Abstract
Circadian physiology is responsible for the temporal regulation of metabolism to optimize energy homeostasis throughout the day. Disturbances in the light/dark cycle, sleep/wake schedule, or feeding/activity behavior can affect the circadian function of the clocks located in the brain and peripheral tissues. These alterations have been associated with impaired glucose tolerance and type 2 diabetes. Animal models with molecular manipulation of clock genes and genetic studies in humans also support these links. It has been demonstrated that the endocrine pancreas has an intrinsic self-sustained clock, and recent studies have revealed an important role of clock genes in pancreatic β cells, glucose homeostasis, and diabetes.
Keywords: clock genes, diabetes, pancreas, β cell, insulin
Type 2 diabetes and the circadian rhythm
Type 2 diabetes has become one of the most important health problems of modern society, affecting millions of people worldwide. Obesity and excessive weight gain play a major role in the onset of diabetes, along with other risk factors including genetics and lack of physical activity. In recent years, circadian disturbances have also been identified as contributors to this metabolic disease. Alterations in circadian rhythm derived from our current lifestyle, such as mistimed sleeping, shift working, or eating at abnormal night-time hours, have been related to type 2 diabetes, obesity, and metabolic syndrome [1–3]. The discovery of these associations opens new possibilities for the design of novel treatments for these metabolic disorders based on the appropriate alignment or resetting of the circadian system [4–6]. In the case of type 2 diabetes, an association to circadian disruption was originally related to an altered function of the central clock located in the hypothalamus. However, recent findings show that this disease can also be attributed to an impaired function of the endocrine pancreatic clock [7–10]. Thus, the study of the pancreatic clock biology is important not only to understand circadian rhythms and their relationship with metabolism but also to develop tools for the prevention and therapy of metabolic diseases such as type 2 diabetes.
Central and peripheral clocks
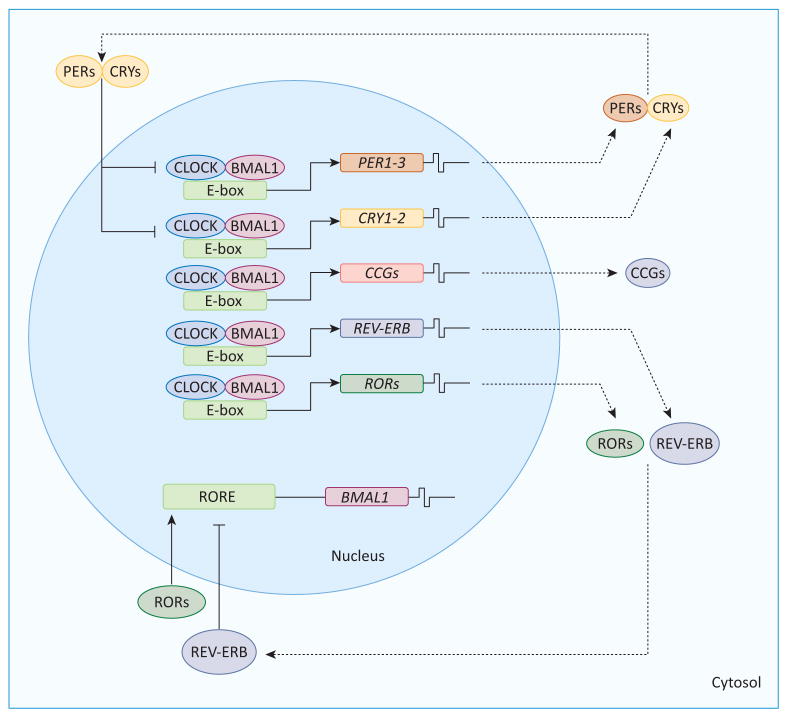
In mammals, the central pacemaker is located in the suprachiasmatic nuclei (SCN) in the hypothalamus. It is controlled by a transcriptional/translational autoregulatory feedback loop involving a set of clock genes (Figure 1). The core clock is composed of circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein-1 (BMAL1), transcription factors which bind to E-box enhancers in the period (Per) and cryptochrome (Cry) promoters, inducing PER and CRY expression [11,12]. PER and CRY proteins interact to form a complex that translocates to the nucleus and inhibits their own CLOCK:BMAL1-induced transcription. Phosphorylation of both proteins can also trigger their degradation by 26S proteasomes. The turnover of PER and CRY allows this cycle to continue, and this clock network forms a self-sustained feedback mechanism with an oscillatory pattern of approximately 24 h (Figure 1). Several proteins encoded by clock genes regulate the expression of downstream targets that further regulate the clock machinery, function as clock-controlled output genes relaying the clock information to downstream proteins, or function in cellular processes such as metabolism. Another regulatory loop is composed of two nuclear receptor genes, which also possess an E-box enhancer activated by the CLOCK:-BMAL1 heterodimer. REV-ERB ALPHA, so named because it is encoded by the reverse strand of the c-erb-A oncogene, can negatively regulate BMAL1 expression. By contrast, the retinoic acid receptor-related orphan receptor α (ROR ALPHA) exerts a positive modulation of BMAL1 (Figure 1). Although the main input signal for this oscillator is the light/dark cycle, the SCN can receive additional information about nutrients and hormones [12]. It is also connected to a complex neural network with projections to different brain centers that regulate temperature, sleep, feeding, hormonal secretion, and glucose homeostasis (Box 1).
Figure 1.
