Abstract
Objectives:
Most current prescription labels fail to meet print guidelines, especially in print size. We therefore compared the legibility of current prescription medication labels against the legibility of prototype labels, based on current guidelines for legibility.
Method:
Sample medication labels were obtained from pharmacies, and prototype medication labels were developed according to legibility guidelines from nongovernmental organizations and pharmacy organizations. Three groups of participants, consisting of older adults with normal vision, older adults with visual impairment and younger adults with visual impairment (total N = 71) took part. Participants were asked to read and rank the labels. Reading speed and accuracy were determined.
Results:
Accuracies were high (75%–100%), and there were no significant differences between samples or prototypes or between groups. Prototypes, however, were read faster than samples (p < 0.001). Subjectively, participants preferred the largest print option (p < 0.001) and instructions with the numbers written in highlighted uppercase words (p < 0.001).
Discussion:
The results indicate that improvements to the label would include larger print size, a consistent layout with left justification and using upper case with highlighting for emphasis of the numbers in the instructions.
Knowledge into Practice.
Legibility of prescription medication labels is important to enhance correct medication use and adherence.
Legibility can be improved without changing current technology or label size.
A combination of larger print, consistent layout, left justification, overall lowercase lettering (but with upper case for numbers in the instructions) and highlighting in yellow can improve reading speed and patient preference of older adults and people with visual impairment, thus improving universal accessibility.
Mise En Pratique Des Connaissances.
Il est important que l’étiquette des médicaments sur ordonnance soit lisible pour améliorer leur bon usage et l’observance.
Il est possible d’améliorer la lisibilité sans changer la technologie actuelle ou la taille des étiquettes.
On peut améliorer la vitesse de lecture et la préférence des patients âgés et des personnes présentant un déficit visuel, et ainsi l’accessibilité universelle, en utilisant de larges caractères, une mise en page uniforme, une justification à gauche, des lettres minuscules sauf pour les chiffres dans les instructions, et en surlignant en jaune les éléments importants.
Prescription medication label legibility is important to ensure patients take their medication appropriately and avoid medication errors. Older adults are particularly at risk, as many visual functions decrease with age and age-related ocular disorders are common.1,2 Those with visual impairment (most of whom are older adults) are another at-risk group. The number of people with visual impairment is increasing3 and rises sharply with age; 13.4% of persons 75 years and older reported a vision disability.3
Older adults are also at risk because of higher rates of medication use.4,5 Medication errors can result from misreading a prescription drug name that sounds similar to a different drug or misreading dosages, warnings and other critical information.6 Furthermore, the inability to read prescription labels clearly can cause patients anxiety because of the potential increased dependence on others, loss of confidentiality and preventable illness.7
It would be anticipated that the legibility of labels can be improved by adopting guidelines for print legibility established by vision-related nongovernmental organizations and pharmaceutical and health organizations.8-15 Yet, in a previous study, we found that many labels fail to meet these guidelines, especially in terms of print size, case and modes of emphasis.16 We concluded that labels could more often meet guidelines without changing technology or the size of labels. Latham et al. similarly found that current labels in the United Kingdom do not meet existing guidelines and that reading speed and accuracy improved with ideal and larger print labels.17
The purpose of the current study is to determine the legibility of medication labels for patients who are more vulnerable (i.e., older participants and those with vision loss). To achieve this goal, the legibility of current prescription medication labels was compared with the legibility of prototype labels, based on current guidelines for legibility, to determine if legibility can be improved with such prototype labels.
Methods
All aspects of the study received clearance through the Office of Research Ethics at the University of Waterloo. All participants gave informed consent.
This project included 3 phases: first, the preparation phase, in which fictional prescriptions (including instructions, drug names and fictional patient names) were developed and tested for equal readability (i.e., equal difficulty of the text itself); second, the acquisition of the sample labels and design of the prototype labels; and third, the main study, which involved 3 patient groups who read the final labels and ranked them for legibility.
Phase 1: Preparation phase: Development and testing of prescription texts
In preparation for the study, hypothetical prescriptions were generated and tested so that wording for patient-critical information (patient name, drug name and drug instructions16,18) had equal (or approximately equal) readability. Forty sample prescriptions were generated with unique drug names and fictional patient names. The patient names were chosen to be of approximately equal length, with just 1 first and 1 family name. Regarding the drugs, controlled substances were avoided. The prescriptions were typed in a similar format to actual prescription information on medication vials and glued onto cue cards.
