Abstract
Interactions between tissue factor and factor VIIa are the primary initiators of coagulation in hemostasis and certain thrombotic diseases. Tissue factor, an integral membrane protein expressed extensively outside of the vasculature, is the regulatory protein cofactor for coagulation factor VIIa. Factor VIIa, a trypsin-like serine protease homologous with other blood coagulation proteases, is weakly active when free in solution and must bind its membrane-bound cofactor for physiologically-relevant activity. Tissue factor allosterically activates factor VIIa by several mechanisms such as active site positioning, spatial stabilization, and direct interactions with the substrate. Protein-membrane interactions between tissue factor, factor VIIa, and substrates all play critical roles in modulating the activity of this enzyme complex. Additionally, divalent cations such as Ca2+ and Mg2+ are critical for correct protein folding, as well as protein-membrane and protein-protein interactions. The contributions of these factors towards tissue factor-factor VIIa activity are discussed in this review.
Keywords: Tissue factor, factor VIIa, protein-membrane interactions, extrinsic tenase, metal ions
INTRODUCTION
The “extrinsic tenase” complex, comprised of tissue factor (TF) and factor VIIa (FVIIa), is a two-subunit enzyme that initiates the coagulation cascade under most in vivo conditions (depicted in Figure 1).1,2 The regulatory subunit of this complex, TF (also known as thromboplastin, CD142, or coagulation factor III), is a cell-surface, transmembrane protein of the class II cytokine receptor family that is extensively expressed amongst adventitial and epithelial tissues; however, tissues exposed to the vessel lumen such as endothelial cells, platelets, and leukocytes constitutively express little or no TF.3–5 The enzymatic subunit is a plasma protein, FVIIa, that is a trypsin-like serine protease demonstrating homology with several other coagulation proteins, including its cognate substrates factors IX (FIX) and X (FX). Total FVII (active enzyme and zymogen) circulates in plasma at a concentration of approximately 10 nM; however, only about 1% is in the active form.6 Due to its poor enzymatic activity, free FVIIa in plasma largely escapes recognition by protease inhibitors and therefore circulates with a half-life of ~90 minutes.7,8 The extended half-life afforded to FVIIa may serve an important function, as low basal concentrations of pre-formed FVIIa may serve to “prime” the coagulation cascade for a rapid response to injury.6
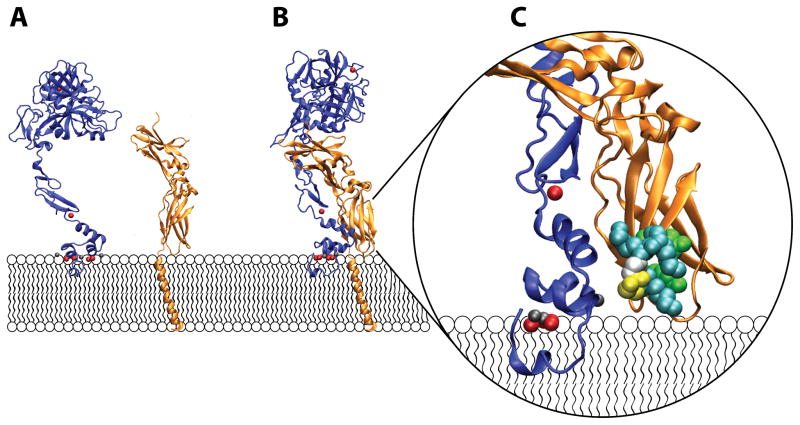
Figure 1.
Crystal structures of FVIIa and sTF arranged on a membrane surface. A) FVIIa (blue) has an extended conformation and binds to anionic phospholipids in membrane bilayers through its N-terminal GLA-domain (depicted here in contact with the membrane). Coordination of divalent cations such as Ca2+ (red spheres) and Mg2+ (gray spheres) by the GLA domain is critical for proper domain folding and function. In addition, a Ca2+ ion is bound to the first EGF-like domain of FVIIa and also to the protease domain of this protein (the domain farthest from the membrane). The isolated ectodomain of TF (sTF, orange) is depicted here as anchored to the membrane surface via a single transmembrane helix, which has been modeled in. Full-length TF also contains a 21 amino acid-long cytoplasmic tail (not shown) which is implicated in interactions with the cytoskeleton. B) Crystal structure of the sTF-FVIIa complex, with the transmembrane helix of TF modeled in. FVIIa interacts extensively with sTF, with a binding interface that spans all domains of FVIIa and sTF. C) Close-up of TF residues putatively involved in substrate recognition (i.e., the substrate-binding exosite region of TF). In addition to allosterically activating FVIIa, TF is thought to directly interact with the protein substrates, FIX and FX, through membrane-proximal residues. Thus, TF residues Tyr157, Lys159, Ser163, Gly164, Lys165, Lys166, and Tyr185 (shown as van Der Waals radii and colored according to identity as follows; Teal: Lysine, White: Glycine, Yellow: Serine, Green: Tyrosine) contribute significantly to interactions with substrate as demonstrated by mutagenesis studies. (Panel C is rotated ~45° from panel B.) The structure of the sTF-FVIIa complex in panels B and C is rendered from pdb file 3TH273 using VMD Molecular Graphics Viewer.118 The isolated structures of FVIIa and sTF shown in panel A are a separation of the two from the TF-FVIIa complex. The transmembrane helix attached to sTF is adopted from pdb file 1A11.119
Under homeostatic conditions, TF and blood are physically separated, but vascular injury exposes plasma (which contains FVII and FVIIa) to a variety of TF-expressing cells. Once zymogen FVII binds to TF, it is rapidly converted to FVIIa by limited proteolysis, thereby generating the active TF-FVIIa complex. Formation of the TF-FVIIa complex greatly increases the enzymatic activity of FVIIa via allosteric interactions between TF and FVIIa, as revealed by about a 20- to 100-fold increase in the rate of hydrolysis of small, chromogenic peptidyl substrates (termed its amidolytic activity),9,10 and nearly a million-fold increase in the rate of activation of the macromolecular substrates, FIX and FX.11 Subsequently FIXa, in association with its regulatory subunit FVIIIa, further activates FX; FXa in turn complexes with its cofactor FVa, to proteolytically cleave prothrombin to thrombin, leading to fibrin clot formation. Widespread, constitutive expression of TF on cells surrounding organs, blood vessels, and skin serves to create a “hemostatic envelope” that initiates clotting upon vascular injury.3
An additional point of modulation of TF-FVIIa is via the phospholipid bilayer. In concert with allosteric activation of the active site of FVIIa upon binding to TF, formation of the extrinsic tenase on a suitably procoagulant phospholipid bilayer increases the rate of FIX or FX activation, in a Ca2+-dependent manner, an additional 1,000-fold.11 In fact, nearly all reactions of the coagulation cascade are reliant upon exposure of phosphatidyl-L-serine (PS) on membrane surfaces.12 The roughly million-fold overall increase in FX activation by the TF-FVIIa-phospholipid complex relative to free FVIIa is critical regulatory point for the coagulation cascade.13 The roles of TF-FVIIa in hemostasis, thrombosis and other biological processes are numerous, and the structural underpinnings comprising the foundation of their activities continue to be a point of focus and investigation.
