunogenicity of degradation products or longer-term healing. Functional assessment of regeneration was also not performed.
Li et al. developed porous PEG-PCL polyurethane scaffolds for peripheral nerve regeneration.163,164 In vitro studies found that these soft elastomeric scaffolds supported the growth of mouse fibroblasts and rat glial cells.163 Recently Niu et al. examined their performance as peripheral nerve guide scaffolds in rats and compared their performance to that of autografts, PCL scaffolds, and silicone tubes.164 Of note, functional measurements such as walking track analysis, electrophysiological analysis, and muscle atrophy were used to assess scaffold performance in vivo. Functional assessment of the scaffolds in this study was performed up to 14 weeks post-op, at which time scaffold weight loss was ~20% in vitro and accompanied with a sharp pH drop. The authors observed that the PEG-PCL scaffolds appeared to have completely degraded at 20 weeks post-op, but no functional assessments were performed at this stage. Functional assessment of healing was performed 14 weeks post-op by walking track analysis with semi-quantitative Sciatic Function Index (SFI) measurements. Based on the SFI, the PCL-PEG-based scaffolds, without the addition of exogenous cells or growth factors, supported nerve regeneration comparable to that achievable with autografts and better than PCL or silicone tube controls (Figure 9). This is an encouraging finding given the significant donor site morbidity associated with harvesting nerve autografts. The importance of the amphiphilic PEG-PCL polymer is evident in this study, reflected by better attachment and proliferation of glial cells on the amphiphilic scaffold than on PCL control in vitro, which in turn improved its in vivo performance as nerve guide scaffolds. The authors attributed the improved performance to the increased hydrophilicity of PEG-PCL.
Figure 9.
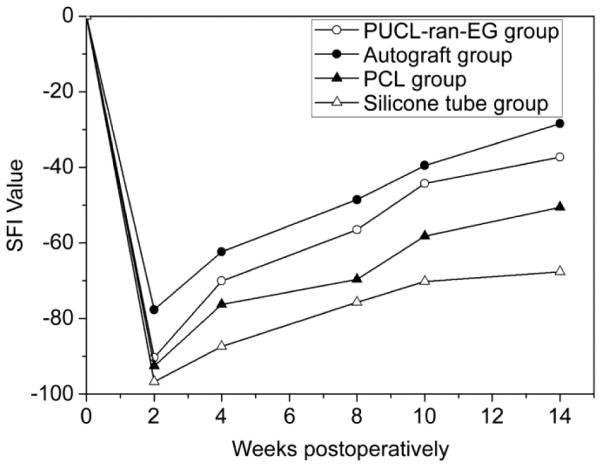
Functional assessment of nerve regeneration (SFI value) over 14 weeks of rats treated with the amphiphilic PCL-PEG (PUCL-ran-EG) copolymer tube, autograft, unmodified PCL, or silicone tubes. A SFI value of 0 is normal and a value of 100 indicates total functional impairment. Reproduced with permission from ref 164. Copyright 2014 Elsevier.
6. CONCLUSIONS
Biodegradable PEG-based amphiphilic copolymers are versatile materials as tissue engineering scaffolds. The hydrophobic polyester components provide biodegradation, aqueous stability, and protein adhesion; meanwhile, the hydrophilic PEG blocks help tune the degradation rate, increase elasticity and hydrophilicity, introduce novel mechanical properties (i.e. self-fixation, shape memory), and help retain the native conformation of adhered proteins. When appropriately designed, the hydrophobic and hydrophilic blocks complement each other to produce a scaffold with improved physical and biological properties.
It should be noted that a number of unresolved issues remain in the design of optimally performing amphiphilic tissue engineering scaffolds. For instance, how to achieve the hydrophobic/hydrophilic balance to either reduce or encourage cell adhesion for specific applications is yet to be fully elucidated. Furthermore, control over scaffold degradation rate and the reduction of inflammatory reactions to scaffold degradation by-products remains a challenge. The discrepancies observed between animal studies, including large animal preclinical studies, and human clinical trials often lie in the choice of suboptimal animal models and the lack of adequate controls, as well as inadequate longitudinal follow-ups to examine long-term performance. Rational design of biodegradable amphiphilic materials for tissue engineering applications can significantly benefit from the understanding of the physical/mechanical requirements for their facile surgical delivery and long-term in vivo safety and functional performance.
ACKNOWLEDGMENT
This research was supported in part by the National Institutes of Health grants R01AR055615 and R01GM088678 and by the Department of Defense Congressionally Directed Medical Research Programs under award number W81XWH-10-0574.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Langer R, Tirrell DA. Designing Materials for Biology and Medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- (2).Langer R, Vacanti J. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- (3).Nair LS, Laurencin CT. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007;32:762–798. [Google Scholar]
- (4).Place ES, Evans ND, Stevens MM. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- (5).Schultz GS, Wysocki A. Interactions between Extracellular Matrix and Growth Factors in Wound Healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- (6).Cai L, Heilshorn SC. Designing ECM-Mimetic Materials Using Protein Engineering. Acta Biomater. 2014;10:1751–1760. doi: 10.1016/j.actbio.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Cui H, Webber MJ, Stupp SI. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Veronese FM, Pasut G. PEGylation, Successful Approach to Drug Delivery. Drug Discov. Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- (9).Latour R. Biomaterials: Protein–Surface Interactions. Encycl. Biomater. Biomed. Eng. 2005:1–15. [Google Scholar]
