Abstract
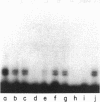
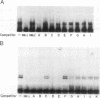
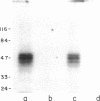
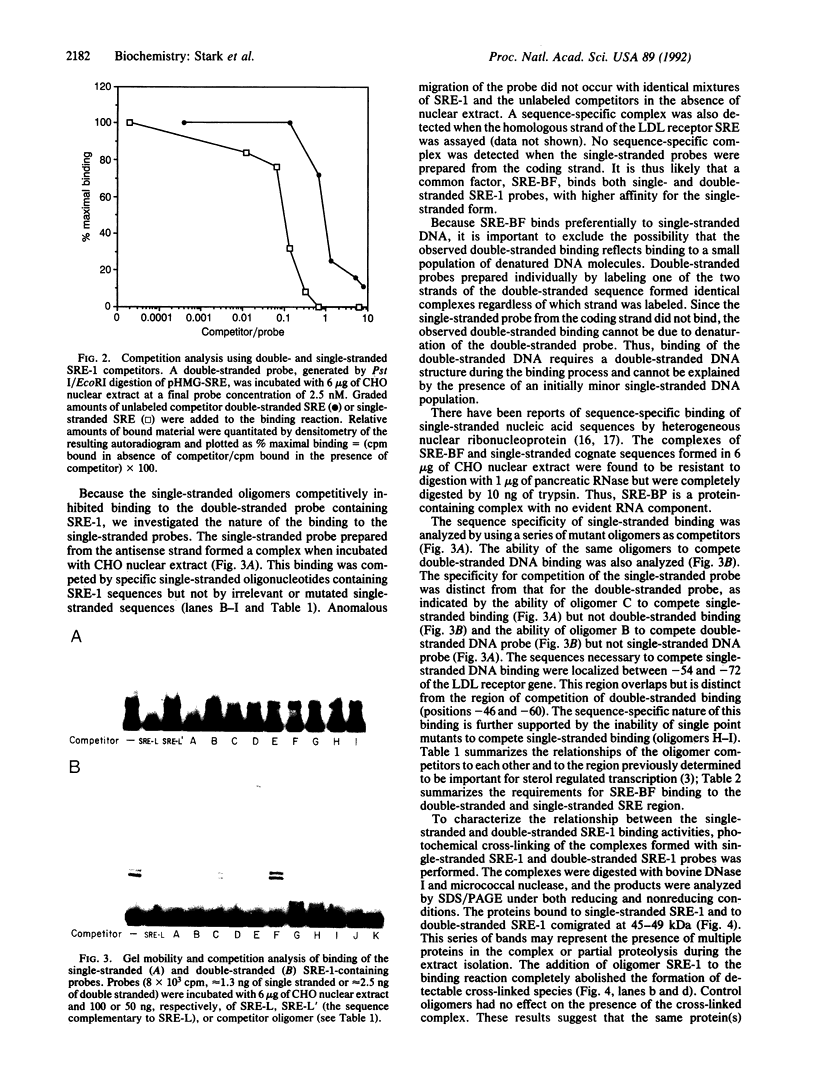
A cis-acting element necessary for sterol regulation, SRE-1, has previously been identified in the promoters of the low density lipoprotein receptor, hydroxymethylglutaryl (HMG)-CoA reductase, and HMG-CoA synthase genes. In this report we describe a nuclear factor, SRE-BF, isolated from Chinese hamster ovary nuclear extracts, that binds to the SRE-1 octanucleotide sequence. In addition to sequence-specific binding to SRE-1, as indicated by competition analysis with double-stranded DNA fragments, single-stranded oligomer DNA sequences also compete for binding in a sequence-specific fashion. Photochemical cross-linking experiments suggest that a common protein factor, with apparent molecular mass of 45-49 kDa, recognizes both single-stranded and double-stranded SRE-1. The binding specificity of SRE-BF to single-stranded SRE-1 closely correlates with the reported in vivo ability of SRE-1 to direct sterol responsiveness of transcription.
