Abstract
Findings from epidemiologic studies indicate that there are associations between breakfast consumption and a lower risk of type 2 diabetes mellitus (T2DM) and metabolic syndrome, prompting interest in the influence of breakfast on carbohydrate metabolism and indicators of T2DM risk. The objective of this review was to summarize the available evidence from randomized controlled trials assessing the impact of breakfast on variables related to carbohydrate metabolism and metabolic wellness. Consuming compared with skipping breakfast appeared to improve glucose and insulin responses throughout the day. Breakfast composition may also be important. Dietary patterns high in rapidly available carbohydrate were associated with elevated T2DM risk. Therefore, partial replacement of rapidly available carbohydrate with other dietary components, such as whole grains and cereal fibers, proteins, and unsaturated fatty acids (UFAs), at breakfast may be a useful strategy for producing favorable metabolic outcomes. Consumption of fermentable and viscous dietary fibers at breakfast lowers glycemia and insulinemia. Fermentable fibers likely act through enhancing insulin sensitivity later in the day, and viscous fibers have an acute effect to slow the rate of carbohydrate absorption. Partially substituting protein for rapidly available carbohydrate enhances satiety and diet-induced thermogenesis, and also favorably affects lipoprotein lipids and blood pressure. Partially substituting UFA for carbohydrate has been associated with improved insulin sensitivity, lipoprotein lipids, and blood pressure. Overall, the available evidence suggests that consuming breakfast foods high in whole grains and cereal fiber, while limiting rapidly available carbohydrate, is a promising strategy for metabolic health promotion.
Keywords: carbohydrate metabolism, dietary patterns, energy metabolism, breakfast, diabetes, metabolic syndrome
Introduction
Diabetes mellitus affects ∼9.3% of people (26 million) in the United States (1), and an additional 79 million people have prediabetes (2). Type 2 diabetes mellitus (T2DM)5, which accounts for 90–95% of all diagnosed cases of diabetes (3), is a multifaceted condition characterized by prolonged insulin resistance and eventual pancreatic β cell exhaustion, which is accelerated by the demand to produce compensatory hyperinsulinemia to overcome insulin resistance (4). Although there is a genetic contribution to its development (5), diabetes in the Western world is related to the twin pandemics of obesity and physical inactivity (6). Much focus has been placed on weight loss and regular physical activity for lowering T2DM risk (7–9). However, beyond energy content, the composition and timing of dietary intake may have important influences on metabolic health and risk factors for T2DM.
Although the breakfast meal is considered by some to be the most important meal of the day, its contributions to various facets of health and wellness are controversial. Epidemiologic investigation suggests that breakfast consumption, compared with breakfast skipping, is associated with a lower risk of T2DM and metabolic syndrome [reviewed in detail by Odegaard et al. (10)]. This paper summarizes the findings of a comprehensive literature review of randomized controlled trials that was conducted to assess the potential impact of breakfast consumption and breakfast type on metabolic health, with a focus on carbohydrate metabolism and T2DM risk factors. For a detailed methodology of the literature review, refer to the Supplemental Material. A flow diagram of the study selection process is depicted in Figure 1. The studies identified for glucose and insulin responses and the major outcomes of these studies are outlined in Table 1. The strength of the available evidence based on the Academy of Nutrition and Dietetics Evidence Analysis criteria (76) is summarized in Table 2.
FIGURE 1.
Flow diagram of the study selection process.
TABLE 1.
