Abstract
Proteases in Vibrio cholerae have been shown to play a role in its pathogenesis. V. cholerae secretes Zn-dependent hemagglutinin protease (HAP) and calcium-dependent trypsin-like serine protease (VesC) by using the type II secretion system (TIISS). Our present studies demonstrated that these proteases are also secreted in association with outer membrane vesicles (OMVs) and transported to human intestinal epithelial cells in an active form. OMV-associated HAP induces dose-dependent apoptosis in Int407 cells and an enterotoxic response in the mouse ileal loop (MIL) assay, whereas OMV-associated VesC showed a hemorrhagic fluid response in the MIL assay, necrosis in Int407 cells, and an increased interleukin-8 (IL-8) response in T84 cells, which were significantly reduced in OMVs from VesC mutant strain. Our results also showed that serine protease VesC plays a role in intestinal colonization of V. cholerae strains in adult mice. In conclusion, our study shows that V. cholerae OMVs secrete biologically active proteases which may play a role in cytotoxic and inflammatory responses.
INTRODUCTION
Vibrio cholerae is the causative agent of the life-threatening disease cholera. Cholera epidemics in Haiti in 2010 provide evidence that it still remains an ongoing public health threat (1). Strains of the El Tor biotype O1 serogroup are responsible for seventh pandemic and recent cholera outbreaks (2). Cholera toxin (CT) and toxin-coregulated pilus (TCP) have been identified as the major virulence factors for V. cholerae pathogenesis. CT is responsible for profuse watery diarrhea, whereas TCP is essential for sustaining colonization of human small intestine. V. cholerae also secretes several proteases which may also play a role in its pathogenesis (3).
The major protease secreted by V. cholerae strains is the 35-kDa hemagglutinin protease (HAP) (4). HAP has been reported to accelerate the bacterial detachment from cultured cells by digestion of V. cholerae adhesins (5). HAP modulates the enterotoxigenicity of cholera toxin by nicking the A subunit of CT (6). HAP also plays a role in processing hemolysin to its mature form by removal of a 15-kDa N-terminal peptide (7). As shown in the above-mentioned studies, HAP plays an indirect role in V. cholerae pathogenesis. The possibility of its direct role in pathogenesis has been shown by Ghosh et al. (8). They reported that purified HAP from a V. cholerae non-O1, non-O139 strain showed a hemorrhagic response in rabbit ileal loops (RILs) and an increase in intestinal short-circuit current in Ussing's chamber (8). Besides HAP, the other major well-characterized metalloprotease is a 97-kDa Vibrio cholerae protease, PrtV which has been shown to play a role in virulence in the Caenorhabditis elegans infection model (9). In an earlier study, we also reported the presence of a novel serine protease encoded by VC1649 (VesC) in a hapA prtV mutant Vibrio cholerae O1 strain and showed its role in the hemorrhagic response in the rabbit ileal loop model (10).
Gram-negative bacteria, including V. cholerae, use 6 different secretion systems (type I to type VI) to transport important virulence factors to the cell envelope and the extracellular milieu (11). Outer membrane vesicles (OMVs) may also serve as a mechanism of delivering active virulence factors into host cells. The virulence factors are protected from digestion by host proteases and are secreted not individually but as multiple factors delivered in a discrete package (12). Toxin delivery mediated by OMVs is recognized as a potent virulence mechanism for many bacterial pathogens. Association of toxins with OMVs have been reported for many pathogenic Gram-negative bacteria. Toxins such as ClyA cytotoxin, α-hemolysin, and CNF1 of Escherichia coli, cytolethal distending toxin (CDT) of Campylobacter jejuni, and vacuolating cytotoxin A (VacA) of Helicobacter pylori are secreted through OMVs (13, 14, 15, 16, 17). Bomberger et al. showed that multiple virulence factors, such as β-lactamase, alkaline phosphatase, hemolytic phospholipase C, and Cif, are directly delivered into the host cytoplasm via fusion of OMVs with lipid rafts in the host plasma membrane and that these toxins are rapidly distributed to specific subcellular locations to affect host cell biology (18). OMV-mediated secretion of biologically active CT, Vibrio cholerae cytolysin (VCC), and PrtV has been reported in V. cholerae (19, 20, 21).
Recent studies on proteomic analysis of V. cholerae OMVs have the shown presence of both HAP and the 59-kDa serine protease VesC (22). However, the functions of these vesicle-associated proteases have not been elucidated. Our study shows that V. cholerae OMVs secrete biologically active proteases which may play a role in cytotoxic and inflammatory responses.
MATERIALS AND METHODS
Ethics statement.
Animal experiments were performed after obtaining necessary permission from the Institutional Animal Ethical Committee (IAEC). The IAEC/CPCSEA approval number is APRO 7624/11/2010.
Bacterial strains.
The bacterial strains, and oligonucleotides used in this study are listed in Table 1. V. cholerae cells were routinely grown in tryptic soy broth (TSB) (Difco, USA) at 37°C with shaking as described earlier (10). E. coli TOP 10 cells were grown overnight in Luria broth (LB) at 37°C with shaking at 200 rpm. Antibiotics were used at the following concentrations: streptomycin, 100 μg/ml; kanamycin, 40 μg/ml; and ampicillin, 100 μg/ml. Bacterial cells, including mutants, were maintained at −70°C in LB containing 20% sterile glycerol.
TABLE 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Relevant characteristics | Source or reference |
|---|---|---|
| Vibrio cholerae strains | ||
| C6709 | Wild type (O1 El Tor); Smr | 56 |
| CHA6.8 | C6709 ΔhapA::Kan; Smr Kmr | 10 |
| CHA6.8 ΔprtV | CHA6.8 ΔprtV; Smr Kmr | 10 |
| CHA6.8 ΔprtV ΔVC1649 (VesC mutant) | CHA6.8 ΔprtV ΔVC1649; Smr Kmr | 10 |
| Comp VC1649 | CHA6.8 ΔprtV ΔVC1649::pVC1649; Smr Kmr Ampr | This study |
| E. coli strain TOP 10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen, USA |
| Plasmids | ||
| pBAD TOPO-TA | Amp+ TA cloning vector plasmid | Invitrogen, USA |
| pVC1649 | pBAD TOPO with Vibrio cholerae VC1649 (VesC) | This study |
| Primers | ||
| IL 8 F1 | 5′-ATGGGGAAGGTGAAGGTCGG-3′ | 57 |
| IL 8 R1 | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | 57 |
| GAPDH F1 | 5′-ATGGGGAAGGTGAAGGTCGG-3′ | 58 |
| GAPDH R1 | 5′-GGATGCTAAGCAGTTGGT-3′ | 58 |
| pBAD F | 5′-ATGCCATAGCATTTTTATCC-3′ | Invitrogen, USA |
| pBAD R | 5′-GATTTAATCTGTATCAGG-3′ | Invitrogen, USA |
| VesC F | 5′-ATGAACAAAACATTTTTATC-3′ | This study |
| VesC R | 5′-TCAGACGCGTTGACGACG-3′ | This study |
Complementation of the VC1649 gene in V. cholerae strain CHA6.8 ΔprtV ΔVC1649.
The VC1649 gene was amplified from genomic DNA isolated from CHA6.8 ΔprtV using VesC forward and reverse primers (Table 1). Cloning was performed as described by Gosink et al. (23). The amplified VC1649 gene was cloned into the pBAD-TOPO TA (Invitrogen, USA) cloning vector under the control of an arabinose-inducible promoter using a cloning kit from Invitrogen and was transformed in E. coli TOP 10 cells. Transformed colonies (Amp+) were selected in a Luria agar (LA) plate containing 100 μg/ml ampicillin. Positive clones were selected from the transformed colonies by colony PCR with the pBAD forward and VesC reverse primers supplied in the cloning kit, and plasmids were isolated using a plasmid isolation kit (Thermo Fisher, USA). Plasmid pVC1649 was transformed into V. cholerae strain CHA6.8 ΔprtV ΔVC1649 by electroporation (23).
Isolation and purification of OMVs from V. cholerae strains.
V. cholerae cells were grown in 2 liter of TSB for 18 h at 37°C under shaking conditions, and outer membrane vesicles (OMVs) were prepared from late-log-phase culture as described previously (17). Crude vesicles were isolated from strain Comp VC1649 grown in 250 ml of TSB using l-arabinose induction as described by Chen et al. (24). Isolated crude OMVs from C6709, OMVs from its isogenic protease mutants, and OMVs from Comp VC1649 were purified by density gradient centrifugation on a discontinuous Optiprep-iodixanol (Sigma, USA) gradient as described by Elluri et al. (20). Fractions of equal volumes were sequentially removed after density gradient centrifugation and analyzed by Western blotting using anti-OmpU antibody. OmpU is the marker protein for V. cholerae OMVs. The protein content of purified OMVs was estimated using the Bradford assay.
Transmission electron microscopy (TEM).
Twenty micrograms of purified OMVs was placed on carbon-coated nickel grids (300 mesh) (Sigma, USA), stained with 2% aqueous uranyl acetate, rinsed, and examined with a transmission electron microscope operating at 60 kV (FEI Tecnai 12; Bio, Twin, The Netherlands).
Raising of antisera against HAP, VesC, and OMVs.
