Abstract
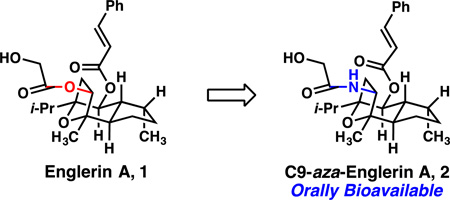
Synthesis of analogues of englerin A with a reduced propensity for hydrolysis of the glycolate moiety led to a compound which possessed the renal cancer cell selectivity of the parent and was orally bioavailable in mice.
Graphical Abstract
Renal cancer is an ongoing and critical medical problem in the United States, with alarming annual increases in incidence and death.1 In 2015 alone, it is estimated that the U.S. will see 61,560 new patients and 14,080 deaths resulting from renal cancer.2 Patients with nonresectable or metastatic tumors are faced with several chemotherapy options, including immunomodulatory therapies, and target-based approaches that owe their success to the discovery of the von Hippel-Lindau (VHL) tumor suppressor gene. The target-based approaches include receptor tyrosine kinase-based therapies that interrupt or block VEGF or TGF-α signaling or related pathways, and are collectively a multi-billion dollar annual health burden in the United States alone. Unfortunately, these treatments can have serious adverse side effects,3 and provide only modest survival advantages,4 driving intense interest in effective new chemotherapeutic drug leads for the treatment of renal cancer.
We isolated the guaiane sesquiterpene ester englerin A from an extract of the Tanzanian plant Phyllanthus engleri Pax on the basis of its high potency and selectivity for inhibiting renal cancer cell growth.5 This compound has stimulated wide interest and activity in both the synthetic chemistry6 and cancer biology7 communities. We report here a stable and orally bioavailable analogue of the natural product englerin A.8
Initial intraperitoneal administration of englerin A to mice was done in DMSO solution.7b We developed more suitable vehicles for both parenteral and oral administration. A cosolvent approach with hydroxypropyl-β-cyclodextrin for parenteral use provided a clear and refrigerator-stable preparation with a concentration of up to 5.7 mg/mL (see Supplemental Information for details). An oral formulation utilizing Labrasol™ was able to dissolve englerin A directly at 20 mg/mL at room temperature.
