The likely vector was Amblyomma triste, a Neotropical tick species only recently recognized in the United States.
Keywords: rickettsial, rickettsiosis, Rickettsia parkeri, Amblyomma triste, Amblyomma maculatum, eschar, vector-borne infections, ticks, Arizona, bacterial infection, bacteria
Abstract
In the United States, all previously reported cases of Rickettsia parkeri rickettsiosis have been linked to transmission by the Gulf Coast tick (Amblyomma maculatum). Here we describe 1 confirmed and 1 probable case of R. parkeri rickettsiosis acquired in a mountainous region of southern Arizona, well beyond the recognized geographic range of A. maculatum ticks. The likely vector for these 2 infections was identified as the Amblyomma triste tick, a Neotropical species only recently recognized in the United States. Identification of R. parkeri rickettsiosis in southern Arizona demonstrates a need for local ecologic and epidemiologic assessments to better understand geographic distribution and define public health risk. Education and outreach aimed at persons recreating or working in this region of southern Arizona would improve awareness and promote prevention of tickborne rickettsioses.
Rickettsia parkeri, a tickborne bacterium that causes a febrile, eschar-associated illness throughout many countries of the Western Hemisphere, is transmitted by Amblyomma ticks. In the United States, ≈40 cases of R. parkeri rickettsiosis have been reported since its recognition in 2004 (1). The Gulf Coast tick (Amblyomma maculatum) is the principal vector of R. parkeri in the United States (2), and all previously documented US infections arose within the known geographic range of these ticks (1). Confirmed cases of R. parkeri rickettsiosis also have been reported from Uruguay and Argentina, where A. triste and A. tigrinum ticks serve as the principal vector species (3–6). Recent reviews of tick collection records and archived specimens documented and identified the presence of ticks very closely related to A. triste in several regions of the southwestern United States and adjacent regions of Mexico since at least 1942 (7,8). Here we report 1 confirmed and 1 probable case of R. parkeri rickettsiosis, each acquired in southern Arizona after bites from A. triste ticks.
Case Histories
Patient 1
Patient 1 was a 49-year-old male resident of Arizona. In July 2014, he was hiking in the Pajarito Mountains of Santa Cruz County, Arizona. This remote and semi-arid region receives a mean annual precipitation of 430 mm and is situated at ≈1,200 m above sea level (Figure 1, panel A). During the hike, the man removed and discarded an adult tick he found attached to his right arm. The tick had been attached for <3 hours. A similar tick found crawling on the patient was photographed on the same day (Figure 1, panel B). An ulcerated lesion appeared at the site of the tick bite ≈5 days later. Ten days after the tick bite, the man had onset of fever with a temperature reaching 38.7°C, which was accompanied by headache, myalgia, and scalp tenderness. On day 11, his physician noted a 1-cm eschar, surrounded by a ring of erythema, lateral to the antecubital fossa of his right arm (Figure 2, panel A). No rash or lymphadenopathy was noted. The patient was prescribed doxycycline (100 mg 2×/d for 10 days), and his temperature returned to normal within 24 hours. However, a sparse maculopapular rash subsequently developed on his back, flank, abdomen, and feet; this rash improved within 4 days. The patient reported no recent out-of-state travel or other tick exposures during the several weeks preceding his illness. A medical entomologist (J.W.M.), expert in Amblyomma tick identification and familiar with previous specimens in this genus collected from southern Arizona, reviewed the tick photograph associated with the case and, on the basis of the distinctive dorsal ornamentation of the tick and its geographic origin, presumptively determined the specimen to be an adult male tick of the A. triste species. In July 2015, the patient hiked with several other persons in the Pajarito Mountains, ≈5 miles south of where he had sustained a tick bite the preceding year. He and 1 of his hiking companions (patient 2) were bitten by ticks that visually resembled those observed in 2014. The tick that bit patient 1 in 2015 was attached for <8 hours before it was removed. Patient 1 developed a small, erythematous papule with a central depressed scab at the bite site that healed within several days but remained otherwise asymptomatic.
