Abstract
Purpose
We examined the role of NMDAR in the regulation of bladder hypertrophy and function in a rat model of cyclophosphamide induced cystitis.
Materials and Methods
Cystitis was induced by intraperitoneal injection of cyclophosphamide (150 mg/kg body weight). NMDAR phosphorylation (activity) and signal transduction pathways were examined by direct measurement and by specific inhibitors in vivo. Bladder hypertrophy was measured by bladder weight/body weight and type I collagen expression. Bladder function was examined by metabolic recording, conscious cystometry and detrusor muscle strip contractility in response to carbachol.
Results
NMDAR activity measured by the phosphorylation level of the NMDAR1 (NR1) subunit was expressed in the spinal cord but not in the bladder at 48 hours of cystitis. NMDAR inhibition with dizocilpine (MK-801) reduced the cystitis induced increment of bladder weight and type I collagen up-regulation in the bladder. NMDAR regulated type I collagen up-regulation was mediated by the PI3K/Akt pathway. NMDAR inhibition also attenuated cystitis induced urinary frequency measured by metabolic cage and cystometry. Cystitis decreased the responsiveness of detrusor muscle strips to carbachol, which was reversed by MK-801 in vivo. Unlike MK-801 the NMDAR antagonist D-AP5, which could not block central NMDAR activity, had no effect on bladder hypertrophy, type I collagen up-regulation or Akt activation caused by cystitis in the bladder.
Conclusions
Findings suggest that NMDAR activity has a role in cystitis induced bladder hypertrophy and overactivity. NMDAR mediated Akt activation may underlie the mechanism of bladder dysfunction.
Keywords: urinary bladder, overactive, receptors, N-methyl-D-aspartate, collagen, cystitis, interstitial, hypertrophy
The role of NMDAR in synaptic plasticity is well established. Emerging evidence shows that NMDAR also has an irreplaceable role in the transmission of visceral and inflammatory pain.1–3 In irritable bowel syndrome and overactive bladder NMDAR activity was found in the central nervous system and it mediates visceral hyperactivity.3,4 Blockading NMDAR activity with the noncompetitive antagonist MK-801 inhibits bladder overactivity caused by cerebral infarction5 and attenuates hyperreflexia in the micturition reflex caused by crystalluria, partial BOO and nerve injury.6–9 The common pathogenesis of irritable bowel syndrome and overactive bladder also involves smooth muscle overactivity.10,11 However, to our knowledge the role of NMDAR in regulating cytological changes in the bladder, thereby regulating bladder function, has not been investigated.
NMDAR has an ionotropic property that regulates Ca2+ influx and Ca2+ dependent physiological effects such as presynaptic neurotransmitter and neuropeptide release.1 Our recent study showed that according to the phosphorylation level of the NR1 subunit the activity level of NMDAR was increased in the lumbosacral spinal cord in CYP induced cystitis.12 NMDAR mediated neurotransmitter release to the bladder may possibly facilitate molecular and physiological changes in the viscera.
In CYP induced cystitis the expression level of type I collagen is increased in the inflamed bladder, acting as one of the major factors contributing to bladder hypertrophy.13 It was suggested that collagen production and extracellular matrix remodeling in peripheral organs may be facilitated by neurotransmitter release from the central and peripheral nervous systems.14,15 In addition to neuronal expression of NMDAR, recent studies showed that NMDAR activity is also present in peripheral tissues, including macrophages, bone marrow and the cardiovascular system.16,17 The effects of central and peripheral NMDAR are often differentiated using the specific antagonists MK-801 and D-AP5. MK-801 is used to block overall NMDAR activity for its ability to cross the blood-brain barrier18,19 and D-AP5 is used to study the role of peripheral NMDAR.16
In the current study we used these tools to investigate whether NMDAR has a role in the development of bladder hypertrophy. At the functional level the increase in detrusor wall thickness can reduce bladder capacity, thereby decreasing urine volume per void and increasing urinary frequency. In addition, bladder wall thickening causes poor detrusor smooth muscle compliance and affects smooth muscle contractility.20 Thus, the role of NMDAR in regulating bladder function during cystitis was also investigated.
MATERIALS AND METHODS
Experimental Animals and Reagents
We used adult male Sprague Dawley® rats at ages 6 to 8 weeks. All experimental procedures were approved by the Virginia Commonwealth University institutional animal care and use committee. Animal care was done in accordance with AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) and NIH (National Institutes of Health) guidelines. CYP, β-actin antibody and other chemicals used in this experiment were obtained from Sigma-Aldrich®. Primary antibodies against type I collagen (sc-28657, 1:1,000), phospho-NR1 (sc-31669, Santa Cruz Biotechnology, Houston, Texas, 1:1,000), phospho-Akt (4060, 1:1,000) and Akt (9272, Cell Signaling Technology®, 1:1,000) were used for Western blot. Secondary antibodies for Western blot were obtained from Pierce®. MK-801 and D-AP5 were obtained from Tocris Bioscience, Bristol, United Kingdom.
CYP Induced Cystitis
Cystitis was induced in rats by intraperitoneal injection of CYP at a single dose of 150 mg/kg body weight. Rats were survived for 48 hours and then sacrificed by isoflurane overdose. Control rats received volume matched saline injection. All injections were performed using isoflurane (2%) anesthesia.
Drug Treatment
Immediately after CYP injection the MK-801 treated cystitis group received a single administration of MK-801 intravenously at a dose of 3 mg/kg body weight. This dose was tested in our previous study and attenuated cystitis induced spinal plasticity in vivo.12 D-AP5 was injected in rats with cystitis at a single dose of 5 mg/kg body weight in the same manner as MK-801. This dose was chosen based on a recent study showing that it inhibits peripheral NMDAR activity with no effect on central NMDAR activity.16 The PI3K inhibitor LY294002 was injected at a single dose of 50 µg/kg body weight. This dose and treatment regimen inhibited Akt activity in our previous study.12 All inhibitor stocks were stored in dimethyl sulfoxide solution and diluted with saline before injection. Control and cystitis rats received vehicle, that is the same amount of dimethyl sulfoxide diluted in saline.