Feedback loop in the core circadian clocks. The clock machinery is composed of different feedback loops. In the primary loop, BMAL1 and CLOCK drive the transcriptional expression of PER and CRY through the activation of E-box enhancers. PER and CRY proteins form heterodimers that translocate to the nucleus, repressing their own transcription by interaction with CLOCK:BMAL1 complexes. The turnover of PER and CRY is also regulated by protein casein kinase 1 and AMP kinase, which phosphorylate these proteins, inducing their degradation in the cytosol by the 26S proteasome complex. This feedback in the primary loop occurs during 24 h and then, a new cycle starts. In another loop, CLOCK:BMAL1 heterodimers drive the expression of REV-ERB and ROR, which inhibit and activate BMAL1 expression, respectively. Some clock-controlled genes (CCGs), which are involved in metabolic pathways, are also under the modulation of the clock machinery. Abbreviations: BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles protein kaput; PER, period homolog Drosophila; CRY, cryptochrome; REV-ERB, reverse-eritroblastosis virus; ROR, retinoic acid receptor-related orphan receptor; RORE, ROR response element.
Box 1. The central and peripheral clocks.
Numerous biological processes, such as body temperature, feeding/ fasting, and sleep/wake behavior, as well as hormonal release, display circadian patterns which are mainly driven by the central clock located in the SCN of the hypothalamus. These circadian rhythms are responsible for the optimization of energy homeostasis throughout the light/dark cycle of the day. The neuronal population that forms this master clock works as a self-sustained circadian oscillator that is controlled by autoregulated transcriptional feedback loops. The SCN also sends neural projections to other brain areas that are important for the regulation of sleep, feeding, energy homeostasis, and temperature [22]. Several external cues (also called zeitgebers) synchronize the internal clocks to 24-h cycles. The change of environmental light throughout the day/night cycle is the main entraining signal for the SCN, which in turn also synchronizes other clocks located in extra-SCN brain areas and peripheral tissues to the photic signals [103]. Additionally, other external cues, such as the time of feeding/fasting or food restriction, can also affect the circadian rhythm of internal clocks. Different metabolic sensors can integrate information from the nutritional state and transfer it to the clock machinery. In this way, changes in NAD/NADH or AMP/ATP during exercise, fasting, or caloric restriction can be sensed by the clocks [22].
Peripheral tissues are also equipped with autonomous clock oscillators with similar molecular components as those of the SCN. The circadian activity of these peripheral clocks is temporally aligned to the central SCN clock mainly by autonomic innervations, hormonal signals (i.e., glucocorticoids), and feeding-related cues [103]. Just as in the central pacemaker, peripheral clocks contribute to global energy metabolism but also participate in local metabolic functions such as those involved in glucose and lipid homeostasis. Although light-dependent time alignment with the SCN is important, the fasting/feeding cycles and changes in several nutritional factors also play an important role in the entrainment of peripheral clocks [22]. For instance, a HFD and caloric restriction affect both peripheral and central clocks, while temporal feeding restriction only entrains peripheral clocks [95].
In addition to the central clock in the brain, peripheral clocks with similar molecular components exist in several organs, such as liver, kidney, heart, adipose tissue, and pancreas [8,10,11,13–18]. The link between the central and peripheral clocks was shown in studies in which the ablation of the SCN eliminated the synchrony of peripheral tissues, suggesting that the SCN maintains the phase alignment of the peripheral clocks [19,20]. The temporal alignment of the peripheral clocks is governed hierarchically by the central clock dependent upon the light/dark cycles, optimizing metabolism and energy homeostasis throughout the day. The alteration of circadian rhythms and/or the misalignment of peripheral clocks with the SCN may be related to the onset of different metabolic diseases including type 2 diabetes [3,21].
Circadian rhythms, metabolism, and health
Circadian variations in behavior and physiology are governed by internal biological clocks that are mainly regulated by light/dark inputs (sleep/activity cycles), and also by nutrient and hormonal signals [15,22,23] (Box 1). In addition to controlling the sleep/wake and feeding cycles, the circadian system regulates physiological processes such as lipid and glucose metabolism, body temperature, locomotor activity, and hormone secretion [24], allowing the optimization of energy acquisition, use, and storage throughout the day. Several parameters related to glucose metabolism, such as glucose tolerance, insulin sensitivity, as well as glucose, glucagon, and insulin plasma levels are known to exhibit circadian variations along the day [25–30]. However, the regulation of this rhythmicity in glucose metabolism is still not well understood. Particularly, it is not totally clear how the interplay of all these factors that undergo 24-h variations can modulate daily changes in plasma glucose levels [31]. For instance, plasma glucose concentrations and glucose uptake are maximal at the beginning of the activity period in animals or around awakening in humans and, almost simultaneously, glucose production is high [31]. At night, however, glucose tolerance in humans is decreased compared with the daytime, probably because of reduced insulin sensitivity [27,30,31].
Although feeding activity is one of the most important factors in the control of plasma insulin levels (Box 2), oscillations in plasma insulin are also reported during day/night cycles in animals and humans independent of fasting/feeding behavior [32–34]. In humans, during the daytime there is a peak of insulin secretion to increase energy storage and utilization [24,32,33]. Reduced insulin secretion and increased glucose production are favored at night [24]. In rodents, this pattern is shifted 12 h according to their nocturnal activity [25].
Box 2. Endocrine pancreas function and type 2 diabetes.
The endocrine pancreas is composed of a heterogeneous collection of cell types that form the islets of Langerhans, which are dispersed throughout the whole pancreatic tissue. The main cell types are the insulin-secreting β cells and the glucagon-releasing α cells. These cell populations respond reciprocally to plasma glucose levels [104]. While β cells secrete insulin at increasing glucose concentrations, α cells release glucagon at lower glucose levels. Insulin mainly acts through the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathway in muscle and adipose tissue to induce glucose uptake, and in the liver to reduce hepatic glucose production. These processes result in decreased plasma glucose levels. By contrast, glucagon mainly binds to liver receptors, activating gluconeogenesis and glycogenolysis via the protein kinase A pathway, which allows for an increase in plasma glucose levels. Thus, glucose is maintained within a physiological range by the fine regulation of islet secretion [104].
