Abstract
The blood coagulation cascade is initiated when the cell-surface complex of factor VIIa (FVIIa, a trypsin-like serine protease) and tissue factor (TF, an integral-membrane protein) proteolytically activates factor X (FX). Both FVIIa and FX bind to membranes via their γ-carboxyglutamate-rich domains (GLA domains). GLA domains contain seven to nine bound Ca2+ ions that are critical for their folding and function, and most biochemical studies of blood clotting have employed supraphysiologic Ca2+ concentrations to ensure saturation of these domains with bound Ca2+. Recently, it has become clear that, at plasma concentrations of metal ions, Mg2+ actually occupies two or three of the divalent metal ion-binding sites in GLA domains, and that these bound Mg2+ ions are required for full function of these clotting proteins. In this study, we investigated how Mg2+ influences FVIIa enzymatic activity. We found that the presence of TF was required for Mg2+ to enhance the rate of FX activation by FVIIa, and we used alanine-scanning mutagenesis to identify TF residues important for mediating this response to Mg2+. Several TF mutations, including those at residues G164, K166, and Y185, blunted the ability of Mg2+ to enhance the activity of the TF/FVIIa conplex. Our results suggest that these TF residues interact with the GLA domain of FX in a Mg2+-dependent manner (although effects of Mg2+ on the FVIIa GLA domain cannot be ruled out). Notably, these TF residues are located within or immediately adjacent to the putative substrate-binding exosite of TF.
Keywords: Tissue Factor, Factor VIIa, Factor X, Factor IX, Magnesium, Calcium, Extrinsic Tenase Complex
The importance of divalent metal ions in blood coagulation reactions is well-documented, as they play key roles in both protein-membrane and protein-protein interactions.2,3 Seven blood coagulation proteins interact reversibly with anionic membranes via their GLA domains, which are rich in the post-translationally modified amino acid, γ-carboxyglutamate (Gla). GLA domains bind multiple divalent metal ions,4 causing them to fold and form a characteristic ω-loop structure, exposing hydrophobic residues that are believed to facilitate penetration of the membrane bilayer.5
The vast majority of biochemical studies of blood clotting proteins have employed supraphysiologic concentrations of Ca2+, which can occupy seven to nine metal ion-binding sites in the GLA domains of factors VII (FVII), IX (FIX), and X (FX).4, 6-8 Ca2+ is the most prevalent divalent metal ion in plasma, with a free concentration of ∼1.25 mM.9 However, biochemical studies of blood clotting proteins using 1.25 mM Ca2+ result in submaximal enzymatic activity and membrane binding, apparently because of incomplete saturation of the metal ion-binding sites in the GLA domains of these proteins. For this reason, supraphysiologic Ca2+ concentrations (typically, 2.5-5 mM Ca2+) are typically employed in studies of blood clotting reactions. Recent studies, however, have shown that using the plasma concentrations of free Ca2+ (1.25 mM) together with the plasma concentration of free Mg2+ (∼0.6 mM) results in clotting factor activities that are at least as high as those observed in the presence of supraphysiologic Ca2+ concentrations.10-14 These studies therefore indicate that Mg2+ plays important, albeit somewhat overlooked, roles in blood clotting reactions.
In the study presented here, we examined how Mg2+ modulates the initiation phase of the blood clotting cascade. Blood clotting is triggered when the integral membrane protein, tissue factor (TF), binds FVIIa to form the TF/FVIIa complex. This complex activates FIX and FX by limited proteolysis. The mechanism(s) by which Mg2+ modulates the activity of clotting proteases is unclear, but recent crystal structures of FVIIa indicate that Mg2+ occupies two or three of the metal ion-binding sites in its GLA domain at physiologic concentrations of Ca2+ and Mg2+,13 with structural implications.1 Persson et al.14 reported that removal of the FX GLA domain eliminated the ability of Mg2+ to increase the rate of FX activation by TF/FVIIa, suggesting that incorporation of Mg2+ into the GLA domain of FX may be the basis by which Mg2+ enhances FX activation.
We utilized site-directed mutagenesis of TF to investigate how Mg2+ modulates the activity of the TF/FVIIa complex (TF/FVIIa). We confirm that TF is a key mediator of the Mg2+ response, despite its lack of direct interaction with either Ca2+ or Mg2+. We now identify individual TF residues within or near its putative substrate-binding exosite that are necessary for Mg2+ to enhance of the rate of FX activation by TF/FVIIa. Furthermore, we demonstrate that physiologic concentrations of Ca2+ and Mg2+ support TF/FVIIa enzymatic activities higher than those in the presence of Ca2+ alone, an effect that was more pronounced when more physiologic lipid compositions were utilized. Our findings support the idea that TF is an integral component of macromolecular substrate recognition by the TF/FVIIa complex, and that Mg2+ contributes to TF/FVIIa function.
Experimental Procedures
Materials
Materials were from the following sources: phospholipids 1-palmitoyl-2-oleoylphosphatidylcholine (PC) and 1-palmitoyl-2-oleoylphosphatidyl-L-serine (PS), from Avanti Polar Lipids (Alabaster, AL), Bio-Beads SM-2 absorbent from Bio-Rad, human FVIIa from American Diagnostica (now Sekisui Diagnostics, Lexington, MA), human FX, FXa, FIX, and FIXa from Haematologic Technologies (Essex Junction, VT), Spectrozyme Xa from Bachem (Bubendorf, Switzerland), Pefachrome FIXa from Enzyme Research Laboratories (South Bend, IN), and Chromozym t-PA from Roche Diagnostics (Mannheim, Germany).