Western Blot
The bladder was freshly dissected out and homogenized in T-PER buffer (Pierce®) supplemented with protease and phosphatase inhibitor cocktails (Sigma). The homogenate was centrifuged at 20,200 × gravity for 10 minutes at 4C. The protein concentration in the supernatant was determined using the DC™ Protein Assay Kit. Proteins were separated on 10% sodium dodecyl sulfatepolyacrylamide electrophoresis gel and transferred to nitrocellulose membrane by a semidry transfer technique. The membrane was blocked with 5% milk in tris-buffered saline for 1 hour and incubated with a specific primary antibody followed by horseradish peroxidase conjugated secondary antibody. Bands were identified by echochemiluminescence and x-ray film exposure. Band density was digitized with FluorChem™ and normalized to nonphospho-protein or β-actin as the internal control. The L6 spinal cord was extracted from rats with cystitis to compare with phospho-NR1 in the bladder.
Immunohistochemistry
The bladder was freshly dissected out and postfixed with 4% paraformaldehyde. After dehydration 7 µm transverse sections were obtained and immunostained with type I collagen antibody (1:200) followed by Cy3 conjugated secondary antibody. The slides were rinsed and cover-slipped with Citifluor™. Images were captured with a fluorescence photomicroscope (Carl Zeiss, Oberkochen, Germany). Primary antibody was used for Western blot, which revealed specific immunoreactive bands in the correct molecular weight for type I collagen.
Conscious Cystometry
Two days after drug injection a lower midline abdominal incision was made in all experimental rats under anesthesia (2.5% isoflurane). The bladder was exposed. A polyethylene-50 catheter with the end flared was inserted in the bladder through a small incision made by an 18 gauge needle at the tip of the bladder dome and secured. The other end was externalized at the back. On the day of surgery after the rats were awake they were placed in the recording cage for about 1 hour for habituation to the new environment. Urodynamic recording of bladder function in nonrestrained awake rats was done for 1 hour using a commercially available system (Med Associates, St. Albans, Vermont) according to manufacturer instruction. Bladder compliance was determined using the formula, (VN − VM)/(PN − PM), where V represents bladder capacity, P represents bladder pressure, M represents initiation of bladder filling and N represents initiation of voiding.20
Smooth Muscle Strip Contraction
The analysis of smooth muscle strip contraction was based on our previously published protocol.21 Briefly, the bladders from controls and rats treated with CYP and/or inhibitors for 48 hours were freshly isolated and cut open from the neck to the top of the dome. The bladder body was cut longitudinally into 3 × 10 mm strips, which were suspended in organ baths (Radnoti, Monrovia, California). The muscle strips were tied to glass hooks at each end with surgical silk and placed in a vertical orientation. The hook was connected to a stretch transducer (Grass Technologies, Quincy, Massachusetts). Force recordings were amplified by a Model 15A12 amplifier contained in a Model 15LT amplifier system and relayed to a PVA-16 PolyVIEW™ adaptor. Muscle strip contractility was tested by the response to CCh. Results were analyzed with PolyVIEW software.
