Abstract
Obesity continues to be one of the most prominent public health dilemmas in the world. The complex interaction among the varied causes of obesity makes it a particularly challenging problem to address. While typical high-fat purified diets successfully induce weight gain in rodents, we have described a more robust model of diet-induced obesity based on feeding rats a diet consisting of highly palatable, energy-dense human junk foods – the “cafeteria” diet (CAF, 45-53% kcal from fat). We previously reported that CAF-fed rats became hyperphagic, gained more weight, and developed more severe hyperinsulinemia, hyperglycemia, and glucose intolerance compared to the lard-based 45% kcal from fat high fat diet–fed group. In addition, the CAF diet-fed group displayed a higher degree of inflammation in adipose and liver, mitochondrial dysfunction, and an increased concentration of lipid-derived, pro-inflammatory mediators. Building upon our previous findings, we aimed to determine mechanisms that underlie physiologic findings in the CAF diet. We investigated the effect of CAF diet-induced obesity on adipose tissue specifically using expression arrays and immunohistochemistry. Genomic evidence indicated the CAF diet induced alterations in the white adipose gene transcriptome, with notable suppression of glutathione-related genes and pathways involved in mitigating oxidative stress. Immunohistochemical analysis indicated a doubling in adipose lipid peroxidation marker 4-HNE levels compared to rats that remained lean on control standard chow diet. Our data indicates that the CAF diet drives an increase in oxidative damage in white adipose tissue that may affect tissue homeostasis. Oxidative stress drives activation of inflammatory kinases that can perturb insulin signaling leading to glucose intolerance and diabetes.
Keywords: obesity, inflammation, oxidative stress, genomics, microarray, 4-HNE
INTRODUCTION
Obesity poses an alarming global public health concern [1,2]. Despite efforts aimed at ameliorating obesity, recent projections have estimated that 51% of the United States population will be obese by 2030 [3]. While typical high-fat purified pellet diets are successful in promoting obesity in mice and rats, we have described a diet-induced obesity model that incorporates a variety of highly palatable, energy-dense foods regularly consumed by humans – the “cafeteria” style (CAF) diet [4,5] that recapitulates obesity-like findings in humans. The CAF diet included a smorgasbord style offering of standard chow pellets, plus 3 human food choices offered daily. We previously reported that rats who ate the CAF diet developed hyperphagia and displayed significantly increased weight gain compared to other diet groups, including a commonly used lard-based high fat diet, consisting of 45% kilocalories-derived from fat and added sucrose. CAF-fed rats developed severe hyperinsulinemia, hyperglycemia, and glucose intolerance. Using a combination of metabolomic strategies and histological tissue analysis, we previously observed a higher degree of inflammation in white and brown adipose and liver compared to rats fed a traditional, purified high-fat diet, low-fat diet, and SC-fed rats [5]. In addition, concentrations of mitochondrial-derived lipid mediators that promote obesity-associated inflammation were found to be significantly elevated in CAF fed rats [4,5]. Metabolomic and immunohistologic measures suggested dramatic adipose tissue dysfunction however, underlying mechanisms remained unclear. Thus, we sought to investigate mechanistic underpinnings of CAF diet-induced inflammation and adipose dysfunction using global gene expression profiling to identify relevant genes and pathways altered with ingestion of the CAF diet. We now report that CAF diet-induced obesity resulted in significant alterations in white adipose tissue gene expression profiles that were associated with blunted protection from oxidative stress and an increase in oxidative damage. Thus, oxidative damage is one CAF diet-mediated pathway that could be responsible for greater adipose inflammation and systemic metabolic dysfunction in this model of diet induced obesity.
