Abstract
Study Objectives:
Modafinil is a non-amphetaminic wake-promoting compound used as therapy against sleepiness and narcolepsy. Its mode of action is complex, but modafinil has been recently proposed to act as a cellular-coupling enhancer in glial cells, through modulation of gap junctions constituted by connexins. The present study investigated in mice the impact of connexins on the effects of modafinil using connexin inhibitors.
Methods:
Modafinil was administered alone or combined with inhibitors of astrocyte connexin, meclofenamic acid, or flecainide, respectively, acting on Cx30 and Cx43. Sleep-wake states were monitored in wild-type and narcoleptic orexin knockout mice. A spontaneous alternation task was used to evaluate working memory in wild-type mice. The effects of the compounds on astroglial intercellular coupling were determined using dye transfer in acute cortical slices.
Results:
Meclofenamic acid had little modulation on the effects of modafinil, but flecainide enhanced the wake-promoting and pro-cognitive effects of modafinil. Co-administration of modafinil/flecainide resulted in a marked decrease in the number and duration of direct transitions to rapid eye movement sleep, which are characteristic of narcoleptic episodes in orexin knockout mice. Furthermore, modafinil enhanced the connexin-mediated astroglial cell coupling, whereas flecainide reduced it. Finally, this modafinil-induced effect was reversed by co-administration with flecainide.
Conclusions:
Our study indicates that flecainide impacts the pharmacological effects of modafinil, likely through the normalization of Cx30-dependent gap junctional coupling in astroglial networks. The enhancement of the wake-promoting, behavioral, and cognitive outcomes of modafinil demonstrated here with flecainide would open new perspectives in the management of sleep disorders such as narcolepsy.
Commentary:
A commentary on this article appears in this issue on page 1175.
Citation:
Duchêne A, Perier M, Zhao Y, Liu X, Thomasson J, Chauveau F, Piérard C, Lagarde D, Picoli C, Jeanson T, Mouthon F, Dauvilliers Y, Giaume C, Lin JS, Charvériat M. Impact of astroglial connexins on modafinil pharmacological properties. SLEEP 2016;39(6):1283–1292.
Keywords: connexin, gap junction, modafinil, sleep, narcolepsy
Significance.
This study focuses on the pharmacological properties of modafinil, a wake-promoting compound used worldwide in sleep and cognitive disorders. The wake-promoting and pro-cognitive effects of modafinil have been linked with complex monoaminergic and gabaergic neuronal mechanisms. Recently, effects of modafinil on astroglial connexins also have drawn attention. Although modafinil improves behavioral activity and excessive daytime sleepiness, it does not prevent the occurrence of cataplexy, a major syndrome of narcolepsy. We found in mice that an astroglial-connexin-30 inhibitor impacts the pharmacological properties of modafinil by enhancing its awakening and pro-cognitive activities and by decreasing the cataplexy-like phenotype in orexin-knockout narcoleptic mice. Our findings thus open new perspectives in the management of sleep and cognitive disorders.
INTRODUCTION
Modafinil is a wake-promoting compound now widely used in the treatment of excessive daytime sleepiness associated with narcolepsy and other central disorders of hypersomnolence.1,2 Recent studies also support a role for modafinil in enhancing cognitive activities.3 Indeed, neuro-imaging studies show activating effects of modafinil in the rodent fronto-cortical areas4 that are involved in both arousal5,6 and cognitive enhancement.7,8
The wake-promoting and pro-cognitive properties of modafinil have been linked with complex neuronal mechanisms of action. Indeed, the effects of modafinil is modulated notably through monoaminergic and GABAergic systems (for review, see Minzenberg3). Recent studies have also extended to possible effects of modafinil on astrocytes. These glial cells express high densities of gap junction proteins, named connexins,9,10 which are involved in sleep regulation.11,12 In addition, we recently found that modafinil increases the cortical expression of Cx30, a major astroglial connexin.13 This increase correlates with an enhancement of intercellular dye coupling in astrocytes and is abolished when neuronal activity is silenced.13 Based on these data, we suggested that the astroglial network interconnected by connexins exerts an impact on modafinil pharmacological actions.
To test this hypothesis, we examined the impact of connexin inhibitors on the wake-promoting and pro-cognitive effects of modafinil using blood brain barrier-penetrating modulators of gap junctional communication that we recently identified, such as flecainide.14 For comparison, meclofenamic acid, a known gap junction modulator15 was also used. Particularly, an orexin knockout mouse model16 was used to examine the impact of connexin modulators on the anti-narcoleptic effect of modafinil.
METHODS
Animals
Prepro-orexin knockout (KO) male mice (Ox−/−) were off-spring of the mouse strain generated by Chemelli et al.16 and kept on C57BL/6J genomic background. Littermate wild-type (WT) male mice were used as control. Genotype was determined with tail biopsies, as presented elsewhere.17
Brain slices were obtained from C57BL/6J mice (Charles River). All experiments in acute slices were prepared from animals aged between P15 to P25. Animals for working memory assessment and pharmacokinetic determination were 3-month-old C57BL/6J or Swiss male mice obtained from Janvier Labs.
Mice were housed in standard laboratory conditions under a 12h light-dark cycle with an access to food and water ad libitum. All animal experimentation reported in the present paper has been conducted in accordance with the guidelines laid down by the European Committees Council Directive (86/609/EEC) and all efforts were made to minimize the number of animals used and their suffering.
The experimenter was blind to treatment.
Drugs
All drugs were prepared in order to obtain an orally applied volume of 10 mL/kg body weight. Modafinil (Sequoia Chemicals) was suspended in saline containing Arabic gum at 2.5% to reach the doses of 32, 64, and 128 mg/kg (respectively MOD32, MOD64, and MOD128). Meclofenamic acid (Sigma-Aldrich) and flecainide acetate salt (FLE, Sigma-Aldrich) were diluted in DMSO. Venlafaxine (VEN, Sigma-Aldrich) was diluted in saline solution (0.9% NaCl).