- (10).Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against Polyethylene Glycol in Healthy Subjects and in Patients Treated with PEG-Conjugated Agents. Expert Opin. Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- (11).Schellekens H, Hennink WE, Brinks V. The Immunogenicity of Polyethylene Glycol: Facts and Fiction. Pharm. Res. 2013;30:1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- (12).Yamaoka T, Tabata Y, Ikada Y. Distribution and Tissue Uptake of Poly(ethylene Glycol) with Different Molecular Weights after Intravenous Administration to Mice. J. Pharm. Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- (13).Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- (14).Hans M, Lowman A. Biodegradable Nanoparticles for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002;6:319–327. [Google Scholar]
- (15).Oh JK. Polylactide (PLA)-Based Amphiphilic Block Copolymers: Synthesis, Self-Assembly, and Biomedical Applications. Soft Matter. 2011;7:5096. [Google Scholar]
- (16).Riley T, Stolnik S, Heald CR, Xiong CD, Garnett MC, Illum L, Davis SS, Purkiss SC, Barlow RJ, Gellert PR. Physicochemical Evaluation of Nanoparticles Assembled from Poly(lactic Acid)-Poly(ethylene Glycol) (PLA-PEG) Block Copolymers as Drug Delivery Vehicles. Langmuir. 2001;17:3168–3174. [Google Scholar]
- (17).Lee JH, Go AK, Oh SH, Lee KE, Yuk SH. Tissue Anti-Adhesion Potential of Ibuprofen-Loaded PLLA-PEG Diblock Copolymer Films. Biomaterials. 2005;26:671–678. doi: 10.1016/j.biomaterials.2004.03.009. [DOI] [PubMed] [Google Scholar]
- (18).Liu S, Hu C, Li F, Li X, Cui W, Fan C. Prevention of Peritendinous Adhesions with Electrospun Ibuprofen-Loaded PELA Fibrous Membranes. Tissue Eng., Part A. 2012:19. doi: 10.1089/ten.TEA.2012.0208. [DOI] [PubMed] [Google Scholar]
- (19).Yang D-J, Chen F, Xiong Z-C, Xiong C-D, Wang Y-Z. Tissue Anti-Adhesion Potential of Biodegradable PELA Electrospun Membranes. Acta Biomater. 2009;5:2467–2474. doi: 10.1016/j.actbio.2009.03.034. [DOI] [PubMed] [Google Scholar]
- (20).Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J. Biodegradable and Thermoreversible PCLA-PEG-PCLA Hydrogel as a Barrier for Prevention of Post-Operative Adhesion. Biomaterials. 2011;32:4725–4736. doi: 10.1016/j.biomaterials.2011.03.046. [DOI] [PubMed] [Google Scholar]
- (21).Park S-N, Jang HJ, Choi YS, Cha JM, Son SY, Han SH, Kim JH, Lee WJ, Suh H. Preparation and Characterization of Biodegradable Anti-Adhesive Membrane for Peritoneal Wound Healing. J. Mater. Sci. Mater. Med. 2007;18:475–482. doi: 10.1007/s10856-007-2007-z. [DOI] [PubMed] [Google Scholar]
- (22).Tirelli N, Lutolf MP, Napoli a, Hubbell J. a. Poly(ethylene Glycol) Block Copolymers. J. Biotechnol. 2002;90:3–15. doi: 10.1016/s1389-0352(01)00057-5. [DOI] [PubMed] [Google Scholar]
- (23).Kumar N, Ravikumar MN, Domb a J. Biodegradable Block Copolymers. Adv. Drug Deliv. Rev. 2001;53:23–44. doi: 10.1016/s0169-409x(01)00219-8. [DOI] [PubMed] [Google Scholar]
- (24).Li Z, Tan BH. Towards the Development of Polycaprolactone Based Amphiphilic Block Copolymers: Molecular Design, Self-Assembly and Biomedical Applications. Mater. Sci. Eng. C. 2014:1–15. doi: 10.1016/j.msec.2014.06.003. [DOI] [PubMed] [Google Scholar]
- (25).Tessmar JK, Göpferich AM. Customized PEG-Derived Copolymers for Tissue-Engineering Applications. Macromol. Biosci. 2007;7:23–39. doi: 10.1002/mabi.200600096. [DOI] [PubMed] [Google Scholar]
- (26).Martina M, Hutmacher DW. Biodegradable Polymers Applied in Tissue Engineering Research: A Review. Polym. Int. 2007;56:145–157. [Google Scholar]
- (27).Filion TM, Xu J, Prasad ML, Song J. In Vivo Tissue Responses to Thermal-Responsive Shape Memory Polymer Nanocomposites. Biomaterials. 2011;32:985–991. doi: 10.1016/j.biomaterials.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bergsma JE, de Bruijn WC, Rozema FR, Bos RR, Boering G. Late Degradation Tissue Response to poly(L-Lactide) Bone Plates and Screws. Biomaterials. 1995;16:25–31. doi: 10.1016/0142-9612(95)91092-d. [DOI] [PubMed] [Google Scholar]
- (29).Engelberg I, Kohn J. Physico-Mechanical Properties of Degradable Polymers Used in Medical Applications: A Comparative Study. Biomaterials. 1991;12:292–304. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- (30).Maurus PB, Kaeding CC. Bioabsorbable Implant Material Review. Oper. Tech. Sports Med. 2004;12:158–160. [Google Scholar]
- (31).Miller R. a, Brady JM, Cutright DE. Degradation Rates of Oral Resorbable Implants (polylactates and Polyglycolates): Rate Modification with Changes in PLA/PGA Copolymer Ratios. J. Biomed. Mater. Res. 1977;11:711–719. doi: 10.1002/jbm.820110507. [DOI] [PubMed] [Google Scholar]
- (32).Woodruff MA, Hutmacher DW. The Return of a Forgotten polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010;35:1217–1256. [Google Scholar]
- (33).Middleton JC, Tipton a J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- (34).Radusch H-J. Poly(Butylene Terephthalate) In: Fakirov S, editor. Handbook of Thermoplastic Polyesters. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, FRG: 2002. pp. 389–419. [Google Scholar]
- (35).Shi R, Chen D, Liu Q, Wu Y, Xu X, Zhang L, Tian W. Recent Advances in Synthetic Bioelastomers. Int. J. Mol. Sci. 2009;10:4223–4256. doi: 10.3390/ijms10104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Henton DE, Gruber P, Lunt J, Randall J. Polylactic Acid Technology. In: Mohanty AK, Misra M, Drzal LT, editors. Natural Fibers, Biopolymers, and Biocomposites. Taylor & Francis; Boca Raton, FL: 2005. pp. 527–578. [Google Scholar]