Full text
PDF

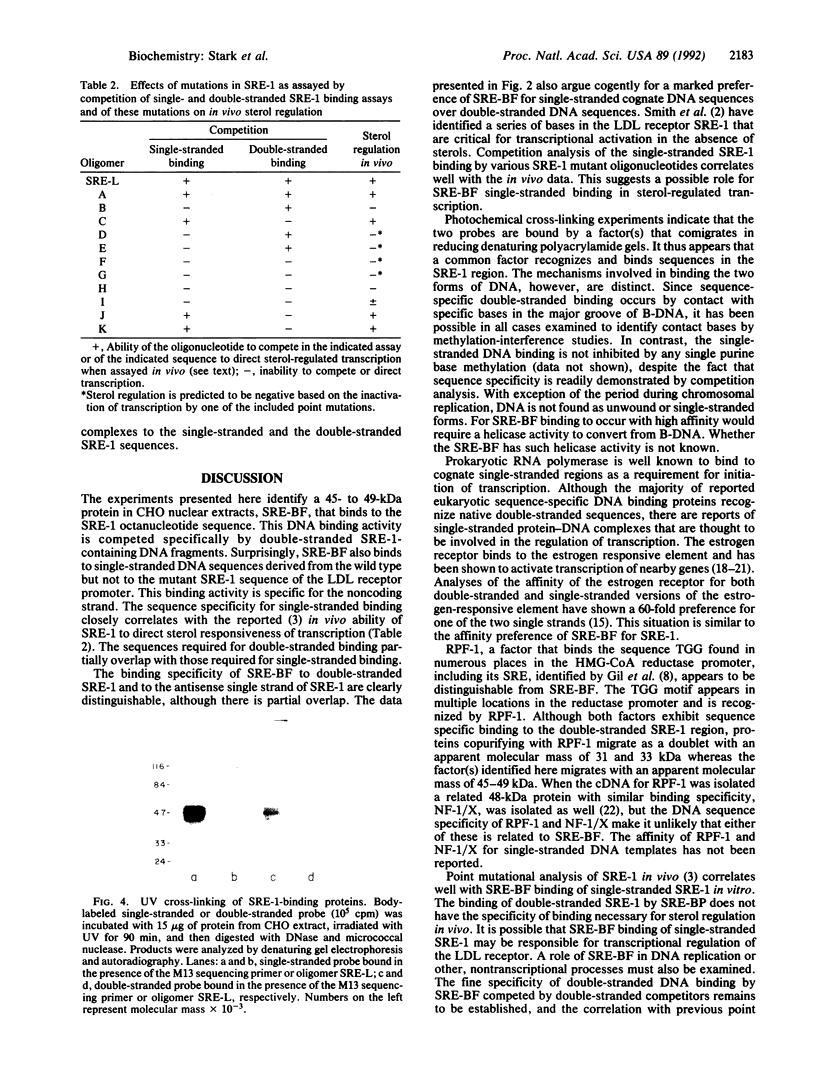

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch J. B., Evans M. I., Friedman T. M., O'Malley P. J. Two functional estrogen response elements are located upstream of the major chicken vitellogenin gene. Mol Cell Biol. 1988 Mar;8(3):1123–1131. doi: 10.1128/mcb.8.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., Hofmann S. L., van der Westhuyzen D. R., Südhof T. C., Brown M. S., Goldstein J. L. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988 Mar 5;263(7):3372–3379. [PubMed] [Google Scholar]
- Gil G., Osborne T. F., Goldstein J. L., Brown M. S. Purification of a protein doublet that binds to six TGG-containing sequences in the promoter for hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1988 Dec 15;263(35):19009–19019. [PubMed] [Google Scholar]
- Gil G., Smith J. R., Goldstein J. L., Slaughter C. A., Orth K., Brown M. S., Osborne T. F. Multiple genes encode nuclear factor 1-like proteins that bind to the promoter for 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8963–8967. doi: 10.1073/pnas.85.23.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Kumar A., Williams K. R., Szer W. Purification and domain structure of core hnRNP proteins A1 and A2 and their relationship to single-stranded DNA-binding proteins. J Biol Chem. 1986 Aug 25;261(24):11266–11273. [PubMed] [Google Scholar]
- Lannigan D. A., Notides A. C. Estrogen receptor selectively binds the "coding strand" of an estrogen responsive element. Proc Natl Acad Sci U S A. 1989 Feb;86(3):863–867. doi: 10.1073/pnas.86.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Notides A. C. Identification of an estrogen-responsive element from the 5'-flanking region of the rat prolactin gene. Mol Cell Biol. 1987 Dec;7(12):4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherall J. E., Goldstein J. L., Luskey K. L., Brown M. S. Loss of transcriptional repression of three sterol-regulated genes in mutant hamster cells. J Biol Chem. 1989 Sep 15;264(26):15634–15641. [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Brown M. S., Kowal R. C., Goldstein J. L. Identification of promoter elements required for in vitro transcription of hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3614–3618. doi: 10.1073/pnas.84.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Goldstein J. L., Brown M. S. Operator constitutive mutation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter abolishes protein binding to sterol regulatory element. J Biol Chem. 1988 Mar 5;263(7):3380–3387. [PubMed] [Google Scholar]
- Osborne T. F., Goldstein J. L., Brown M. S. 5' end of HMG CoA reductase gene contains sequences responsible for cholesterol-mediated inhibition of transcription. Cell. 1985 Aug;42(1):203–212. doi: 10.1016/s0092-8674(85)80116-1. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Orth K., Calame K. L. Binding in vitro of multiple cellular proteins to immunoglobulin heavy-chain enhancer DNA. Mol Cell Biol. 1986 Dec;6(12):4168–4178. doi: 10.1128/mcb.6.12.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Taylor A. K., Andalibi A., Svenson K. L., Lusis A. J. Identification of a zinc finger protein that binds to the sterol regulatory element. Science. 1989 Aug 11;245(4918):640–643. doi: 10.1126/science.2562787. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Osborne T. F., Brown M. S., Goldstein J. L., Gil G. Multiple sterol regulatory elements in promoter for hamster 3-hydroxy-3-methylglutaryl-coenzyme A synthase. J Biol Chem. 1988 Dec 5;263(34):18480–18487. [PubMed] [Google Scholar]
- Smith J. R., Osborne T. F., Goldstein J. L., Brown M. S. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem. 1990 Feb 5;265(4):2306–2310. [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Brown M. S., Goldstein J. L. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987 Mar 27;48(6):1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Shenk T. A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990 Dec;10(12):6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]