Gap Junction Functionality Evaluation
Cell Culture, Transfection, and Selection
The rat insulinoma RIN cell line, deficient in gap junction intercellular communication,18 was grown in OptiMem medium, supplemented with 10% fetal calf serum. GJB6 (Cx30) and GJA1 (Cx43) open reading frames were amplified from human cDNA. The open reading frames were cloned in pcDNA3.1/ V5-His-TOPO and cells were transfected using Lipofectamine and selected using geneticin. Cell culture reagents were purchased from Life Technologies.
Dye Transfer Experiments
Confluent cells were loaded with two fluorochromes, calcein acetoxymethyl ester (Calcein-AM, Sigma-Aldrich), a gap junction permeable dye, and Vybrant Dil (Life Technologies) a membrane lipophilic dye, as previously described.14 Cells were dissociated and incubated for 3 hours on previously seeded non-loaded cells and in the presence of chemical compounds (n = 3 independent trials). Flow cytometry was conducted on a FACScan (BD Biosciences). Inhibition was quantified as the proportion of receiver cells that gained fluorescence; this ratio of cellular coupling was further normalized on the vehicle one.14 IC50 were determined using GraphPad Prism, log-transformed concentration values, and those ratios were fitted to a two-parameter logistic equation.
Working Memory Assessment
Animals were housed individually. All procedures were carried out during the light phase of the cycle between 08:00 and 00:00 a.m. Mice were injected 30 min prior to T-maze experiment. The working memory experiment took place in a T-maze (50 cm × 10 cm × 25 cm). Mice (n = 6–23 per group, with a total of 77) were given 6 successive trials separated by a 120-s intertrial interval.19 An alternation response was considered each time the subject entered the arm opposite to the one visited on the previous trial. Alternation rate was calculated in percentage for the 6 successive trials.
Quantification of Sleep-Wake Episodes
Surgery
At the age of 12 weeks, 70 mice were implanted with 6 cortical electrodes and 3 muscle electrodes to record the EEG and EMG and to monitor the sleep-wake cycle, as presented in previous studies.17 Cortical electrodes were inserted into the dura through 3 pairs of holes made in the skull. Muscle electrodes were inserted into the neck muscles. This implantation allows stable and long-lasting polysomnographic recordings.20
Polysomnographic Recording
After surgery, the animals were housed individually, on a 12 h light/dark cycle (lights-on at 07:00) with ambient temperature maintained at 23.5 ± 1.0°C. Polysomnographic records were visually scored by 10-s epochs for wakefulness (W), slow wave sleep (SWS), and paradoxical or rapid eye movement (REM) sleep according to previously described criteria validated for mice.20,21 Direct REM sleep onset (DREMs) episodes were quantified as the occurrence of REM sleep directly from W, namely a REM episode that follows directly a wake episode lasting more than 60 s without being preceded by any cortical slow activity of > 5 s during the 60 s.16
Drug Administration and Experimental Procedures
Each mouse was subjected to a recording session of 2 continuous days, beginning at 07:00. Administrations were performed at 18:45, just before lights-off (19:00 p.m.), since Ox−/− mice display DREM episodes only during lights-off phase.16 For wakefulness assessment, oral deliveries were performed at 09:45, after light onset (07:00). The order of delivery was randomized. Polysomnographic recordings were maintained during the whole lights-off period (12 h). Two administrations were separated by a period of washout of 7 days.
Pharmacokinetics of Modafinil in Plasma and Brain
Mice (n = 35 in total) received orally either modafinil or modafinil + flecainide, vehicle application served as a control (n = 3–4/group/endpoint). At 30 min, 1 h, 2 h, or 4 h, mice were anesthetized with pentobarbital, and blood was collected by cardiac puncture, on EDTA-DiSodium buffer. Blood was centrifuged and plasma was stored at −80°C. Brains were dissected, homogenized in methanol 1:2.5 (weight/volume) and stored at −80°C.
Modafinil was determined after protein precipitation followed by reverse phase liquid chromatography with tandem mass spectrometry detection (LC/MS-MS), a technique adapted from previously described methods.22,23 The concentration range for the detection of modafinil was confirmed to be linear between 100 ng/mL to 100 μg/mL.
Electrophysiology and Dye Coupling Experiments
Mouse brains were dissected and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2, bubbled with 95% O2/5% CO2. Coronal brain slices (300 μm) containing the somatosensory cortex were cut using a vibratome. The slices were incubated at 33°C for 30 min and transferred at room temperature before use. Thereafter, the slices were placed in a recording chamber and perfused continuously with oxygenated ACSF (pH 7.4) at a rate of 2 mL/min. Cells were identified as astrocytes based on morphological criteria and electrophysiological properties.13 Slices were treated with 200 μM modafinil and 500 μM flecainide during 2 h before and during recording. To assess the level of gap junction coupling, cells were loaded with sulforhodamine B (Molecular Probes) for 10 min.13 Intercellular diffusion of sulforhodamine B was captured thereafter with a CCD camera (Pixelfly QE) on successive focal planes and the number of sulforhodamine B positive cells (i.e., cells coupled to the recorded astrocyte) was determined using ImageJ software.
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using 1-way ANOVAs followed by Tukey post hoc test or 2-way ANOVAs followed by Bonferroni post hoc test when appropriated, with Prism 6 (Graph Pad Software Inc.). For 2-way ANOVAs, if there was no significant interaction between factors, no multiple comparison test was performed. Significant levels were considered for P < 0.05.