- (37).Huang M-H, Li S, Hutmacher DW, Coudane J, Vert M. Degradation Characteristics of Poly(ε-Caprolactone)-Based Copolymers and Blends. J. Appl. Polym. Sci. 2006;102:1681–1687. [Google Scholar]
- (38).Bulstra SK, Geesink RG, Bakker D, Bulstra TH, Bouwmeester SJ, van der Linden a J. Femoral Canal Occlusion in Total Hip Replacement Using a Resorbable and Flexible Cement Restrictor. J. Bone Joint Surg. Br. 1996;78:892–898. doi: 10.1302/0301-620x78b6.6806. [DOI] [PubMed] [Google Scholar]
- (39).Grote JJ, Bakker D, Hesseling SC, van Blitterswijk CA. New Alloplastic Tympanic Membrane Material. Am. J. Otol. 1991;12:329–335. [PubMed] [Google Scholar]
- (40).Beumer GJ, van Blitterswijk C. a, Ponec M. Biocompatibility of a Biodegradable Matrix Used as a Skin Substitute: An in Vivo Evaluation. J. Biomed. Mater. Res. 1994;28:545–552. doi: 10.1002/jbm.820280504. [DOI] [PubMed] [Google Scholar]
- (41).Deschamps AA, van Apeldoorn AA, Hayen H, de Bruijn JD, Karst U, Grijpma DW, Feijen J. In Vivo and in Vitro Degradation of Poly(ether Ester) Block Copolymers Based on Poly(ethylene Glycol) and Poly(butylene Terephthalate) Biomaterials. 2004;25:247–258. doi: 10.1016/s0142-9612(03)00495-2. [DOI] [PubMed] [Google Scholar]
- (42).Wan Y, Chen W, Yang J, Bei J, Wang S. Biodegradable Poly(l-Lactide)-Poly(ethylene Glycol) Multiblock Copolymer: Synthesis and Evaluation of Cell Affinity. Biomaterials. 2003;24:2195–2203. doi: 10.1016/s0142-9612(03)00107-8. [DOI] [PubMed] [Google Scholar]
- (43).Wurth JJ, Blumenthal NR, Shastri VP. Hydrophilization of Poly(Caprolactone) Copolymers through Introduction of Oligo(Ethylene Glycol) Moieties. PLoS One. 2014;9:e99157. doi: 10.1371/journal.pone.0099157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tang ZG, Black R. a, Curran JM, Hunt J. a, Rhodes NP, Williams DF. Surface Properties and Biocompatibility of Solvent-Cast Poly[e-Caprolactone] Films. Biomaterials. 2004;25:4741–4748. doi: 10.1016/j.biomaterials.2003.12.003. [DOI] [PubMed] [Google Scholar]
- (45).Kutikov AB, Song J. An Amphiphilic Degradable Polymer/hydroxyapatite Composite with Enhanced Handling Characteristics Promotes Osteogenic Gene Expression in Bone Marrow Stromal Cells. Acta Biomater. 2013;9:8354–8364. doi: 10.1016/j.actbio.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Grafahrend D, Heffels K-H, Beer MV, Gasteier P, Möller M, Boehm G, Dalton PD, Groll J. Degradable Polyester Scaffolds with Controlled Surface Chemistry Combining Minimal Protein Adsorption with Specific Bioactivation. Nat. Mater. 2011;10:67–73. doi: 10.1038/nmat2904. [DOI] [PubMed] [Google Scholar]
- (47).Grafahrend D, Calvet JL, Klinkhammer K, Salber J, Dalton PD, Möller M, Klee D. Control of Protein Adsorption on Functionalized Electrospun Fibers. Biotechnol. Bioeng. 2008;101:609–621. doi: 10.1002/bit.21928. [DOI] [PubMed] [Google Scholar]
- (48).Park JS, Woo DG, Sun BK, Chung H-M, Im SJ, Choi YM, Park K, Huh KM, Park K-H. In Vitro and in Vivo Test of PEG/PCL-Based Hydrogel Scaffold for Cell Delivery Application. J. Control. Release. 2007;124:51–59. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- (49).Radder a M., Leenders H, van Blitterswijk C. a. Interface Reactions to PEO/PBT Copolymers (Polyactive) after Implantation in Cortical Bone. J. Biomed. Mater. Res. 1994;28:141–151. doi: 10.1002/jbm.820280202. [DOI] [PubMed] [Google Scholar]
- (50).Williams DF. On the Mechanisms of Biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- (51).Burkersroda F. von, Schedl L, Göpferich A. Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- (52).Dong Y, Liao S, Ngiam M, Chan CK, Ramakrishna S. Degradation Behaviors of Electrospun Resorbable Polyester Nanofibers. Tissue Eng., Part B. 2009;15:333–351. doi: 10.1089/ten.TEB.2008.0619. [DOI] [PubMed] [Google Scholar]
- (53).Li S, Rashkov I, Espartero J, Vert M, Manolova N. Synthesis, Characterization, and Hydrolytic Degradation of PLA/PEO/PLA Triblock Copolymers with Short Poly(L-Lactic Acid) Chains. Macromolecules. 1996;29:50–56. [Google Scholar]
- (54).Li S, Rashkov I, Espartero J, Vert M, Manolova N. Synthesis , Characterization , and Hydrolytic Degradation of PLA/PEO/PLA Triblock Copolymers with Long Poly (L-Lactic Acid) Blocks. Macromolecules. 1996;29:50–56. [Google Scholar]
- (55).Cohn D, Younes H. Compositional and Structural Analysis of PELA Biodegradable Block Copolymers Degrading under in Vitro Conditions. Biomaterials. 1989;10:466–474. doi: 10.1016/0142-9612(89)90088-4. [DOI] [PubMed] [Google Scholar]
- (56).Huang M-H, Li S, Hutmacher DW, Schantz J-T, Vacanti CA, Braud C, Vert M. Degradation and Cell Culture Studies on Block Copolymers Prepared by Ring Opening Polymerization of Epsilon-Caprolactone in the Presence of Poly(ethylene Glycol) J. Biomed. Mater. Res., Part A. 2004;69:417–427. doi: 10.1002/jbm.a.30008. [DOI] [PubMed] [Google Scholar]
- (57).Hu D, Liu H. Structural Analysis and Degradation Behavior in Polyethylene Glycol/Poly(L-Lactide) Copolymers. J. Appl. Polym. Sci. 1994;51:473–482. [Google Scholar]
- (58).Bei J, Li J, Wang Z, Le J, Wang S-G. Polycaprolactone-Poly(ethylene-Glycol) Block Copolymer. IV: Biodegradation Behaviorin Vitro Andin Vivo. Polym. Adv. Technol. 1997;8:693–696. [Google Scholar]
- (59).Nagata M, Kitazima I. Photocurable Biodegradable Poly(ε-Caprolactone)/poly(ethylene Glycol) Multiblock Copolymers Showing Shape-Memory Properties. Colloid Polym. Sci. 2005;284:380–386. [Google Scholar]