Type 2 diabetes results from insulin resistance along with impaired β cell function. In this condition, insulin action is attenuated, leading to decreased insulin-induced glucose uptake in the adipose tissue and muscleas, well as enhanced hepatic glucose production [105]. In the majority of individuals, insulin resistance induces a compensatory adaptation in the pancreas, resulting in an augmented β cell mass and function to increase insulin release, which ensures the maintenance of normoglycemia. However, when this β cell compensation is insufficient, hyperglycemia may progressively develop. This deficient adaptive response can be also accompanied by β cell failure and loss. It has been suggested that hyperglucagonemia and α cell dysfunction are also involved in the pathophysiology of type 2 diabetes, and may exacerbate the high plasma glucose levels.
Circadian rhythms can be disrupted by impaired function of biological clocks as well as by desynchronization between the SCN and the external environment or the peripheral clocks. This may lead to unfavorable health effects and the development of diseases such as metabolic syndrome, obesity, and type 2 diabetes [35]. Current lifestyle and social habits, such as eating or working at night, being exposed to artificial light at night, and altered sleeping schedules are among the factors that can cause circadian disruption. Shift workers, who are a paradigmatic model of circadian misalignment, have alterations in pancreatic β cell responses and in glucose and lipid metabolism [3,36,37], as well as an increased risk of developing metabolic syndrome, cardiovascular disease, cancer, obesity, and type 2 diabetes [37–41]. Individuals with altered or restricted sleep exhibit an increased body mass index, impaired glucose tolerance, and reduced insulin responsiveness [42–44]. In obese individuals or patients with type 1 or type 2 diabetes, the circadian rhythms and magnitude of insulin secretion and sensitivity, as well as glucose tolerance, are impaired [45]. Some of these changes have also been observed in diabetic patients subjected to sleep deprivation [42,46,47]. Thus, alterations in circadian rhythms may lead to metabolic disturbances that could predispose to diabetes.
Clock genes and metabolism: implications for glucose homeostasis and type 2 diabetes
Animal models with manipulated clock genes have revealed that circadian rhythms have a key regulatory function in metabolism and glucose homeostasis. These animal models have also shown that impaired function of clock genes may result in prediabetic conditions or overt diabetes. Some evidence of this was first found in Clock mutant mice studies [48]. These mice had disrupted rhythms in feeding and locomotor activity, hyperphagia, hyperlipidemia, hyperglycemia, and hypoinsulinemia. Studies performed in mice lacking Bmal1 showed altered adipogenesis and hepatic carbohydrate metabolism [49–51]. While global inactivation of Bmal1 suppressed the diurnal variation in glucose and triglycerides and led to impaired gluconeogenesis and recovery from insulin-induced hypoglycemia [50], liver-specific deletion of this clock gene resulted in hepatic clock dysfunction, fasting hypoglycemia, and altered glucose clearance throughout the day [49]. Clear diabetic phenotypes characterized by hyperglycemia and hypoinsulinemia were demonstrated in Clock and Bmal1 mutants, as well as pancreas-specific Bmal1 knockout (KO) mice [8]. In these animal models, circadian clock gene expression was impaired in the endocrine pancreas.
Several metabolic alterations have also been found in genetically manipulated animal models of CLOCK/BMAL1 downstream target genes. For instance, Per2 KO mice displayed normal circadian clock function in white adipose tissue, but altered lipid metabolism [52]. Per3 single and Per1/2/3 triple KO mice showed altered body mass regulation when fed a high-fat diet (HFD) [53]. When both Cry1 and Cry2 were deleted, behavioral and molecular circadian rhythms were impaired, lipid metabolism and glucose metabolism were modified, and glucose intolerance emerged [54–56]. Conversely, hepatic Cry1 overexpression in diabetic (db/db) mice led to increased insulin sensitivity and lower blood glucose levels [57]. It was also reported that cryptochrome proteins downregulate liver gluconeogenesis during fasting by inhibition of glucagon-induced cAMP/protein kinase A signaling and cAMP response element-binding protein phosphorylation [57]. The use of synthetic CRY1/2 agonists inhibited glucagon-induced gluconeogenesis in primary hepatocytes [58].
Studies on mice lacking the nuclear receptors Rev-erbα or Rorα revealed alterations in bile and lipid metabolism, as well as decreased adiposity and high-density lipoprotein (HDL) levels [59,60]. In the case of Rev-erbα KO mice, although these animals displayed alterations in the molecular clock oscillatory properties, they did not show arrhythmic behavior in a constant environment [60]. The link with lipid metabolism was also found in Rev-erbα and β double KO mice [61]. These animals presented altered circadian wheel running behavior and disrupted circadian clock gene expression in the liver. When diet-induced obese mice were treated with Rev-erb agonists, these animals decreased fat mass and weight gain, as well as triglyceride and cholesterol levels [6]. Interestingly, Rev-erbα overexpression in the liver also altered energy, carbohydrate metabolism, and lipid metabolism [62]. Thus, several studies using animal models support the role of clock genes in lipid and glucose homeostasis, and also indicate that their impaired function may lead to metabolic pathologies.
