Abstract
The investigation of the hydrogen-bonding effect on the aggregation tendency of ruthenium compounds [(η6-p-cymene)Ru(κNHR,κNOH)Cl]Cl (R = Ph (1a), Bn (1b)) and [(η6-p-cymene)Ru(κ2NH(2-pic),κNOH)][PF6]2 (1c), [(η6-p-cymene)Ru(κNHBn,κNO)Cl] (2b) and [(η6-p-cymene)Ru(κNBn,κ2NO)] (3b), has been performed by means of concentration dependence 1H NMR chemical shifts and DOSY experiments. The synthesis and full characterization of new compounds 1c, [(η6-p-cymene)Ru(κNPh,κ2NO)] (3a) and 3b are also reported. The effect of the water soluble ruthenium complexes 1a-1c on cytotoxicity, cell adhesion and cell migration of the androgen-independent prostate cancer PC3 cells have been assessed by MTT, adhesion to type-I-collagen and recovery of monolayer wounds assays, respectively. Interactions of 1a-1c with DNA and human serum albumin have also been studied. Altogether, the properties reported herein suggest that ruthenium compounds 1a-1c have considerable potential as anticancer agents against advanced prostate cancer.
Keywords: arene ruthenium compounds, oxime, chiral syntheses, non-covalent interactions, anticancer
Introduction
Although cisplatin and its derivatives are still widely used in the clinic, their side effects, general low solubility and intrinsic or acquired resistance in some cancer types have encouraged research for the discovery of new metal compounds with improved properties.[1,2] From the plethora of transition metal-based compounds synthesized and studied as potential antitumor agents during the last decade, ruthenium derivatives have emerged as promising candidates.[2,3,4–8] Some Ru(III) compounds, NAMI-A (imidazolium trans-[tetrachloridobis(dimethylsulfoxide)(1H-imidazole)-ruthenate(III)),[9] KP1019 (indazolium trans-[tetrachloridobis(1H-indazole)-ruthenate(III)]),[10] and KP1339 (sodium trans-[tetrachloridobis(1H-indazole)-ruthenate(III)])[11] are currently in phase II clinical trials. Organometallic ruthenium(II) arene Supporting information for this article is given via a link at the end of the document complexes such as [(η6-C6H5Ph)Ru(en)Cl][PF6] (RM175, en = ethylenediamine)[12] and [(η6-arene)Ru(pta)Cl2] (pta = 1,3,5-triaza-7-phosphatricyclo[3.3.1]decane; arene = toluene RAPTA-T; arene = p-cymene RAPTA-C)[13,14] are also showing great therapeutic promise. In particular, RAPTA-T and RAPTA-C have been shown to have some similar effects to NAMI-A, namely low cell cytotoxicity in vitro, selective anti-tumor activity towards solid metastasizing tumors in vivo and remarkable anti-angiogenic properties.[9,14–16] The differences in the anti-carcinogenic properties of structurally similar compounds such as NAMI-A and KP1019, KP1339 or RM175 and RAPTA derivatives show that the ligands bound to the metal assume an important relevance for the activity of the metal based-drugs.[17,18] NAMI-A and RAPTA-T, RAPTA-C compounds seem to act via molecular targets other than DNA,[2,4,19] with different mechanisms of action from those of classical platinum anticancer drugs. In recent years, it has been argued that even for cisplatin, DNA binding is not the only mechanism towards cisplatin-induced apoptosis. The research of possible interactions of metal-based anticancer agents with protein targets that are selective for tumor malignancy are considered a more effective approach to new antitumor drug design.[17,20]
Non-covalent interactions play key roles in biological molecular recognition. Hydrogen bonding, hydrophobic and electrostatic interactions can effectively enhance site-and base-recognition, not only of nucleic acids but also of proteins and enzymes crucial to both metastatic and angiogenic processes.[21,22,23] In this context, oxime groups offer significant advantages for biological applications. They possess stronger hydrogen-bonding capabilities than alcohols or carboxylic acids and have received considerable attention with respect to directional non-covalent intermolecular interactions.[24,25] The presence of hydrogen bonding can also favor solubility of the resulting metal compounds in biological media. In addition, some oxime organic derivatives have been reported to have anticarcinogenic activities, with biological effects such as endothelium-independent relaxation in blood-vessels, an increase in the targeting of specific nuclear bases of DNA and oxidative DNA cleavage.[26] Although oxime and oximate metal derivatives have been extensively studied,[24] their antitumoral properties have received little attention. Oxime-containing Pt(II) compounds have been recently reported as a novel class of nonclassic platinum-based complexes with interesting antitumor properties, different DNA binding behavior and a different pattern of protein interaction from those found in classical cisplatin.[27,28] A variety of Rh(III) and Ir(III) compounds with oximate ligands have also been studied.[29] The results show a strong cytotoxic effect toward HeLa and HL60 cancer lines, whereas the compounds do not modify DNA in a similar way to that of cisplatin. DNA-binding studies of oxime-containing ruthenium compounds have been previously investigated, but only a weak, non-intercalative in nature interaction was found.[30] However, to the best of our knowledge, the anti-carcinogenic properties of ruthenium oxime compounds have not been reported before.[31]
Examples of enantiopure arene ruthenium anticancer derivatives are scarce in the literature,[32,33,34] probably due to the difficult isolation of unique stereoisomers or enantiomers of organometallic compounds.[34–38] Within this context, enantiomerically pure, naturally occurring terpenes are useful building blocks for asymmetric synthesis.[39,40] They are inexpensive starting reagents, commercially available in optically pure form and easily tailored by stereoselective functionalization.[41] Some of us recently published the stereoselective synthesis of ruthenium compounds with amino-oxime ligands derived from R-limonene.[42] In this work we report a detailed synthetic and NMR study of those and analogous ruthenium compounds, together with a preliminary biological study of the anticarcinogenic properties they have shown against the androgen-unresponsive PC3 cell line.
Results and Discussion
Synthesis and characterization
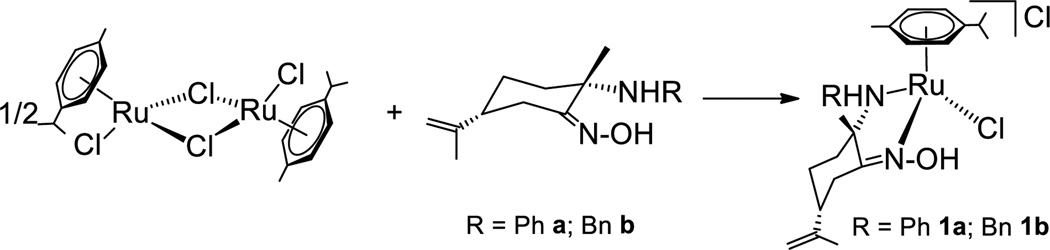
The reactions of dimer [(η6-p-cymene)RuCl2]2 with enantiomerically pure amino-oxime derivatives (2S,5R)-[NHR,NOH] (R = Ph a, Bn b)[41,43] afford previously described ruthenium compounds [(η6-p-cymene)Ru(kNHR,kNOH)Cl]Cl,[42] 1a or 1b, respectively (Scheme 1). Since compounds 1a and 1b are chiral-at-metal derivatives with a new stereogenic center at the amino ligand, four different diastereomers distinguishable by NMR spectroscopy could be formed, namely RRuSN-(2S,5R)-[NHR,NOH], RRuRN-(2S,5R)-[NHR,NOH], SRuSN-(2S,5R)-[NHR,NOH] or SRuRN-(2S,5R)-[NHR,NOH]. 1H and 13C NMR spectra of 1a and 1b showed the existence of only one diastereomer in solution, namely SRuRN-(2S,5R)-[NHR,NOH]. Reaction of dimer [(η6-p-cymene)RuCl2]2 with a or b was monitored by NMR spectroscopy in chloroform-d1. 1H NMR spectra showed the complete conversion of the reactants to only one diastereomer compound after only 5 minutes at room temperature. Epimerization[32,35,36] was never observed in CDCl3, acetone-d6, methanol-d4 or D2O (see supporting information), suggesting a preferred mode of the ligand chelation.[37,44] Stereoselective coordination of potential chelating ligands derived from natural terpenes has been observed before.[40,45]
Scheme 1.
Synthesis of chiral amino-oxime ruthenium compounds.
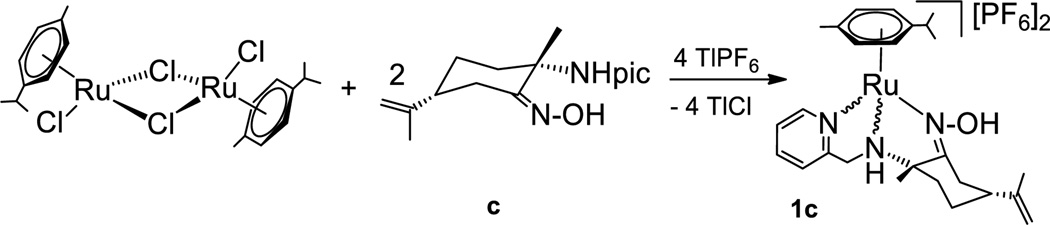
Following a similar procedure, dimer [(η6-p-cymene)RuCl2]2 was reacted with two equivalents of (2S,5R)-[NH(2-pic),NOH] (c). The 1H NMR spectrum in acetone-d6 or tetrahydrofurane-d8(THF-d8) of the resulting solid residue showed a complicated set of resonances which could not be assigned to a unique organometallic derivative. The downfield =NOH proton region showed four broad signals of relative intensities 1:1:1.7:0.8, which could be tentatively assigned to the =NOH proton resonances of four different diastereomers. Having in mind the possible formation of four different diastereomers, as well as the potential κ2N,N,N/κ3N,N,N coordination of the 2-picolylamino-oxime ligand that could produce mono and/or dicationic ruthenium compounds,[8,46] we carried out the reaction in the presence of TlPF6 (Scheme 2). The reaction proceeds in two hours at room temperature to afford a brown suspension, from which a brown solid residue can be extracted with THF or acetone. 1H, 13C and 15N NMR spectra of the solid in acetone-d6 or THF-d8 indicate the existence of two different diastereomers of the dicationic complex [(η6-p-cymene)Ru{κ2NH(2-pic),κNOH}][PF6]2 (1c), in a molar ratio of ca. 2:1. The lack of stereoselectivity of the synthesis reaction of 1c compared to 1a, 1b analogues is probably due to the potential trihapto coordination of the 2-picolylamino-oxime ligand. Such a coordination mode implies displacement of both chloride ligands from the metal center, which could favor formation of configurationally labile solvate derivatives after dissociation of the chlorido ligands and thus, epimerization of the ruthenium compounds.[35]
Scheme 2.
Synthesis of picolylamino-oxime ruthenium stereoisomers
Both isomers are insoluble in chloroform, dichloromethane or toluene which agrees well with their dicationic character. The relative intensities of each diastereomer do not change in THF-d8 or acetone-d6 solution at room temperature or after heating up to 70 °C for several days. While 1H NMR spectrum of the mixture shows a complicated set of overlapped proton signals, 13C NMR displays two well defined set of resonances. Bidimensional 15N-1H HMBC, 13C-1H HSQC and HMBC NMR experiments allowed a full assignment of the proton, carbon and nitrogen resonances displayed (see Supporting Information).
Coordination of the organic derivative c to the ruthenium(II) center shifts the 13C NMR signals due to Cq=NOH, CqNH and −CH2-NH groups (δ 179.4, 73.6 and 61.2 (1c-major); 175.4, 71.6 and 59.4 (1c-minor)) to lower fields in comparison to those signals assigned to the same fragments in the amino-oxime ligand precursor (δ 162.4, 56.9 and 48.1 (c)). Upfield shift of the nitrogen resonances arising from the oxime, picoline and amine fragments found in 1c-major (δ 258.8, 232.7 and 29.9) and 1c-minor (265.2, 236.4 and 28.9) relative to those observed in c (δ 343.3, 305.3 and 51.8) supports the proposed structure for the dicationic ruthenium compounds, with a trihapto-coordination of the picolylamino-oxime derivative to the ruthenium center. A similar upfield shift of the nitrogen signals relative to those observed in the NMR spectra of the ligand precursors was observed for the previously described[42] compounds 1a and 1b (see Supporting Information). 19F and 31P NMR spectra of the mixture of 1c-major and 1c-minor showed a septuplet and a doublet at δ −144.2 and −72.2 (JP-F = 708 Hz), respectively, due to PF6 counter-anions of both isomers, while CHN elemental analysis of the sample agreed well with the proposed composition. The IR spectrum of compound 1c exhibits strong, broad ν(N-H/O-H) bands at 3642-3500, 3290-3100 cm−1 and C=N stretching frequency at 1649, 1614 cm−1, with similar wavenumbers to those bands observed in the IR spectrum of organic derivative c (see Supporting Information).