- (60).Zhang A, Feng Z, Xie Z. Long-Term Investigation on Hydrolytic Degradation and Morphology of Poly ( Ethylene Glycol Terephthalate ) - B - Poly ( Butylene Terephthalate ) Copolymer Films. J. Appl. Polym. Sci. 2009;111:1462–1470. [Google Scholar]
- (61).Patel A, Gaharwar AK, Iviglia G, Zhang H, Mukundan S, Mihaila SM, Demarchi D, Khademhosseini A. Highly Elastomeric Poly(glycerol Sebacate)-Co-Poly(ethylene Glycol) Amphiphilic Block Copolymers. Biomaterials. 2013;34:3970–3983. doi: 10.1016/j.biomaterials.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Lutolf MP, Hubbell JA. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- (63).Brandl F, Sommer F, Goepferich A. Rational Design of Hydrogels for Tissue Engineering: Impact of Physical Factors on Cell Behavior. Biomaterials. 2007;28:134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- (64).Fonseca KB, Granja PL, Barrias CC. Engineering Proteolytically-Degradable Artificial Extracellular Matrices. Prog. Polym. Sci. 2013;39:2010–2029. [Google Scholar]
- (65).Kulinski Z, Piorkowska E. Crystallization, Structure and Properties of Plasticized Poly(l-Lactide) Polymer. 2005;46:10290–10300. [Google Scholar]
- (66).Deschamps A. a, Claase MB, Sleijster WJ, de Bruijn JD, Grijpma DW, Feijen J. Design of Segmented Poly(ether Ester) Materials and Structures for the Tissue Engineering of Bone. J. Control. Release. 2002;78:175–186. doi: 10.1016/s0168-3659(01)00497-7. [DOI] [PubMed] [Google Scholar]
- (67).Cohn D, Younes H. Biodegradable PEO/PLA Block Copolymers. J. Biomed. Mater. Res. 1988;22:993–1009. doi: 10.1002/jbm.820221104. [DOI] [PubMed] [Google Scholar]
- (68).Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- (69).Kutikov AB, Gurijala A, Song J. Rapid Prototyping Amphiphilic Polymer/Hydroxyapatite Composite Scaffolds with Hydration-Induced Self-Fixation Behavior. Tissue Eng., Part C. 2014 doi: 10.1089/ten.tec.2014.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Bedoui F, Widjaja LK, Luk A, Bolikal D, Murthy NS, Kohn J. Anomalous Increase in Modulus upon Hydration in Random Copolymers with Hydrophobic Segments and Hydrophilic Blocks. Soft Matter. 2012;8:2230. [Google Scholar]
- (71).Xu J, Bohnsack D. a, Mackay ME, Wooley KL. Unusual Mechanical Performance of Amphiphilic Crosslinked Polymer Networks. J. Am. Chem. Soc. 2007;129:506–507. doi: 10.1021/ja067986i. [DOI] [PubMed] [Google Scholar]
- (72).Murthy NS, Wang W, Kohn J. Microphase Separation in Copolymers of Hydrophilic PEG Blocks and Hydrophobic Tyrosine-Derived Segments Using Simultaneous SAXS/WAXS/DSC. Polymer. 2010;51:3978–3988. doi: 10.1016/j.polymer.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Vemuganti A, Siegler S, Abusafieh A, Kalidindi S. Development of Self-Anchoring Bone Implants. II. Bone-Implant Interface Characteristics in Vitro. J. Biomed. Mater. Res. 1997;38:328–336. doi: 10.1002/(sici)1097-4636(199724)38:4<328::aid-jbm4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- (74).Phillips JH, Rahn BA. Fixation Effects on Membranous and Endochondral Onlay Bone-Graft Resorption. Plast. Reconstr. Surg. 1988;82:872–877. doi: 10.1097/00006534-198811000-00023. [DOI] [PubMed] [Google Scholar]
- (75).Lendlein A, Langer R. Biodegradable, Elastic Shape-Memory Polymers for Potential Biomedical Applications. Science. 2002;296:1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- (76).Liu C, Qin H, Mather PT. Review of Progress in Shape-Memory Polymers. J. Mater. Chem. 2007;17:1543. [Google Scholar]
- (77).Tseng L-F, Mather PT, Henderson JH. Shape-Memory-Actuated Change in Scaffold Fiber Alignment Directs Stem Cell Morphology. Acta Biomater. 2013;9:8790–8801. doi: 10.1016/j.actbio.2013.06.043. [DOI] [PubMed] [Google Scholar]
- (78).Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally Invasive Approach to the Repair of Injured Skeletal Muscle With a Shape-Memory Scaffold. Mol. Ther. 2014;22:1441–1449. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Baker RM, Henderson JH, Mather PT. Shape Memory Poly(ε-Caprolactone)-Co-Poly(ethylene Glycol) Foams with Body Temperature Triggering and Two-Way Actuation. J. Mater. Chem. B. 2013;1:4916. doi: 10.1039/c3tb20810a. [DOI] [PubMed] [Google Scholar]
- (80).Feng Y, Zhang S, Zhang L, Guo J, Xu Y. Synthesis and Characterization of Hydrophilic Polyester-PEO Networks with Shape-Memory Properties. Polym. Adv. Technol. 2011;22:2430–2438. [Google Scholar]
- (81).Gu X, Mather PT. Entanglement-Based Shape Memory Polyurethanes: Synthesis and Characterization. Polymer. 2012;53:5924–5934. [Google Scholar]
- (82).Kutikov AB, Reyer KA, Song J. Shape-Memory Performance of Thermoplastic Amphiphilic Triblock Copolymer Poly(d,l-Lactic Acid-Co-Ethylene Glycol-Co-D,l-Lactic Acid) (PELA)/Hydroxyapatite Composites. Macromol. Chem. Phys. 2014;215:2482–2490. doi: 10.1002/macp.201400340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. State of the Art and Future Directions of Scaffold-Based Bone Engineering from a Biomaterials Perspective. J. Tissue Eng. Regen. Med. 2007;1:245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- (84).Dorozhkin SV. Calcium Orthophosphate-Based Biocomposites and Hybrid Biomaterials. J. Mater. Sci. 2009;44:2343–2387. [Google Scholar]
- (85).Neuendorf RE, Saiz E, Tomsia a P., Ritchie RO. Adhesion between Biodegradable Polymers and Hydroxyapatite: Relevance to Synthetic Bone-like Materials and Tissue Engineering Scaffolds. Acta Biomater. 2008;4:1288–1296. doi: 10.1016/j.actbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- (86).Zhou H, Lawrence JG, Bhaduri SB. Fabrication Aspects of PLA-CaP/PLGA-CaP Composites for Orthopedic Applications: A Review. Acta Biomater. 2012;8:1999–2016. doi: 10.1016/j.actbio.2012.01.031. [DOI] [PubMed] [Google Scholar]