Using the above mentioned vehicles, we attempted to determine maximum tolerated doses (MTD) in mice by intraperitoneal (i.p.), intravenous (i.v.), and oral (p.o.) routes. The i.p. MTD for englerin A was approximately 10 mg/kg. Intravenous administration was rapidly lethal,7e with an estimated MTD of 50 µg/kg. Mice died almost instantaneously at doses from 1–10 mg/kg. In contrast, p.o. administration of englerin A by gavage was tolerated up to 100 mg/kg. While englerin A was detected in mouse serum after i.p. injection, the oral route did not produce detectable levels of the compound (see Supplemental Information for details). We reasoned that the glycolate moiety of englerin A was hydrolyzed by gastric acid, and thus sought to prepare a more stable series of analogues.
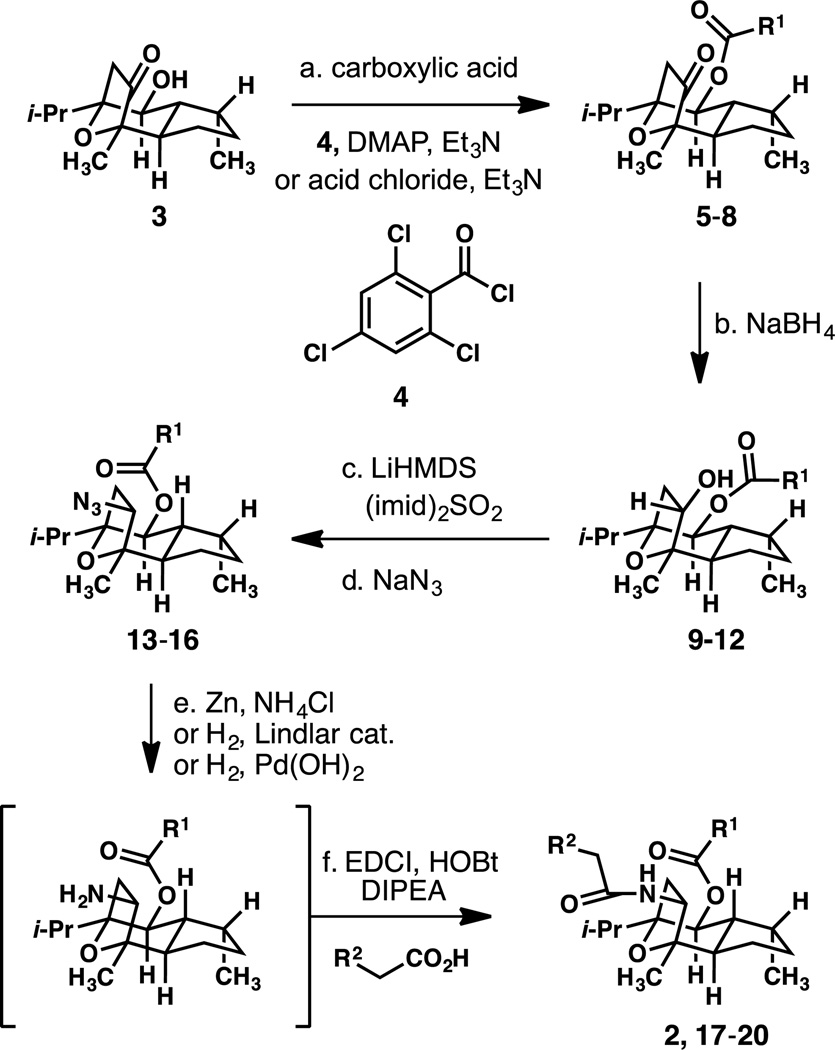
The incorporation of nitrogen at C9 of the natural product was a straightforward process requiring minimal modification to the synthesis of englerin A described by our laboratory in 2011.6f The production of aza-englerin analogues employed the hydroxyketone 3 as a starting point. The C6 hydroxyl function was acylated using the Yamaguchi protocol or commercially available acid chloride under standard conditions to give esters 5–8 in 41–93% yield. Reduction of the C9 ketone functions to the corresponding alcohols 9–12 under the action of methanolic sodium borohydride proceeded as previously described in 67–99% yield. Conversion of the alcohols to the imidizolium sulfonates was similarly straightforward, and proceeded under the action of lithium bis(trimethylsilyl)amide and sulfuryl diimidazole in 55–100% yield. In each case, the nitrogen atom was introduced in the form of azide under standard substitution conditions (NaN3 in warm DMF) and gave the azides 13–16 cleanly in 75–99% yield. Chemoselective reduction of the azides to the corresponding primary amines was achieved under hydrogenative conditions or under the action of zinc powder and ammonium chloride in methanol (See Supplementary Information for details). In each case, the primary amines were acylated directly without further purification utilizing glycolic acid or fluoroacetic acid and N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride to give a series of C9-aza-englerin A analogues 2 and 17–20 in 29–72% yield (two steps).