RESULTS
Flecainide Inhibited Gap Junctional Communication Mediated by Cx43 and Cx30
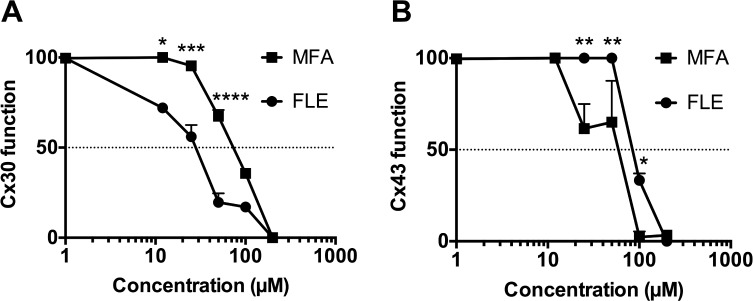
Two cellular models were designed to assess the functionality of Cx30 and Cx43. We found that glycyrrhizic acid did not inhibit those gap junctions (data not shown, confirmation of data presented elsewhere24,25) and that the tested compounds, flecainide and meclofenamic acid, differed in their inhibition potency on Cx30 (IC50s respectively 25 and 70 μM for flecainide and meclofenamic acid) and Cx43 (IC50s respectively 100 and 50 μM for flecainide and meclofenamic acid) (Figure 1A and 1B), indicating that flecainide and meclofenamic acid act preferentially at Cx30 or Cx43, respectively.
Figure 1.
Flecainide and meclofenamic acid differentially reduce Cx43 and Cx30 gap junction functionality. The gap junction functionality was assessed in 2 cell lines (n = 3 separate experiments per condition), stably transfected with plasmids respectively coding for astrocyte connexin 30 (Cx30) (A) and connexin 43 (Cx43) (B), using a parachute assay. Data are normalized on gap junction functionality after treatment with vehicle. Note that treatments with flecainide (FLE) decreased Cx30 more efficiently than meclofenamic acid (MFA); meanwhile Cx43-coupling is more intensely inhibited by meclofenamic acid than flecainide. Two-way ANOVA followed by Bonferroni post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 MFA vs. FLE. Dotted line represents a 50% inhibition. Estimated IC50 values for Cx30 functionality are 25 μM and 70 μM, respectively for FLE and MFA. And IC50 values for Cx43 functionality are 100 μM and 50 μM, respectively for FLE and MFA.
Flecainide, but Not Meclofenamic Acid, Significantly Enhanced the Awakening Effects of Modafinil in Wild-Type Mice
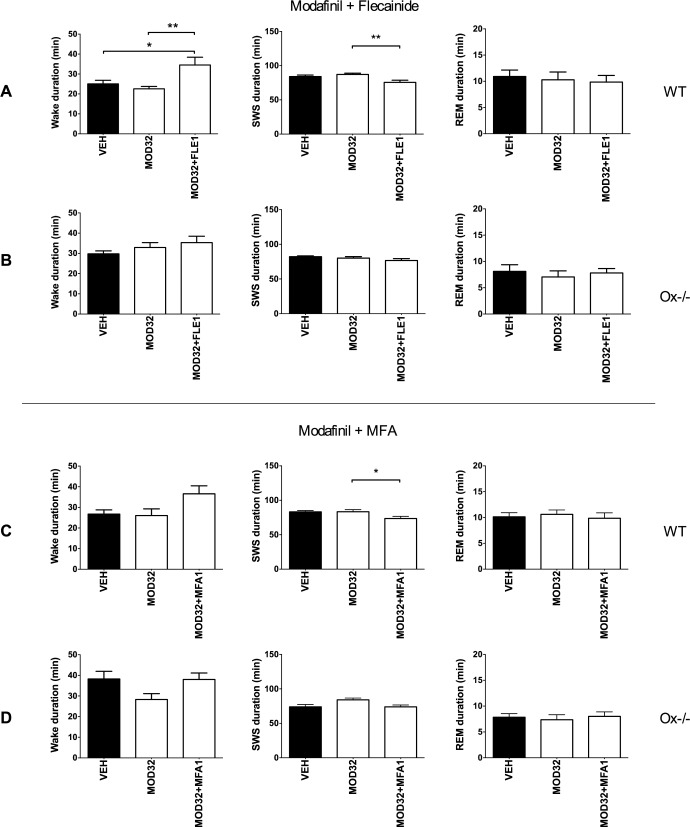
During the lights-on period, while modafinil alone (32 mg/kg) had no significant effects on the sleep-wake states, the combination between modafinil and flecainide enhanced wakefulness (+60%, F2,24 = 6.03, P = 0.0084) compared to modafinil alone (Figure 2A), with smaller tendency for meclofenamic acid (+35% of wake duration, F2,27 = 3.50, P = 0.0631; Figure 2C). Meclofenamic acid and flecainide alone did not modulate sleep-wake cycles, but when combined with modafinil reduced SWS compared to modafinil alone (−13% and −12%, F 2,27 = 4.04 and F2,24 = 5.89, P = 0.0483 and P = 0.0079, respectively). The same pharmacological dosing was performed in Ox−/− mice; however, no significant sleep-wake effects were noted between modafinil alone or combined with flecainide (Figure 2B; respectively, for WK, SMS, and REM sleep, F 2,27 = 1.29, F2,27 = 1.74, and F2,27 = 0.25).
Figure 2.
Flecainide improves the effect of a sub-efficient dose of modafinil on wakefulness, in WT but not in Ox−/− mice. Sleep and wake durations after oral administration with modafinil 32 mg/kg alone or combined flecainide 1 mg/kg (MOD32+FLE1) or meclofenamic acid 1 mg/kg (MOD32+MFA1) were evaluated in WT (respectively, (A) and (C)) and Ox−/− (respectively, (B) and (D)) mice using polysomnography analysis, during 2 hours. Note that at 32 mg/kg, modafinil had no significant effects on the sleep-wake states but enhanced wakefulness at the expense of SWS when combined with flecainide. n = 9–10 mice per group. One-way ANOVA followed by a Tukey post hoc test *P < 0.05 and **P < 0.01. Ox−/−, orexin knock-out mice; VEH, vehicle; WT, wild-type.
