Abstract
Background:
Acute myeloid leukemia (AML) is curable in a subset of cases. The DNA methylation regulator TET2 is frequently mutated in AML, and we hypothesized that studying TET2-specific differentially methylated CpGs (tet2-DMCs) improves AML classification.
Methods:
We used bisulfite pyrosequencing to analyze the methylation status of four tet2-DMCs (SP140, MCCC1, EHMT1, and MTSS1) in a test group of 94 consecutive patients and a validation group of 92 consecutive patients treated with cytarabine-based chemotherapy. Data were analyzed with hierarchical clustering, Cox proportional hazards regression, and Kaplan-Meier analyses. All statistical tests were two-sided.
Results:
In the test cohort, hierarchical clustering analysis identified low levels of tet2-DMC methylation in 31 of 94 (33%) cases, and these had markedly longer overall survival (median survival 72+ vs 14 months, P = .002). Similar results were seen in the validation cohort. tet2-DMC–low status was shown to be an independent predictor of overall survival (hazard ratio = 0.29, P = .0002). In The Cancer Genome Atlas (TCGA) dataset where DNA methylation was analyzed by a different platform, tet2-DMC–low methylation was also associated with improved outcome (median survival = 55 vs 15 months, P = .0003) and was a better predictor of survival than mutations in TET2, IDH1, or IDH2, individually or combined.
Conclusions:
Low levels of tet2-DMC methylation define a subgroup of AML that is highly curable and cannot be identified solely by genetic and cytogenetic analyses.
Acute myeloid leukemia (AML) is a highly heterogeneous hematologic malignancy. Most genetic and cytogenetic changes in AML have now been identified. Although these genetic changes are useful for classification and prognostication (1–3), they do not fully explain the clinical heterogeneity in outcomes. About half of patients with AML have an intermediate cytogenetic risk where heterogeneity remains problematic. Recent advances in the treatment for AML have improved outcomes for young patients through chemo-intensification and/or the use of allogeneic bone marrow transplantation (4). About half of young patients can be cured by chemotherapy alone, and identifying this curable subset will facilitate management of AML.
Epigenetic mechanisms such as DNA methylation are important in control of gene expression in stem cell physiology, normal differentiation, and cancer development (5,6). DNA methylation is frequently abnormal in AML as examined by studies of individual genes and genome wide (7–10). DNA methylation patterns can also be prognostic in AML, either when studied genome-wide (11) or in a gene-specific manner (12,13). In addition, clinical studies with DNA methyltransferase inhibitors have shown impressive responses in some patients (14), suggesting that aberrant DNA methylation may be a hallmark of the disease. Several genes encoding DNA methylation enzymes (DNMT3A) (15), DNA demethylating enzymes (TET2) (16), and related genes (IDH1/2) are mutated in AML (17). TET2 and IDH1/2 mutations are potentially important because animal models of these replicate aspects of the human phenotype (18,19). TET2, IDH1, and IDH2 mutations tend to be mutually exclusive and are thought to cause leukemia by inducing aberrant DNA methylation at specific targets (10).
The prognostic impact of TET2, IDH1, and IDH2 status has been difficult to ascertain because of contradictory findings in different studies. For example, a bad outcome for TET2 mutations was seen in some studies (16,20,21), whereas no difference was seen in others (22,23). Similarly, IDH mutations were shown to be unfavorable in one study (IDH1) (24), favorable in one (IDH2) (25), and had no effect on survival in others (IDH2 and IDH1) (26,27). Because all three genes are thought to affect a common number of targets, we hypothesized that DNA methylation analysis of these targets may be more useful for prognostic purpose.
We previously identified differentially methylated CpG sites in TET2-mutant cases of CMML (TET2-specific differentially methylated CpGs; tet2-DMCs) (28,29). Most of them were found to be outside CpG islands and have enrichment at hematopoietic-specific enhancers marked by H3K4me1 and at binding sites for the transcription factor p300. These tet2-DMCs have not previously been characterized in AML. In this study, we used bisulfite pyrosequencing to determine the DNA methylation status of four tet2-DMCs and found that low levels of methylation define a curable subset of AML that cannot be identified with cytogenetics and genetics alone.
Methods
Patients
We analyzed whole bone marrow samples collected prior to treatment from 94 AML patients for the test cohort and 92 AML patients for the validation cohort. All patients were seen and treated with chemotherapy at the University of Texas MD Anderson Cancer Center (MDACC). The Institutional Review Board at MDACC and Temple University approved these studies, and all patients gave informed consent for the collection of residual tissues as per institutional guidelines and in accordance with the Declaration of Helsinki. In this study, we only included patients treated with MDACC-standard cytarabine-based induction regimens. The patients were selected solely based on sample availability. There was no difference in survival between patients with or without available samples in the MDACC leukemia bank. The two cohorts (test and validation) consisted of consecutive patients with AML and excluded good risk cytogenetics (if known). The patients were treated on four main chemotherapy regimens (idarubicin + cytarabine, 65 patients; idarubicin + cytarabine + vorinostat, 43 patients; idarubicin + cytarabine + tipifarnib, 34 patients; idarubicin + cytarabine + sorafenib, 42 patients; fludarabine + cytarabine + G-CSF + gemtuzumab, two patients). The four regimens gave equivalent survival in this cohort. Post remission therapy generally included six to eight additional cycles of the same therapy at the same or reduced doses depending on toxicity. Eighty-two percent and 90% of the patients achieved CR with one course in the test and validation cohorts, respectively. Standard diagnostic and remission criteria were used (30). DNA was extracted by standard methods.