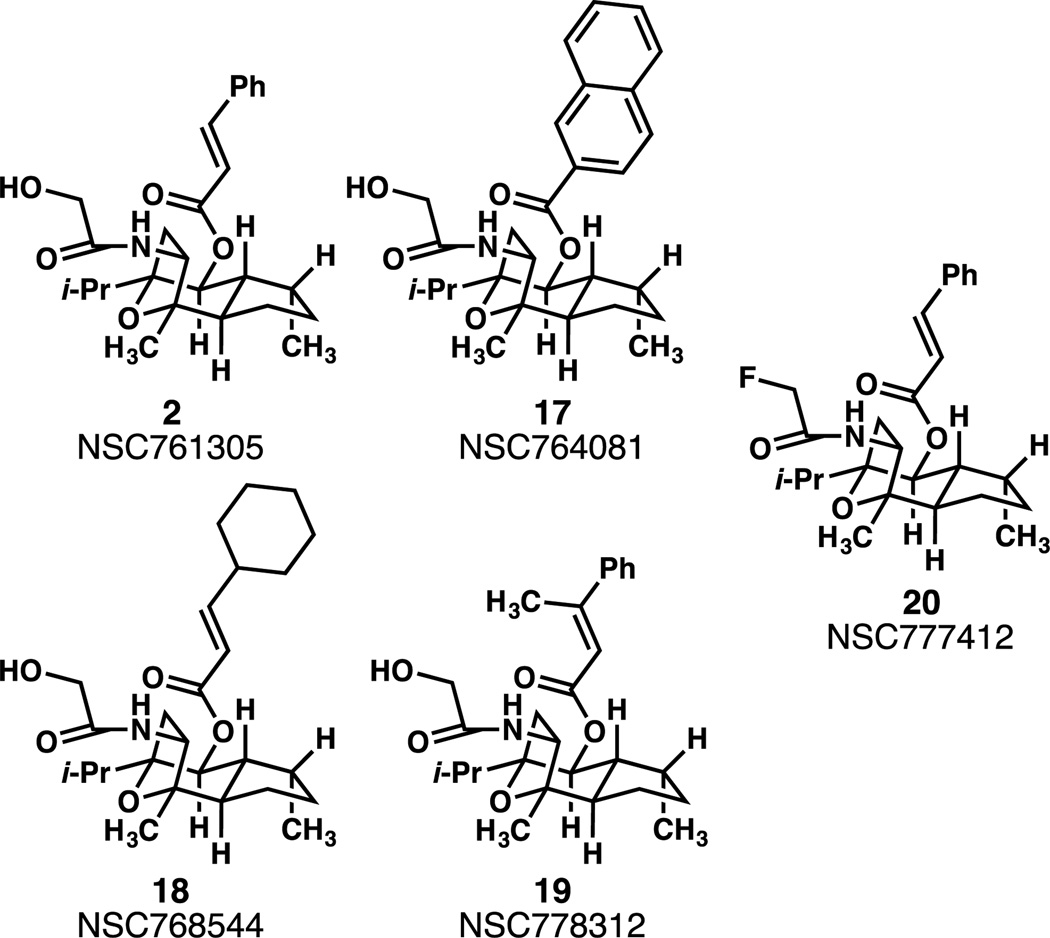
Five analogues 2, 17, 18, 19, and 20 were evaluated in the NCI 60 cell screening assay.9 Compound 20 was inactive in preliminary screening at 10−5M, and was not tested further. The results are shown in Tables 1, 2, and 3, and in the Supplemental Information.
Table 1.
NCI 60 data one dose data for aza-englerins at 10−5M.
| Compound | One Dose Mean Pct |
One Dose Range Pct |
A498 Pct |
HS 578T Pct |
|---|---|---|---|---|
| 1 | 81 | 236 | 39 | 29 |
| 2 | 84 | 127 | −15 | 9 |
| 17 | 79 | 128 | −15 | −18 |
| 18 | 100 | 92 | 41 | 73 |
| 19 | 93 | 150 | −25 | −9 |
| 20 | 99 | 41 | 83 | 93 |
Table 2.
NCI 60 data five dose data for aza-englerins.
| Compound | Mean GI50 µM |
Range log10 units |
A498 GI50 nM |
HS 578T GI50 nM |
|---|---|---|---|---|
| 1 | 3.7 | 3.30 | 10 | 10 |
| 2 | 13 | 2.49 | 162 | 832 |
| 17 | 14 | 1.10 | 1620 | 2230 |
| 18 | 39 | 0.9 | 5620 | 6170 |
| 19 | 27 | 1.94 | 1150 | 2240 |
Table 3.
Matrix COMPARE of selected analogues versus englerin A at the GI50 level, Pearson correlation coefficients.
| Compound | 1 | 2 | 17 | 19 |
|---|---|---|---|---|
| 1 | 1 | 0.731 | 0.748 | 0.833 |
| 2 | 0.731 | 1 | 0.856 | 0.846 |
| 17 | 0.748 | 0.856 | 1 | 0.782 |
| 19 | 0.833 | 0.846 | 0.782 | 1 |
Even the most active englerins (e.g., 1) display relatively modest average growth inhibition at 10−5M in the one dose initial tests (Table 1), since only 8 cell lines of 60 display substantial sensitivity. The range of response at 10−5M gives a better estimate of potency; by this criterion, none of the aza-englerin analogues is as potent as 1. Compounds 2, 17 and 19 come the closest to englerin A. Inspecting the response of the cell lines which are most sensitive to englerin A (A498 and HS 578T), the same conclusion can be reached. One dose data is of limited precision compared to the five dose data, therefore we tested the five compounds in the latter format.