Production and Relipidation of TF
Recombinant human membrane-anchored TF (memTF, residues 3-244)15 and soluble TF (sTF, residues 3-219)16 were expressed in Escherichia coli and purified as previously described. TF mutants were prepared using the Q5 site-directed mutagenesis kit from New England Biolabs (Ipswich, MA). TF liposomes were prepared by incorporating memTF into phospholipid vesicles of varying composition as described previously.17
FVIIa Amidolytic Activity and Quantifying TF/FVIIa Binding
Initial rates of hydrolysis of the Chromozym-tPA substrate (FVIIa amidolytic activity) were measured essentially as previously described,18 in buffer containing either 1.25 mM CaCl2, 1.85 mM CaCl2, or 1.25 mM CaCl2 and 0.6 mM MgCl2. The affinity of FVIIa for TF was measured as described previously,19 using 5 nM FVIIa, 0-20 nM memTF, 1 mM Chromozym-tPA, and 0.1% Triton X-100 in HBSA [20 mM HEPES (pH 7.4), 100 mM NaCl, 0.02% sodium azide, and 0.1% bovine serum albumin], either with 1.85 mM CaCl2 or with 1.25 mM CaCl2 and 0.6 mM MgCl2.
Rates of Activation of FX and FIX
Initial rates of FX activation by TF/FVIIa using memTF, either in solution or relipidated into phospholipid vesicles, were quantified using a continuous FX activation assay as described previously,19,20 with slight modifications. Briefly, memTF and FVIIa were incubated essentially as described, but in buffers whose divalent metal ion concentrations were either 1.25 mM Ca2+, 1.85 mM Ca2+, or 1.25 mM Ca2+ and 0.6 mM Mg2+. Reactions were initiated by addition of 100 nM FX and 1 mM Spectrozyme Xa. Reactions without membranes included 0.1% Triton X-100 and typically employed 10 nM FVIIa and 500 nM memTF. Reactions with membranes typically employed 30-100 pM FVIIa and excess relipidated memTF (>1 nM memTF, with 25 μM total phospholipid).
Initial rates of FIX activation were quantified in a two-stage assay as described previously,21 with some variations. Briefly, FVIIa and memTF were incubated at 37°C for 5 min in buffer containing 0.06% Triton X-100 together with either 1.25 mM Ca2+ or 1.25 mM Ca2+ and 0.6 mM Mg2+. FIX was then added and incubated at 37°C, yielding typical final concentrations of 20-40 nM FVIIa, 500 nM TF, and 1 μM FIX. Timed 10 μL aliquots were removed and quenched on ice using 10 μL of 20 mM EDTA in 10×HBSA. To quantify the FIXa generated, final concentrations of 40% ethylene glycol and 1 mM Pefachrome FIXa were added to quenched samples (total volume, 100 μL), and the rate of change in A405 was measured at 35°C.
Solution Nuclear Magnetic Resonance (NMR) Spectroscopy
Purified sTF was concentrated using Amicon Ultra-15 centrifugal filters (EMD Millipore, Billerica, MA) with an Mr cutoff of 10 kDa and then dialyzed into 50 mM NaCl and 50 mM sodium phosphate (pH 6.5). Solution NMR samples were prepared with 100 μM sTF, 1 mM DSS (4,4,-dimethyl-4-silapentane-1-sulfonic acid), and 10% D2O along with 1.25 mM Ca2+, 0.5 mM Mg2+, or no divalent cations. 15N-1H two-dimensional (2D) HSQC correlation spectra were acquired on a Varian/Agilent VNMRS 17.6 T (750 MHz 1H frequency) spectrometer for 16.6 h each at ∼30°C. The measured pH values were 6.74, 6.78, and 6.79 for the samples with 1.25 mM Ca2+, 0.5 mM Mg2+, and no divalent cations, respectively. Spectra were processed using NMRPipe22 and chemical shifts were analyzed in SPARKY.23
Results
Components of the TF/FVIIa Complex Required for Mg2+-Dependent Rate Enhancements
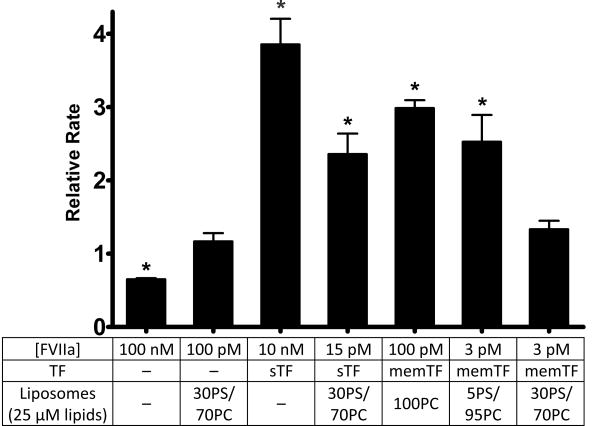
Although Mg2+ has been shown to modulate the enzymatic activity of FVIIa toward its cognate substrates, FIX11 and FX,13,24 and removal of the FX GLA domain abrogates this effect,14 a thorough understanding of how each component of the TF/FVIIa/membrane complex responds to Mg2+ has not yet been achieved. We therefore varied the constituents of this complex and examined the ability of Mg2+ to modulate the rate of FX activation by FVIIa. Figure 1 shows the relative rates of FX activation by various components of the TF/FVIIa/membrane complex in the presence of physiologic concentrations of Ca2+ and Mg2+ (1.25 and 0.6 mM, respectively), normalized to the rates observed with physiologic (1.25 mM) Ca2+ alone. For FVIIa in solution (no membranes or TF), the presence of Mg2+ and Ca2+ actually decreased the rate of FX activation by 35% relative to that with Ca2+ alone. Mg2+ did not significantly influence the rate of FX activation by FVIIa in the presence of 30% PS/70% PC liposomes [but in the absence of TF (second bar in Figure 1)]. In the presence of sTF but without membranes, Mg2+ enhanced the rate of FX activation by FVIIa by approximately 4-fold, consistent with previous findings.13,14 In the presence of sTF and 30% PS/70% PC liposomes, Mg2+ still enhanced the rate of FX activation by FVIIa, but to a lesser extent than in the absence of membranes. Mg2+ also enhanced FX activation by FVIIa bound to relipidated TF, with the magnitude of the enhancement dependent on the lipid composition. Thus, Mg2+ enhanced the rate of FX activation in the presence of TF liposomes containing either 100% PC or 5% PS and 95% PC but did not significantly enhance the rate of FX activation when TF-liposomes contained very high levels of PS (i.e., 30% PS and 70% PC).