During our experiments, we noticed a strong dependence of the proton NMR chemical shifts of compounds 1a, 1b and, in a much less degree, 1c, on the solution concentration. Gradual changes of the chemical shifts of 1H NMR resonances with the variation of concentration have been well documented as a signature of non-covalent interactions leading to self-aggregation behavior in solution.[23,47,48]
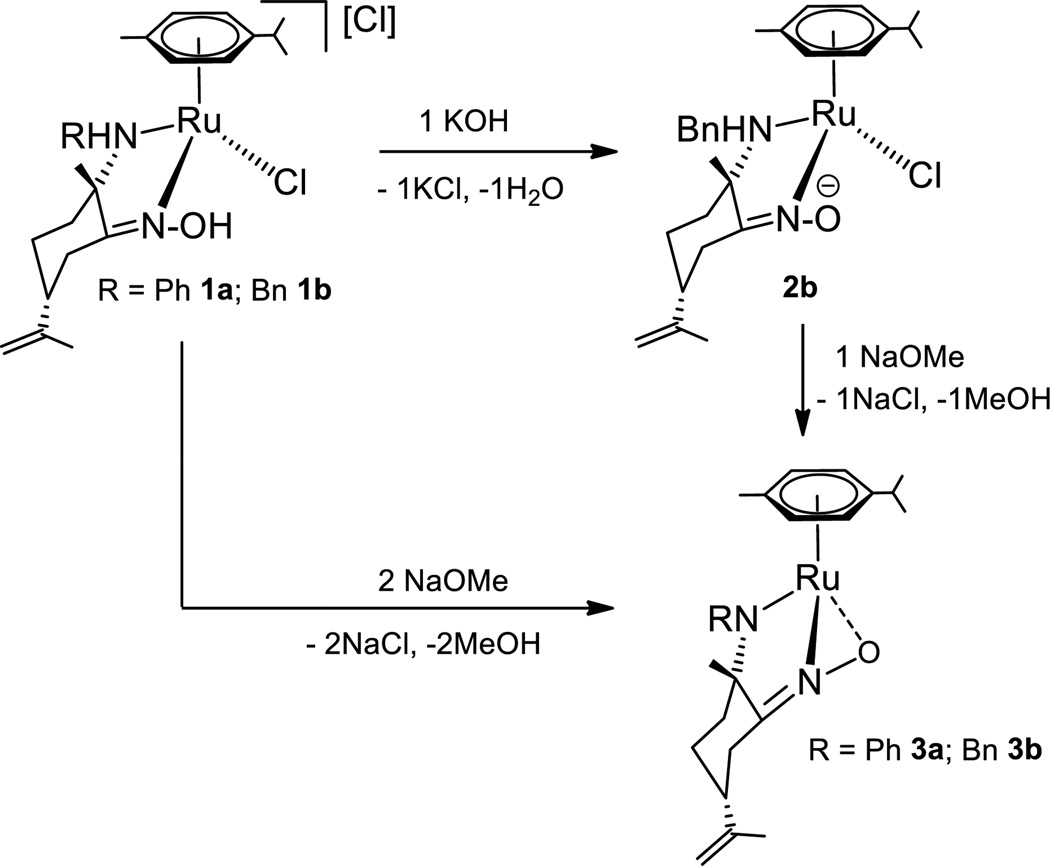
Since we were interested in the role of hydrogen bonds on these self-association processes, we synthesized the corresponding amido-oximate compound, which lacks any traditional HB donors, =NOH and −NH. The synthesis of the neutral oximate derivative [(η6-p-cymene)Ru(kNHBn,kNO)Cl][42] (2b) was already reported by a selective deprotonation reaction of equimolar amounts of 1b and OH (Scheme 3). We performed the reaction of 1b with NaOMe in a stoichiometric ratio of 1:2, which afforded [(η6-p-cymene)Ru(kNBn, k2NO)] (3b), as described in Scheme 3. Treatment of toluene solutions of 2b with NaOMe in a stoichiometric ratio of 1:1 also afforded pure derivative 3b. Compound 3b has been fully characterized by NMR, IR spectroscopy and CHN elemental analysis. The analogous amido derivative [(η6-p-cymene)Ru(k NPh, k2NO)] (3a) can be prepared following a similar procedure (see Supporting Information).
Scheme 3.
Synthesis of amido-oximate ruthenium compounds 3a, 3b
Confirmation of the amine deprotonation was provided by IR and 15N-1H HMBC NMR spectroscopic data. IR spectra show the disappearance of the broad absorption in the 3400-3040 cm−1 region assigned to v(NH/NOH), observed in the corresponding spectra of cationic compounds 1a or 1b (see supporting information). The C=N stretching frequencies (1622, 1590 cm−13a; 1620, 1601 cm−13b) shift to lower wavenumbers when compared to those found in 1a; 1b or 2b, respectively, indicating reduced C=N bond strengths.
Deprotonation of the =NOH oxime function of 1b to afford derivative 2b,[42] shifts the 15N NMR resonances from δ 272.0 (1b) to 291.7 (2b), while keeping the amine nitrogen signal unaltered (δ 50.4 (1b), 50.4 (2b)) (see Supporting Information). In contrast, 15N-1H HMBC NMR spectra of amido-oximate compounds showed nitrogen resonances at δ 329.0 and 263.5 (3a), 328.4 and 256.4 (3b) unambiguously assigned to =NO and −RN-Ru nitrogen atoms, respectively.
The strong downfield chemical shift of the nitrogen resonance confirms structural changes on the amine nitrogen atom of 1a or 1b, and supports the proposed structure for 3a or 3b. The versatility of the oxime groups allows mono kN,kO, or dihapto k2NO coordination of nitrogen and oxygen atoms to the metal centers, depending on the nature of the ligands and the metal.[24,27,49] While X-ray structure of compound 2b[42] and other 18 electron ruthenium oximate derivatives showed a kN coordination of the oxime unit,[31,50] a k2NO coordination is preferred when 16 electron compounds would be formed otherwise[51]
While compounds 1a-C and 2b are highly soluble in water[52] (see Supporting Information and Experimental Section) and air-stable for months, 3a and 3b remains insoluble in protic solvents and slowly decomposes within a two days period in the presence of air. Derivatives 1a-C are stable in D2O for weeks at room temperature. Partial decomposition has only been observed for 1b after heating the D2O solutions above 60 °C.
Self-aggregation Behaviour
It is well known that non-covalent interactions play key roles in biological molecular recognition. Thus, we investigated the tendency of these ruthenium compounds to aggregate, considering that they can undergo several non-covalent interactions. H-bond (HB) acceptors (Cl, N and O atoms), HB donors (amino and oxime groups) and carbon atoms with a partial positive charge (aromatic C-H or aliphatic C-H in α position relative to the C=NOH fragment) are present in 1a-C. Thus, classical hydrogen bonding and C-H·X interactions can be established. In addition, arene fragments can be involved in π-π stacking interactions.
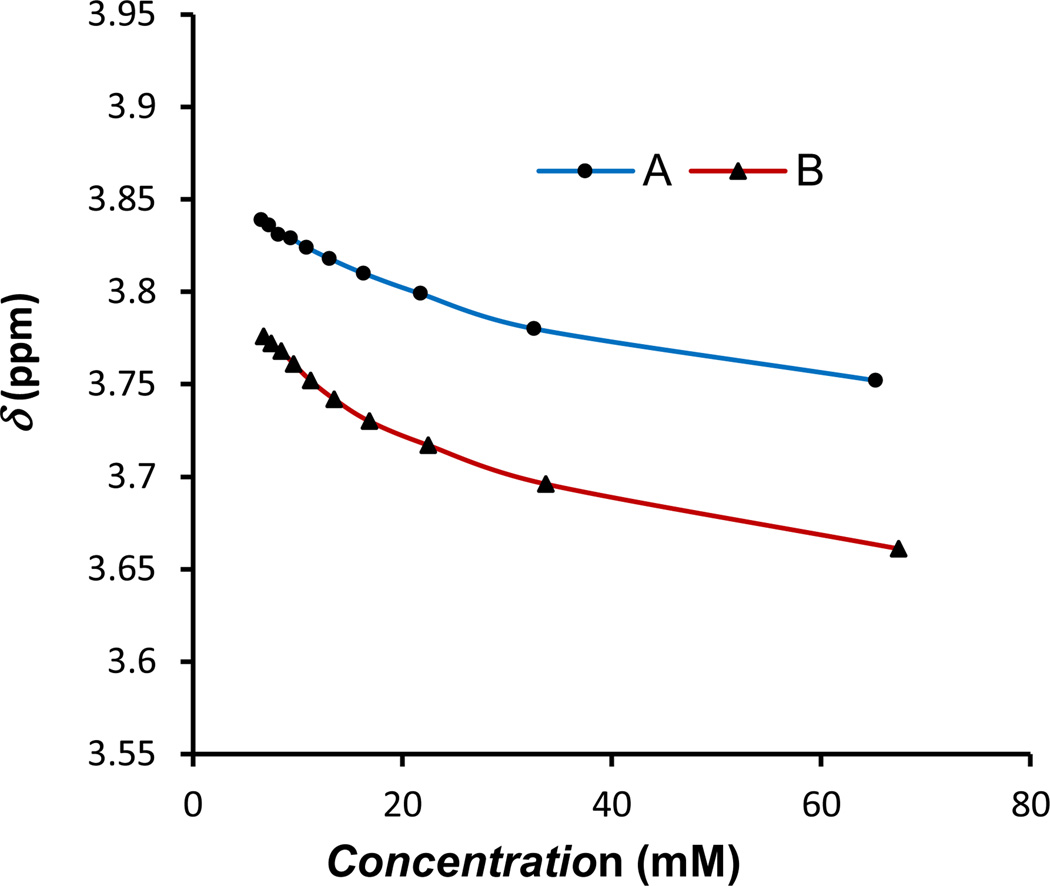
The concentration dependence of 1H NMR spectral changes was studied for compounds 1a and 1b, at 25 °C in CDCl3. Self-association of aromatic frameworks has been studied before by least-squares curve fitting performed on the concentration-dependent 1H NMR resonances, assuming an indefinite self-association or a monomer-dimer model.[48,53]
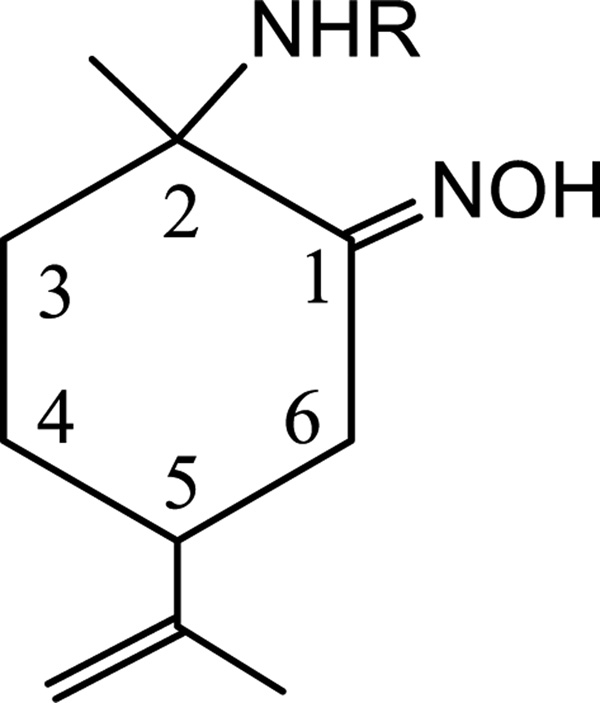
Least squares curve fitting was performed on the concentration dependent 1H NMR of compounds 1a and 1b assuming a dimerization model.[54] Unequivocal 1H NMR assignments of the p-cymene resonances were made on the basis of COSY, HSQC and HMBC NMR experiments (see Supporting Information). The selection of the resonances to be studied was a difficult task due to overlapping of some of the aromatic resonances at certain concentrations and broadening of −NH and =NOH corresponding signals. Thus, association constants (Ka) were determined by the least squares curve fitting of the concentration dependent NMR chemical shifts of -CH26 (Figure 1 and 2). Table 1 summarizes the determined association constants. The complicated set of resonances of the 1H NMR spectrum of 1c precluded the study of the 2-picolylamino-oxime ruthenium compound by this method.
Figure 1.
Numbering of some of the different protons of amino-oxime ligands present in 1a, 1b
Figure 2.
Concentration dependence of 1H NMR chemical shifts for one of the −CH26 protons of A) compound 1a in CDCl3, B) compound 1b in CDCl3.
Table 1.
Dimerization Constants and Thermodynamic Parameters for Self-Aggregation of compounds 1a and 1b at 295 K.
| Compound | Solvent | Ka (M−1) | −ΔG (Kj·mol−1)a |
|---|---|---|---|
| 1a | CDCl3 | 13.27 ± 1.54 | 6.34 |
| 1b | CDCl3 | 29.95 ± 3.78 | 8.34 |
Calculated by means of equation ΔG = −RTlnK.