Modafinil Combined with Flecainide Reduced the Narcoleptic Phenotype DREMs Episodes in Orexin −/− Mice
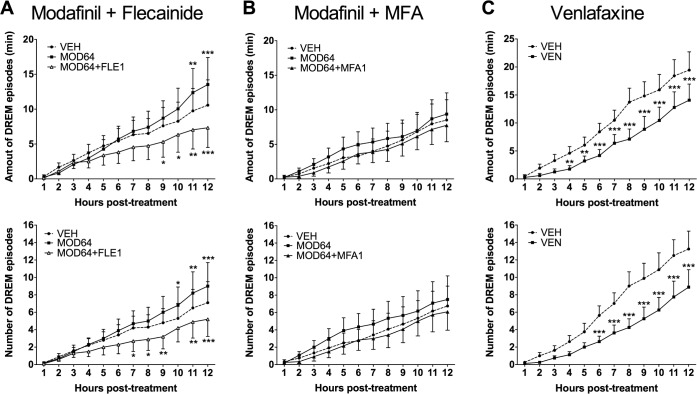
Orexin−/− mice presented, during the dark phase under baseline conditions, typical DREMs episodes that persisted with modafinil 64 mg/kg (Figure 3). Interestingly, adding flecainide (1 mg/kg) to the modafinil treatment (MOD64+FLE1) significantly decreased the DREMs phenotype (F22,198 = 3.84, P = 0.0002 for total duration and F22,198 = 3.20, P = 0.0008 for total number as compared to VEH group). The effect, starting at the 4th to 5th hour post dosing, was characterized by a reduction of DREMs both in terms of total duration (by 45%), and number (by 42%) as compared to modafinil alone during the 12-h recording section after dosing (Figure 3A). Meclofenamic acid combined to modafinil had no effect on DREMs occurrence (F22,242 = 0.67 and F22,242 = 1.15, respectively for duration and number of DREMs; Figure 3B). Venlafaxine (VEN, 5 mg/kg), a serotoninergic and noradrenergic reuptake inhibitor known to reduce DREMs episodes in mice and cataplectic episodes in humans, significantly reduced DREMs total duration by 28% (F11,77 = 6.86, P < 0.0001) and number of episodes by 33% (F11,77 = 7.18, P < 0.0001) as compared with vehicle (Figure 3C).
Figure 3.
Modafinil combined with flecainide, and venlafaxine reduces the DREM duration and episode numbers in Ox−/− narcoleptic mice. Modafinil 64 mg/kg combined with flecainide 1 mg/kg (MOD64+FLE1) (A), or meclofenamic acid (MOD64+MFA1) (B) or alone (MOD64), or venlafaxine 5 mg/kg (VEN (C) were orally administrated to Ox−/− mice, and DREM episodes were scored during 12 h post-treatment. Note the absence of significant effect of modafinil alone or combined with meclofenamic acid, while MOD64+FLE1 reduced significantly DREM duration and episode numbers. Venlafaxine significantly reduced both DREM duration and episode number. n = 8–12 mice per group. Two-way repeated ANOVA followed by Bonferroni post hoc test: *P < 0.05, **P < 0.01 and ***P < 0.001 vs. vehicle (VEH). Ox−/−, orexin knock-out mice.
Flecainide, but Not Meclofenamic Acid, Significantly Improved the Pro-Cognitive Effects of Modafinil
Working memory performance was assessed by the 6-trial mean of alternation percentage in the T-Maze after vehicle or the treatment with either modafinil alone, or combined with flecainide or meclofenamic acid (Figure 4). While modafinil (64 mg/kg) alone had no effect, the same dose of modafinil combined with flecainide 1 mg/kg (MOD64+FLE1) significantly increased the alternation rate compared with vehicle (F4,72 = 5.94, P = 0.0345), with a significant difference between treatments (P = 0.0096). In contrast, modafinil combined with meclofenamic acid (MOD64+MFA1) produced no significant effect compared with vehicle or modafinil alone. Interestingly, the use of flecainide did allow modafinil to gain the increased alternation (+ 74%) produced by the dose of 128 mg/kg (+ 76%) with a half dose, namely 64 mg/kg and as a result, both treatments reached the above chance level, i.e., 50% alternation score (P < 0.0001; Figure 4). It should be mentioned here that flecainide 1 mg/kg had no effect on the spontaneous alternation (data not shown) suggesting that the compound at the dose used had no direct effects, but likely acted via interaction with modafinil.
Figure 4.
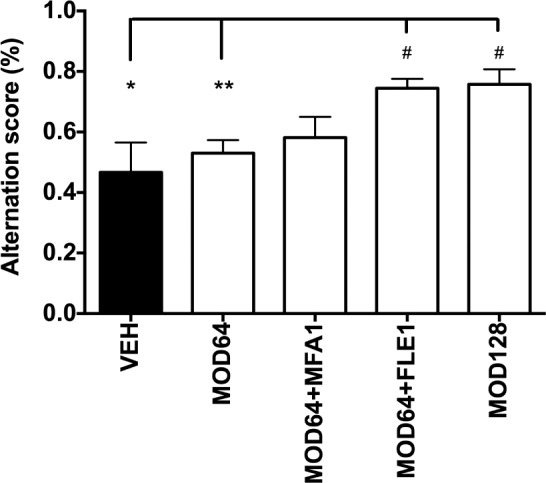
Flecainide improves the effects of modafinil on working memory in wild-type mice. Working memory is evaluated in mice using alternation measured in T-maze apparatus. The graphic shows that (i) modafinil 64 mg/kg alone (MOD64) had no effect; (ii) modafinil 64 mg/kg + flecainide 1 mg/kg (MOD64+FLE1) significantly enhanced the alternation rate, as compared to vehicle or modafinil 64 mg/kg alone (MOD64); iii) the combination between modafinil 64 mg/kg and meclofenamic acid 1 mg/kg (MOD64+MFA1) had no effect. Note also that the modafinil and flecainide combination results in the same increased alternation produced by modafinil using a higher dose, i.e., 128 mg/ kg (MOD128). n = 6–23 mice per group. One-way ANOVA followed by a Tukey post hoc test *P < 0.05 vs. vehicle (VEH) and **P < 0.01 vs MOD64 and One sample t-test # P < 0.0001 vs. random 50% alternation.