Figure 1.
Effect of Mg2+ on FX activation by FVIIa in the presence or absence of TF or membranes. FX activation by FVIIa was measured in the presence of the components listed, with a dash (—) indicating the component was not present. Data are mean initial rates of FX activation in the presence of 1.25 mM Ca2+ and 0.6 mM Mg2+ normalized to rates using 1.25 mM Ca2+ alone. Error bars are one standard error (n ≥ 3). Normalized rates that are statistically significantly different from 1.0 are indicated with an asterisk (one-sample t-tests; p < 0.05).
TF Mutations That Diminish the Ability of Mg2+ To Enhance FX Activation by TF/FVIIa in Solution
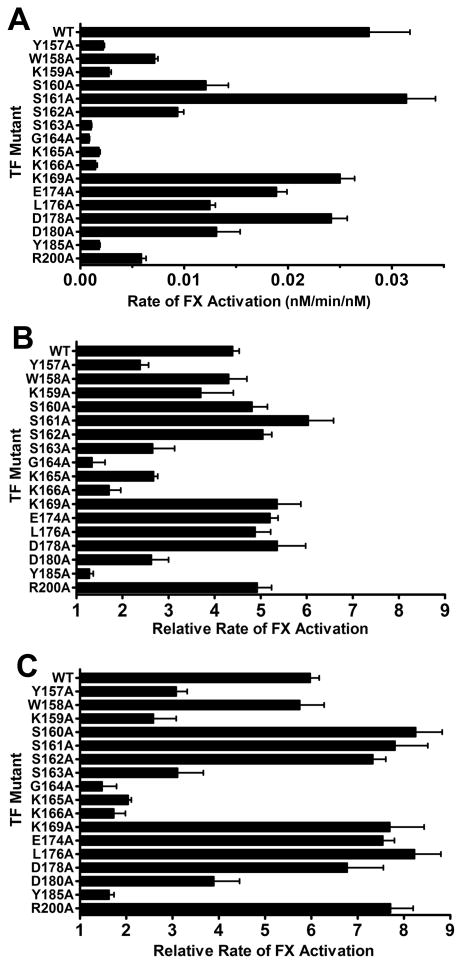
Previous studies identified a putative substrate-binding region (exosite) in TF, consisting of a patch of surface-exposed residues in the C-terminal fibronectin type III domain of this protein that are critical for recognition of macromolecular substrates (FVII, FIX, and FX) by the TF/FVIIa complex. In particular, mutating TF residue S163, K166, or Y185 to alanine substantially decreased the rates of FX activation by TF/FVIIa, while having little or no effect on the affinity of FVIIa for TF or on FVIIa amidolytic activity.21 We previously showed that if the GLA domain of FX is removed, mutations in the TF exosite no longer influence the rate of FX activation, suggesting that the FX GLA domain interacts with the TF exosite.25 Furthermore, Persson et al.14 showed that removing the FX GLA domain abrogated the ability of Mg2+ to enhance FX activation by TF/FVIIa. We therefore hypothesized that Mg2+ enhances the rate of FX action by TF/FVIIa largely by enhancing the interaction between FX and the substrate-binding exosite region of TF. To test this idea, we mutated 17 amino acids in memTF individually to alanine, chosen by their location within or adjacent to the putative substrate-binding exosite.21 We expressed and purified these memTF mutants and then assessed the ability of Mg2+ to enhance the rate of FX activation by the resulting TF/FVIIa complexes. In particular, we compared three divalent metal ion conditions: 1.25 mM Ca2+ alone, 1.85 mM Ca2+ alone, and 1.25 mM Ca2+ and 0.6 mM Mg2+. When we measured the rates of FX activation by memTF/FVIIa complexes in solution (i.e., with Triton X-100 and no membranes) in the presence of 1.85 mM Ca2+, we found that 13 of the 17 single amino acid mutations decreased the initial rate of FX activation by at least 50% relative to that of wild-type (WT) memTF, and that six of the mutations (Y157A, K159A, S163A, G164A, K166A, and Y185A) decreased the rate by at least 90% (Figure 2A). These results are in good agreement with those previously determined by Kirchhofer et al,21 which were obtained in the presence of phospholipid membrane surfaces. The absolute rates of FX activation obtained under each divalent metal ion condition that we tested are given in Table S1 of Supporting Information.
Figure 2.