MATERIALS AND METHODS
Animals and diet treatments
All procedures were performed with the approval of the Duke University Institutional Animal Care and Use Committee. Male Wistar rats (~200g, 7-8 weeks old), purchased from Harlan Laboratories (Dublin, VA), were housed 2 rats/cage in the Duke University animal housing facility. Rats were maintained on a 12 hour light/dark cycle and given ad libitum access to standard chow (SC, Harlan Teklan 7001, Dublin, VA) for 2 weeks before being randomized onto experimental diets. At 9-10 weeks of age (~300g body weight, N = 5 for CAF and N = 4 for SC), rats were either maintained on the SC diet, or were switched to a CAF diet consisting of human snack food provided along with the SC pellet diet (Table 1). For a detailed description of the CAF diet components, refer to Sampey et al [5]. Briefly, the CAF diet included human food purchased at a supermarket and was provided in excess, including cookies, cereals, cheese, processed meats, crackers, etc. [5]. To estimate total gram and overall caloric intake of the CAF diet, items were weighed prior to and after consumption, and corrected for drying. The snack food items varied daily according to the fat, protein, and carbohydrate content as listed in Supplementary Table S2 of Sampey et al. [5]. Fat intake was the most drastically altered macronutrient with an estimated intake of 55% kcal from fat per day in CAF-fed rats. In addition, kilocalories from simple carbohydrate consumption was increased 500% (from 36 kcal to 180 kcal) in the CAF-fed group compared to the SC-fed groups. There were no added sugars to the SC diet; any sugars present were derived from whole-grains (corn, oats, etc.). Simple sugars were <5% of total carbohydrates in SC7001 (see Supplementary Table S3 from [5]). After 15 weeks on diet, rats were fasted for 6 hours and epididymal white adipose tissue (WAT) was collected. A portion of the WAT was isolated for mRNA isolation and another portion was fixed and paraffin-embedded for IHC.
Table 1. Composition of standard chow (SC) and cafeteria (CAF) diets.
| SC7001 | Cafeteria | |
|---|---|---|
| Name | Standard chow (SC) | Cafeteria (CAF) |
| Manufacturer | Harlan Teklad | Misc |
| Catalog number | SC7001 | 3 items + SC7001 |
|
| ||
| FAT | ||
|
| ||
| kcal/gm | 3.83 | varies daily |
| % fat/weight | 4% | |
| % fat/kcal | 12% | 45-53% |
| Fat sources: | Porcine fat Linoleic acid (1%/wt) |
See Table 2 of ref. 3 |
|
| ||
| PROTEIN | ||
|
| ||
| % protein /wt | 25% | ~20% |
| % prot/kcal | 34% | |
| Dehulled soybean meal, porcine meat, dehydrated alfalfa |
||
| Protein sources: | meal, dried whey, casein, purified amino acids |
See Table 2 of ref. 3 |
|
| ||
| CARBOHYDRATE | ||
|
| ||
| %carb/wt | 66% | ~35% |
| % carb/kcal | 54% | |
|
Carbohydrate
sources |
corn, wheat, barley, oats |
See Table 2 of ref. 3 |
RNA isolation
QIAzol Lysis Reagent was used to isolate mRNA from 100mg WAT samples (Qiagen, Valencia, CA, USA and [5,6]). mRNA quantity and quality were analyzed by Nanodrop (Thermoscientific, Wilmington, DE) and Bioanalyzer 2100 (Agilent, Wilmington, DE), respectively. For microarray analyses, cDNA was synthesized at the Functional Genomics Core at UNC-Chapel Hill. N = 5 for CAF diet group and 4 for SC diet group.
Gene expression microarrays
Gene expression quantification was conducted at the Functional Genomics Core Facility at UNC-Chapel Hill using genome-wide transcript microarrays (Affymetrix Rat Gene 1.0 ST, Santa Clara, CA). Microarrays where subjected to quality assessment and processed by robust multi-array average to produce transcript-level expression estimates using the aroma.affymetrix R package [7]. Transcripts were annotated with rat gene symbols using Affymetrix’s annotation (RaGene-1_0-st-v1.na28.rn4.transcript.csv). Expression values were then log2 transformed. See Supplemental table (“S”) Tables 1 and 2 for the complete gene expression data sets. Full gene names are in S Table 3.