Mutation Analysis
Mutation status of FLT3 (internal tandem duplications [FLT3-ITD] and tyrosine kinase domain [FLT3-TKD]), NPM1, and RAS were obtained from clinical records and were tested in Clinical Laboratory Improvement Amendments (CLIA)-approved clinical laboratories. We used pyrosequencing to analyze mutations of the R132 residue in IDH1 and residues R140 and R172 in IDH2, which have been reported as mutated in AML (17,31). Mutations affecting the amino acid R882 residue in the DNMT3A gene (15,32) were analyzed by pyrosequencing. Primer sequences and polymerase chain reaction (PCR) conditions are listed in Supplementary Table 1 (available online).
Quantitative DNA Methylation Analyses by Bisulfite Pyrosequencing
We used bisulfite pyrosequencing to quantitatively assess DNA methylation (33) for four regions (CpG sites in SP140, MCCC1, EHMT1, and MTSS1), and higher than 95% success rates were obtained for DNA methylation data from patients. The EpiTect Bisulfite Kit (QIAGEN) was used for bisulfite conversion of 0.5 to 1 µg of DNA. After bisulfite conversion, pyrosequencing was performed on the PyroMark Q96 MD platform (QIAGEN). Success rates in pyrosequencing were 100%, 100%, 98%, and 97% for SP140, MCCC1, EHMT1, and MTSS1. Primer sequences and PCR conditions are listed in Supplementary Table 1 (available online).
Statistical Analysis
Statistical analyses were performed using PRISM (GraphPad Software, Inc., La Jolla, CA) and the statistical computing language R (www.r-project.org). We used the Mann-Whitney test to compare continuous variables of DNA methylation levels. Fisher’s exact test was used for two-by-two contingency analyses. All P values were two-tailed, and the threshold of statistical significance was a P value of less than .05. Survival data are presented using the Kaplan-Meier method, and P values for different groups were generated with the log-rank test, with surviving patients being censored with a median follow-up of 48 months (2 to 72 months) and 44 months (16 to 82 months) in the test and validation cohorts, respectively. The Cox proportional hazards model was used for multiple regression analyses. Multiple regression analyses were performed with covariates which were shown to be statistically significant in univariate analyses, including age and antecedent hematologic disorder. European LeukemiaNet (ELN) (34), IDH/DNMT3A mutation status, and tet2-DMC status were also included in multiple regression analyses. The Cox proportional hazards assumption was tested for each covariate analytically using Schoenfeld residuals. There was no evidence of nonproportional hazards. Hazard ratios (HRs) are shown with 95% confidence intervals (CIs). Hierarchical clustering analyses were performed by ArrayTrack (http://edkb.fda.gov/webstart/arraytrack/) with the Euclidean distance dissimilarities and Ward’s method.
Results
Patients
We studied consecutive patients with adult (age 17 years and older) AML enrolled in front-line chemotherapy studies at MDACC. These clinical trials included patients up to the age of 73 years and excluded favorable-risk AML patients when known. The clinical characteristics of the test (n = 94) and validation (n = 92) cohorts are shown in Table 1. The patients in the test and validation cohorts were accrued consecutively and were enrolled on four main clinical trials, all of which had a cytarabine and idarubicin backbone. Complete remission (CR) was obtained in 73% and 78% of the patients from the test and validation cohorts, respectively, and median overall survival (OS) was 17 and 19 months in the two cohorts. Genetic alterations were identified in 81 (43%) out of 186 AML patients included in the test and validation cohort (Table 1; Supplementary Figure 1, available online). Univariate analyses revealed that age, cytogenetics, antecedent hematologic disorder (AHD), and mutations in NPM1 were associated with OS (P < .0001 for all comparisons except for NPM1 mutations, with P = .01) (Supplementary Figure 2, available online). Mutations in IDH1/2 and DNMT3A did not affect OS statistically significantly (Supplementary Figure 2B, available online).
Table 1.
Patient characteristics*
| Characteristics | Test | Validation | TCGA | P | P | P |
|---|---|---|---|---|---|---|
| (test vs validation) | (test vs TCGA) | (validation vs TCGA) | ||||
| Total No. | 94 | 92 | 194 | |||
| Age, mean (range), y | 49 (18–73) | 50 (17–66) | 55 (18–88) | .59 | .0002 | .0007 |
| Male sex, No. (%) | 48 (51) | 38 (41) | 54 (28) | .19 | .71 | .06 |
| Bone marrow blasts at diagnosis, mean (range), % | 58 (8–96) | 53 (10–99) | 54 (0–98) | .24 | <.0001 | <.0001 |
| WBC at diagnosis, mean (range), 103/uL | 34 (1–228) | 15 (1–162) | 38 (1–298) | .01 | .18 | <.0001 |
| Cytogenetic risk group, No. (%) | .13 | <.0001 | <.0001 | |||
| Favorable | 3 (3) | 0 (0) | 36 (19) | |||
| Intermediate | 50 (55) | 58 (64) | 113 (59) | |||
| Poor | 38 (42) | 32 (36) | 42 (22) | |||
| Antecedent hematologic disorder, No. (%) | 29 (31) | 9 (10) | NA | .0005 | NA | NA |
| Complete remission rate, No. (%) | 69 (73) | 72 (78) | NA | .5 | NA | NA |
| Overall survival, median (range), mo | 17 (0–72+) | 19 (0–82+) | 12 (0–95+) | .98 | .002 | .002 |
| Mutations, No. (%) | ||||||
| FLT3-ITD | 22 (24) | 12 (14) | 57 (30) | .09 | .26 | .003 |
| FLT3-TKD | 8 (9) | 5 (6) | NA | .57 | NA | NA |
| RAS | 12 (14) | 10 (13) | 11 (6) | .82 | .03 | .08 |
| NPM1 | 21 (24) | 12 (18) | 45 (24) | .43 | 1 | .39 |
| IDH1 | 3 (3) | 5 (5) | 17 (9) | .72 | .13 | .48 |
| IDH2 | 8 (9) | 5 (6) | 17 (9) | .57 | 1 | .48 |
| IDH1/2 | 10 (11) | 10 (12) | 34 (18) | .82 | .16 | .28 |
| DNMT3A | 9 (10) | 12 (13) | 18 (25) | .64 | .02 | .07 |
* The P values were computed from Fisher’s exact test for two-by-two contingency analyses, Mann-Whitney test to compare continuous variables (age, bone marrow blasts at diagnosis, and WBC at diagnosis), and the log-rank test for survival data. All P values were two-tailed, and the threshold of statistical significance was P < .05. NA = not analyzed; WBC = white blood cells; TCGA = the Cancer Genome Atlas.