- (87).Supová M. Problem of Hydroxyapatite Dispersion in Polymer Matrices: A Review. J. Mater. Sci. Mater. Med. 2009;20:1201–1213. doi: 10.1007/s10856-009-3696-2. [DOI] [PubMed] [Google Scholar]
- (88).Gaharwar AK, Dammu S. a, Canter JM, Wu C-J, Schmidt G. Highly Extensible, Tough, and Elastomeric Nanocomposite Hydrogels from Poly(ethylene Glycol) and Hydroxyapatite Nanoparticles. Biomacromolecules. 2011;12:1641–1650. doi: 10.1021/bm200027z. [DOI] [PubMed] [Google Scholar]
- (89).Song J, Xu J, Filion T, Saiz E, Tomsia AP, Lian JB, Stein GS, Ayers DC, Bertozzi CR. Elastomeric High-Mineral Content Hydrogel-Hydroxyapatite Composites for Orthopedic Applications. J. Biomed. Mater. Res., Part A. 2009;89:1098–1107. doi: 10.1002/jbm.a.32110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Filion TM, Li X, Mason-Savas A, Kreider JM, Goldstein SA, Ayers DC, Song J. Elastomeric Osteoconductive Synthetic Scaffolds with Acquired Osteoinductivity Expedite the Repair of Critical Femoral Defects in Rats. Tissue Eng., Part A. 2011;17:503–511. doi: 10.1089/ten.tea.2010.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Skelly JD, Lange J, Filion TM, Li X, Ayers DC, Song J. Vancomycin-Bearing Synthetic Bone Graft Delivers rhBMP-2 and Promotes Healing of Critical Rat Femoral Segmental Defects. Clin. Orthop. Relat. Res. 2014 doi: 10.1007/s11999-014-3841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Fu S, Wang X, Guo G, Shi S, Liang H, Luo F, Wei Y, Qian Z. Preparation and Characterization of Nano-Hydroxyapatite/Poly(ε-caprolactone)−Poly(ethylene glycol)−Poly(ε-Caprolactone) Composite Fibers for Tissue Engineering. J. Phys. Chem. C. 2010;114:18372–18378. [Google Scholar]
- (93).Fu SZ, Wang XH, Guo G, Shi S, Fan M, Liang H, Luo F, Qian ZY. Preparation and Properties of Nano-hydroxyapatite/PCL-PEG-PCL Composite Membranes for Tissue Engineering Applications. J. Biomed. Mater. Res., Part B. 2011;97:74–83. doi: 10.1002/jbm.b.31788. [DOI] [PubMed] [Google Scholar]
- (94).Fu S, Ni P, Wang B, Chu B, Zheng L, Luo F, Luo J, Qian Z. Injectable and Thermo-Sensitive PEG-PCL-PEG Copolymer/collagen/n-HA Hydrogel Composite for Guided Bone Regeneration. Biomaterials. 2012;33:4801–4809. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- (95).Fu S, Ni P, Wang B, Chu B, Peng J, Zheng L, Zhao X, Luo F, Wei Y, Qian Z. In Vivo Biocompatibility and Osteogenesis of Electrospun Poly(ε-Caprolactone)-Poly(ethylene Glycol)-Poly(ε-Caprolactone)/nano-Hydroxyapatite Composite Scaffold. Biomaterials. 2012;33:8363–8371. doi: 10.1016/j.biomaterials.2012.08.023. [DOI] [PubMed] [Google Scholar]
- (96).Kaito T, Myoui A, Takaoka K, Saito N, Nishikawa M, Tamai N, Ohgushi H, Yoshikawa H. Potentiation of the Activity of Bone Morphogenetic Protein-2 in Bone Regeneration by a PLA-PEG/hydroxyapatite Composite. Biomaterials. 2005;26:73–79. doi: 10.1016/j.biomaterials.2004.02.010. [DOI] [PubMed] [Google Scholar]
- (97).Yoneda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K. Repair of an Intercalated Long Bone Defect with a Synthetic Biodegradable Bone-Inducing Implant. Biomaterials. 2005;26:5145–5152. doi: 10.1016/j.biomaterials.2005.01.054. [DOI] [PubMed] [Google Scholar]
- (98).Lin G, Cosimbescu L, Karin NJ, Tarasevich BJ. Injectable and Thermosensitive PLGA-G-PEG Hydrogels Containing Hydroxyapatite: Preparation, Characterization and in Vitro Release Behavior. Biomed. Mater. 2012;7:024107. doi: 10.1088/1748-6041/7/2/024107. [DOI] [PubMed] [Google Scholar]
- (99).Arima Y, Iwata H. Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-Assembled Monolayers. Biomaterials. 2007;28:3074–3082. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- (100).Lee J, Khang G, Lee J, Lee H. Interaction of Different Types of Cells on Polymer Surfaces with Wettability Gradient. J. Colloid Interface Sci. 1998;205:323–330. doi: 10.1006/jcis.1998.5688. [DOI] [PubMed] [Google Scholar]
- (101).Bhushan B, Schricker SR. A Review of Block Copolymer-Based Biomaterials That Control Protein and Cell Interactions. J. Biomed. Mater. Res., Part A. 2014;102:2467–2480. doi: 10.1002/jbm.a.34887. [DOI] [PubMed] [Google Scholar]
- (102).Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- (103).Keselowsky BG, Collard DM, García AJ. Surface Chemistry Modulates Fibronectin Conformation and Directs Integrin Binding and Specificity to Control Cell Adhesion. J. Biomed. Mater. Res., Part A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- (104).Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S. Engineering the Cell-Material Interface for Controlling Stem Cell Adhesion, Migration, and Differentiation. Biomaterials. 2011;32:3700–3711. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]
- (105).Tziampazis E, Kohn J, Moghe PV. PEG-Variant Biomaterials as Selectively Adhesive Protein Templates: Model Surfaces for Controlled Cell Adhesion and Migration. Biomaterials. 2000;21:511–520. doi: 10.1016/s0142-9612(99)00212-4. [DOI] [PubMed] [Google Scholar]