Association constants calculated from aliphatic −CH26 for compound 1b gave larger Ka than that calculated for compound 1a (Table 1). Values obtained for ΔG are consistent with the energy of hydrogen bonding.[55]
The self-aggregation tendency of cytotoxic RAPTA and half sandwich diamino Ru(II) salts derivatives has been demonstrated by means of diffusion NMR spectroscopy.[56] In order to further investigate the kind and strength of the non-covalent interactions which are responsible for the self-association processes in our compounds, the translational self-diffusion coefficient (Dt) of the species present in solution was determined by using DOSY NMR measurements. The latter allowed the hydrodynamic radius of the diffusing compounds to be evaluated by taking advantage of the Stockes-Einstein equation.[57] To avoid the measurement of T and η, an internal standard can be used. Thus, only the ratio between self-diffusion coefficients of the sample and the reference is considered, Dref/Ds= cs·rHs/cref·rH(ref).[47,58] The numerical factor c differs significantly with the solvent and the size and shape of the molecule. According to the Zuccaccia results,[56.57] the hydrodynamic radius and the numerical factor c of the reference compound tetrakis(trimethylsilyl)silane (TMSS) can be considered constant in a given solvent within the range of the concentrations studied. Thus, any changes in the translational diffusion coefficients ratio, DTMSS/Ds, can be attributed to modifications in the product csrHs of the sample considered, in a given solvent. In order to check the quality of the results, we used two spherical internal standards, namely TMSS and tetrakis(trimethylsilyloxy)silane (TMSO), which are assumed not to aggregate in solution, thus, the ratios between both, DTMSO/DTMSS should be constant in solutions of different concentration.[58] Saturated solutions of the ruthenium compounds set the upper limit of concentration ranges used, for each given solvent. Table 2 summarizes the data obtained from the DOSY NMR experiments.
Table 2.
Diffussion coefficients (D, m2·s−1) for solutions of TMSS (1mM) and TMSO (1mM) at various concentrations (C, mM) of compounds 1a-c, 2b and 3b at 295 K.
| Entry | Compound (solvent) | C | 1010·Ds | DTMSS/Ds | DTMSO/DTMSS | Δrs |
|---|---|---|---|---|---|---|
| 1 | 1a (CDCl3) | 11.7 | 6.957 | 1.626 | 0.951 | 1.00 |
| 2 | 1a (CDCl3) | 63.0 | 5.180 | 2.135 | 0.955 | 1.31 |
| 3 | 1a (CDCl3:C6D6)a | 5.31 | 6.685 | 1.627 | 0.931 | 1.00 |
| 4 | 1a (CDCl3:C6D6)a | 65.5 | 3.676 | 2.889 | 0.937 | 1.77 |
| 5 | 1a (methanol-d4) | 14.5 | 6.966 | 1.385 | 0.939 | 1.00 |
| 6 | 1a (methanol-d4) | 76.5 | 6.280 | 1.475 | 0.935 | 1.06 |
| 7 | 1a (acetone-d6) | 7.75 | 11.67 | 1.584 | 0.904 | 1.00 |
| 8 | 1a (acetone-d6) | 64.9 | 10.02 | 1.704 | 0.913 | 1.07 |
| 9 | 1c (acetone-d6) | 3.25 | 10.59 | 1.852 | 0.903 | 1.00 |
| 10 | 1c (acetone-d6) | 62.1 | 10.42 | 1.882 | 0.903 | 1.02 |
| 11 | 1b (CDCl3) | 6.92 | 7.894 | 1.599 | 0.930 | 1.19 |
| 12 | 1b (CDCl3) | 30.7 | 4.442 | 2.413 | 0.945 | 1.79 |
| 13 | 1b (CDCl3) | 62.5 | 3.362 | 3.147 | 0.931 | 2.34 |
| 14 | 1b·PF6 (CDCl3) | 6.93 | 5.473 | 1.957 | 0.949 | 1.45 |
| 15 | 1b·PF6 (CDCl3) | 52.3 | 4.346 | 2.444 | 0.948 | 1.82 |
| 16 | 2b (CDCl3) | 7.38 | 5.739 | 1.922 | 0.948 | 1.43 |
| 17 | 2b (CDCl3) | 81.2 | 4.830 | 2.284 | 0.931 | 1.70 |
| 18 | 3b (CDCl3) | 5.82 | 8.542 | 1.345 | 0.928 | 1.00 |
| 19 | 3b (CDCl3) | 87.1 | 7.341 | 1.453 | 0.929 | 1.08 |
CDCl3:C6D6 = 8:2
Table 2 shows that the calculated relative standard deviation of DTMSS/Ds values is always less than 3% for each given solvent. Since the numerical factor c differs slightly in different solvents,[57] DTMSS/Ds values are expected to vary in some extension when solutions of different solvents are considered. The different DTMSS/Ds values of 1a and 1b in CDCl3 or CDCl3:C6D6 (8:2) at different concentrations (Table 2, entries 1–4, 11–13) support the conclusion that the compounds self-aggregate in solution.
Although the absolute level of aggregation cannot be inferred without a complete calculation of rs, Δrs (see Experimental Part) provide a simplified method for obtaining an indication of the changes on the hydrodynamic radius of the sample considered with the increasing concentrations, and thus, a confirmation of their self-association behavior in solution.
The data indicate smaller variations of Δrs for 1a than 1b (Table 2, entries 1, 2, 11, 13) which is in accordance with the Ka values calculated before.
The effect of solvent was investigated for complex 1a. Aromatic solvents are known to significantly reduce π-stacking interactions because the solvent molecules effectively solvate the solute.[59] In a similar way, protic solvents decrease hydrogen bond interactions between solute molecules. On the other hand, HB or ion pairs interactions are expected to increase with solvents of low permittivity.[57] Using of CDCl3:C6D6 solutions increases variation of Δrs values at different concentrations of 1a relative to that found in pure CDCl3 (entries 1–4 in Table 2). This is in line with π-stacking interactions not being the main factor of the aggregation process, which is supported, in the solid state, by the molecular X-ray structure of compound 1a published before.[42] Stacking interactions of the aromatic fragments are not observed in the ordered extended structures observed for derivative 1a.
The tendency of 1a to self-associate with increasing concentration decreases as permittivity solvent increases (as can be inferred from the entries 1–8, of Table 2) which agrees with the main aggregation motif being ion pairing and/or hydrogen bonds interactions.
Since compound 1c is only soluble in high permittivity solvents, self-aggregation behavior of 1c could not be assessed in other solvents but acetone-d6. Variation of Δrs values for 1c were similar to that obtained for derivative 1a, (Table 2, entries 7–10) indicating low changes on their aggregation behavior in that solvent. Accurate DOSY NMR of 1a-1c in deuterated water at the concentrations range required could not be obtained. Reference compounds TMSS and TMSO are only slightly soluble in water, and their solutions decompose during the DOSY experiments, precluding accurate measurement of their translational diffusion coefficients. However, methanol-d4 solutions of 1a could model the aggregation behavior in water of the compounds under study.
The effect of the counter-ion was investigated for cationic complex 1b. Substitution of chloride by the less coordinating counter-ion [PF6]− decreases the variation of Δrs with solution concentration (Table 2, entries 11–15 ) while DTMSS/Ds ratios and thus, absolute values of Δrs, are higher for 1b-PF6 (see Supporting Information) than 1b at low concentrations, as it has been already demonstrated for other cationic arene ruthenium derivatives with ion pairing effects.[57]
The effect of HB donors =NOH and −NH on the self-association process was studied with derivatives 1b, 2b and 3b. Since neutral ruthenium compounds 2b and 3b only differ from cation 1b in one and two hydrogen atoms, respectively, we have assumed that the hydrodynamic radius of the organometallic fragment should be similar for the three compounds, and calculated Δrs are referred to the compound which affords the lowest DTMSS/Ds values, namely compound 3b in a 5.82 mM CDCl3 solution.
Aggregation is less affected by increasing concentrations for compound 2b than 1b, but DTMSS/Ds ratios and thus, absolute values of Δrs, are higher for 2b than 1b at low concentrations, as it can be deduced from DOSY data summarized in Table 2 (entries 11, 13, 16, 17). Almost no variation on Δrs was found for compound 3b at different concentrations (Table 2, entries 18, 19). That fact agrees well with the hydrogen bond interactions being a key factor of self-association, since derivative 2b, for which no ion pairing contribution can exist, possess stronger HB acceptors (=NO−) than 1b, while 3b lacks of traditional HB donors. The molecular X-ray structure of compound 2b published before[42] shows intermolecular contacts between the oxygen of the oximate unit and one of the hydrogen atoms of the CH3-CNH of an adjacent molecule, leading to the formation of dimers in the solid state.
Hydrogen bonding has been found to be an important factor in some ion-pair formation of cationic ruthenium compounds.[57,60] A similar behavior might be taking place in compounds 1a,b, since NMR data allow us to confirm that both interactions are present in solution.
Lipophilicity
Lipophilicity/hydrophobicity is one of the most important physicochemical properties related to the pharmacokinetic behavior of drug-like molecules.[7,61,62] The partition coefficient between water and n-octanol (logP) is one of the most commonly used parameters, affording relevant information about the hydrophobicity of compounds. In order to correlate the hydrogen-bonding ability of the compounds and their hydrophilicity, we decided to determine the n-octanol/water partition coefficient of derivatives 1a and 1b using the shake-flask method[63] at room temperature. The estimated values (logPo/w = 0.40 ± 0.01 (1a) and −0.14 ± 0.05 (1b)) indicates that 1b is more hydrophilic than 1a. This fact agrees with the water solubility of the ruthenium compounds, which follows the order 1a < 1b (ca. 21, 28 mM in water, respectively) and correlates with the stronger association capability of 1b relative to 1a demonstrated by concentration dependence 1H NMR chemical shifts and DOSY experiments.
NMR Studies under physiological conditions
To elucidate the solution behavior of the compounds under physiological relevant conditions, time-dependent 1H NMR experiments were conducted with 1a-1c at pH 7.4 in a phosphate buffer saline solution in D2O.[64] All three compounds proved to be stable under these conditions. Thus, no apparent changes in the NMR spectra were observed during a measurement time of 24, 48 and 72 hours at room temperature or 36 °C (see Supporting Information).
In vitro cell studies
Prostate cancer (PCa) is the most common non-skin cancer among men in developed countries and the second leading cause of cancer death.[65] Although early PCa is generally treatable, most advanced cases eventually progress to a stage characterized by resistance to drugs such as cisplatin and by androgen independence, which jointly contribute to the lack of treatment response and the high mortality rates among patients with advanced PCa.[66] In this regard, bone metastases, with an incidence of 80–90 % in patients at advanced stages of the disease, are the most common cause of death.[67] The in vitro effect of the arene ruthenium complexes 1a-1c on cytotoxicity, cell adhesion and migration of the androgen-independent prostate cancer PC3 cells was assessed by MTT, adhesion to type-I-collagen and recovery of monolayer wounds assays, respectively.
Cytotoxicity activity
The cytotoxic activity of the highly water soluble 1b, b·HCl and [(η6-p-cymene)RuCl2]2 were evaluated as the IC50 values after 72 h of incubation (Table 3).
Table 3.
IC50 (µM) ± S.E.M.a for compounds 1a-1c, b·HCl and [(η6-p-cymene)RuCl2]2 in the PC3 cells.
| Complex | IC50 (µM) ± S.E.M.a |
|---|---|
| 1a | 24.4 ± 0.75 (3 h) |
| 1b | 14.8 ± 0.40 (3 h) |
| 8.70 ± 1.50 (24 h) | |
| 9.40 ± 4.50 (72 h) | |
| 1c | 21.5 ± 0.80 (3 h) |
| b·HCl | 177 ± 5.50 (72 h) |
| [(η6-p-cymene)RuCl2]2 | 213 ± 6.90 (72 h) |
Each value represents the mean ± S.E.M. of three independent experiments.
While starting organometallic and proligand products are poorly cytotoxic (IC50 > 170 µM), the water soluble ruthenium amino-oxime compound shows considerable higher cytotoxicity than cisplatin (51 ± 0.10 µM, under the same experimental conditions[68,69]).
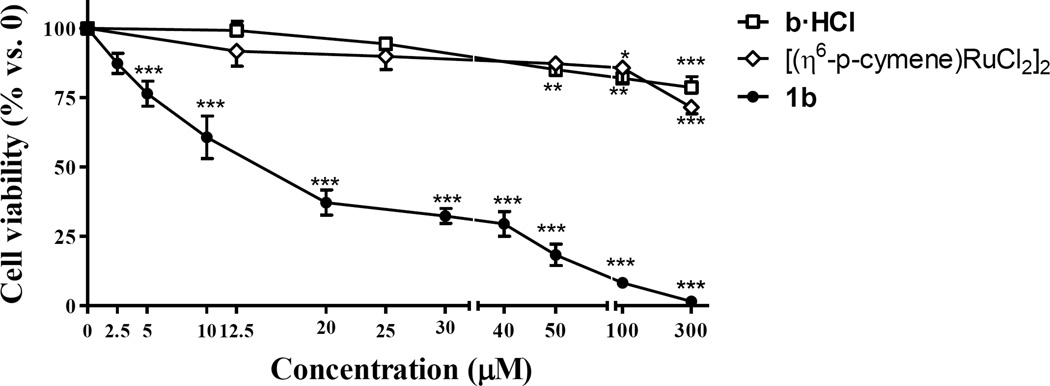
Antiproliferative effect of derivative 1b was also assessed at different exposure times (3 h and 24 h), revealing a high cytotoxic activity against PC3 cells after 3h of incubation (IC50 = 14.8 ± 0.40, Table 3). Treatment with ruthenium compounds 1a, 1b and 1c for 3 h significantly decreased cell viability in PC3 cells as compared with control conditions, showing an inhibitory activity of 75–82% at concentrations of 50 µM, with calculated IC50 values in the low micromolar concentration range (Figure 3, Table 3). In order to compare the effect on cell viability of the starting material [(η6-p-cymene)RuCl2]2 and of the ligand precursors, with that of organometallic compounds 1a- 1c, the ruthenium dimer and water soluble organic ammonium-oximes were also studied under the same experimental conditions (Figure 3 and supporting information). They showed a decrease of only 13–18% on PC3 cell viability at the same concentrations (50 µM) which confirms that coordination of amino-oxime derivatives to ruthenium lead to compounds with significantly enhanced activity.