Figure 1.
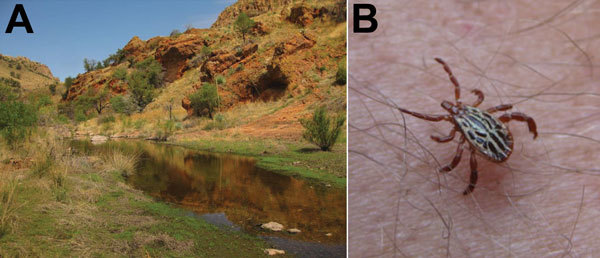
A) Typical habitat in the Pajarito Mountains in Santa Cruz County, Arizona, USA, near the location where patient 1 sustained a bite from a tick that resulted in Rickettsia parkeri rickettsiosis in July 2014. B) Male tick identical to the tick that bit patient 1. The distinctive white ornamentation on the scutum and disjunct geographic origin strongly support its presumptive identification as Amblyomma triste.
Figure 2.
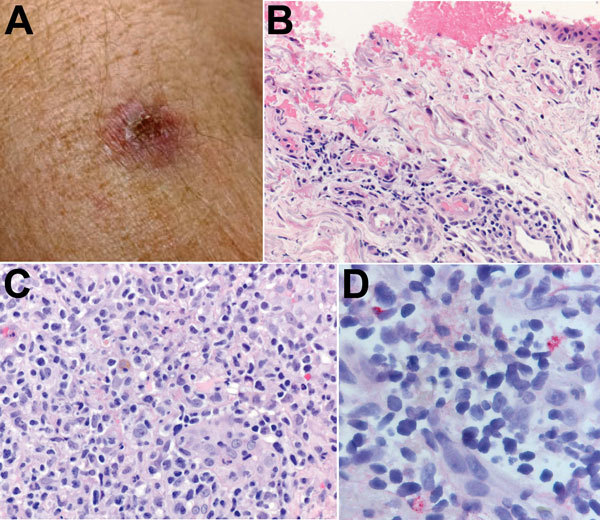
A) Eschar on the right arm of patient 1 at the site of tick bite sustained in Santa Cruz County, Arizona, USA. B) Histological appearance of the eschar biopsy specimen showing ulcerated epidermis with hemorrhage and perivascular lymphohistiocytic inflammatory infiltrates in the superficial dermis. Hematoxylin-eosin staining; original magnification ×50. C) Dense lymphohistiocytic infiltrates around eccrine ducts in the deep dermis of the biopsy specimen. Hematoxylin-eosin staining; original magnification ×100. D) Sparsely distributed intracellular antigens of Rickettsia parkeri (red) within the inflammatory infiltrates, detected by immunohistochemistry. Alkaline phosphatase with naphthol-fast red and hematoxylin counterstaining; original magnification ×158.
Patient 2
Patient 2 was a 42-year-old female resident of Arizona. While hiking, she discovered a tick attached to her scalp behind her right ear. The tick was attached for <8 hours before it was removed. A small ulcer surrounded by a narrow rim of erythema developed at the bite site. Four days after the tick bite, the patient had onset of fever with a temperature of 37.7°C, myalgia, and fatigue. Two days later, a sparse maculopapular rash appeared on her lower legs and arms; this rash lasted for ≈3 days. The patient was prescribed doxycycline (100 mg 2×/d for 10 days) on the first day of fever, and her constitutional symptoms resolved within 48 hours. She did not report any out-of-state or other outdoor exposures during the weeks before her illness.