Spontaneous Urination
An electrical scale connected to a computer program was used to record rat voiding frequency and amount. The rat was place in a recording cage above the scale. After a 30-minute exploration and accommodation period each void was collected by the scale and recorded by the computer automatically. The time dependent voids were plotted as a function of each voiding amount at the recorded time point. Frequency is shown as the average number of voids per hour. Voiding volume is shown as the average amount per void collected by the scale.
Statistical Analysis
Results of each study are shown as the mean ± SD. Control and experimental groups were compared by Kruskal-Wallis nonparametric 1-way ANOVA with differences between means considered significant at p ≤0.05.
RESULTS
N-methyl-d-aspartate Recptor
Inhibition with MK-801 attenuated bladder hypertrophy and type I collagen up-regulation in cystitis bladder
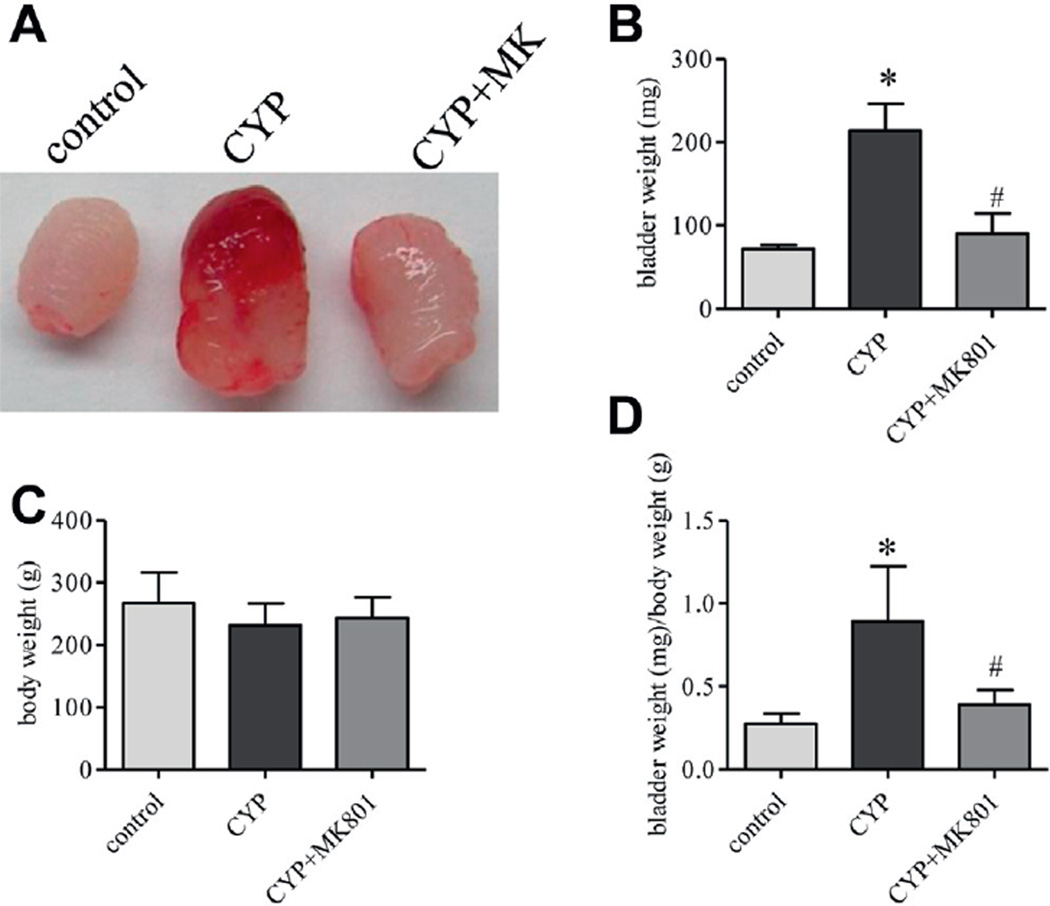
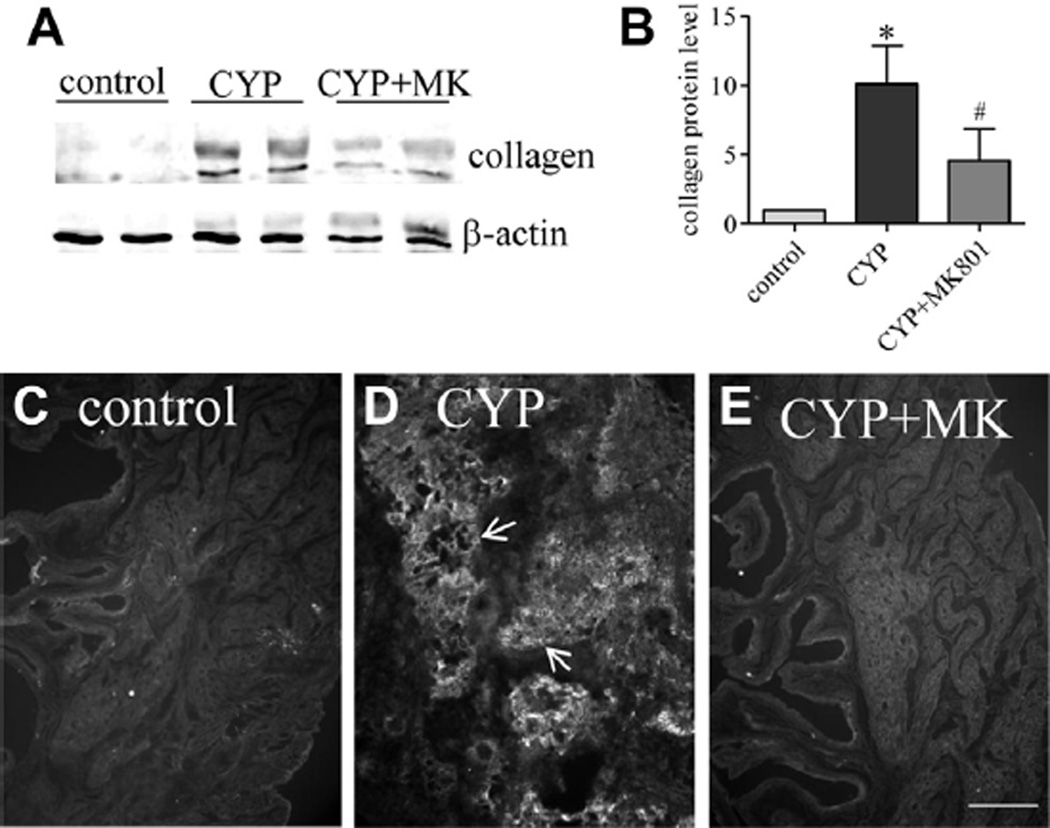
After MK-801 treatment bladder size and weight were less than those in the rat with cystitis (fig. 1, A and B). Body weight in each drug treatment group did not significantly change (fig. 1, C). In vehicle treated rats with cystitis the ratio of bladder weight to body weight was significantly higher than in controls (mean 0.89 ± 0.12 vs 0.28 ± 0.03 mg/gm) (fig. 1, D). Inhibition of NMDAR with MK-801 markedly reduced bladder size (mean 0.39 ± 0.03 mg/gm body weight) (fig. 1, A). Western blot of bladder samples showed that type I collagen expression was up-regulated in rats with cystitis (fig. 2, A and B). MK-801 treatment decreased the type I collagen up-regulation in the bladder caused by cystitis (fig. 2, B). Results were confirmed by immunostaining bladder samples with type I collagen antibody, which revealed that CYP cystitis induced robust formation of type I collagen fibers compared to controls (fig. 2, C and D). MK-801 greatly decreased bladder type I collagen production (fig. 2, E).
Figure 1.
Cystitis induced bladder hypertrophy was blocked by inhibiting NMDAR activity with MK-801 (MK). After CYP and MK-801 treatment bladder was freshly dissected (A). Bladder weight was expressed as ratio of bladder weight to body weight (B to D). Results represent 9 or 10 rats per group. Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP.
Figure 2.
Type I collagen up-regulation induced by cystitis in bladder was blocked by inhibiting NMDAR activity with MK-801 (MK). Western blot (A) shows type I collagen expression in 2 CYP and 2 CYP plus MK-801 rats. Note mean ± SD collagen protein (B). Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP. Immunostaining for type I collagen (arrows) confirmed Western blot results (C to E). Control and CYP rats received same amount of vehicle as MK-801 amount. Results represent 5 rats per group. Scale bar indicates 50 µm.