STRUCTURE
Tissue Factor
TF, a 263 amino acid glycoprotein with a molecular weight of ~46kDa and member of the cytokine class II receptor family, is composed of three domains: a 219 amino acid N-terminal extracellular domain (residues 1–219, whose crystal structure is shown in Figure 1); a 22 amino acid transmembrane domain (residues 220–242); and a 21 amino acid cytoplasmic C-terminal tail (residues 242–263). The cytoplasmic tail contains two phosphorylation sites at Ser253 and Ser258, and one S-palmitoylation site at Cys245. Removal of the cytoplasmic domain has no deleterious effects on TF coagulant activity. The TF transmembrane domain is composed of a single-spanning alpha-helix, the precise identity of which has been shown unimportant for TF procoagulant function;14 anchoring of a histidine-tagged extracellular domain of TF to the membrane using nickel-chelating lipids resulted in full restoration of procoagulant activity of TF.15 The extracellular domain of TF (sTF) is composed of two fibronectin type III domains, and is connected to the transmembrane domain through a six-amino acid linker. This linker likely exhibits sufficient flexibility to conformationally decouple the extracellular domain of TF from the transmembrane and cytoplasmic domains.14,16 The fibronectin type III domain structure, composed mainly of beta-strands connected by β-loops, is a member of the immunoglobulin-like family of protein folds and is conserved amongst a wide variety of extracellular proteins.17
The procoagulant activity of TF does not necessarily correlate with its levels of cell-surface expression. Much of the TF expressed on a cell surface is ‘encrypted’, and must first be ‘decrypted’ to participate fully in coagulation reactions. The process by which this occurs has yet to be fully explained, and is likely a combination of several mechanisms. One clear contributor is exposure of anionic phospholipids. Healthy cells actively sequester anionic phospholipids such as PS to the inner leaflet of the plasma membrane,18,19 but following cellular damage, activation, or increased levels of cytosolic Ca2+ this bilayer asymmetry is lost, resulting in increased PS exposure on the outer leaflet which increases the specific activity of cell-surface TF-FVIIa complexes. PS exposure is well known to decrease the apparent Km for activation of FIX and FX, but additional mechanisms could include conformational rearrangement of TF or the TF-FVIIa complex and subsequent exposure of substrate binding sites.16,20 Expression levels of GRP78, a molecular chaperone protein, have also been shown to mediate TF procoagulant activity in a Ca2+-dependent manner and thus may also play a role in its decryption.21,22
A fascinating suggestion is that disulfide linkages play a role in TF encryption/decryption, and in particular, that the membrane-proximal cysteine pair in TF (Cys186-Cys209) is an ‘allosteric’ disulfide that is subject to redox control, leading to TF encryption/decryption.23–25 Others have disputed this conclusion, however, and have proposed alternative explanations for the observed effects (reviewed in 26,27). Though the importance of TF disulfide bond formation towards its cofactor activity has yet to be resolved, FVIIa binding to TF is not dependent on the oxidation state of Cys186 and Cys209.23 A number of insightful and detailed reviews focus on controversies surrounding TF decryption are available.28–32
Factor VII/VIIa
The trypsin-like serine protease FVII (in the inactive precursor, or zymogen form) is a ~50KDa, single-chain polypeptide consisting of 406 residues, with an N-terminal γ-carboxyglutamate-rich (GLA) domain, two epidermal growth factor-like domains (EGF1 and EGF2), and a C-terminal serine protease domain.33–36 Activation of FVII to FVIIa is accomplished via specific proteolytic cleavage of the Ile153-Arg152 bond in the short linker region between the EGF2 and protease domain, with the resultant light and heavy chains held together by a single disulfide bond (Cys135-Cys262). The crystal structure of FVIIa is shown in Figure 1. FVII has significant structural and sequence homology to coagulation factors IX, X, and protein C.37,38
FVIIa binds the phospholipid membrane in a Ca2+-dependent manner through its N-terminal GLA-domain. Containing 10 vitamin K-dependent, posttranslationally modified γ-carboxyglutamate (Gla) residues, GLA-domains coordinate 7–9 divalent metal ions such as Ca2+ and Mg2+, inducing conformational rearrangements that are requisite for interaction with membrane surfaces.39,40
Immediately C-terminal to the GLA domain is an aromatic stack and two epidermal growth factor (EGF) domains (EGF1 and EGF2). The aromatic stack connects the GLA to EGF1 domain, which binds a single Ca2+ ion with moderately high affinity.41,42 Occupancy of this Ca2+-binding site increases FVIIa amidolytic activity and TF association.43 The FVIIa heavy chain comprises the trypsin-like protease domain, which is also homologous to other coagulation serine proteases such as FX, FIX, protein C, and prothrombin. The catalytic triad consists of His193, Asp242 and Ser344, and binding of a single Ca2+ ion within the FVIIa protease domain is critical for catalytic activity.41,44 Additionally, proteolytic activation of FVII to FVIIa frees the newly formed amino terminus at Ile153 to fold back and insert into the activation pocket, forming a salt bridge with the carboxylate of Asp343 to generate the oxyanion hole.45 Formation of this salt bridge is critical for FVIIa activity; indeed, FVIIa with the mutation V154G is cleaved to the two-chain form normally and with wild-type macromolecular substrate affinity,46 but with significantly reduced ability of the resultant FVIIa to activate FX.46,47 Reduced N-terminal hydrogen-deuterium exchange upon TF binding to FVIIa supports the hypothesis that the N-terminal Ile153 is not fully inserted into the activation pocket when free in solution.48 Additionally, unlike most other serine proteases, oxyanion hole formation in free FVIIa does not occur upon proteolytic activation, but instead upon substrate interaction.49 As a result, FVIIa circulates in a zymogen-like state that is poorly recognized by plasma protease inhibitors,49 allowing it to circulate with a half-life of approximately 90 minutes.7,8 This is far longer than other coagulation enzymes such as FIXa, FXa and thrombin, whose plasma half-lives are on the order of seconds to minutes.50,51
STRUCTURE-FUNCTION RELATIONSHIP
Association of TF with FVIIa allosterically activates the protease, creating what is essentially a dimeric enzyme in which TF is the regulatory subunit and FVIIa the catalytic subunit. The ability to cleave very small, tripeptidyl-amide substrates (amidolytic activity) of FVIIa is increased approximately 50-fold upon binding of TF, with the largest change being increased kcat,9,10 indicating that TF association induces conformational changes within the active site of the FVIIa protease domain.45,52–54 Additionally, pKa values of the catalytic triad are altered upon TF binding.55 It has been hypothesized that FVIIa in solution exists in an equilibrium between two states; one in which the heavy-chain N-terminus has inserted into the active site pocket, and one that is more zymogen-like with the N-terminus incorrectly or incompletely inserted. TF may preferentially bind FVIIa when it is in the catalytically active form,45 shifting the equilibrium towards the active (N-terminal buried) conformation. Hydrogen-deuterium exchange experiments coupled with mass spectrometry have demonstrated that several loop regions within the protease domain of FVIIa are stabilized upon binding to TF, through rearrangement and strengthening of an extensive hydrogen bonding network.48,52 These structural changes are not limited to the protease domain but extend throughout most of FVIIa,48 indicating widespread allosteric modulation of FVIIa by TF.