HAP was purified from the culture supernatant of C6709 as described by Ghosh et al. (8). Antiserum to purified HAP was prepared by immunizing a New Zealand White rabbit by intramuscular injection with 100 μg of HAP emulsified with an equal volume of Freund's complete adjuvant (Sigma, USA). This was followed by four booster injections with 100 μg of HAP and incomplete adjuvant (Sigma, USA) at 7-day intervals. Blood samples were collected from rabbits on day 0 and 3 days after the final injections and were allowed to clot at room temperature for 30 min. Sera were collected, followed by centrifugation (1,000 rpm, 10 min), dilution in phosphate-buffered saline (PBS) (Sigma, USA), and storage at −80°C (25). Antiserum against the 59-kDa serine protease (VesC) was also raised in a New Zealand White rabbit. VesC was partially purified as described earlier (10). The mass spectrometrically identified band of VesC in a native polyacrylamide gel was excised, homogenized in PBS, and administered into an intramuscular region of a New Zealand White rabbit. Antiserum against VesC was drawn from the rabbit after four booster injections as described above. Antiserum against OMVs was prepared by immunizing 7-week-old mice (BALB/c). Mice were immunized subcutaneously with 50 μg of OMVs isolated from C6709 at the first, second, and third weeks, followed by a booster dose of 75 μg of the OMVs at the fourth week. The serum was collected, and the antibody titer was determined by enzyme-linked immunosorbent assay (ELISA) with the preimmune serum as a control.
SDS-PAGE and Western blot analysis.
V. cholerae OMVs were separated by 10% SDS-PAGE. Briefly, 20 μg each of purified OMVs and whole-cell lysates of C6709 and CHA6.8 were mixed with Laemmli sample buffer (1:1) and boiled for 10 min prior to electrophoresis. Proteins with known molecular weights (Thermo Fisher, USA) were used as markers. Gels were stained with Coomassie brilliant blue stain after electrophoresis. For immunoblotting, the separated proteins were transferred onto a nitrocellulose membrane (Bio-Rad, USA) by the wet transfer method using a Trans-Blot apparatus (Bio-Rad, USA). The membrane was blocked at room temperature for 1 h in 5% nonfat dry milk powder (Bio-Rad, USA)–PBS–Tween 20. After incubation, the membrane was washed and incubated with rabbit anti-HAP (1:1,000 dilution in 5% [wt/vol] nonfat dry milk), and anti-VesC (1:10,000 dilution in 5% [wt/vol] nonfat dry milk) antisera, followed by incubation for 1 h at room temperature with a 1:10,000 dilution of anti-rabbit horseradish peroxidase-conjugated secondary antibody (Thermo Fisher, USA). The blots were visualized with ECL Western blotting detection reagent (Millipore, USA).
Proteolytic digestion of prohemolysin.
Proteolytic digestion of El Tor prohemolysin was performed as described earlier (7). Briefly, 10 μl of 79-kDa prohemolysin (1 mg/ml) was mixed with 10 μl (100 μg/ml) of purified C6709 OMVs and incubated at 37°C for 30 min, and SDS-PAGE was performed. Untreated purified prohemolysin and HAP-treated prohemolysin were used as negative and positive controls, respectively.
Proteinase K susceptibility assay.
The proteinase K susceptibility assay was performed as described previously (26). Briefly, OMVs were treated with proteinase K (1 μg/ml) for 30 min at 37°C in either the presence or absence of 1% SDS, and samples were analyzed by immunoblotting against anti-HAP and anti-VesC antibodies raised in rabbit.
Media for cell lines and culture conditions.
Int407 and T84 human intestinal epithelial cells were used in this study. Int407 cells were grown in 25-ml tissue culture flasks (BD Biosciences) containing minimal essential medium (MEM) (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) along with penicillin G and streptomycin sulfate (Sigma, USA) at 37°C in a CO2 incubator (Heraeus, Germany). Cells at 80 to 90% confluence were seeded in 6-well and 24-well tissue culture plates (BD Biosciences) at a concentration of approximately 105 cells/well. Cells were permitted to reach confluence by allowing them to grow for 24 h in a CO2 incubator. T84 cells were used for interleukin-8 (IL-8) assay, and the cells were maintained in Dulbecco's modified Eagle's Medium-nutrient F12 (DMEM-F12) (Gibco, USA) supplemented with antibiotic solution as described above.
Tissue culture assay.
The tissue culture assay was performed as described by Ghosh et al. (8). Briefly, OMVs from C6709 and its isogenic knockout strains were filter sterilized and serially diluted in serum-free MEM from 0.5 μg/ml to 100 μg/ml. The exhaust media were aspirated from 24-well tissue culture plates containing Int407 cells grown to confluence and washed with PBS, and serially diluted OMVs were added and grown at 37°C in a CO2 incubator for 24 h. Purified HAP was used as positive control. Results were analyzed by phase-contrast microscopy and photographs taken (CK 40; Olympus).
OMV internalization assay.
Int407 cells were incubated with 20 μg/ml of purified OMVs from C6709 and its isogenic protease mutants for 1 h at 37°C in a 5% CO2 atmospheric chamber to monitor internalization of V. cholerae OMVs. The effects of several endocytic inhibitors (nystatin, dynasore, and methyl-β-cyclodextrin [MβCD]) on OMV uptake were examined by pretreating Int407 cells with nystatin (25 μg/ml) and dynasore (80 μM) for 1 h and with MβCD (1 mg/ml) (Sigma, USA) for 30 min as described by Parker et al. (27). This was followed by incubation with purified OMVs for 1 h. Cells were then fixed with 4% paraformaldehyde at 4°C for 20 min, followed by permeabilization with 1% Triton X-100 in PBS for 20 min at room temperature. Mouse antiserum against OMVs (1:2,000) was added to OMV-treated cells and incubated overnight at 4°C in a moist chamber. Cells were washed with sterile PBS and incubated with tetramethyl rhodamine isocyanate (TRITC)-conjugated anti-mouse secondary antibody (1:500) and were kept in the dark at 37°C for 90 min.
For colocalization studies, primary antibodies against HAP (1:1,000) and OMVs (1:2,000) raised in rabbit and mouse, respectively, were added to C6709 and CHA6.8 OMV-treated cells. Primary antibodies against VesC (1:2,500) and OMVs (1:2,000) were added to cells treated with OMVs from C6709, isogenic protease mutants of C6709, and the VC1649 complemented strain and incubated overnight as described above. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (1:500) and TRITC-conjugated anti-mouse antibody (1:500) and were kept in the dark at 37°C for 90 min after incubation. Cells were washed and resuspended in 100 μl of PBS. Approximately 20 μl of resuspended sample was used to make a thin film on a glass slide. One drop of 90% glycerol was added, a thin glass coverslip was placed on it, and the slide was viewed under a confocal microscope (model LSM 510 META; Zeiss, Germany) and excited at 488 nm and 543 nm for FITC and TRITC, respectively. The emission filters used were 475 nm to 525 nm for FITC and 558 nm to 601 nm for TRITC. Experiments were also done with PBS as negative control. Merged images were developed using Image J software.
Mouse intestinal fluid accumulation assay.
The mouse intestinal fluid accumulation assay was performed as described earlier (28). Briefly, 20 μg of purified OMVs from V. cholerae and its isogenic protease mutant strains was injected into ligated intestinal segments of about 4 cm in length from 4- to 5-week-old adult BALB/c mice. All experimental procedures were performed under anesthesia with ketamine. After 6 h, mice were sacrificed and loops were excised. The enterotoxic activity was determined by measuring the fluid accumulation (FA) ratio, which is the ratio of fluid accumulated in the intestinal loop to the length of the loop (g/cm). PBS was used as a negative control. Values were expressed as mean ± standard deviation (SD) of the mean (n = 5 mice per group).
Histopathological studies of mouse ileal loops (MILs).
Mouse ileal tissues were collected and fixed in 10% neutral buffered formalin solution for histopathological studies. The tissues were further embedded in paraffin and processed following the standard protocol. Three- to four-micrometer thin sections were cut in a microtomy rotor (Leica, Germany). The sections were strained with hematoxylin and eosin and observed under a light microscope. Photographs were taken under a magnification of ×40 with a Leica DMLB microscope (Solms, Germany) equipped with a digital imaging system.
Flow cytometric analysis.
Flow cytometric analysis was performed as described by Thay et al. (29). Briefly, Int 407 cells were treated with OMVs from V. cholerae strain C6709 and its protease knockout mutant strains at concentrations of 20 μg/ml and 40 μg/ml for 4 h and then stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) (BD Pharmingen) according to the manufacturer's instructions. The cells were stained with FITC-annexin V in annexin V binding buffer for 10 min, followed by addition of PI, and analyzed by flow cytometry using Cell Quest Pro software (BD Biosciences). A total of 104 cells were acquired for data analysis in each set of experiments. Values were expressed as mean ± SD of the mean for 3 different observations (n = 3).
CT-bead ELISA.
CT concentrations in OMVs and culture supernatants of C6709 and its isogenic protease mutants grown in TSB were measured by bead ELISA as described by Oku et al. (30). Culture supernatant of V. cholerae N16961 grown in Syncase medium was used as a positive control.
Mouse intestinal colonization assay.