Ten graduate or undergraduate optometry students, aged 22 to 27 years with normal corrected visual acuity (a measure of the finest detail that the eye can see, usually measured with letters of decreasing size), took part in this phase. Low-vision simulators, which decreased both contrast sensitivity and visual acuity, were used to decrease reading speed and increase errors so that differences between the texts would become evident. Visual acuity was reduced by 3 lines (to 20/36, a factor of 2× poorer) on a standard Early Treatment Diabetic Retinopathy Study (ETDRS) logMAR (logarithm of the Minimum Angle of Resolution) letter chart,19 and log contrast sensitivity was reduced to 1.05 measured on the Mars letter contrast sensitivity test.20 The Mars chart has letters of the same size but decreasing contrast and so measures the minimum contrast that a person requires to read the letters. Participants were asked to read each cue card as fast as possible without making mistakes. The order of the cue cards was randomized for each participant. Because many drug names may be difficult to pronounce and because many patients are likely to recognize their medications by the first syllable, participants were asked to spell the first 5 letters of the drug name. The accuracy and time taken to read the patient name, drug name and instructions were determined separately. For each measure, outliers beyond 2 standard deviations were flagged and removed. The remaining patient names, drug trade names and instructions were randomly recombined to compose 24 equally readable prescriptions (see the appendix available online at cph.sagepub.com/supplemental) to be used in phase 3, the main study. Twelve were randomly assigned to the pharmacy samples and 12 to be used for the prototypes.
Phase 2: Acquisition of the sample labels and design of prototype labels
Pharmacy selection and acquisition of sample labels
Sample labels from 48 pharmacies had been collected as part of a previous study.16 Note that the text for these previously collected samples was different from that in the current study. These were used to select a sample of pharmacies from which to recruit and that would give a good representation of current label print sizes. The 48 labels were arranged in order of print size of the instructions. Every fourth pharmacy in the set of 48 was contacted (to select a total of 12 pharmacies to supply sample labels for the study). If a pharmacy was not willing to participate, we contacted the next pharmacy on the list. Twelve of the 24 prescriptions were sent to participating pharmacies, and the pharmacist was asked to print a label for each prescription (i.e., 12 labels) as they normally would for a regular medication vial, as if they had received that prescription. Pharmacists were asked to use numerals for the instructions if they normally did so and were asked to keep the patient names and trade names exactly as indicated. All other information (pharmacy contact information, DIN numbers, prescription numbers, physician information) was to be printed as they normally would. A total of 144 unique pharmacy labels were collected (12 prescriptions × 12 pharmacies; see Figure 1A for a sample standard prescription label). The 12 pharmacies consisted of 3 chain/mass merchandizer/groceries, 3 banners and 6 independents. The labels were pasted onto empty standard-sized medicine 20-dram vials.
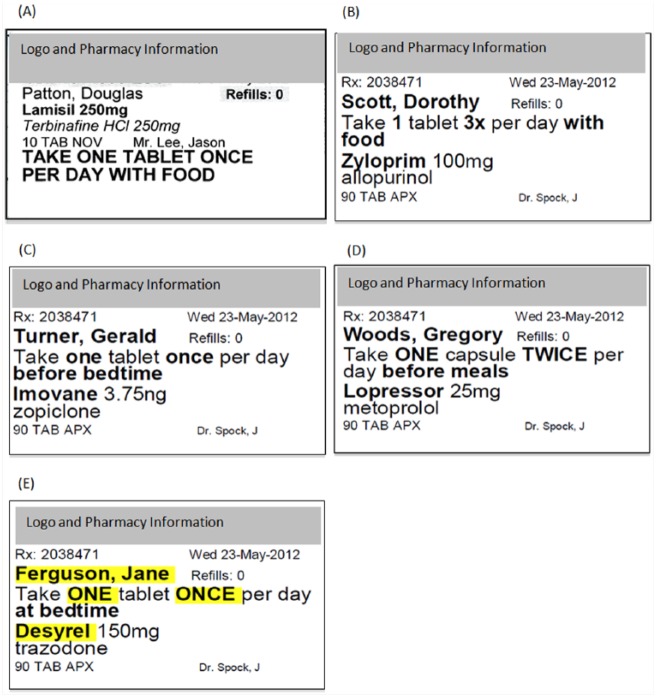
Figure 1.
Examples of labels
(A) Pharmacy sample label. (B) Prototype label, instructions numbers in numerals. (C) Prototype label instructions numbers in lowercase words. (D) Prototype label, instruction numbers in uppercase words. (E) Prototype label, instruction numbers in uppercase words with highlighting (note that highlighting was in yellow). All of these versions of the prototypes were in 12-, 14- or 16-point print. The layout shown is with 14 point.