Active Site Positioning
In vivo enzymatic activity of the TF-FVIIa complex occurs exclusively on the phospholipid membrane surface, and is dependent upon the interaction of FVIIa, FIX and FX with the membrane through their membrane-binding GLA-domains. Fluorescence resonance energy transfer (FRET) experiments indicate that free FVIIa adopts a stable, extended structure when bound to the membrane, with its active site positioned ~80 Å above the membrane surface.56 This distance is in good agreement with those seen for the homologous proteins FIXa57 and FXa58. Upon FVIIa binding to TF, the FVIIa active site is repositioned ~6 Å closer to the membrane, a modulation that may aid in proper alignment of the FVIIa catalytic triad with the target substrate cleavage site.56 In comparable FRET experiments using GLA-domainless FVIIa, the active site was still positioned a similar distance above the membrane, demonstrating that TF is able to fully support FVIIa active site positioning even in the absence of FVIIa-membrane interactions.59 Further, experiments using multiple approaches have shown that TF supports full FVIIa proteolytic activity as long as the TF extracellular domain is tethered in some way to the membrane surface, while the exact nature of this membrane tether is essentially irrelevant.14,15,60 In contrast, raising the active site of FVIIa greater than 80 Å above the membrane surface using TF/P-selectin chimeras greatly reduced the ability of the TF-FVIIa complex to activate FX, but did not diminish TF-FVIIa amidolytic activity. This indicates that TF-mediated positioning of the FVIIa active site above the membrane surface is important for its activity towards cognate substrates.61
Molecular dynamics (MD) simulations of TF-FVIIa in the presence of membrane surfaces indicate TF reduces FVIIa inter-domain flexibility. Both in solution and on the membrane surface, the hinge-like motion of FVIIa and its Cα RMSD values are significantly reduced in the presence of TF.16
Spatial Stabilization
Free FVIIa is an inherently dynamic molecule, with MD simulations, fluorescence anisotropy, and hydrogen-deuterium exchange data indicating intra- and inter- domain flexibility.16,52,62–64 A major component of TF allosteric modulation of FVIIa activity is the stabilization and reduced flexibility of FVIIa upon TF binding. In the protease domain, stabilization of the 170-loop located near the TF interaction site appears to be important for FVIIa amidolytic activity.63,64 Replacement of the loop with that of a similar but truncated loop from trypsin results in an increase in FVIIa amidolytic activity even in the absence of TF, suggesting that stabilization of the 170-loop plays a significant role in TF-mediated allosteric activation of FVIIa.65 Additionally, hydrogen exchange experiments and MD simulations show that TF binding aids the insertion of Ile153 into the activation pocket of FVIIa and stabilizes its structure, even after removal of the N-terminal insertion.52,63 Specific TF and FVIIa residues have been identified that contribute to stabilization of FVIIa within the TF-FVIIa complex. Alanine scanning mutagenesis studies66 and crystallography data41 demonstrated that Met306 in FVIIa plays a pivotal role in TF interactions, restricting the flexibility of FVIIa’s 170-loop upon FVIIa-TF complex formation. The crystal structure of free FVIIa,67 in which the 170-loop and precluding α-helix (containing Met306) are more disordered, also supports this idea.
TF-Substrate Interactions
In addition to the role of TF in allosterically activating FVIIa, binding interactions between the ‘exosite’ region of TF and macromolecular substrates are also implicated in TF-FVIIa catalytic activity (shown in Figure 1C). The known physiologic substrates of TF-FVIIa are FVII, FIX, FX and certain protease activated receptors (PARs). TF mutational analysis has identified a number of residues that, when mutated, support full FVIIa amidolytic activity towards small peptidyl substrates but are deficient in their ability to support macromolecular substrate (FVII, FIX, FX) activation.68–70 Several crystal structures have shown disorder in the TF loop region at residues 159–165,41,49 and residues in or adjacent to this flexible loop have been shown to be especially critical for proteolytic activity of the TF-FVIIa complex, thereby defining the proposed substrate-binding exosite region of TF that is quite distant from the FVIIa active site.68,69 Interestingly, mutation of Gly164 of TF to a marginally more bulky alanine significantly impairs TF-FVIIa proteolytic activity, suggesting the flexibility afforded by glycine is critical for macromolecular substrate recognition.68,70
TF residues Lys165 and Lys166 have also been demonstrated to be important for substrate recognition and binding; mutation of either of these residues to alanine results in a significant decrease in the cofactor function of TF.68,69,71,72 However, TF with mutations at K165A and K166A activated GLA-domainless FX at rates comparable to that of the wild-type TF, and utilization of GLA-domainless FVIIa greatly muted the effects of these TF mutations on FX activation.69 Crystal structures have indicated that Lys165 and Lys166 face away from each other, with Lys165 pointing towards FVIIa in most TF-FVIIa structures, and Lys166 pointing into the substrate binding exosite region.41,73 Putative salt bridge formation between Lys165 of TF and Gla35 of FVIIa would support the notion that TF interaction with the GLA-domain of FVIIa modulate substrate recognition.73 Taken together, these results suggest that the C-terminal portion of the TF ectodomain directly interacts with the GLA-domains (and possibly the adjacent EGF1 domains) of FIX and FX, and that the presence of the FVIIa GLA-domain may modulate these interactions, either directly or indirectly. Furthermore, the TF residues involved in substrate interactions with FIX and FX are similar or identical to those that interact with FVII during TF-mediated FVII autoactivation,53 indicating a similar mechanism of substrate binding.
A number of monoclonal anti-TF antibodies have been raised, with the vast majority blocking association between TF and FVII.74 However, two monoclonal antibodies, TF8-5G9 and TF8-11D12 (which came from the same fusion and which are probably identical) were shown not to inhibit TF-FVIIa binding, but to strongly inhibit activation of FIX and FX by TF-FVIIa.75,76 Crystal structures of TF in complex with the antibody TF-5G977 indicates that the epitope on TF for this antibody overlaps significantly with the substrate interaction (exosites) region identified by Kirchhofer et al.70 More recently, two additional monoclonal anti-TF antibodies (D3 and 5G6) with similar properties have also been reported.78
Monoclonal antibody TF9-10H10 binds to TF but does not inhibit its procoagulant activity, which is unusual as almost all anti-TF antibodies are inhibitory.74 More recent studies using this antibody have shown that it does inhibit the ability of the TF-FVIIa complex to participate in signaling.79 These results suggest that TF-FVIIa signaling via integrins and PAR-2 is mediated by exosite-like interactions on TF distinct from those involved in TF-FVIIa procoagulant functions.
