Abstract
The apicomplexan parasite Cryptosporidium causes significant diarrheal disease worldwide. Effective anticryptosporidial agents are lacking, in part because the molecular mechanisms underlying Cryptosporidium-host cell interactions are poorly understood. Previously, we identified and characterized a novel Cryptosporidium parvum C-type lectin domain-containing mucin-like glycoprotein, CpClec. In this study, we evaluated the mechanisms underlying interactions of CpClec with intestinal epithelial cells by using an Fc-tagged recombinant protein. CpClec-Fc displayed Ca2+-dependent, saturable binding to HCT-8 and Caco-2 cells and competitively inhibited C. parvum attachment to and infection of HCT-8 cells. Binding of CpClec-Fc was specifically inhibited by sulfated glycosaminoglycans, particularly heparin and heparan sulfate. Binding was reduced after the removal of heparan sulfate and following the inhibition of glycosaminoglycan synthesis or sulfation in HCT-8 cells. Like CpClec-Fc binding, C. parvum attachment to and infection of HCT-8 cells were inhibited by glycosaminoglycans and were reduced after heparan sulfate removal or inhibition of glycosaminoglycan synthesis or sulfation. Lastly, CpClec-Fc binding and C. parvum sporozoite attachment were significantly decreased in CHO cell mutants defective in glycosaminoglycan synthesis. Together, these results indicate that CpClec is a novel C-type lectin that mediates C. parvum attachment and infection via Ca2+-dependent binding to sulfated proteoglycans on intestinal epithelial cells.
INTRODUCTION
Cryptosporidium is an apicomplexan parasite that causes significant diarrheal disease worldwide (1). It is endemic to many resource-limited countries and causes recreational water outbreaks in industrialized nations (2). Disease is self-limiting in immunocompetent hosts but can be debilitating, even fatal, in immunocompromised individuals, particularly untreated AIDS patients (3) and malnourished children (1) in resource-limited areas. Cryptosporidium is one of four pathogens responsible for most cases of moderate-to-severe diarrhea in young children in Asia and Africa and is the second leading cause of diarrheal disease and death in these children (4). Still, no consistently effective therapies exist for these vulnerable populations (5), making it urgent to identify molecular targets for the development of novel interventions.
Proteins involved in mediating Cryptosporidium-host cell interactions are potential targets for intervention, but few have been characterized. Until recently (6, 7), the inability to propagate Cryptosporidium in vitro and the lack of a system for genetic manipulation have hindered the discovery and validation of new molecular targets. Still, many studies, including our own, have demonstrated the importance of mucin-like glycoproteins and lectins in mediating Cryptosporidium infection in vitro and in vivo (8, 9).
Previously, we reported the identification and characterization of a C-type lectin domain (CTLD)-containing protein from Cryptosporidium parvum named CpClec (10). CTLD-containing proteins are calcium-dependent, glycan-binding proteins ubiquitous among both vertebrates and invertebrates (11). They play essential roles in cell-cell interactions, with diverse functions ranging from pathogen recognition and immune activation to microbial adhesion and host cell invasion. CpClec is the first CTLD-containing protein reported in a protozoan. It is a type 1 transmembrane protein that contains, in addition to a CTLD, a mucin-like domain predicted to be O glycosylated and a tyrosine-based sorting motif in the cytoplasmic tail (10). Native CpClec is ∼120 kDa, larger than the predicted size of 86 kDa, likely because of glycosylation. Expression of CpClec is developmentally regulated, and the protein localizes to the apical region and dense granules in sporozoites and merozoites, as well as to the feeder organelle in intracellular stages, suggesting possible roles in host cell attachment, invasion, and/or intracellular development. We identified a single CTLD-containing protein in multiple Cryptosporidium spp. and in all cyst-forming, gut-invading apicomplexans (10), including the early-branching gregarines (J. G. Ludington and H. D. Ward, unpublished data), suggesting that these are evolutionarily conserved proteins that may be important in infection of the intestine.
Proteoglycans consist of a core protein attached to a glycosaminoglycan (GAG) (12). They can be membrane bound, intracellular, or secreted into the extracellular matrix. Differences in core proteins, along with variations in the type(s) and stoichiometry of attached GAG chains, create significant structural and functional diversity (12). Most relevant to this study are the heparan sulfate-containing proteoglycans (HSPGs) in the small intestine (13). These can be secreted into the overlying mucus layer or function as membrane-bound components of the intestinal glycocalyx.
Many pathogens utilize proteoglycans during infection (14), including HIV (15), Borrelia burgdorferi (16, 17), Plasmodium spp. (18, 19), and Toxoplasma gondii (20–23). Recently, Inomata et al. reported that heparin mediates C. parvum invasion in vitro via interaction with elongation factor 1α (24). Still, the precise role of GAGs during C. parvum infection and the mechanisms underlying these interactions are poorly understood.
In this report, we characterize the mechanisms underlying CpClec interactions with host cells by using an Fc-tagged recombinant protein. Our results indicate that CpClec is a novel C-type lectin that mediates C. parvum infection by binding to HSPGs on intestinal epithelial cells.
MATERIALS AND METHODS
C. parvum.
C. parvum (Iowa isolate) oocysts were obtained from Bunch Grass Farms, Deary, ID. Prior to use, oocysts were surface sterilized with a 10% (vol/vol) commercial bleach solution (sodium hypochlorite).
Cell lines.
HEK 293T cells were provided by Linden Hu (Tufts University, Boston, MA). CHO cell lines K1 (wild type), pgsA-745 (deficient in xylosyl transferase I) (25), and pgsD-677 (deficient in N-acetylglucosaminyltransferase and glucuronosyltransferase) (26) were provided by John Leong (Tufts University, Boston, MA). The intestinal epithelial cell lines HCT-8 and Caco-2 were provided by Doug Jefferson (Tufts University, Boston, MA). CHO cell lines were maintained at 37°C in 5% CO2 in Ham's F12 medium (Life Technologies, Grand Island, NY) containing 10% fetal bovine serum (FBS), 2 mM glutamine, and 25 mM HEPES. All other cell lines were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle medium (DMEM; Life Technologies) containing 10% FBS and 25 mM HEPES.
CpClec-Fc cloning, expression, and purification.
The DNA sequence encoding the extracellular C-type lectin and mucin-like domains of CpClec were amplified from a T. gondii pHLEM expression vector containing the full-length sequence (S. Bhalchandra and H. D. Ward, unpublished data) with the following primers (restriction sites are underlined): forward, GGGGATATCAGGAGATACTGGCCATGCAAT; reverse, GGGAGATCTTGGCCTAAGAGTGGTTGT. The sequence was amplified with an Mx3000P thermocycler (Stratagene, San Diego, CA) with cycling conditions of 95°C for 10 min; 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s; and 72°C for 10 min. The product was cloned into the pCR-2.1-TOPO vector (Life Technologies, Grand Island, NY). Constructs containing the correct sequences were inserted into the pFUSE-mIgG2a-Fc vector (InvivoGen, San Diego, CA) by using EcoRV and BglII (New England BioLabs, Ipswich, MA). DNA sequencing was performed on a Perkin-Elmer ABI 377 sequencer at the Tufts University Core Facility.