Design of prototype labels
Twelve different prototype versions were designed based on the guidelines from nongovernmental organizations for accessible print and pharmaceutical and health organizations for prescription medication labels.8-15 The prototype instructions were written in sentence case (as is recommended, but different from all current prescription medication labels16). Four style variations included the numbers of the instructions written in 1) numerals, 2) lowercase words, 3) uppercase words or 4) highlighted uppercase words (see Figures 1B–E). For the last option (with highlighting), the patient name, the trade drug name and numbers of the instructions were highlighted in yellow. Each style option was printed in print size 12, 14 or 16 point, thus giving rise to 12 different versions (4 styles each in 3 different print sizes). All prototypes had patient critical information bolded (patient name, number of tablets/capsules, times per day, additional instructions and drug trade name). All prototype labels were designed to maximize the use of space on the label for important information. The remaining 12 of the 24 prescriptions were printed in each of the 12 style combinations to make 144 unique labels (12 prescriptions × 12 prototype versions). Each prototype label was pasted onto the same type of vial as the sample pharmacy labels. Examples of each of the 4 prototype label styles are shown in Figure 1 for the 14-point print size. Only the label used for 16-point print required a label size 11% taller than a standard label. The prescription information for both 14 point and 12 point fit on a standard label.
Phase 3: Main study
Sample size calculation
The sample size calculation was based on preliminary data of participants with age-related maculopathy, and their speed of reading the dosage and patient name on medicine labels for which the standard deviation of the difference between 2 labels was 11 seconds. The average time taken to read this information was 21 seconds, and we assumed that an improvement of time taken of 8 seconds would represent a significant improvement (i.e., an improvement of more than 33%). This results in a sample size of 17 in each group (paired t test for 80% power). We therefore recruited 24 participants in each group, to ensure that we had adequate power and to be able to form two 12 × 12 Latin square design for each group (see description below). A 12 × 12 Latin square was chosen, as it was decided to test 3 different font sizes of the prototypes and 4 different versions (see above), resulting in 12 variants. A Latin square is a method of controlling for order effects, similar to randomizing, when there are 2 variables of interest (in this case, the text and pharmacy for the pharmacy samples or the text and the style/font size of the prototype labels). It efficiently balances out factors that are of less interest (in this case, the individual subject’s performance and the prescription text). The variables that are of interest are each presented the same number of times in a specific place in the sequence (thus helping to control for fatigue or practice effects).
Participant recruitment
For the main study, participants were recruited from the University of Waterloo (UW) Optometry Primary Care and Low Vision Clinics, and some participants with visual impairment were recruited from CNIB (formerly Canadian National Institute for the Blind), Toronto. The UW Low Vision Clinic and CNIB are available to serve the whole visually impaired populations in their respective areas. Inclusion criteria were specified for 3 different groups. The first group consisted of 24 individuals aged ≥65 years who had distance visual acuity of 20/40 or better (older normal vision). The second group consisted of 24 individuals aged ≥65 years who had distance visual acuity poorer than 20/40 to 20/400 (older visually impaired).21,22 The last group consisted of 23 individuals aged 20 to 64 years with visual acuity poorer than 20/40 to 20/400 (younger visually impaired). We excluded individuals who were legally blind according to the World Health Organization, who would probably require other means to read prescription labels. Other exclusion criteria were any mention of dementia, cognitive impairment or physical challenges in the patient file; those who did not read regularly in English; and those who achieved <21 on the modified Telephone Interview of Cognitive Status (TICS-M), a screening questionnaire for cognitive impairment, which was designed to be delivered by telephone. The TICS-M has been shown to be well correlated with the Mini-Mental State Examination.23
Experimental design
For the sample labels, a 12 × 12 Latin square was used to balance prescription information so that each participant read different prescription information for each pharmacy label. Although prescription information was selected for equal readability, this allowed a balancing out of the prescription information in order to study differences in pharmacy labelling. The first 12 participants in each group were assigned in the Latin square, which was repeated for the next 12 participants in each group. Similar Latin squares were used for the prototype labels. This results in a label from each pharmacy being read by each participant, but with different prescription information. Thus, a sample label from each pharmacy was read by all 71 subjects (24, 24 and 23 in each group, respectively), but the prescription information was varied.