Materials and Methods
Two tissue biopsy specimens were collected in July 2014 from the eschar of patient 1. DNA was extracted from 1 sample using a QIAamp DNA Mini Kit (QIAGEN, Valencia, CA, USA) and eluted in a final volume of 200 μL. Extracted DNA was tested in duplicate by using Rickettsia genus–specific, R. rickettsii–specific, and R. parkeri–specific real-time PCR assays (9,10). Cycle threshold (Ct) values <40 were considered positive. A nested PCR assay was used to amplify a segment of the ompA antigen gene by using 3 μL of purified DNA template and 0.8 μmol/L each of primer 190–70 and 190–701 in the primary reaction and 1 μL of the completed primary PCR reaction and 0.8 μmol/L each of primer 190-FN1 and 190-RN1 in the nested reaction (2). The amplified DNA fragment was sequenced by using a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequence alignments were made by using SeqMan Pro in the DNASTAR Lasergene 12 suite (DNASTAR, Inc., Madison, WI, USA) and evaluated with BLAST (http://blast.ncbi.nlm.nih.gov/blast.cgi). A second biopsy specimen was fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections cut at 3 μm in thickness were stained with hematoxylin-eosin and tested by using an immunoalkaline phosphatase technique with a polyclonal anti–R. rickettsii antiserum, diluted 1:500 (11).
Acute- and convalescent-phase serum samples were collected from patient 1 in 2014 and 2015 and from patient 2 in 2015. These specimens were tested for IgG and IgM reactive with antigens of R. parkeri and R. rickettsii by using an indirect immunofluorescence antibody assay. All specimens were diluted initially at 1:32, and antibody titers were expressed as the reciprocal of the last subsequent dilution that provided specific fluorescence. An antibody titer >64 was considered evidence of past exposure to a spotted fever group Rickettsia species, and a >4-fold change in titer between specimens collected separately was considered evidence of a recent infection or exposure (11). Serum samples were processed by using the Zorba IgG Removal Kit (Zeus Scientific, Branchburg, NJ, USA) before evaluating for IgM.
In 2015, the 2 male ticks that had bitten patients 1 and 2, respectively, and an additional 4 female and male specimens found crawling on clothing of the patients and their hiking companions were placed in 70% ethanol. These specimens were sent to the US Department of Agriculture National Veterinary Services Laboratories (Ames, Iowa, USA) for morphologic identification, and they were subsequently tested at the Centers for Disease Control and Prevention (Atlanta, Georgia, USA) by molecular techniques for evidence of infection with R. parkeri. Ticks were minced individually by using sterile scalpel blades. DNA was extracted by using a QIAamp DNA Mini Kit, and each sample was eluted in a final volume of 100 μL. Extracted DNA was evaluated by using a Rickettsia genus–specific real-time PCR assay and a nested ompA antigen gene PCR assay followed by sequencing, as described previously. An additional collection of 1 male and 1 female A. triste tick taken from clothing of the same hikers in Santa Cruz County, Arizona, was retained as a voucher in the parasitology reference collection at the National Veterinary Services Laboratories (accession no. 15-023437).
Results
Real-time PCR of DNA extracted from the eschar of patient 1 yielded positive results when evaluated using the Rickettsia genus–specific assay (averaged Ct 36.86, SD 0.74) and the R. parkeri–specific assay (Ct 35.51) and yielded a negative result when evaluated using the R. rickettsii–specific assay. A 540-bp segment of the ompA gene amplified by a nested PCR assay demonstrated complete identity with the corresponding segment of the ompA gene of R. parkeri strain Portsmouth (GenBank accession no. CP003341.1). Microscopic examination of the formalin-fixed skin biopsy specimen demonstrated ulceration of the epidermis and lymphohistiocytic inflammatory cell infiltrates distributed predominantly around small blood vessels and eccrine glands and ducts in the superficial and deep dermis. Inflamed vessels revealed focally swollen endothelial cells but no fibrin thrombi (Figure 2, panels B and C). Immunohistochemical staining for spotted fever group Rickettsia spp. revealed sparse intracellular antigens of R. parkeri within macrophages in the inflammatory infiltrates (Figure 2, panel D).