Summary of findings for the effect of breakfast on glucose and insulin responses1
| Breakfast consumption vs. skipping |
Protein level comparisons |
Fiber level comparisons |
Glycemic index comparisons |
Fat level comparisons |
|||||||||||
| Variable | Advantage of breakfast, n (refs) | Equivocal,n (refs) | Advantage of skipping, n (refs) | Advantage of higher protein,n (refs) | Equivocal,n (refs) | Advantage of lower protein,n (refs) | Advantage of higher fiber,n (refs) | Equivocal,n (refs) | Advantage of lower fiber,n (refs) | Advantage of lower glycemic index,n (refs) | Equivocal,n (refs) | Advantage of higher glycemic index,n (refs) | Advantage of higher fat,n (refs) | Equivocal,n (refs) | Advantage of lower fat,n (refs) |
| Acute effects | |||||||||||||||
| Glucose response studies | 3 (11–13) | 6 (14–18) | 1 (19)2 | 3 (20–22) | 1 (23) | 0 (NA) | 20 (24–43) | 10 (44–53) | 0 (NA) | 15 (20, 23, 29, 54–65) | 2 (53, 66) | 0 (NA) | 5 (46, 67–70) | 1 (71) | 1 (72) |
| Insulin response studies | 1 (11) | 6 (13–15, 17, 18, 73) | 0 (NA) | 2 (20, 21) | 2 (22, 23) | 0 (NA) | 13 (24–27, 29–31, 36–38, 41, 44, 46) | 7 (32, 33, 35, 39, 47, 49, 53) | 0 (NA) | 10 (20, 23, 29, 54, 56, 58, 62–65) | 5 (53, 57, 59, 61, 66) | 0 (NA) | 5 (46, 67–70) | 1 (71) | 1 (72) |
| Second meal and later effects | |||||||||||||||
| Glucose response studies | 4 (13–15, 73) | 1 (11) | 0 (NA) | 0 (NA) | 1 (20) | 0 (NA) | 4 (27, 28, 45, 74) | 3 (29, 30, 47) | 0 (NA) | 5 (54–56, 74, 75) | 3 (20, 29, 57) | 0 (NA) | 1 (68) | 1 (67) | 1 (69) |
| Insulin response studies | 2 (15, 73) | 3 (11, 13, 14)3 | 0 (NA) | 0 (NA) | 1 (20) | 0 (NA) | 1 (45) | 4 (29, 30, 47, 74) | 0 (NA) | 2 (74, 75) | 5 (53, 57, 59, 61, 66) | 0 (NA) | 0 (NA) | 3 (67–69) | 0 (NA) |
Glucose and insulin responses generally refer to 24-h glycemia or insulinemia, respectively, or a fixed period comprising morning and/or morning plus postlunch timeframes. NA, not applicable; refs, references.
Benefit of breakfast skipping observed in subjects with type 2 diabetes mellitus (and small sample size, n = 13).
TABLE 2.
Strength of available evidence for breakfast and glucose/insulin response1
| Breakfast consumption vs. skipping | Protein level comparisons | Fiber level comparisons | Glycemic index comparisons | Fat level comparisons | |
| Acute effects | |||||
| Quality | Fair | Fair | Good | Good | Fair |
| Consistency | Fair | Fair | Fair | Good | Fair |
| Quantity | Fair | Fair | Good | Good | Fair |
| Clinical impact | Fair | Fair | Fair | Good | Fair |
| Generalizability | Fair | Good | Good | Good | Good |
| Overall | Fair | Fair | Good | Good | Fair |
| Second and later meal effects | |||||
| Quality | Good | Limited | Fair | Good | Fair |
| Consistency | Good | Limited | Fair | Fair | Limited |
| Quantity | Limited | Limited | Limited | Limited | Limited |
| Clinical impact | Good | Limited | Fair | Fair | Limited |
| Generalizability | Good | Limited | Good | Good | Fair |
| Overall | Good | Limited | Fair | Fair-Good | Limited |
Based on the Academy of Nutrition and Dietetics Evidence Analysis Manual (76).
Breakfast Consumption Compared with Breakfast Skipping
Breakfast skipping is associated with weight gain and other adverse outcomes (10, 77), including increased risk of T2DM and metabolic syndrome (78–86). The relation observed between breakfast skipping and increased T2DM and metabolic syndrome risk may relate to a prolonged elevation in FFAs throughout the morning hours in breakfast skippers, resulting in reduced insulin sensitivity later in the day (11, 12). A second hypothesis is that there may be differences in the ability to handle carbohydrate loads in the morning compared with later in the day because of 1) differences in sympathetic nervous system activity, or 2) diurnal patterns in the release of incretin hormones (e.g., glucagon-like peptide 1 and gastric inhibitory polypeptide) in response to a meal (87–90).