Study protocol
Participants were shown 6 demonstration vials and were instructed to read only the patient’s name, instructions or trade name of the drug and to read as fast as possible without making mistakes. They were asked to read the first 5 letters of the trade drug name. They used their habitual spectacles or reading device at the reading distance that was optimal for them. The demonstration vials were used to accustom the participant to the task. The results were not recorded or included in the analysis. Participants then read the 12 pharmacy vials in order, according to the Latin square. These readings were timed with a stopwatch, and the entire session was videotaped as a check for errors and to determine the final time. Errors that occurred while reading were recorded. If there was any error in the reading of the patient name, drug name or instructions, a score of zero was given for that component (drug name, patient name or instructions). If participants corrected themselves, this was not counted as an error. For the pharmacy samples, reading the first 5 letters of either the generic or trade name was counted as correct. To provide a break from reading, participants were next asked about their general health, ocular health and their current glasses or low-vision devices. The participants then read the 12 prototype vials as described above. For the prototypes, all participants read the trade drug names.
The final task was to rank each set of 12 sample labels in terms of legibility, by physically arranging the vials in order of most to least legible. The participant was asked to judge based on the overall label (including the layout, print size, colouring, pharmacy logo and contact information, etc.). Participants often used an iterative process (i.e., comparing one by one until they were satisfied they were in the correct order). The 12 prototype labels were similarly ranked.
Analysis
Since we were not interested in the prescription information, we were able to take the average for each pharmacy sample with different prescription information. Similarly, we calculated the average for each prototype version (which had different prescription information) as read by each participant. For the samples, the data for each pharmacy (accuracy, time or ranking) were analyzed separately. For the prototypes, the data for each style variant were analyzed separately and then were grouped according to style or font size. When comparing the samples and the prototypes, the average data from the samples versus the prototypes for each participant were compared.
Accuracy was recorded as 1 if totally correct or 0 if any error was made, as it was decided that any error in the patient-critical information could potentially result in a medication error. Accuracy was analyzed with nonparametric tests. The Friedman test was used to analyze differences in reading accuracy among the sample and prototype labels, and the Mann-Whitney test was used to compare between the samples and the prototypes. The time taken to read information was transformed to a log scale, and parametric statistics were used for analysis. One-way ANOVA with post hoc analysis corrected for multiple comparisons by the adjusted Bonferroni method24 was used to compare the time differences between the groups for the samples and the prototypes. Ranking data were recorded as 1 = most preferred and 12 = least preferred and were analyzed by determining the median for each participant and then applying repeated-measures analysis of variance (ANOVA).
Results
Participants
The demographics of the participant groups are given in Table 1. Apart from planned differences, there was a significant difference in visual acuity between the older and younger participants with low vision (t test, p = 0.014). There was no significant difference in the age of the older participants with and without low vision (t test, p = 0.067) or in the percentage of females.
Table 1.
Demographics of the participants*
| Percentage female | Age (years) | Mean visual acuity | |
|---|---|---|---|
| Older normal vision | 71 | 75 ± 6.8 | 20/24 ± 1.2 letter chart lines (0.12 logMAR) |
| Older low vision | 71 | 79 ± 7.8 | 20/83 ± 2 lines (0.2 logMAR) |
| Younger low vision | 52 | 45 ± 11.3 | 20/120 ± 2.3 lines (0.23 logMAR) |
logMAR = logarithm of the minimum angle of resolution.
Reading accuracy
Overall, there were no significant differences in accuracy of the reading of patient name, instructions or drug name between the samples and the prototypes for any participant group. The accuracy of reading each piece of information (the patient name, the drug name and the instructions) was then considered separately. For all groups, there was a significant difference in the patient name accuracy among the samples (Friedman test, p < 0.01). Post hoc testing revealed this was due to one label (label 9), which had a different layout and a pharmacy name that was similar to a patient’s name, such that participants frequently read the pharmacist name for the patient name. There were no other significant differences in patient name accuracy for the sample labels in any of the groups. There were no other significant differences in accuracy (among samples or prototypes for patient name, drug name or instructions [Friedman p > 0.05], between samples and prototypes [Mann-Whitney test, p > 0.05] or between participant groups [Friedman p > 0.05]). Apart from the patient name in label 9, accuracy was high, ranging from 75% to 100% (average = 92% ± 7.3) of each piece of information being read correctly for the samples and from 79% to 100% (average 95% ± 4.7) for the prototypes.