The five dose tests support the compound potency estimates (Table 2). Compound 2 shows substantial selectivity for kidney cancer cell lines, but is clearly at least 15-fold weaker than englerin A. In the breast cancer cell line HS 578T, which is very sensitive to englerin A, 2 is ~80-fold less potent. Compounds 17 and 19 are 5–10-fold weaker than 2, while 18 is another several-fold weaker.
Examination of the patterns of selectivity confirm that all of the more potent analogues share the renal selectivity profile of the natural product (Table 3). Compound 18 was not included due to the limited dynamic range of its data.
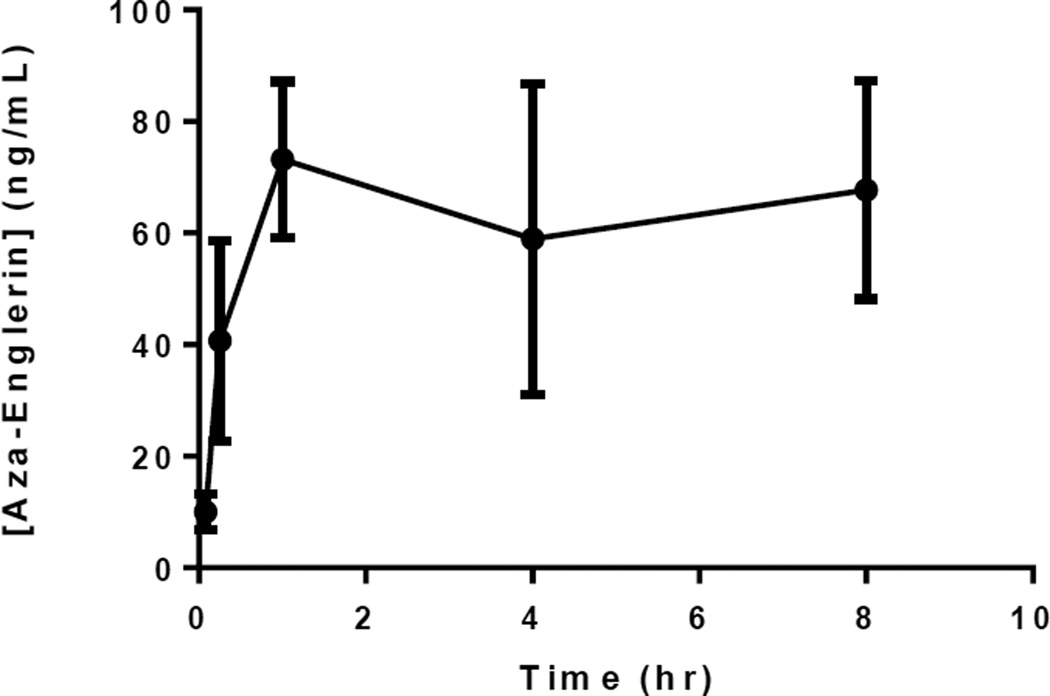
The most potent analogue 2 was chosen for preliminary pharmacokinetic evaluation in mice, using the oral route. The compound was administered in Labrasol vehicle at 50 mg/kg, and was well tolerated. Analysis of serum samples taken at different time points demonstrated that 2 was bioavailable, and serum levels were maintained much longer and at higher concentrations than they were with englerin A given i.p. (Figure 2).
Figure 2.
Serum levels of 2 after oral administration at 50 mg/kg in mice (n=3) measured by HPLC-MS
This result supports the theory that englerin A is not orally bioavailable due to cleavage of the glycolate ester in the acid conditions of the stomach, which would produce englerin B, which we5 and others6c have previously shown to have no activity on cancer cell growth. Furthermore, recent data show that englerin B can be detected in serum of rats after oral administration of englerin A.7e
While 2 is bioavailable by the oral route, its intrinsic activity in cellular systems such as the NCI 60 assay is much weaker than englerin A, as noted above. Further evidence for its weaker intrinsic activity was obtained with a panel of 4 VHL-deficient kidney cancer cell lines, in which the IC50 values ranged from 10 to 100 µM (see Supplemental Information) and in stimulation of generic PKC activity in lysates of 786-0 cells, where 2 had no activity at 1 µM, while 1 was active at 10 nM, and 2’-chloro-englerin A12 and tonantzitlolone13 were active at 1 µM (see Supplementary Information). It is not clear why analogues 17–19 are even weaker than 2, since replacement of the cinnamate with alternative ester groups has been reported by others to give high cellular potency in the glycolate series.10,11 Although purely speculative at this time, it is possible that the amide N-H presents a sufficiently different hydrogen bonding landscape relative to the natural product to alter its conformation (perhaps due to internal hydrogen bonding with the endocyclic oxygen atom) or otherwise disrupt the nature of its binding to the cellular target, which remains unknown as of this writing. Current efforts are focused on design and synthesis of aza-englerin analogues with improved cell growth inhibition that address this other structural speculation.