The pFUSE-mIgG2a-Fc plasmid encoding CpClec-Fc or Fc alone was transfected into HEK 293T cells with polyethyleneimine (Sigma, St. Louis, MO). Recombinant proteins were purified from supernatants of transfected cells by protein G affinity chromatography (GE Healthcare Life Sciences, Pittsburgh, PA). Dialyzed recombinant proteins were stored at −80°C in phosphate-buffered saline (PBS) containing an EDTA-free protease inhibitor cocktail (Sigma) and 0.02% sodium azide.
Protein concentration was determined with a BCA assay kit (Life Technologies), and purity and molecular weight were determined by 10% SDS-PAGE under reducing conditions, followed by Coomassie blue staining and/or immunoblotting with horseradish peroxidase-conjugated anti-mouse IgG (Vector Laboratories).
For deglycosylation, 10 μg of CpClec-Fc or Fc alone was denatured at 95°C for 10 min; this was followed by incubation with neuraminidase and O-glycosidase (Sigma) or peptide N-glycosidase F (Sigma) for 4 h at 37°C. Changes in protein size were evaluated by SDS-PAGE and Coomassie blue staining.
Glycan array analysis.
To determine if CpClec binds to known mammalian glycans, 40 μg of purified CpClec in PBS was submitted to the Consortium for Functional Glycomics (CFG; http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp) for analysis on the CFG mammalian glycan array, version 5.2 (27).
CpClec-Fc binding assays.
For immunofluorescence-based binding assays, HCT-8, CHO K1, CHO pgsA-745, or CHO pgsD-677 cells were grown to confluence on 12-mm coverslips in 24-well plates at 37°C in 5% CO2. Cells were incubated with 200 nM CpClec-Fc or Fc alone in serum-free DMEM containing 0.1% normal goat serum (NGS), 1 mM CaCl2, 1 mM MnCl2, and 0.2% sodium azide for 1 h at 4°C. Cells were washed three times with PBS and fixed with ice-cold 100% methanol for 10 min at room temperature (RT). After three washes with PBS, cells were incubated with goat anti-mouse IgG conjugated to Alexa Fluor 488 (Life Technologies). The coverslips were mounted on microscopy slides with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and examined by fluorescence microscopy with a Zeiss Axio Imager Z.1 fluorescence microscope (Carl Zeiss Microscopy, Jena, Germany). Images were captured with an IEEE 1394 digital charge-coupled device camera (Hamamatsu, Hamamatsu City, Japan), and localization of fluorescence was achieved with Volocity software (Improvision Inc., Lexington, MA).
For enzyme-linked immunosorbent assay (ELISA)-based binding assays, cells were grown to confluence in 96-well plates at 37°C in 5% CO2. For live-cell assays, HCT-8 cells were incubated with various concentrations of CpClec-Fc or Fc alone in serum-free DMEM containing 0.1% NGS, 1 mM CaCl2, 1 mM MnCl2, and 0.2% sodium azide for 1 h at 4°C. For fixed-cell assays, HCT-8, Caco-2, or CHO cells were fixed with 1% glutaraldehyde in PBS for 30 min at RT, quenched with 0.1 M glycine in PBS, and washed twice with serum-free DMEM. Nonspecific binding was blocked with serum-free DMEM containing 5% NGS for 4 h at RT. Cells were washed once with serum-free DMEM and incubated with various concentrations of CpClec-Fc or Fc alone in serum-free DMEM containing 0.1% NGS, 1 mM CaCl2, and 1 mM MnCl2 for 1 h at RT. Following incubation, cells were washed three times with serum-free DMEM and fixed with ice-cold 100% methanol for 10 min at RT. Cells were washed three times with Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl, pH 7.4), and nonspecific binding was blocked with TBS containing 2% NGS overnight at 4°C. Cells were washed three times with TBS, and bound proteins were quantified by ELISA with biotinylated anti-mouse Fc IgG (Vector Laboratories), followed by streptavidin-conjugated alkaline phosphatase (Vector Laboratories) and p-nitrophenylphosphate (Sigma) substrate. Binding was detected by measuring A405 with a Wallac Victor2 1420 multilabel counter (PerkinElmer, Waltham, MA).
To evaluate calcium dependence, 200 nM CpClec-Fc or Fc alone was incubated with increasing concentrations of EGTA for 30 min at RT and binding was measured by ELISA. To assess whether Ca2+ could reverse inhibition by EGTA, 200 nM CpClec-Fc or Fc alone was incubated with 5 mM EGTA for 30 min at RT; this was followed by incubation with increasing concentrations of CaCl2 for an additional 10 min at RT, and binding was measured by ELISA. To control for a possible effect of calcium on the binding of biotinylated anti-mouse Fc IgG to Fc-tagged proteins, 10 μg/ml mouse IgG (Sigma) diluted in 0.05 M sodium carbonate-bicarbonate buffer, pH 9.6, was allowed to adsorb to Nunc-Immuno MaxiSorp 96-well plates (Thermo Fisher Scientific, Waltham, MA) overnight at 4°C. Wells were washed three times with TBS, and nonspecific binding was blocked with TBS containing 5% NGS for 2 h at RT. Wells were washed three times with TBS, and binding of biotinylated anti-mouse Fc IgG preincubated with increasing concentrations of EGTA for 30 min at RT was measured by ELISA.
For glycan-binding assays, 200 nM CpClec-Fc or Fc alone was preincubated with 500 mM monosaccharides (glucose, fructose, galactose, N-acetylgalactosamine, sialic acid) or disaccharides (sucrose, lactose, maltose); increasing concentrations of heparan sulfate, heparin, chondroitin sulfate A, chondroitin sulfate B, hyaluronic acid, or fucoidan (all from Sigma); or medium alone as a control for 20 min at RT. This was followed by incubation with fixed HCT-8 or Caco-2 cells grown to confluence in 96-well plates. Binding was measured by ELISA.
HCT-8 cells grown in 96-well plates were incubated with 0.5 U/ml heparinase II (Sigma) in lyase buffer (serum-free DMEM containing 1% BSA and EDTA-free protease inhibitor cocktail (Fisher Scientific, Pittsburgh, PA) or with lyase buffer alone as a control for 2 h at 37°C or with 30 mM NaClO3, 30 mM NaCl, 2 mM 4-nitrophenyl-β-d-xylopyranoside (β-xyloside; Sigma), or 2 mM 4-nitrophenyl-α-d-xylopyranoside (α-xyloside; Sigma) in complete DMEM as a control for 12 h at 37°C in 5% CO2. Cells were fixed, and CpClec-Fc or Fc binding was measured by ELISA. CHO cell lines grown to confluence in 96-well plates at 37°C in 5% CO2 were fixed as described above; this was followed by incubation with 200 nM CpClec-Fc or Fc alone, and binding was measured by ELISA.