Time taken to read labels
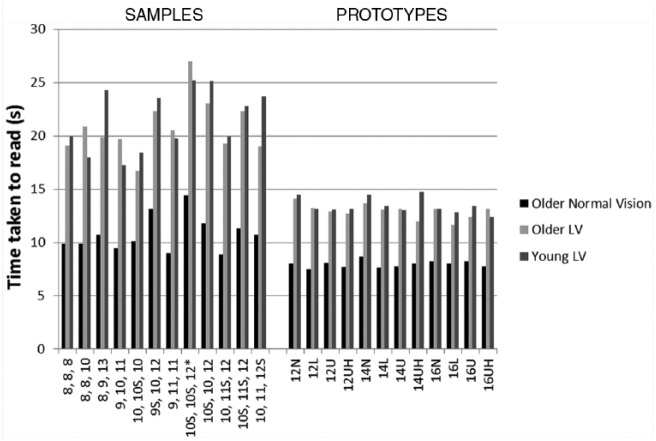
The average times to read individual labels are shown in Figure 2. Overall, the prototypes were read faster than the samples (p < 0.0001), and this was true for each participant group (p < 0.0001). To account for the possibility of a practice effect resulting from reading the samples before the prototypes, the data of both samples and prototypes were rearranged into the order that they were read for each participant. Repeated-measures ANOVA (2 × samples/prototype × 12 order of reading) showed a significant effect of sample versus prototype (p < 0.001) and order of reading (p < 0.05) for all participant groups (i.e., there is a practice effect). The speed for the samples plateaued for the last 3 data points. The average of these points was therefore calculated for each participant and compared with the average of the time taken for the prototypes. There was a significant difference for all groups (p < 0.001, 2-way paired t test), that is, the prototypes were read significantly faster, despite the practice effect. Participants with low vision took longer to read both samples and prototypes compared with the older normal vision group, but there was no difference between the groups with low vision (1-way ANOVA, p < 0.05). The average times for reading of the samples and prototypes are shown in Table 2.
Figure 2.
Mean time taken to read the sample labels and prototype labels plotted against the label print size
The characteristics of the print for each label are shown. For the samples, the values are plotted in order of average print size for the instructions, drug name and patient name going from smallest to largest font size. When these averages were the same, they were further ordered in terms of font size for 1) the instructions, 2) drug name and 3) patient name. All were printed in all capitals unless there is an “S,” which indicates sentence case. The asterisk indicates label 9. For the prototypes, all instructions were printed in sentence case. “U” indicates that the instruction numbers were in uppercase words. “L” indicates the numbers in lowercase words, “UH” represents the numbers written in uppercase words with highlighting, and “N” indicates they were written in numerals.
Table 2.
Time taken in seconds (average ± SD) to read sample and prototype prescription medication labels
| Older group with normal vision | Older group with low vision | Younger group with low vision | |
|---|---|---|---|
| Pharmacy samples | 10.79 ± 2.65 | 20.81 ± 10.56 | 21.50 ± 15.86 |
| Prototypes | 7.98 ± 1.79 | 12.94 ± 5.19 | 13.44 ± 8.16 |
Among sample labels, there was no overall relationship between print size of the patient critical information and the time taken to read them. However, all 3 groups showed significant differences among a few of the individual sample labels (repeated-measures ANOVA, p < 0.001). When we qualitatively considered the characteristics of the labels that were either read faster or slower (as identified with post hoc testing), there were no obvious consistent differences in the use of italics versus upright, print size or uppercase versus lowercase or sentence case, although 2 of the fastest read appeared to be less crowded than others, with more white space.
Among prototype labels, there was no effect of print size on the time taken to read the labels for any group (p > 0.05, repeated-measures ANOVA, 3 sizes). Repeated-measures ANOVA (4 styles) showed that there was a significant effect of style (p = 0.018) for the older normal vision group. Post hoc analysis showed that lowercase words for the numbers were read faster than when numerals were used (p = 0.009). There was no effect of style in the other 2 groups (p > 0.05).
Ranking
Repeated-measures ANOVA for print size (3× print size) showed a significant effect of print size in all groups (p < 0.001). Post hoc analysis showed that there was a significant preference for larger print; 14 point was preferred to 12 point, and 16 point was preferred to 14 point; Figure 3). There was a main effect of print style (repeated-measures ANOVA [4 styles], p < 0.001), and post hoc analysis showed that upper case with highlighting was significantly preferred to all the other styles in all the groups of participants (Figure 4).
Figure 3.