Pathogen-free adult 4- to 5-week-old BALB/c mice were used for the colonization assay. Prior to the experiment, all mice were fasted for 24 h with free access to sterile water. The experiment was performed as described by Olivier et al. (31). Briefly, mice were injected intraperitoneally with a mixture of 35 mg/kg ketamine and 5 mg/kg xylazine for anesthesia. Two hundred microliters of suspensions of ∼109 viable bacterial cells was administered orally with two boluses of 0.5 ml of 5% (wt/vol) NaHCO3. All mice were kept in microisolator cages with free access to food and sterile water after inoculation. Mice were euthanized with Euthanasia 6 solution after 18 h of oral inoculation. The small intestine above the cecum was collected after dissection, homogenized, and then serially diluted in sterile PBS (pH 7.4). Homogenized materials were plated on appropriate antibiotic-containing tryptic soy agar (TSA) plates and incubated for 24 h. All experiments were performed in triplicates, and values were expressed as mean ± SD (n = 10 mice per group) (*, P < 0.05; **, P < 0.01) and compared with the CFU obtained for C6709.
ELISA and semiquantitative RT-PCR for IL-8 estimation.
For enzyme-linked immunosorbent assays (ELISAs), the growth medium was removed from the plates. Cells were washed with PBS (Sigma, USA) and maintained in antibiotic- and serum-free DMEM-F12. The cells were treated with 10 μg/ml of V. cholerae OMVs from C6709 and its isogenic knockout mutant strains for IL-8 stimulation. Supernatants were collected after 24 h and centrifuged to remove dead cells, and IL-8 concentrations were determined by ELISA using an ELISA kit from Invitrogen (USA) according to the instructions of the manufacturer. For reverse transcription-PCR (RT-PCR), total RNA was isolated from the OMV-treated T84 cells using TRIzol reagent (Invitrogen, USA). RNA was quantified by spectrophotometric analysis and the quality of the RNA samples determined by estimating the A260/A280 ratio. cDNA was prepared from 1.5 μg of total RNA using the Superscript II first-strand synthesis system for RT-PCR (Invitrogen, USA). A 2.5-μl portion of cDNA was PCR amplified in a 25-μl reaction volume containing 10 mM Tris-Cl (pH 8.3), 50 mM KCl, 2.0 mM MgCl2, 2.5 mM each deoxynucleoside triphosphate (dNTP), 20 pmol of each primer, and 1 U of Taq DNA polymerase (Roche, Germany). The primers used for IL-8 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) are listed in Table 1. The PCR conditions were as follows: an initial denaturation step at 95°C for 5 min followed by 35 cycles of 95°C (1 min), 55°C and 60°C for IL-8 and GAPDH, respectively (1 min), and 72°C (30 s). The final extension step was done at 72°C for 7 min. The products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized using a gel documentation system (Bio-Rad, USA). The data from ELISA and RT-PCR was recorded as mean ± SD from at least three groups of experiments. Densitometric quantification of RT-PCR bands was performed with Quantity One software (Bio-Rad, USA).
Statistical analysis.
All experiments were performed in triplicates. Values were expressed as mean ± standard deviation of the mean. Variables were compared for significance using two-way analysis of variance and the Bonferroni test, with one asterisk indicating a P value between 0.01 to 0.05 and two asterisks indicating a P value between 0.01 to 0.001.
RESULTS
Fractions of HAP and VesC are associated with OMVs of Vibrio cholerae C6709 and hapA mutant strains, respectively.
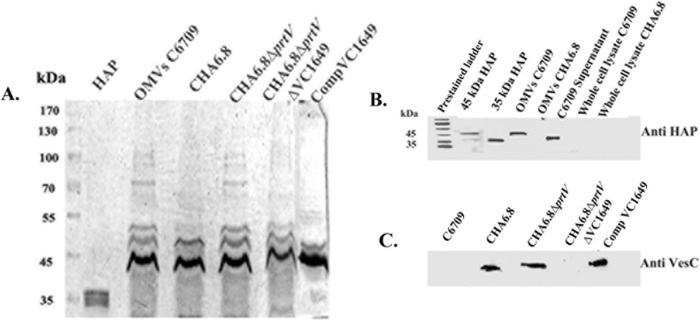
V. cholerae secretes abundant vesicles during its normal course of growth (32). To investigate the association of proteases with outer membrane vesicles (OMVs), crude OMVs were isolated from culture supernatants of the pathogenic O1 El Tor strain C6709 and its protease mutants by ultracentrifugation, followed by purification using Optiprep density gradient centrifugation. Fractions obtained from the gradient centrifugation of OMVs were analyzed by immunoblotting using OmpU antiserum. Fractions showing reactivity with OmpU antiserum were examined by transmission electron microscopy for ultrastructural analysis and used for further experiments. OMVs of C6709 appeared as spherical electron-dense materials of heterogeneous sizes with a diameter in the range of 25 to 150 nm. Major morphological differences were not observed in OMVs from the isogenic protease mutant strains and OMVs from Comp VC1649. To investigate OMV-associated secretion of HAP, OMVs from HAP-producing C6709 and ΔhapA strain CHA6.8 were subjected to SDS-PAGE along with whole-cell lysates from the respective strains, and immunoblotting was performed using polyclonal antiserum against HAP. Concentrated OMV-free culture supernatants from C6709 and purified HAP were used as positive controls. An immunoreactive band corresponding to HAP was observed only in C6709 OMVs. The presence of HAP was not observed in whole-cell lysate prepared from strain C6709. The immunoblot results showed the presence of the mature 45-kDa form of HAP in OMVs instead of its processed 35-kDa form, which was present in OMV-free culture supernatant of C6709 (Fig. 1B). The presence of VesC in the OMVs was detected by immunoblotting using VesC antiserum (1:10,000). VesC was detected in the OMVs from the CHA6.8 and CHA6.8 ΔprtV strains and the VC1649 complemented construct. In contrast, its presence was not observed in OMVs from C6709 and CHA6.8 ΔprtV ΔVC1649 (Fig. 1C). The above results demonstrated that HAP is secreted in association with OMVs from the V. cholerae wild-type strain C6709, whereas the serine protease VesC is secreted in association with OMVs from ΔhapA strains.
FIG 1.
Association of HAP and VesC with V. cholerae OMVs by immunoblot analysis. (A) Ten percent SDS-PAGE profile of the OMVs of Vibrio cholerae strains C6709 (lane 3), CHA6.8 (lane 4), CHA6.8 ΔprtV (lane 5), CHA6.8 ΔprtV ΔVC1649 (lane 6), and Comp VC1649 (complemented) (lane 7). Five micrograms of purified HAP was resolved in lane 2, and a prestained protein ladder (Thermo Fisher, USA) is in lane 1. (B) Immunoblot analyses with OMVs from C6709 and CHA6.8 and whole-cell lysates from the respective strains against HAP antiserum. Purified 45-kDa and 35-kDa HAP and concentrated OMV-free culture supernatant of C6709 were used as positive controls. (C) Immunoblot analyses with OMVs of C6709 and its isogenic protease mutants against VesC antiserum.
Localization of protease within the OMVs.
Localization of the HAP and VesC within the OMVs was confirmed by proteinase K susceptibility assay. Clear proteolytic digestion of HAP was observed only when OMVs from C6709 were incubated with proteinase K in the presence of 1% SDS, and this proteolytic digestion was inhibited in the presence of phenylmethylsulfonyl fluoride (PMSF) (Fig. 2A). A similar result was observed with OMV-associated VesC in the CHA6.8 ΔprtV strain (Fig. 2B). These results confirmed the luminal localization of HAP and VesC within the vesicles.
FIG 2.
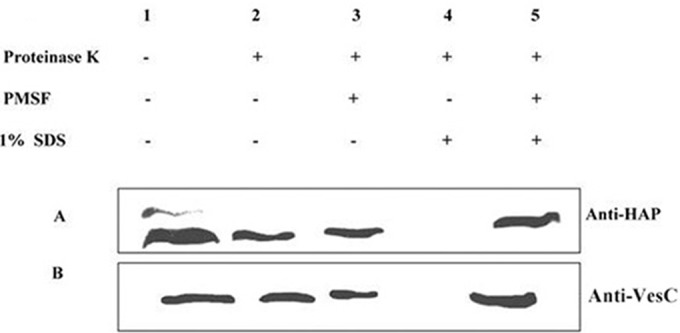
Localization of proteases in OMVs by proteinase K susceptibility assay. OMVs from C6709 and CHA6.8 ΔprtV were incubated at 37°C for 30 min with or without 1 μg/ml proteinase K alone or with 1% SDS or 1 mM PMSF. Lane 1, control reaction; lane 2, sensitivity to extracellular protease; lane 3, inhibition of extracellular proteases; lane 4, protease sensitivity to luminal proteins; lane 5, inhibition of protease activity by PMSF (control). OMVs were incubated under the above-described conditions, precipitated with acetone, dried, electrophoresed, and immunoblotted against HAP (A) or VesC (B) antiserum.
Protease-associated OMVs bind to and are internalized by human intestinal epithelial cells in a protease-independent manner.