C. parvum attachment and infection assays.
For attachment assays, hypochlorite-treated oocysts were incubated in 0.75% sodium taurocholate in PBS for 30 min at 37°C. Excysted sporozoites were labeled with 10 μM Vybrant carboxyfluorescein diacetate succinimidyl ester (CFSE; Life Technologies) (28). Sporozoites were purified by filtration through a 3-μm filter (Millipore, Billerica, MA). For attachment inhibition assays with CpClec-Fc, HCT-8 cells grown to confluence on 12-mm coverslips in 24-well plates were fixed with 2% paraformaldehyde (PFA) for 15 min at RT, nonspecific binding was blocked with serum-free DMEM containing 4% BSA for 4 h at RT, and fixed cells were incubated with increasing concentrations of CpClec-Fc or Fc alone in serum-free DMEM containing 0.1% BSA, 1 mM CaCl2, and 1 mM MnCl2 for 1 h at RT. For attachment inhibition assays with GAGs, sporozoites were incubated with increasing concentrations of GAGs in serum-free DMEM containing 0.1% BSA for 20 min at 4°C prior to incubation with HCT-8 cells. In addition, HCT-8 cells were treated with 0.5 U/ml heparinase II, 30 mM NaClO3, 30 mM NaCl, 2 mM β-xyloside, or 2 mM α-xyloside; washed three times with serum-free DMEM; and fixed with 2% PFA. CHO cell lines were grown to confluence and fixed as described above. A total of 4 × 105 sporozoites were incubated with HCT-8 or CHO cells for 1 h at 37°C in 5% CO2, washed twice with PBS, and fixed with ice-cold methanol for 10 min at RT. Cells were washed three times with PBS, and coverslips were mounted on slides with Vectashield mounting medium containing DAPI (Vector Laboratories) and examined by fluorescence microscopy. For quantitation of attachment, images of three separate 10× fields were captured per slide and numbers of CFSE-labeled sporozoites per field were quantitated by Volocity point measurement analysis.
Infection assays were adapted from those of Bessoff et al. (29) and Gut and Nelson (30). Hypochlorite-treated oocysts were incubated with 0.75% sodium taurocholate in DMEM containing 2% FBS, 2 mM glutamine, and 25 mM HEPES (infection medium) for 10 min at 15°C (30) and diluted in infection medium prior to use. HCT-8 cells were grown to confluence in complete DMEM on 12-mm coverslips in 24-well plates at 37°C in 5% CO2. For infection inhibition assays with CpClec-Fc, cells were preincubated with 200 nM CpClec-Fc or Fc alone in complete DMEM or medium alone as a control for 1 h at 37°C in 5% CO2 and washed three times with infection medium. For infection inhibition assays with GAGs, oocysts were incubated with increasing concentrations of GAGs in infection medium or in infection medium alone as a control for 30 min at 37°C prior to incubation with HCT-8 cells. HCT-8 cells were treated with 0.5 U/ml heparinase II, 30 mM NaClO3, 30 mM NaCl, 2 mM β-xyloside, or 2 mM α-xyloside and washed three times with infection medium. For all experiments, HCT-8 cells were incubated with 1 × 105 oocysts in infection medium for 3 h at 37°C in 5% CO2. Cells were washed three times with complete DMEM and incubated in complete DMEM for another 24 h at 37°C in 5% CO2. Cells were washed once with PBS, fixed with 4% PFA in PBS for 15 min at RT, permeabilized with 0.25% Triton X-100 in PBS for 10 min at 37°C, and washed three times with 0.1% Tween 20 in PBS. Nonspecific binding was blocked with PBS containing 4% BSA for 2 h at RT. Cells were washed three times with PBS and incubated with biotinylated Vicia villosa lectin (VVL; Vector Laboratories), which recognizes sporozoites and intracellular stages (30), and streptavidin-conjugated Alexa Fluor 568 (Life Technologies) in PBS containing 0.1% Tween 20 and 1% BSA for 1 h at RT. Cells were washed three times with PBS, mounted with Vectashield mounting medium containing DAPI (Vector Laboratories), and examined by fluorescence microscopy. For quantitation of infection, images of three separate 10× fields per slide were captured and numbers of VVL-stained parasites per field were quantified by Volocity point measurement analysis.
To compare C. parvum infection of HCT-8 and CHO K1 cells, cells were grown to confluence in complete DMEM or Ham's F12 medium, respectively, in 24-well plates at 37°C in 5% CO2, and infection was evaluated as described above.
Statistical analysis.
Data presented are representative of at least two independent experiments, each performed with three or more replicates. Data are expressed as the mean ± the standard error of the mean (SEM). Statistical analyses were performed in GraphPad Prism version 6 for Mac (GraphPad Software, San Diego, CA). Statistical significance was determined with Student's t test.
RESULTS
CpClec-Fc binding to intestinal epithelial cells is specific, saturable, and calcium dependent.
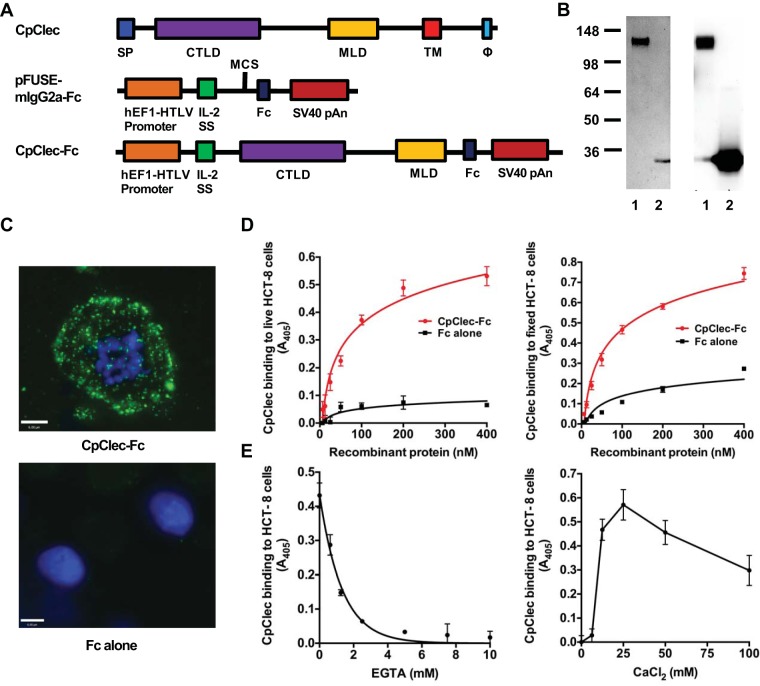
In order to characterize interactions of CpClec with intestinal epithelial cells, we generated, expressed, and purified an Fc-tagged recombinant protein containing the extracellular C-type lectin and mucin-like domains of CpClec (Fig. 1A). As a control, we expressed and purified the Fc tag alone. CpClec-Fc and Fc migrated with molecular masses of ∼140 and ∼30 kDa, respectively, on SDS-PAGE and immunoblotting (Fig. 1B), larger than the predicted sizes of ∼80 and ∼25 kDa, suggesting that the proteins undergo posttranslational modification. CpClec contains 40 residues predicted to be O-glycosylated and 7 residues predicted to be N-glycosylated (10). The Fc tag contains a single N-glycosylation site (31). It is therefore possible that the increased sizes of native CpClec and recombinant CpClec-Fc are due to O-glycosylation and/or N-glycosylation. Removal of O-glycans decreased the molecular mass to ∼130 kDa, while removal of N glycans decreased the molecular mass to ∼110 kDa (see Fig. S1 in the supplemental material). Removal of O-glycans from recombinant Fc alone had no effect on protein size, while removal of N-glycans decreased the molecular mass to ∼25 kDa. Together, these data demonstrate that CpClec-Fc undergoes N-glycosylation of both the Fc and CpClec regions and O-glycosylation of the CpClec region. Still, deglycosylation did not reduce CpClec-Fc to the predicted size of ∼80 kDa. This may be due to protein charge, which can affect mobility during SDS-PAGE, or to other forms of posttranslational modification.