Flecainide did Not Modify the Concentration of Modafinil in the Plasma and Brain
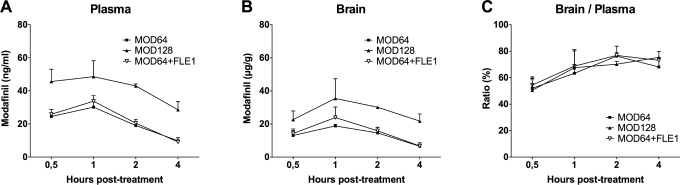
The concentration of modafinil in plasma and cerebral tissue was measured respectively at 30 min, 1 h, 2 h, and 4 h after acute oral administrations. Modafinil was not detectable in the vehicle or the flecainide group. As shown in Figure 5, modafinil was detectable in the plasma as early as 30 min after the treatment with either modafinil alone (64 and 128 mg/kg) or combined with flecainide. Modafinil reached its maximal concentration (Cmax) 1 h after dosing with the values of 30.2 ± 3.3 μg/mL for MOD64, 48.6 ± 9.6 μg/ mL for MOD128 and 33.7 ± 3.4 μg/mL for MOD64+FLE1. In the brain (Figure 5), similarly to the plasma, Cmax was found at 1 h after dosing and estimated as 18.8 ± 0.9 μg/g for MOD64, 35.5 ± 11.9 μg/g for MOD128 and 23.9 ± 6.4 μg/g for MOD64+FLE1. The plasma and brain concentrations of modafinil decreased from 2 h after all treatments. In addition, brain to plasma concentration ratio reached approximately 50% in all the groups at 30 min and 70% at 1 to 4 h for all treatments, without any effect between groups. Thus, the plasma and brain concentrations of modafinil as well as its brain/plasma ratio depended on the dose used and time post-treatment, but not on flecainide co-treatment, which had no impact on the pharmacokinetic parameters and bioavailability of modafinil.
Figure 5.
The plasma and brain concentration and the brain/blood ratio of modafinil following its acute treatment alone or combined with flecainide in wild-type mice. Modafinil (MOD) was measured in the plasma (A) and brain tissue (B) after oral dosing using LC/MS-MS method. Ratio of blood/plasma was calculated (C). Note that the plasma and brain concentrations of modafinil as well as its brain/plasma ratio depended on the dose used and time post-treatment, but not on flecainide (FLE). As shown in the three panels, flecainide did not modify the pharmacokinetic parameters of modafinil. Modafinil was not detectable in the vehicle or flecainide group. n = 2–3 mice per condition.
Flecainide Abolished the Increase in Astrocyte Dye Coupling Induced by Modafinil
Astrocytes from the mouse somatosensory cortex were selected based on their morphology and once whole-cell recording configuration was established by their electrophysiological properties: very negative resting membrane potential (−79 ± 2.2 mV), low input resistance (22 ± 1.5 MΩ), typical linear current-voltage relationship. In control condition, dye coupling was detected in neighboring cells (mean of coupled cells, m = 50.0 ± 1.1; n = 6 experiments). Modafinil at a concentration of 200 μM significantly increased by 27% (P = 0.0023) the number of coupled cells (m = 63.8 ± 3.6; n = 5, Figure 6). In contrast, flecainide at a concentration of 500 μM reduced dye coupling by 18% compared to control (m = 32.0 ± 1.5; n = 3; F 3,14 = 28.9, P = 0.0009) and by 46% compared to modafinil. Co-treatment with modafinil 200 μM and flecainide 500 μM resulted in a cellular coupling comparable to the control level and inferior to that of modafinil alone (m = 41.5 ± 1.8; n = 4).
Figure 6.
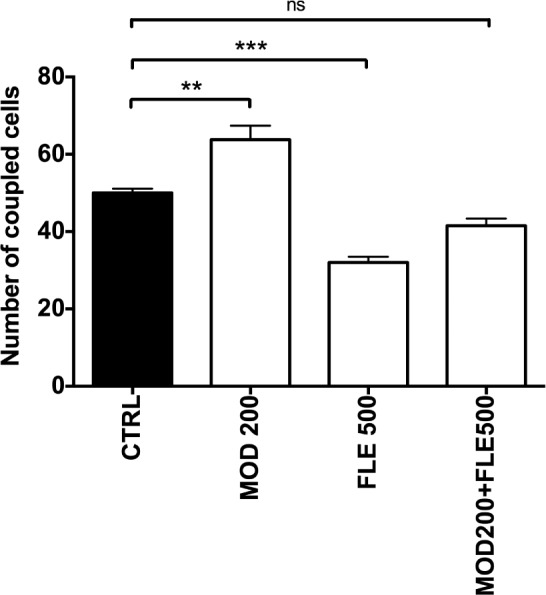
Modafinil enhances dye coupling in astrocytes studied in acute cortical slices, this effect is reversed by flecainide. Summary diagram showing the numbers of dye coupled astrocytes under the indicated conditions (n ranging from 3 to 6 independent experiments). Modafinil 200 μM (MOD 200) significantly increases cellular coupling compared to vehicle, and the combination modafinil/flecainide (MOD200+FLE500) restored a cellular coupling level comparable to the vehicle group. Oneway ANOVA followed by Tukey post hoc test: *P < 0.05 and **P < 0.01 vs. control (CTRL).
DISCUSSION
The present study investigated the impact of astroglial connexins on the pharmacological outputs of modafinil on behavior and sleep-wake cycle in wild-type and narcoleptic Ox−/− mice. We demonstrated here that flecainide, an astroglial connexin inhibitor was able to enhance the awakening and pro-cognitive effects of modafinil. More importantly, we established that modafinil combined with flecainide reduced the narcoleptic DREMs phenotype in Ox−/− mice, effect that is not seen with modafinil used alone. Finally, electrophysiological and dye coupling experiments showed that the gap junction enhancing effects of modafinil were reversed by flecainide.