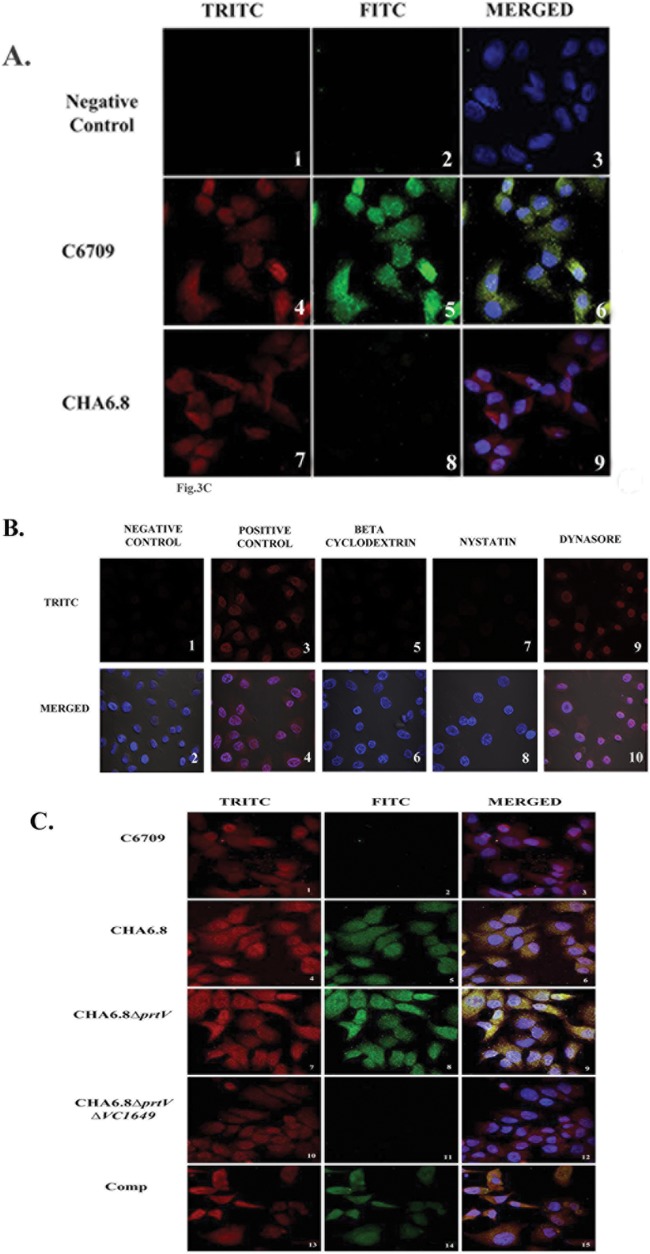
The interaction of OMVs with Int407 cells was studied by confocal microscopy. OMV-specific red fluorescence was observed throughout Int407 cells treated with OMVs from C6709 and its isogenic protease mutant strains (Fig. 3A, panels 4 and 7, and C, panels 1, 4, 7, and 10). In contrast, no fluorescence was observed in cells treated with buffer (Fig. 3A, panel 1). Internalization of OMVs in Int407 cells was confirmed by treatment with different endocytic inhibitors prior to addition of OMVs. The cholesterol-sequestering agent MβCD inhibited the entry of V. cholerae OMVs in Int407 cells (Fig. 3B, panels 5 and 6). This result suggests that V. cholerae OMVs may be internalized into the cells by cholesterol-enriched lipid rafts. MβCD has also been reported to inhibit clathrin-mediated endocytosis when applied at high concentrations (33). Inhibition of vesicular endocytosis was further confirmed by using nystatin, a cholesterol binding agent, which disrupts lipid rafts but does not affect clathrin-mediated endocytosis. Our results showed that nystatin also inhibits the entry of V. cholerae OMVs in Int407 cells (Fig. 3B, panels 7 and 8). Interestingly, dynasore (an inhibitor of the dynamin-dependent endocytic pathway) failed to inhibit internalization of OMVs into Int407 cells (Fig. 3B, panels 9 and 10). The inhibition of internalization of C6709 OMVs is shown in Fig. 3B, which shows that C6709 OMVs failed to enter Int407 cells upon treatment with MβCD and nystatin. The above results suggested that V. cholerae OMVs are internalized into In407 cells by lipid raft-dependent endocytosis.
FIG 3.
Colocalization of proteases and OMVs in the Int407 cell line. (A) Int407 cells were treated with OMVs from strain C6709 (panels 4, 5, and 6) or CHA6.8 (panels 7, 8, and 9) or with sterile PBS as a negative control (panels 1, 2, and 3), incubated with HAP and OMV antisera, and stained with TRITC (red)-conjugated anti-mouse antibody (panels 1, 4, and 7). Red fluorescence was detected in all OMVs except the negative control. HAP was detected using anti-HAP and FITC-conjugated (green) anti-rabbit antibody in panel 5. Merged images are shown in panels 3, 6, and 9. The nucleus was stained with DAPI (4′,6′-diamidino-2-phenylindole). (B) Inhibition of internalization of C6709 OMVs in Int407 cells treated with dynasore, methyl-β-cyclodextrin (MβCD), and nystatin prior to addition of OMVs. Cells with untreated OMVs were used as a negative control and cells without inhibition as a positive control. OMVs were labeled with TRITC-conjugated antibody, and the nucleus was stained with DAPI. (C) Int407 cells were treated with C6709 OMVs (panels 1, 2, and 3), CHA6.8 OMVs (panels 4, 5, and 6), CHA6.8 ΔprtV OMVs (panels 7, 8, and 9), CHA6.8 ΔprtV Δ VC1649 OMVs (panels 10, 11, and 12), and Comp VC1649 (panels 13, 14, and 15), incubated with VesC and OMVs antisera, and stained with red TRITC-conjugated anti-mouse antibody (panels 1, 4, 7, 10, and 13). Red fluorescence were detected in all OMVs. The presence of VesC was detected using anti-VesC and FITC-conjugated (green) anti-rabbit antibodies (panels 5, 8, and 14). Merged images are shown in panels 3, 6, 9, 12, and 15. The nucleus was stained with DAPI.
To investigate OMV-mediated transport of HAP in Int407 cells, confocal microscopy was performed, using HAP antiserum and FITC-conjugated secondary antibody. The merged images showed colocalization of HAP and OMVs in Int407 cells when treated with HAP-associated C6709 OMVs (Fig. 3A, panel 6), whereas cells treated with CHA6.8 showed only OMV-specific red fluorescence (Fig. 3A, panel 9). OMVs were labeled with VesC antiserum and FITC-conjugated secondary antibody to assess whether VesC is transported into cells. Our results with the colocalization assay clearly showed that VesC is transported into Int407 cells through OMVs from hapA mutant strains CHA6.8 and CHA6.8 ΔprtV (Fig. 3C, panels 6 and 9). Colocalization of OMVs and VesC was not observed when cells were treated with CHA6.8 ΔprtV ΔVC1649 OMVs. However, the merged image showed adherence and internalization of OMVs in the absence of VesC (Fig. 3C, panel 12), and this suggests that VesC is not responsible for adherence of OMVs in Int407 cells. OMV-mediated transport of VesC was further confirmed with OMVs from strain Comp VC1649, which showed both OMV- and VesC-specific signals in the cells (Fig. 3C, panels 13 and 14).
OMV-associated proteases are biologically active.