DNA Methylation of tet2-DMCs in AML
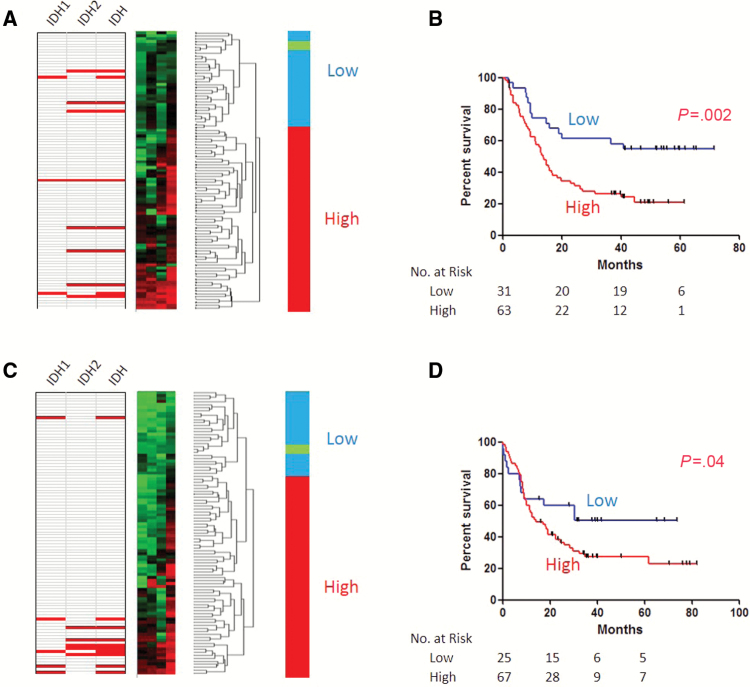
We measured methylation status of 4 tet2-DMCs (a CpG site close to the transcription start site of SP140 and CpG sites in gene-bodies of MCCC1, EHMT1, and MTSS1). All four loci showed highly variable methylation compared with normal peripheral blood (NPB) and also compared with normal bone marrow–derived CD34+ or CD34- cells (Supplementary Figure 3, available online). For each locus, a subset of cases had methylation levels equivalent to or lower than normal, while many cases were substantially higher than normal. DNA methylation of these four tet2-DMCs was highly concordant in AML (R = 0.4–0.6, P < .0001 for all correlations, data not shown), consistent with shared DNA methylation regulation. We therefore used hierarchical clustering analysis to define tet2-DMC methylation status. In the test cohort, a subset of 31 of 94 (33%) patients had low DNA methylation levels for all four tet2-DMCs (Figure 1A) and clustered with NPB (“normal like tet2-DMC”). This group of patients showed statistically significantly longer survival compared with those with higher DNA methylation (median survival = 72+ vs 14 months, P = .002) (Figure 1B). Multiple regression analysis revealed that tet2-DMC–low status, along with ELN-adverse and AHD, was an independent predictor of OS (tet2-DMC–low: HR = 0.29, P = .0002) (Table 2).
Figure 1.
DNA methylation signatures for TET2-specific differentially methylated CpGs (tet2-DMC)-low and -high patients in acute myeloid leukemia (AML). Hierarchical clustering analyses (left) in the test cohort (A) and the validation cohort (C) were used for classifying tet2-DMC–low (blue) and –high (red) patients. Note that normal peripheral blood (green) clustered with tet2-DMC–low patients. Mutation status for IDH1, IDH2, or IDH1/2 are shown on the left for each case (red indicates mutation). Tet2-DMC–low patients showed statistically longer overall survival compared with tet2-DMC–high in the test cohort (B) and the validation cohort (D). P values are derived from the two-sided log-rank test.
Table 2.
Multiple regression analyses of survival*
| Covariate | P | HR (95% CI) |
|---|---|---|
| Test cohort | ||
| ELN-adverse | <.0001 | 3.40 (1.93 to 5.98) |
| AHD | .0107 | 2.09 (1.19 to 3.68) |
| tet2-DMC–low | .0002 | 0.29 (0.15 to 0.55) |
| Combined cohort | ||
| Age, y | .031 | 1.02 (1.00 to 1.04) |
| ELN-favorable | <.0001 | 0.14 (0.05 to 0.35) |
| ELN-intermediate–1 | <.0001 | 0.31 (0.20 to 0.48) |
| ELN-intermediate–2 | .0014 | 0.44 (0.26 to 0.72) |
| AHD | .012 | 1.84 (1.14 to 2.96) |
| tet2-DMC–low | .0008 | 0.45 (0.28 to 0.71) |
| Cohort with a clinically applicable tet2-DMC signature | ||
| Age, y | .02 | 1.02 (1.00 to 1.04) |
| ELN-favorable | <.0001 | 0.11 (0.04 to 0.27) |
| ELN-intermediate–1 | <.0001 | 0.29 (0.19 to 0.47) |
| ELN-intermediate–2 | .001 | 0.43 (0.26 to 0.77) |
| tet2-DMC–low | .001 | 0.51 (0.34 to 0.77) |
* Age (>60 years [42]), AHD, ELN, IDH/DNMT3A mutation status, and tet2-DMC status were included as categorical variables. The Cox proportional hazards model was used. AHD = antecedent hematologic disorder; CI = confidence interval; ELN = European LeukemiaNet; HR = hazard ratio; tet2-DMCs = TET2-specific differentially methylated CpGs.