Figure 3.
Effect of derivative 1b on PC3 cells viability, compared to that of ammonium-oxime compound b·HCl and ruthenium dimer [(η6-p-cymene)RuCl2]2. Cells were treated with increasing doses of organic and ruthenium compounds for 3 hours. Cell viability was measured by means of MTT assay. The results are expressed as a percentage of live cells compared to control. Data are the mean ± S.E.M. of at least three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus Control.
Some other ruthenium compounds have proved their antitumor activity against PC3 cells line, known for their chemoresistance.[62,69,70] To the best of our knowledge, the most successful ruthenium derivative tested in vitro is the cyclopentadienyl cationic derivative [(η5-C5H5)Ru(bipy)(PPh3)][CF3SO3] (TM34,[69] bipy = bipyridine), with IC50 values of 0.54 ± 0.10 µM after 72 hours of incubation, almost 100-folds more cytotoxic against PC3 when compared to cisplatin.[68,69] The water-soluble version of the compound, [(η5-C5H5)Ru(bipy)(mTPPMSNa)][CF3SO3] (TM85, bipy = bipyridine, mTPPMS = diphenylphosphane-benzene-3-sulfonate) was evaluated in a variety of cell lines. The IC50 value found for the PC3 cell line was 25.8 ± 8.5 µM after a 72 h exposure.[62]
Adhesion to collagen
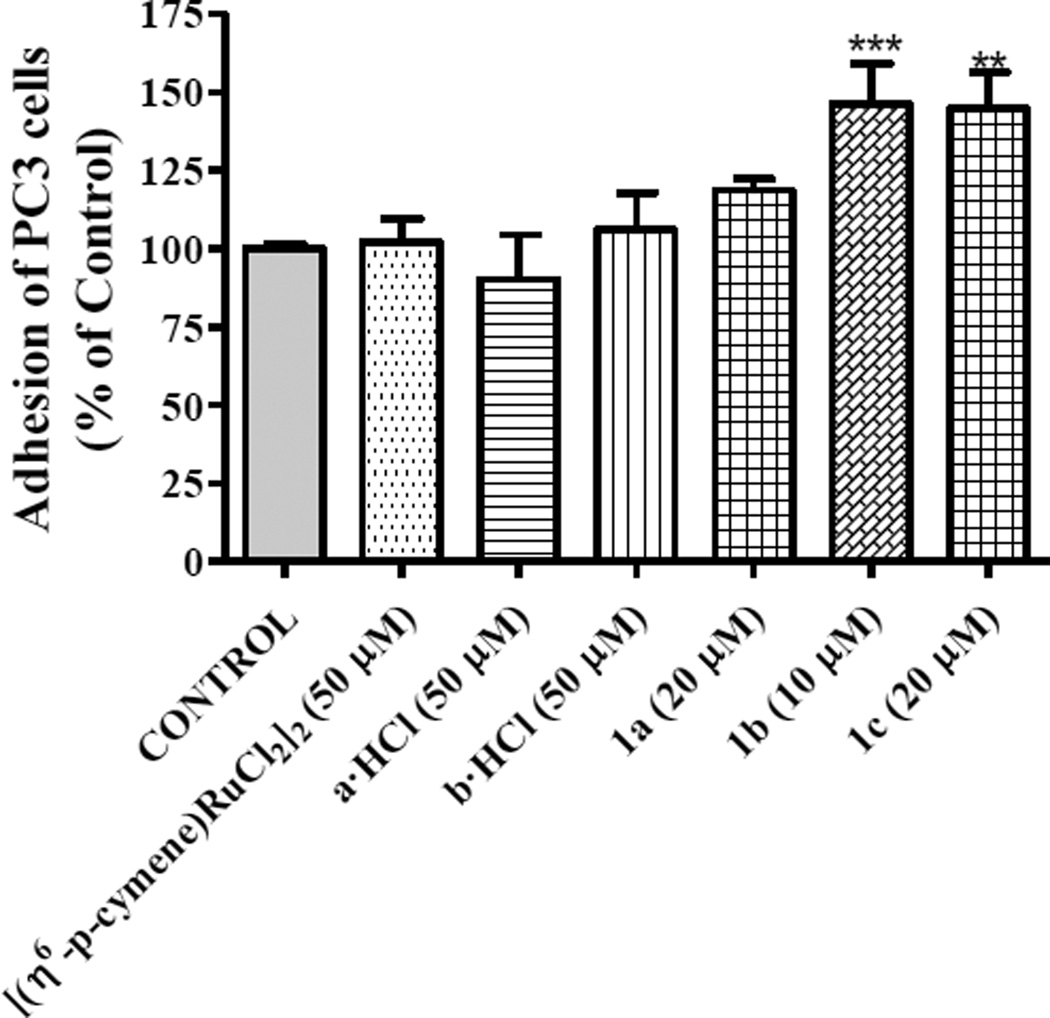
We performed experiments on cell adhesion to type-I collagen in order to determine the effect of 1a, 1b and 1c on metastatic capability of androgen-independent prostate cancer cells under conditions which did not cause cell death. To investigate the cell adhesion in vitro, we incubated PC3 cells in the absence or presence of 20 µM of 1a and 1c and 10 µM of 1b on a collagen plate. PC3 cells rapidly adhered to collagen basement in 40 min and showed a significant increase of the cell adhesion to collagen after treatment with 1a (18 %), 1b (46 %) and 1c (45 %) compared to that observed for control cells. The arene ruthenium dimer and ammonium-oxime derivatives-treated cells exhibited an adhesion pattern similar to that of control cells (Figure 4).
Figure 4.
Effect of 1a-1c complexes on adhesion of PC3 cells to type-I collagen was studied after treatment with organic and organometallic compounds for 40 min. Data are the mean ± S.E.M. of at least three experiments. **, P < 0.01; ***, P < 0.001 versus Control.
Wound-healing assay
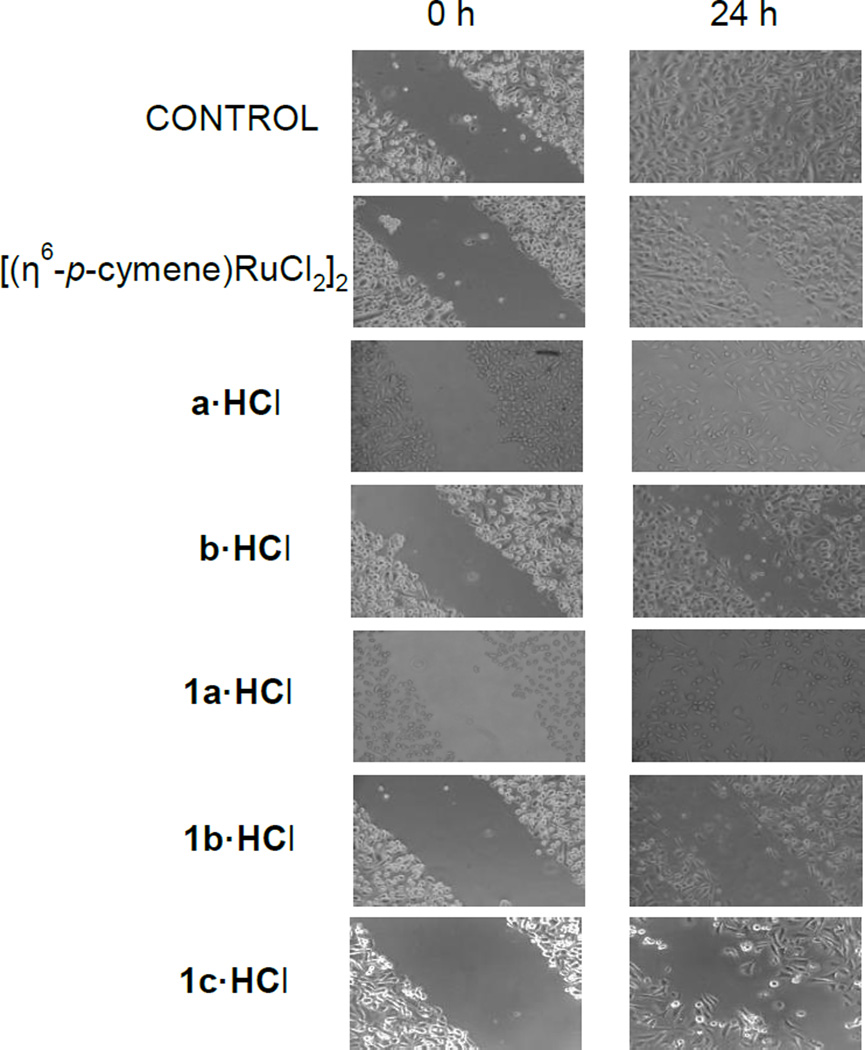
The wound-healing assay is a useful method for gauging the anti-migratory activity of drug candidates.[71] In order to evaluate cell migration we performed wound-healing assays, in which a small wound area was made on the plate with a confluent monolayer of cells (Figure 5). After 24 h, the cells treated with the ruthenium complexes 1a, 1b and 1c (5 µM) showed a lower migration capability (60% of wound healing) than that of control cells. The ruthenium dimer and ammonium-oxime treated cells showed a migration pattern similar to that of control cells (5 % of wound healing). Our results confirmed the inhibitory effect of compounds 1a-1c on prostate tumor cell migration. In this regard, the treatment of human umbilical vein endothelial cells with organometallic ruthenium(II) compounds RAPTA-C, DAPTA-C and DAPTA-T for 6 h reveals a similar migratory capability using a concentration ten times higher than that used in our study.[16]
Figure 5.
The effect of organic and organometallic compounds on cell migration was studied in human prostate cancer PC3 cell line. Microscopic analysis of the cell-free area was carried out at the indicated time (24 h) after the addition of the neuropeptide and the width of the area invaded by prostate cells was estimated.
The amino-oxime Ru(II) complexes seem to modulate both adhesion and migratory capabilities of PC3 cells affecting metastatic phenotype. It is worth noting that compound 1b, which has demonstrated higher self-aggregation than 1a in solution, showed also the best anti-cancer activity.
Reactivity with biomolecules
Interactions with plasmid DNA
Since DNA replication is a key event for cell division, it is among critically important targets in cancer chemotherapy. Most cytotoxic platinum drugs form strong covalent bonds with the DNA bases.[72] However, a variety of platinum compounds act as DNA intercalators upon coordination to the appropriate ancillary ligands.[73] The more thoroughly studied ruthenium antitumor agents have displayed differences with respect to their interactions with DNA depending on their structure.[5] Thus, while NAMI-A is known to have fewer and weaker interactions with DNA than cisplatin,[5] KP1019 undergoes interactions similar to cisplatin but with a lower intensity in terms of DNA-DNA and DNA-protein crosslinks.[74] Organometallic piano-stool ruthenium(II) compounds based on bicyclic arenes RM175 interact strongly with DNA binding to guanines and by intercalation.[21,75] Organometallic ruthenium(II) RAPTA derivatives, characterized by the presence of water soluble PTA phosphine, exhibit pH-dependent DNA damage: at the pH typical of hypoxic tumor cells DNA was damaged, whereas at the pH characteristic of healthy cells little or no damage was detected.[76] It is also known that some metal compounds containing oxime ligands cause oxidative DNA cleavage,[77] and an increase in the targeting of specific nuclear bases of DNA[78]
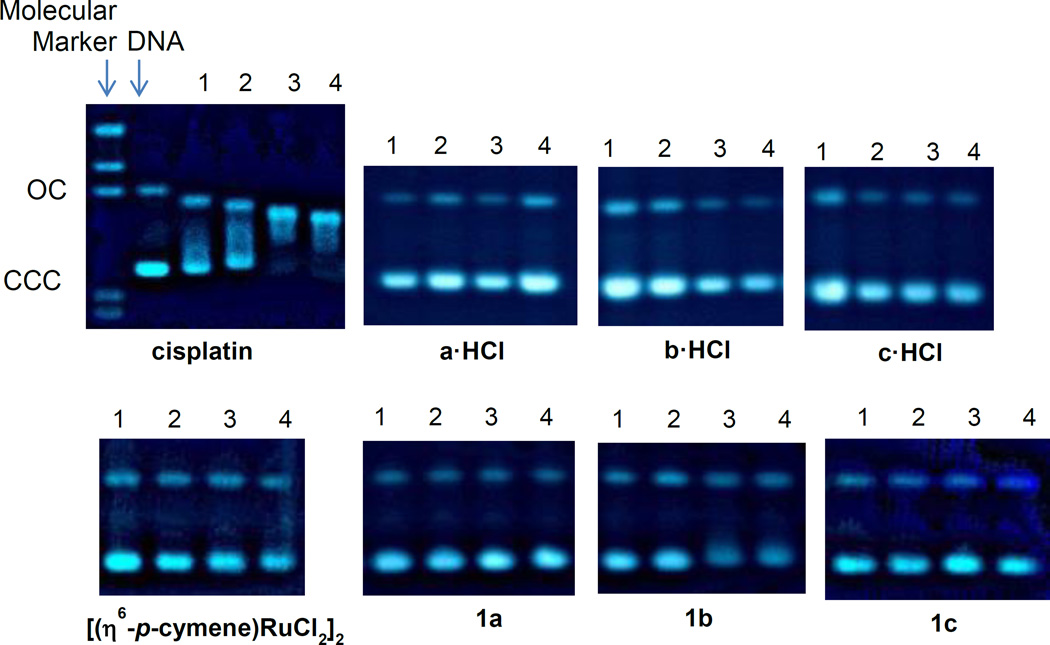
In this context, we performed agarose gel electrophoresis studies to unravel the effects of the water soluble ammonium-oxime ligands a·HCl-c·HCl and ruthenium complexes 1a-1c on plasmid (pBR322) DNA (Figure 6). Cisplatin and the starting dimeric organometallic ruthenium complex [(η6-p-cymene)RuCl2]2 were also measured as controls.