Regulated collagen up-regulation mediated by PI3K/Akt pathway in vivo
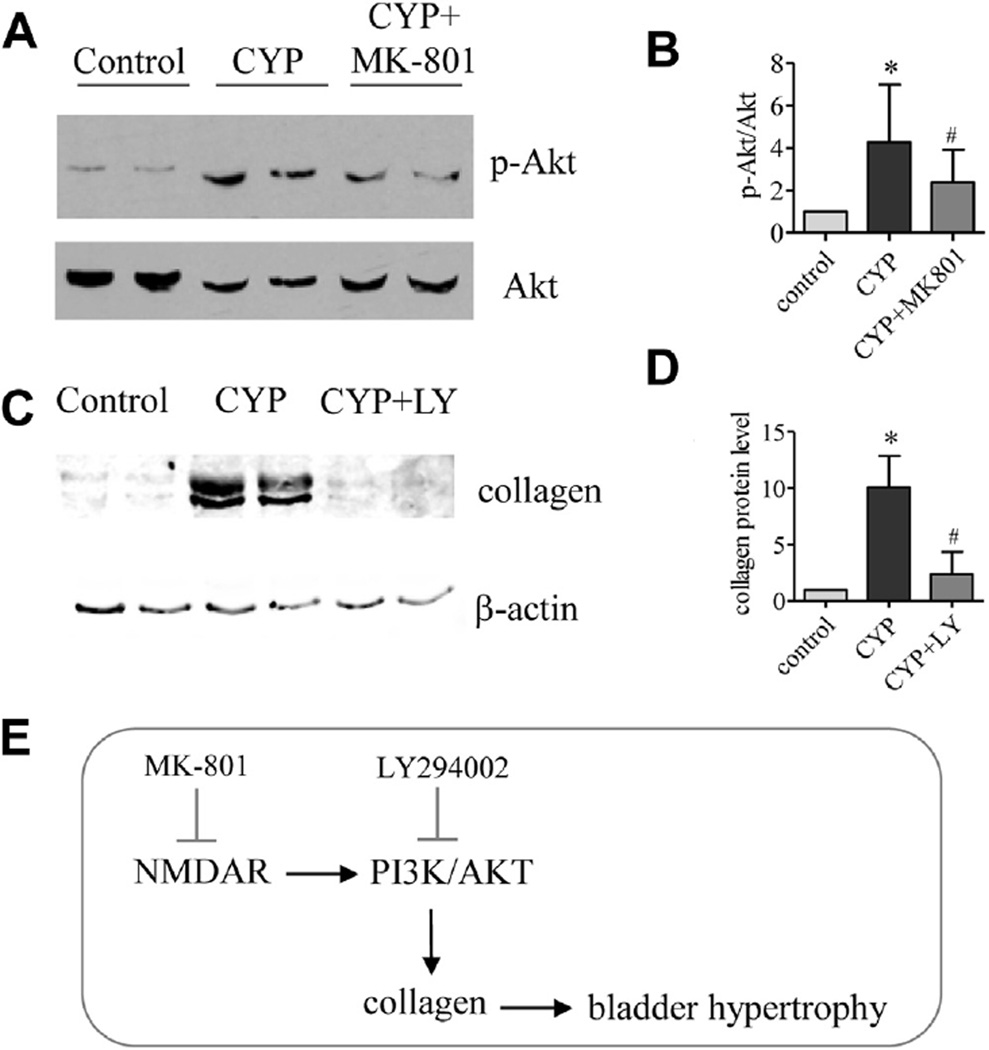
Cystitis increased the phosphorylation (activation) level of Akt in the bladder (fig. 3, A and B). During cystitis the increases in bladder phospho-Akt were decreased by MK-801 (fig. 3, A and B). Inhibiting Akt activity with the inhibitor LY294002, which blocks the activity of PI3K, an upstream kinase of Akt, decreased cystitis induced type I collagen up-regulation in the bladder (fig. 3, C and D). Briefly, NMDAR inhibition reduced collagen expression and bladder hypertrophy via the PI3K/Akt pathway (fig. 3, E).
Figure 3.
In bladder PI3K/Akt pathway was involved in NMDAR mediated collagen production. Akt activity was examined by Akt phosphorylation level (A) normalized to nonphospho-Akt (B). Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP. Western blots show samples from 2 CYP and 2 CYP plus LY294002 (LY) treated rats (A and C). Relative type I collagen was normalized to β-actin (C and D). PI3K/Akt pathway was involved in NMDAR regulated bladder hypertrophy (E). Control and CYP rats received same amount of vehicle as MK-801 (A and B) and LY294002 amount (C and D). Results represent 4 or 5 rats per group.
Activity in spinal cord but not in bladder during cystitis
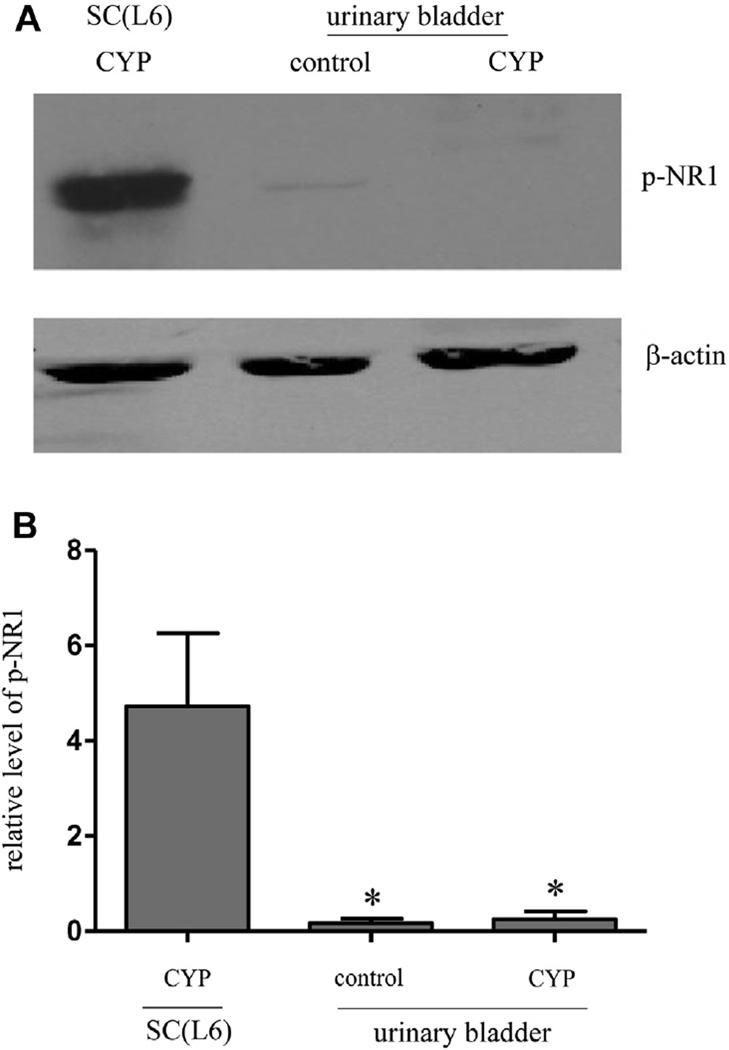
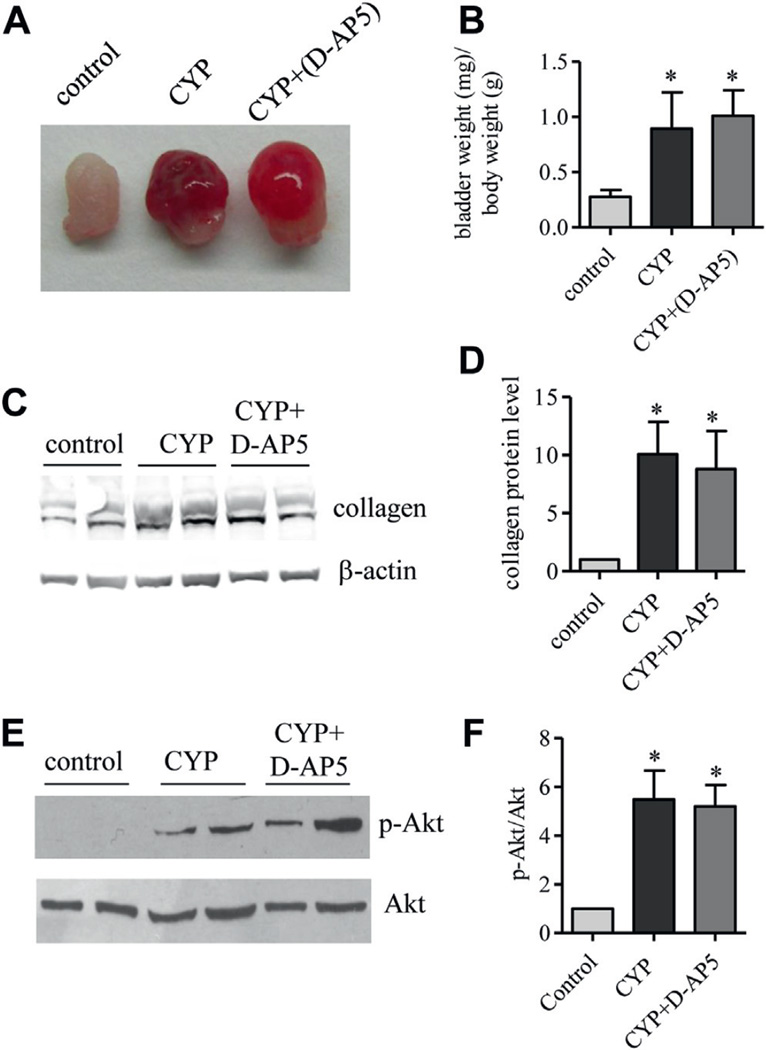
According to the readout of the NR1 phosphorylation level bladder NMDAR activity was at an extremely low level with no change during cystitis when using the L6 spinal cord from rats with cystitis for comparison (fig. 4). Results suggested that cystitis induced NMDAR activity was primarily present in the central nervous system. This notion was further supported by using the NMDAR inhibitor D-AP5, which could not cross the blood-brain barrier and failed to reduce the bladder enlargement caused by cystitis (fig. 5, A and B). Western blot demonstrated that D-AP5 also failed to decrease cystitis induced type I collagen up-regulation or cystitis induced Akt phosphorylation in the bladder (fig. 5, C to F).