Modafinil has been used in sleep medicine worldwide for more than two decades. Numerous preclinical studies have led to several hypotheses regarding its mode of action. The noradrenergic hypothesis has been supported by data showing that adrenergic antagonists or deletion of alpha1B-receptors are able to attenuate the waking effects of modafinil.3,6,26 The dopaminergic hypothesis has been prevailing since the identification of an affinity of modafinil toward dopamine transporter27,28 and a role for D1 and D2 receptors.29,30 Nevertheless, modafinil differs from dopaminergic psychostimulants by induction of quiet waking, weak addiction and tolerance and an absence of clear neuronal and behavioral excitation,31–33 char acteristics that could be explained by a decrease in GABA in brain areas involved in sleep-waking control.34
More recently, effects of modafinil on regulation of astroglial connexins have drawn attention.13,39 Astrocyte connexins are highly organized and regulated to form astroglial functionally plastic networks35 and they impact neuronal activity.36 In particular, astroglial connexins and notably Cx43 have been involved in sleep and homeostasis regulation,37,38 as sleep deprivation and subsequent sleep rebound modify their expression11 and their inhibition causes sleep loss.12 Finally, our recent study suggested that astroglial connexins and among them Cx30 could constitute a non-neuronal contributor affecting the pharmacological profile of modafinil.13 We then have examined the impact of astroglial connexin modulators on the wake-promoting and pro-cognitive effects of modafinil in WT and narcoleptic orexin KO mice.
We first characterized the properties of two connexin inhibitors on human Cx30 and Cx43 gap junctions using two cell lines. Meclofenamic acid15 inhibited Cx43 more efficiently than flecainide14 but on the opposite flecainide was a more potent inhibitor of Cx30. Therefore, flecainide was chosen to determine whether Cx30 has impact on modafinil both in our tests on astroglial cells and behavioral studies. Meclofenamic acid was used for comparison with flecainide, and similar effects for those compounds are expected on murine isoforms due to high conservation of those proteins between vertebrates.40,41
Modafinil is well known to alter the sleep-wake cycle by promoting wakefulness in both animals and humans.42,43 Here we tested in mice whether such a characteristic arousal effect can be mediated by connexins using the Cx30 inhibitor flecainide we have characterized here. For this purpose, we used a sub-efficient dose of modafinil, 32 mg/kg3,33,44 rather than 64 mg/kg, a quite efficient dose. During the lights-off period when mice are sleepy, modafinil at 32 mg/kg induces a subtle arousing effect,33 thus leaving a margin for improvement by connexin modulation. The 32-mg/kg dose was combined with flecainide at 1 mg/kg, a dose that was determined in our pilot study and also according to previous studies to have no impact on the cardiovascular function in rodents45 and dogs.46,47 Meanwhile this dose is below efficient dose in humans48 and leads to plasma and brain exposure in rodents in the micromolar order of magnitude. Meclofenamic acid was used at similar doses for comparison. We found in WT mice that the modafinil/flecainide combination significantly increased the total duration of wakefulness while, importantly, modafinil at a sub-efficient dose or flecainide alone did not. However, no significant enhancement on wake was found with meclofenamic acid combined with modafinil, only a reduction of SWS was observed.
Modafinil at 64 mg/kg but not 32 mg/kg is known to enhance cognitive activities,7,49 we therefore tested the impact of flecainide and meclofenamic acid using the alternating sequential test, to assess spatial working memory.50 Our results showed that flecainide 1 mg/kg significantly enhanced the pro-cognitive effect of modafinil at a sub-efficient dose, while at this dose range, both modafinil and flecainide are devoid of their own pro-cognitive effect (data not shown). Thus, the use of a low dose of flecainide (1 mg/kg) did allow reducing the dose of modafinil by 50% (from 128 mg/kg to 64 mg/kg) in terms of the pro-cognitive effect. Furthermore, the effect of flecainide on modafinil appeared selective, as modafinil combined with meclofenamic acid produced no significant cognitive enhancement.
The impact of flecainide on the pharmacological action of modafinil is not limited to its effect on wakefulness and cognitive activities, but also on narcoleptic phenotype. Previous studies reported that Ox−/− mice display behavior/motor arrests during waking, namely cataplexy, a pathognomonic behavioral phenotype related to DREMs episodes as defined by several research groups.16,17,42,51–54 Using this murine narcoleptic model, we previously reported that modafinil allows DREMs episodes to persist,42 a finding we confirmed in the present study. However, we found no between treatment differences in W, SWS, and REM sleep duration that may reflect a differential pharmacological response to modafinil in Ox−/− mice requiring doses to be adapted.55 More importantly, we found that co-administration of modafinil and flecainide, but not meclofenamic acid, significantly decreased the occurrence of DREMs episodes, both in terms of total duration and episode number. Interestingly, this DREMs-decreasing effect of modafinil/flecainide is similar to that produced in the same mouse genotype by venlafaxine, an antidepressant widely used to treat cataplexy in humans.56 Moreover, pitolisant, an inverse agonist of the histamine H3 receptor, has also been shown to reduce the number of DREMs episodes (alone or co-administrated with modafinil) in Ox−/− mice.42
As flecainide has higher potency at astroglial Cx30 than meclofenamic acid, our data indicate that Cx30 rather than Cx43 should mediate the enhancement of flecainide on modafinil. In support of this, it was recently shown that the effect of modafinil on astrocytes involves Cx30 rather than Cx43.13 These results suggest that astroglial network mediated by Cx30 could have an impact on neuronal pathways controlling sleep-wake, DREMs, and cognitive activities. Further studies are required to determine how the modafinil/flecainide combination affects such neuronal pathways.
It should be emphasized here that flecainide administrated at the dose of 1 mg/kg did not modify the pharmacokinetic profile of modafinil in either the plasmatic compartment or the whole brain tissue as attested by the similar Cmax and Tmax of modafinil post-treatment, confirming different pathways of metabolism for those drugs.57,58 The lack of own effect of flecainide alone and the lack of pharmacokinetic interaction between modafinil and flecainide indicate a post-pharmacokinetic interaction between modafinil and flecainide occurring at the neural and glial level controlling sleep-wake and cognitive functions. This interaction is also supported by ex vivo data obtained from our study on astroglial cell coupling. Previous study reported that modafinil increases the number of coupled cortical astrocytes.13 Here, we confirmed these data from mouse cortical astrocytes and further found that flecainide restored a basal level of gap junctional communication enhanced by modafinil. Apparent discrepancy between Figure 1 and Figure 6 on the levels of coupling inhibition are presumably due to the difference between the used techniques: parachute assay measures the kinetics of formation of new gap junctions and their function, while microinjection in slices quantifies the effects of a drug on established gap junctions.