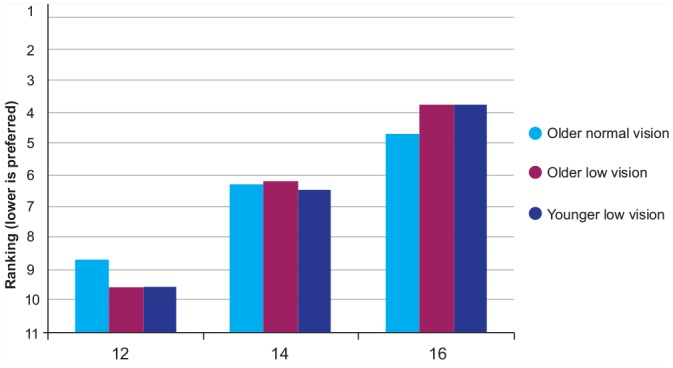
Median ranking for label print size for print size, according to group; all groups preferred 16 point
Figure 4.
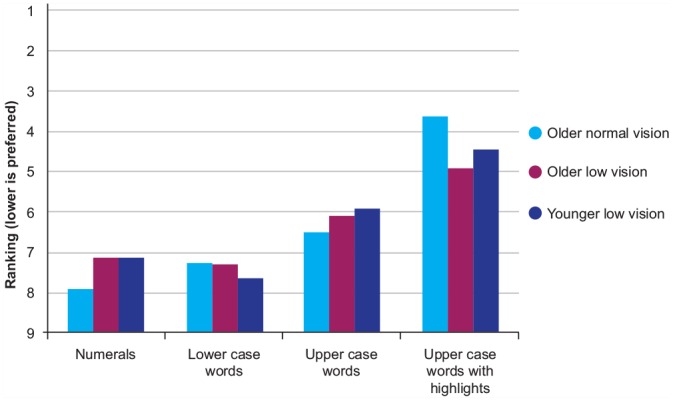
Median ranking for label print size for each print style, according to group; all groups preferred capitals with highlighting
For the pharmacy samples, there was no significant association between the size of the patient-critical information and the participant’s ranking of the samples. All 3 groups chose label 10, on average, to be the best label. This label had a print size of 13 point for the instructions (a large-print option from one of the pharmacies).
Discussion
The results of this study show that accuracy was quite high for most of the labels, even for those participants with low vision, although it must be remembered that they were using their low-vision devices. However, the prototypes were read significantly faster than the pharmacy samples (about 3 seconds faster for older people with normal vision and 8 seconds faster for those with low vision). Larger print was consistently preferred by all participant groups, as was the style version that included upper case for the numbers of the instructions.
Lorenc and Branthwaite25 reported that a patient’s being able to read the label was an independent factor for improved adherence. Although our results show that accuracy of reading was high among all groups with both the sample prescription labels and the prototype labels, all groups took longer to read sample labels than prototype labels, suggesting that the prototype labels are easier to read. Subjectively, participants preferred upper case and highlighting for the numbers in the instructions with larger print.
Our findings are similar to a UK study by Latham et al.,17 which showed improved reading speed, but not accuracy, in the “mild” simulated visually impaired group with labels that were designed according to the UK National Patient Safety Agency guidelines and with large-print labels compared with actual sample labels. Legibility was worse in the “moderate” visually impaired group, with most participants not being able to read fonts smaller than 14 point. Latham’s study was performed with visual impairment simulators, not with participants with visual impairment who used reading aids, as in the present study. These differences may explain why we did not find a decrease in accuracy for the visually impaired groups. However, our current major finding, that those with visual impairment took longer to read labels than those with normal vision, is in agreement with the findings of Latham et al. Participants with visual impairment took about 10 seconds longer for the pharmacy samples and about 5 seconds longer for the prototypes (Table 2). Despite high accuracy (92%–95%), reading a medication label slowly would still present a potential barrier for people with visual impairment.
There was a significant improvement in reading speed with the prototypes. This improved by 3 seconds for the older normal vision group and 8 seconds for both groups with visual impairment, which is a 37% improvement. However, when both samples and prototypes were analyzed separately, it was not clear which features of the prototypes resulted in that improvement. Among the samples, there was no consistent feature of the print that resulted in faster or slower reading. It is important to note, however, that layout may be an important factor. The 2 labels that were read the fastest appeared to be less crowded. Label 9 was consistently read more slowly and less accurately by all groups. The information on this label was spread horizontally, the instructions were grouped at the bottom and the pharmacy name was where the patient name would be anticipated. Most other labels placed the important information on the left of the label in a more vertically linear fashion. Malkinson6 has suggested the use of a hierarchical approach for providing information to the patient about the medication. This can be applied to information on a label. It seems that important information should be maximally left-aligned to avoid issues with a curved vial.