Figure 6.
Electrophoresis mobility shift assays for cisplatin, [(η6-p-cymene)RuCl2]2, derivatives a·HCl-c·HCl and compounds 1a-1c (see Experimental for details). DNA refers to untreated plasmid pBR322. 1, 2, 3 and 4 correspond to metal/DNAbp ratios of 0.25, 0.5, 1.0 and 2.0 respectively.
Plasmid (pBR322) DNA has two main forms: OC (open circular or relaxed form, Form II) and CCC (covalently closed or supercoiled form, Form I). Changes in electrophoretic mobility of both forms are usually taken as evidence of metal-DNA binding. Generally, the larger the retardation of supercoiled DNA (CCC, Form I), the greater the DNA unwinding produced by the drug.[79] Binding of cisplatin to plasmid DNA, for instance, results in a decrease in mobility of the CCC form and an increase in mobility of the OC form (see lanes 1–4 for cisplatin in Figure 6).
Treatment with increasing amounts of derivatives a·HCl-c·HCl and ruthenium compounds 1a-1c do not affect the mobility of the faster-running supercoiled form (Form I) even at the highest molar ratios (lane 4, Figure 6). In conclusion, the experiments probing DNA-drug interactions showed that the biologically active ruthenium complexes have no or very little interaction with plasmid (pBR322) DNA, pointing to an alternative biomolecular target for these compounds.
This is also in accordance with previous reports on other coordination Ru(II) complexes[30] and organometallic Rh(III) and Os(III)[29] derivatives containing oxime groups which showed no or weak interaction with DNA by different techniques.
In addition, to evaluate the possible interaction of the new ruthenium complexes 1a-1c with DNA some Thermal Denaturation experiments were carried out. The melting technique is a sensitive and easy tool to detect even slight DNA conformational changes. It is known that a destabilizing interaction with the double helix (typically, covalent) produces a decrease in the Tm, while a stabilizing interaction (usually by intercalation or by electrostatic attraction) induces an increase of the Tm. Bearing that in mind, Calf Thymus DNA was incubated for 1 hour with each drug at a DNA:drug ratio of 2:1. The results are summarized in Table 4.
Table 4.
Changes in the Tm of CT DNA after incubation with complexes 1a, 1b, and 1c for 1h in 5mM tris/NaClO4 buffer at pH 7.39 and r = 0.5.
| Complex | ΔT (Tm DNA/Complex-Tm DNA) °C |
|---|---|
| 1a | + 0.4 |
| 1ba | --- |
| 1c | + 1.0 |
Compound 1b did not afford a single Tm value most plausibly due to its decomposition while heating it in the buffer solution containing DNA.
Complex 1a was not able to modify the melting temperature of a solution of calf thymus DNA beyond the experimental error while the modification of the temperature with 1c (1 °C) indicates a very weak interaction with double-stranded DNA, most likely of electrostatic nature.[80] This fact suggests that these compounds do not interact with DNA or that interaction is so weak that cannot be detected by this technique, supporting the results obtained in the study of the interaction of these compounds with plasmid (pBR322) DNA. In summary, it seems that the cytotoxic effects of the ruthenium compounds 1a-1c are not solely related to DNA damage which suggests that an alternative cellular death pathway may be taking place.
Interactions with HSA
Human serum albumin (HSA) is the most abundant carrier protein in plasma and is able to bind a variety of substrates including metal cations, hormones and most therapeutic drugs. It has been demonstrated that the distribution, the free concentration and the metabolism of various drugs can be significantly altered as a result of their binding to the protein.[74]
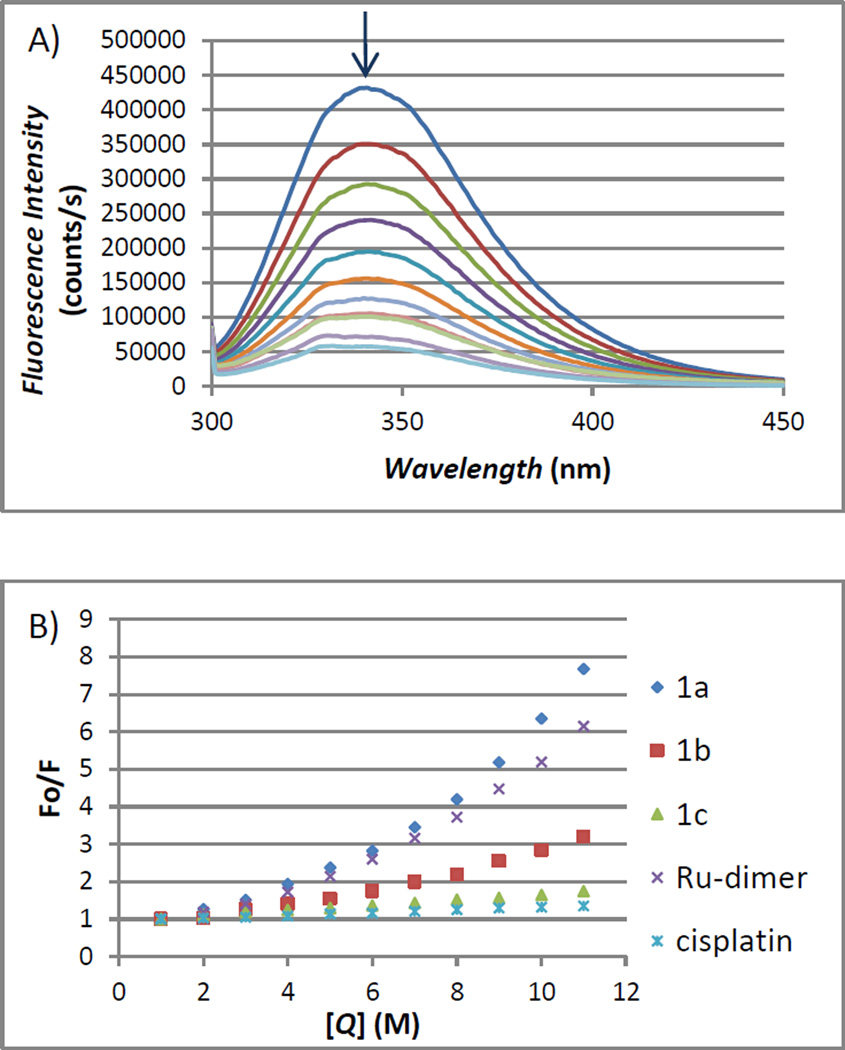
HSA possesses three fluorophores, these being tryptophan (Trp), tyrosine (Tyr) and phenylalanine (Phe) residues, with Trp214 being the major contributor to the intrinsic fluorescence of HSA. This Trp fluorescence is sensitive to the environment and binding of substrates, as well as changes in conformation that can result in quenching (either dynamic or static). Thus, the fluorescence spectra of HSA in the presence of increasing amounts of compound 1a-1c, the ruthenium starting material [(η6-p-cymene)RuCl2]2 and cisplatin were recorded in the range of 300–450 nm upon excitation of the tryptophan residue at 295 nm. All the compounds caused a concentration dependent quenching of fluorescence without changing the emission maximum or shape of the peaks as seen in Figure 7(A) for compound 1a.
Figure 7.
(A) Fluorescence titration curve of HSA with compound 1a. Arrow indicates the increase of quencher concentration (10–100 µM). (B) Stern-Volmer plot for HSA fluorescence quenching observed with compounds 1a-1c, [(η6-p-cymene)RuCl2]2 and cisplatin.
The quenching was more significant for the dimer [(η6-p-cymene)RuCl2]2 and Ru(II) complexes 1a-1c than for cisplatin under the chosen conditions. This is most likely due to a faster reactivity of the organometallic ruthenium compounds with HSA compared to cisplatin.
The fluorescence data was analyzed by the Stern-Volmer equation. While a linear Stern-Volmer plot is indicative of a single quenching mechanism, either dynamic or static, the positive deviation observed in the plots of F0/F versus [Q] of 1a or 1b (Figure 7(B)) suggests the presence of different binding sites in the protein.[81] A similar behavior was observed for iminophosphorane complexes of Ru(II),[6] Pd(II) or Pt(II)[82] reported by some of us. In the case of [MCl2(TPA=N-C(O)-2-NC5H4)] (M = Pd, Pt), isothermal titration calorimetry (ITC)[82] showed two different binding interactions which explained the lack of linearity observed in the fluorescence quenching studies, as the Stern-Volmer method assumes all binding sites to be equivalent.
We believe that a similar reactivity takes place for the compounds described here. In contrast, the Stern-Volmer plot for complex 1c shows a linear relationship, suggesting the existence of a single quenching mechanism and a single binding affinity. The Stern-Volmer constant for 1c is 1.17×106 M−1.
Conclusions
The self-association tendency of the compounds 1a-1c has been studied by means of concentration dependence and DOSY 1H NMR spectroscopy experiments. Hydrogen bond interactions seem to be a key factor in the observed process in solution. Furthermore, our study has shown that the use of amino-oxime ligands is a useful strategy to design water-soluble metal compounds. The oxime-containing Ru(II) derivatives did not display an interaction with plasmid (pBR322) DNA and their interaction with calf-thymus DNA seems very weak or nonexistent. The compounds 1a and 1b interact with HSA faster than cisplatin and most plausibly through more than one binding site. This different behavior along with very promising in vitro results on cytotoxicity, cell adhesion and migration of the androgen-independent cisplatin-resistant prostate cancer PC3 cell line makes these compounds attractive candidates for further testing as potential prostate chemotherapeutic agents.
Experimental Section
Synthesis of metal complexes 1a-C and 2b were performed without exclusion of moisture or air. Manipulations involving synthesis of metal complexes 3a and 3b were performed at an argon/vacuum manifold using standard Schlenk techniques or in a glove-box MBraun MOD System. Solvents were dried by known procedures and used freshly distilled. (2S,5R)-[NHR,NOH], (R = Ph a, Bn b, 2-pic c), corresponding adducts (2S,5R)-[NHRHCl,NOH], (R = Ph a·HCl, Bn b·HCl, 2-pic c·HCl), [(η6-p-cymene)Ru(kNHR,kNOH)Cl]Cl (R = Ph 1a, Bn, 1b) and [(η6-p-cymene)Ru(kNHBn,kNO)Cl] 2b were prepared according to previous reports.[41,42] Tetrakis(trimethylsilyl)silane (TMSS), tetrakis(trimethylsilyloxy)silane (TMSO), sodium methoxide (NaOMe), [(η6-p-cymene)RuCl2]2, cisplatin, KCl, NaCl, Na2HPO4, KH2PO4 and DCl solution in D2O were purchased from Sigma-Aldrich. Commercially available reagents were used without further purification. NMR spectra were recorded on a Bruker 400 Ultrashield. 1H and 13C chemical shifts are reported relative to tetramethylsilane. 15N chemical shifts are reported relative to liquid ammonia (25 °C). Coupling constants J are given in Hertz. Elemental analyses were performed in our laboratories (UAH) on a Perkin Elmer 2400 CHNS/O Analyzer, Series II. IR spectra were recorded on IR FT Perkin Elmer (Spectrum 2000) spectrophotometer on KBr pellets; only significant bands are cited in the text. pH was measured in a HANNA HI208 pHmeter in distilled water solutions.