FIG 1.
CpClec-Fc binding to intestinal epithelial cells is specific, saturable, and calcium dependent. (A) CpClec contains a signal peptide (SP), a CTLD, a mucin-like domain (MLD), a transmembrane domain (TM), and a sorting motif (Φ) in the cytoplasmic tail. The CTLD and MLD were cloned into the pFUSE-mIgG2a-Fc vector. (B) Recombinant CpClec-Fc and Fc alone were expressed in HEK 293T cells, purified by protein G affinity chromatography, and analyzed by SDS-PAGE (left) and immunoblotting (right). Lanes: 1, CpClec-Fc; 2, Fc alone. The values to the left are molecular sizes in kilodaltons. (C) Live HCT-8 cells were incubated with CpClec-Fc or Fc alone, fixed, and visualized by fluorescence microscopy. Scale bars = 6 μm. (D) Live (left) or fixed (right) HCT-8 cells were incubated with CpClec-Fc or Fc alone, and binding was quantified by ELISA. (E, left) CpClec-Fc or Fc alone was incubated with EGTA, and binding to fixed HCT-8 cells was quantified by ELISA. (E, right) CpClec-Fc or Fc alone preincubated with EGTA was incubated with CaCl2, and binding to fixed HCT-8 cells was quantified by ELISA. CpClec-Fc binding was normalized to binding of Fc alone. Data represent the mean ± the SEM.
Binding of CpClec-Fc to live and fixed intestinal epithelial HCT-8 cells was punctate, dose dependent, and saturable, in contrast to the negligible binding observed with Fc alone (Fig. 1C and D). To determine whether CpClec requires calcium for host cell binding, similar to traditional C-type lectins (11), CpClec-Fc was preincubated with EGTA, a Ca2+-specific chelator, before incubation with HCT-8 cells. EGTA inhibited the binding of CpClec-Fc in a dose-dependent manner, with complete inhibition observed at a concentration of 5 mM. Binding was completely reconstituted upon the addition of 25 mM calcium chloride (CaCl2) (Fig. 1E), though binding decreased in the presence of higher concentrations of CaCl2, possibly because of the changes in ionic strength affecting protein conformation and/or binding (32). EGTA treatment did not affect the binding of anti-mouse IgG to mouse IgG Fc (data not shown). Binding of CpClec-Fc to Caco-2 cells, another intestinal epithelial cell line, was also dose and Ca2+ dependent (see Fig. S2A and B in the supplemental material). Taken together, these results suggest that CpClec binds to a specific ligand(s) on the intestinal epithelial cell surface and requires calcium for binding.
CpClec-Fc competitively inhibits attachment and infection of C. parvum in vitro.
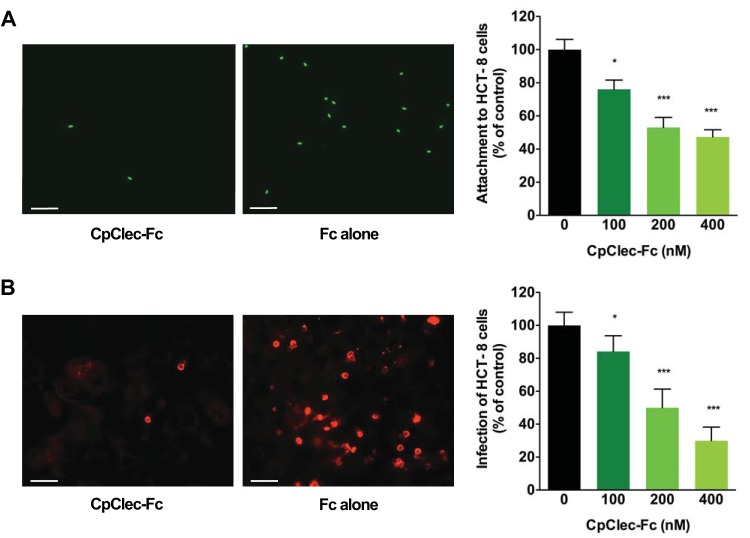
To determine whether CpClec mediates C. parvum attachment to and infection of intestinal epithelial cells, we attempted to competitively inhibit these processes in the presence of CpClec-Fc. Attachment of C. parvum sporozoites to HCT-8 cells was inhibited in a dose-dependent manner, with ∼50% inhibition observed in the presence of 400 nM CpClec-Fc (Fig. 2A). C. parvum infection of HCT-8 cells was reduced to an even greater extent than attachment alone, with ∼70% inhibition observed at the highest concentration of CpClec-Fc tested (Fig. 2B). Inhibition of attachment or infection in the presence of Fc alone was insignificant, indicating that the effect of CpClec-Fc was specific. These data show that CpClec plays a role in the attachment and invasion of C. parvum in vitro.
FIG 2.
CpClec-Fc inhibits C. parvum attachment and infection. (A) Fixed HCT-8 cells were incubated with CpClec-Fc, Fc alone, or medium; this was followed by incubation with CFSE-labeled C. parvum sporozoites. Attached sporozoites were quantified by fluorescence microscopy. Attachment in the presence of CpClec-Fc was normalized to attachment in the presence of Fc alone. (B) HCT-8 cells were incubated with CpClec-Fc, Fc alone, or medium; infected with C. parvum oocysts; fixed; and permeabilized; and infection was quantified by fluorescence microscopy. Infection in the presence of CpClec-Fc was normalized to infection in the presence of Fc alone. Scale bars = 100 μm. Data represent the mean ± the SEM. *, P < 0.05; ***, P < 0.0005 (compared to attachment or infection in the control [0 nM CpClec-Fc = medium alone]).
CpClec-Fc binds to sulfated proteoglycans.