Similarly, among the prototypes, print characteristics did not influence the time taken to read, with the exception that reading was statistically faster with lowercase words than with numerals for the adults with normal vision. However, this difference was only 0.03 second, which is probably not practically or clinically significant. It is therefore possible that prototypes were easier to read, not because of one particular feature but because of a combination of features, including increased print size, spacing, justification, consistent layout and emphasis on important information.
Although reading speed and accuracy did not identify which specific feature improves legibility, the ranking results showed consistent preferences. All participant groups preferred upper case with highlighting (which was yellow) for the numbers in the instructions to all other styles and larger font among the prototypes. All 3 groups preferred sample label 10, which had the largest font (13 point) for the instructions. Whereas it has been suggested that sentence case is more easily distinguishable because of variations in the unique shape of each word,6,26 upper case and highlighting add emphasis on important pieces of information such as numbers and special instructions that are crucial for proper dosing. Latham et al.17 also showed that use of upper case for emphasis was preferred by most observers.
Limitation
One limitation of this study is that it was possible to test only a certain number of prototype variants. We chose 4 different print styles with 3 different font sizes. These choices were based on the nongovernmental agency and pharmaceutical and health organizations’ guidelines for prescription medication labels, and we chose those that we expected to be easiest to read. However, there are other combinations of styles/sizes and layouts that could have been tested, and this could be the focus of future studies.
Clinical relevance
Because legibility is a contributing factor to correct medication use and possibly to adherence, legibility of medicine labels is an issue of concern to individual pharmacists, pharmacy technicians and the pharmaceutical industry as a whole. This study shows that a combination of print characteristics allowed quicker reading and was preferred overall, indicating that these characteristics give increased accessibility to the patient-critical information. These characteristics included larger print, a consistent layout, left justification, overall lowercase lettering but with uppercase lettering for numbers in the instructions and highlighting (in yellow; Box 1).
Box 1. Recommendations for medication labelling.
Whenever possible:
Use as large a label as possible (to allow larger print).
Use the largest print possible.
Use lower case for the patient-critical information but use uppercase words for the numbers in the instructions (see Figure 1D, E).
Use left justification (alignment) for the patient-critical information (all examples in Figure 1 show this).
Use yellow highlighting for the numbers of the instructions (Figure 1E).
Consistent layout of information between all labelling companies would be helpful.
Conclusion
This study supports the findings of previous studies demonstrating that making the print as large as possible will likely decrease barriers for patients and be preferred by them. In addition, there are indications that using a consistent layout with justification and printing the numbers for the instructions as uppercase words and highlighting may also be helpful ways to improve the accessibility of the information on the label. These changes can be made without moving to different types of technology (e.g., technologies other than printing, such as Braille or electronic chips). Further, larger-print labels would allow even larger print size, which was preferred by all the participant groups and may allow a greater percentage of the population to be self-reliant, having decreased need for adaptations and reading aids and thereby improving patient compliance and ease of self-medicating.■
Supplementary Material
Footnotes
Author Contributions:S. J. Leat initiated the project and was responsible for project design and methods, data collection and analysis and reviewing and final editing of the manuscript. A. Krishnamoorthy was responsible for data collection and data analysis and wrote the final manuscript draft. A. Carbonara was responsible for data collection and data analysis and editing the final manuscript. D. Gold initiated the project, was responsible for project design and data collection at CNIB and edited the manuscript. C. Rojas-Fernandez initiated the project, was responsible for project design, consulted regarding aspects of the study and edited the final manuscript.
Funding:This study was funded by the Canadian National Institute for the Blind E.A Baker fund.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Haegerstrom-Portnoy G. The Glenn A. Fry Award Lecture 2003: vision in elders—summary of findings of the SKI study. Optom Vis Sci 2005;82:87-93. [DOI] [PubMed] [Google Scholar]
- 2. Lott LA, Schneck ME, Haegerstrom-Portnoy G, et al. Reading performance in older adults with good acuity. Optom Vis Sci 2001;78:316-24. [DOI] [PubMed] [Google Scholar]
- 3. Statistics Canada. The 2006 participation and activity limitation survey: disability in Canada. Available: www.statcan.gc.ca/bsolc/olc-cel/olc-cel?catno=89-628-X&CHROPG=1&lang=eng (accessed Dec. 22, 2014).