Serum samples from patient 1, collected 6 and 24 days after the onset of his illness in 2014, reacted with R. parkeri antigens at IgG titers of 64 and 512, respectively, and with R. rickettsii antigens at IgG titers of <32 and 128, respectively. Samples from these 2 collection dates demonstrated IgM titers of 1,024 and 1,024 when these were reacted with R. parkeri and R. rickettsii, antigens, respectively. Serum samples collected from this patient in 2015, at 12 and 34 days after his second tick bite, reacted with R. parkeri antigens at IgG titers of 32 and 128, respectively, and with R. rickettsii antigens at IgG titers of 32 and 128, respectively. No IgM reactive with R. parkeri or R. rickettsii antigens was detected in either sample. Serum specimens collected from patient 2 at 1 and 32 days after the onset of fever reacted with R. parkeri antigens at IgG titers of 64 and 64, respectively, and with R. rickettsii antigens at IgG titers of <32 and <32, respectively. No IgM reactive with R. parkeri or R. rickettsii antigens was detected in either sample.
By using the only published taxonomic key that includes A. triste among North American Amblyomma spp. ticks (8) in addition to 2 widely accepted taxonomic keys to Neotropical ticks (12,13), each of 2 female and 4 male tick specimens collected in 2015 were identified as A. triste on the basis of details of scutal ornamentation, leg armature, and festoons. The Rickettsia genus–specific real-time PCR assay was positive for 3 male ticks (average Ct 18.36–19.93, SD 0.05–0.09), including the 2 ticks that bit patients 1 and 2. A 540-bp segment of the ompA gene was amplified by using nested PCR on each of these same specimens, and sequence analysis demonstrated complete identity with the corresponding segment of the ompA gene of R. parkeri strain Portsmouth.
Discussion
Before this report, all documented US cases of R. parkeri rickettsiosis occurred within the known geographic range of A. maculatum ticks, predominantly in coastal states of the Eastern Seaboard and along the Gulf of Mexico (1). The patients described in this report were infected with R. parkeri in southern Arizona after bites from ticks identified photographically and morphologically as A. triste. To our knowledge, established US populations of the Gulf Coast tick do not occur west of the 100th meridian. In contrast, collection records from multiple sources documented historical attachments of A. triste ticks to humans in Cochise (1942) and Santa Cruz (1992) Counties in southern Arizona (7). Adult A. triste tick collections have been reported from these counties during July–September, corresponding with the local monsoon season.
A. triste is an aggressive, human-biting tick species related closely to A. maculatum (14) and is recognized as a potential vector of R. parkeri in Argentina, Brazil, and Uruguay, where rates of rickettsial infection in this tick species range from ≈6% to 20% (15–20). The distribution of A. triste ticks in North America is less well-characterized, with validated collection records from the edges of the Chihuahuan Desert, generally at higher altitudes, and in the Mexican Highlands section of the Basin and Range Province, within the US states of Arizona and Texas (7) and the states of Sonora, Durango, and Coahuila in Mexico (7,21). Our findings indicate that the A. triste tick is also a vector of R. parkeri in southern Arizona. Although A. triste ticks have probably adapted to certain semi-arid environments of the southwest, preliminary observations from this investigation and archival collection records suggest that host-seeking adult A. triste ticks are most active during July–September, corresponding to the monsoon season in this region and the period of highest risk for human exposure to R. parkeri. R. rickettsii, the agent of Rocky Mountain spotted fever (RMSF), is also endemic to southern Arizona and northern Mexico, where it is transmitted to humans by Rhipicephalus sanguineus ticks (22,23).
The clinical characteristics of the confirmed and probable cases of R. parkeri rickettsiosis described in this report are similar to previous descriptions of the disease (4–6,11). Of particular interest is the re-exposure of patient 1 to an R. parkeri–infected tick ≈1 year after primary infection with this agent. During his initial infection in 2014, the patient generated substantial titers of IgG and IgM reactive with antigens of R. parkeri and R. rickettsii. In 2015, after the bite of another infected tick, this patient had a small and rapidly healing lesion at the inoculation site and demonstrated an IgG seroconversion to these same antigens, but did not otherwise become ill and did not mount a measurable IgM response to either antigen. In this context, these data identified an anamnestic antibody response after exposure to an infected tick in 2015 and suggest that some level of protective immunity to R. parkeri persisted in patient 1 for at least 1 year after his primary infection.