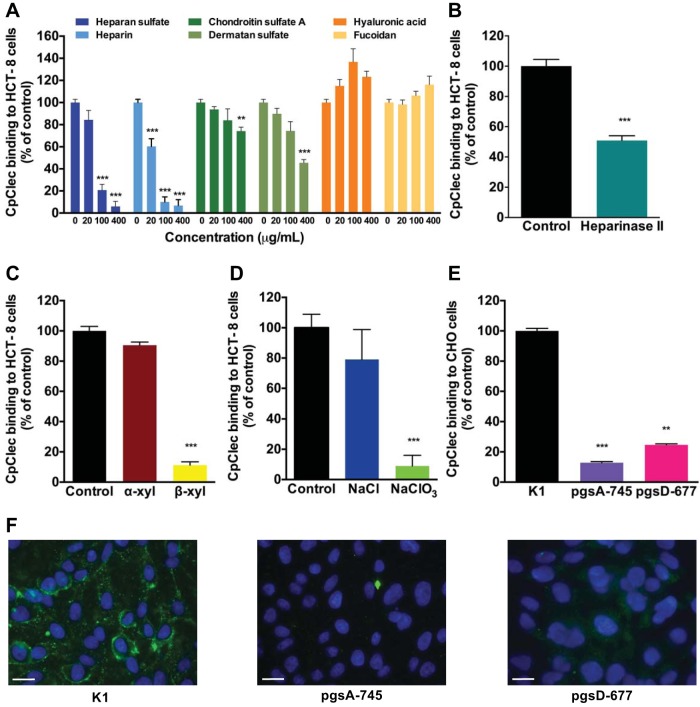
C-type lectins traditionally bind to glycans in a Ca2+-dependent manner (11). Since CpClec-Fc has a CTLD and displayed Ca2+-dependent binding to intestinal epithelial cells, it seemed likely that its ligand was a glycan on the cell surface. None of the monosaccharides or disaccharides tested inhibited CpClec-Fc binding to HCT-8 cells (data not shown), and CpClec-Fc did not display any appreciable binding to the CFG mammalian glycan array (version 5.2), which contains >600 known mammalian glycans (see Table S1 in the supplemental material). Several mammalian C-type lectins, particularly the selectins, interact with proteoglycans (11). Interestingly, GAGs are not present on the CFG glycan array (see Table S1). With this in mind, the ability of GAGs to competitively inhibit CpClec-Fc binding to host cells was evaluated. Both heparin and heparan sulfate significantly inhibited the binding of CpClec-Fc to HCT-8 cells at 100 μg/ml (Fig. 3A). Heparin also inhibited the binding of CpClec-Fc to Caco-2 cells (see Fig. S2C in the supplemental material). Dermatan sulfate (chondroitin sulfate B) and chondroitin sulfate A inhibited binding by ∼50% and ∼25%, respectively, but only at 400 μg/ml (Fig. 3A). Neither hyaluronic acid (a nonsulfated GAG) nor fucoidan (a sulfated non-GAG polysaccharide) inhibited binding at any concentration.
FIG 3.
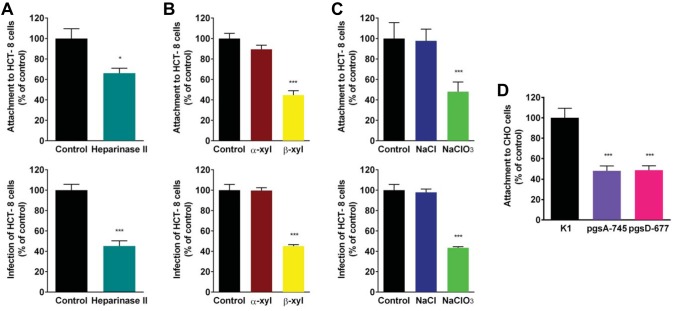
CpClec-Fc interacts with sulfated proteoglycans on intestinal epithelial cells. (A) Fixed HCT-8 cells were incubated with CpClec-Fc or Fc alone in the presence of GAGs or fucoidan. (B) HCT-8 cells were treated with heparinase II or lyase buffer, fixed, and incubated with CpClec-Fc or Fc alone. (C) HCT-8 cells were incubated with α-xyloside, β-xyloside, or medium alone; fixed; and incubated with CpClec-Fc or Fc alone. (D) HCT-8 cells were incubated with NaCl, NaClO3, or medium alone; fixed; and incubated with CpClec-Fc or Fc alone. (A to D) Binding was quantified by ELISA. (E and F) Fixed CHO K1, pgsA-745, and pgsD-677 cells were incubated with CpClec-Fc or Fc alone. Binding was quantified by ELISA (E) or visualized by fluorescence microscopy (F). Scale bars = 100 μm. For all quantification experiments, binding of CpClec-Fc was normalized to the binding of Fc alone. Quantification data represent the mean ± the SEM. **, P < 0.05; ***, P < 0.0005 (compared to binding in the control [buffer or medium alone or CHO K1 cells]).
We then evaluated whether alteration of host cell GAGs would affect CpClec-Fc binding. Treatment of HCT-8 cells with heparinase II to remove heparan sulfate reduced CpClec-Fc binding by ∼50% (Fig. 3B), while treatment of HCT-8 cells with β-xyloside (an inhibitor of GAG production) (33) or sodium chlorate (an inhibitor of sulfation) (34) reduced binding significantly more than treatment with the controls α-xyloside and sodium chloride, respectively (Fig. 3C and D).
To validate our findings, we evaluated the binding of CpClec-Fc to CHO cell lines containing genetic defects in GAG synthesis (25, 26). Binding to CHO pgsA-745 (no GAGs) and pgsD-677 (no heparan sulfate, three times as much chondroitin sulfate) cells was significantly weaker than binding to the wild-type CHO K1 cell line (Fig. 3E and F). Together, these results suggest that CpClec is a C-type lectin that binds to HSPGs on host cells.
C. parvum attachment and infection in vitro are mediated by sulfated proteoglycans.
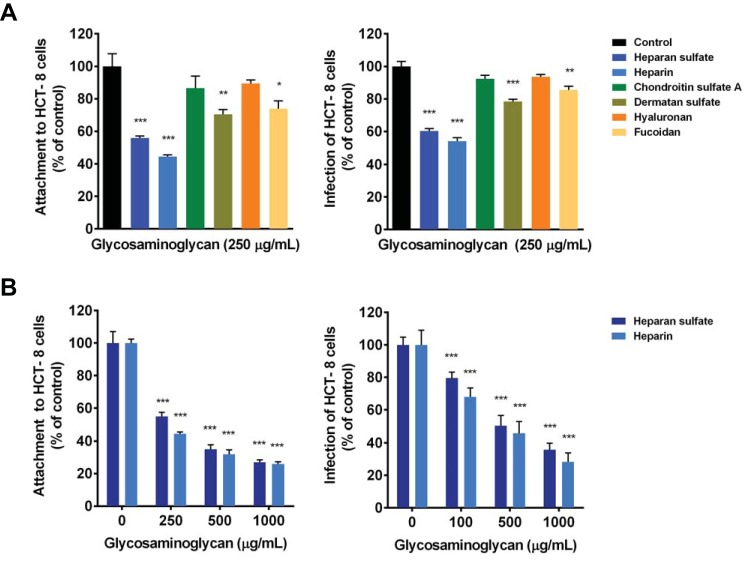
To determine whether proteoglycans mediate C. parvum attachment, we evaluated the ability of GAGs to inhibit the attachment of sporozoites to HCT-8 cells. Attachment was inhibited by ∼40% and 50% in the presence of 250 μg/ml heparan sulfate and heparin, respectively (Fig. 4A). Dermatan sulfate and fucoidan inhibited attachment by ∼25%, while there was no significant inhibition of attachment by chondroitin sulfate A or hyaluronan at the same concentration. Heparan sulfate and heparin displayed dose-dependent inhibition of attachment, with ∼70% inhibition at the highest concentration tested (Fig. 4B). Treatment of HCT-8 cells with heparinase II reduced attachment by ∼30% (Fig. 5A), while treatment of HCT-8 cells with β-xyloside or sodium chlorate reduced sporozoite attachment by ∼50% (Fig. 5B and C). As with CpClec-Fc binding, sporozoite attachment to both CHO pgsA-745 and pgsD-677 cells was ∼50% less than that to CHO K1 cells (Fig. 5D).