Supplementary Material
Figure 1.
Structures of target aza-englerins.
Scheme 1.
Synthesis of Aza-englerin analogues.
Conditions: (a) carboxylic acid, 2,4,6-trichlorobenzoyl chloride, DMAP, Et3N, toluene, 23 °C or carboxylic acid chloride, DMAP, Et3N, toluene, 23 °C. (b) NaBH4, CH3OH, 0 °C. (c) LiHMDS, (imid)2SO2, THF, −10 → 23 °C. (d) NaN3, DMF, 80 °C. (e) Zn dust, NH4Cl, CH3OH, 23 °C or H2, Lindlar catalyst, EtOH, 23 °C or H2, Pd(OH)2, CH3OH, 23 °C (f) R2CH2CO2H, EDCI, HOBt, i-Pr2NEt, DMF, 23 °C. See Supplementary Information for detailed procedures.
Acknowledgments
J.A.B., C.J.P., C.S., L.N., and W.D. F. are supported by the Intramural Program of the National Cancer Institute (1ZIABC01147003, 1ZIABC01068311, and 1Z1CSC00653621). W.J.C. gratefully acknowledges financial support from the National Institutes of Health (R01CA163287), the University of Hawaii, the University of Hawaii Cancer Center, and the University of Delaware. Some data were acquired at UD on instruments obtained with the assistance of NSF and NIS funding (NSF CHE0421224, CHE1229234, and CHE0840401; NIH P20GM103541, P20GM104316, P30GM110758, S10RR02692, and S10OD016267). W.J.C. gratefully acknowledges the assistance of Michael F. Wisthoff and Senzhi Zhao in the preparation of the Supplemental Information and spectral data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ASSOCIATED CONTENT
Supplementary Information
Experimental procedures, characterization data, and 1H and 13C spectra for all new compounds. Bioloigical evaluation data for compounds 1, 2, and 17–20.
Author Contributions
W.J.C., J.A.B., L.N., and W.D.F. designed research; W.D.F., C.P., S.H., Z.L., C.S., I.T., and F.J.S. performed research; J.A.B., W.J.C., L.N., and W.D.F. analyzed data, and W.J.C. and J.A.B. wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References and Notes
- 1.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S. Cancer. 2015;121:1827. doi: 10.1002/cncr.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US National Cancer Institute. [accessed 02-29-2016];Surveillance, Epidemiology, and End Results Program (SEER) http://seer.cancer.gov.