Figure 4.
Western blot demonstrates that NMDAR NR1 subunit phosphorylation was expressed in spinal cord (SC) but not in bladder during cystitis (A). L6 after CYP injection was used for comparison. Note mean ± SD relative phospho-NR1 level in 3 rats (B). Asterisk indicates p <0.05 vs spinal cord.
Figure 5.
Cystitis induced bladder hypertrophy and collagen up-regulation in bladder (A) were not affected by D-AP5. After CYP and D-AP5 treatment bladder weight was measured and expressed as ratio of bladder weight to body weight (B). Asterisk indicates p <0.05 vs control. Western blot of bladder protein extracts was done to analyze type I collagen (C and D) and phospho-Akt (E and F). Control and CYP rats received same amount of vehicle as D-AP5 amount. Results represent 4 rats per group. Western blot shows samples from 2 CYP and 2 CYP plus D-AP5 treated rats (C and E).
Inhibition improved bladder function
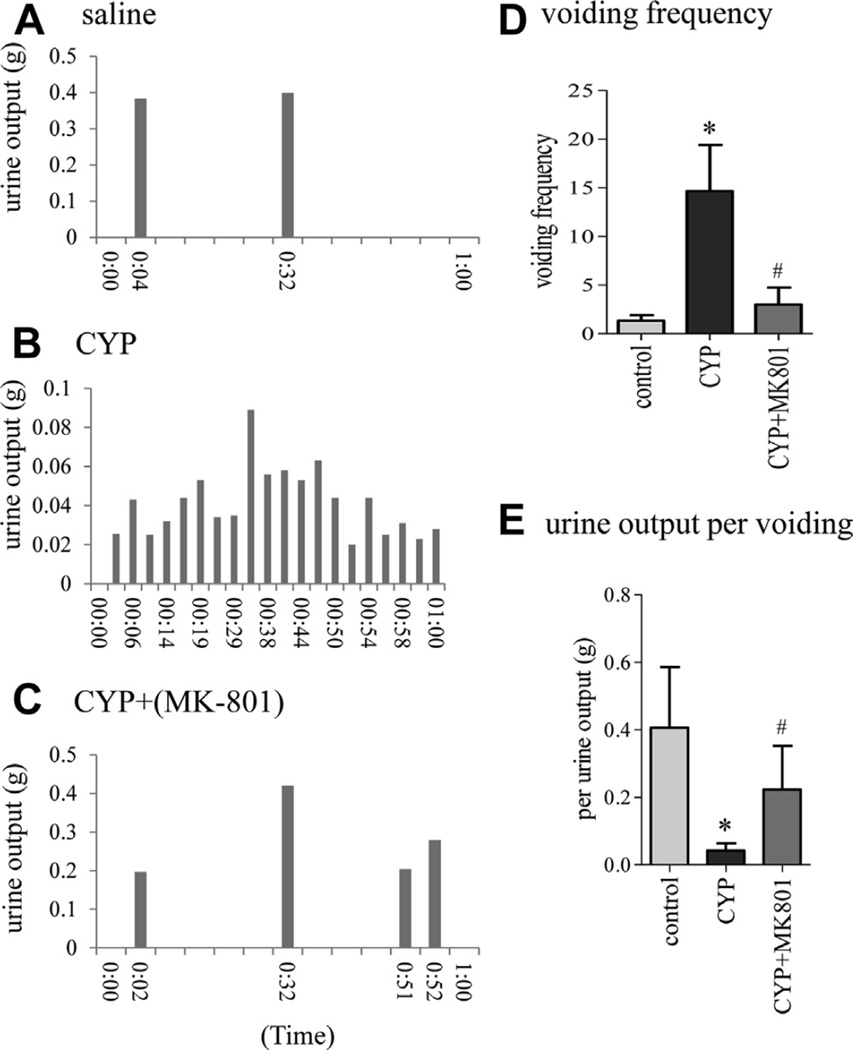
Micturition patterns were recorded by evaluating spontaneous urination using a metabolic cage complemented by cystometry (figs. 6 and 7). In the noninvasive metabolic recording each vertical bar represented 1 void and the height of the bar represented the amount of voided urine measured by an electronic scale in gm (fig. 6, A to C). Results showed an increased number of voids in rats with cystitis (fig. 6, A, B and D). Cystitis induced increases in micturition frequency were attenuated by MK-801 (fig. 6, B, C and D). Urine output also significantly changed among the groups (fig. 6, E). Less urine was excreted by rats with cystitis compared to controls (fig. 6, A, B and E). MK-801 reversed micturition frequency and increased the urine output (fig. 6, B, C and E).