FIG 4.
C. parvum attachment to and infection of intestinal epithelial cells are mediated by GAGs. (A and B) HCT-8 cells were incubated with CFSE-labeled sporozoites or infected with oocysts in the presence of GAGs or fucoidan (A) or increasing concentrations of heparin or heparan sulfate (B), or medium alone. For all experiments, attachment and infection were quantified by fluorescence microscopy. Data represent the mean ± the SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 (compared to the control [buffer or medium alone]).
FIG 5.
Disruption of cell surface GAGs reduces C. parvum attachment and infection. (A) HCT-8 cells were treated with heparinase II or lyase buffer; this was followed by incubation with CFSE-labeled sporozoites or infection with oocysts. (B) HCT-8 cells were incubated with α-xyloside, β-xyloside, or medium alone and incubated with CFSE-labeled sporozoites or infected with oocysts. (C) HCT-8 cells were incubated with NaCl, NaClO3, or medium alone and incubated with CFSE-labeled sporozoites or infected with oocysts. (D) CHO K1, pgsA-745, and pgsD-677 cells were incubated with CFSE-labeled sporozoites. For all experiments, attachment and infection were quantified by fluorescence microscopy. Data represent the mean ± the SEM. *, P < 0.05; ***, P < 0.0005 (compared to the control [buffer or medium alone or CHO K1 cells]).
To determine if the attachment pathway mediated by GAGs is one that leads to productive infection, we measured the infection of HCT-8 cells in the presence of GAGs. We found that C. parvum infection was inhibited by ∼40 and 50% in the presence of 250 μg/ml heparan sulfate and heparin, respectively. Dermatan sulfate and fucoidan inhibited infection minimally, while there was no significant inhibition by chondroitin sulfate A or hyaluronan (Fig. 4A). Inhibition of infection by heparan sulfate and heparin was dose dependent, with maximal inhibition of ∼60% and 70%, respectively, at the highest concentration tested (Fig. 4B). Treatment of HCT-8 cells with heparinase II, β-xyloside, or sodium chlorate reduced infection by ∼50% (Fig. 5A to C). We were unable to assess infection of the mutant CHO cells since the parent K1 cell line did not support C. parvum infection (see Fig. S3 in the supplemental material). Taken together, these data confirm the role of sulfated proteoglycans, particularly HSPGs, during C. parvum attachment to and infection of intestinal epithelial cells.
DISCUSSION
GAGs are the glycan component of proteoglycans. They consist of linear polysaccharide chains containing repeating disaccharide units of an amino sugar (N-acetylgalactosamine [GalNAc] or N-acetylglucosamine [GlcNAc]) and a uronic acid (glucuronic acid [GlcA] or iduronic acid [IdoA]) or galactose (Gal) (35). GAGs include hyaluronan, keratan sulfate, chondroitin sulfate A, dermatan sulfate/chondroitin sulfate B, heparin, and heparan sulfate. Structural variations and binding specificities among GAGs are due to differences in the composition of the disaccharide subunits, epimerization of GlcA to IdoA, and the degree and pattern of sulfation. We found CpClec binding to host cells to be mediated primarily through HSPGs. Heparan sulfate and heparin almost completely inhibited the binding of CpClec-Fc to HCT-8 cells. These GAGs are more heavily sulfated and carry a greater negative charge than the other GAGs tested in this study. Therefore, it seemed possible that CpClec-Fc binding to host cells could be mediated through electrostatic interactions, especially since binding of CpClec-Fc was significantly reduced following the treatment of HCT-8 cells with sodium chlorate, an inhibitor of sulfation. Interestingly, dermatan sulfate inhibited the binding of CpClec-Fc to a greater extent than chondroitin sulfate A did, though both are comparable in charge. Dermatan sulfate contains repeating subunits of GalNAc and IdoA, whereas chondroitin sulfate A contains repeating subunits of GalNAc and GlcA (35). Both heparan sulfate and heparin contain various amounts of IdoA as well, suggesting that the binding of CpClec-Fc to HCT-8 cells is dependent on a combination of a sulfate-mediated charge and the glycan backbone composition, particularly the presence of IdoA. This idea is further supported by the lack of inhibition seen with fucoidan, which carries a negative charge but is not a GAG, as well as the comparably reduced binding of CpClec-Fc to CHO pgsA-745 and pgsD-677 cells.
Binding of CpClec-Fc to host cells was dependent on both calcium and cell surface sulfated GAGs, suggesting that CpClec binds sulfated proteoglycans in a Ca2+-dependent manner. There are four Ca2+ coordination sites conserved among CTLDs of C-type lectins (36). Sites 1 and 4 help maintain the CTLD structure, while site 2 has a dual role in maintaining structure and facilitating carbohydrate binding. The function of site 3 is unknown. Calcium and glycan binding within site 2 is mediated by three groups of residues—the EPN/QPD motif, the WND motif, and a glutamic acid residue directly preceding a conserved cysteine. CpClec does not contain the EPN/QPD or WND motif (10) but does contain the glutamic acid residue, suggesting that it may have a novel Ca2+-dependent glycan-binding motif, a finding reported for other CTLD-containing proteins (36).
Both CpClec-Fc and sulfated GAGs inhibited C. parvum attachment to and infection of HCT-8 cells, suggesting that CpClec mediates C. parvum infection through interactions with sulfated proteoglycans. We previously demonstrated inhibition of C. parvum attachment in the presence of gastrointestinal mucins (37), which can also be heavily sulfated (38). In addition to mediating C. parvum infection, it is also possible that sulfated glycans or proteoglycans may serve a protective role by sequestering sporozoites in the lumen of the intestine to facilitate clearance.
Neither attachment nor infection was completely inhibited in the presence of CpClec-Fc or sulfated GAGs. This is not surprising, considering that C. parvum, like other apicomplexans, appears to utilize multiple ligand-receptor interactions for host cell attachment and invasion (8, 9). CpClec-Fc inhibited C. parvum infection to a greater extent than attachment alone. The reason for this is unclear, but considering that CpClec localizes to subcellular locations such as dense granules and the feeder organelle (10), both of which are thought to be important during intracellular development (39, 40), it is possible that the role of CpClec during infection extends beyond interactions at the cell surface. This idea is further supported by the finding that treatment of HCT-8 cells with heparinase II, β-xyloside, or sodium chlorate reduced C. parvum infection more than attachment alone. Proteoglycans have been implicated in the mediation of intracellular development of T. gondii as well (41). Similar to CpClec-Fc, C. parvum attachment to CHO pgsA-745 and pgsD-677 was comparably reduced, a finding also reported for T. gondii (21), suggesting that intestinal apicomplexan parasites display a higher affinity for HSPGs during infection.