4-hydroxynonenal immunohistochemistry
Sections of WAT from rats fed SC or CAF diets were stained for 4-hydroxynonenal (4-HNE), a marker of cellular lipid peroxidation [8]. 5μm sections were stained using an anti-4-HNE primary antibody (1:800 dilution in Dako antibody diluent, Abcam, Cambridge, MA) and biotinylated goat anti-mouse secondary antibody (1:500 dilution in Dako antibody diluent, Jackson ImmunonResearch, West Grove, PA). Positive 4-HNE staining was visualized using a Vectastain© Elite ABC peroxidase system (1:50 dilution, Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine (DAB, Thermo Fisher Scientific, Pittsburgh, PA) development. N=3/group. Digital images of stained sections were generated by scanning slides using an Aperio Image Scope Digital Slide Scanner (Vista, CA). Total positive DAB staining was quantified in 5 random microscopic fields using Aperio ImageScope Software (Vista, CA and [6,9]).
Statistical methods
Statistical analysis of microarrays (SAM) analysis was used to identify genes that were significantly different between CAF and SC diet groups with a false discovery rate (FDR) of < 0.05 [10]. Pathway enrichment and functional clusters of gene ontology were identified from differentially expressed genes between CAF and SC using DAVID [11]. DAVID functional pathway analysis was used to identify the genes whose expression changes were responsible for defining whether a pathway was modulated in the CAF diet group. For 4-HNE staining, unpaired Student’s t-test was used using GraphPad Prism 6 (La Jolla, CA). A p-value < 0.05 was considered significant.
RESULTS & DISCUSSION
Genomic profiling of WAT revealed that CAF diet-fed rats displayed blunted anti-oxidant capacity and reduced anti-inflammatory mediators compared to control SC-fed rats
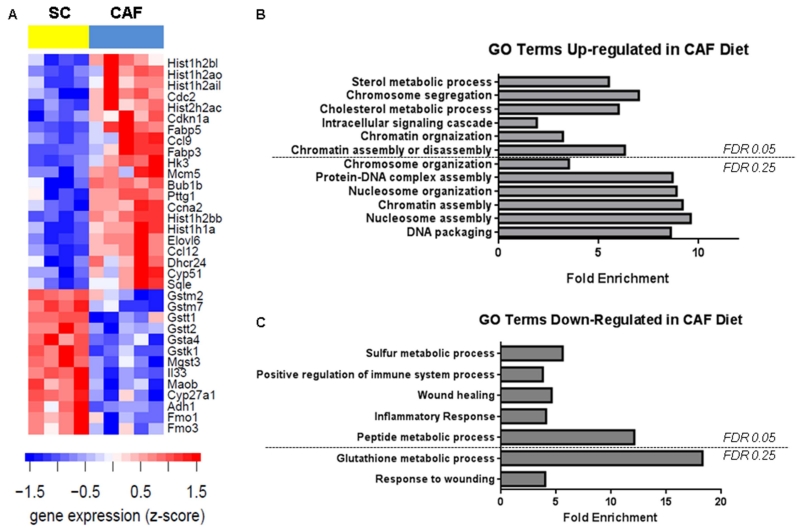
Obesity is recognized as a state of low-grade, chronic inflammation which may be perpetuated, in part, by increased oxidative stress [12,13]. The most striking finding through expression analysis was significantly blunted anti-oxidant pathways in CAF-fed WAT compared to SC-fed controls. Gene expression analysis of WAT mRNA from rats fed either SC or CAF revealed that expression of 377 genes were significantly elevated and 392 genes were significantly decreased in the CAF diet group compared to the SC group (S Table 1 and S Table 2, respectively; full gene names can be found in S Table 3). A subset of genes are up- and down- regulated genes are highlighted in Table 2 and Table 3, respectively. The 34 most regulated genes are highlighted in Figure 1A. DAVID gene ontology (GO) analysis of differentially expressed genes between CAF diet and SC diet-fed groups revealed several enriched functional clusters of GO pathways of importance to the Metabolic Syndrome and inflammatory state of the WAT tissue. A total of 12 GO pathways were up-regulated in the CAF diet rats compared to the SC control group (FDR ≤ 0.25) (Figure 1B). Seven functional pathways were down-regulated in the CAF-fed WAT tissue compared to SC controls (FDR ≤ 0.25) including Drug metabolism, Glutathione metabolism, and Metabolism of xenobiotics by cytochrome P450, mainly driven by decreased expression of glutathione S-transferases (Figure 1C).