Figure 6.
Representative results of spontaneous urination metabolic test show that MK-801 inhibited cystitis induced urinary frequency and restored voided volume. Urine was collected by scale connected to computer to record real-time micturition frequency (bars) and voided volume in controls (A), rats with cystitis (B) and rats with cystitis treated with MK- 801 (C). Voiding frequency (D) and voided volume (E) were determined. Control and CYP rats received same amount of vehicle as MK-801 amount. Results represent 3 or 4 rats per group. Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP.
Figure 7.
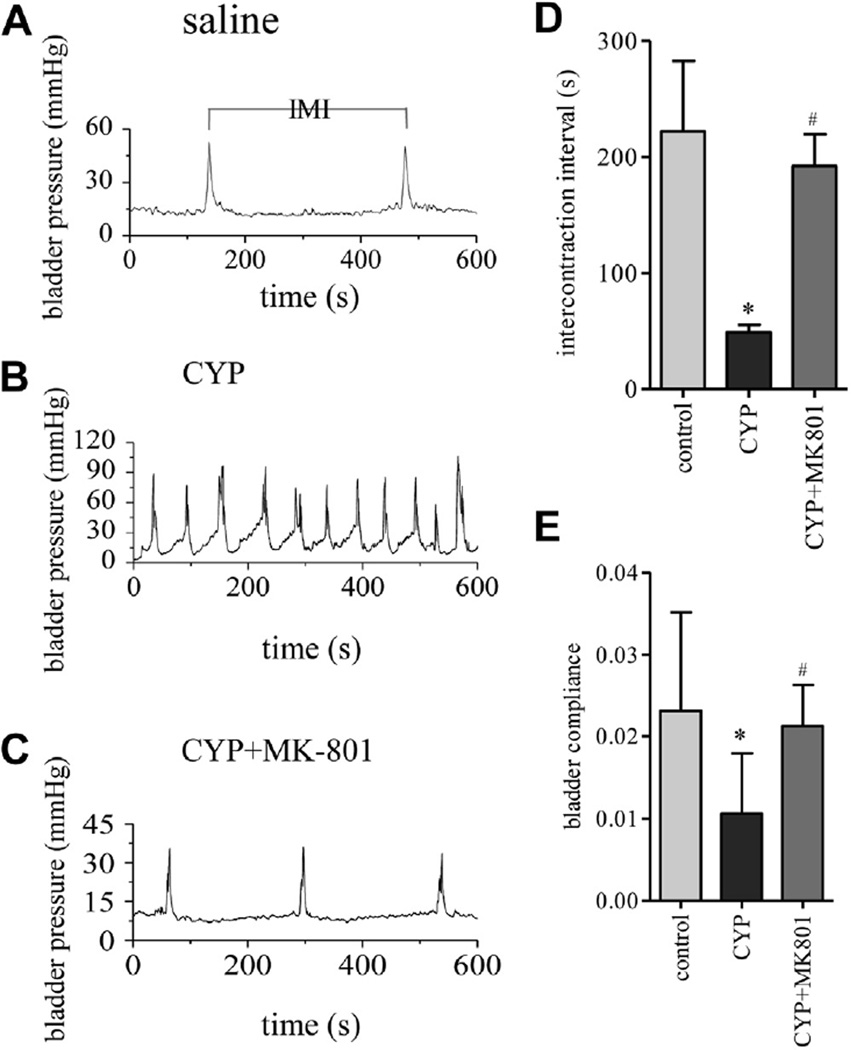
Urodynamics demonstrated decreased intermicturition interval (IMI) during cystitis, which was reversed by MK-801. Conscious cystometry was performed in controls, and rats with cystitis with and without MK-801 (A to C). s, seconds. Intermicturition interval was recorded and analyzed (D) and bladder compliance was calculated (E). Control and CYP rats received same amount of vehicle as MK-801 amount. Results represent 3 or 4 rats per group. Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP.
The effect of MK-801 on improving bladder function was further examined by conscious cystometry. In controls the average intermicturition interval was 222.1 ± 12.1 seconds at an infusion rate of 9 ml per hour. In rats with cystitis the intermicturition interval was significantly decreased (mean 48.9 ± 4.7 seconds), reflecting increased micturition frequency (fig. 7, A, B and D). MK-801 markedly improved bladder function by increasing the intermicturition intervals (mean 192.0 ± 15.9 seconds), thereby decreasing micturition frequency (fig. 7, B to D). After CYP treatment the bladder showed poor compliance, which was restored by MK-801 (fig. 7, E).