Several apicomplexans interact with proteoglycans during infection (14). Heparin has been reported to inhibit Plasmodium sp., Babesia sp., and Theileria sergenti growth in vitro and/or in vivo via inhibition of merozoite invasion of erythrocytes (42–45). P. falciparum is reported to express a large GAG-binding proteome consisting primarily of surface proteins involved in attachment, invasion, and tissue sequestration (44, 46). For example, VAR2CSA, a membrane-associated adhesin, binds a lightly sulfated form of chondroitin sulfate A present in the placenta, thereby mediating the establishment of placental malaria (47). Circumsporozoite protein, another adhesin, binds to HSPGs on hepatocytes and mediates infection of the liver (19).
T. gondii infection of host cells has been shown to be both enhanced and inhibited by GAGs at low and high concentrations, respectively (20–23, 48). Similar to P. falciparum, it is reported to contain a large GAG-binding proteome (46), including proteins involved in host cell attachment and invasion, like SAG1 (48), SAG3 (23), and P104 (22), as well as proteins believed to be involved in parasitophorous vacuole formation and intracellular development, like GRA2 (48). The mechanisms underlying the affinities of these proteins for specific GAGs remain poorly understood, but it has been suggested that they involve (i) a combination of glycan composition and sulfate charge and (ii) presentation (21).
Recently, Inomata and colleagues reported that sulfated GAGs inhibit C. parvum infection in a dose-dependent manner (24). They also demonstrated binding of heparin to elongation factor 1α, an adhesin previously implicated in the mediation of C. parvum invasion in vitro (49). In this study, we employed a more comprehensive methodology and independently demonstrated the role of sulfated GAGs during C. parvum attachment and infection.
This is the first report to demonstrate the binding of a pathogen-associated C-type lectin to sulfated proteoglycans on host cells. However, several mammalian C-type lectins are known to bind to proteoglycans (11). The selectins (E-, L-, and P-selectin) are type I transmembrane C-type lectins that bind to sulfated GAGs such as heparin and heparan sulfate (50). Lecticans are a group of chondroitin sulfate proteoglycans that also contain a CTLD and have been shown to bind both sulfated (heparan sulfate) and nonsulfated (hyaluronan) GAGs (51, 52).
In conclusion, our results indicate that CpClec is a novel C-type lectin that mediates C. parvum attachment and infection via Ca2+-dependent binding of HSPGs on intestinal epithelial cells. Ongoing studies are directed at determining whether CpClec interacts with specific HSPGs, such as syndecan 1 (53), on intestinal epithelial cells and elucidating how these interactions mediate subsequent invasion and/or intracellular development. The recent development of a system for genetically modifying C. parvum (7) will be an invaluable tool for studying CpClec and, in combination with the use of human primary intestinal epithelial cells (54) and murine models, will be essential for validating the importance of CpClec interactions with proteoglycans during C. parvum infection. Our findings provide further insight into the complexity of Cryptosporidium-host cell interactions and may aid in the development of novel GAG-based interventions for cryptosporidiosis.
Supplementary Material
ACKNOWLEDGMENTS
J.G.L. is supported by NIH T32 AI07077 to the Tufts University Sackler School of Graduate Biomedical Sciences Program in Immunology and T32 GM008448 to the Tufts University School of Medicine Medical Scientist Training Program. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Jamie Heimburg-Molinaro and David F. Smith and the Consortium for Functional Glycomics (Emory University) for the glycan array assays. We also thank John Leong, Linden Hu, Doug Jefferson (Tufts University), Shiv Pillai, and Vinay Mahajan (Harvard University) for reagents, cell lines, and technical advice and John Leong, Mercio Perrin (Tufts University), and Richard Cummings (Emory University) for helpful suggestions.
Funding Statement
Jacob Ludington was funded by NIH grants T32 GM008448 and T32 AI07077. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01410-15.
REFERENCES
- 1.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen X, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hlavsa MC, Roberts VA, Kahler AM, Hilborn ED, Wade TJ, Backer LC, Yoder JS, Centers for Disease Control and Prevention . 2014. Recreational water-associated disease outbreaks—United States, 2009–2010. MMWR Morb Mortal Wkly Rep 63:6–10. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6301a2.htm. [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connor RM, Shaffie R, Kang G, Ward HD. 2011. Cryptosporidiosis in patients with HIV/AIDS. AIDS 25:549–560. doi: 10.1097/QAD.0b013e3283437e88. [DOI] [PubMed] [Google Scholar]
- 4.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 5.Cabada MM, White AC Jr. 2010. Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis 23:494–499. doi: 10.1097/QCO.0b013e32833de052. [DOI] [PubMed] [Google Scholar]
- 6.Morada M, Lee S, Gunther-Cummins L, Weiss LM, Widmer G, Tzipori S, Yarlett N. 2016. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Vinayak S, Pawlowic MC, Sateriale A, Brooks CF, Studstill CJ, Bar-Peled Y, Cipriano MJ, Striepen B. 2015. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523:477–480. doi: 10.1038/nature14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lendner M, Daugschies A. 2014. Cryptosporidium infections: molecular advances. Parasitology 141:1511–1532. doi: 10.1017/S0031182014000237. [DOI] [PubMed] [Google Scholar]
- 9.Wanyiri J, Ward H. 2006. Molecular basis of Cryptosporidium-host cell interactions: recent advances and future prospects. Future Microbiol 1:201–208. doi: 10.2217/17460913.1.2.201. [DOI] [PubMed] [Google Scholar]
- 10.Bhalchandra S, Ludington J, Coppens I, Ward HD. 2013. Identification and characterization of Cryptosporidium parvum Clec, a novel C-type lectin domain-containing mucin-like glycoprotein. Infect Immun 81:3356–3365. doi: 10.1128/IAI.00436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings RD, McEver RP. 2009. C-type lectins, p 439–457. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 12.Esko JD, Linhardt RJ. 2009. Proteins that bind sulfated glycosaminoglycans, p 501–511. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 13.Prydz K, Vuong TT, Kolset SO. 2009. Glycosaminoglycan secretion in xyloside treated polarized human colon carcinoma Caco-2 cells. Glycoconj J 26:1117–1124. doi: 10.1007/s10719-009-9232-2. [DOI] [PubMed] [Google Scholar]
- 14.Kamhi E, Joo EJ, Dordick JS, Linhardt RJ. 2013. Glycosaminoglycans in infectious disease. Biol Rev Camb Philos Soc 88:928–943. doi: 10.1111/brv.12034. [DOI] [PubMed] [Google Scholar]