Protein-Membrane Interactions
Lipid bilayer composition plays an important role in activity of the TF-FVIIa complex. GLA-domain containing coagulation proteins are well-known to preferentially bind anionic phospholipids in general, and PS in particular.12 PS is, however, actively sequestered to the inner leaflet of the plasma membrane, serving as an important point of regulation of blood clotting.12,18,19,80 Exposure of PS on the outer leaflet occurs either via physical disruption of the cell membrane as a consequence trauma, or via regulated cellular processes such as those that occur upon platelet activation. Interestingly, despite the high degree of sequence homology between GLA-domains of different clotting proteins, their membrane binding affinities vary by three orders of magnitude,81,82 with FVIIa and activated protein C (APC) displaying the weakest affinities for PS-containing bilayers. We recently reported that FVIIa and APC preferentially bind to bilayers containing phosphatidic acid (PA), a minor anionic lipid in cell membranes.83 PA has minimal effect on TF-FVIIa activity in vitro, however, likely due to the fact that protein-protein interactions between TF-FVIIa dominate the recruitment of FVIIa to the membrane.84 Further, when used pharmacologically to treat bleeding disorders, the mechanism of action of recombinant FVIIa has been shown to be independent of TF.85,86 Thus, the membrane binding characteristics of “free” (non-TF bound) FVIIa may be an important component of its in vivo efficacy, especially when high concentrations of recombinant FVIIa are employed to treat bleeding.
Direct interactions between the TF ectodomain and the membrane surface may also contribute to the activity of the TF-FVIIa complex. It is thought that there is considerable freedom of motion and autonomy of the TF ectodomain relative to the membrane due to the structural flexibility of the short peptide linker between the ectodomain and the transmembrane domain.14 Furthermore, MD simulations have identified a number of TF residues in the C-terminal portion of the ectodomain that directly contact the phospholipid membrane surface. These residues maintained association of sTF (i.e., the isolated ectodoman) with the membrane surface, suggesting that TF residues may associate directly with PS headgroups.16 Additionally, simulations suggest the orientation of TF with respect to the membrane is altered upon FVIIa binding, with TF leaning toward FVIIa. As a result, the TF residues in contact with the phospholipid membrane are proposed to change.16 This region of putative membrane-interacting TF residues is immediately adjacent to the proposed substrate-binding exosite, and alanine scanning mutagenesis studies have identified mutations in this region that alter the ability of membrane-anchored TF-FVIIa to activate FX.20 Interestingly, increasing the PS content of TF-liposomes partially overcomes these deficiencies, suggesting that direct PS-TF interactions may either stabilize the complex or induce ideal conformational arrangements, promoting interaction of FIX and FX with the TF exosite.16,20
Biochemical studies of protein-membrane interactions in blood clotting often utilize liposomes with non-physiological membrane compositions. Thus, it typically requires 30% or more PS to achieve maximal TF-FVIIa enzymatic activity in vitro,87 while only ~10% of the total plasma membrane bilayer is composed PS in eukaryotic cells.88 Incorporation of phosphatidylethanolamine (PE, a plasma membrane phospholipid that is much more abundant than PS) into TF-liposomes markedly decreases the required PS content, although PE by itself does little to promote TF activity.87 Other lipids such as phosphatidic acid, phosphatidylglycerol, and phosphatidylinositol also reduce the PS requirement, indicating ‘synergy’ between PS and PE is not a unique property of PE but a more broadly encompassing mechanism. This ‘ABC hypothesis’ (Anything But Choline) postulates that any lipid not containing the bulky choline headgroup of PC or sphingomyelin can ‘synergize’ with PS and decrease the required PS content for maximal enzymatic activity.
Divalent Metal Ions
Calcium ions are required for assembly and function of TF-FVIIa. Although TF lacks any known divalent metal ion binding-sites, Ca2+ can occupy up to nine metal binding sites within FVIIa (Figure 1). Of these, seven reside in the GLA-domain of FVIIa and are critical for both structure and function of this domain. In particular, Gla7 and Gla9 coordination of Ca2+ induces formation of the ω-loop, which exposes hydrophobic residues that are believed to insert into the bilayer.87,89–91 Recent crystallographic, enzymatic, and equilibrium dialysis studies have indicated that the FVIIa GLA-domain actually binds a combination of 4–5 Ca2+ and 2–3 Mg2+ under physiologic divalent metal ion conditions.49,73 Ca2+ is absolutely required for GLA-domain structure and function, and can occupy all GLA-domain metal binding sites when it is the only divalent metal ion present (especially at supraphysiologic concentrations of Ca2+), but Mg2+ alone is unable to induce the correct GLA-domain structure. This suggests that a subset of metal binding sites in GLA domains are occupied with Mg2+ in vivo,40,73 and occupancy of these sites by Mg2+ even in the presence of vast excesses of Ca2+ suggest these sites preferentially bind Mg2+ in plasma.49,73 The reasons for differential metal ion specificity of GLA domains remain unclear, but are likely due to differences in coordination geometries of each binding site along with the ‘hardness’ properties of Ca2+ versus Mg2+.73,92 Mg2+ has been demonstrated to modulate both the membrane binding73 and enzymatic properties93 of FVIIa and FIX94.
One Ca2+ binds to the EGF1 domain of FVIIa, for which Mg2+ cannot substitute. 49 This Ca2+ is implicated in optimizing TF-FVIIa binding interactions, likely through modulating the orientation of the FVIIa GLA-domain relative to the EGF1 domain.95 The protease domain of FVIIa also contains one Ca2+ binding site; its occupancy results in allosteric activation through reregistration of the Ca2+ binding loop.52 Additionally, two Zn2+ binding sites have been identified in the protease domain, although Zn2+ occupancy of these sites inhibits both FVIIa enzymatic activity and TF binding.49,96
The Ca2+ binding sites in FVIIa are incompletely saturated at plasma concentrations of free Ca2+ (~1.25 mM).97,98 However, using the plasma concentrations of free Ca2+ and Mg2+ (~1.25 mM and ~0.6 mM, respectively) restores activity to maximal levels, indicating that Mg2+ likely plays a role in vivo.73,93,94,99 The GLA-domains of both FVIIa and FX mediate enzymatic rate enhancements due to Mg2+, suggesting that conformational changes of the FX GLA-domain upon Mg2+ occupancy of metal binding sites modifies its interactions with TF.99
Signaling
The role of TF in cellular signaling is incompletely understood, though it is known to play roles in tumor growth,79 metastasis,100,101 angiogenesis, and anti-apoptotic signaling;102 additionally, TF is known to be upregulated in many malignant tumor cell types.103–105 FVIIa binding to TF has been implicated in directly mediating PAR-2 cleavage via mechanisms both dependent106–109 and independent110,111 of the cytoplasmic domain of TF.112 Phosphorylation of the TF cytoplasmic domain subsequent to TF-FVIIa cleavage of PAR-2 is thought to release TF-dependent negative regulation of PAR-2 mediated signaling.79,103,108 This results in mitogen-activated protein kinase (MAPK) pathway activation and downstream effects, including the upregulation of cytokines and proangiogenic factors.103,113 Activation of the p44/42 MAPK pathway, as well as JAK2, p70/p85S6K, and p90RSK, can occur independent of the TF cytoplasmic domain.110,114,115 Though the cytoplasmic domain is not necessary for activation of these proteins, proteolytically active FVIIa is required. In addition, TF can also mediate signaling through the enzymatic activity of FXa in complex with TF-FVIIa, which plays an important role in the regulation of several pathways. TF-FVIIa-FXa-mediated cleavage of PARs, particularly PAR-2, has been shown to up-regulate IL-8 expression, resulting in increased cell migration.101 A number of detailed reviews can be found regarding TF-FVIIa-mediated signaling.101,103,116,117
CONCLUSION
The interaction between TF and FVIIa is a critical component of hemostasis. TF is expressed extensively on cells outside the vasculature, creating a ‘hemostatic envelope.’ Disruption of the blood vessel endothelium results in exposure of blood coagulation proteins, including FVIIa, to TF-bearing cells. Subsequent to TF-FVIIa complex formation, two blood clotting zymogens, FIX and FX, are proteolytically activated, propagating the coagulation cascade and resulting in fibrin deposition and clot formation. The physical separation of TF from the plasma clotting enzymes is undoubtedly a regulatory mechanism; without its protein cofactor, FVIIa is a very weakly enzyme and will not initiate coagulation at physiologic concentrations. Expression of TF inside the vasculature results in aberrant coagulation cascade activity and is believed to play an important role in many thrombotic disorders.