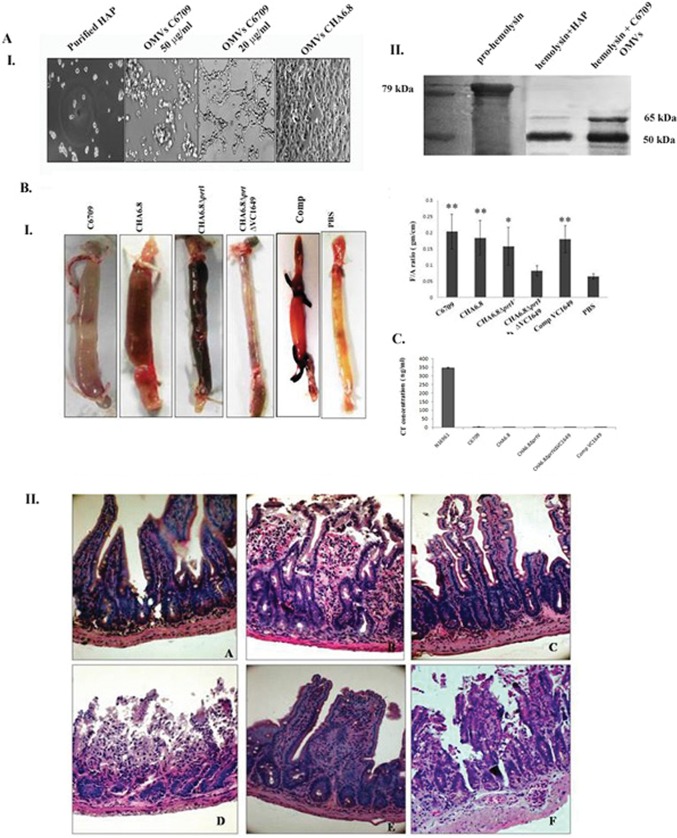
Studies have shown that OMVs deliver cargo to the specific targets in an active form (34). In the present study, we showed that both HAP and VesC are transported into intestinal epithelial cells through OMVs of their producing strains. HAP-associated C6709 OMVs induced morphological changes of Int407 cells in a dose-dependent manner when treated for 24 h (Fig. 4A, panel I). C6709 OMVs showed a cell-distending effect at a low concentration (20 μg/ml), whereas they showed a cell-rounding effect similar to that for purified HAP at a higher concentration (50 μg/ml) (Fig. 4A, panel I). In contrast, such morphological changes were not observed when cells were treated with OMVs from CHA6.8 and other protease mutant strains. The proteolytic activity of HAP-associated OMVs was studied on 79-kDa prohemolysin to confirm its biological activity. HAP has been reported to proteolytically cleave prohemolysin by digestion of the N-terminal polypeptide chain to its 65-kDa mature form (7). Our results showed that OMV-associated HAP digests 79-kDa prohemolysin to produce 65-kDa and 50-kDa fragments, similar to the effect observed with purified HAP (Fig. 4A, panel II). Our results confirmed that OMV-associated HAP is biologically active. In earlier studies, we have reported that purified HAP and partially purified VesC induce a hemorrhagic fluid response in the RIL assay (8, 10). The biological activities of OMV-associated proteases were evaluated in vivo by fluid accumulation assay in the ilea of adult mice. OMVs from wild-type strain C6709 showed significant fluid accumulation (FA ratio, 0.20 ± 0.03; n = 5) (**, P ≤ 0.01) compared to the control (PBS treated) (Fig. 4B, panel I). Histopathological studies of ileal tissues revealed widely dilated and ruptured villi and the presence of inflammatory cells in the mucosa (magnification, ×40) (Fig. 4B, panel IIB). Interestingly, hemorrhagic fluid accumulation was observed in the intestinal segments when they were treated with OMVs from CHA6.8 and CHA6.8 ΔprtV. Histopathological studies of the ileal tissues showed an altered villous structure and mild hemorrhage in the submucosal membrane when treated with CHA6.8 OMVs (Fig. 4B, panel IIC). However, CHA6.8 ΔprtV OMVs caused maximum damage of the ileal tissues (Fig. 4B, panel IID). The ileal tissues showed grossly disrupted villi with hemorrhage in all layers of the mucosa. In contrast, ileal tissues treated with CHA6.8 ΔprtV ΔVC1649 OMVs showed an almost normal villous structure with minimum hemorrhage in the mucosa and submucosa in histopathological studies (Fig. 4B, panel IIE). Fluid accumulation in the intestinal segments was also significantly reduced (FA ratio, 0.07 ± 0.03; n = 5). These results may suggest that OMV-associated VesC may be responsible for the hemorrhagic fluid response in the mouse ileal loop assay. The MIL assay with OMVs from Comp VC1649 showed restoration of hemorrhagic fluid in intestinal segments, and histopathological studies showed mucosal damage and the presence of inflammatory cells in the mucosa and submucosa (Fig. 4B, panel IIF). The presence of VesC in the OMVs may responsible for the hemorrhagic fluid responses. However, it was reported earlier that CT is responsible for fluid accumulation in the mouse ileal loop assay (35). We measured the CT titers of V. cholerae OMVs and culture supernatants by CT-bead ELISA to confirm whether the OMV-mediated inflammatory responses is due to the presence of CT in the OMVs. Both cell-free culture supernatants and OMVs from V. cholerae strains grown in TSB showed almost negligible CT titers compared to that of the positive-control strain N16961 grown in Syncase medium (Fig. 4C) Therefore, the hemorrhagic response by OMVs in the MIL assay is not due to CT. The above results confirmed that OMV-associated VesC is responsible for hemorrhagic fluid accumulation in mouse ileal loops.
FIG 4.
Protease-associated OMVs are biologically active. (A) Biological activities of HAP-associated OMVs. Panel I, Int407 cells were treated with OMVs from C6709 at 50 μg/ml and 20 μg/ml and OMVs from CHA6.8 for 24 h at 37°C. The higher concentration of C6709 OMVs showed a cell-rounding effect, whereas the lower concentration showed a cell-distending effect. No morphological changes were observed in CHA6.8. Panel II, immunoblot analyses against hemolysin antiserum with 10 μg purified hemolysin treated with HAP and C6709 OMVs and purified hemolysin as positive control. (B) Panel I, effect of OMVs of Vibrio cholerae strains in the mouse ileal loop assay. Twenty micrograms (100 μg/ml) of OMVs from wild-type strain C6709 and its knockout derivatives was introduced into ligated ileal loops of adult mice. The mice were sacrificed after 6 h, loops were excised, and fluid accumulation was calculated as the loop weight/length ratio. Images of mouse ileal loops treated with V. cholerae OMVs are shown. The graph shows a summary of the data, represented as mean ± SD (n = 5 mice per group). Variables were compared for significance using two-way analysis of variance and the Bonferroni test (*, P value between 0.01 and 0.05; **, P value between 0.01 and 0.001). Panel II, histopathological studies of mouse ileal loops. Twenty micrograms of OMV-treated ileal tissues was processed for histopathological analysis, and photomicrographs were taken at a magnification of ×40. A, PBS-treated ileal tissues showed normal villi with mucosal structure; B, a magnified image of ileal tissues treated with C6709 OMVs shows dilated and ruptured villi with accumulation of inflammatory cells in mucosal layers; C, CHA6.8 shows an altered villous structure with mild hemorrhage in the submucosa; D, CHA 6.8 ΔprtV OMV-treated ileal tissues show grossly disrupted villi with hemorrhage in all layers of the mucosa; E, ileal tissues treated with OMVs of strain CHA6.8 ΔprtV ΔVC1649 show an almost-normal villous structure with minimum hemorrhage in the mucosa, submucosa, and lamina propria; F, OMVs from the VC1649 complemented strain show accumulation of inflammatory cells and hemorrhage in mucosal layers. (C) CT-beads ELISA results for OMVs from the C6709, CHA6.8, CHA6.8 ΔprtV, CHA6.8ΔprtV ΔVC1649, and Comp VC1649 strains. Culture supernatant of strain N16961 was used as a positive control.
Host cell death induced by V. cholerae OMVs is dependent on proteases.
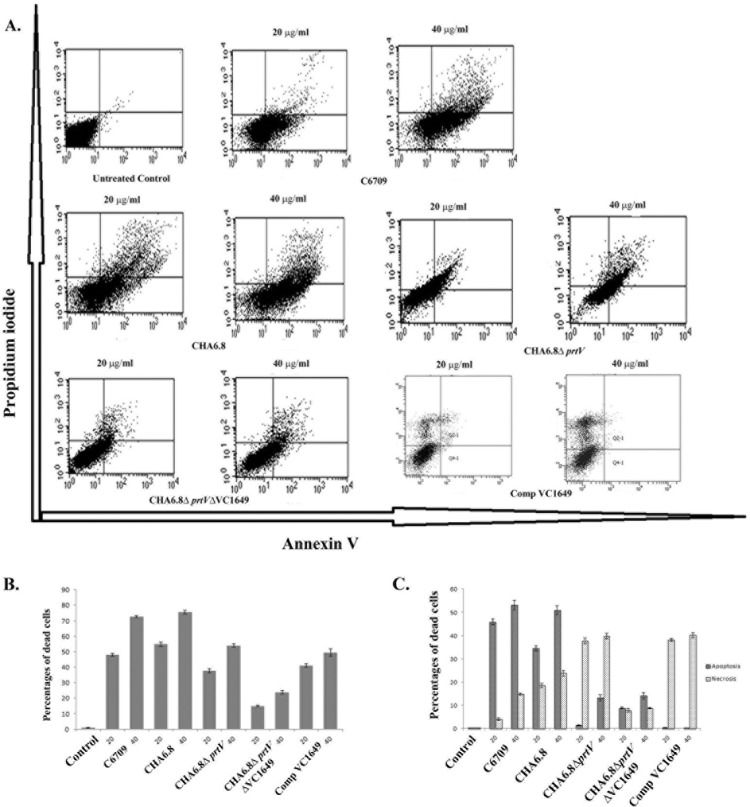
The cell death mechanisms induced by V. cholerae OMVs were analyzed by flow cytometry. Flow cytometric analysis with C6709 OMVs showed a dose-dependent apoptotic response in Int407 cells (annexin V+/PI− fraction). More than 50% cell death was observed at 20 μg/ml of C6709 OMVs compared to the untreated control. However, CHA6.8 OMVs induced greater cytotoxicity than C6709 OMVs, and cell death occurred by both apoptosis and necrosis. Interestingly 30% reduced cell death was observed when cell were treated with CHA6.8 ΔprtV OMVs compared to CHA6.8 OMVs. However, a 2-fold increase in the necrotic cell population was observed compared to that with CHA6.8 OMVs (Fig. 5C). CHA6.8 ΔprtV ΔVC1649 OMVs showed reduced cytotoxicity compared to C6709, CHA6.8, and CHA6.8 ΔprtV OMVs. OMVs from the VC1649 complemented strain restored the cytotoxicity in Int407 cells. These results clearly demonstrated that VesC is responsible for necrotic damage of Int407 cells (Fig. 5B and C).
FIG 5.