Effect of TF mutations on rates of FX activation by the memTF/FVIIa complex in solution (with 0.1% Triton X-100), measured using different divalent metal ion concentrations. (A) FX activation by the memTF/FVIIa complex using 1.85 mM Ca2+ alone, graphed as initial rates of FX activation divided by the memTF/FVIIa complex concentration. (B) Relative rates of FX activation by memTF/FVIIa in solution using 1.25 mM Ca2+ and 0.6 mM Mg2+, normalized to rates using 1.85 mM Ca2+ alone. (C) Relative rates of FX activation by the memTF/FVIIa complex in solution using 1.25 mM Ca2+ and 0.6 mM Mg2+, normalized to rates using 1.25 mM Ca2+ alone. Data are means ± the standard error (n ≥ 3).
To determine how these TF mutations affected the ability of Mg2+ to enhance the enzymatic activity of the memTF/FVIIa complex (memTF/FVIIa), we quantified initial rates of FX activation in the presence of 1.25 mM Ca2+ and 0.6 mM Mg2+ and normalized those data to the rates using 1.85 mM Ca2+ alone (Figure 2B) or 1.25 mM Ca2+ alone (Figure 2C). The purpose of the former condition is to compare the combination of Ca2+ and Mg2+ to an identical concentration of Ca2+ alone, such that the total divalent metal ion concentration is held constant at 1.85 mM.
Similar to the effect of Mg2+ we observed with sTF in solution, the combination of 1.25 mM Ca2+ and 0.6 mM Mg2+ enhanced the rate of FX activation by FVIIa bound to WT memTF in detergent solution by 4.4-fold compared to that with 1.85 mM Ca2+ alone (Figure 2B) and 6.0-fold compared to that with 1.25 mM Ca2+ alone (Figure 2C). The TF exosite mutants fell into two main categories with regard to their sensitivity to Mg2+. The first category included mutations that were highly deleterious for FX activation but that nevertheless showed essentially the same ability of Mg2+ to enhance the rate of FX activation observed with WT memTF/FVIIa complexes. Thus, TF mutants W158A, S160A, S162A, E174A, L176A, and R200A exhibited absolute rates of FX activation that were <75% of that of WT memTF (Figure 2A) yet showed essentially the same response to the combination of Ca2+ and Mg2+ versus Ca2+ alone that was observed for WT memTF (Figure 2B, C). The second category included mutants Y157A, S163A, G164A, K159A, K165A, K166A and Y185A that exhibited both substantially decreased absolute rates of FX activation (Figure 2A) and substantially blunted ability of Mg2+ to enhance their rates of FX activation (Figures 2B, C). On the other hand, no TF mutants that retained WT absolute rates of FX activation yet were deficient in their response to Mg2+ were identified. A similar pattern of results was obtained when we examined the ability of Mg2+ to enhance the rate of FIX activation by this collection of memTF mutants (Figure S1 of Supporting Information).
To better understand our results, we also investigated the effect of simply increasing the Ca2+ concentration from 1.25 to 1.85 mM on the rate of FX activation by memTF/FVIIa complexes in solution (Figure S2 of Supporting Information). With WT TF, increasing the Ca2+ concentration resulted in an approximately 1.3-fold increase in the rate of FX activation. Within experimental error, most of the TF mutants supported similar increases in the rate of FX activation with an increase in the Ca2+ concentration. Two TF mutants (K159A and K165A) were significantly different, exhibiting approximately 30% slower rates of FX activation at 1.85 mM Ca2+ than at 1.25 mM Ca2+ (Figure S2 of the Supporting Information).
TF Mutations That Diminish the Ability of Mg2+ To Enhance FX Activation by TF/FVIIa on Membranes
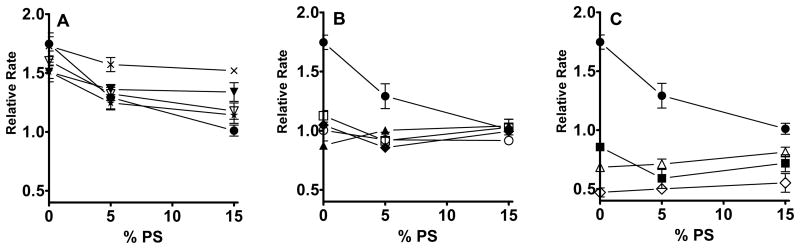
Our studies thus far have examined the ability of Mg2+ to increase the rate of FX by TF/FVIIa complexes in solution. We next examined the effects of 11 selected TF mutants in experiments in which memTF was incorporated into liposomes of varying the phospholipid composition (Figure 3). As was also seen in Figure 1, the ability of the combination of 1.25 mM Ca2+ and 0.6 mM Mg2+ to enhance the rate of FX activation relative to that with 1.85 mM Ca2+ alone using WT TF was a function of the PS content of the TF liposomes. Thus, as the PS content increased, the ability of Mg2+ to enhance the rate of FX activation was decreased, reaching an essentially negligible level of enhancement by Mg2+ at 15% PS. For the sake of clarity, we grouped the TF mutants in three panels of Figure 3 according to their ability to support Mg2+-dependent enhancement of the rate of FX activation in solution. Thus, those memTF mutants that supported essentially WT responses to Mg2+ in solution (W158A, S162A, E174A, and D178A) also exhibited increased rates of FX activation in the presence of Mg2+ when employed in liposomes (Figure 3A). Although increasing the PS content of the TF liposomes tended to blunt the Mg2+ response in these mutants, the effect of increasing the PS content on the Mg2+ response was generally less extensive than that observed with WT TF. This was most particularly true of the S162A mutant.
Figure 3.