Future studies should aim to better identify the geographic and host ranges of A. triste ticks in the southwestern United States and the frequency with which these ticks are infected with R. parkeri. Nonetheless, our data suggest that at least some of the ≈330 cases of RMSF reported from Arizona during the past 10 years (http://www.azdhs.gov/phs/oids/data/stats-archive.htm) might actually represent infections with R. parkeri. Because the geographic distribution of A. triste ticks also includes several states of northern Mexico, some cases of spotted fever group rickettsiosis in this region might be attributable to infections with R. parkeri. Commonly used serologic tests do not distinguish between these clinically similar tickborne diseases, and molecular assays are necessary to provide an etiologic diagnosis (11). RMSF is a life-threatening infection that was associated with a 7% case-fatality rate in Arizona during 2002–2011 (24) and a 20% case-fatality rate among patients <19 years of age in Sonora, Mexico, during 2004–2013 (25). By comparison, no deaths have been attributed to R. parkeri rickettsiosis (1,4,6,11). Although RMSF and R. parkeri rickettsiosis both respond rapidly to therapy with doxycycline, species-specific diagnoses are crucial to accurately define the epidemiologies of the individual diseases in regions where both pathogens might be endemic.
Identification of R. parkeri rickettsiosis in southern Arizona demonstrates a need for local ecologic and epidemiologic assessments to better understand geographic distribution and define public health risk. Education and outreach aimed at persons recreating or working in this region of southern Arizona would improve awareness and promote prevention of tickborne rickettsioses.
Biography
Ms. Herrick is an infectious disease epidemiologist at the Arizona Department of Health Services. She is interested in the public health response to and epidemiology of communicable diseases.
Footnotes
Suggested citation for this article: Herrick KL, Pena SA, Yaglom HD, Layton BJ, Moors A, Loftis AD, et al. Rickettsia parkeri rickettsiosis, Arizona, USA. Emerg Infect Dis. 2016 May [date cited]. http://dx.doi.org/10.3201/eid2205.151824
References
- 1.Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J Med Entomol. 2015;52:230–52 . 10.1093/jme/tju022 [DOI] [PubMed] [Google Scholar]
- 2.Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, et al. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–3. 10.3201/eid1305.061468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti-Díaz IA, Moraes-Filho J, Pacheco RC, Labruna MB. Serological evidence of Rickettsia parkeri as the etiological agent of rickettsiosis in Uruguay. Rev Inst Med Trop Sao Paulo. 2009;51:337–9. 10.1590/S0036-46652009000600005 [DOI] [PubMed] [Google Scholar]
- 4.Romer Y, Seijo AC, Crudo F, Nicholson WL, Varela-Stokes A, Lash RR, et al. Rickettsia parkeri rickettsiosis, Argentina. Emerg Infect Dis. 2011;17:1169–73. 10.3201/eid1707.101857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portillo A, García-García C, Sanz MM, Santibáñez S, Venzal JM, Oteo JA. A confirmed case of Rickettsia parkeri infection in a traveler from Uruguay. Am J Trop Med Hyg. 2013;89:1203–5. 10.4269/ajtmh.13-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romer Y, Nava S, Govedic F, Cicuttin G, Denison AM, Singleton J, et al. Rickettsia parkeri rickettsiosis in different ecological regions of Argentina and its association with Amblyomma tigrinum as a potential vector. Am J Trop Med Hyg. 2014;91:1156–60. 10.4269/ajtmh.14-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertins JW, Moorhouse AS, Alfred JT, Hutcheson HJ. Amblyomma triste (Acari: Ixodidae): new North American collection records, including the first from the United States. J Med Entomol. 2010;47:536–42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzmán-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Pérez TM. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: identification keys, distribution and hosts. Zootaxa. 2011;2998:16–38. [Google Scholar]