In humans, genetic studies have also shown a link between clock genes and metabolism. For instance, genetic variants of CLOCK have been associated with increased susceptibility to total energy intake, obesity, and metabolic syndrome [63–65]. BMAL1 genetic variants have been correlated with hypertension, gestational diabetes, and type 2 diabetes [66,67], while PER2 single nucleotide polymorphisms have been associated with high fasting blood glucose levels and abdominal obesity [68,69]. CRY2 variants have been related to type 2 diabetes and impaired fasting glucose [70,71]. A genetic polymorphism in the melatonin receptor, which plays a critical role in the modulation of the circadian rhythms, is also associated with increased susceptibility to impaired insulin secretion, gestational diabetes, and type 2 diabetes [72,73]. Thus, several genetic observations in humans point to the existence of a close link between clock gene dysfunction and pathological conditions, such as metabolic syndrome, obesity, and type 2 diabetes.
The role of clock genes in pancreatic β cell function
The endocrine pancreas is a complex organ composed of different cell types that regulate glucose metabolism according to physiological demands. The main pancreatic cells involved in glucose homeostasis are the insulin-secreting β cells and the glucagon-secreting α cells (Box 2). The secretion of these two hormones is pulsatile and occurs within minutes after a nutrient challenge [74]. However, plasma insulin and glucagon levels have also been shown to exhibit 24-h oscillations independent of feeding, as mentioned earlier [25,26,28,32,33]. Moreover, the deregulation of circadian insulin secretion and action has been identified in first-degree relatives of patients with type 2 diabetes, who exhibited decreased insulin secretion and glucose uptake [45]. The exact mechanism behind these circadian oscillations is still unknown. Although this pattern was originally believed to be exclusively regulated by neuronal signaling and the SCN [75], an intrinsic circadian oscillator controlling insulin secretion was discovered in the rat pancreas [76]. Later, it was shown that the mRNA levels of PER1, PER2, BMAL1, CLOCK, and REV-ERB ALPHA in the pancreas oscillated throughout a 24-h period [11]. Additionally, a high expression of the transcription factors and clock-controlled output genes, d-binding protein (DBP) and thyrotroph embryonic factor (TEF), were shown to exist in human pancreatic islets and to have a circadian variation in insulin-secreting cells [77].
The islet clock appears to consist of the same components as those of other peripheral clocks, exhibiting oscillations in their gene expression throughout the day in different species. A circadian clock gene expression has been shown in rat pancreas [11], mouse pancreas [78], mouse islets [8,10], human islets, and dispersed human islet cells [79]. Additionally, it has also been observed in pancreatic cell lines, such as MIN6 cells [10], INS-1 cells [80], and αTC1-9 cells [81]. DBP has also been found to oscillate in the pancreatic cell line MIN6 and in mouse islets [82]. These findings support the existence of an intrinsic clock within the pancreatic islet.
Studies performed in whole-body, pancreas-specific, and β cell-specific KO mice have revealed the importance of islet clock genes in the control of insulin secretion and the development of diabetes. Additionally, these investigations have shown some of the potential mechanisms involved. ClockΔ19/Δ19 mutant mice, as well as pancreas-specific Bmal1 KO mice, exhibit hyperglycemia, hypoinsulinemia, and glucose intolerance [8]. This diabetic phenotype has been associated with altered insulin secretory responses. Pancreatic islets isolated from Clock mutant mice had reduced size and impaired insulin release [8]. Consistent with these findings, these pancreatic islets presented decreased β cell proliferation as well as reduced expression or changes in the circadian pattern of different genes involved in glucose uptake and metabolism, insulin signaling, cell cycle, and β cell growth [8] (Figure 2). In the same way, Clock antisense treatment during pancreatic embryogenesis resulted in changes of different cell cycle proteins via Wnt and Notch signaling [83]. Analysis of insulin release in isolated islets from ClockΔ19/Δ19 mice revealed impaired secretion in response to glucose but also to nonmetabolic stimuli, indicating that defective exocytosis rather than altered glucose metabolism was likely to be involved [8]. The lack of changes in glucose-induced Ca2+ signaling (which precedes insulin secretion) as well as the decreased expression of proteins involved in vesicle transport and docking, such as Vamp3 and Syntaxin6, further supported this specific defect in β cell exocytosis.
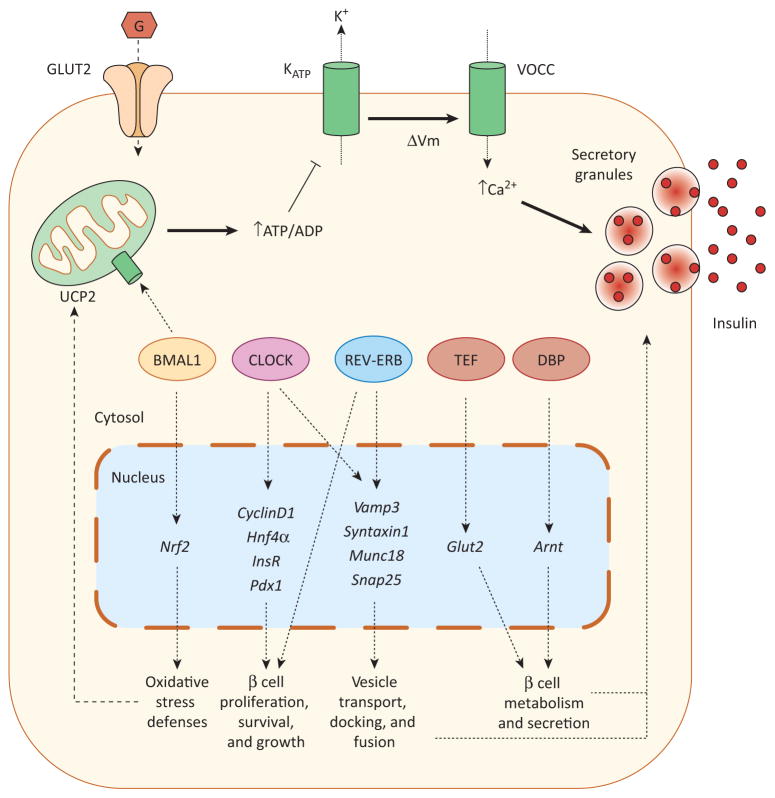
Figure 2.