- 3.Launay-Vacher V, Aapro M, De CG, Jr, Cohen E, Deray G, Dooley M, Humphreys B, Lichtman S, Rey J, Scotte F, Wildiers H, Sprangers B. Ann. Oncol. 2015;26:1677. doi: 10.1093/annonc/mdv136. [DOI] [PubMed] [Google Scholar]
- 4.Macleod LC, Tykodi SS, Holt SK, Wright JL, Lin DW, Tretiakova MS, True LD, Gore JL. Urology. 2015;86:262. doi: 10.1016/j.urology.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Ratnayake R, Covell DG, Ransom TT, Gustafson KR, Beutler JA. Org. Lett. 2009;11:57. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Willot M, Radtke L, Könning D, Fröhlich R, Gessner VH, Strohmann C, Christmann M. Angew. Chem. Int. Ed. Engl. 2009;48:9105. doi: 10.1002/anie.200905032. [DOI] [PubMed] [Google Scholar]; (b) Molawai K, Delpont N, Echavarren AM. Angew. Chem. Int. Ed. Engl. 2010;49:3517. doi: 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Kang Q, Ng SY, Chen DY. J. Am. Chem. Soc. 2010;132:8219. doi: 10.1021/ja102927n. [DOI] [PubMed] [Google Scholar]; (d) Xu J, Caro-Diaz EJ, Theodorakis EA. Org. Lett. 2010;12:3708. doi: 10.1021/ol1015652. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhou Q, Chen X, Ma D. Angew. Chem. Int. Ed. Engl. 2010;49:3513. doi: 10.1002/anie.201000888. [DOI] [PubMed] [Google Scholar]; (f) Li Z, Nakashige M, Chain WJ. J. Am. Chem. Soc. 2011;133:6553. doi: 10.1021/ja201921j. [DOI] [PubMed] [Google Scholar]; (g) Lee J, Parker KA. Org. Lett. 2012;14:2682. doi: 10.1021/ol3007524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Takahashi K, Komine K, Yokoi Y, Ishihara J, Hatakeyama SJ. Org. Chem. 2012;77:7364. doi: 10.1021/jo301145r. [DOI] [PubMed] [Google Scholar]; (i) Zahel M, Kessberg A, Metz P. Angew. Chem. Int. Ed Engl. 2013 doi: 10.1002/anie.201301247. [DOI] [PubMed] [Google Scholar]; (j) Zhang J, Zheng S, Peng W, Shen Z. Tetrahedron Lett. 2014;55:1339. [Google Scholar]
- 7.(a) Sulzmaier FJ, Li Z, Nakashige ML, Fash DM, Chain WJ, Ramos JW. PLoS. One. 2012;7:e48032. doi: 10.1371/journal.pone.0048032. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sourbier C, Scroggins BT, Ratnayake R, Prince TL, Lee S, Lee JM, Trepel JB, Beutler JA, Linehan WM, Neckers LM. Cancer Cell. 2013;23:228. doi: 10.1016/j.ccr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Williams RT, Yu AL, Diccianni MB, Theodorakis EA, Batova AJ. Exp. Clin. Cancer Res. 2013;32:1. doi: 10.1186/1756-9966-32-57. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. Angew. Chem. Int Ed Engl. 2015;54:3787. doi: 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, Yeola S, Lao J, McPate M, Verkuyl JM, Marsh G, Sarber J, Amaral A, Bailey S, Lubicka D, Pham H, Miranda N, Ding J, Tang HM, Ju H, Tranter P, Ji N, Krastel P, Jain RK, Schumacher AM, Loureiro JJ, George E, Berellini G, Ross NT, Bushell SM, Erdemli G, Solomon JM. PLoS One. 2015;10:e0127498. doi: 10.1371/journal.pone.0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) López-Suárez L, Riesgo L, Bravo F, Ransom TT, Beutler JA, Echavarren AM. Chem Med Chem. 2016 doi: 10.1002/cmdc.201600040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Caropreso V, Darvishi E, Turbyville TJ, Ratnayake R, Grohar PJ, McMahon JB, Woldemichael G. J. Biol. Chem. 2016 doi: 10.1074/jbc.M115.701375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chain WJ, Beutler JA, Fash DM, Figg WD, Li Z, Peer CJ, Ramos JW, Sulzmaier FJ. Aza-epoxy-guaiane Derivatives and Treatment of Cancer. PCT/US2015/014601. U. S. Patent Application. 2015 Feb 5;
- 9.Shoemaker RH. Nat. Rev. Cancer. 2006;6:813. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 10.Chan KP, Chen DY. Chem Med Chem. 2011;6:420. doi: 10.1002/cmdc.201000544. [DOI] [PubMed] [Google Scholar]
- 11.Radtke L, Willot M, Sun H, Ziegler S, Sauerland S, Strohmann C, Frohlich R, Habenberger P, Waldmann H, Christmann M. Angew. Chem. Int. Ed Engl. 2011;50:3998. doi: 10.1002/anie.201007790. [DOI] [PubMed] [Google Scholar]
- 12.Akee RK, Ransom TT, Ratnayake R, McMahon JB, Beutler JA. J. Nat. Prod. 2012;75:45. doi: 10.1021/np200905u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sourbier C, Scroggins B, Mannes PZ, Liao PJ, Siems K, Wolf D, Beutler JA, Linehan WM, Neckers L. Oncotarget. 2015;6:29963. doi: 10.18632/oncotarget.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.