Table 2. Selected adipose genes significantly up-regulated with CAF diet exposure.
Selected genes of interest with increased expression in WAT from rats fed CAF diet are listed. An FDR < 0.05 (q value ≤ 5), determined by SAM analysis, was considered significant. See Supplemental table S Table 1 for a complete list of gene expression data.
| Gene Name | Gene Symbol | q-value |
|---|---|---|
| ELOVL family member 6, elongation of long chain fatty acids | Elovl6 | 0.0 |
| Fatty acid binding protein 3 | Fabp3 | 1.5 |
| Fatty acid binding protein 5 | Fabp5 | 1.5 |
| Hexokinase 3 (white cell) | Hk3 | 1.5 |
| Chemokine (C-C-motif) Ligand 9 | Ccl9 | 2.3 |
| Lipin 2 | Lpin2 | 2.5 |
| Chemokine (C-C-motif) Ligand 12 | Ccl12 | 3.7 |
| 24-dehydrocholesterol reductase | Dhcr24 | 3.8 |
| Acyl-coenzyme A dehydrogenase, long chain | Acadl | 3.8 |
| Cytochrome P450-51 | Cyp51 | 4.1 |
| MAPK-KK 11 | Mapk11 | 4.1 |
| Adipose differentiation-related protein | Adfp | 4.7 |
| Acyl-coenzyme A dehydrogenase, C-2 to C-3 short chain | Acads | 4.7 |
Table 3. Selected adipose gene significantly down-regulated with CAF diet exposure.
Selected genes of interest with decreased expression in WAT from rats fed CAF diet are listed. An FDR < 0.05 (q value ≤ 5), determined by SAM analysis, was considered significant. See Supplemental table S Table 2 for a complete list of gene expression data.
| Gene Name | Gene Symbol | q-value |
|---|---|---|
| Glutathione S-transferase, theta 3 | Gstt3 | 0.0 |
| Interleukin 33 | Il33 | 0.0 |
| Glutathione S-transferase, mu 7 | Gstm7 | 0.9 |
| Glutathione S-transferase, kappa 1 | Gstk1 | 0.9 |
| Glutathione S-transferase, a4 | Gsta4 | 0.9 |
| Glutathione S-transferase, theta 1 | Gstt1 | 1.7 |
| Glutathione S-transferase, mu 2 | Gstm2 | 2.5 |
| Glutathione S-transferase, theta 2 | Gstt2 | 3.4 |
| Cytochrome P450, family 27, subfamily a, polypeptide 1 |
Cyp27a1 | 3.7 |
| Glutathione S-transferase, mu 6-like | Gstm6l | 4.1 |
| CD22 molecule | Cd22 | 4.7 |
Figure 1. A. Gene expression and ontology analysis revealed CAF-diet downregulation of genes limiting oxidative stress and inflammation in WAT.
A. Gene expression is displayed as a heat map in which columns are samples, rows are genes and shading indicates expression level according to the legend. Gene expression values were transformed to z-scores. Representative genes were selected from the significantly differentially gene lists (Table S1 and S2). DAVID Gene Ontology analysis of genes differentially expressed between CAF versus SC was used to identify functional clusters of gene ontology (GO) categories significantly over-represented and upregulated (B) or downregulated (C) in CAF-fed adipose versus SC-fed; an False discovery rate (FDR) cutoff of either 0.05 or 0.25 (as indicated) was used. Data are presented as the fold enrichment for each ontology group. Data presented are the comparison of changes in gene expression in CAF group with respect to the SC group.