TF modulates FVIIa activity through a number of mechanisms. FVIIa interaction with TF has been demonstrated to position its active site ~75 Å above the membrane surface, ~6 Å closer than FVIIa in the absence of TF. Additionally, MD simulations have suggested that TF restricts both FVIIa inter- and intra- domain flexibility, particularly within the protease domain. TF has been shown to stabilize a number of loop regions in the protease domain of FVIIa as well as facilitate the insertion of the N-terminus of the FVIIa heavy chain into the activation pocket, which is critical for its enzymatic activity. In addition, an extended substrate binding exosite has been identified on TF, which has been shown to interact directly with both extrinsic tenase complex substrates, FIX and FX. The importance of the phospholipid membrane in mediating the activity of TF-FVIIa cannot be understated; recruitment of FX to the membrane surface is dependent upon the exposure of anionic phospholipids, particularly PS, which may interact with substrates (FIX and FX), enzyme (FVIIa) and protein cofactor (TF). All the interactions between TF, FVIIa, protein substrates and membrane surfaces are dependent upon divalent cations. Although several structures of TF-FVIIa have been solved, as of yet no tertiary TF-FVIIa-FX or TF-FVIIa-FIX structures have been determined, nor has the role of the membrane surface in the formation of these complexes been determined structurally. Such results would provide significant information regarding the structure-function relationship of the extrinsic tenase in blood clotting.
Acknowledgments
Studies in the authors’ laboratory were supported by grants R01 HL047014 and R01 HL103999 from the National Heart, Lung and Blood Institute of the NIH.
References
- 1.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey JH. Tissue factor: a key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004;79(2):103–108. doi: 10.1532/ijh97.03167. [DOI] [PubMed] [Google Scholar]
- 3.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 4.Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59(2):421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81(3):734–744. [PubMed] [Google Scholar]
- 7.Kondo S, Kisiel W. Regulation of factor VIIa activity in plasma: Evidence that antithrombin III is the sole plasma protease inhibitor of human factor VIIa. Thromb Res. 1987;46(2):325–335. doi: 10.1016/0049-3848(87)90294-5. [DOI] [PubMed] [Google Scholar]
- 8.Seligsohn U, Kasper CK, Osterud B, Rapaport SI. Activated factor VII: Presence in factor IX concentrates and persistence in the circulation after infusion. Blood. 1979;53(5):828–837. [PubMed] [Google Scholar]
- 9.Lawson JH, Butenas S, Mann KG. The evaluation of complex-dependent alterations in human factor VIIa. J Biol Chem. 1992;267(7):4834–4843. [PubMed] [Google Scholar]
- 10.Neuenschwander PF, Branam DE, Morrissey JH. Importance of substrate composition, pH and other variables on tissue factor enhancement of factor VIIa activity. Thromb Haemost. 1993;70(6):970–977. [PubMed] [Google Scholar]
- 11.Ruf W, Rehemtulla A, Morrissey JH, Edgington TS. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991;266(4):2158–2166. [PubMed] [Google Scholar]
- 12.Zwaal RFA, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376(3):433–453. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 13.Morrissey JH, Neuenschwander PF, Huang Q, et al. Factor VIIa-tissue factor: functional importance of protein-membrane interactions. Thromb Haemost. 1997;78(1):112–116. [PubMed] [Google Scholar]
- 14.Paborsky LR, Caras IW, Fisher KL, Gorman CM. Lipid association, but not the transmembrane domain, is required for tissue factor activity. Substitution of the transmembrane domain with a phosphatidylinositol anchor. J Biol Chem. 1991;266(32):21911–21916. [PubMed] [Google Scholar]
- 15.Waters EK, Morrissey JH. Restoring full biological activity to the isolated ectodomain of an integral membrane protein. Biochemistry. 2006;45(11):3769–3774. doi: 10.1021/bi052600m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkubo YZ, Morrissey JH, Tajkhorshid E. Dynamical view of membrane binding and complex formation of human factor VIIa and tissue factor. J Thromb Haemost. 2010;8(5):1044–1053. doi: 10.1111/j.1538-7836.2010.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lhermusier T, Chap H, Payrastre B. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J Thromb Haemost. 2011;9(10):1883–1891. doi: 10.1111/j.1538-7836.2011.04478.x. [DOI] [PubMed] [Google Scholar]
- 19.Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol. 2009;44(5):264–277. doi: 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke K, Yuan J, Morrissey JH. Tissue factor residues that putatively interact with membrane phospholipids. PLoS ONE. 2014;9(2):e88675. doi: 10.1371/journal.pone.0088675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hashimi AA, Caldwell J, Gonzalez-Gronow M, et al. Binding of anti-GRP78 autoantibodies to cell surface GRP78 increases tissue factor procoagulant activity via the release of calcium from endoplasmic reticulum stores. J Biol Chem. 2010;285(37):28912–28923. doi: 10.1074/jbc.M110.119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozza LM, Austin RC. Getting a GRP on tissue factor activation. Arterioscler Thromb Vasc Biol. 2005;25(8):1529–1531. doi: 10.1161/01.ATV.0000177041.47444.e2. [DOI] [PubMed] [Google Scholar]
- 23.Butenas S. Tissue factor structure and function. Scientifica. 2012;2012:964862. doi: 10.6064/2012/964862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen VM, Ahamed J, Versteeg HH, et al. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45(39):12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 25.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282(35):25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 26.Bach RR, Monroe D. What is wrong with the allosteric disulfide bond hypothesis? Arterioscler Thromb Vasc Biol. 2009;29(12):1997–1998. doi: 10.1161/ATVBAHA.109.194985. [DOI] [PubMed] [Google Scholar]
- 27.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123(1):171–176. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110(12):3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao LV, Kothari H, Pendurthi UR. Tissue factor: mechanisms of decryption. Front Biosci. 2012;4:1513–1527. doi: 10.2741/477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pendurthi UR, Rao LV. Role of tissue factor disulfides and lipid rafts in signaling. Thromb Res. 2008;122(Suppl 1):S14–18. doi: 10.1016/S0049-3848(08)70012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butenas S, Krudysz-Amblo J. Decryption of tissue factor. Thromb Res. 2012;129(Suppl 2):S18–20. doi: 10.1016/j.thromres.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao LV, Kothari H, Pendurthi UR. Tissue factor encryption and decryption: facts and controversies. Thromb Res. 2012;129(Suppl 2):S13–17. doi: 10.1016/j.thromres.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radcliffe R, Nemerson Y. Activation and control of factor VII by activated factor X and thrombin: Isolation and characterization of a single chain form of factor VII. J Biol Chem. 1975;250(2):388–395. [PubMed] [Google Scholar]