Clock genes in the regulation of pancreatic β cell function. Glucose enters the pancreatic β cell via glucose transporter 2 (GLUT2) and is metabolized by mitochondria, resulting in the rapid generation of ATP and the subsequent closure of ATP-sensitive K+ (KATP) channels. The closure of these channels leads to plasma membrane depolarization (ΔVm), opening voltage-gated Ca2+ channels (VOCC). The consequent Ca2+ influx triggers insulin release through exocytosis of insulin secretory granules. Pancreatic β cell regulation by clock genes may involve several processes and mechanisms. Impaired glucose-induced insulin secretion (GSIS) has been shown in islets deficient in CLOCK, BMAL1, or REV-ERB. CLOCK and REV-ERB may regulate insulin secretion by modulating the expression of genes involved in exocytosis (Vamp3, Syntaxin1, Munc18, Snap25). Additionally, CLOCK and REV-ERB regulate β cell proliferation, probably through the modulation of different genes involved in β cell survival and growth (CyclinD1, Hnf4α, InsR, Pdx1). BMAL1 is also required for normal β cell GSIS by reducing UCP2-mediated mitochondrial uncoupling and oxidative stress and by increasing antioxidant defenses. This latter action is mediated by direct transcriptional modulation of Nrf2 by BMAL1. The clock-controlled output proteins TEF and DBP have also been shown to directly modulate the transcriptional activity of the glucose transporter 2 (Glut2) and Arnt, respectively. This latter gene plays a key role in β cell metabolism and GSIS. Abbreviations: BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles protein kaput; CRY, cryptochrome; DBP, d-binding protein; PER, period homolog Drosophila; REV-ERB ALPHA, reverse-eritroblastosis virus α; TEF, thyrotroph embryonic factor; UCP2, uncoupling protein 2.
Similar alterations in insulin secretion were observed in isolated islets of whole-body Bmal1 KO and pancreas-specific Bmal1 KO mice [8]. Another study reported hyperglycemia and hypoinsulinemia in global Bmal1 KO mice and demonstrated that the impaired glucose tolerance in this animal model was due to defective first phase insulin secretion [84]. Both isolated islets from these KO mice and INS-1 cells with Bmal1 knockdown exhibited a disrupted glucose-induced increase in the mitochondrial membrane potential gradient due to enhanced uncoupling protein 2 (Ucp2) expression, which led to decreased ATP levels and insulin secretion [84]. Inhibition of Ucp2 partially reversed these defects. In contrast to the findings in ClockΔ19/Δ19 mice [8], these latter results pointed to defects in glucose metabolism rather than alterations in exocytosis.
These dissimilar effects might be due to different CLOCK and BMAL1 pleiotropic actions in addition to their circadian role. A similar diabetic phenotype and secretion defects were reported in β cell-specific Bmal1 KO mice [85]. This latter study indicated that Bmal1 is important for the prevention of oxidative stress. Pancreatic islets isolated from these mice displayed augmented reactive oxygen species, increased Ucp2 expression, and impaired glucose-induced insulin release [85] (Figure 2). Oxidative stress prevention or Ucp2 suppression were able to reverse these defects. Additionally, it was shown that the key antioxidant regulatory factor Nrf2 was a direct target of Bmal1. In agreement with these reports, selective pancreas deletion of Bmal1 also produced glucose intolerance and defective insulin secretion in mice and isolated islets [86].
In addition to the core clock components Clock and Bmal1, other clock genes such as Rev-erbα are also important for pancreatic β cell function. Rev-erbα is expressed in pancreatic islets and exhibits circadian oscillations in MIN6 cells [10]. When this gene was silenced in mouse pancreatic islets, glucose-induced insulin secretion and β cell proliferation decreased [10]. Similarly, Rev-erbα expression exhibited circadian changes in αTC1-9 cells and, when it was downregulated, glucose-modulated glucagon secretion became impaired [81]. The effect of Rev-erbα on both β and α cell secretion may be related to the exocytotic process, as expression of exocytotic genes, such as Vamp3, Syntaxin1, Munc18, and Snap25, were decreased when Rev-erbα expression was silenced [81], in agreement with previous results in ClockΔ19/Δ19 mice [8]. Thus, although further work is necessary to understand the regulatory networks, it seems that clock genes may influence β cell mass and function by modulation of different processes (Figure 2).
Interestingly, in addition to clock genes, the expression of several other genes exhibits a circadian rhythm in the pancreas, isolated islets, and β cell lines. These genes include Glut2, Glucokinase, Syntaxin1A, Insulin, Ucp2, Nrf2, CyclinD1, Pdx1, and other genes involved in β cell metabolism, secretion, growth, and insulin signaling [77,87]. It has been reported that Nrf2 is a transcriptional target of Bmal1 [85], TEF transactivates Glut2 by direct interaction with the promoter [77], and DBP directly increases the transcription of Arnt/Hif1β [82], a key gene in β cell metabolism and secretion (Figure 2). It would be interesting to know whether the circadian expression of all these above-mentioned genes is coupled to an oscillatory functional output in different β cell processes. In this regard, there is a high correlation between the diurnal changes in insulin secretion and the glucose-induced reduction in β cell K+ conductance, most likely via ATP-dependent K+ channel inhibition, which was measured at the same time of the day [88]. Likewise, a circadian expression of functional K+ channels has been reported in rat pancreatic islets [89].