Host cell death induced by OMVs from V. cholerae strains. (A) Flow cytometric analysis of cell death induced by V. cholerae OMVs. The graphical displays show data from a representative experiment. In each display the lower right quadrant represents apoptotic cells (annexin V+/PI−), and the upper left and right quadrants represent necrotic cells (annexin V+/PI+ and annexin V−/PI+ fractions). Upper panel, untreated Int407 cells and Int407 cells treated with 20 μg/ml and 40 μg/ml of OMVs from V. cholerae strain C6709. Lower panel, Int407 cells treated with 20 μg/ml and 40 μg/ml OMVs from isogenic protease mutants of the C6709 and Comp VC1649 strains. (B) Results from three independent flow cytometry experiments. Data are represented as mean (±SD) percentage of dead cells after treatment with 20 and 40 μg/ml of OMVs from V. cholerae strains (n = 3). (C) Fractions of apoptotic and necrotic cells of the same experiment. Data are represented as mean (±SD) percentages of apoptotic and necrotic cells (n = 3).
VesC plays a role in intestinal colonization of V. cholerae in the adult mouse model.
Assessment of colonization of V. cholerae strains in mouse intestinal epithelium showed significantly reduced bacterial colonization of the triple knockout mutant strain CHA6.8 ΔprtV ΔVC1649 (CFU 3 × 103 cells/g of intestine) compared with the ΔhapA ΔprtV strain CHA6.8 ΔprtV (CFU 8 × 105 cells/g of intestine) (Fig. 6). The ΔhapA strain CHA6.8 showed increased colonization (CFU 1 × 108 cells/g of intestine) compared to wild-type strain C6709 (CFU 3 × 107 cells/g of intestine). There was a significant decrease in colonization in the CHA6.8 ΔprtV ΔVC1649 strain compared to the CHA6.8 ΔprtV strain. Interestingly, when the VC1649 gene was complemented in CHA6.8 ΔprtV ΔVC1649, the strain showed 3-fold-increased colonization. These results suggest that VesC may play a role in colonization in adult mice.
FIG 6.
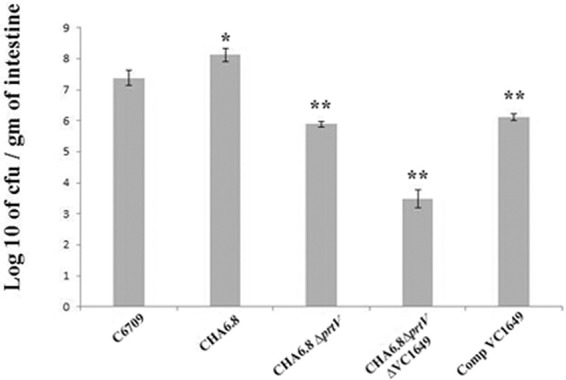
Comparative analysis of colonization of Vibrio cholerae strain C6709 and its isogenic knockout mutants in mouse intestine. A summary of the data, represented as mean ± SD (n = 10 mice per group), is shown. Variables were compared for significance using two-way analysis of variance and the Bonferroni test (*, P value between 0.01 and 0.05; **, P value between 0.01 to 0.001).
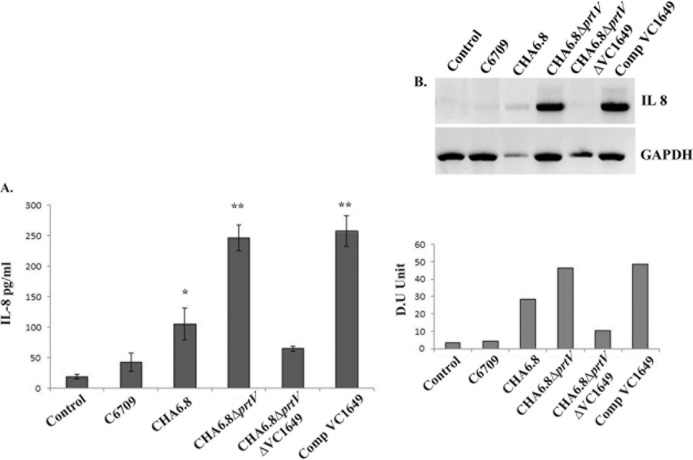
OMV-mediated IL-8 secretion from T84 cells is dependent on VesC.
OMVs isolated from C6709 and its protease mutant Vibrio cholerae strains were incubated with T84 cells for 24 h, and culture supernatants were taken and assayed for IL-8 expression by ELISA and semiquantitative RT-PCR. Significant IL-8 mRNA expression was observed when cells were treated with CHA6.8, CHA6.8 ΔprtV, and Comp VC1649, as visible IL-8-specific bands were observed in agarose gel electrophoresis. OMVs from the CHA6.8 ΔprtV and Comp VC1649 strains induced maximum mRNA expression (Fig. 7B). Protein expression in the same set of experiments was studied by IL-8 ELISA to assess whether the IL-8 mRNA level is correlated at the protein level. The ELISA results showed that OMVs from CHA6.8 induced more than a 2.5-fold increase in IL-8 secretion compared to those from wild-type strain C6709 and a ∼10-fold increase compared to those from untreated cells. Interestingly, IL-8 secretion was increased ∼25-fold compared to that in untreated cells when cells were treated with CHA6.8 ΔprtV OMVs in 24 h. This increased IL-8 secretion was reduced 4-fold when cells were treated with OMVs from the VesC mutant strain (Fig. 7A). Upon VesC complementation in the hapA prtV VC1649 triple knockout mutant strain, IL-8 induction by OMVs was significantly increased. These results suggest that VesC may act as an inducer for OMV-mediated IL-8 secretion in human intestinal epithelial cells.
FIG 7.
IL-8 induction by OMVs of V. cholerae O1 strains in the T84 cell line. (A) Comparative analysis of IL-8 secretion from T84 cells coincubated with OMVs from V. cholerae O1 El Tor strain C6709, its isogenic protease mutant strains CHA6.8, CHA6.8 ΔprtV, and CHA6.8 ΔprtV ΔVC1649, and CHA6.8 ΔprtV ΔVC1649::VC1649 (Comp VC1649) determined by ELISA. Data represent mean ± SD from three independent experiments performed under similar conditions. Variables were compared for significance using two-way analysis of variance and the Bonferroni test (*, P value between 0.01 and0.05; **, P value between 0.01 and 0.001). (B) IL-8 production from T84 cells was analyzed at the mRNA and protein levels by RT-PCR and resolved in a 2% agarose gel. IL-8 expression in T84 cells treated with OMVs from O1 El Tor strain C6709 and its protease knockout mutant strains CHA6.8, CHA6.8 ΔprtV, CHA6.8 ΔprtV ΔVC1649, and Comp VC1649 is displayed in lanes 2 to 5, respectively, and the untreated control is in lane 1. Densitometric quantification in densitometric units (DU) for IL-8 after normalization to GAPDH is shown below the agarose gel.
DISCUSSION
Proteases produced by microorganisms play an important role in virulence (3). Vibrio cholerae secretes hemagglutinin protease (HAP), V. cholerae protease (PrtV), and serine proteases through the type II secretion system (TIISS) (21, 36). Earlier we have reported that purified HAP from a ctx-negative V. cholerae non-O1, non-O139 strain and VesC from a ΔhapA ΔprtV Vibrio cholerae strain may play a role in pathogenesis by inducing a hemorrhagic response in the rabbit ileal loop (RIL) assay (8, 10). Our present study demonstrated that HAP and VesC are also secreted in association with outer membrane vesicles (OMVs). Our results showed that HAP is secreted in association with OMVs from wild-type strain C6709, whereas the 59-kDa serine protease VesC is secreted in association with OMVs from hapA mutant strains but not from wild-type strain C6709. The absence of VesC in C6709 OMVs could be due to its proteolytic degradation in the presence of HAP, as it has been reported to mask the secretion of other proteases from V. cholerae (3, 10). Earlier studies have shown that secretion of serine proteases is regulated by metalloproteases in Vibrio species (3, 37). Syngkon et al. showed that 90% of the protease activity in strain C6709 is due to HAP, as only residual protease activity was detected in hapA and prtV mutant strains (10). Sikora et al. reported the presence of several serine proteases, i.e., VesA, VesB, and VesC, in V. cholerae O1 El Tor strain N16961, in which significantly less HAP is produced due to a frameshift mutation in HapR (38). However, Altindis et al. showed the presence of VesC in the OMVs of V. cholerae wild-type strain C6706 when grown under conditions which activate TCP (22). HAP was not detected in the OMVs of the wild-type strain under these conditions, as production of HAP is coordinated in a fashion reciprocal to that for cholera toxin and TCP (39). Secretion of HAP from OMVs of V. cholerae is influenced by serine protease DegP, and the degP mutant strains have been shown to secrete HAP and 8 other proteins from outer membrane vesicles (22).
Immunoblotting results showed the presence of mature 45-kDa HAP in C6709 OMVs instead of its processed 35-kDa form secreted through the TIISS. The mature 45-kDa form of HAP was purified earlier by Ghosh et al. in the presence of the metalloprotease inhibitor EDTA, whereas in its absence the processed 35-kDa form is purified from culture supernatant (8). The protease undergoes several steps of processing, including cleavage of the signal peptide and further processing of the N terminus and C terminus to generate the 35-kDa protein (4). The presence of the 45-kDa form of HAP within the C6709 OMVs could be due to its luminal localization within the vesicles. Earlier studies have shown that luminal localization of proteins within OMVs protects them from proteolytic digestions (40). Therefore, it may be suggested that the 45-kDa HAP is protected within the OMVs from further processing.