It has been proposed that a confined astroglial networks may facilitate neuroglial interactions, and, as a result, promote neuronal signaling.36 Hence, we hypothesize that astroglial connexins upregulated by modafinil may tend to disorganize astroglial networks and their function, which, in turn, might affect the efficacy of modafinil. By modulating the size of this network and reorganizing it, astroglial connexin inhibition might therefore enhance the pharmacological effects of modafinil. Further studies are required in order to test this hypothesis and to better characterize how this glial action of modafinil contributes to its arousal and behavioral effects. The effects of modafinil and flecainide alone and combined could be monitored on neuronal connexins as it has been demonstrated that modafinil enhancement of cellular coupling is neuron-dependent.13,39 Moreover, the ratio between flecainide and modafinil should be optimized to gain more efficacy in sleep-wake cycle of wild-type mice but also in sleep deprived animals.
In summary, we demonstrated here that flecainide impacts the pharmacological action of modafinil by enhancing its wake-promoting and pro-cognitive effects and notably by a marked decrease in narcoleptic phenotype in Ox−/− mice. The involved mechanisms remain to be further investigated but would likely include a restoration of the functionality of the astroglial connexins. A potential modified neuronal indirect signaling (through, for instance, sodium and potassium channel blocking activity of flecainide) might also be involved and should be further explored. Whatever the mechanisms of this modafinil/flecainide combination might be, these findings from animal models are worthy of evaluation in human narcolepsy to better manage both excessive daytime sleepiness and cataplexy, and would open new perspectives in the management of sleep disorders.
DISCLOSURE STATEMENT
This work was supported by the CEA (Commissariat à l'Energie Atomique), Theranexus Company and the ANR (14-CE16-0022). Dr. Dauvilliers has received funds for speaking, board engagements, and travel to conferences from UCB Pharma, Jazz, Bioprojet and Theranexus. Adeline Duchêne, Christèle Picoli, Tiffany Jeanson, Franck Mouthon, and Mathieu Charvériat are employees of Theranexus Company. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Pr Michel Hamon, Dr Françoise Brunner, Dr Nathalie Plaud, Dr Joëlle Adrien and Dr Véronique Fabre for their experimental, technical, and scientific expertise. We also thank Prof. Masashi Yanagisawa (Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, TX 75390-8584, USA) for the original supply of Ox−/− mouse strain.
ABBREVIATIONS
- Cx
connexin
- DREM
direct REM sleep onset
- LC/MS-MS
liquid chromatography with tandem mass spectrometry
- MFA
meclofenamic acid
- Ox−/−
orexin knock-out
- REM
rapid eye movement
- SWS
slow wave sleep
- W
wakefulness
REFERENCES
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16:9–18. doi: 10.1016/j.sleep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 4.Gozzi A, Colavito V, Seke Etet PF, et al. Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology. 2011;37:822–37. doi: 10.1038/npp.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E. Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- 6.Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–26. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- 7.Beracochea D, Celerier A, Peres M, Pierard C. Enhancement of learning processes following an acute modafinil injection in mice. Pharmacology Biochem Behav. 2003;76:473–9. doi: 10.1016/j.pbb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 99:116–29. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ransom B, Giaume C. Gap Junctions and hemichannels: neuroglia, 3rd ed. Oxford University Press. 2012 [Google Scholar]
- 10.Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev. 2011;63:160–76. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Perez J, Ballesteros-Zebadua P, Fernandez-Figueroa EA, Ruiz-Olmedo I, Reyes-Grajeda P, Paz C. Sleep deprivation and sleep recovery modifies connexin36 and connexin43 protein levels in rat brain. Neuroreport. 2012;23:103–7. doi: 10.1097/WNR.0b013e32834e8fcb. [DOI] [PubMed] [Google Scholar]
- 12.Franco-Perez J, Paz C. Quinine, a selective gap junction blocker, decreases REM sleep in rats. Pharmacol Biochem Behav. 2009;94:250–4. doi: 10.1016/j.pbb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Petit JM, Ezan P, Gyger J, Magistretti P, Giaume C. The psychostimulant modafinil enhances gap junctional communication in cortical astrocytes. Neuropharmacology. 2013;75:533–8. doi: 10.1016/j.neuropharm.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Picoli C, Nouvel V, Aubry F, et al. Human connexin channel specificity of classical and new gap junction inhibitors. J Biomol Screen. 2012;17:1339–47. doi: 10.1177/1087057112452594. [DOI] [PubMed] [Google Scholar]
- 15.Harks EG, de Roos AD, Peters PH, et al. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther. 2001;298:1033–41. [PubMed] [Google Scholar]
- 16.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 17.Anaclet C, Parmentier R, Ouk K, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–38. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Corsso C, Srinivas M, Urban-Maldonado M, et al. Transfection of mammalian cells with connexins and measurement of voltage sensitivity of their gap junctions. Nat Protoc. 2006;1:1799–809. doi: 10.1038/nprot.2006.266. [DOI] [PubMed] [Google Scholar]
- 19.Pierard C, Liscia P, Philippin JN, et al. Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav. 2007;88:55–63. doi: 10.1016/j.pbb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gondard E, Anaclet C, Zhang M, et al. Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice. Neuropsychopharmacology. 2013;38:1015–31. doi: 10.1038/npp.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzarino M, Botre F. A fast liquid chromatographic/mass spectrometric screening method for the simultaneous detection of synthetic glucocorticoids, some stimulants, anti-oestrogen drugs and synthetic anabolic steroids. Rapid Commun Mass Spectrom. 2006;20:3465–76. doi: 10.1002/rcm.2729. [DOI] [PubMed] [Google Scholar]