Cystitis Impaired Detrusor Muscle Strip Contractility Restored by NMDAR Activity Inhibition with MK-801 In Vivo
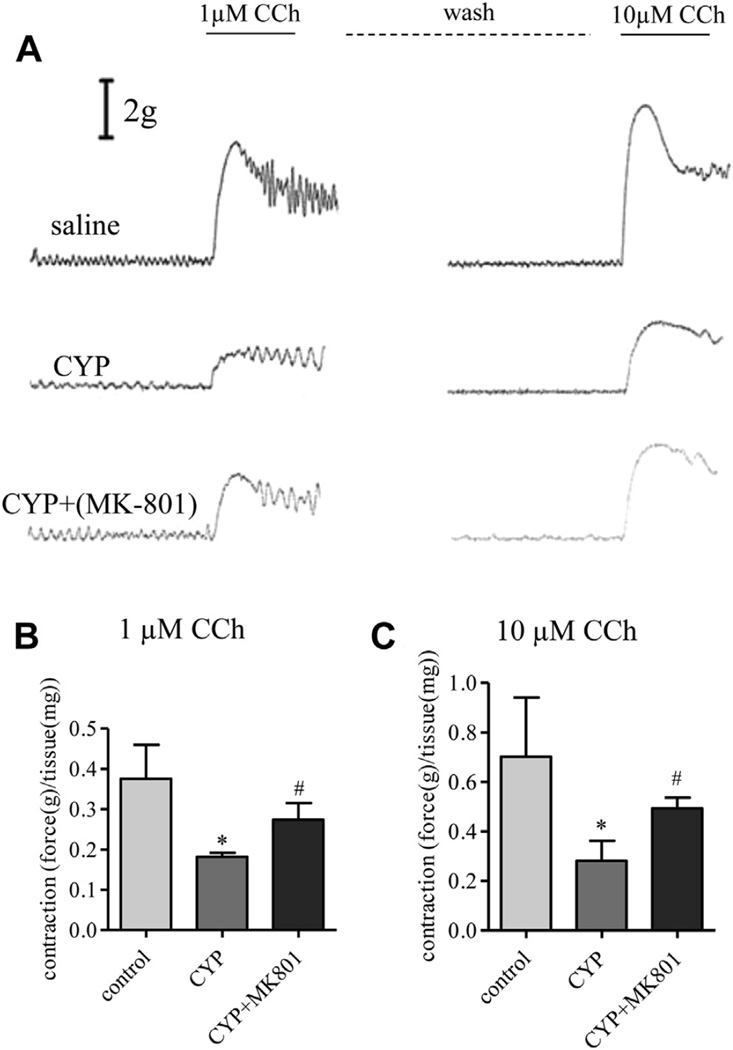
CCh elicited contractile force was examined in isolated detrusor muscle strips from controls, rats with cystitis and rats with MK-801 treatment. The average weight of muscle strips was 8.8 ± 2.5, 8.7 ± 2.7 and 7.0 ± 1.6 mg in controls, rats with cystitis and rats with cystitis treated with MK-801, respectively. There was no significant difference in the average weight of muscle strips among the groups. Using organ bath techniques we found that the bladders of CYP treated rats showed fewer contractile responses after treatment with 1 and 10 µM CCh (fig. 8). Mean contraction amplitude in CYP rats was 0.19 ± 0.01 gm/mg tissue in response to the 1 µM CCh stimulus, significantly lower than in controls (0.38 ± 0.03 gm/mg tissue). Similar trends were seen in response to 10 µM CCh (control and CYP mean 0.70 ± 0.09 and 0.28 ± 0.03 gm/mg tissue, respectively). Bladders from CYP plus MK-801 treated rats performed better after 1 and 10 µM CCh treatment (mean 0.25 ± 0.02 and 0.49 ± 0.02 gm/mg tissue) than after CYP treatment alone.
Figure 8.
MK-801 in vivo restored contractility impaired by cystitis in detrusor muscle strips from controls, and rats with cystitis with and without MK-801 subjected to 1 µM CCh stimulation (A). Strip tension was expressed as force (B). Asterisk indicates p <0.05 vs control. Pound sign indicates p <0.05 vs CYP. After washing until contractile tone reached baseline 10 µM CCh was applied (A and C). Control and CYP rats received same amount of vehicle as MK-801 amount. Results represent 3 or 4 rats with 2 or 3 strips per rat averaged as 1 point.
DISCUSSION
We investigated the role of NMDAR in the regulation of bladder hypertrophy and overactivity caused by cystitis. Blockading the NMDAR mediated signaling pathway reduced cystitis induced bladder enlargement and reversed bladder frequency. Inhibiting NMDAR also improved bladder compliance and restored detrusor smooth muscle contractility in rats with cystitis. Exploration of the underlying mechanisms showed that treatment with the NMDAR antagonist MK-801 blocked NMDAR mediated Akt activation and type I collagen up-regulation in the bladder, ultimately leading to a reduction in bladder weight and micturition frequency. These results suggest that NMDAR activity has a critical role in regulating bladder hypertrophy and function during cystitis, and the role is mediated by the PI3K/Akt pathway.
Bladder enlargement is a severe medical condition that develops in many diseases and disorders in humans and animals with bladder inflammation, neurological impairment and bladder outlet obstruction of various origins, social stress or as a natural effect of aging.22–24 At the anatomical level bladder organ hypertrophy can result from edema, an enlarged lamina propria space and the thickening of bladder layers, including the urothelium and detrusor smooth muscle.12 At the molecular physiological level bladder wall thickening can be due to excessive extracellular matrix production and deposition, detrusor cellular growth, hyperplasia and hypertrophy, as well as increased inflammatory responses.13,25,26 Type I collagen, one of the main constitutive proteins contributing to bladder hypertrophy, is regulated at least in part by increased endogenous NGF in the bladder during cystitis.13 This study shows that NMDAR activation also contributes to type I collagen up-regulation in the bladder during cystitis.
The mechanism by which NMDAR regulates changes in the bladder may include its role in activating the PI3K/Akt pathway. Our previous series showed that Akt activity is increased in the bladder during cystitis and involved in type I collagen production by bladder cells.13 Blockade of NMDAR activity with MK-801 consistently reverses cystitis induced Akt activation in the bladder. Inhibition of endogenous Akt activity by blocking the activity of Akt kinase (PI3K) also decreases type I collagen during cystitis. These results suggest that NMDAR regulated cytological changes more likely occur via activation of the PI3K/Akt pathway. An explanation is that NMDAR may facilitate neurotransmitter release at the nerve terminals in the bladder, thereby regulating Akt activation and type I collagen expression. The PI3K/Akt pathway is also activated by NGF in the bladder during cystitis.13 Thus, it may act as a convergent point in NGF and NMDAR mediated actions in vivo.
Excessive production and accumulation of extracellular matrix result in hypertrophy and poor compliance of contractile organs.27,28 During cystitis up-regulation of type I collagen, the most abundant collagen in the organ, can stiffen the organ and decrease the tension force of detrusor wall strips to stimuli. Other examples are in patients with BOO and in animal models with a higher collagen level in the bladder. Human BOO bladder strips demonstrated decreased tension as the CCh induced response.29 In rats oxybutynin induced relaxant responses of partial BOO bladder 4 weeks postoperatively were significantly higher than in controls.30 In cystitis we found that the responsiveness of detrusor muscle strips to CCh was decreased due to bladder wall thickening as a result of excessive build-up of type I collagen in the bladder. Our urodynamic recording, metabolic test and muscle strip contractility studies revealed that MK-801 treatment improved but could not completely reverse bladder function to the normal level. This is consistent with molecular findings that MK-801 partially decreased up-regulation of Akt activity and type I collagen expression in the bladder. NMDAR antagonistic therapy might be useful but not sufficient to treat bladder disease.
CONCLUSIONS
The current study shows that NMDAR inhibition with MK-801 was effective in improving bladder function at least in part. The underlying mechanism includes the regulatory role of NMDAR in collagen up-regulation in the bladder during cystitis.
Acknowledgments
Study received Virginia Commonwealth University institutional animal care and use committee approval.
Supported by NIH Grant DK077917 (LYQ).