- 34.Broze GJ, Jr, Majerus PW. Purification and properties of human coagulation factor VII. J Biol Chem. 1980;255(4):1242–1247. [PubMed] [Google Scholar]
- 35.Kisiel W, Davie EW. Isolation and characterization of bovine factor VII. Biochemistry. 1975;14(22):4928–4934. doi: 10.1021/bi00693a023. [DOI] [PubMed] [Google Scholar]
- 36.Gladhaug A, Prydz H. Purification of the coagulation factors VII and X from human serum. Some properties of factor VII. Biochim Biophys Acta. 1970;215(1):105–111. doi: 10.1016/0304-4165(70)90392-2. [DOI] [PubMed] [Google Scholar]
- 37.Hagen FS, Gray CL, O’Hara P, et al. Characterization of a cDNA coding for human factor VII. Proc Natl Acad Sci U S A. 1986;83:2412–2416. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hara PJ, Grant FJ, Haldeman BA, et al. Nucleotide sequence of the gene coding for human factor VII, a vitamin K-dependent protein participating in blood coagulation. Proc Natl Acad Sci U S A. 1987;84(15):5158–5162. doi: 10.1073/pnas.84.15.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloom JW, Mann KG. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978;17(21):4430–4438. doi: 10.1021/bi00614a012. [DOI] [PubMed] [Google Scholar]
- 40.Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995;270(14):7980–7987. doi: 10.1074/jbc.270.14.7980. [DOI] [PubMed] [Google Scholar]
- 41.Banner DW, D’Arcy A, Chène C, et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380(6569):41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 42.Schiodt J, Harrit N, Christensen U, Petersen LC. Two different Ca2+ ion binding sites in factor VIIa and in des(1–38) factor VIIa. FEBS Lett. 1992;306(2–3):265–268. doi: 10.1016/0014-5793(92)81014-d. [DOI] [PubMed] [Google Scholar]
- 43.Persson E, Nielsen LS. Ca2+ in the first epidermal growth factor-like domain of activated factor VII. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S79–S81. [PubMed] [Google Scholar]
- 44.Sabharwal AK, Birktoft JJ, Gorka J, et al. High affinity Ca2+-binding site in the serine protease domain of human factor VIIa and its role in tissue factor binding and development of catalytic activity. J Biol Chem. 1995;270(26):15523–15530. doi: 10.1074/jbc.270.26.15523. [DOI] [PubMed] [Google Scholar]
- 45.Higashi S, Matsumoto N, Iwanaga S. Molecular mechanism of tissue factor-mediated acceleration of factor VIIa activity. J Biol Chem. 1996;271(43):26569–26574. doi: 10.1074/jbc.271.43.26569. [DOI] [PubMed] [Google Scholar]
- 46.Persson E. Macromolecular substrate affinity for free factor VIIa is independent of a buried protease domain N-terminus. Biochem Biophys Res Commun. 2006;341(1):28–32. doi: 10.1016/j.bbrc.2005.12.146. [DOI] [PubMed] [Google Scholar]
- 47.Toso R, Bernardi F, Tidd T, et al. Factor VII mutant V154G models a zymogen-like form of factor VIIa. Biochem J. 2003;369(3):563–571. doi: 10.1042/BJ20020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song H, Olsen OH, Persson E, Rand KD. Sites involved in intra- and interdomain allostery associated with the activation of factor VIIa pinpointed by hydrogen-deuterium exchange and electron transfer dissociation mass spectrometry. J Biol Chem. 2014;289(51):35388–35396. doi: 10.1074/jbc.M114.614297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajaj SP, Schmidt AE, Agah S, Bajaj MS, Padmanabhan K. High resolution structures of p-aminobenzamidine- and benzamidine-VIIa/soluble tissue factor: unpredicted conformation of the 192–193 peptide bond and mapping of Ca2+, Mg2+, Na+, and Zn2+ sites in factor VIIa. J Biol Chem. 2006;281(34):24873–24888. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]
- 50.Mather T, Oganessyan V, Hof P, et al. The 2. 8 Å crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15(24):6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 51.Walker CP, Royston D. Thrombin generation and its inhibition: a review of the scientific basis and mechanism of action of anticoagulant therapies. Br J Anaesth. 2002;88(6):848–863. doi: 10.1093/bja/88.6.848. [DOI] [PubMed] [Google Scholar]
- 52.Rand KD, Jorgensen TJ, Olsen OH, et al. Allosteric activation of coagulation factor VIIa visualized by hydrogen exchange. J Biol Chem. 2006;281(32):23018–23024. doi: 10.1074/jbc.M602968200. [DOI] [PubMed] [Google Scholar]
- 53.Kirchhofer D, Eigenbrot C, Lipari MT, et al. The tissue factor region that interacts with factor Xa in the activation of factor VII. Biochemistry. 2001;40(3):675–682. doi: 10.1021/bi002013v. [DOI] [PubMed] [Google Scholar]
- 54.Persson E, Kjalke M, Olsen OH. Rational design of coagulation factor VIIa variants with substantially increased intrinsic activity. Proc Natl Acad Sci U S A. 2001;98(24):13583–13588. doi: 10.1073/pnas.241339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuenschwander PF, Vernon JT, Morrissey JH. Tissue factor alters the pKa values of catalytically important factor VIIa residues. Biochemistry. 2002;41(10):3364–3371. doi: 10.1021/bi0110847. [DOI] [PubMed] [Google Scholar]
- 56.McCallum CD, Hapak RC, Neuenschwander PF, Morrissey JH, Johnson AE. The location of the active site of blood coagulation factor VIIa above the membrane surface and its reorientation upon association with tissue factor. A fluorescence energy transfer study. J Biol Chem. 1996;271(45):28168–28175. doi: 10.1074/jbc.271.45.28168. [DOI] [PubMed] [Google Scholar]
- 57.Mutucumarana VP, Duffy EJ, Lollar P, Johnson AE. The active site of factor IXa is located far above the membrane surface and its conformation is altered upon association with factor VIIIa. A fluorescence study. J Biol Chem. 1992;267(24):17012–17021. [PubMed] [Google Scholar]
- 58.Husten EJ, Esmon CT, Johnson AE. The active site of blood coagulation factor Xa. Its distance from the phospholipid surface and its conformational sensitivity to components of the prothrombinase complex. J Biol Chem. 1987;262(27):12953–12961. [PubMed] [Google Scholar]
- 59.McCallum CD, Su B, Neuenschwander PF, Morrissey JH, Johnson AE. Tissue factor positions and maintains the factor VIIa active site far above the membrane surface even in the absence of the factor VIIa Gla domain. A fluorescence resonance energy transfer study. J Biol Chem. 1997;272(48):30160–30166. doi: 10.1074/jbc.272.48.30160. [DOI] [PubMed] [Google Scholar]