Effect of 11 selected TF mutations on the ability of Mg2+ to enhance the rate of FX activation by TF/FVIIa/membrane complexes. WT or mutant memTF was incorporated into liposomes containing 0-15% PS, with the balance being PC. Initial rates of FX activation by the resulting memTF/FVIIa complexes were measured with 1.25 mM Ca2+ and 0.6 mM Mg2+ and normalized to the rates observed with 1.85 mM Ca2+ alone. The memTF mutants are grouped in the three panels as described in the text, with the same data for WT memTF (●) plotted in each panel for comparison. (A) Normalized rates of FX activation observed with memTF mutants S162A (⨯), W158A (▼), E174A (∇), and D178A (✯). (B) Normalized rates of FX activation observed with memTF mutants S163A (□), K165A (◆), K159A (○), and Y157A (▲). (C) Normalized rates of FX activation observed with memTF mutants Y185A (∆), K166A (■), and G164A (◊). Data are means ± the standard error (n ≥ 3).
On the other hand, the memTF mutants that supported intermediate responses to Mg2+ in solution (Y157A, K159A, S163A, and K165A) exhibited essentially no response to Mg2+ in liposomes (Figure 3B). Finally, the memTF mutants that exhibited the most deficient responses to Mg2+ in solution (G164A, K166A and Y185A) actually exhibited, in liposomes, lower rates of FX activation in the presence of Mg2+ and Ca2+ compared to the rates with Ca2+ alone (Figure 3C). Furthermore, the blunted responses to Mg2+ by the TF mutants in panels B and C of Figure 3 were observed irrespective of the PS content of the memTF liposomes.
Solution NMR Spectroscopy of sTF in the Presence of Divalent Cations
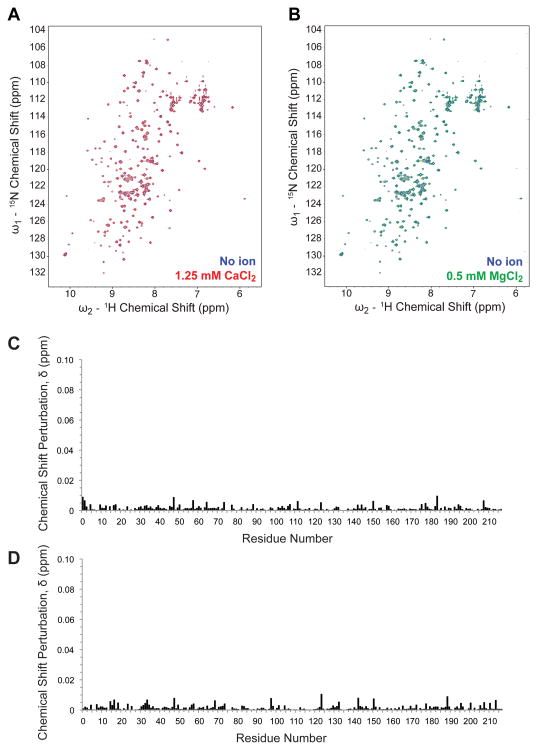
To investigate whether TF interacts directly with the divalent cations Ca2+ and Mg2+, we utilized solution NMR spectroscopy to compare 15N-1H 2D heteronuclear single-quantum coherence (HSQC) correlation spectra in the presence and absence of these ions. As shown in Figure 4, addition of a physiologic divalent cation (either 1.25 mM Ca2+ or 0.5 mM Mg2+) to 100 μM sTF samples resulted in no significant shifts in peak positions in 15N-1H 2D correlation spectra. These results are consistent with the idea that neither Ca2+ nor Mg2+ interacts directly with sTF in a specific manner, which in turn is consistent with available x-ray crystal structures in which neither Ca2+ nor Mg2+ has been observed bound to sTF.8,13
Figure 4.
Lack of a detectable effect of divalent metal ions on NMR spectra of sTF. (A) 15N-1H 2D HSQC correlation spectrum of 100 μM sTF in 50 mM sodium phosphate, 50 mM NaCl, 1 mM DSS, 10% D2O and no divalent metal ions (blue) overlaid with the sTF spectrum in the same buffer with 1.25 mM Ca2+ (red). (B) 15N-1H 2D HSQC correlation spectrum of 100 μM sTF in the same buffer as in panel A, without divalent metal ions (blue) overlaid with the spectrum in the presence of 0.5 mM Mg2+ (green). (C) Chemical shift perturbations upon titration with 1.25 mM Ca2+ (from panel A) calculated as δ = ([0.1δN]2 + δH2)1/2), with an average chemical shift perturbation of 0.0014 ppm. (D) Chemical shift perturbations upon titration with 0.5 mM Mg2+ (from panel B), with an average chemical shift perturbation of 0.0015 ppm. Of the 208 expected non-proline resonances in sTF, 196 resonances were assigned.