NMR experiments
Dilutions experiments were carried out from an initial 500 µL stock solution. This initial stock solution was diluted with CDCl3 (100 µL), and the dilution process sequentially repeated for the next 9 samples. OriginLab Software was used for least-squares curve fitting to the theoretical equation of the dimerization model (Supporting Information). DOSY experiments were acquired in a Bruker Ultra Shield 400 spectrometer, using the ledbpgp2s pulse program. The gradient strength (g) was the variable parameter, while Δ (diffusion time) and δ (diffusion gradient length) were kept constant during the 2D-DOSY study. Appropriate Δ and δ values were selected for each sample by optimization of the attenuation of the 1H NMR signals in 1D-versions of the diffusing ledbpgp1s pulse program. The values of Δ and δ were 40–100 ms and 1.5–2.5 ms, respectively; depending on the sample and the solution concentration (eddy current delay was set to 5 ms in all the experiments). The pulse gradients (g) were incremented from 2 to 95% of the maximum gradient strength in a linear ramp. The diffusion dimension was processed with Bruker topspin T1/T2 software. Δrs values were calculated according to the equation 1, considering Ci and C0 the highest and lowest concentration studied for each compound in a given solvent, respectively, and assuming that the product CTMSS·rTMSS is not concentration-dependent.
| (1) |
Synthesis of [(η6-p-cymene)Ru{k2NH(2-pic),kNOH}][PF6]2 (1c)
A THF (10 mL) solution of (2S,5R)-{NH(2-pic),NOH} (0.27 g, 0.98 mmol) and [(η6-p-cymene)RuCl2]2 (0.30 g, 0.49 mmol) was stirred for 30 minutes at room temperature and then treated with TlPF6 (0.68 g, 1.96 mmol). After stirring of the mixture for two hours, the brown suspension was filtered to afford a brown solution. Evaporation of the solvent affords a brown-deep solid. The solid was purified by column chromatography. Elution with acetone allowed isolation of derivative 1c (yield 0.25 g, 63 %). NMR data confirmed the presence of two different isomers, 1c-major and 1c-minor in a 2:1 ratio. Solubility in H2O at 24 °C (mM): 9.0 ± 1. Value of pH ([9.0 mM]) in H2O at 24 °C: 4.00. C26H37N3ORuP2F12 (798,56): calcd. C 39.10, H 4.67, N 5.26; found: C 38.81, H 4.58, N 5.11. IR (KBr): ν = 3642-3500, 3290-3100 (NH/NOH), 1649, 1614 (C=N) cm−1. 19F NMR (376.5 MHz, 293 K, acetone-d6): δ = −72.2 (d, JP-F = 708, PF6) ppm. 31P NMR (161.9 MHz, 293 K, acetone-d6): δ = −144.2 (spt, JP-F = 708, PF6) ppm. Since many of the 1H NMR resonances were overlapped, full assignment was only possible with bidimensional 1H-13C experiments (see Supporting Information for details). 1c-major: 1H NMR (plus HSQC, plus HMBC, 400.1 MHz, 293 K, acetone-d6): δ = 11.76 (br, overlapped, NOH), 9.45, 8.21, 7.90, 7.75 (all m, overlapped, C5H4N), 7.76 (overlapped, NH), 6.49, 6.33, 6.26, 6.19 (all m, overlapped, p-cymene-C6H4), 5.06, 5.16 (both s, each 1H, =CH2), 5.16 (d, JHH = 17, 1H, pic-CH2), 4.76 (dd, JHH = 5, JHH = 17, 1H, pic-CH2), 3.61, 2.37, 2.24, 2.15, 2.13, 1.97 (all m, overlapped, -CH23,4,6), 2.77 (m, 1H, −CH-C=), 2.75 (spt, JHH = 6, overlapped, p-cymene-CHMe2), 2.19, 1.85, 1.43 (all s, p-cymene-CH3 + picNC-CH3 + CH3C=), 1.16, 1.09 (both d, JHH = 6, overlapped, p-cymene-CH(CH3)2) ppm. 13C- NMR (plus APT, plus gHSQC, plus HMBC, 100.6 MHz, 293 K, acetone-d6): δ = 179.4 (−, Cq=N), 162.0 (−, Cipso-C5H4N), 156.8, 142.9, 128.1, 124.5 (+, −NC5H4), 147.2 (−, =Cq-Me), 113.6 (−, =CH2), 110.4, 105.5 (both −, Cipso-p-cymene), 89.0, 88.2, 87.6, 86.6 (+, −C6H4), 73.6 (−, Cq-NH), 61.2 (−, pic-CH2), 43.2 (+, −CH5), 38.9, 25.3, 29.6 (all −, − CH23,4,6), 32.2 (+, p-cymene-CHMe2), 23.2, 21.9, 19.4 (all +, CH3-CNH + CH3-C= + CH3-C6H4), 23.4, 22.9 (+, p-cymene-CH(CH3)2) ppm. 15N NMR (gHMBC, 40.5 MHz, 293 K, acetone-d6): δ = 258.8 (C=N), 232.7 (NC5H4), 29.9 (NH) ppm. 1c-minor: 1H NMR (plus HSQC, plus HMBC, 400.1 MHz, 293 K, acetone-d6): δ = 11.76 (br, NOH), 9.45, 8.21, 7.90, 7.75 (all m, overlapped, C5H4N), 7.76 (overlapped, NH), 6.53, 6.41, 6.41, 6.33 (all m, overlapped, p-cymene-C6H4), 3.98, 3.32 (both s, each 1 H, =CH2), 5.02 (d, JHH = 17, 1H, pic-CH2), 4.59 (dd, JHH = 5, JHH = 17, 1H, pic-CH2), 3.37, 2.57, 2.15, 1.97, 1.97, 1.87 (all m, overlapped, −CH23,4,6 ), 2.82 (spt, JHH = 7, overlapped, p-cymene-CHMe2), 2.51 (m, 1H, −CH-C=), 2.26, 1.77, 1.48 (all s, each 3H, p-cymene-CH3 + picNC-CH3 + CH3C=), 1.23, 1.16 (both d, JHH = 7, overlapped, p-cymene-CH(CH3)2) ppm. 13C- NMR (plus APT, plus gHSQC, plus HMBC, 100.6 MHz, 293 K, acetone-d6): δ = 175.4 (−, Cq=N), 161.1 (−, Cipso-C5H4N), 156.4, 142.9, 127.9, 125.3 (+, −NC5H4), 146.0 (−, =Cq-Me), 111.2 (−, =CH2), 110.7, 104.6 (both −, Cipso-p-cymene), 89.1, 88.7, 87.9, 86.7 (+, −C6H4), 71.6 (−, Cq-NH), 59.4 (−, pic-CH2), 40.3 (+, −CH5), 32.1, 29.5, 26.8 (all −, −CH23,4,6), 32.9 (+, p-cymene-CHMe2), 27.5, 21.7, 19.1 (all +, CH3-CNH + CH3-C= + CH3-C6H4), 24.5, 22.3 (+, p-cymene-CH(CH3)2) ppm. 15N NMR (gHMBC, 40.5 MHz, 293 K, acetone-d6): δ = 265.2 (C=N), 236.4 (NC5H4), 28.9 (NH) ppm.
Synthesis of [(η6-p-cymene)Ru(kNBn,k2NO)] (3b)
A THF solution of 1b (0.27 g, 0.47 mmol) was treated with NaOMe (0.05 g, 0.98 mmol) at room temperature. After stirring of the mixture during 2 hours, evaporation of the THF and extraction from the solid residue with toluene affords an orange solution from which an orange solid was isolated, washed with hexane (5×2 mL) and fully characterized as derivative 3b (yield 0.22 g, 92 %). Alternatively, a toluene solution of 2b (0.20 g, 0.37 mmol) was treated with NaOMe (0.02 g, 0.37 mmol) at room temperature. After stirring of the mixture during 2 hours, the resulting suspension was filtered and the solvent evaporated to dryness to afford an orange solid which was identified as derivative 3b. C27H36N2ORu (505.66): calcd. C 64.13, H 7.18, N 5.54; found C 63.81, H 6.87, N 5.09. IR (KBr): ν = 1620, 1600 (νC=N) cm−1. 1H NMR (plus COSY, plus HSQC, 400.1 MHz, 293 K, CDCl3): δ = 7.37 (overlapped, 5H, −C6H5), 5.20 (d, 1H, 3JHH = 6, p-cymene-C6H4), 5.09 (br, 1H, =CH2), 4.95, 4.84 (both d, each 1H, 3JHH = 6, p-cymene-C6H4), 4.72 (br, 1H, =CH2), 4.66 (d, 1H, 3JHH = 6, p-cymene-C6H4), 4.47 (m, 2H, −CH2Ph), 3.87 (d, 1H, 2JHH = 14, −CH26), 2.57 (spt, 1H, 3JHH = 6, p-cymene-CHMe2), 2.40 (br, 1H, −CH5), 2.08 (s, 3H, p-cymene-CH3), 1.85 (dd, 1H, 2JHH = 16, 3JHH= 6, −CH26), 1.79 (m, 1H, −CH23), 1.64 (m, 1H, −CH24), 1.60 (s, 3H, CH3-C=), 1.55 (m, 1H, −CH24), 1.24, 1.22 (both d, each 3H, 3JHH = 6, p-cymene-CH(CH3)2), 1.10 (overlapped, 4H, NC-CH3 + −CH23) ppm. 13C-NMR (plus APT, plus gHSQC, 100.6 MHz, 293 K, CDCl3): δ = 163.4 (−, C=NO), 144.9, 144.2 (both −, C=CH2 + Cipso-Bn), 128.6, 128.5, 127.0 (all +, −C6H5o,m,p), 112.2 (−, =CH2), 98.9, 88.8 (both −, Cipso-p-cymene), 84.6, 84.0, 83.5, 80.7 (+, p-cymene-C6H4), 80.4 (−, C-NBn), 64.0 (−, CH2Ph), 42.1 (+, −CH5), 32.7 (−, −CH23), 31.8 (+, p-cymene-CHMe2), 26.5 (−, −CH24), 25.3 (−CH26), 24.1, 24.1 (both +, p-cymene-CH(CH3)2), 23.4 (+, −CH3-CNBn), 22.8 (+, CH3-C=), 19.8 (+, p-cymene-CH3) ppm. 15N NMR (gHMBC, 40.5 MHz, 293 K, CDCl3): δ = 328.4 (C=NO), 256.4 (Ru-NBn) ppm.
Biological assays
n-octanol-water partition coefficients
The n-octanol-water partition coefficient was measured using the shake-flask method.[63] Distilled water and n-octanol were stirred together for 72 h at 25 °C, to promote saturation of both phases. The solvents were separated and freshly used. Aliquots of stock solutions (1.5mM) of 1a and 1b in the n-octanol saturated aqueous phase were added to equal volumes of water saturated n-octanol and shaken on a mechanical shaker for 1 h. The resultant biphasic solution was centrifuged to separate the layers, and UV-vis absorption spectra of both solutions were registered in both phases in a Cary 100 Bio UV-visible spectrophotometer at 411 nm and compared with a calibration curve to obtain the compound concentration of 1a and 1b in both phases. LogP was defined as the logarithm of the ratio [Ru]ocanol/[Ru]water; values reported are the means of three separate experiments.
Cell line and culture conditions
The androgen-unresponsive cell line PC3 was obtained from the American Type Culture Collection (Manassas, VA) and may be related to recurrent prostate cancers that have achieved androgen independence. All culture media were supplemented with 1% penicillin/streptomycin/amphoterycin B (Life Technologies, Barcelona, Spain). The culture was performed in a humidified 5% CO2 environment at 37 °C. After the cells reached 70–80% confluence, they were washed with phosphate buffered saline (PBS), detached with 0.25% trypsin/0.2% EDTA and seeded at 30,000–40,000 cells/cm2. The culture medium was changed every 3 days.
Cytotoxicity assays
PC-3 (4 × 104) cells were grown in 24-well plates. After 24 h, the culture medium was removed and replaced with RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin B for 24 h. Stock solutions of compounds 1a-1c (up to 300 µM) were prepared in complete medium and used for sequential dilutions to desired concentrations. Stock solutions of compounds a·HCl-c·HCl and [(η6-p-cymene)RuCl2]2 were freshly prepared in DMSO (100 µL), diluted in complete medium (50 mL) up to 800 and 1000 µM, respectively, and used for sequential dilutions to desired concentrations. The final concentration of DMSO in the cell culture medium did never exceed 0.5%. Control groups with and without DMSO (0.5%) were included in the assays.
The cytotoxic activity of the chemical compounds was screened against PC3 cell line within a wide concentration range depending on the exposure time, using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay. Cell growth was determined by a tetrazolium assay, which measures the reduction of substrate MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to a dark blue formazan product by mitochondrial dehydrogenases in living cells. Isopropanol was added to each well to dissolve the formazan precipitates and absorbances were read at 570 nm using the plate reader ELX 800 (Bio-Tek Instruments, Winooski, VT) with a reference wavelength at 620 nm. Each experiment was repeated at least three times and each concentration was tested in at least six replicates. Results are expressed as a percentage of survival with respect to control cells in the absence of the compound. IC50 values (half-inhibitory concentration) were calculated from curves constructed by plotting cell survival (%) versus compound concentration (M). The IC50 values were calculated with the GraphPad Prism software.