- 9.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51:314–7. 10.1128/JCM.01723-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denison AM, Amin BJ, Nicholson WL, Paddock CD. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis. 2014;59:635–42. 10.1093/cid/ciu358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–96. 10.1086/592254 [DOI] [PubMed] [Google Scholar]
- 12.Jones EK, Clifford CM, Keirans JE, Kohls G. Ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young Univ Sci Bull Biol Ser. 1972;17:1–40. [Google Scholar]
- 13.Martins TF, Lado P, Labruna MB, Venzal JM. El género Amblyomma (Acari: Ixodidae) en Uruguay: especies, distribución, hospedadores, importancia sanitaria y claves para la determinación de adultos y ninfas. Veterinaria (Montevideo). 2014;50:26–41. [Google Scholar]
- 14.Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 2005;60:99–112. 10.1007/s11230-004-1382-9 [DOI] [PubMed] [Google Scholar]
- 15.Venzal JM, Portillo A, Estrada-Peña A, Castro O, Cabrera PA, Oteo JA. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg Infect Dis. 2004;10:1493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nava S, Elshenawy Y, Eremeeva ME, Sumner JW, Mastropaolo M, Paddock CD. Rickettsia parkeri in Argentina. Emerg Infect Dis. 2008;14:1894–7. 10.3201/eid1412.080860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicuttin G, Nava S. Molecular identification of Rickettsia parkeri infecting Amblyomma triste ticks in an area of Argentina where cases of rickettsiosis were diagnosed. Mem Inst Oswaldo Cruz. 2013;108:123–5. 10.1590/S0074-02762013000100022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monje LD, Nava S, Antoniazzi LR, Colombo VC, Beldomenico PM. In vitro isolation and infection intensity of Rickettsia parkeri in Amblyomma triste ticks from the Paraná River Delta region, Argentina. Ticks Tick Borne Dis. 2014;5:924–7. [DOI] [PubMed]
- 19.Melo ALT, Alves AS, Nieri-Bastos FA, Martins TF, Witter R, Pacheco TA, et al. Rickettsia parkeri infecting free-living Amblyomma triste ticks in the Brazilian Pantanal. Ticks Tick Borne Dis. 2015;6:237–41. [DOI] [PubMed]
- 20.Lado P, Costa FB, Verdes JM, Labruna MB, Venzal JM. Seroepidemiological survey of Rickettsia spp. in dogs from the endemic area of Rickettsia parkeri rickettsiosis in Uruguay. Acta Trop. 2015;146:7–10. 10.1016/j.actatropica.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Guzman-Cornejo CT, Pérez TM, Nava S, Guglielmone AA. Confirmation of the presence of Amblyomma triste Koch, 1844 (Acari: Ixodidae) in Mexico. Syst Appl Acarol. 2006;11:47–50.
- 22.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–94. 10.1056/NEJMoa050043 [DOI] [PubMed] [Google Scholar]
- 23.Eremeeva ME, Zambrano ML, Anaya L, Beati L, Karpathy SE, Santos-Silva MM, et al. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J Med Entomol. 2011;48:418–21. 10.1603/ME10181 [DOI] [PubMed] [Google Scholar]
- 24.Regan JJ, Traeger MS, Humpherys D, Mahoney DL, Martinez M, Emerson GL, et al. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis. 2015;60:1659–66. 10.1093/cid/civ116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez-Hernandez G, Murillo-Benitez C, Candia-Plata MC, Moro M. Clinical profile and predictors of fatal Rocky Mountain spotted fever in children from Sonora, Mexico. Pediatr Infect Dis J. 2015;34:125–30. 10.1097/INF.0000000000000496 [DOI] [PubMed] [Google Scholar]