Discussion
Divalent cations such as Ca2+ and Mg2+ are critical components of blood coagulation, supporting both protein-protein and protein-membrane interactions. While the contributions of Ca2+ to blood coagulation reactions have been extensively studied, the roles of Mg2+ are less well understood. In this study, we confirmed that TF is required for Mg2+ to enhance the rate of activation of FIX or FX by FVIIa,14 and we showed that specific residues in the putative substrate-binding region of TF contribute to this Mg2+-dependent rate enhancement. Although TF is an allosteric regulator of the general catalytic activity of FVIIa, this function is independent of the presence of Mg2+ as long as Ca2+ is available, because adding Mg2+ has no significant effect on FVIIa amidolytic activity14 and does not enhance the affinity of FVIIa for TF (Table S2 of the Supporting Information). Thus, enhancement of TF/FVIIa catalytic activity by Mg2+ is restricted to macromolecular substrates (FIX and FX). Importantly, a previous study showed that removing the FX GLA domain eliminated the ability of Mg2+ to enhance the rate of FX activation by TF/FVIIa,14 and our lab previously demonstrated that the GLA domain of FX must be intact for mutations in the TF exosite to have any effect on the rate of FX activation by TF/FVIIa.25 These results support the idea that the FX GLA domain interacts directly with the TF exosite and is of critical importance for Mg2+ recognition. This does not preclude the involvement of FVIIa in mediating these interactions; indeed, a recent study reported that the FVIIa GLA domain must also be intact for Mg2+ to enhance the rate of FX activation by TF/FVIIa.26
Our working hypothesis to explain all these findings is that Mg2+ promotes the binding of FX (or FIX) to the exosite region of TF. To accomplish this, Mg2+ could, in principle, be contributing to the increased rates of FX activation via its binding to the GLA domains of either FVIIa or FX, or perhaps even binding to TF. (Because we observe the effect of Mg2+ in solution, it is not necessary to invoke association of Mg2+ with phospholipids, and therefore, Mg2+ must be promoting protein-protein interactions.) Our NMR results, together with a wealth of X-ray crystal structures, argue strongly that Mg2+ does not associate measurably with TF. At physiologic concentrations of divalent metal ions, Mg2+ is thought to occupy at least two, and possibly three or four metal ion-binding sites in the GLA domains of both FVIIa and FX.27 Although our results do not allow us to determine whether binding of Mg2+ to FVIIa or FX is more important for enhancing the rate of FX activation, one can speculate that occupancy of the FX GLA domain with Mg2+ may increase the affinity of this domain for the TF exosite. Binding of Mg2+ to the FVIIa GLA domain may also play a regulatory role, as comparison of crystal structures in the presence or absence of Mg2+ indicates that conformational changes occur in the FVIIa GLA domain upon Mg2+ occupancy.1 Interestingly, the 159-165 loop of sTF was disordered when the sTF/FVIIa complex was crystallized with Ca2+ only but was ordered when the complex was crystallized in the presence of both Ca2+ and Mg2+. How these structural changes might affect TF/FVIIa activity toward its cognate substrates is currently unclear.
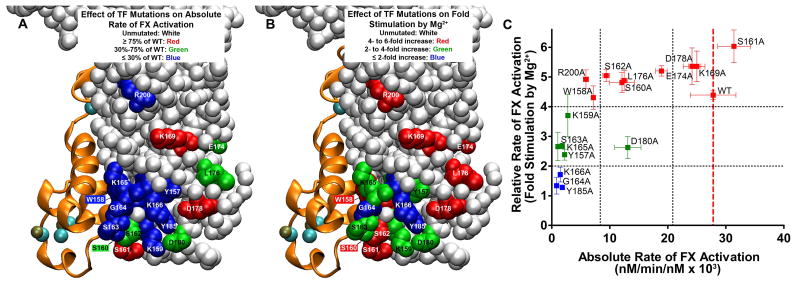
For better visual interpretation, we mapped the mutations in this study onto the X-ray crystal structure of the FVIIa/sTF complex, colored according to their impact either on the absolute rate of FX activation (Figure 5A) or on the ability of Mg2+ to enhance the rate of FX activation (Figure 5B) by memTF/FVIIa in solution. In Figure 5C, we also graphed the effects of these mutations, plotted as the ability of Mg2+ to enhance the rate of FX activation (y-axis) versus the absolute rate of FX activation (x-axis), with data points colored using the same scheme as in Figure 5B. The deleterious effects of these TF exosite mutations on the absolute rate of FX activation (Figure 5A) largely confirm the findings of Kirchhofer et al.21 Only a subset of these mutations, however, weakened the ability of Mg2+ to enhance the rate of FX activation, with mutations G164A, K166A, and Y185A (colored blue in Figure 5B) essentially eliminating the Mg2+ effect; notably, these three mutations also severely reduced the absolute rate of FX activation (colored blue in Figure 5A). TF mutations Y157A, S163A, K165A, and D180A (colored green in Figure 5B) exhibited a more moderate yet still significant reduction in the Mg2+ effect; of these, three (Y157A, S163A, and K165A) greatly reduced the absolute rate of FX activation (colored blue in Figure 5A), while one (D180A) produced a more moderate reduction in the absolute rate of FX activation (colored green in Figure 5A). TF mutant K159A severely reduced the absolute rate of FX activation but did not significantly diminish the Mg2+ effect relative to that of WT TF. The 10 remaining mutations also did not significantly weaken the ability of Mg2+ to enhance the rate of FX activation relative to that of WT TF, irrespective of whether they decreased the absolute rate of FX activation.
Figure 5.