Cell adhesion assay
Concentrated type-I collagen solution was diluted in 10 mM glacial acetic acid and coated onto 96-well plates for 1 h at 37°C Plates were washed twice with PBS (pH 7.4). Cells were harvested with 0.25% trypsin/0.2% EDTA and collected by centrifugation. They were suspended in RPMI medium/0.1% (w/v) bovine serum albumin (BSA) (pH 7.4) and treated with the complexes for 30 min. Then, cells plated at 2.5 × 104 cells per 100 µl. The assay was terminated at indicated time intervals by aspiration of the wells. Cell adhesion was quantified by MTT colorimetric assay as mentioned above.
Wound-healing assay
PC3 cells were incubated in 24-well plates and a small wound area was made with a scraper in the confluent monolayer. Afterwards, cells were incubated in the absence or presence of the complexes. Four representative fields of each wound were captured using a Nikon Diaphot 300 inverted microscopy at different times (0–24 h). Wound areas of untreated samples were averaged and assigned a value of 100%.
Data analysis
Data were subjected to one-way ANOVA and differences were determined by Bonferroni’s multiple comparison test. Each experiment was repeated at least three times. Data are shown as the means of individual experiments and presented as the mean 6 S.E.M.; P < 0.05 was considered statistically significant.
Interactions with biomolecules
Calf Thymus DNA, plasmid (pBR322) DNA, HSA and buffers were purchased from Sigma-Aldrich. Electrophoresis experiments were carried out in a Bio-Rad Mini sub-cell GT horizontal electrophoresis system connected to a Bio-Rad Power Pac 300 power supply. Photographs of the gels were taken with an Alpha Innotech FluorChem 8900 camera. Thermal denaturation experiments were performed on an Agilent 8453 diode-array spectrophotometer equipped with a HP 89090 Peltier temperature control accessory. Fluorescence intensity measurements were carried out on a PTI QM-4/206 SE Spectrofluorometer (PTI, Birmingham, NJ) with right angle detection of fluorescence using a 1 cm path length quartz cuvette.
Mobility Shift Assay
10 µL aliquots of pBR322 plasmid DNA (20 µg/mL) in buffer (5 mM Tris/HCl, 50 mM NaClO4, pH = 7.39) were incubated with different concentrations of the compounds (a·HCl-c·HCl, 1a-1c and [(η6-p-cymene)RuCl2]2) (in the range 0.25 and 4.0 metal complex:DNAbp) at 37 °C for 20 h in the dark. Samples of free DNA and cisplatin-DNA were prepared as controls. After the incubation period, the samples were loaded onto the 1 % agarose gel. The samples were separated by electrophoresis for 1.5 h at 80 V in Tris-acetate/EDTA buffer (TAE). Afterwards, the gel was stained for 30 min with a solution of GelRed Nucleic Acid stain.
Thermal Denaturation Experiments
Melting curves were recorded in media containing 50 mM NaClO4 and 5 mM Tris/HCl buffer (pH = 7.39). The absorbance at 260 nm was monitored for solutions of Calf Thymus DNA (35 µM) before and after incubation with a solution of the drug under study (17.5 µM in Tris/HCl buffer) for 1 h at room temperature. The temperature was increased by 0.5 °C/min between 65 and 82 °C and by 3 °C/min between 25 and 65 °C and between 82 and 97 °C.
Fluorescence Spectroscopy
An solution of each compound (8 mM) was prepared and ten aliquots of 2.5 µL were added successively to a solution of HSA (10 µM) in phosphate buffer (pH = 7.4) to achieve final metal complex concentrations in the range 10–100 µM. The excitation wavelength was set to 295 nm, and the emission spectra of HSA samples were recorded at room temperature in the range of 300 to 450 nm. The fluorescence intensities of the metal compounds and the buffer are negligible under these conditions. The fluorescence was measured 240 s after each addition of compound solution. The data were analyzed using the classical Stern-Volmer equation F0/F = 1 + KSV[Q].
Supplementary Material
Acknowledgments
Financial support from the Ministerio de Economía y Competitividad of Spain (MINECO, I3 Program, Project 3090XF067) and the Universidad de Alcalá (UAH, Project CCG2013/EXP-056) is acknowledged. Y.B. acknowledges Agencia Española de Cooperación Internacional (AECI) for fellowship. Brooklyn College (The City University of New York) authors thank the National Cancer Institute (NCI) for grant 1SC1CA182844 (M.C.). E.R. would like to thank Dr. M. del Camino González-Arellano (UAH) for fruitful discussions on the aggregation studies in solution.
Footnotes
Supporting information for this article is given via a link at the end of the document
References
- 1.Keppler BK. Metal Complexes in Cancer Chemotherapy. Weinheim: Wiley VCH; 1993. [Google Scholar]; Barnes KR, Lippard SJ. In: Metal Ions in Biological Systems. Sigel A, Sigel H, editors. Vol. 42. Marcel Dekker Inc; 2004. pp. 179–208. [Google Scholar]; van Rijt SH, Sadler PJ. Drug Discov. Today. 2009;14:1089–1097. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gasser G, Ott I, Metzler-Nolte N. J. Med. Chem. 2011;54:3–25. doi: 10.1021/jm100020w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hartinger CG, Metzler-Nolte N, Dyson PJ. Organometallics. 2012;31:5677–5685. [Google Scholar]
- 2.Sava G, Bergamo A, Dyson PJ. Dalton Trans. 2011;40:9069–9075. doi: 10.1039/c1dt10522a. [DOI] [PubMed] [Google Scholar]
- 3.Melchart M, Sadler PJ. In: Bioorganometallics. Jaouen G, editor. Weinheim: Wiley VCH Verlag GmbH & Co. KGaA; 2006. pp. 39–64. [Google Scholar]; Bergamo A, Sava G. Dalton Trans. 2011;40:7817–7823. doi: 10.1039/c0dt01816c. [DOI] [PubMed] [Google Scholar]; Smith GS, Therrien B. Dalton Trans. 2011;40:10793–10800. doi: 10.1039/c1dt11007a. [DOI] [PubMed] [Google Scholar]; Clavel CM, Paunescu E, Nowak-Sliwinska P, Griffioen AW, Scopelliti R, Dyson PJ. J. Med. Chem. 2014;57:3546–3558. doi: 10.1021/jm5002748. [DOI] [PubMed] [Google Scholar]; Nazarov AA, Hartinger CG, Dyson PJ. Journal of Organometallic Chemistry. 2014;751:251–260. [Google Scholar]; Pettinari R, Marchetti F, Condello F, Pettinari C, Lupidi G, Scopelliti R, Mukhopadhyay S, Riedel T, Dyson PJ. Organometallics. 2014;33:3709–3715. [Google Scholar]
- 4.Ang WH, Casini A, Sava G, Dyson PJ. J. Organomet. Chem. 2011;696:989–998. [Google Scholar]
- 5.Bergamo A, Gaiddon C, Schellens JHM, Beijnen JH, Sava G. J. Inorg. Biochem. 2012;106:90–99. doi: 10.1016/j.jinorgbio.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Frik M, Martinez A, Elie BT, Gonzalo O, de Mingo DR, Sanau M, Sanchez-Delgado R, Sadhukha T, Prabha S, Ramos JW, Marzo I, Contel M. J. Med. Chem. 2014;57:9995–10012. doi: 10.1021/jm5012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aman F, Hanif M, Siddiqui WA, Ashraf A, Filak LK, Reynisson J, Sohnel T, Jamieson SMF, Hartinger CG. Organometallics. 2014;33:5546–5553. [Google Scholar]
- 8.Sommer MG, Kureljak P, Urankar D, Schweinfurth D, Stojanovic N, Bubrin M, Gazvoda M, Osmak M, Sarkar B, Kosmrlj J. Chem.-Eur. J. 2014;20:17296–17299. doi: 10.1002/chem.201404448. [DOI] [PubMed] [Google Scholar]
- 9.Sava G, Zorzet S, Turrin C, Vita F, Soranzo M, Zabucchi G, Cocchietto M, Bergamo A, DiGiovine S, Pezzoni G, Sartor L, Garbisa S. Clin. Cancer Res. 2003;9:1898–1905. [PubMed] [Google Scholar]
- 10.Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, Zorbas H, Dyson PJ, Keppler BK. Chem. Biodivers. 2008;5:2140–2155. doi: 10.1002/cbdv.200890195. [DOI] [PubMed] [Google Scholar]
- 11.Trondl R, Heffeter P, Kowol CR, Jakupec MA, Berger W, Keppler BK. Chem. Sci. 2014;5:2925–2932. [Google Scholar]; Kuhn PS, Pichler V, Roller A, Hejl M, Jakupec MA, Kandioller W, Keppler BK. Dalton Trans. 2015;44:659–668. doi: 10.1039/c4dt01645a. [DOI] [PubMed] [Google Scholar]
- 12.Aird RE, Cummings J, Ritchie AA, Muir M, Morris RE, Chen H, Sadler PJ, Jodrell DI. Br. J. Cancer. 2002;86:1652–1657. doi: 10.1038/sj.bjc.6600290. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bergamo A, Masi A, Peacock AFA, Habtemariam A, Sadler PJ, Sava G. J. Inorg. Biochem. 2010;104:79–86. doi: 10.1016/j.jinorgbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Scolaro C, Bergamo A, Brescacin L, Delfino R, Cocchietto M, Laurenczy G, Geldbach TJ, Sava G, Dyson PJ. J. Med. Chem. 2005;48:4161–4171. doi: 10.1021/jm050015d. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Berndsen RH, Dubois M, Müller C, Schibli R, Griffioen AW, Dyson PJ, Nowak-Sliwinska P. Chem. Sci. 2014;5:4742–4748. [Google Scholar]
- 15.Bergamo A, Masi A, Dyson PJ, Sava G. Int. J. Oncol. 2008;33:1281–1289. [PubMed] [Google Scholar]
- 16.Nowak-Sliwinska P, van Beijnum JR, Casini A, Nazarov AA, Wagnieres G, van den Bergh H, Dyson PJ, Griffioen AW. J. Med. Chem. 2011;54:3895–3902. doi: 10.1021/jm2002074. [DOI] [PubMed] [Google Scholar]
- 17.Sava G, Jaouen G, Hillard EA, Bergamo A. Dalton Trans. 2012;41:8226–8234. doi: 10.1039/c2dt30075c. [DOI] [PubMed] [Google Scholar]
- 18.Adhireksan Z, Davey GE, Campomanes P, Groessl M, Clavel CM, Yu HJ, Nazarov AA, Yeo CHF, Ang WH, Droge P, Rothlisberger U, Dyson PJ, Davey CA. Nat. Commun. 2014;5:3462. doi: 10.1038/ncomms4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyson PJ, Sava G. Dalton Trans. 2006:1929–1933. doi: 10.1039/b601840h. [DOI] [PubMed] [Google Scholar]; Guidi F, Modesti A, Landini I, Nobili S, Mini E, Bini L, Puglia M, Casini A, Dyson PJ, Gabbiani C, Messori L. J. Inorg. Biochem. 2013;118:94–99. doi: 10.1016/j.jinorgbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Barry NPE, Sadler PJ. Chem. Commun. 2013;49:5106–5131. doi: 10.1039/c3cc41143e. [DOI] [PubMed] [Google Scholar]; Kilpin KJ, Dyson PJ. Chem. Sci. 2013;4:1410–1419. [Google Scholar]
- 21.Chen HM, Parkinson JA, Morris RE, Sadler PJ. J. Am. Chem. Soc. 2003;125:173–186. doi: 10.1021/ja027719m. [DOI] [PubMed] [Google Scholar]
- 22.Xie P, Streu C, Qin J, Bregman H, Pagano N, Meggers E, Marmorstein R. Biochemistry. 2009;48:5187–5198. doi: 10.1021/bi802067u. [DOI] [PMC free article] [PubMed] [Google Scholar]; Das S, Sinha S, Britto R, Somasundaram K, Samuelson AG. J. Inorg. Biochem. 2010;104:93–104. doi: 10.1016/j.jinorgbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Bhat SS, Kumbhar AS, Lonnecke P, Hey-Hawkins E. Inorg. Chem. 2010;49:4843–4853. doi: 10.1021/ic902374t. [DOI] [PubMed] [Google Scholar]
- 24.Kukushkin VY, Pombeiro AJL. Coord. Chem. Rev. 1999;181:147–175. and references therein. [Google Scholar]
- 25.Colak AT, Irez G, Mutlu H, Hokelek T, Caylak N. J. Coord. Chem. 2009;62:1005–1014. [Google Scholar]