To confirm these findings, another set of 92 AML patients was investigated for validation and was found to have very similar methylation distribution at all four loci (Supplementary Figure 3, available online). Once again, a subset of cases had methylation levels equivalent to or lower than normal, and methylation was highly concordant. A subset of 25 patients (27%) was found to have tet2-DMC–low methylation by hierarchical clustering analysis (Figure 1C). These patients also showed statistically significantly improved OS (median survival = 74+ vs 14 months, P = .04) (Figure 1D).
Based on the remarkably consistent data between the two cohorts, we combined them to improve accuracy of the analyses. We found no statistical difference between tet2-DMC–low (n = 56) and –high (n = 130) in the combined data (Table 3) in any of the clinical characteristics examined other than OS (median survival = 74+ vs 14 months, P = .0004) (Figure 2). We looked at effects of various mutations on DNA methylation status for the four tet2-DMCs and found that only IDH1/2 mutations were associated with statistically significantly higher DNA methylation status (data not shown). Multiple regression analysis of the combined dataset revealed that tet2-DMC–low status was independently associated with a prolonged OS (HR = 0.45, P = .0008) along with age, ELN-favorable, -intermediate–1, -intermediate–2, and AHD (Table 2).
Table 3.
Patient characteristics for tet2-DMC–low and –high*
| Characteristics | tet2-DMC– low | tet2-DMC– high | P |
|---|---|---|---|
| Total No. | 56 | 130 | |
| Age, mean (range), y | 50 (19–68) | 49 (17–73) | .96 |
| Male sex, No. (%) | 22 (39) | 64 (49) | .26 |
| Bone marrow blasts at diagnosis, mean (range), % | 53 (8–96) | 57 (10–99) | .32 |
| WBC at diagnosis, mean (range), 103/uL | 22 (1–148) | 26 (1–228) | .71 |
| Cytogenetic risk group, No. (%) | .34 | ||
| Favorable | 2 (4) | 1 (1) | |
| Intermediate | 31 (55) | 77 (62) | |
| Poor | 23 (41) | 47 (38) | |
| Antecedent hematologic disorder, No. (%) | 13 (23) | 25 (19) | .56 |
| Complete remission rate, No. (%) | 46 (82) | 95 (73) | .2 |
| Overall survival, median (range), mo | 74+ (0–79+) | 14 (0–82+) | .0004 |
| Mutations, No. (%) | |||
| FLT3-ITD | 9 (17) | 25 (20) | .68 |
| FLT3-TKD | 3 (6) | 10 (8) | .76 |
| RAS | 5 (10) | 17 (15) | .46 |
| NPM1 | 13 (28) | 20 (19) | .21 |
| IDH1 | 2 (4) | 6 (5) | 1 |
| IDH2 | 3 (6) | 10 (8) | .76 |
| IDH1/2 | 5 (9) | 15 (12) | .8 |
| DNMT3A | 7 (13) | 14 (11) | .8 |
* The P values were computed from Fisher’s exact test for two-by-two contingency analyses, Mann-Whitney test to compare continuous variables (age, bone marrow blasts at diagnosis, and WBC at diagnosis), and the log-rank test for survival data. All P values were two-tailed, and the threshold of statistical significance was P < .05. tet2-DMCs = TET2-specific differentially methylated CpGs.
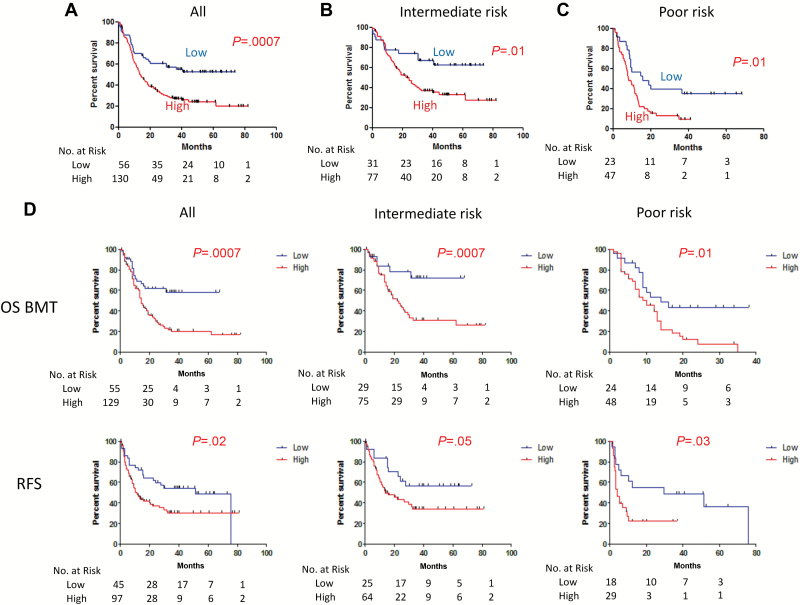
Figure 2.
