Abstract
OBJECTIVES--To test the hypotheses that Parkinson's disease can differentially produce deficits in voluntary and rhythmic jaw movements, which involve different neuronal circuits, and that levodopa treatment improves specific components of the motor deficit. METHODS--Patients with idiopathic Parkinson's disease and control subjects were tested on a series of jaw motor tasks that included simple voluntary movement, isometric clenching, and natural and paced rhythmic movements. Jaw movements were measured by changes in electromagnetic fields and EMG activity. Patients with Parkinson's disease with fluctuations in motor responses to levodopa were tested while off and on. RESULTS--During the off state, patients with Parkinson's disease were significantly worse than the control subjects on most tasks. The deficits included a decrease in amplitude and velocity during jaw opening and closing, aberrant patterns and low amplitude of EMG activity during clenching, and low vertical amplitude and prolonged durations of occlusion during rhythmic movements. No decrements were found in the amplitude of voluntary lateral jaw movements or the frequency of rhythmic movements. During the on state, improvements occurred in the patterns and level of EMG activity during clenching and in the vertical amplitude and duration of occlusion during rhythmic movements, although a significant decrement occurred in the lateral excursion of the jaw. CONCLUSIONS--Parkinson's disease affects the central programming of functionally related muscles involved in voluntary and rhythmic jaw movements and levodopa replacement influences only certain aspects of jaw movement, most likely those requiring sensory feedback.
Full text
PDF

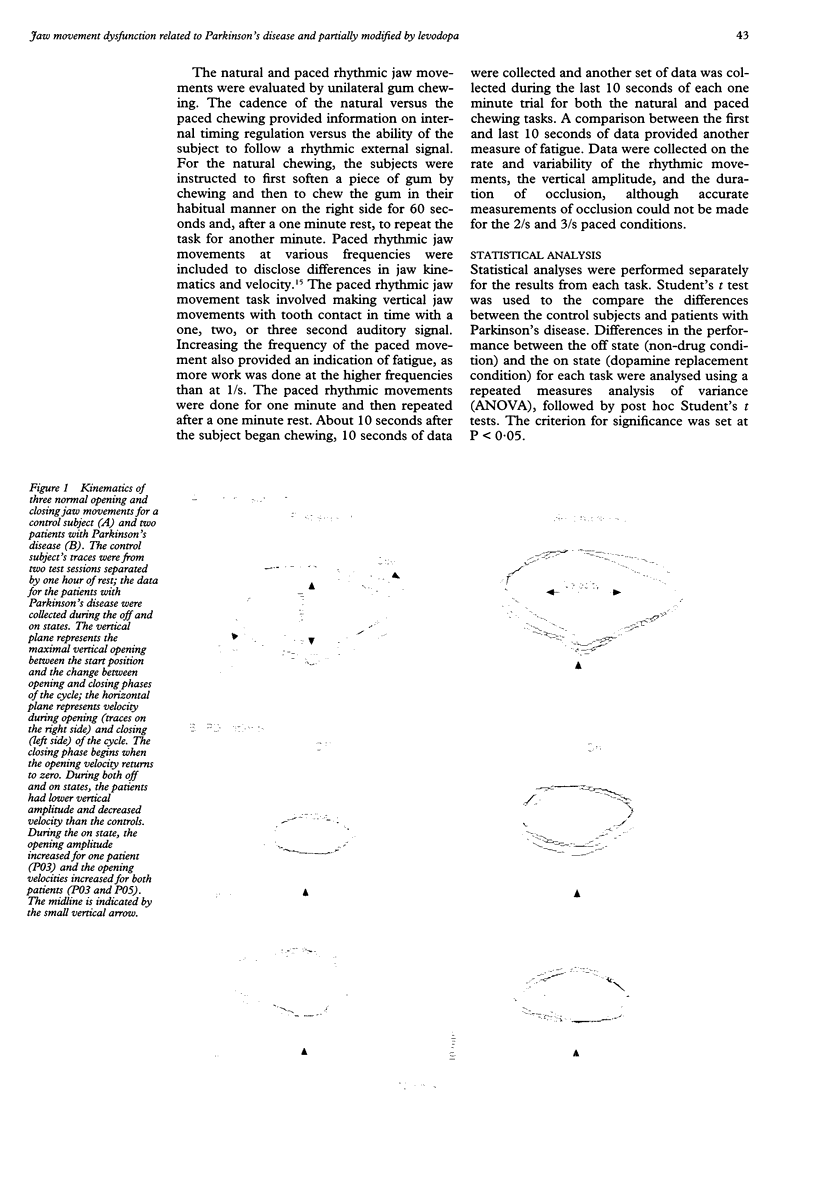

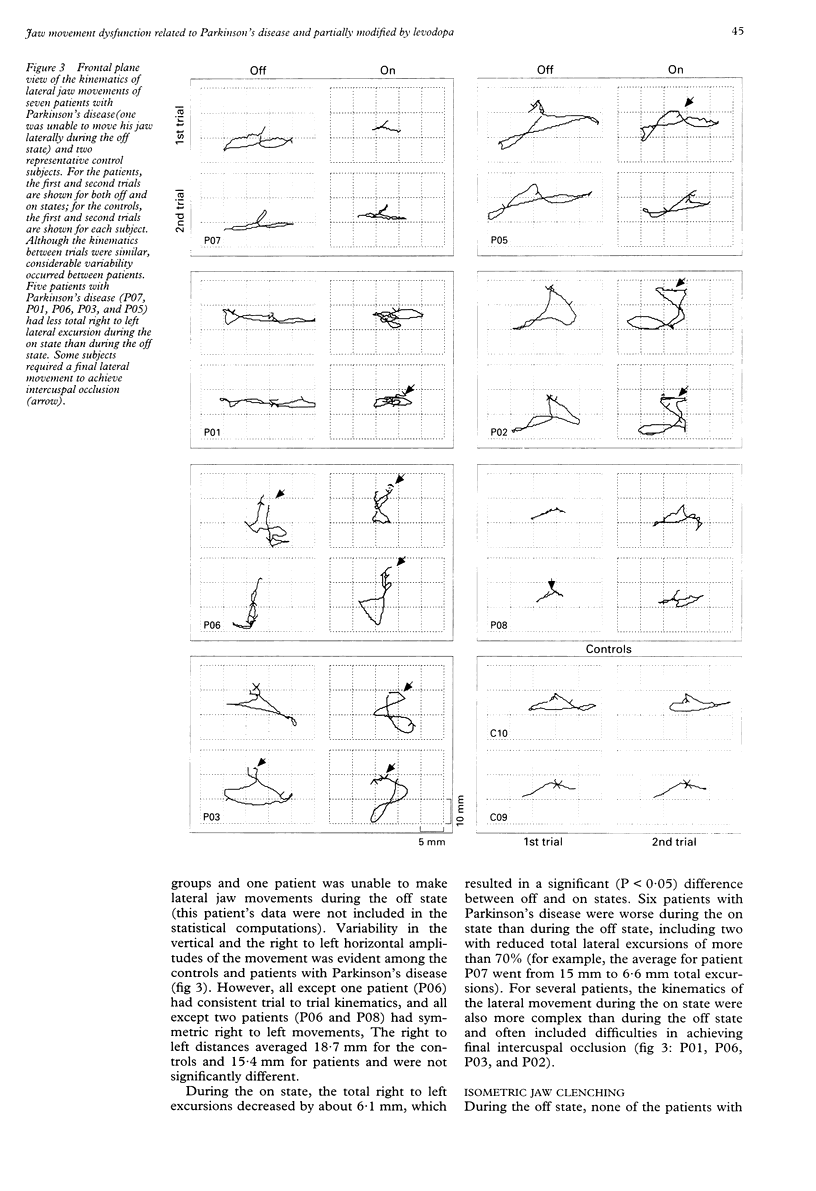




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbs J. H., Hartman D. E., Vishwanat B. Orofacial motor control impairment in Parkinson's disease. Neurology. 1987 Mar;37(3):394–398. doi: 10.1212/wnl.37.3.394. [DOI] [PubMed] [Google Scholar]