- 26.Scaffidi-Domianello YY, Meelich K, Jakupec MA, Arion VB, Kukushkin VY, Galanski M, Keppler BK. Inorg. Chem. 2010;49:5669–5678. doi: 10.1021/ic100584b. [DOI] [PubMed] [Google Scholar]
- 27.Bartel C, Bytzek AK, Scaffidi-Domianello YY, Grabmann G, Jakupec MA, Hartinger CG, Galanski M, Keppler BK. J. Biol. Inorg. Chem. 2012;17:465–474. doi: 10.1007/s00775-011-0869-5. [DOI] [PubMed] [Google Scholar]
- 28.Ossipov K, Scaffidi-Domianello YY, Seregina IF, Galanski M, Keppler BK, Timerbaev AR, Bolshov MA. J. Inorg. Biochem. 2014;137:40–45. doi: 10.1016/j.jinorgbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Wirth S, Rohbogner CJ, Cieslak M, Kazmierczak-Baranska J, Donevski S, Nawrot B, Lorenz IP. J. Biol. Inorg. Chem. 2010;15:429–440. doi: 10.1007/s00775-009-0615-4. [DOI] [PubMed] [Google Scholar]
- 30.Chitrapriya N, Mahalingam V, Zeller M, Lee H, Natarajan K. J. Mol. Struct. 2010;984:30–38. [Google Scholar]
- 31.Chitrapriya N, Mahalingam V, Channels LC, Zeller M, Fronczek FR, Natarajan K. Inorg. Chim. Acta. 2008;361:2841–2850. [Google Scholar]
- 32.Mendoza-Ferri MG, Hartinger CG, Eichinger RE, Stolyarova N, Severin K, Jakupec MA, Nazarov AA, Keppler BK. Organometallics. 2008;27:2405–2407. [Google Scholar]
- 33.Atilla-Gokcumen GE, Di Costanzo L, Meggers E. J. Biol. Inorg. Chem. 2011;16:45–50. doi: 10.1007/s00775-010-0699-x. [DOI] [PubMed] [Google Scholar]; Fu Y, Soni R, Romero MJ, Pizarro AM, Salassa L, Clarkson GJ, Hearn JM, Habtemariam A, Wills M, Sadler PJ. Chem.-Eur. J. 2013;19:15199–15209. doi: 10.1002/chem.201302183. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kilpin KJ, Cammack SM, Clavel CM, Dyson PJ. Dalton Trans. 2013;42:2008–2014. doi: 10.1039/c2dt32333h. [DOI] [PubMed] [Google Scholar]
- 34.Blanck S, Maksimoska J, Baumeister J, Harms K, Marmorstein R, Meggers E. Angew. Chem.-Int. Edit. 2012;51:5244–5246. doi: 10.1002/anie.201108865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner H, Oeschey R, Nuber B. Organometallics. 1996;15:3616–3624. [Google Scholar]
- 36.Ward TR, Schafer O, Daul C, Hofmann P. Organometallics. 1997;16:3207–3215. [Google Scholar]
- 37.Faller JW, Patel BP, Albrizzio MA, Curtis M. Organometallics. 1999;18:3096–3104. [Google Scholar]
- 38.Therrien B, Ward TR. Angew. Chem.-Int. Edit. 1999;38:405–408. doi: 10.1002/(SICI)1521-3773(19990201)38:3<405::AID-ANIE405>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Larionov SV. Russ. J. Coord. Chem. 2012;38:1–23. and references therein. [Google Scholar]
- 40.Chahboun G, Brito JA, Royo B, El Amrani MA, Gomez-Bengoa E, Mosquera MEG, Cuenca T, Royo E. Eur. J. Inorg. Chem. 2012:2940–2949. [Google Scholar]; Zelewsky A. Coord. Chem. Rev. 1999;190–192:811–825. [Google Scholar]
- 41.Brecknell DJ, Carman RM, Singaram B, Verghese J. Aust. J. Chem. 1977;30:195–203. [Google Scholar]
- 42.Ibn El Alami MS, El Amrani MA, Dahdouh A, Roussel P, Suisse I, Mortreux A. Chirality. 2012;24:675–682. doi: 10.1002/chir.22073. [DOI] [PubMed] [Google Scholar]
- 43.Tkachev AV, Rukavishnikov AV, Chibiryaev AM, Denisov AY, Gatilov YV, Bagryanskaya IY. Aust. J. Chem. 1992;45:1077–1086. [Google Scholar]
- 44.Noyori R, Hashiguchi S. Accounts Chem. Res. 1997;30:97–102. [Google Scholar]; Davenport AJ, Davies DL, Fawcett J, Russell DR. Dalton Trans. 2004:1481–1492. doi: 10.1039/b400747f. [DOI] [PubMed] [Google Scholar]; Touge T, Hakamata T, Nara H, Kobayashi T, Sayo N, Saito T, Kayaki Y, Ikariya T. J. Am. Chem. Soc. 2011;133:14960–14963. doi: 10.1021/ja207283t. [DOI] [PubMed] [Google Scholar]
- 45.Shabalina IY, Kirin VP, Maksakov VA, Virovets AV, Golovin AV, Agafontsev AM, Tkachev AV. Russ. J. Coord. Chem. 2008;34:286–294. [Google Scholar]; Kirin VP, Prikhod’ko IY, Maksakov VA, Virovets AV, Agafontsev AM, Golovin BA. Russ. Chem. Bull. 2009;58:1371–1382. [Google Scholar]
- 46.Butenschon H. Chem. Rev. 2000;100:1527–1564. doi: 10.1021/cr940265u. [DOI] [PubMed] [Google Scholar]
- 47.Cabrita EJ, Berger S. Magn. Reson. Chem. 2001;39:S142–S148. [Google Scholar]
- 48.Garcia-Frutos EM, Hennrich G, Gutierrez E, Monge A, Gomez-Lor B. J. Org. Chem. 2010;75:1070–1076. doi: 10.1021/jo902013v. [DOI] [PubMed] [Google Scholar]; Dossel LF, Kamm V, Howard IA, Laquai F, Pisula W, Feng XL, Li C, Takase M, Kudernac T, De Feyter S, Mullen K. J. Am. Chem. Soc. 2012;134:5876–5886. doi: 10.1021/ja211504a. [DOI] [PubMed] [Google Scholar]
- 49.Baumann SO, Bendova M, Fric H, Puchberger M, Visinescu C, Schubert U. Eur. J. Inorg. Chem. 2009:3333–3340. [Google Scholar]
- 50.Singh SK, Sharma S, Dwivedi SD, Zou RQ, Xu Q, Pandey DS. Inorg. Chem. 2008;47:11942–11949. doi: 10.1021/ic8009699. [DOI] [PubMed] [Google Scholar]
- 51.Werner H, Daniel T, Knaup W, Nurnberg O. Journal of Organometallic Chemistry. 1993;462:309–318. [Google Scholar]
- 52.Li YQ, de Kock C, Smith PJ, Chibale K, Smith GS. Organometallics. 2014;33:4345–4348. [Google Scholar]
- 53.Martin RB. Chem. Rev. 2005;96:3043–3064. doi: 10.1021/cr960037v. [DOI] [PubMed] [Google Scholar]; Kastler M, Pisula W, Wasserfallen D, Pakula T, Mullen K. J. Am. Chem. Soc. 2005;127:4286–4296. doi: 10.1021/ja0430696. [DOI] [PubMed] [Google Scholar]
- 54.All the NMR dilution data fit nicely to the assumptions of both equations derived from the infinite association and dimerization models. Thus, we did not examine the model in which the association constant for dimerization is different from those for the formation of higher aggregates.
- 55.Ciancaleoni G, Di Maio I, Zuccaccia D, Macchioni A. Organometallics. 2007;26:489–496. and references therein. [Google Scholar]
- 56.Bolano S, Ciancaleoni G, Bravo J, Gonsalvi L, Macchioni A, Peruzzini M. Organometallics. 2008;27:1649–1652. [Google Scholar]
- 57.Zuccaccia D, Macchioni A. Organometallics. 2005;24:3476–3486. [Google Scholar]
- 58.Burini A, Fackler JP, Galassi R, Macchioni A, Omary MA, Rawashdeh-Omary MA, Pietroni BR, Sabatini S, Zuccaccia C. J. Am. Chem. Soc. 2002;124:4570–4571. doi: 10.1021/ja0174837. [DOI] [PubMed] [Google Scholar]
- 59.Tobe Y, Utsumi N, Kawabata K, Nagano A, Adachi K, Araki S, Sonoda M, Hirose K, Naemura K. J. Am. Chem. Soc. 2002;124:5350–5364. doi: 10.1021/ja012458m. [DOI] [PubMed] [Google Scholar]
- 60.Zuccaccia D, Foresti E, Pettirossi S, Sabatino P, Zuccaccia C, Macchioni A. Organometallics. 2007;26:6099–6105. [Google Scholar]
- 61.Varbanov HP, Goschl S, Heffeter P, Theiner S, Roller A, Jensen F, Jakupec MA, Berger W, Galanski M, Keppler BK. J. Med. Chem. 2014;57:6751–6764. doi: 10.1021/jm500791c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morais TS, Santos FC, Jorge TF, Corte-Real L, Madeira PJA, Marques F, Robalo MP, Matos A, Santos I, Garcia MH. J. Inorg. Biochem. 2014;130:1–14. doi: 10.1016/j.jinorgbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Takacsnovak K, Avdeef A, Box KJ, Podanyi B, Szasz G. J. Pharm. Biomed. Anal. 1994;12:1369–1377. doi: 10.1016/0731-7085(94)00090-5. [DOI] [PubMed] [Google Scholar]
- 64.Scaffidi-Domianello YY, Legin AA, Jakupec MA, Arion VB, Kukushkin VY, Galanski M, Keppler BK. Inorg. Chem. 2011;50:10673–10681. doi: 10.1021/ic2010612. [DOI] [PubMed] [Google Scholar]
- 65.Siegel R, Miller K, Jemal A. CA-Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 66.Oh WK, Tay MH, Huang JT. Cancer. 2007;109:477–486. doi: 10.1002/cncr.22439. [DOI] [PubMed] [Google Scholar]
- 67.Saylor PJ. Asian J. Androl. 2014;16:341–347. doi: 10.4103/1008-682X.122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gama S, Mendes F, Marques F, Santos IC, Carvalho MF, Correia I, Pessoa JC, Santos I, Paulo A. J. Inorg. Biochem. 2011;105:637–644. doi: 10.1016/j.jinorgbio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Tomaz AI, Jakusch T, Morais TS, Marques F, de Almeida RFM, Mendes F, Enyedy EA, Santos I, Pessoa JC, Kiss T, Garcia MH. J. Inorg. Biochem. 2012;117:261–269. doi: 10.1016/j.jinorgbio.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Wei Y, Au JS. In: Integration/Interaction of Oncologic Growth. Meadows G, editor. Vol. 15. Netherlands: Springer; 2005. [Google Scholar]; Demoro B, de Almeida RFM, Marques F, Matos CP, Otero L, Pessoa JC, Santos I, Rodriguez A, Moreno V, Lorenzo J, Gambino D, Tomaz AI. Dalton Trans. 2013;42:7131–7146. doi: 10.1039/c3dt00028a. [DOI] [PubMed] [Google Scholar]; Ceresa C, Bravin A, Cavaletti G, Pellei M, Santini C. Curr. Med. Chem. 2014;21:2237–2265. doi: 10.2174/0929867321666140216125721. [DOI] [PubMed] [Google Scholar]
- 71.Biersack B, Effenberger K, Knauer S, Ocker M, Schobert R. Eur. J. Med. Chem. 2010;45:4890–4896. doi: 10.1016/j.ejmech.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 72.Dabrowiak JC. Metals in Medicine. Chichester, UK: John Wiley and Sons, Ltd; 2009. [Google Scholar]
- 73.Liu HK, Sadler PJ. Accounts Chem. Res. 2011;44:349–359. doi: 10.1021/ar100140e. [DOI] [PubMed] [Google Scholar]
- 74.Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK. Chem. Rev. 2006;106:2224–2248. doi: 10.1021/cr040704h. [DOI] [PubMed] [Google Scholar]
- 75.Chen HM, Parkinson JA, Parsons S, Coxall RA, Gould RO, Sadler PJ. J. Am. Chem. Soc. 2002;124:3064–3082. doi: 10.1021/ja017482e. [DOI] [PubMed] [Google Scholar]
- 76.Allardyce CS, Dyson PJ, Ellis DJ, Heath SL. Chem. Commun. 2001:1396–1397. [Google Scholar]; Allardyce CS, Dyson PJ, Ellis DJ, Salter PA, Scopelliti R. Journal of Organometallic Chemistry. 2003;668:35–42. [Google Scholar]
- 77.Saglam N, Colak A, Serbest K, Dulger S, Guner S, Karabocek S, Belduz AO. Biometals. 2002;15:357–365. doi: 10.1023/a:1020228723299. [DOI] [PubMed] [Google Scholar]
- 78.Hambley TW, Ling ECH, O’Mara S, McKeage MJ, Russell PJ. J. Biol. Inorg. Chem. 2000;5:675–681. doi: 10.1007/s007750000151. [DOI] [PubMed] [Google Scholar]
- 79.Fox KR. Drug-DNA interactions protocols. Methods in Molecular Biology. New York, USA: Humana Press Inc; 1997. [Google Scholar]
- 80.Martinez A, Rajapakse CSK, Sanchez-Delgado RA, Varela-Ramirez A, Lema C, Aguilera RJ. J. Inorg. Biochem. 2010;104:967–977. doi: 10.1016/j.jinorgbio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lacowicz JR. Principles of Fluorescence Spectroscopy. New York, USA: Kluwer Academy; 1999. [Google Scholar]
- 82.Carreira M, Calvo-Sanjuan R, Sanau M, Zhao XB, Magliozzo RS, Marzo I, Contel M. J. Inorg. Biochem. 2012;116:204–214. doi: 10.1016/j.jinorgbio.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.