Kaplan-Meier survival curves for TET2-specific differentially methylated CpGs (tet2-DMC)-low (blue) and -high (red) patients in the combined cohorts. Tet2-DMC–low patients showed statistically longer overall survival (OS) compared with tet2-DMC–high in the analysis with all patients (A) in the intermediate-risk cytogenetics group (B) and the poor-risk cytogenetics group (C). D) Kaplan-Meier survival curves for OS adjusted based on bone marrow transplantations censoring and recurrence-free survival are shown in the upper and lower rows, respectively, in the analysis with all patients (left column), in the intermediate-risk cytogenetics group (center column), and the poor-risk cytogenetics group (right column), respectively. P values are derived from two-sided log-rank test. OS BMT = overall survival adjusted for bone marrow transplantations censoring; RFS = recurrence-free survival.
Cytogenetic status is the most consistent predictor of outcome in AML, and the multiple regression analysis supported that tet2-DMC status considerably refines the classification. To illustrate this, we analyzed patients with only intermediate-risk or poor-risk groups. Figure 2, B and C, shows that median survival was 74+ vs 23 months (P = .01) for the intermediate-risk group, and median survival was 16 vs 8 months (P = .01) for the poor-risk group, in the tet2-DMC–low and –high groups, respectively (Figure 2, B and C). Figure 2D shows Kaplan-Meier survival curves censored at stem-cell transplantation, and again tet2-DMC–low status was associated with statistically significantly longer survival in all patients, intermediate-risk groups, or poor-risk groups (P = .0007 for all cases, P = .007 for intermediate-risk cases, or P = .01 for poor-risk cases, respectively). This was also the case for recurrence-free survival (P = .02 for all cases, P = .05 for intermediate-risk cases, or P = .03 for poor-risk cases, respectively) (Figure 2D). Finally, we also examined the subset of patients with NPM1 mutation but no FLT3-ITD (N+F-). The tet2-DMC–low group had 11 patients out of 48 (23%) with this signature, whereas the tet2-DMC–high group had 15 of 107 (14%) with this signature (P = .24). N+F- patients had a better outcome (as previously reported), but this was modulated further by tet2-DMC status (Supplementary Figure 4, available online), suggesting that they mark independent biological subsets.
A Clinically Applicable tet2-DMC Signature
A hierarchical clustering analysis requires a certain number of patients with DNA methylation status for tet2-DMCs to properly classify patients, and this is not practical to guide treatment of individual patients. We therefore sought to establish a method to define tet2-DMC status prospectively. To do this, we set thresholds for each tet2-DMC calculated by mean+SD for the tet2-DMC–low group. The levels of mean+SD for each tet2-DMC were 31.5%, 38.0%, 32.3%, and 42.4% for SP140, MCCC1, EHMT1, and MTSS1, respectively, and methylation above these thresholds was called “positive” for each gene (Supplementary Figure 5, available online). We then classified AML patients into tet2-DMC–low and –high based on having zero to one or more than one genes methylated, respectively. Kaplan-Meier curves showed that patients with tet2-DMC–low (n = 67) defined by this classification survived statistically significantly longer than those who were tet2-DMC–high (median survival = 79+ vs 14 months, P = .0006). Patients with tet2-DMC–low by this classification showed higher complete remission rate (P = .03) (Supplementary Table 2, available online), and multiple regression analysis showed that tet2-DMC–low status was associated with a prolonged OS (HR = 0.51, P = .001), along with ELN-favorable, -intermediate–1, -intermediate–2, and age (Table 2).
Validation in the Cancer Genome Atlas Research Network Dataset
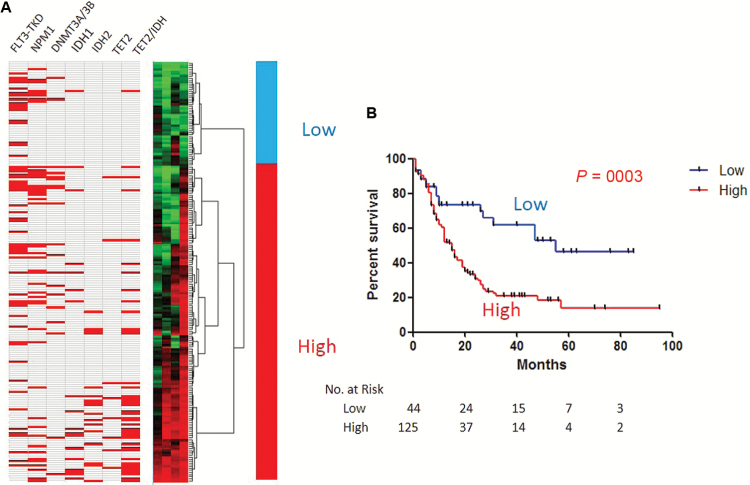
To further validate the prognostic impact of tet2-DMCs in AML, we utilized the dataset from TCGA (35) where genome-wide mutation and methylation status is available. Because the Illumina 450K Infinium platform used in TCGA analysis has probes for DNA methylation detection mostly at gene promoters, we looked at the few tet2-DMCs at promoters found in our previous study. To match results from both studies, we searched for CpG probes on the Infinium platform closest (<50bp) to the sites analyzed in our restriction enzyme-based method (36). We found three tet2-DMCs at gene promoters (SP140, LSP1, and UNC93B1) whose sites analyzed by both methods nearly coincide. We also found and added one out of the three nonpromoter tet2-DMCs used in our study (EHMT1). We used methylation status of these four tet2-DMCs for a hierarchical clustering analysis (Figure 3A). In the 194-patient TCGA dataset with available outcome, tet2-DMC–low status also showed statistically significantly longer OS (median survival 55 vs 15 months, P = .0003) (Figure 3B). Interestingly, these tet2-DMC–low patients were younger (P < .001) and had the M3 subtype and/or favorable-risk cytogenetics more often than the other groups (both, P < .0001) (Table 4). When only patients with M3 or favorable-risk cytogenetics were analyzed, the tet2-DMC–low subgroup showed statistically significantly longer survival than the tet2-DMC–high subgroup (P = .0018) (Supplementary Figure 6A, available online). On the other hand, M3 or favorable-risk cytogenetics patients were also found to have longer survival than the rest of the patients when only tet2-DMC–low patients were analyzed (P = .023) (Supplementary Figure 6B, available online), suggesting that tet2-DMC–low status has additive beneficial effects on survival in AML, along with known favorable-risk status such as cytogenetics and M3. Finally, when we excluded APL and good-risk AMLs from the TCGA dataset, tet2-DMC methylation status showed exactly the same trend as the MDACC patients, with a large difference in survival favoring the tet2-DMC–low group, though the survival difference did not reach statistical significance (P = .09) (Supplementary Figure 6C, available online).