OMVs have been shown to deliver different proteins and toxins into host cells in an active form (40). Several studies have shown that toxins such as CT, RTX, VCC, and metalloprotease PrtV in V. cholerae are transported to host cells through OMVs in a biologically active form (19, 20, 41). Our present study showed that both HAP- and VesC-associated OMVs are internalized into human intestinal epithelial cells (Int407) in a protease-independent manner. Confocal microscopic images revealed that these OMVs may be internalized into host cells through lipid rafts, as vesicular endocytosis was completely inhibited by MβCD and nystatin.
The proteases in OMVs are transported in biologically active forms. OMV-associated HAP induces dose-dependent morphological changes in Int407 cells similar to those shown by purified HAP in our earlier study (8). HAP has been shown to proteolytically activate El Tor hemolysin/cytolysin by nicking at its N-terminal peptide to generate the 65-kDa mature form (7). Our results showed that OMV-associated HAP also digests the 79-kDa prohemolysin to generate 65-kDa and 50-kDa mature forms. The 50-kDa mature hemolysin is a C-terminally processed, identically truncated monomer of the 65-kDa mature hemolysin reported by Ekigai et al. in V. cholerae O1 strains (42).
The roles of OMV-associated proteases in pathogenesis were studied in the mouse ileal loop (MIL) model. This is the first study to show fluid accumulation in mouse ileal loops caused by V. cholerae OMV-associated proteases. HAP-associated OMVs showed an enterotoxic response in the MIL assay. VesC-associated CHA6.8 ΔprtV OMVs showed a visible hemorrhagic fluid response in MILs. Histopathological analysis of the mice ileum showed grossly disrupted villi with hemorrhage in all layers of the mucosa, which was significantly reduced upon treatment with CHA6. 8ΔprtV ΔVC1649 OMVs. These effects were restored with OMVs from the VC1649 complemented strain. A similar hemorrhagic fluid response was observed with partially purified VesC and culture supernatants from CHA6.8 ΔprtV in rabbit ileal loop (RIL) assay by Syngkon et al. They showed significant reduction of hemorrhagic fluid when inhibited with PMSF (10). This hemorrhagic fluid accumulation was not due to cholera toxin, as CT-bead ELISA showed the absence of CT in V. cholerae OMVs (Fig. 4C). The OMV-free culture supernatants also showed the absence of CT in bead ELISA (data not shown). This may be due to different conditions for production of CT and HAP. Benitez et al. showed that growth conditions that favor production of HAP downregulate CT and TCP and vice versa (43).
OMV-associated proteases of V. cholerae induced cytotoxic effects in Int407 cells in a dose-dependent manner. C6709 OMVs induced an apoptotic response in Int407 cells. Thay et al. have shown that membrane-derived vesicles from Staphylococcus aureus induce apoptosis in a dose-dependent manner by an α-toxin-dependent mechanism (29). However, the apoptotic cell population was not significantly reduced when treated with CHA6.8 OMVs. CHA6.8 OMVs induced a greater cytotoxic response with an increase in necrotic cells compared to HAP-associated C6709 OMVs. Studies have shown that there is an increased virulence in hapA-deleted V. cholerae strains. Benitez et al. showed that inactivation of hapA results in increased intestinal colonization and adherence of V. cholerae in human intestinal epithelial cells (44). Zhou et al. also reported that hapA-defective cholera vaccine candidate strains showed reactogenicity. Culture supernatants of these strains can stimulate IL-8 secretion from T-84 cells (45). Therefore, it may be suggested that the presence of metalloprotease PrtV in CHA6.8 OMVs may be responsible for increased OMV-mediated cytotoxicity. However, earlier studies have shown that PrtV modulates IL-8 secretion from T84 cells (46). Interestingly, when cells were treated with CHA6.8 ΔprtV, a shift from an apoptotic population to a necrotic population was observed. In CHA6.8 ΔprtV ΔVC1649 OMVs, the cytotoxicity was significantly reduced, and increased cytotoxicity in Int407 cells was restored with OMVs from the VC1649 complemented strain. These results are very similar to the effect observed with OMVs on MIL assay.
Earlier studies on the pathogenicity of OMVs in bacterial infections have shown the role of lipopolysaccharide (LPS)- and OMV pathogen-associated molecular patterns (47, 48). LPS in the OMVs is sensed by the Toll-like receptor 4 (TLR4) complex to trigger inflammatory responses (49). OMVs isolated from Salmonella enterica serovar Typhimurium activate macrophages and dendritic cells to increase levels of surface major histocompatibility complex (MHC) class II expression as well as the production of the proinflammatory mediators tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) (50). Helicobacter pylori and Pseudomonas aeruginosa OMVs show a proinflammatory response by IL-8 secretion (51, 52). IL-8 is a major proinflammatory cytokine which act as a potent chemoattractant for polymorphonuclear leukocytes (PMN) and recruits them into infected sites of the epithelial layer, which results in opening of epithelial tight junctions (53). The IL-8 response of Vibrio cholerae OMVs was shown by Chatterjee and Chaudhuri. They showed that OMVs from classical V. cholerae strain O395 induce IL-8 secretion from intestinal epithelial cells, which activates dendritic cells to promote T-cell polarization (54). However, our results showed that HAP-associated OMVs from El Tor strain C6709 failed to induce significant IL-8 secretion from T84 cells. Earlier studies have also shown that HAP does not induce IL-8 secretion from intestinal epithelial cells (45). OMVs from ΔhapA strain CHA6.8 showed ∼10-fold-increased IL-8 secretion compared to untreated controls, whereas IL-8 secretion was increased ∼25-fold upon treatment with CHA6.8 ΔprtV OMVs. The greater IL-8 response by OMVs from the ΔhapA ΔprtV double mutant strain could be due to the absence of PrtV, as it has been reported to reduce IL-8 secretion from intestinal epithelial cells (46). This increased IL-8 secretion by CHA6.8 ΔprtV was significantly reduced upon treatment with OMVs from the VesC mutant strain. Interestingly, OMVs from Comp VC1649 showed significant induction of IL-8 secretion. These results show that VesC within the V. cholerae OMVs is responsible for the increased IL-8 response in T84 cells.
Our results revealed 3-fold-increased colonization of the CHA6.8 ΔprtV strain compared to the VesC-deleted CHA6.8ΔprtVΔVC1649 strain in adult mice. Interestingly increased colonization of V. cholerae was restored with the VC1649 complemented strain. Earlier, Silva et al. (59) also reported an increased colonization of hapA mutant strains in infant mouse model. However, Sikora et al. reported that VesC is not responsible for colonization of V. cholerae in infant mouse model (38). Our results showed that VesC may play an important role in colonization in adult mice.
OMVs have recently been used to develop acellular vaccines for several diseases related to Gram-negative bacteria. The most successful use of OMVs as a vaccine candidate has been against serogroup B Neisseria meningitides infection, for which over 55 million doses have been administered to date against serogroup B infections (55). Schild et al. showed that OMVs from V. cholerae can generate a protective immune response against cholera infection in the adult mouse model (25). However, our results suggest that the presence of proteases in the OMVs may affect the use of OMVs as a vaccine candidate for cholera.
In conclusion, we showed that the proteases HAP and VesC are secreted in association with V. cholerae OMVs in an active form. OMV-associated VesC induces a hemorrhagic response in the MIL assay and a proinflammatory response in human cultured intestinal epithelial cells, and VesC may play an important role in colonization in the adult mouse model.
ACKNOWLEDGMENTS
This work was supported by intramural funding from the Indian Council of Medical Research. A.M. was supported by a fellowship from the Indian Council of Medical Research.
We thank Somnath Chatterjee, confocal microscope operator, NICED central facility (Carl Zeiss, India), for technical help with confocal microscopic studies, Somdatta Chatterjee, Division of Bacteriology, NICED, for crucial assistance with VC1649 complementation, and Amarshi Mukherjee, Division of Biochemistry, NICED, for providing purified hemolysin.