The profound loss of multiple glutathione-S transferase isoforms in WAT from rats fed a CAF style diet suggested that this rapid model of obesity promoted severe oxidative stress. Glutathione (GSH), the major anti-oxidant produced by cells [14], is a free radical scavenger that plays a vital role in protecting cells from oxidative damage. Glutathione S-transferases catalyze the addition of GSH to molecules – for example, peroxidized lipids and xenobiotics -- for the purpose of making the molecule water-soluble and, thus, able to be excreted [15]. Suppressed GST family gene expression, like that observed in the CAF-fed group, leads to down-regulation of multiple functional pathways, namely drug metabolism and metabolism of xenobiotics by cytochrome P450, as determined by DAVID bioinformatics analysis.
Furthermore, expression profiling and DAVID pathway analysis demonstrated increased expression of fatty acid binding proteins and a reduction in key anti-inflammatory genes. CAF-diet increased expression of several genes related to lipid metabolism, including Elvol6, fatty acid binding protein 3 and fatty acid binding protein 5. Elvol6 is a microsomal enzyme that catalyzes the elongation of C12, 14, and 16 saturated and monounsaturated fatty acids, and in this way, can modulate intracellular fatty acid composition [16]. Elevated Elvol6 may lead to accumulation of stearate (18:0), a pro-inflammatory saturated fatty acid [17] and blocked insulin-stimulated activation of AKT, thus impairing insulin signaling [18]. Elvol6 can also induce endoplasmic reticulum stress and apoptosis [19]. Fatty acid binding proteins are intracellular lipid chaperones that play central roles in fatty acid transport, trafficking, and export [20,21,22]. Fabp3 (H-FABP) and Fabp5 (E-FABP) regulate glucose uptake or non-alcoholic fatty liver disease, respectively [23,24]. In addition, Fabp5 expression increases with reactive oxygen species production and oxidative stress [25,26]. Thus, CAF-diet upregulated important mediators of lipid biology that lead to altered metabolism and insulin resistance. In addition, CAF-induced obesity also significantly decreased expression of anti-inflammatory cytokines Il-33 and cd22 in WAT. IL-33 induces helper T cells (Th2), mast cells, eosinophils, and basophils to produce type 2 cytokines and is negatively associated with BMI and diabetes [27,28]. Type 2 cytokines promote alternative activation of macrophages which is central to maintenance of insulin sensitivity [29,30]. CD22 is a lectin that blunts the immune response in B cells [31]. Together, downregulation of these cytokines may lead to a lack of control of inflammation, leading to chronic inflammation associated with obesity.
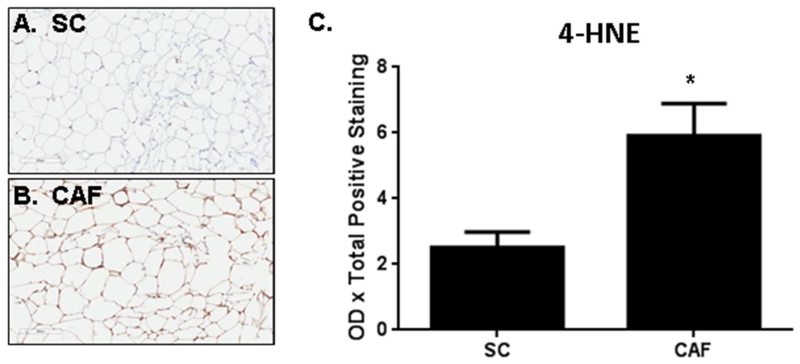
Lipid peroxidation was significantly increased in WAT from CAF-fed rats compared to SC-fed controls potentially due to reduced 4-HNE excretion secondary to CAF down-regulation of glutathione S-transferase alpha 4 (Gsta4)
To test for evidence of oxidative stress based on genomic findings, 4-hydroxynonenal (4-HNE) was used as a marker to detect oxidative damage in the WAT tissue. 4-HNE is an α,β-unsaturated hydroxyalkenal which is produced during the process of lipid peroxidation; therefore, 4-HNE is a specific marker of polyunsaturated omega-6 lipid (e.g. linoleic and arachidonic acids) damage with oxidative stress [32]. IHC staining for 4-HNE, a marker of cellular oxidative stress, demonstrated that WAT from CAF-fed rats had more than double 4-HNE staining than the SC controls (Figure 2 A-C, p = 0.03). Curtis et al. reported that increased 4-HNE resulted in impaired adipocyte mitochondrial function and increased superoxide