- 23.Dubey S, Ahi S, Reddy IM, Kaur T, Beotra A, Jain S. A novel study of screening and confirmation of modafinil, adrafinil and their metabolite modafinilic acid under EI-GC-MS and ESI-LC-MS-MS ionization. Indian J Pharmacol. 2009;41:278–83. doi: 10.4103/0253-7613.59928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picoli C, Nouvel V, Aubry F, et al. Human connexin channel specificity of classical and new gap junction inhibitors. J Biomol Screen. 2012;17:1339–47. doi: 10.1177/1087057112452594. [DOI] [PubMed] [Google Scholar]
- 25.Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–7. [PubMed] [Google Scholar]
- 26.Stone EA, Cotecchia S, Lin Y, Quartermain D. Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse. 2002;46:269–70. doi: 10.1002/syn.10127. [DOI] [PubMed] [Google Scholar]
- 27.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Fowler JS, Logan J, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626–33. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Lin JS, Gervasoni D, Hou Y, et al. Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J Sleep Res. 2000;9:89–96. doi: 10.1046/j.1365-2869.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A. 1996;93:14128–33. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parmentier R, Anaclet C, Guhennec C, et al. The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders. Biochem Pharmacol. 2007;73:1157–71. doi: 10.1016/j.bcp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- 35.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 36.Escartin C, Rouach N. Astroglial networking contributes to neurometabolic coupling. Front Neuroenergetics. 2013;5:4. doi: 10.3389/fnene.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Giaume C. Astroglial connexins as elements of sleep-wake cycle regulation and dysfunction. In: Parpura AV, editor. Pathological potential of neuroglia. Springer; 2014. [Google Scholar]
- 39.Beck P, Odle A, Wallace-Huitt T, Skinner RD, Garcia-Rill E. Modafinil increases arousal determined by P13 potential amplitude: an effect blocked by gap junction antagonists. Sleep. 2008;31:1647–54. doi: 10.1093/sleep/31.12.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–40. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willecke K, Eiberger J, Degen J, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 42.Lin JS, Dauvilliers Y, Arnulf I, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol Dis. 2008;30:74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Kopp C, Petit JM, Magistretti P, Borbely AA, Tobler I. Comparison of the effects of modafinil and sleep deprivation on sleep and cortical EEG spectra in mice. Neuropharmacology. 2002;43:110–8. doi: 10.1016/s0028-3908(02)00070-9. [DOI] [PubMed] [Google Scholar]
- 44.Rambert F, Pessonnier J, Duteil J. Modafinil-, amphetamine-and methylphenidate-induced hyperactivities in mice involve different mechanisms. Eur J Pharmacol. 1990;183:455–6. [Google Scholar]
- 45.Barrett TD, Hayes ES, Walker MJ. Lack of selectivity for ventricular and ischaemic tissue limits the antiarrhythmic actions of lidocaine, quinidine and flecainide against ischaemia-induced arrhythmias. Eur J Pharmacol. 1995;285:229–38. doi: 10.1016/0014-2999(95)00406-b. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama K, Hashimoto K. Antiarrhythmic effects of the class 1c antiarrhythmic drug, flecainide, on canine ventricular arrhythmia models. Jpn Heart J. 1989;30:487–95. doi: 10.1536/ihj.30.487. [DOI] [PubMed] [Google Scholar]
- 47.Heath BM, Cui Y, Worton S, et al. Translation of flecainide- and mexiletine-induced cardiac sodium channel inhibition and ventricular conduction slowing from nonclinical models to clinical. J Pharmacol Toxicol Methods. 2011;63:258–68. doi: 10.1016/j.vascn.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Somani P. Antiarrhythmic effects of flecainide. Clin Pharmacol Ther. 1980;27:464–70. doi: 10.1038/clpt.1980.65. [DOI] [PubMed] [Google Scholar]
- 49.Beracochea D, Cagnard B, Celerier A, le Merrer J, Peres M, Pierard C. First evidence of a delay-dependent working memory-enhancing effect of modafinil in mice. Neuroreport. 2001;12:375–8. doi: 10.1097/00001756-200102120-00038. [DOI] [PubMed] [Google Scholar]
- 50.Beracochea DJ, Jaffard R. Impairment of spontaneous alternation behavior in sequential test procedures following mammillary body lesions in mice: evidence for time-dependent interference-related memory deficits. Behav Neurosci. 1987;101:187–97. doi: 10.1037//0735-7044.101.2.187. [DOI] [PubMed] [Google Scholar]
- 51.Mignot E, Nishino S. Emerging therapies in narcolepsy-cataplexy. Sleep. 2005;28:754–63. doi: 10.1093/sleep/28.6.754. [DOI] [PubMed] [Google Scholar]
- 52.Fujiki N. Associated Professional Sleep Societies--SLEEP 2006 20th Anniversary Meeting. 17-22 June 2006, Salt Lake City, UT, USA. IDrugs. 2006;9:610–3. [PubMed] [Google Scholar]
- 53.Scammell TE, Willie JT, Guilleminault C, Siegel JM International Working Group on Rodent Models of Narcolepsy. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Guo RX, Anaclet C, Roberts JC, et al. Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox-/- mice. Br J Pharmacol. 2009;157:104–17. doi: 10.1111/j.1476-5381.2009.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willie JT, Renthal W, Chemelli RM, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–95. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Lopez R, Dauvilliers Y. Pharmacotherapy options for cataplexy. Expert Opin Pharmacother. 2013;14:895–903. doi: 10.1517/14656566.2013.783021. [DOI] [PubMed] [Google Scholar]
- 57.Robertson P, Jr., Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–37. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- 58.Conard GJ, Ober RE. Metabolism of flecainide. Am J Cardiol. 1984;53:41B–51B. doi: 10.1016/0002-9149(84)90501-0. [DOI] [PubMed] [Google Scholar]