- (106).Göpferich A, Peter SJ, Lucke A, Lu L, Mikos AG. Modulation of Marrow Stromal Cell Function Using poly(D,L-Lactic Acid)-Block-Poly(ethylene Glycol)-Monomethyl Ether Surfaces. J. Biomed. Mater. Res. 1999;46:390–398. doi: 10.1002/(sici)1097-4636(19990905)46:3<390::aid-jbm12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- (107).Lieb E, Tessmar J, Hacker M, Fischbach C, Rose D, Blunk T, Mikos AG, Göpferich A, Schulz MMB, Tessmar J, Gopferich A. Poly (D, L-Lactic Acid)-Poly (ethylene Glycol)-Monomethyl Ether Diblock Copolymers Control Adhesion and Osteoblastic Differentiation of Marrow Stromal Cells. Tissue Eng. 2003;9:71–84. doi: 10.1089/107632703762687555. [DOI] [PubMed] [Google Scholar]
- (108).Gupta MK, Walthall JM, Venkataraman R, Crowder SW, Jung DK, Yu SS, Feaster TK, Wang X, Giorgio TD, Hong CC, Baudenbacher FJ, Hatzopoulos AK, Sung H. Combinatorial Polymer Electrospun Matrices Promote Physiologically-Relevant Cardiomyogenic Stem Cell Differentiation. PLoS One. 2011;6:e28935. doi: 10.1371/journal.pone.0028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Ma C, Pang D, Xiong Z, Bai W, Xiong C. Cellular Responses to Electrospun Membranes Made from Blends of PLLGA with PEG and PLLGA-B-PEG. J. Biomed. Mater. Res., Part A. 2012:1–8. doi: 10.1002/jbm.a.34226. [DOI] [PubMed] [Google Scholar]
- (110).Peng H, Xiao Y, Mao X, Chen L, Crawford R, Whittaker AK. Amphiphilic Triblock Copolymers of Methoxy-Poly(ethylene Glycol)-B-poly(L-Lactide)-B-poly(L-Lysine) for Enhancement of Osteoblast Attachment and Growth. Biomacromolecules. 2009;10:95–104. doi: 10.1021/bm800937g. [DOI] [PubMed] [Google Scholar]
- (111).Mahmood T. a, de Jong R, Riesle J, Langer R, van Blitterswijk C. a. Adhesion-Mediated Signal Transduction in Human Articular Chondrocytes: The Influence of Biomaterial Chemistry and Tenascin-C. Exp. Cell Res. 2004;301:179–188. doi: 10.1016/j.yexcr.2004.07.027. [DOI] [PubMed] [Google Scholar]
- (112).Moroni L, Hendriks J. a a, Schotel R, de Wijn JR, van Blitterswijk C. a. Design of Biphasic Polymeric 3-Dimensional Fiber Deposited Scaffolds for Cartilage Tissue Engineering Applications. Tissue Eng. 2007;13:361–371. doi: 10.1089/ten.2006.0127. [DOI] [PubMed] [Google Scholar]
- (113).Hendriks J. a a, Moroni L, Riesle J, de Wijn JR, van Blitterswijk C. a. The Effect of Scaffold-Cell Entrapment Capacity and Physico-Chemical Properties on Cartilage Regeneration. Biomaterials. 2013;34:4259–4265. doi: 10.1016/j.biomaterials.2013.02.060. [DOI] [PubMed] [Google Scholar]
- (114).Patel N, Padera R, Sanders GH, Cannizzaro SM, Davies MC, Langer R, Roberts CJ, Tendler SJ, Williams PM, Shakesheff KM. Spatially Controlled Cell Engineering on Biodegradable Polymer Surfaces. FASEB J. 1998;12:1447–1454. doi: 10.1096/fasebj.12.14.1447. [DOI] [PubMed] [Google Scholar]
- (115).Adams ML, Lavasanifar A, Kwon GS. Amphiphilic Block Copolymers for Drug Delivery. J. Pharm. Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- (116).Tessmar JK, Göpferich AM. Matrices and Scaffolds for Protein Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- (117).Lissenberg-Thunnissen SN, de Gorter DJJ, Sier CFM, Schipper IB. Use and Efficacy of Bone Morphogenetic Proteins in Fracture Healing. Int. Orthop. 2011;35:1271–1280. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Miyamoto S, Takaoka K, Okada T, Yoshikawa H, Hashimoto J, Suzuki S, Ono K. Polylactic Acid-Polyethylene Glycol Block Copolymer. A New Biodegradable Synthetic Carrier for Bone Morphogenetic Protein. Clin. Orthop. Relat. Res. 1993:333–343. [PubMed] [Google Scholar]
- (119).Saito N, Okada T, Toba S, Miyamoto S, Takaoka K. New Synthetic Absorbable Polymers as BMP Carriers: Plastic Properties of Poly-D,L-Lactic Acid-Polyethylene Glycol Block Copolymers. J. Biomed. Mater. Res. 1999;47:104–110. doi: 10.1002/(sici)1097-4636(199910)47:1<104::aid-jbm15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- (120).Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K. A Biodegradable Polymer as a Cytokine Delivery System for Inducing Bone Formation. Nat. Biotechnol. 2001;19:332–335. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- (121).Kato M, Toyoda H, Namikawa T, Hoshino M, Terai H, Miyamoto S, Takaoka K. Optimized Use of a Biodegradable Polymer as a Carrier Material for the Local Delivery of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Biomaterials. 2006;27:2035–2041. doi: 10.1016/j.biomaterials.2005.10.007. [DOI] [PubMed] [Google Scholar]
- (122).Moroni L, Licht R, de Boer J, de Wijn JR, van Blitterswijk C. a. Fiber Diameter and Texture of Electrospun PEOT/PBT Scaffolds Influence Human Mesenchymal Stem Cell Proliferation and Morphology, and the Release of Incorporated Compounds. Biomaterials. 2006;27:4911–4922. doi: 10.1016/j.biomaterials.2006.05.027. [DOI] [PubMed] [Google Scholar]
- (123).Gaharwar AK, Mihaila SM, Kulkarni A. a, Patel A, Di Luca A, Reis RL, Gomes ME, van Blitterswijk C, Moroni L, Khademhosseini A. Amphiphilic Beads as Depots for Sustained Drug Release Integrated into Fibrillar Scaffolds. J. Control. Release. 2014;187:66–73. doi: 10.1016/j.jconrel.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and Controlled Release of a Hydrophilic Antibiotic Using Poly(lactide-Co-Glycolide)-Based Electrospun Nanofibrous Scaffolds. J. Control. Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- (125).Zhang J, Song J. Amphiphilic Degradable Polymers for Immobilization and Sustained Delivery of Sphingosine 1-Phosphate. Acta Biomater. 2014;10:3079–3090. doi: 10.1016/j.actbio.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-Phosphate Accelerates Wound Healing in Diabetic Mice. J. Dermatol. Sci. 2007;48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- (127).Qi X, Okamoto Y, Murakawa T, Wang F, Oyama O, Ohkawa R, Yoshioka K, Du W, Sugimoto N, Yatomi Y, Takuwa N, Takuwa Y. Sustained Delivery of Sphingosine-1-Phosphate Using Poly(lactic-Co-Glycolic Acid)-Based Microparticles Stimulates Akt/ERK-eNOS Mediated Angiogenesis and Vascular Maturation Restoring Blood Flow in Ischemic Limbs of Mice. Eur. J. Pharmacol. 2010;634:121–131. doi: 10.1016/j.ejphar.2010.02.038. [DOI] [PubMed] [Google Scholar]