Figure 3.
DNA methylation signatures for TET2-specific differentially methylated CpGs (tet2-DMC)-low and -high patients in The Cancer Genome Atlas (TCGA) dataset. A) Hierarchical clustering analysis was used for classifying tet2-DMC–low and –high patients. Mutations of multiple genes are shown in red. The TET2/IDH combined column indicates mutations of any of the three genes. B) Kaplan-Meier survival curves for tet2-DMC–low (blue) and –high (red) patients in the TCGA dataset. Tet2-DMC–low patients showed statistically longer overall survival compared with tet2-DMC–high in the analysis with all patients. P values are derived from the two-sided log-rank test.
Table 4.
Patient characteristics for tet2-DMC–low and –high in the TCGA dataset*
| Characteristics | tet2-DMC–low | tet2-DMC–high | P |
|---|---|---|---|
| Total No. | 48 | 146 | |
| Age, mean (range), y | 49 (21–76) | 57 (18–88) | .0011 |
| Male sex, No. (%) | 25 (52) | 80 (55) | .87 |
| Bone marrow blasts at diagnosis, mean (range), % | 30 (0–94) | 39 (0–98) | .07 |
| WBC at diagnosis, mean (range), 103/uL | 29 (1–134) | 41 (1–298) | .16 |
| Cytogenetic risk group, No. (%) | <.0001 | ||
| Favorable | 31 (67) | 5 (3) | |
| Intermediate | 14 (30) | 99 (68) | |
| Poor | 1 (2) | 41 (28) | |
| Acute promyelocytic leukemia (M3), No. (%) | 18 (38) | 1 (1) | <.0001 |
| Overall survival, median (range), mo | 55 (0–85+) | 15 (0–95+) | .0003 |
| Mutations, No. (%) | |||
| ASXL1 | 0 (0) | 3 (6) | 1 |
| DNMT3A | 2 (12) | 17 (31) | .13 |
| FLT3-TKD | 17 (35) | 40 (29) | .47 |
| RAS | 2 (4) | 9 (6) | .73 |
| NPM | 8 (17) | 37 (26) | .24 |
| IDH1 | 1 (2) | 17 (12) | .08 |
| IDH2 | 0 (0) | 17 (12) | .008 |
| TET2 | 0 (0) | 8 (15) | .18 |
| TET2/IDH | 1 (2) | 41 (28) | <.0001 |
* The P values were computed from Fisher’s exact test for two-by-two contingency analyses, Mann-Whitney test to compare continuous variables (age, bone marrow blasts at diagnosis, and WBC at diagnosis), and the log-rank test for survival data. All P values were two-tailed, and the threshold of statistical significance was P < .05. TCGA = the Cancer Genome Atlas; tet2-DMCs = TET2-specific differentially methylated CpGs.
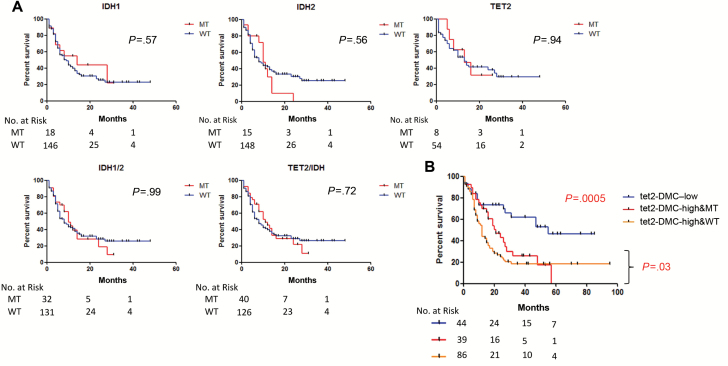
As expected, IDH2 mutations were statistically significantly higher in the tet2-DMC–high groups and TET2, and IDH1 mutations were exclusive to the tet2-DMC–high group. No clinical or genetic characteristic studied was found to be statistically different between tet2-DMC–high with or without TET2/IDH mutation (Supplementary Table 3, available online). We also examined the complement of genes mutated in each of the subsets by gene ontology analysis and found relatively minor differences (data not shown). Mutations of IDH1, IDH2, or TET2 had no effect on survival individually or in combination in the TCGA dataset (Figure 4A), in contrast to the tet2-DMC–low status, which was highly statistically significant. When combining mutation and methylation data, we found that tet2-DMC–low status (all but one patient had no mutation of TET2/IDH) is predictive of the longest survival (median survival = 55 months, P = .0005), followed by tet2-DMC–high with TET2/IDH mutations (median survival = 21 months), and tet2-DMC–high without TET2/IDH mutations (median survival = 12 months, P = .03 for tet2-DMC–high with and without TET2/IDH) (Figure 4B). Thus, tet2-DMC methylation was a better predictor of outcome than mutations in the TET2/IDH axis, but a combined methylation/mutation analysis was best for delineation of prognostically distinct subgroups.