Localization and properties of TF residues investigated. The structure is from Protein Data Bank entry 3TH2, in which sTF/FVIIa was crystallized in the presence of 5 mM Ca2+ and 2.5 mM Mg2+.1 FVIIa is depicted as orange ribbons, with bound Ca2+ ions colored teal and Mg2+ ions beige. (A) Localization of the TF residues tested in this study for their effect on the absolute rate of FX activation by memTF/FVIIa in solution. TF residues are color-coded according to their rate of FX activation in the presence of 1.85 mM Ca2+ alone (from Figure 2A). Unmutated TF residues are colored white. TF residues are colored red, which, when mutated, retained essentially WT activity (i.e., ≥ 75% of the WT rate of FX activation). Residues with a moderate effect on the FX activation rate are colored green (30-75% of the WT rate); residues with severe defects are colored blue (≤ 30% of the WT rate). Residues W158 and S160, obstructed in this view, are colored blue and green, respectively. (B) Localization of TF residues tested in this study as being important for the ability of Mg2+ to enhance the rate of FX activation by memTF/FVIIa in solution. TF residues are color-coded according to their rate of FX activation in the presence of Ca2+ and Mg2+ normalized to the rate at 1.85 mM Ca2+ alone (from Figure 2B). Unmutated TF residues are colored white. TF residues are colored red, which, when mutated, retained essentially WT responses to Mg2+ (i.e., 4-6-fold enhancement of the FX activation rate). Residues with blunted responses to Mg2+ are colored green (2-4-fold enhancement by Mg2+) or blue (1-2-fold enhancement by Mg2+). Residues W158 and S160, obstructed in this view are colored red. (C) Relative rate of FX activation (rate with 1.25 mM Ca2+ and 0.6 mM Mg2+, divided by the rate with 1.85 mM Ca2+) vs the absolute rate of FX activation, in both cases by memTF/FVIIa in solution (i.e., data replotted from panels A and B of Figure 2, respectively). Residues are color-coded as in panel B. Vertical black dotted lines correspond to the color-coding cutoff values from panel A; horizontal black dotted lines correspond to the color-coding cutoff values from panel B. A vertical dashed red line marks the location of WT TF.
The location of TF residues that mediate Mg2+-dependent rate enhancement suggests that a critical component of the TF/FVIIa interaction with Mg2+-bound FX substrate involves the flexible TF loop located at residues 160-165, with the adjacent residues Y185, K166, K159, and Y157 also involved. These residues, in particular residues 164-166, are highly conserved across mammalian species (Figure S3 of Supporting Information). The importance of G164 to Mg2+ recognition is particularly interesting, as glycine lacks a functional side chain with which to interact with macromolecular substrates. A possible explanation is that this highly conserved glycine permits flexibility or orientations of the adjacent loop(s) that are otherwise not possible with amino acids containing bulkier side chains.
When WT memTF was incorporated into liposomes, we found that increasing the PS content of the membranes blunted the response to Mg2+, confirming our previous findings.24 We also found that increasing the PS content of TF liposomes blunted the Mg2+ response in these TF mutants. With several of these mutants (especially S162A), this blunting effect of PS was less extensive than that observed with WT TF. We also found that the TF mutants that were most deficient in response to Mg2+ when tested in solution (G164A, K166A, and Y185A) actually had lower rates of FX activation in TF liposomes in the presence of Mg2+ and Ca2+ than in the presence of Ca2+ alone. Furthermore, the essentially negative effects of Mg2+ on this class of TF mutants were observed irrespective of the PS content of the memTF liposomes.
Messer et al. have shown that occupancy of the FIXa GLA domain with Mg2+ enhances its affinity for PS-containing membranes and have proposed that Mg2+ generally enhances the membrane binding of GLA domain-containing clotting proteins.28 If this is part of the mechanism by which Mg2+ enhances the rate of FX activation by TF/FVIIa (i.e., by better recruiting FX to the membrane surface in the vicinity of TF/FVIIa and thereby increasing the local substrate concentration), then it follows that increasing the PS content may blunt the Mg2+ effect, because greatly increasing the PS content will likewise increase the affinity of FX for the membrane surface and thus overshadow the relative contribution from Mg2+. However, the fact that some TF mutations selectively abolish the ability of Mg2+ to enhance the rate of FX activation by the TF/FVIIa/membrane complex argues in favor of protein-protein interactions (presumably, between the TF exosite and the GLA domain of FX) being also very important in mediating the ability of Mg2+ to enhance the proteolytic activity of TF/FVIIa toward FIX and FX.
Supplementary Material
Acknowledgments
Funding: This study was supported by National Institutes of Health Grant R01 HL103999 from the National Heart, Lung and Blood Institute.
Abbreviations
- FX
factor X
- FIX
factor IX
- FVII
factor VII
- HSQC
heteronuclear single quantum coherence
- memTF
tissue factor (residues 3-244) containing its membrane-anchoring helix
- NMR
nuclear magnetic resonance
- sTF
soluble tissue factor (residues 3-219)
- TF
tissue factor
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information: A table listing the initial rates of FX activation supported by all TF mutants under three different divalent metal ion conditions, graphs of the effects of Mg2+ on rates of FIX activation by TF/FVIIa (for WT and mutant TF), a graph showing the effects of increasing the Ca2+ concentration on FX activation by TF/FVIIa (for WT and mutant TF), a sequence alignment of the mutated portion of TF from several mammalian species, and the Kd values for binding of FVIIa to TF (both WT and selected mutants). The Supporting Information is available free of charge on the ACS Publications website DOI: 10.1021/acs.biochem.5b00608.