- (128).Sakkers RJ, Dalmeyer R. a, de Wijn JR, van Blitterswijk C. a. Use of Bone-Bonding Hydrogel Copolymers in Bone: An in Vitro and in Vivo Study of Expanding PEO-PBT Copolymers in Goat Femora. J. Biomed. Mater. Res. 2000;49:312–318. doi: 10.1002/(sici)1097-4636(20000305)49:3<312::aid-jbm3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- (129).Roessler M, Wilke a, Griss P, Kienapfel H. Missing Osteoconductive Effect of a Resorbable PEO/PBT Copolymer in Human Bone Defects: A Clinically Relevant Pilot Study with Contrary Results to Previous Animal Studies. J. Biomed. Mater. Res. 2000;53:167–173. doi: 10.1002/(sici)1097-4636(2000)53:2<167::aid-jbm6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- (130).Waris E, Ashammakhi N, Lehtimäki M, Tulamo R-M, Törmälä P, Kellomäki M, Konttinen YT. Long-Term Bone Tissue Reaction to Polyethylene Oxide/polybutylene Terephthalate Copolymer (Polyactive) in Metacarpophalangeal Joint Reconstruction. Biomaterials. 2008;29:2509–2515. doi: 10.1016/j.biomaterials.2008.02.013. [DOI] [PubMed] [Google Scholar]
- (131).Du C, Meijer GJ, van de Valk C, Haan RE, Bezemer JM, Hesseling SC, Cui FZ, de Groot K, Layrolle P. Bone Growth in Biomimetic Apatite Coated Porous Polyactive 1000PEGT70PBT30 Implants. Biomaterials. 2002;23:4649–4656. doi: 10.1016/s0142-9612(02)00214-4. [DOI] [PubMed] [Google Scholar]
- (132).Nandakumar A, Fernandes H, de Boer J, Moroni L, Habibovic P, van Blitterswijk C. a. Fabrication of Bioactive Composite Scaffolds by Electrospinning for Bone Regeneration. Macromol. Biosci. 2010;10:1365–1373. doi: 10.1002/mabi.201000145. [DOI] [PubMed] [Google Scholar]
- (133).Nandakumar A, Yang L, Habibovic P, van Blitterswijk C. Calcium Phosphate Coated Electrospun Fiber Matrices as Scaffolds for Bone Tissue Engineering. Langmuir. 2010;26:7380–7387. doi: 10.1021/la904406b. [DOI] [PubMed] [Google Scholar]
- (134).Ren J, Ren T, Zhao P, Huang Y, Pan K. Repair of Mandibular Defects Using MSCs-Seeded Biodegradable Polyester Porous Scaffolds. J. Biomater. Sci. Polym. Ed. 2007;18:505–517. doi: 10.1163/156856207780852578. [DOI] [PubMed] [Google Scholar]
- (135).Lin Z-Y, Duan Z-X, Guo X-D, Li J-F, Lu H-W, Zheng Q-X, Quan D-P, Yang S-H. Bone Induction by Biomimetic PLGA-(PEG-ASP)n Copolymer Loaded with a Novel Synthetic BMP-2-Related Peptide in Vitro and in Vivo. J. Control. Release. 2010;144:190–195. doi: 10.1016/j.jconrel.2010.02.016. [DOI] [PubMed] [Google Scholar]
- (136).Kutikov AB, Skelly JD, Ayers DC, Song J. Templated Repair of Long Bone Defects in Rats with Bioactive Spiral-Wrapped Electrospun Amphiphilic Polymer/Hydroxyapatite Scaffolds. ACS Appl. Mater. Interfaces. 2015 doi: 10.1021/am508984y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Kim MS, Kim SK, Kim SH, Hyun H, Khang G, Lee HB. In Vivo Osteogenic Differentiation of Rat Bone Marrow Stromal Cells in Thermosensitive MPEG-PCL Diblock Copolymer Gels. Tissue Eng. 2006;12:2863–2873. doi: 10.1089/ten.2006.12.2863. [DOI] [PubMed] [Google Scholar]
- (138).Ni P-Y, Fan M, Qian Z-Y, Luo J-C, Gong C-Y, Fu S-Z, Shi S, Luo F, Yang Z-M. Synthesis and Characterization of Injectable, Thermosensitive, and Biocompatible Acellular Bone Matrix/poly(ethylene Glycol)-Poly (ε-Caprolactone)-Poly(ethylene Glycol) Hydrogel Composite. J. Biomed. Mater. Res., Part A. 2012;100:171–179. doi: 10.1002/jbm.a.33262. [DOI] [PubMed] [Google Scholar]
- (139).Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J, Li X, Luo F, Yang Z, Wei Y. Injectable Thermosensitive PEG-PCL-PEG Hydrogel/acellular Bone Matrix Composite for Bone Regeneration in Cranial Defects. Biomaterials. 2014;35:236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- (140).Fu S, Guo G, Gong C, Zeng S, Liang H, Luo F, Zhang X, Zhao X, Wei Y, Qian Z. Injectable Biodegradable Thermosensitive Hydrogel Composite for Orthopedic Tissue Engineering. 1. Preparation and Characterization of Nanohydroxyapatite/poly(ethylene Glycol)-Poly(epsilon-Caprolactone)-Poly(ethylene Glycol) Hydrogel Nanocomposites. J. Phys. Chem. B. 2009;113:16518–16525. doi: 10.1021/jp907974d. [DOI] [PubMed] [Google Scholar]
- (141).Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Temenoff JS, Mikos AG. Review: Tissue Engineering for Regeneration of Articular Cartilage. Biomaterials. 2000;21:431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- (143).Hansen OM, Foldager CB, Christensen BB, Everland H, Lind M. Increased Chondrocyte Seeding Density Has No Positive Effect on Cartilage Repair in an MPEG-PLGA Scaffold. Knee Surg. Sports Traumatol. Arthrosc. 2013;21:485–493. doi: 10.1007/s00167-012-1996-4. [DOI] [PubMed] [Google Scholar]
- (144).Keeney M, Lai JH, Yang F. Recent Progress in Cartilage Tissue Engineering. Curr. Opin. Biotechnol. 2011;22:734–740. doi: 10.1016/j.copbio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- (145).Sims CD, Butler PE, Casanova R, Lee BT, Randolph MA, Lee WP, Vacanti CA, Yaremchuk MJ. Injectable Cartilage Using Polyethylene Oxide Polymer Substrates. Plast. Reconstr. Surg. 1996;98:843–850. doi: 10.1097/00006534-199610000-00015. [DOI] [PubMed] [Google Scholar]