- Alexander G. E., Crutcher M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990 Jul;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. P., Abbs J. H. Response properties of the perioral reflex in Parkinson's disease. Exp Neurol. 1987 Dec;98(3):563–572. doi: 10.1016/0014-4886(87)90265-2. [DOI] [PubMed] [Google Scholar]
- Chandler S. H., Goldberg L. J. Effects of pontomedullary reticular formation stimulation on the neuronal networks responsible for rhythmical jaw movements in the guinea pig. J Neurophysiol. 1988 Mar;59(3):819–832. doi: 10.1152/jn.1988.59.3.819. [DOI] [PubMed] [Google Scholar]
- Connor N. P., Abbs J. H. Task-dependent variations in parkinsonian motor impairments. Brain. 1991 Feb;114(Pt 1A):321–332. [PubMed] [Google Scholar]
- Cruccu G., Pauletti G., Agostino R., Berardelli A., Manfredi M. Masseter inhibitory reflex in movement disorders. Huntington's chorea, Parkinson's disease, dystonia, and unilateral masticatory spasm. Electroencephalogr Clin Neurophysiol. 1991 Feb;81(1):24–30. doi: 10.1016/0168-5597(91)90100-c. [DOI] [PubMed] [Google Scholar]
- DeLong M. R., Crutcher M. D., Georgopoulos A. P. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985 Feb;53(2):530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Fromm C., Kröller J., Jennings V. A. Motor Cortex control of finely graded forces. J Neurophysiol. 1983 May;49(5):1199–1215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Gardiner T. W., Nelson R. J. Striatal neuronal activity during the initiation and execution of hand movements made in response to visual and vibratory cues. Exp Brain Res. 1992;92(1):15–26. doi: 10.1007/BF00230379. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., Luschei E. S. Effects of destroying spindle afferents from jaw muscles on mastication in monkeys. J Neurophysiol. 1974 Sep;37(5):967–981. doi: 10.1152/jn.1974.37.5.967. [DOI] [PubMed] [Google Scholar]
- Haraldson T., Carlsson G. E., Dahlström L., Jansson T. Relationship between myoelectric activity in masticatory muscles and bite force. Scand J Dent Res. 1985 Dec;93(6):539–545. doi: 10.1111/j.1600-0722.1985.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Hoffman D. S., Luschei E. S. Responses of monkey precentral cortical cells during a controlled jaw bite task. J Neurophysiol. 1980 Aug;44(2):333–348. doi: 10.1152/jn.1980.44.2.333. [DOI] [PubMed] [Google Scholar]