- 4. Reason B, Terner M, Moses McKeag A, et al. The impact of polypharmacy on the health of Canadian seniors. Fam Pract 2012;29:427-32. [DOI] [PubMed] [Google Scholar]
- 5. Meredith S, Feldman PH, Frey D, et al. Possible medication errors in home healthcare patients. J Am Geriatr Soc 2001;49:719-24. [DOI] [PubMed] [Google Scholar]
- 6. Malkinson TJ. Age related changes in vision and its implications for medication labeling. IEEE International Prof Comm Conf 2001:101-16. [Google Scholar]
- 7. American Foundation for the Blind. Access to drug labels survey report. Available: www.afb.org/section.aspx?FolderID=3&SectionID=3&TopicID=135&DocumentID=4520 (accessed Jun. 14, 2013).
- 8. The United States Pharmacopeial Convention. Recommendations on prescription container labeling. Available: www.usp.org/usp-nf/notices/retired-compendial-notices/recommendations-prescription-container-labeling (accessed Jun. 5, 2013).
- 9. American Society of Consultant Pharmacists. Guidelines for prescription labeling and consumer medication information for people with vision loss. Available: www.ascpfoundation.org/programs/visuallyimpaired.cfm (accessed Jun. 5, 2013).
- 10. Läkemedelsverket Medical Products Agency. Guideline to the Medical Products Agency’s regulation on labelling and package leaflets for medicinal products. Available: www.lakemedelsverket.se/english/All-news/NYHETER—2009/Guideline-in-English–labelling-and-package-leaflets-for-medicinal-products/ (accessed Jun. 5, 2013).
- 11. National Safety Patient Agency. Design for patient safety: a guide to the design of dispensed medicines. Available: www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety/?entryid45=59829 (accessed Jun. 5, 2013).
- 12. American Foundation for the Blind. Guidelines for prescription labeling and consumer medication information for people with vision loss. Available: www.afb.org/section.aspx?FolderID=3&SectionID=3&TopicID=403&SubTopicID=256&DocumentID=4064 (accessed Jun. 5, 2013).
- 13. Canadian National Institute for the Blind. Clear print design standard. Available: www.cnib.ca/en/services/resources/clearprint/Pages/default.aspx (accessed Jun. 5, 2013).
- 14. Royal National Institute of Blind People. Clear print. Available: www.rnib.org.uk/professionals/accessibleinformation/text/Pages/clear_print.aspx (accessed Jun. 5, 2013).
- 15. American Council of the Blind. Best practices and guidelines for the large print documents used by the low vision community. Available: http://acb.org/node/750 (accessed Jun. 5, 2013).
- 16. Leat SJ, Ahrens K, Krishnamoorthy A, et al. The legibility of prescription medication labelling in Canada: moving from pharmacy-centred to patient-centred labels. Can Pharm J (Ott) 2014;147:179-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Latham K, Waller S, Schaitel J. Do best practice guidelines improve the legibility of pharmacy labels for the visually impaired? Ophthal Physiol Opt 2011;31:275-82. [DOI] [PubMed] [Google Scholar]
- 18. National Association of Boards of Pharmacy. Report of the task force on uniform prescription labeling requirements. Available: www.nabp.net/news/assets/08TF_Uniform_Presc_Labeling_Req.pdf (accessed Dec. 22, 2014).
- 19. Ferris F, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol 1982;94:91-6. [PubMed] [Google Scholar]
- 20. Dougherty BE, Flom RE, Bullimore MA. An evaluation of the Mars letter contrast sensitivity test. Optom Vis Sci 2005;82:970-5. [DOI] [PubMed] [Google Scholar]
- 21. Maberley DA, Hollands H, Chuo J, et al. The prevalence of low vision and blindness in Canada. Eye 2006;20:341-6. [DOI] [PubMed] [Google Scholar]
- 22. Leat SJ, Legge GE, Bullimore MA. What is low vision? A re-evaluation of definitions. Optom Vis Sci 1999;76:198-211. [DOI] [PubMed] [Google Scholar]
- 23. de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 2003;18:318-24. [DOI] [PubMed] [Google Scholar]
- 24. Jaccard J, Wan CK. LISREL approaches to interaction effects in multiple regressions. Thousand Oaks (CA): SAGE Publications; 1996. [Google Scholar]
- 25. Lorenc L, Branthwaite A. Are older adults less compliant with prescribed medication than younger adults? Br J Clin Psychol 1993;32:485-92. [DOI] [PubMed] [Google Scholar]
- 26. Tinker M. Legibility of print. Ames (IA): Iowa State University Press; 1963. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.