production [33]. Certain glutathione S-transferases are responsible for mitigating and maintaining 4-HNE levels within cells. Herein, we provide evidence that CAF-diet exposure induced a striking decrease in glutathione metabolism-related genes (Table 3 and S Table 2). Notably, glutathione S-transferase alpha 4 (Gsta4) was decreased by CAF-diet compared to SC-fed WAT. Gsta4 catalyzes the addition of a glutathione molecule onto 4-HNE to facilitate its excretion. Importantly, Gsta4 is the glutathione S-transferase with the highest affinity for 4-HNE, and therefore, the glutathione S-transferase primarily responsible for metabolizing 4-HNE to reduce oxidative stress burden [34,35,36]. Down-regulation of Gsta4 resulted in increased mitochondrial reactive oxygen species production, as well as increased carbonylation of metabolism-associated proteins including fatty acid binding proteins [37,38]. Taken together, CAF-diet down-regulated Gsta4 expression may have elevated levels of 4-HNE, as detected in the WAT. Similar to our findings, other gene expression analyses of white adipose from diet-induced obesity models, including one utilizing a slightly different CAF style diet, have reported decreased expression of glutathione-associated genes with obesity [39,40], lending support to the relevance of this antioxidant pathway to obesity-induced defects.
Figure 2. Oxidative stress is increased in WAT from CAF diet-exposed rats.
Anti-4-HNE IHC was performed in WAT collected from rats fed (A) SC or (B) CAF diet for 15 weeks. Representative 20× images are shown. C. Quantification of mean optical density x total positive staining ± SEM. N = 3. *p-value = 0.03.
A limitation to using the CAF diet is that nutritional information may only be obtained from what is provided on packaging of the items that were purchased. Since the CAF diet is not a defined, purified diet, there are many unknowns that may contribute to the oxidative stress detected in adipose tissue. Despite these limitations, studies in humans parallel our results. Indeed, in obese adults and children, elevated markers of oxidative stress and suppressed glutathione metabolites correlated with dysregulated adipokine levels, which contribute to the development of Metabolic Syndrome [41,42,43]. Furthermore, elevated 4-HNE has been reported in obese humans [41,44,45]. Importantly obesity-induced oxidative damage may be reversible: reducing inflammation through anti-inflammatory supplementation or weight loss reduced the expression of oxidative damage-promoting genes and oxidative damage [46,47]
In conclusion, feeding rodents a cafeteria-style diet consisting of highly palatable, energy dense human junk foods resulted in significant adipose oxidative damage potentially due to a down-regulation of glutathione metabolic pathways. Our results corroborate observations of enhanced oxidative burden in adipose tissue of obese humans therefore demonstrating that the cafeteria-style model of diet-induced obesity is a relevant model of human disease.
Supplementary Material
Supplemental Table 1: Genes over-expressed in CAF-fed WAT compared to SC-fed. Gene expression analysis of WAT mRNA from rats fed either SC or CAF revealed that expression of 377 genes were significantly elevated in the CAF diet group compared to the SC group.
Supplemental Table 2. Genes under-expressed in CAF-fed WAT compared to SC-fed. Gene expression analysis of WAT mRNA from rats fed either SC or CAF revealed that expression of 392 genes were significantly decreased in the CAF diet group compared to the SC group.
Supplemental Table 3. Gene symbols. Gene symbols and full names for genes over- and under-expressed in CAF-fed WAT compared to SC-fed.