- (146).Jansen EJP, Pieper J, Gijbels MJJ, Guldemond N. a, Riesle J, Van Rhijn LW, Bulstra SK, Kuijer R. PEOT/PBT Based Scaffolds with Low Mechanical Properties Improve Cartilage Repair Tissue Formation in Osteochondral Defects. J. Biomed. Mater. Res., Part A. 2009;89:444–452. doi: 10.1002/jbm.a.31986. [DOI] [PubMed] [Google Scholar]
- (147).Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, Takaoka K, Yoshikawa H. A New Biotechnology for Articular Cartilage Repair: Subchondral Implantation of a Composite of Interconnected Porous Hydroxyapatite, Synthetic Polymer (PLA-PEG), and Bone Morphogenetic Protein-2 (rhBMP-2) Osteoarthritis Cartilage. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- (148).Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M. Transforming Growth Factor Beta-Releasing Scaffolds for Cartilage Tissue Engineering. Tissue Eng., Part B. 2014;20:106–125. doi: 10.1089/ten.TEB.2013.0271. [DOI] [PubMed] [Google Scholar]
- (149).Lind M, Larsen A, Clausen C, Osther K, Everland H. Cartilage Repair with Chondrocytes in Fibrin Hydrogel and MPEG Polylactide Scaffold: An in Vivo Study in Goats. Knee Surg. Sports Traumatol. Arthrosc. 2008;16:690–698. doi: 10.1007/s00167-008-0522-1. [DOI] [PubMed] [Google Scholar]
- (150).Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Søballe K, Bünger C, Lind M. Combined 3D and Hypoxic Culture Improves Cartilage-Specific Gene Expression in Human Chondrocytes. Acta Orthop. 2011;82:234–240. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical Animal Models in Single Site Cartilage Defect Testing: A Systematic Review. Osteoarthritis Cartilage. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- (152).Chu CR, Szczodry M, Bruno S. Animal Models for Cartilage Regeneration and Repair. Tissue Eng., Part B. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Bakker D, van Blitterswijk C. a, Hesseling SC, Koerten HK, Kuijpers W, Grote JJ. Biocompatibility of a Polyether Urethane, Polypropylene Oxide, and a Polyether Polyester Copolymer. A Qualitative and Quantitative Study of Three Alloplastic Tympanic Membrane Materials in the Rat Middle Ear. J. Biomed. Mater. Res. 1990;24:489–515. doi: 10.1002/jbm.820240407. [DOI] [PubMed] [Google Scholar]
- (154).Bakker D, van Blitterswijk C. a, Hesseling SC, Daems WT, Grote JJ. The Behavior of Alloplastic Tympanic Membranes in Staphylococcus Aureus-Induced Middle Ear Infection. II. Morphological Study of Epithelial Reactions. J. Biomed. Mater. Res. 1990;24:809–828. doi: 10.1002/jbm.820240703. [DOI] [PubMed] [Google Scholar]
- (155).Bakker D, van Blitterswijk C. a, Hesseling SC, Daems WT, Kuijpers W, Grote JJ. The Behavior of Alloplastic Tympanic Membranes in Staphylococcus Aureus-Induced Middle Ear Infection. I. Quantitative Biocompatibility Evaluation. J. Biomed. Mater. Res. 1990;24:669–688. doi: 10.1002/jbm.820240604. [DOI] [PubMed] [Google Scholar]
- (156).Beumer GJ, van Blitterswijk C. a, Bakker D, Ponec M. Cell-Seeding and in Vitro Biocompatibility Evaluation of Polymeric Matrices of PEO/PBT Copolymers and PLLA. Biomaterials. 1993;14:598–604. doi: 10.1016/0142-9612(93)90178-5. [DOI] [PubMed] [Google Scholar]
- (157).Van Dorp a G., Verhoeven MC, Koerten HK, Van Der Nat-Van Der Meij TH, Van Blitterswijk C. a, Ponec M. Dermal Regeneration in Full-Thickness Wounds in Yucatan Miniature Pigs Using a Biodegradable Copolymer. Wound repair Regen. 1998;6:556–568. doi: 10.1046/j.1524-475x.1998.60608.x. [DOI] [PubMed] [Google Scholar]
- (158).Mensik I, Lamme EN, Riesle J, Brychta P. Effectiveness and Safety of the PEGT/PBT Copolymer Scaffold as Dermal Substitute in Scar Reconstruction Wounds (Feasibility Trial) Cell Tissue Bank. 2002;3:245–253. doi: 10.1023/A:1024674325253. [DOI] [PubMed] [Google Scholar]
- (159).Choi JS, Leong KW, Yoo HS. In Vivo Wound Healing of Diabetic Ulcers Using Electrospun Nanofibers Immobilized with Human Epidermal Growth Factor (EGF) Biomaterials. 2008;29:587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- (160).Madigan NN, McMahon S, O’Brien T, Yaszemski MJ, Windebank AJ. Current Tissue Engineering and Novel Therapeutic Approaches to Axonal Regeneration Following Spinal Cord Injury Using Polymer Scaffolds. Respir. Physiol. Neurobiol. 2009;169:183–199. doi: 10.1016/j.resp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Pabari A, Yang SY, Seifalian AM, Mosahebi A. Modern Surgical Management of Peripheral Nerve Gap. J. Plast. Reconstr. Aesthet. Surg. 2010;63:1941–1948. doi: 10.1016/j.bjps.2009.12.010. [DOI] [PubMed] [Google Scholar]
- (162).Maquet V, Martin D, Scholtes F, Franzen R, Schoenen J, Moonen G, Jér me R. Poly(D,L-Lactide) Foams Modified by Poly(ethylene Oxide)-Block-poly(D,L-Lactide) Copolymers and a-FGF: In Vitro and in Vivo Evaluation for Spinal Cord Regeneration. Biomaterials. 2001;22:1137–1146. doi: 10.1016/s0142-9612(00)00357-4. [DOI] [PubMed] [Google Scholar]
- (163).Li G, Li D, Niu Y, He T, Chen KC, Xu K. Alternating Block Polyurethanes Based on PCL and PEG as Potential Nerve Regeneration Materials. J. Biomed. Mater. Res., Part A. 2014;102:685–697. doi: 10.1002/jbm.a.34732. [DOI] [PubMed] [Google Scholar]
- (164).Niu Y, Chen KC, He T, Yu W, Huang S, Xu K. Scaffolds from Block Polyurethanes Based on Poly(ε-Caprolactone) (PCL) and Poly(ethylene Glycol) (PEG) for Peripheral Nerve Regeneration. Biomaterials. 2014;35:4266–4277. doi: 10.1016/j.biomaterials.2014.02.013. [DOI] [PubMed] [Google Scholar]