References
- 1.Vadivel K, Agah S, Messer AS, Cascio D, Bajaj MS, Krishnaswamy S, Esmon CT, Padmanabhan K, Bajaj SP. Structural and functional studies of γ-carboxyglutamic acid domains of factor VIIa and activated protein C: Role of magnesium at physiological calcium. J Mol Biol. 2013;425:1961–1981. doi: 10.1016/j.jmb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom JW, Mann KG. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978;17:4430–4438. doi: 10.1021/bi00614a012. [DOI] [PubMed] [Google Scholar]
- 3.Zwaal RFA, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376:433–453. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 4.Sunnerhagen M, Forsén S, Hoffrén AM, Drakenberg T, Teleman O, Stenflo J. Structure of the Ca2+-free GLA domain sheds light on membrane binding of blood coagulation proteins. Nat Struct Biol. 1995;2:504–509. doi: 10.1038/nsb0695-504. [DOI] [PubMed] [Google Scholar]
- 5.Falls LA, Furie BC, Jacobs M, Furie B, Rigby AC. The ω-loop region of the human prothrombin γ-carboxyglutamic acid domain penetrates anionic phospholipid membranes. J Biol Chem. 2001;276:23895–23902. doi: 10.1074/jbc.M008332200. [DOI] [PubMed] [Google Scholar]
- 6.Stenflo J, Suttie JW. Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–172. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- 7.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 8.Banner DW, D'Arcy A, Chène C, Winkler FK, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, McDonnell EH, Sedor FA, Toffaletti JG. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med. 2002;126:947–950. doi: 10.5858/2002-126-0947-PEOMOI. [DOI] [PubMed] [Google Scholar]
- 10.Sekiya F, Yamashita T, Atoda H, Komiyama Y, Morita T. Regulation of the tertiary structure and function of coagulation factor IX by magnesium(II) ions. J Biol Chem. 1995;270:14325–14331. doi: 10.1074/jbc.270.24.14325. [DOI] [PubMed] [Google Scholar]
- 11.Sekiya F, Yoshida M, Yamashita T, Morita T. Magnesium(II) is a crucial constituent of the blood coagulation cascade - Potentiation of coagulant activities of factor IX by Mg2+ ions. J Biol Chem. 1996;271:8541–8544. doi: 10.1074/jbc.271.15.8541. [DOI] [PubMed] [Google Scholar]
- 12.van den Besselaar AMHP. Magnesium and manganese ions accelerate tissue factor-induced coagulation independently of factor IX. Blood Coagul Fibrinolysis. 2002;13:19–23. doi: 10.1097/00001721-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj SP, Schmidt AE, Agah S, Bajaj MS, Padmanabhan K. High resolution structures of p-aminobenzamidine- and benzamidine-VIIa/soluble tissue factor: unpredicted conformation of the 192-193 peptide bond and mapping of Ca2+, Mg2+, Na+, and Zn2+ sites in factor VIIa. J Biol Chem. 2006;281:24873–24888. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]
- 14.Persson E, Østergaard A. Mg2+ binding to the Gla domain of factor X influences the interaction with tissue factor. J Thromb Haemost. 2007;5:1977–1978. doi: 10.1111/j.1538-7836.2007.02661.x. [DOI] [PubMed] [Google Scholar]
- 15.Neuenschwander PF, Bianco-Fisher E, Rezaie AR, Morrissey JH. Phosphatidylethanolamine augments factor VIIa-tissue factor activity: enhancement of sensitivity to phosphatidylserine. Biochemistry. 1995;34:13988–13993. doi: 10.1021/bi00043a004. [DOI] [PubMed] [Google Scholar]
- 16.Rezaie AR, Fiore MM, Neuenschwander PF, Esmon CT, Morrissey JH. Expression and purification of a soluble tissue factor fusion protein with an epitope for an unusual calcium-dependent antibody. Protein Expr Purif. 1992;3:453–460. doi: 10.1016/1046-5928(92)90062-2. [DOI] [PubMed] [Google Scholar]
- 17.Smith SA, Morrissey JH. Rapid and efficient incorporation of tissue factor into liposomes. J Thromb Haemost. 2004;2:1155–1162. doi: 10.1111/j.1538-7836.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 18.Waters EK, Yegneswaran S, Morrissey JH. Raising the active site of factor VIIa above the membrane surface reduces its procoagulant activity but not factor VII autoactivation. J Biol Chem. 2006;281:26062–26068. doi: 10.1074/jbc.M604915200. [DOI] [PubMed] [Google Scholar]
- 19.Waters EK, Morrissey JH. Restoring full biological activity to the isolated ectodomain of an integral membrane protein. Biochemistry. 2006;45:3769–3774. doi: 10.1021/bi052600m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. The local phospholipid environment modulates the activation of blood clotting. J Biol Chem. 2007;282:6556–6563. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhofer D, Lipari MT, Moran P, Eigenbrot C, Kelley RF. The tissue factor region that interacts with substrates factor IX and factor X. Biochemistry. 2000;39:7380–7387. doi: 10.1021/bi000182+. [DOI] [PubMed] [Google Scholar]
- 22.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 23.Goddard TD, Kneller DG. SPARKY 3. University of California; San Francisco: 2006. [Google Scholar]
- 24.Tavoosi N, Morrissey JH. Influence of membrane composition on the enhancement of factor VIIa/tissue factor activity by magnesium ions. Thromb Haemost. 2014;111:770–772. doi: 10.1160/TH13-07-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q, Neuenschwander PF, Rezaie AR, Morrissey JH. Substrate recognition by tissue factor-factor VIIa. Evidence for interaction of residues Lys165 and Lys166 of tissue factor with the 4-carboxyglutamate-rich domain of factor X. J Biol Chem. 1996;271:21752–21757. doi: 10.1074/jbc.271.36.21752. [DOI] [PubMed] [Google Scholar]
- 26.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–176. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Vadivel K, Bajaj SP. Structural biology of factor VIIa/tissue factor initiated coagulation. Front Biosci. 2012;17:2476–2494. doi: 10.2741/4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messer AS, Velander WH, Bajaj SP. Contribution of magnesium in binding of factor IXa to the phospholipid surface: implications for vitamin K-dependent coagulation proteins. J Thromb Haemost. 2009;7:2151–2153. doi: 10.1111/j.1538-7836.2009.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.