The information about clock genes in human islets is scarce. Human islets also express BMAL1, CLOCK, PER1, PER2, PER3, CRY1, and CRY2, and have been found to exhibit self-sustained circadian oscillations of Bmal1–luciferase expression [79]. Islets from patients with type 2 diabetes have decreased CRY2, PER2, and PER3 expression, which positively correlated with insulin content [90]. Additionally, the exposure of human pancreatic islets to high glucose and palmitate levels decreased PER3 expression [90]. Although various animal studies support that clock genes have an essential function in the endocrine pancreas, further research will be required to unravel their role in the human pancreas.
Environmental conditions that alter circadian rhythms and the pancreatic clock
Current lifestyle frequently involves alterations in the natural rhythms governed by the circadian system and the daily light/dark cycle. Diet composition as well as eating and activity at inappropriate times, which can disrupt circadian clocks, have been related to type 2 diabetes [40,41,91]. Although sleep loss has also been associated with this disease [43,44,47], recent studies indicate that circadian misalignment induced by changing the sleeping schedules can also increase diabetes risk independent of sleep loss [21]. SCN ablation affects the circadian pattern of glucose tolerance, as well as glucose, insulin, and glucagon plasma levels [26–28], suggesting that this central clock plays an important role in glucose homeostasis. However, several environmental factors can also affect the function of these circadian oscillators not only in the SCN but also in peripheral locations (Box 1).
In recent mouse studies, constant exposure to light or a HFD, or a combination of both, altered SCN function, affecting the circadian patterns of feeding, energy expenditure, and insulin sensitivity [92]. Experiments with mice fed a HFD have shown changes in the circadian expression of clock genes and clock-regulated metabolic genes in the hypothalamus, liver, and adipose tissue [16]. Additionally, mice fed a HFD during the light phase gained more weight than those fed during the dark phase [93]. Temporal feeding restriction in mice produced a misalignment between the SCN and the peripheral clocks in liver, kidney, heart, and pancreas, but did not affect the circadian behavior of the central clock [94]. By contrast, caloric restriction can affect both central and peripheral clocks [95]. Simulated shift work in rats led to abdominal obesity and dampened glucose rhythms. Both alterations were reversed when the feeding time was shifted back to the activity phase [96]. The majority of these results show the importance of appropriate feeding timing for the maintenance of circadian function. Thus, feeding schedules uncoupled from natural day/night cycles can lead to altered circadian rhythms and lack of synchrony between central and peripheral clocks that may favor metabolic diseases.
The effect of some of these environmental factors has also been analyzed in the endocrine pancreas. The effect of light exposure at night has been investigated in the rat pancreas using Per1-driven luciferase [97]. While changes in the light/dark cycle in vivo entrained the phase of islet clock transcriptional oscillations, 10 weeks of continued exposure to light at night (constant light regime over 24 h) impaired the amplitude, phase, and interislet synchrony of clock transcriptional oscillations. In these circumstances, glucose-stimulated insulin secretion was impaired due to a decrease in the insulin secretory pulse mass [97]. Interestingly, it was also observed that glucose regulates the amplitude and period of Per1 circadian oscillations, indicating a nutrient-sensing mechanism in the islet clock. It has also been reported that constant light regime or 6-h advance of the light cycle every 3 days accelerates the development of diabetes in Sprague–Dawley rats transgenic for the human islet amyloid polypeptide [7]. Exposure to a HFD has been shown to change the circadian expression pattern of Rev-erbα, Clock, Per1, and Per2 in mouse pancreatic islets, as well as circadian insulin secretion [10]. Circadian misalignment by simulation of shift work induced oxidative stress and altered insulin release in pancreatic islets similar to that observed in β cell-specific Bmal1 KO mice [85]. Thus, several studies have demonstrated that environmental conditions that affect circadian rhythms can also impair the pancreatic clock and the function of this endocrine organ.
Concluding remarks and future perspectives
Numerous studies have revealed an association between alterations in human circadian rhythms and metabolic diseases, such as type 2 diabetes. Moreover, animal models with genetically manipulated clock genes, as well as genetic studies in humans, have further demonstrated a link between clock genes and glucose homeostasis. Although glucose metabolism and its circadian variations may depend on several tissues and factors, which also present daily activity oscillations, the demonstration of an existing self-sustained circadian clock in the endocrine pancreas is of critical importance. Indeed, several animal models with an ablated function of different clock genes, either in the whole body, the pancreas, or β cells, exhibit diabetic phenotype features, including glucose intolerance, hyperglycemia, and/ or hypoinsulinemia. Additionally, the pancreatic islets from these animal models show impaired exocytosis and insulin release, altered mitochondrial function, and alterations in cell proliferation or cell size. Several of these observations have also been reported in human islets. It has been recently demonstrated that the pancreatic clock and islet function are also affected by some environmental factors known to disrupt circadian rhythms. It could therefore be concluded that the pancreatic clock plays an important role in endocrine pancreas function and in glucose homeostasis, and that its malfunction may lead to glucose intolerance and type 2 diabetes. Since the research focused on the pancreatic clock is relatively new, there are yet important questions to be answered (Box 3).
Box 3. Outstanding questions.
What is the exact contribution of the pancreatic clock to glucose homeostasis and the daily variations in plasma glucose and insulin?
Which mechanisms allow for the communication between the SCN and the pancreatic clock?