Highlights.
Cafeteria diet is a robust model of human obesity.
Cafeteria diet down-regulated genes involved in glutathione metabolism.
Elevated oxidative damage in white adipose tissue was detected.
ACKNOWLEDGEMENTS
We acknowledge the support of Dr. Amanda M. Vanhoose, Ph.D. and Ms. Helena Winfield in assisting with rat experiments. ARJ was supported by NIH F32H117616. LM, MM, and DNH were supported by UNC University Cancer Research Fund, NIH AA017376; LM and MT were supported by the UNC Nutrition Obesity Research Consortium (NIH P30DK056350).
ABBREVIATIONS
- CAF
cafeteria diet
- FDR
false discovery rate
- SAM
significance analysis of microarrays
- SC
standard control diet
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Flegal KM, Panagiotou OA, Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol. 2015;25:201–207. doi: 10.1016/j.annepidem.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- [3].Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and Severe Obesity Forecasts Through 2030. American journal of preventive medicine. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- [4].Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Newgard CB, Brauer HA, Troester MA, Makowski L. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One. 2012;7:e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sundaram S, Freemerman AJ, Johnson AR, Milner JJ, McNaughton KK, Galanko JA, Bendt KM, Darr DB, Perou CM, Troester MA, Makowski L. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bengtsson SKH, Bullard J, Hansen K. aroma.affymetrix: A generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory. B. University of California, Department of Statistics; 2008. Tech Report. [Google Scholar]
- [8].Evans RM, Scholz RW. Development of renal gluconeogenesis in chicks fed high fat and high protein “carbohydrate-free” diets. J Nutr. 1973;103:242–250. doi: 10.1093/jn/103.2.242. [DOI] [PubMed] [Google Scholar]
- [9].Sundaram S, Le TL, Essaid L, Freemerman AJ, Huang MJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Weight loss reversed obesity-induced HGF/c-Met pathway and basal-like breast cancer progression. Frontiers in Oncology. 2014;4 doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gu W, Hecht NB. Developmental expression of glutathione peroxidase, catalase, and manganese superoxide dismutase mRNAs during spermatogenesis in the mouse. J Androl. 1996;17:256–262. [PubMed] [Google Scholar]
- [12].Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: glucose transporter (GLUT1)-mediated glucose metabolism drives a pro-inflammatory phenotype. J Biol Chem. 2014 doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- [14].Scholz RW, Graham KS, Gumpricht E, Reddy CC. Mechanism of interaction of vitamin E and glutathione in the protections against membrane lipid peroxidation. Ann N Y Acad Sci. 1989;570:514–517. [Google Scholar]
- [15].Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- [16].Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- [17].Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- [19].Green CD, Olson LK. Modulation of palmitate-induced endoplasmic reticulum stress and apoptosis in pancreatic beta-cells by stearoyl-CoA desaturase and Elovl6. Am J Physiol Endocrinol Metab. 2011;300:E640–649. doi: 10.1152/ajpendo.00544.2010. [DOI] [PubMed] [Google Scholar]
- [20].Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kusudo T, Kontani Y, Kataoka N, Ando F, Shimokata H, Yamashita H. Fatty acid-binding protein 3 stimulates glucose uptake by facilitating AS160 phosphorylation in mouse muscle cells. Genes Cells. 2011;16:681–691. doi: 10.1111/j.1365-2443.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- [24].Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Siren J, Hamsten A, Fisher RM, Yki-Jarvinen H. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- [25].Kanda K, Sugama K, Sakuma J, Kawakami Y, Suzuki K. Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc Immunol Rev. 2014;20:39–54. [PubMed] [Google Scholar]
- [26].Bennaars-Eiden A, Higgins L, Hertzel AV, Kapphahn RJ, Ferrington DA, Bernlohr DA. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo. Evidence for a role in antioxidant biology. J Biol Chem. 2002;277:50693–50702. doi: 10.1074/jbc.M209493200. [DOI] [PubMed] [Google Scholar]