- 60.Huang X, Ding WQ, Vaught JL, et al. A soluble tissue factor-annexin V chimeric protein has both procoagulant and anticoagulant properties. Blood. 2006;107(3):980–986. doi: 10.1182/blood-2005-07-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waters EK, Yegneswaran S, Morrissey JH. Raising the active site of factor VIIa above the membrane surface reduces its procoagulant activity but not factor VII autoactivation. J Biol Chem. 2006;281(36):26062–26068. doi: 10.1074/jbc.M604915200. [DOI] [PubMed] [Google Scholar]
- 62.Waxman E, Laws WR, Laue TM, Nemerson Y, Ross JBA. Human factor VIIa and its complex with soluble tissue factor: Evaluation of asymmetry and conformational dynamics by ultracentrifugation and fluorescence anisotropy decay methods. Biochemistry. 1993;32(12):3005–3012. doi: 10.1021/bi00063a011. [DOI] [PubMed] [Google Scholar]
- 63.Olsen OH, Rand KD, Østergaard H, Persson E. A combined structural dynamics approach identifies a putative switch in factor VIIa employed by tissue factor to initiate blood coagulation. Protein Sci. 2007;16(4):671–682. doi: 10.1110/ps.062504907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colina CM, Venkateswarlu D, Duke R, Perera L, Pedersen LG. What causes the enhancement of activity of factor VIIa by tissue factor? J Thromb Haemost. 2006;4(12):2726–2729. doi: 10.1111/j.1538-7836.2006.02222.x. [DOI] [PubMed] [Google Scholar]
- 65.Soejima K, Yuguchi M, Mizuguchi J, et al. The 99 and 170 loop-modified factor VIIa mutants show enhanced catalytic activity without tissue factor. J Biol Chem. 2002;277(50):49027–49035. doi: 10.1074/jbc.M203091200. [DOI] [PubMed] [Google Scholar]
- 66.Dickinson CD, Kelly CR, Ruf W. Identification of surface residues mediating tissue factor binding and catalytic function of the serine protease factor VIIa. Proc Natl Acad Sci U S A. 1996;93(25):14379–14384. doi: 10.1073/pnas.93.25.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pike ACW, Brzozowski AM, Roberts SM, Olsen OH, Persson E. Structure of human factor VIIa and its implications for the triggering of blood coagulation. Proc Natl Acad Sci U S A. 1999;96(16):8925–8930. doi: 10.1073/pnas.96.16.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruf W, Miles DJ, Rehemtulla A, Edgington TS. Tissue factor residues 157–167 are required for efficient proteolytic activation of factor X and factor VII. J Biol Chem. 1992;267(31):22206–22210. [PubMed] [Google Scholar]
- 69.Huang Q, Neuenschwander PF, Rezaie AR, Morrissey JH. Substrate recognition by tissue factor-factor VIIa. Evidence for interaction of residues Lys165 and Lys166 of tissue factor with the 4-carboxyglutamate-rich domain of factor X. J Biol Chem. 1996;271(36):21752–21757. doi: 10.1074/jbc.271.36.21752. [DOI] [PubMed] [Google Scholar]
- 70.Kirchhofer D, Lipari MT, Moran P, Eigenbrot C, Kelley RF. The tissue factor region that interacts with substrates factor IX and factor X. Biochemistry. 2000;39(25):7380–7387. doi: 10.1021/bi000182+. [DOI] [PubMed] [Google Scholar]
- 71.Roy S, Hass PE, Bourell JH, Henzel WJ, Vehar GA. Lysine residues 165 and 166 are essential for the cofactor function of tissue factor. J Biol Chem. 1991;266(32):22063–22066. [PubMed] [Google Scholar]
- 72.Ruf W, Miles DJ, Rehemtulla A, Edgington TS. Cofactor residues lysine 165 and 166 are critical for protein substrate recognition by the tissue factor-factor VIIa protease complex. J Biol Chem. 1992;267(9):6375–6381. [PubMed] [Google Scholar]
- 73.Vadivel K, Agah S, Messer AS, et al. Structural and functional studies of γ-carboxyglutamic acid domains of factor VIIa and activated protein C: Role of magnesium at physiological calcium. J Mol Biol. 2013;425(11):1961–1981. doi: 10.1016/j.jmb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morrissey JH, Fair DS, Edgington TS. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988;52(3):247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 75.Fiore MM, Neuenschwander PF, Morrissey JH. An unusual antibody that blocks tissue factor/factor VIIa function by inhibiting cleavage only of macromolecular substrates. Blood. 1992;80(12):3127–3134. [PubMed] [Google Scholar]
- 76.Ruf W, Edgington TS. An anti-tissue factor monoclonal antibody which inhibits TF. VIIa complex is a potent anticoagulant in plasma. Thromb Haemost. 1991;66:529–533. [PubMed] [Google Scholar]
- 77.Huang M, Syed R, Stura EA, et al. The mechanism of an inhibitory antibody on TF-initiated blood coagulation revealed by the crystal structures of human tissue factor, Fab 5G9 and TF 5G9 complex. J Mol Biol. 1998;275(5):873–894. doi: 10.1006/jmbi.1997.1512. [DOI] [PubMed] [Google Scholar]
- 78.Kirchhofer D, Moran P, Chiang N, et al. Epitope location on tissue factor determines the anticoagulant potency of monoclonal anti-tissue factor antibodies. Thromb Haemost. 2000;84(6):1072–1081. [PubMed] [Google Scholar]
- 79.Versteeg HH, Schaffner F, Kerver M, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111(1):190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Contreras FX, Sánchez-Magraner L, Alonso A, Goñi FM. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010;584(9):1779–1786. doi: 10.1016/j.febslet.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 81.McDonald JF, Shah AM, Schwalbe RA, et al. Comparison of naturally occurring vitamin K-dependent proteins: correlation of amino acid sequences and membrane binding properties suggests a membrane contact site. Biochemistry. 1997;36(17):5120–5127. doi: 10.1021/bi9626160. [DOI] [PubMed] [Google Scholar]
- 82.Nelsestuen GL, Kisiel W, Di Scipio RG. Interaction of vitamin K dependent proteins with membranes. Biochemistry. 1978;17(11):2134–2138. doi: 10.1021/bi00604a017. [DOI] [PubMed] [Google Scholar]
- 83.Tavoosi N, Smith SA, Davis-Harrison RL, Morrissey JH. Factor VII and protein C are phosphatidic acid-binding proteins. Biochemistry. 2013;52(33):5545–5552. doi: 10.1021/bi4006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neuenschwander PF, Morrissey JH. Roles of the membrane-interactive regions of factor VIIa and tissue factor. The factor VIIa Gla domain is dispensable for binding to tissue factor but important for activation of factor X. J Biol Chem. 1994;269(11):8007–8013. [PubMed] [Google Scholar]
- 85.Hedner U. Mechanism of action, development and clinical experience of recombinant FVIIa. J Biotechnol. 2006;124(4):747–757. doi: 10.1016/j.jbiotec.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 86.Feng D, Whinna H, Monroe D, Stafford DW. FVIIa as used pharmacologically is not TF dependent in hemophilia B mice. Blood. 2014;123(11):1764–1766. doi: 10.1182/blood-2013-08-522987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tavoosi N, Davis-Harrison RL, Pogorelov TV, et al. Molecular determinants of phospholipid synergy in blood clotting. J Biol Chem. 2011;286(26):23247–23253. doi: 10.1074/jbc.M111.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kay JG, Koivusalo M, Ma X, Wohland T, Grinstein S. Phosphatidylserine dynamics in cellular membranes. Mol Biol Cell. 2012;23(11):2198–2212. doi: 10.1091/mbc.E11-11-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang M, Rigby AC, Morelli X, et al. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10(9):751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 90.Sunnerhagen M, Forsén S, Hoffrén AM, et al. Structure of the Ca2+-free GLA domain sheds light on membrane binding of blood coagulation proteins. Nat Struct Biol. 1995;2(6):504–509. doi: 10.1038/nsb0695-504. [DOI] [PubMed] [Google Scholar]