- 15.Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 16.Leong JM, Morrissey PE, Ortega-Barria E, Pereira ME, Coburn J. 1995. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 63:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs RD. 1994. Borrelia burgdorferi bind to epithelial cell proteoglycans. J Clin Invest 93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried M, Duffy PE. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 19.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med 177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carruthers VB, Hakansson S, Giddings OK, Sibley LD. 2000. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect Immun 68:4005–4011. doi: 10.1128/IAI.68.7.4005-4011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega-Barria E, Boothroyd JC. 1999. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem 274:1267–1276. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 22.Gong H, Kobayashi K, Sugi T, Takemae H, Kurokawa H, Horimoto T, Akashi H, Kato K. 2012. A novel PAN/apple domain-containing protein from Toxoplasma gondii: characterization and receptor identification. PLoS One 7:e30169. doi: 10.1371/journal.pone.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquet A, Coulon L, De Neve J, Daminet V, Haumont M, Garcia L, Bollen A, Jurado M, Biemans R. 2001. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol 116:35–44. doi: 10.1016/S0166-6851(01)00297-3. [DOI] [PubMed] [Google Scholar]
- 24.Inomata A, Murakoshi F, Ishiwa A, Takano R, Takemae H, Sugi T, Cagayat Recuenco F, Horimoto T, Kato K. 2015. Heparin interacts with elongation factor 1α of Cryptosporidium parvum and inhibits invasion. Sci Rep 5:11599. doi: 10.1038/srep11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esko JD, Stewart TE, Taylor WH. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A 82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, Cheifetz S, Massague J, Lindahl U, Esko JD. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci U S A 89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DF, Song X, Cummings RD. 2010. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol 480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 28.Feng H, Nie W, Bonilla R, Widmer G, Sheoran A, Tzipori S. 2006. Quantitative tracking of Cryptosporidium infection in cell culture with CFSE. J Parasitol 92:1350–1354. doi: 10.1645/GE-853R.1. [DOI] [PubMed] [Google Scholar]
- 29.Bessoff K, Sateriale A, Lee KK, Huston CD. 2013. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother 57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gut J, Nelson RG. 1999. Cryptosporidium parvum: synchronized excystation in vitro and evaluation of sporozoite infectivity with a new lectin-based assay. J Eukaryot Microbiol 46:56S–57S. [PubMed] [Google Scholar]
- 31.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. 2012. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 4:1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papaneophytou CP, Grigoroudis AI, McInnes C, Kontopidis G. 2014. Quantification of the effects of ionic strength, viscosity, and hydrophobicity on protein-ligand binding affinity. ACS Med Chem Lett 5:931–936. doi: 10.1021/ml500204e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esko JD, Montgomery RI. 2001. Synthetic glycosides as primers of oligosaccharide biosynthesis and inhibitors of glycoprotein and proteoglycan assembly. Curr Protoc Mol Biol Chapter 17:Unit17.11. doi: 10.1002/0471142727.mb1711s32. [DOI] [PubMed] [Google Scholar]
- 34.Greve H, Cully Z, Blumberg P, Kresse H. 1988. Influence of chlorate on proteoglycan biosynthesis by cultured human fibroblasts. J Biol Chem 263:12886–12892. [PubMed] [Google Scholar]
- 35.Esko JD, Kimata K, Lindahl U. 2009. Proteoglycans and sulfated glycosaminoglycans, p 229–248. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 36.Zelensky AN, Gready JE. 2005. The C-type lectin-like domain superfamily. FEBS J 272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 37.Joe A, Verdon R, Tzipori S, Keusch GT, Ward HD. 1998. Attachment of Cryptosporidium parvum sporozoites to human intestinal epithelial cells. Infect Immun 66:3429–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison MJ, Packer NH. 2000. Measurement of sulfate in mucins. Methods Mol Biol 125:211–216. [DOI] [PubMed] [Google Scholar]
- 39.Blackman MJ, Bannister LH. 2001. Apical organelles of Apicomplexa: biology and isolation by subcellular fractionation. Mol Biochem Parasitol 117:11–25. doi: 10.1016/S0166-6851(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 40.Tzipori S, Ward H. 2002. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect 4:1047–1058. doi: 10.1016/S1286-4579(02)01629-5. [DOI] [PubMed] [Google Scholar]
- 41.Bishop JR, Crawford BE, Esko JD. 2005. Cell surface heparan sulfate promotes replication of Toxoplasma gondii. Infect Immun 73:5395–5401. doi: 10.1128/IAI.73.9.5395-5401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagiwara K, Takahashi M, Ichikawa T, Tsuji M, Ikuta K, Ishihara C. 1997. Inhibitory effect of heparin on red blood cell invasion by Theileria sergenti merozoites. Int J Parasitol 27:535–539. doi: 10.1016/S0020-7519(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 43.Bork S, Yokoyama N, Ikehara Y, Kumar S, Sugimoto C, Igarashi I. 2004. Growth-inhibitory effect of heparin on Babesia parasites. Antimicrob Agents Chemother 48:236–241. doi: 10.1128/AAC.48.1.236-241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi K, Takano R, Takemae H, Sugi T, Ishiwa A, Gong H, Recuenco FC, Iwanaga T, Horimoto T, Akashi H, Kato K. 2013. Analyses of interactions between heparin and the apical surface proteins of Plasmodium falciparum. Sci Rep 3:3178. doi: 10.1038/srep03178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle MJ, Richards JS, Gilson PR, Chai W, Beeson JG. 2010. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 115:4559–4568. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Jiang N, Jia B, Chang Z, Zhang Y, Wei X, Zhou J, Wang H, Zhao X, Yu S, Song M, Tu Z, Lu H, Yin J, Wahlgren M, Chen Q. 2014. A comparative study on the heparin-binding proteomes of Toxoplasma gondii and Plasmodium falciparum. Proteomics 14:1737–1745. doi: 10.1002/pmic.201400003. [DOI] [PubMed] [Google Scholar]
- 47.Dahlbäck M, Jorgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M, Pinto VV, Arnot DE, Theander TG, Salanti A. 2011. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem 286:15908–15917. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzouz N, Kamena F, Laurino P, Kikkeri R, Mercier C, Cesbron-Delauw MF, Dubremetz JF, De Cola L, Seeberger PH. 2013. Toxoplasma gondii secretory proteins bind to sulfated heparin structures. Glycobiology 23:106–120. doi: 10.1093/glycob/cws134. [DOI] [PubMed] [Google Scholar]
- 49.Matsubayashi M, Teramoto-Kimata I, Uni S, Lillehoj HS, Matsuda H, Furuya M, Tani H, Sasai K. 2013. Elongation factor-1α is a novel protein associated with host cell invasion and a potential protective antigen of Cryptosporidium parvum. J Biol Chem 288:34111–34120. doi: 10.1074/jbc.M113.515544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. 1998. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest 101:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ujita M, Shinomura T, Ito K, Kitagawa Y, Kimata K. 1994. Expression and binding activity of the carboxyl-terminal portion of the core protein of PG-M, a large chondroitin sulfate proteoglycan. J Biol Chem 269:27603–27609. [PubMed] [Google Scholar]
- 52.Yamaguchi Y. 2000. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci 57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 54.Castellanos-Gonzalez A, Cabada MM, Nichols J, Gomez G, White AC Jr. 2013. Human primary intestinal epithelial cells as an improved in vitro model for Cryptosporidium parvum infection. Infect Immun 81:1996–2001. doi: 10.1128/IAI.01131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.