- [27].Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- [28].Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, Al-Arouj M, Behbehani K, Dehbi M, Dermime S. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014;15:19. doi: 10.1186/1471-2172-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- [31].Kawasaki N, Rademacher C, Paulson JC. CD22 regulates adaptive and innate immune responses of B cells. J Innate Immun. 2011;3:411–419. doi: 10.1159/000322375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [33].Curtis JM, Hahn WS, Stone MD, Inda JJ, Droullard DJ, Kuzmicic JP, Donoghue MA, Long EK, Armien AG, Lavandero S, Arriaga E, Griffin TJ, Bernlohr DA. Protein carbonylation and adipocyte mitochondrial function. J Biol Chem. 2012;287:32967–32980. doi: 10.1074/jbc.M112.400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He NG, Singhal SS, Srivastava SK, Zimniak P, Awasthi YC, Awasthi S. Transfection of a 4-hydroxynonenal metabolizing glutathione S-transferase isozyme, mouse GSTA4-4, confers doxorubicin resistance to Chinese hamster ovary cells. Arch Biochem Biophys. 1996;333:214–220. doi: 10.1006/abbi.1996.0383. [DOI] [PubMed] [Google Scholar]
- [35].Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [36].Ostergaard M, Rasmussen HH, Nielsen HV, Vorum H, Orntoft TF, Wolf H, Celis JE. Proteome profiling of bladder squamous cell carcinomas: identification of markers that define their degree of differentiation. Cancer Res. 1997;57:4111–4117. [PubMed] [Google Scholar]
- [37].Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K, Muoio DE, Arriaga EA, Bernlohr DA. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grimsrud PA, Picklo MJ, Sr., Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- [39].Moraes RC, Blondet A, Birkenkampw-Demtroeder K, Sr., Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Begeot M. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology. 2003;144:4773–4782. doi: 10.1210/en.2003-0456. [DOI] [PubMed] [Google Scholar]
- [40].Lopez IP, Marti A, Milagro FI, Zulet L, Md Mde, Moreno-Aliaga MJ, Martinez JA, De Miguel C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes Res. 2003;11:188–194. doi: 10.1038/oby.2003.30. [DOI] [PubMed] [Google Scholar]
- [41].Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- [43].Codoner-Franch P, Pons-Morales S, Boix-Garcia L, Valls-Belles V. Oxidant/antioxidant status in obese children compared to pediatric patients with type 1 diabetes mellitus. Pediatr Diabetes. 2010;11:251–257. doi: 10.1111/j.1399-5448.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- [44].Vincent HK, Bourguignon CM, Weltman AL, Vincent KR, Barrett E, Innes KE, Taylor AG. Effects of antioxidant supplementation on insulin sensitivity, endothelial adhesion molecules, and oxidative stress in normal-weight and overweight young adults. Metabolism. 2009;58:254–262. doi: 10.1016/j.metabol.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, Golay A, Witztum JL, Giacobino JP. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett. 2003;551:104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- [46].Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE, Targeting T. Inflammation Using Salsalate in Type 2 Diabetes Study, Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Crujeiras AB, Parra D, Milagro FI, Goyenechea E, Larrarte E, Margareto J, Martinez JA. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: a nutrigenomics study. OMICS. 2008;12:251–261. doi: 10.1089/omi.2008.0001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Genes over-expressed in CAF-fed WAT compared to SC-fed. Gene expression analysis of WAT mRNA from rats fed either SC or CAF revealed that expression of 377 genes were significantly elevated in the CAF diet group compared to the SC group.
Supplemental Table 2. Genes under-expressed in CAF-fed WAT compared to SC-fed. Gene expression analysis of WAT mRNA from rats fed either SC or CAF revealed that expression of 392 genes were significantly decreased in the CAF diet group compared to the SC group.
Supplemental Table 3. Gene symbols. Gene symbols and full names for genes over- and under-expressed in CAF-fed WAT compared to SC-fed.