Figure 4.
A) Univariate analyses for overall survival (OS) of patients with mutations of IDH1, IDH2, or TET2 individually or in combination in The Cancer Genome Atlas (TCGA) Research Network dataset. Kaplan-Meier survival curves were drawn for each gene. Note that none of them have an effect on the survival. P values are derived from the two-sided log-rank test. B) Kaplan-Meier survival curves for OS of patients based on their TET2-specific differentially methylated CpGs (tet2-DMC) and TET2/IDH mutation status. The tet2-DMC–low status (blue) shows the longest survival, followed by the tet2-DMC–high with TET2/IDH mutations (red) and the tet2-DMC–high without TET2/IDH mutations (orange). MT = mutant; WT = wild type.
Discussion
Personalized medicine requires detailed molecular classification of patients to provide a specific therapy for an individual’s condition. Cytogenetics and genetics have been used for decades in this regard in AML. Cytogenetic classification is useful for predicting prognosis and assigning specific therapies in AML. Mutations in genes such as NPM1 and DNMT3A were previously found to be prognostic as well (15,37). DNA methylation is frequently abnormal in AML, and DNA methylation patterns can be prognostic (11–13), though the underlying mechanism(s) remains to be fully understood. Recently, epigenetic regulators such as TET2 (16) and IDH1/2 (31) have been found to be mutated in AML; however, their effects on prognosis are controversial, and it remains unclear if these could have a notable impact on improving the current classification systems. Because TET2/IDH potentially affect the same molecular pathways to regulate DNA demethylation (10), we hypothesized that DNA methylation status could integrate upstream defects and provide better markers for prognosis and therapy.
In this study, we found that a DNA methylation signature at tet2-DMCs defines clinically distinct groups of adult AML cases. Patients with normal-like tet2-DMC methylation status have statistically longer OS, independently of currently used prognostic factors such as age or cytogenetics. Cure rate in tet2-DMC–low patients treated with chemotherapy exceeded 50%, as compared with less than 20% in tet2-DMC–high patients. Remarkably, this difference could also be seen in AML patients with poor-risk cytogenetics, where cure rates were almost 40% in tet2-DMC–low patients compared with 10% in tet2-DMC–high patients. The cure rate in patients with intermediate-risk cytogenetics and tet2-DMC–low (about 60%) is nearly as good as that seen in patients with favorable-risk cytogenetics. The underlying mechanism of this difference in survival warrants further investigation; we hypothesize that AML cases with low tet2-DMC methylation have a preserved capacity for differentiation, which may explain chemosensitivity. We also developed a clinically applicable tet2-DMC signature with similar findings. There was a small difference in the number of patients labeled tet2-DMC–low by cluster analysis (n = 56) vs the clinical signature (n = 67), suggesting that there is room for further improvements in the prognostic signature.
Prognostic classification impacts treatment in AML. Patients with favorable-risk cytogenetics respond to chemotherapy dose intensification; patients with poor-risk cytogenetics are routinely referred for stem cell transplantation, while management of patients with diploid cytogenetics is variable. Our data suggest testing a prospective new stratification for treatment strategies: chemo-intensification for patients with tet2-DMC–low who are likely to respond well to conventional chemotherapies and new treatments or an early decision for stem cell transplantation for patients with tet2-DMC–high. It would be of interest to find out if DNA methylation inhibitors, now routinely used in treatment of elderly AML, could improve the chemosensitivity of tet2-DMC–high AML cells by hypomethylation of these tet2-DMCs to restore differentiation potential. Indeed, at least one study has reported a high response rate to decitabine chemosensitization in AML (38).
It is worth noting that methylation status of tet2-DMC is a better indicator of outcome than TET2 mutations or a combined TET2/IDH mutation index. This is because of the fact that mutations of TET2/IDH1/2 are identified only in a subset of AML patients with tet2-DMC–high status. It is possible that dysregulated DNA methylation at tet2-DMCs is associated with AML even in the absence of these mutations. More broadly, our data argue that classification schemes that incorporate epigenetic and genetic information may be more efficient for precision medicine. Despite the fact that we used different sets of tet2-DMCs in our initial cohorts than in the TCGA dataset (because of technical differences between the methylation platforms used), we found similar trends regarding a prognostic impact of tet2-DMCs on survival. While we used four markers in this study, it is worth testing whether adding other tet2-DMCs to the panel improves its ability to predict survival in AML.
Recently, Marcucci et al. (39) reported a novel seven gene biomarker set with promoter DMCs whose DNA methylation and gene expression were associated with outcome in AML. Six out of these seven genes were detectable by DREAM, the deep sequencing-based technique we used to identify tet2-DMCs in a CMML cohort (29). None of them showed differential levels of DNA methylation in TET2 mutated cases (the promoters were mostly unmethylated at less than 2% in both MT and WT), which is consistent with our finding that most tet2-DMCs are located at non-CpG island and nonpromoter sites. Broadly, it would be important to integrate models of outcome in AML that incorporate both tet2-DMCs and other differentially methylated sites.