- Hoover J. E., Strick P. L. Multiple output channels in the basal ganglia. Science. 1993 Feb 5;259(5096):819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Persson M., Johnels B. Levodopa induced ON-OFF motor fluctuations in Parkinson's disease related to rhythmical masticatory jaw movements. J Neurol Neurosurg Psychiatry. 1992 Apr;55(4):304–307. doi: 10.1136/jnnp.55.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. P., Widmer C. G., Feine J. S. Validity of diagnostic and monitoring tests used for temporomandibular disorders. J Dent Res. 1995 Apr;74(4):1133–1143. doi: 10.1177/00220345950740041501. [DOI] [PubMed] [Google Scholar]
- MacDonald J. W., Hannam A. G. Relationship between occlusal contacts and jaw-closing muscle activity during tooth clenching: Part I. J Prosthet Dent. 1984 Nov;52(5):718–728. doi: 10.1016/0022-3913(84)90149-5. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Obeso J. A. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994 Aug;117(Pt 4):877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Mink J. W., Thach W. T. Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 1991 Feb;65(2):273–300. doi: 10.1152/jn.1991.65.2.273. [DOI] [PubMed] [Google Scholar]
- Mogoseanu D., Smith A. D., Bolam J. P. Monosynaptic innervation of trigeminal motor neurones involved in mastication by neurones of the parvicellular reticular formation. J Comp Neurol. 1993 Oct 1;336(1):53–65. doi: 10.1002/cne.903360105. [DOI] [PubMed] [Google Scholar]
- Mohl N. D., Dixon D. C. Current status of diagnostic procedures for temporomandibular disorders. J Am Dent Assoc. 1994 Jan;125(1):56–64. doi: 10.14219/jada.archive.1994.0014. [DOI] [PubMed] [Google Scholar]
- Montgomery E. B., Jr, Nuessen J., Gorman D. S. Reaction time and movement velocity abnormalities in Parkinson's disease under different task conditions. Neurology. 1991 Sep;41(9):1476–1481. doi: 10.1212/wnl.41.9.1476. [DOI] [PubMed] [Google Scholar]
- Murray G. M., Lin L. D., Moustafa E. M., Sessle B. J. Effects of reversible inactivation by cooling of the primate face motor cortex on the performance of a trained tongue-protrusion task and a trained biting task. J Neurophysiol. 1991 Mar;65(3):511–530. doi: 10.1152/jn.1991.65.3.511. [DOI] [PubMed] [Google Scholar]