Is the misalignment of the pancreatic clock with the SCN sufficient to induce alterations in glucose homeostasis and diabetes?
Will pharmacological modulation of the clock proteins lead to useful therapeutics to treat metabolic diseases?
Which environmental factors known to produce circadian disruption are able to affect the pancreatic clock and the function of this organ? What are the mechanisms?
Could the reversal of or the intervention in these environmental factors restore the normal circadian pattern in the pancreas?
Understanding the role of the pancreatic clock may help in the design of therapeutic interventions for metabolic diseases, such as type 2 diabetes, when they are a consequence of circadian alterations. For instance, since Cry1 overexpression in diabetic mice increased insulin sensitivity and lowered blood glucose levels [57], it would be interesting to explore the role of CRY1/2 agonists in the treatment of diabetes. In this regard, the agonist KL001 was proved to be useful in preventing the glucagon-mediated activation of hepatic glucose production, which could be beneficial in the control of fasting glucose levels [58]. The use of REV-ERB agonists may also be valuable for the treatment of diabetes and obesity, since these molecules enhance energy expenditure, reduce fat mass, and improve dyslipidemia and hyperglycemia in diet-induced obese mice [6]. ROR GAMMA antagonists have been recently suggested to be a therapeutic strategy in type 2 diabetes by modulation of insulin resistance and glucose tolerance [98]. Other potential drugs to be explored in metabolic diseases could include ligands for ROR ALPHA [99–101], as well as for other components of the central clock [91]. Although several of these ligands, agonists, and antagonists modulate metabolic processes in peripheral clocks located in the liver, adipose tissue, and muscle, their actions in the pancreatic clock remain to be explored.
Taking into consideration the pancreas chronobiology also implies evaluating the appropriate daily times for medical treatments in diabetic patients or for the evaluation of glycemic parameters. In this regard, recent clinical studies have shown that there exists a circadian variation in the response to the glucose challenge test in pregnancy, which may influence the diagnosis of gestational diabetes [102]. In addition, it would be important to analyze whether alterations of circadian rhythms (i.e., shift work) affect the human pancreatic clock and function. If so, it would be also interesting to evaluate whether lifestyle changes adapted to the natural light/dark cycle and to appropriate feeding and activity times have a positive impact on the pancreas clock in terms of preventing and treating metabolic diseases, such as diabetes.
Acknowledgments
We apologize for not including several articles that are important in this field owing to space limitations. The authors thank Ángel Nadal for his comments on the manuscript. I.Q. is supported by grants from the Ministerio de Economía (BFU2013-42789), Generalitat Valenciana (PROMETEO/2011/080), and the European Foundation for the Study of Diabetes (EFSD/BI Basic Programme). CIBERDEM is an initiative of the Instituto de Salud Carlos III.
References
- 1.Barbadoro P, et al. Rotating shift-work as an independent risk factor for overweight Italian workers: a cross-sectional study. PLoS ONE. 2013;8:e63289. doi: 10.1371/journal.pone.0063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esquirol Y, et al. Shiftwork and higher pancreatic secretion: early detection of an intermediate state of insulin resistance? Chronobiol Int. 2012;29:1258–1266. doi: 10.3109/07420528.2012.719959. [DOI] [PubMed] [Google Scholar]
- 3.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cizza G, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010;7:274–285. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens B. Obesity: heavy sleepers. Nature. 2013;497:S8–S9. doi: 10.1038/497S8a. [DOI] [PubMed] [Google Scholar]
- 6.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale JE, et al. Disruption of circadian rhythms accelerates development of diabetes through pancreatic β-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, et al. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Vieira E, et al. The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology. 2012;153:592–601. doi: 10.1210/en.2011-1595. [DOI] [PubMed] [Google Scholar]
- 11.Muhlbauer E, et al. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 13.Ando H, et al. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology. 2011;152:1347–1354. doi: 10.1210/en.2010-1068. [DOI] [PubMed] [Google Scholar]
- 14.Balsalobre A, et al. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 15.Dibner C, et al. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 16.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, et al. Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice. Chronobiol Int. 2007;24:793–820. doi: 10.1080/07420520701672556. [DOI] [PubMed] [Google Scholar]
- 18.Vieira E, et al. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1032–E1037. doi: 10.1152/ajpendo.90510.2008. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A, et al. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto K, et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 21.Leproult R, et al. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, et al. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peek CB, et al. Nutrient sensing and the circadian clock. Trends Endocrinol Metab. 2012;23:312–318. doi: 10.1016/j.tem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcheva B, et al. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;217:127–155. doi: 10.1007/978-3-642-25950-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalsbeek A, et al. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab. 2010;21:402–410. doi: 10.1016/j.tem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 26.La Fleur SE, et al. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 27.La Fleur SE, et al. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 28.Ruiter M, et al. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 29.Shi SQ, et al. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–381. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Cauter E, et al. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 31.La Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003;15:315–322. doi: 10.1046/j.1365-2826.2003.01019.x. [DOI] [PubMed] [Google Scholar]
- 32.Boden G, et al. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 33.Kalsbeek A, et al. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav. 1998;63:553–558. doi: 10.1016/s0031-9384(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 34.Shea SA, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10(Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caciari T, et al. Evaluation of some cardiovascular risk parameters in health professionals exposed to night work. Ann Ig. 2013;25:23–30. doi: 10.7416/ai.2013.1903. [DOI] [PubMed] [Google Scholar]
- 37.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 38.Di Lorenzo L, et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 39.Ellingsen T, et al. Study of shift work and risk of coronary events. J R Soc Promot Health. 2007;127:265–267. doi: 10.1177/1466424007083702. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson B, et al. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan A, et al. Rotatingnight shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donga E, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 43.Spiegel K, et al. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taheri S, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boden G, et al. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 46.Boden G, et al. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45:1044–1050. doi: 10.2337/diab.45.8.1044. [DOI] [PubMed] [Google Scholar]