- 91.Persson E, Petersen LC. Structurally and functionally distinct Ca2+ binding sites in the gamma-carboxyglutamic acid-containing domain of factor VIIa. Eur J Biochem. 1995;234(1):293–300. doi: 10.1111/j.1432-1033.1995.293_c.x. [DOI] [PubMed] [Google Scholar]
- 92.de Courcy B, Pedersen LG, Parisel O, et al. Understanding selectivity of hard and soft metal cations within biological systems using the subvalence concept. I. Application to blood coagulation: direct cation-protein electronic effects vs indirect interactions through water networks. J Chem Theory Comput. 2010;6(4):1048–1063. doi: 10.1021/ct100089s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tavoosi N, Morrissey JH. Influence of membrane composition on the enhancement of factor VIIa/tissue factor activity by magnesium ions. Thromb Haemost. 2014;111(4):770–772. doi: 10.1160/TH13-07-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sekiya F, Yoshida M, Yamashita T, Morita T. Magnesium(II) is a crucial constituent of the blood coagulation cascade - Potentiation of coagulant activities of factor IX by Mg2+ ions. J Biol Chem. 1996;271(15):8541–8544. doi: 10.1074/jbc.271.15.8541. [DOI] [PubMed] [Google Scholar]
- 95.Persson E, Olsen OH, Østergaard A, Nielsen LS. Ca2+ binding to the first epidermal growth factor-like domain of factor VIIa increases amidolytic activity and tissue factor affinity. J Biol Chem. 1997;272(32):19919–19924. doi: 10.1074/jbc.272.32.19919. [DOI] [PubMed] [Google Scholar]
- 96.Petersen LC, Olsen OH, Nielsen LS, Freskgård PO, Persson E. Binding of Zn2+ to a Ca2+ loop allosterically attenuates the activity of factor VIIa and reduces its affinity for tissue factor. Protein Sci. 2000;9(5):859–866. doi: 10.1110/ps.9.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prendergast FG, Mann KG. Differentiation of metal ion-induced transitions of prothrombin fragment 1. J Biol Chem. 1977;252(3):840–850. [PubMed] [Google Scholar]
- 98.Persson E, Bjork I, Stenflo J. Protein structural requirements for Ca2+ binding to the light chain of factor X. Studies using isolated intact fragments containing the gamma-carboxyglutamic acid region and/or the epidermal growth factor-like domains. J Biol Chem. 1991;266(4):2444–2452. [PubMed] [Google Scholar]
- 99.Persson E, Østergaard A. Mg2+ binding to the Gla domain of factor X influences the interaction with tissue factor. J Thromb Haemost. 2007;5(9):1977–1978. doi: 10.1111/j.1538-7836.2007.02661.x. [DOI] [PubMed] [Google Scholar]
- 100.Bromberg ME, Konigsberg WH, Madison JF, Pawashe A, Garen A. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc Natl Acad Sci U S A. 1995;92(18):8205–8209. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hjortoe GM, Petersen LC, Albrektsen T, et al. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103(8):3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hembrough TA, Swartz GM, Papathanassiu A, et al. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003;63(11):2997–3000. [PubMed] [Google Scholar]
- 103.Bluff JE, Brown NJ, Reed MW, Staton CA. Tissue factor, angiogenesis and tumour progression. Breast Cancer Res. 2008;10(2):204. doi: 10.1186/bcr1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer. 2000;83(2):164–170. doi: 10.1054/bjoc.2000.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shoji M, Hancock WW, Abe K, et al. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152(2):399–411. [PMC free article] [PubMed] [Google Scholar]
- 106.Dorfleutner A, Ruf W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 2003;102(12):3998–4005. doi: 10.1182/blood-2003-04-1149. [DOI] [PubMed] [Google Scholar]
- 107.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279(22):23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 108.Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10(5):502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 109.Ryden L, Grabau D, Schaffner F, et al. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126(10):2330–2340. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sorensen BB, Freskgård PO, Nielsen LS, et al. Factor VIIa-induced p44/42 mitogen-activated protein kinase activation requires the proteolytic activity of factor VIIa and is independent of the tissue factor cytoplasmic domain. J Biol Chem. 1999;274(30):21349–21354. doi: 10.1074/jbc.274.30.21349. [DOI] [PubMed] [Google Scholar]
- 111.Camerer E, Rottingen JA, Iversen JG, Prydz H. Coagulation factors VII and X induce Ca2+ oscillations in Madin-Darby canine kidney cells only when proteolytically active. J Biol Chem. 1996;271(46):29034–29042. doi: 10.1074/jbc.271.46.29034. [DOI] [PubMed] [Google Scholar]
- 112.Åberg M, Siegbahn A. Tissue factor non-coagulant signaling – molecular mechanisms and biological consequences with a focus on cell migration and apoptosis. J Thromb Haemost. 2013;11(5):817–825. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- 113.de Jonge E, Friederich PW, Vlasuk GP, et al. Activation of coagulation by administration of recombinant factor VIIa elicits interleukin 6 (IL-6) and IL-8 release in healthy human subjects. Clin Diagn Lab Immunol. 2003;10(3):495–497. doi: 10.1128/CDLI.10.3.495-497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Versteeg HH, Spek CA, Slofstra SH, et al. FVIIa:TF induces cell survival via G12/G13-dependent Jak/STAT activation and BclXL production. Circul Res. 2004;94(8):1032–1040. doi: 10.1161/01.RES.0000125625.18597.AD. [DOI] [PubMed] [Google Scholar]
- 115.Versteeg HH, Sorensen BB, Slofstra SH, et al. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. J Biol Chem. 2002;277(30):27065–27072. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- 116.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27(29):4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rao LV, Pendurthi UR. Tissue factor-factor VIIa signaling. Arterioscler Thromb Vasc Biol. 2005;25(1):47–56. doi: 10.1161/01.ATV.0000151624.45775.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14(1):33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 119.Opella SJ, Marassi FM, Gesell JJ, et al. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nat Struct Biol. 1999;6(4):374–379. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]