- Mushiake H., Inase M., Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991 Sep;66(3):705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Muramatsu S., Yoshida M. Role of the basal ganglia in manifestation of rhythmical jaw movement in rats. Brain Res. 1990 Dec 10;535(2):335–338. doi: 10.1016/0006-8993(90)91620-v. [DOI] [PubMed] [Google Scholar]
- Olsson K. A., Landgren S., Westberg K. G. Location of, and peripheral convergence on, the interneuron in the disynaptic path from the coronal gyrus of the cerebral cortex to the trigeminal motoneurons in the cat. Exp Brain Res. 1986;65(1):83–97. doi: 10.1007/BF00243832. [DOI] [PubMed] [Google Scholar]
- Plesh O., Bishop B., McCall W. Mandibular movements and jaw muscles' activity while voluntarily chewing at different rates. Exp Neurol. 1987 Nov;98(2):285–300. doi: 10.1016/0014-4886(87)90243-3. [DOI] [PubMed] [Google Scholar]
- Schneider J. S., Diamond S. G., Markham C. H. Deficits in orofacial sensorimotor function in Parkinson's disease. Ann Neurol. 1986 Mar;19(3):275–282. doi: 10.1002/ana.410190309. [DOI] [PubMed] [Google Scholar]
- Schultz W., Romo R. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res. 1992;91(3):363–384. doi: 10.1007/BF00227834. [DOI] [PubMed] [Google Scholar]
- Stelmach G. E., Teasdale N., Phillips J., Worringham C. J. Force production characteristics in Parkinson's disease. Exp Brain Res. 1989;76(1):165–172. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- Von Krosigk M., Smith A. D. Descending Projections from the Substantia Nigra and Retrorubral Field to the Medullary and Pontomedullary Reticular Formation. Eur J Neurosci. 1991;3(3):260–273. doi: 10.1111/j.1460-9568.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Wang J. S., Stohler C. S. Textural properties of food used in studies of mastication. J Dent Res. 1990 Sep;69(9):1546–1550. doi: 10.1177/00220345900690090101. [DOI] [PubMed] [Google Scholar]
- Weinrich M., Koch K., Garcia F., Angel R. W. Axial versus distal motor impairment in Parkinson's disease. Neurology. 1988 Apr;38(4):540–545. doi: 10.1212/wnl.38.4.540. [DOI] [PubMed] [Google Scholar]