The interactions between the tet2-DMC–low signature with known prognostic markers in AML is an interesting question to pursue. We did not find a marked enrichment of NPM1 mutant patients in the tet2-DMC–low subgroup, though the patient numbers remain small. The CCAAT/enhancer binding protein α (CEBPA) has gained increasing attention as a favorable prognostic factor in acute myeloid leukemia (AML) (40,41). In the TCGA dataset where CEBPA mutation status is available, there were no tet2-DMC–low patients with CEBPA mutations, implying that the tet2-DMC signature is CEBPA independent. Future studies should address the full complement of genetic/epigenetic interactions with outcome in AML.
In summary, we found that low level methylation of tet2-DMCs defines a subgroup of AML that is curable and which cannot be identified solely by genetic and cytogenetic analyses. This finding may lead to new clinically useful biomarkers for prognosis in AML and to a better risk stratification for treatment.
Funding
This work was supported by National Institutes of Health grants CA100632, CA121104, and CA049639 and supported by a Stand Up to Cancer grant from the American Association for Cancer Research. JPI is an American Cancer Society Clinical Research professor supported by a generous gift from the F. M. Kirby Foundation. The authors declare no competing financial interests.
Supplementary Material
The authors thank the patients who have contributed to our understanding of these disorders.
Authorship Contributions: JY and JPJI designed the study; JY, RT, and NJMR performed mutation analyses; JY performed DNA methylation analysis; JY and MC performed statistical analysis; SAP managed clinical records; JY and JPJI wrote the manuscript with assistance from JJ, SMK, CEBR, FR, and HMK.
References
- 1. Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 2. Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 3. Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–4336. [DOI] [PubMed] [Google Scholar]
- 4. Derolf AR, Kristinsson SY, Andersson TM, et al. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113(16):3666–3672. [DOI] [PubMed] [Google Scholar]
- 5. Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28(10):1079–1088. [DOI] [PubMed] [Google Scholar]
- 6. Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toyota M, Kopecky KJ, Toyota MO, et al. Methylation profiling in acute myeloid leukemia. Blood. 2001;97(9):2823–2829. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y, Dunbar A, Gondek LP, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113(6):1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Figueroa ME, Skrabanek L, Li Y, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114(16):3448–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Figueroa ME, Lugthart S, Li Y, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bullinger L, Ehrich M, Dohner K, et al. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood. 2010;115(3):636–642. [DOI] [PubMed] [Google Scholar]
- 13. Deneberg S, Grovdal M, Karimi M, et al. Gene-specific and global methylation patterns predict outcome in patients with acute myeloid leukemia. Leukemia. 2010;24(5):932–941. [DOI] [PubMed] [Google Scholar]
- 14. Pollyea DA, Zehnder J, Coutre S, et al. Sequential azacitidine plus lenalidomide combination for elderly patients with untreated acute myeloid leukemia. Haematologica. 2013;98(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdel-Wahab O, Mullally A, Hedvat C, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quivoron C, Couronne L, Della Valle V, et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer Cell. 2011;20(1):25–38. [DOI] [PubMed] [Google Scholar]
- 19. Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nibourel O, Kosmider O, Cheok M, et al. Incidence and prognostic value of TET2 alterations in de novo acute myeloid leukemia achieving complete remission. Blood. 2010;116(7):1132–1135. [DOI] [PubMed] [Google Scholar]
- 21. Metzeler KH, Maharry K, Radmacher MD, et al. TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2011;29(10):1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaidzik VI, Paschka P, Spath D, et al. TET2 mutations in acute myeloid leukemia (AML): results from a comprehensive genetic and clinical analysis of the AML study group. J Clin Oncol. 2012;30(12):1350–1357. [DOI] [PubMed] [Google Scholar]
- 23. Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–1152. [DOI] [PubMed] [Google Scholar]
- 24. Schnittger S, Haferlach C, Ulke M, et al. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116(25):5486–5496. [DOI] [PubMed] [Google Scholar]
- 25. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thol F, Damm F, Wagner K, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 2010;116(4):614–616. [DOI] [PubMed] [Google Scholar]
- 27. Wagner K, Damm F, Gohring G, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010;28(14):2356–2364. [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki J, Taby R, Vasanthakumar A, et al. Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics. 2012;7(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamazaki J, Jelinek J, Lu Y, et al. TET2 mutations affect non-CpG island DNA methylation at enhancers and transcription factor binding sites in chronic myelomonocytic leukemia. Cancer Res. 2015;75(14):2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–2302. [DOI] [PubMed] [Google Scholar]
- 31. Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(14):2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walter MJ, Ding L, Shen D, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25(7):1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colella S, Shen L, Baggerly KA, et al. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35(1):146–150. [DOI] [PubMed] [Google Scholar]
- 34. Rollig C, Bornhauser M, Thiede C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758–2765. [DOI] [PubMed] [Google Scholar]
- 35. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jelinek J, Liang S, Lu Y, et al. Conserved DNA methylation patterns in healthy blood cells and extensive changes in leukemia measured by a new quantitative technique. Epigenetics. 2012;7(12):1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(4):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scandura JM, Roboz GJ, Moh M, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011;118(6):1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcucci G, Yan P, Maharry K, et al. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32(6):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Preudhomme C, Sagot C, Boissel N, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA). Blood. 2002;100(8):2717–2723. [DOI] [PubMed] [Google Scholar]
- 41. Green CL, Koo KK, Hills RK, et al. Prognostic significance of CEBPA mutations in a large cohort of younger adult patients with acute myeloid leukemia: impact of double CEBPA mutations and the interaction with FLT3 and NPM1 mutations. J Clin Oncol. 2010;28(16):2739–2747. [DOI] [PubMed] [Google Scholar]
- 42. Stone RM, Berg DT, George SL, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B. N Engl J Med. 1995;332(25):1671–1677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.