REFERENCES
- 1.Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, Bose N, Brewer T, Calderwood SB, Clemens JD, Cravioto A, Eustache E, Jérôme G, Gupta N, Harris JB, Hiatt HH, Holstein C, Hotez PJ, Ivers LC, Kerry VB, Koenig SP, Larocque RC, Léandre F, Lambert W, Lyon E, Mekalanos JJ, Mukherjee JS, Oswald C, Pape JW, Gretchko Prosper A, Rabinovich R, Raymonville M, Réjouit JR, Ronan LJ, Rosenberg ML, Ryan ET, Sachs JD, Sack DA, Surena C, Suri AA, Ternier R, Waldor MK, Walton D, Weigel JL. 2011. Meeting cholera's challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis 5:e1145. doi: 10.1371/journal.pntd.0001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faruque SM, Albert MJ, Mekalanos JJ. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 62:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyoshi S. 2013. Extracellular proteolytic enzymes produced by human pathogenic Vibrio species. Front Microbiol 4:339. doi: 10.3389/fmicb.2013.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häse CC, Finkelstein RA. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol 173:3311–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein RA, Boesman-Finkelstein M, Chang Y, Häse CC. 1992. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun 60:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth BA, Boesman-Finkelstein M, Finkelstein RA. 1984. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun 45:558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagamune K, Yamamoto K, Honda T. 1997. Intramolecular chaperone activity of the pro-region of Vibrio cholerae El Tor cytolysin. J Biol Chem 272:1338–1343. doi: 10.1074/jbc.272.2.1338. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh A, Saha DR, Hoque KM, Asakuna M, Yamasaki S, Koley H, Das SS, Chakrabarti MK, Pal A. 2006. Enterotoxigenicity of mature 45-kilodalton and processed 35-kilodalton forms of hemagglutinin protease purified from a cholera toxin gene-negative Vibrio cholerae non-O1, non-O139 strain. Infect Immun 74:2937–2946. doi: 10.1128/IAI.74.5.2937-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaitkevicius K, Lindmark B, Ou G, Song T, Toma C, Iwanaga M, Zhu J, Andersson A, Hammarström ML, Tuck S, Wai SN. 2006. A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc Natl Acad Sci U S A 103:9280–9285. doi: 10.1073/pnas.0601754103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syngkon A, Elluri S, Koley H, Rompikunata PK, Saha DR, Chakrabarti MK, Bhadra RK, Wai SN, Pal A. 2010. Studies on a novel serine protease of a ΔhapAΔprtV Vibrio cholerae O1 strain and its role in haemorrhagic response in the rabbit ileal loop model. PLoS One 5:e13122. doi: 10.1371/journal.pone.0013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugsley AP. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 57:50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and host pathogen interaction. Genes Dev 19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 13.Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dhalfors A, Mizunoe Y, Uhlin BE. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35. doi: 10.1016/S0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 14.Balsalobre C, Silvan JM, Berglund S, Mizunoe Y, Uhlin BE, Wai SN. 2006. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol Microbiol 59:99–112. doi: 10.1111/j.1365-2958.2005.04938.x. [DOI] [PubMed] [Google Scholar]
- 15.Davis JM, Carvalho HM, Rasmussen SB, O'Brien AD. 2006. Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic E. coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect Immun 74:4401–4408. doi: 10.1128/IAI.00637-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindmark B, Rompikuntal PK, Vaitkevicius K, Song T, Mizunoe Y, Uhlin BE, Guerry P, Wai SN. 2009. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol 9:220. doi: 10.1186/1471-2180-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker H, Chitcholtan K, Hampton MB, Keenan JI. 2010. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect Immun 78:5054–5061. doi: 10.1128/IAI.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomberger JM, MacEachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 5:e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee D, Chaudhuri K. 2011. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett 585:1357–1362. doi: 10.1016/j.febslet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Elluri S, Enow C, Vdovikova S, Rompikunal P, Dongre M, Carlsson S, Pal A, Uhlin BE, Wai SN. 2014. Outer membrane vesicles mediate transport of biologically active Vibrio cholerae cytolysin (VCC) from V. cholerae strains. PLoS One 9:e106731. doi: 10.1371/journal.pone.0106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rompikuntal PK, Vdovikova S, Duperthuy M, Jhonson TL, Ahlund M, Lundmark R, Oscarson J, Sandkvist M, Uhlin BE, Wai SN. 2015. Outer membrane vesicle mediated export of processed PrtV protease from Vibrio cholerae. PLoS One 10:e0134098. doi: 10.1371/journal.pone.0134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altindis E, Fu Y, Mekalanos JJ. 2014. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc Natl Acad Sci U S A 111:E1548–E1556. doi: 10.1073/pnas.1403683111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosink KK, Kobayashi R, Kawagishi I, Hase CC. 2002. Analyses of the roles of three cheA homologs in chemotaxis of Vibrio cholerae. J Bacteriol 184:1767–1771. doi: 10.1128/JB.184.6.1767-1771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. 2010. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci U S A 107:3099–3104. doi: 10.1073/pnas.0805532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schild S, Nelson EJ, Camilli A. 2008. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun 76:4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng LW, Schneewind O. 2000. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol 182:3183–3190. doi: 10.1128/JB.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker H, Chitcholtan K, Hampton MB, Keenan JI. 2010. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect Immun 72:5054–5061. doi: 10.1128/IAI.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawasvirojwong S, Srimanote P, Chatsudthipong V, Muanprasat C. 2013. An adult mouse model of Vibrio cholerae induced diarrhea for studying pathogenesis and potential therapy of cholera. PLoS Negl Trop Dis 7:e2293. doi: 10.1371/journal.pntd.0002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thay B, Wai SN, Oscarsson J. 2013. Staphylococcus aureus α-toxin dependent induction of host cell death by membrane derived vesicles. PLoS One 8:e54661. doi: 10.1371/journal.pone.0054661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oku Y, Uesaka Y, Hirayama T, Takeda Y. 1988. Development of highly sensitive bead-ELISA to detect bacterial protein toxins. Microbiol Immunol 32:807–816. doi: 10.1111/j.1348-0421.1988.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 31.Olivier V, Queen J, Satchell KJF. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. doi: 10.1371/journal.pone.0007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee SN, Das J. 1967. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol 49:1–11. doi: 10.1099/00221287-49-1-1. [DOI] [PubMed] [Google Scholar]
- 33.Rodal SK, Skretting G, Garred F, Vilhardt B, Van Duers B, Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnington KE, Kuehn MJ. 2014. Protein secretion and export via outer membrane vesicles. Biochim Biophys Acta 1843:1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikh IA, Koley H, Chakrabarti MK, Hoque KM. 2013. The Epac signalling pathway regulates Cl− secretion via modulation of apical KCNN4c channels in diarrhea. J Biol Chem 288:20404–20415. doi: 10.1074/jbc.M113.467860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott ME, Dossani ZY, Sandkvist M. 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc Natl Acad Sci U S A 98:13978–13983. doi: 10.1073/pnas.241411198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young DB, Broadbent DA. 1982. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun 37:875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikora AE, Zielke RA, Lawrence DA, Andrews PC, Sandkvist M. 2011. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem 286:16555–16566. doi: 10.1074/jbc.M110.211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva AJ, Pham K, Benitez JA. 2003. Hemaglutinnin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883–1891. doi: 10.1099/mic.0.26086-0. [DOI] [PubMed] [Google Scholar]
- 40.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boardman BK, Meehan BM, Fullner-Satchell KJ. 2007. Growth phase regulation of Vibrio cholerae RTX toxin export. J Bacteriol 189:1827–1835. doi: 10.1128/JB.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikigai H, Ono T, Nakae T, Otsuru H, Shimamura T. 1999. Two form of Vibrio cholerae O1 El Tor hemolysin derived from identical precursor protein. Biochim Biophys Acta 1415:297–305. doi: 10.1016/S0005-2736(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 43.Benitez JA, Silva AJ, Finkelstein RA. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect Immun 69:6549–6553. doi: 10.1128/IAI.69.10.6549-6553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bénitez JA, Spelbrink RG, Silva A, Phillips TE, Stanley CM, Boesman-Finkelstein M, Finkelstein RA. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun 65:3474–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Gao DQ, Michalski J, Benitez JA, Kaper JB. 2004. Induction of interleukin-8 in T84 cells by Vibrio cholerae. Infect Immun 72:389–397. doi: 10.1128/IAI.72.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou G, Rompikuntal PK, Bitar A, Lindmark B, Vaitkevicius K, Wai SN, Hammarström ML. 2009. Vibrio cholerae cytolysin causes an inflammatory response in human intestinal epithelial cells that is modulated by the PrtV protease. PLoS One 4:e7806. doi: 10.1371/journal.pone.0007806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitmire WM, Garon CF. 1993. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdoferi. Infect Immun 61:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opal SM. 2007. The host response to endotoxin, antilipopolysaccaride strategies, and the management of severe sepsis. Int J Med Microbiol 297:365–377. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Ellis TN, Kuehn MJ. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev 74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol 179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 51.Bauman SJ, Kuehn MJ. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect 8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ismail S, Hampton MB, Keenan JI. 2003. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun 71:5670–5675. doi: 10.1128/IAI.71.10.5670-5675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCormick BA, Siber AM, Maurelli AT. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun 66:4237–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatterjee D, Chaudhuri K. 2013. Vibrio cholera O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 dependent manner and induces dendritic cell-mediated Th2/Th17 responses. J Biol Chem 288:4299–4309. doi: 10.1074/jbc.M112.408302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahhan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitides. Vaccine 27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 56.Haralalka S, Nandi S, Bhadra RK. 2003. Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J Bacteriol 185:4672–4682. doi: 10.1128/JB.185.16.4672-4682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar M, Chaudhuri K. 2004. Association of adherence and motility in interleukin 8 induction in human intestinal epithelial cells by Vibrio cholerae. Microbes Infect 6:676–685. doi: 10.1016/j.micinf.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Silva AJ, Leitch GJ, Camilli A, Benitez JA. 2006. Contribution of hemagglutinin/protease and motility to the pathogenesis of El Tor biotype cholera. Infect Immun 74:2072–2079. doi: 10.1128/IAI.74.4.2072-2079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]