Abbreviations and Acronyms
- Akt
protein kinase B
- BOO
bladder outlet obstruction
- CCh
carbachol
- CYP
cyclophosphamide
- D-AP5
D-2-amino-5-phosphonopentanoate
- MK-801
dizocilpine
- NGF
nerve growth factor
- NMDAR
N-methyl-d-aspartate receptor
- phospho
phosphorylated
- PI3K
phosphoinositide 3-kinase
REFERENCES
- 1.McRoberts JA, Coutinho SV, Marvizon JCG, et al. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- 2.Liu XJ, Gingrich JR, Vargas-Caballero M, et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150:185. doi: 10.1016/j.bbr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama O. Pharmacological and genetic analysis of mechanisms underlying detrusor overactivity in rats. Neurourol Urodyn. 2010;29:107. doi: 10.1002/nau.20746. [DOI] [PubMed] [Google Scholar]
- 5.Yotsuyanagi S, Yokoyama O, Komatsu K, et al. Role of cyclooxygenase-2 in the development of bladder overactivity after cerebral infarction in the rat. J Urol. 2005;174:365. doi: 10.1097/01.ju.0000161601.77023.05. [DOI] [PubMed] [Google Scholar]
- 6.Ishida F, Sato T, Imaizumi M, et al. MK-801 inhibits the micturition reflex in chronic bladder irritation caused by crystalluria in the rat. Auton Neurosci. 2003;105:1. doi: 10.1016/S1566-0702(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Kakizaki H, Shibata T, et al. Effects of chronic blockade of N-methyl-D-aspartate receptors by MK-801 on neuroplasticity of the micturition reflex pathway after partial urethral obstruction in the rat. J Urology. 2003;170:1427. doi: 10.1097/01.ju.0000074713.69589.4a. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama O, Mizuno H, Komatsu K, et al. Role of glutamate receptors in the development and maintenance of bladder overactivity after cerebral infarction in the rat. J Urology. 2004;171:1709. doi: 10.1097/01.ju.0000104861.73314.fe. [DOI] [PubMed] [Google Scholar]
- 9.Kontani H, Ueda Y. A method for producing overactive bladder in the rat and investigation of the effects of GABAergic receptor agonists and glutamatergic receptor antagonists on the cystometrogram. J Urol. 2005;173:1805. doi: 10.1097/01.ju.0000154345.87935.a4. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa M, Palsson OS, van Tilburg MA, et al. Motility response to colonic distention is increased in postinfectious irritable bowel syndrome (PI-IBS) Neurogastroenterol Motil. 2014;26:696. doi: 10.1111/nmo.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cukier JM, Cortina-Borja M, Brading AF. A case-control study to examine any association between idiopathic detrusor instability and gastrointestinal tract disorder, and between irritable bowel syndrome and urinary tract disorder. Br J Urol. 1997;79:865. doi: 10.1046/j.1464-410x.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- 12.Kay JC, Xia CM, Liu M, et al. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Exp Neurol. 2013;250:366. doi: 10.1016/j.expneurol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J Biol Chem. 2010;285:4206. doi: 10.1074/jbc.M109.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koon HW, Shih D, Karagiannides I, et al. Substance P modulates colitis-associated fibrosis. Am J Pathol. 2010;177:2300. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chéret J, Lebonvallet N, Buhé V, et al. Influence of sensory neuropeptides on human cutaneous wound healing process. J Dermatol Sci. 2014;74:193. doi: 10.1016/j.jdermsci.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012;342:461. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Lee SJ, Seo KW, et al. Homocysteine induces COX-2 expression in macrophages through ROS generated by NMDA receptor-calcium signaling pathways. Free Radic Res. 2013;47:422. doi: 10.3109/10715762.2013.784965. [DOI] [PubMed] [Google Scholar]
- 18.Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant Mk-801-selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Qiu G, Hao P, et al. Effect of partial bladder outlet obstruction on detrusor compliance, excitability and contractility in rats. Scand J Urol Nephrol. 2006;40:293. doi: 10.1080/00365590600641988. [DOI] [PubMed] [Google Scholar]
- 20.Al-Qudah M, Anderson CD, Mahavadi S, et al. Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C. Am J Physiol Gastrointest Liver Physiol. 2014;306:G328. doi: 10.1152/ajpgi.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan KY, Gupta S, de Vries R, et al. Effects of ionotropic glutamate receptor antagonists on rat dural artery diameter in an intravital microscopy model. Br J Pharmacol. 2010;160:1316. doi: 10.1111/j.1476-5381.2010.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronsson P, Johnsson M, Vesela R, et al. Adenosine receptor antagonism suppresses functional and histological inflammatory changes in the rat urinary bladder. Auton Neurosci. 2012;171:49. doi: 10.1016/j.autneu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Altuntas CZ, Daneshgari F, Izgi K, et al. Connective tissue and its growth factor CTGF distinguish the morphometric and molecular remodeling of the bladder in a model of neurogenic bladder. Am J Physiol Renal Physiol. 2012;303:F1363. doi: 10.1152/ajprenal.00273.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iguchi N, Hou A, Koul HK, et al. Partial bladder outlet obstruction in mice may cause E-cadherin repression through hypoxia induced pathway. J Urol. 2014;16:5347. doi: 10.1016/j.juro.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Dmochowski RR, Gomelsky A. Overactive bladder in males. Ther Adv Urol. 2009;1:209. doi: 10.1177/1756287209350383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiitta O, Wahlström T, Virtanen I, et al. Tenascin in inflammatory conditions and neoplasms of the urinary bladder. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:283. doi: 10.1007/BF02899274. [DOI] [PubMed] [Google Scholar]
- 27.Homer RJ, Elias JA. Airway remodeling in asthma: Therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28. doi: 10.1152/physiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lee SD, Akbal C, Miseeri R, et al. Collagen prolyl 4-hydroxylase is up-regulated in an acute bladder outlet obstruction. J Pediatr Urol. 2006;2:225. doi: 10.1016/j.jpurol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Svalo J, Nordling J, Bouchelouche K, et al. The novel beta3-adrenoceptor agonist mirabegron reduces carbachol-induced contractile activity in detrusor tissue from patients with bladder outflow obstruction with or without detrusor overactivity. Eur J Pharmacol. 2013;699:101. doi: 10.1016/j.ejphar.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 30.Babaoglu M, Zumrutbas AE, Acar IC, et al. Gap junction expression and the effects of gap junction inhibitors in overactive bladder models: does ovariectomy have a role? Int Urol Nephrol. 2013;45:1001. doi: 10.1007/s11255-013-0488-x. [DOI] [PubMed] [Google Scholar]