- 47.Donga E, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33:1573–1577. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamia KA, et al. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimba S, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dallmann R, et al. Altered body mass regulation in male mPeriod mutant mice on high-fat diet. Chronobiol Int. 2010;27:1317–1328. doi: 10.3109/07420528.2010.489166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barclay JL, et al. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304:E1053–E1063. doi: 10.1152/ajpendo.00512.2012. [DOI] [PubMed] [Google Scholar]
- 55.Lamia KA, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamura H, et al. Hypertension due to loss of clock: novel insight from the molecular analysis of Cry1/Cry2-deleted mice. Curr Hypertens Rep. 2011;13:103–108. doi: 10.1007/s11906-011-0181-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirota T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lau P, et al. The orphan nuclear receptor, RORα, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008;283:18411–18421. doi: 10.1074/jbc.M710526200. [DOI] [PubMed] [Google Scholar]
- 60.Preitner N, et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 61.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kornmann B, et al. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garaulet M, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garaulet M, et al. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population) Eur J Hum Genet. 2010;18:364–369. doi: 10.1038/ejhg.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sookoian S, et al. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 66.Pappa KI, et al. The major circadian pacemaker ARNT-like protein-1 (BMAL1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Res Clin Pract. 2013;99:151–157. doi: 10.1016/j.diabres.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Woon PY, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Englund A, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garaulet M, et al. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110:917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu C, et al. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PLoS ONE. 2011;6:e21464. doi: 10.1371/journal.pone.0021464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JY, et al. Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med Genet. 2011;12:82. doi: 10.1186/1471-2350-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellman B, et al. Isolated mouse islets respond to glucose with an initial peak of glucagon release followed by pulses of insulin and somatostatin in antisynchrony with glucagon. Biochem Biophys Res Commun. 2012;417:1219–1223. doi: 10.1016/j.bbrc.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi T, et al. Diurnal changes in sympathetic activity. Relation to food intake and to insulin injected into the ventromedial or suprachiasmatic nucleus. J Clin Invest. 1988;82:282–286. doi: 10.1172/JCI113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peschke E, et al. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41:1085–1092. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- 77.Allaman-Pillet N, et al. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Muhlbauer E, et al. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. doi: 10.1016/j.ejphar.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 79.Pulimeno P, et al. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56:497–507. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muhlbauer E, et al. Differential and day-time dependent expression of nuclear receptors RORα, RORβ, RORγ and RXRα in the rodent pancreas and islet. Mol Cell Endocrinol. 2013;365:129–138. doi: 10.1016/j.mce.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Vieira E, et al. Involvement of the clock gene Rev-erbα in the regulation of glucagon secretion in pancreatic α-cells. PLoS ONE. 2013;8:e69939. doi: 10.1371/journal.pone.0069939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakabayashi H, et al. Clock-controlled output gene Dbp is a regulator of Arnt/Hif-1β gene expression in pancreatic islet β-cells. Biochem Biophys Res Commun. 2013;434:370–375. doi: 10.1016/j.bbrc.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 83.Li Z, et al. Clock controls timing of mouse pancreatic differentiation through regulation of Wnt- and Notch-based and cell division components. Biochem Biophys Res Commun. 2007;359:491–496. doi: 10.1016/j.bbrc.2007.05.156. [DOI] [PubMed] [Google Scholar]
- 84.Lee J, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets. 2011;3:381–388. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J, et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadacca LA, et al. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frese T, et al. Circadian and age-dependent expression patterns of GLUT2 and glucokinase in the pancreatic β-cell of diabetic and nondiabetic rats. Horm Metab Res. 2007;39:567–574. doi: 10.1055/s-2007-984471. [DOI] [PubMed] [Google Scholar]
- 88.Delattre E, et al. Diurnal variations in insulin secretion and K+ permeability in isolated rat islets. Clin Exp Pharmacol Physiol. 1999;26:505–510. doi: 10.1046/j.1440-1681.1999.03073.x. [DOI] [PubMed] [Google Scholar]
- 89.Muhlbauer E, et al. Circadian changes of ether-a-go-go-related-gene (Erg) potassium channel transcripts in the rat pancreas and β-cell. Cell Mol Life Sci. 2007;64:768–780. doi: 10.1007/s00018-007-6478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stamenkovic JA, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985. doi: 10.1016/j.metabol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Schroeder AM, et al. How to fix a broken clock. Trends Pharmacol Sci. 2013;34:605–619. doi: 10.1016/j.tips.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coomans CP, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 93.Arble DM, et al. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Challet E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B. 2010;180:631–644. doi: 10.1007/s00360-010-0451-4. [DOI] [PubMed] [Google Scholar]
- 96.Salgado-Delgado R, et al. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 97.Qian J, et al. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeda Y, et al. Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet. 2014;10:e1004331. doi: 10.1371/journal.pgen.1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kojetin DJ, et al. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar N, et al. Identification of SR 3335 (ML-176): a synthetic RORα selective inverse agonist. ACS Chem Biol. 2011;6:218–222. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldberg RJ, et al. Circadian variation in the response to the glucose challenge test in pregnancy: implications for screening for gestational diabetes mellitus. Diabetes Care. 2012;35:1578–1584. doi: 10.2337/dc11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohawk JA, et al. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quesada I, et al. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 105.Muoio DM, et al. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]