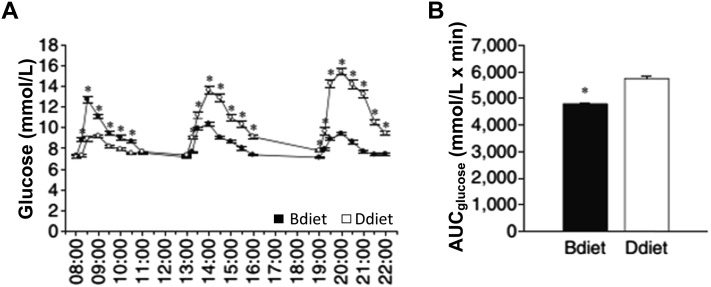
The same foods, distributed differently throughout the day, appear to have differential effects on glycemic control in subjects with T2DM. In a randomized crossover trial conducted by Jakubowicz et al. (87), a meal pattern that included a high-energy breakfast plus a low-energy dinner (breakfast: 2946 kJ, lunch: 2523 kJ, and dinner: 858 kJ) significantly reduced postprandial hyperglycemia over the course of the day compared with a meal pattern with a low-energy breakfast plus a high-energy dinner (breakfast: 858 kJ, lunch: 2523 kJ, and dinner: 2946 kJ) in subjects with diabetes (Figure 2A and B) (87). These results are consistent with those from other studies showing diurnal variations in carbohydrate metabolism, with greater insulin sensitivity in the morning compared with mid-day or evening (88–90).
FIGURE 2.
Differential effects of high-energy breakfast compared with high-energy dinner on blood glucose over the course of the day in individuals with type 2 diabetes mellitus. Glucose (A) and AUC for glucose (B). Bdiet—breakfast: 2946 kJ; lunch: 2523 kJ; dinner: 858 kJ. Ddiet—breakfast: 858 kJ; lunch: 2523 kJ; dinner: 2946 kJ. Values are means ± SEMs, n = 18; *P < 0.05. Bdiet, high-energy breakfast and reduced-energy dinner; Ddiet, higher-energy dinner and reduced-energy breakfast. Adapted from reference 87 with permission.
Breakfast Composition
In addition to breakfast size and skipping, the composition of the breakfast meal is an important consideration with regard to carbohydrate metabolism and metabolic health. For example, a breakfast higher in protein or unsaturated fatty acids (UFAs) may lower glucose and insulin responses by displacing glucose-generating carbohydrate from the meal. In addition, breakfast is a meal that often contributes significantly to the daily consumption of dietary fiber (91–94). Various types of dietary fiber may confer health benefits via effects on colonic fermentation, through displacement of rapidly available carbohydrate, and by slowing intestinal carbohydrate absorption (95). The subsequent sections will consider the effects of breakfast composition on glycemia, insulinemia, and other indicators of glucose homeostasis throughout the day.
Rapidly Available Carbohydrate at Breakfast
It is becoming increasingly clear that dietary patterns high in rapidly available carbohydrate are associated with an increased risk of developing T2DM (96–98). In contrast, greater consumption of both cereal fibers and whole grains has been associated with a reduced risk of developing T2DM (86, 99–101). In a meta-analysis of 22 cohort studies conducted by Alhazmi et al. (102), consumption of total dietary carbohydrate in the highest compared with the lowest quintile was associated with a significant elevation in T2DM risk (RR: 1.11; 95% CI: 1.01, 1.22; P = 0.04). Carbohydrate type and the content of a meal are the principal determinants of the postprandial blood glucose response, and, consequently, the insulin secretion required to dispose of the absorbed glucose. Diets high in rapidly available carbohydrate typically have a high glycemic index (GI) and deliver a large glycemic load (GL), contributing to pancreatic β cell demand (103, 104). Dietary patterns with a high GI and/or GL have been associated with an increased risk of T2DM (104, 105). Methods by which the GL of a meal can be lowered include a reduction in rapidly available carbohydrate consumption through substitution with noncarbohydrate energy sources, such as UFAs and proteins; inclusion of viscous fibers to slow carbohydrate absorption; and/or use of low GI carbohydrate foods, which are often rich in nondigestible or partially digestible polysaccharides (fibers and resistant starches) (103). The 2015–2020 Dietary Guidelines for Americans recommend substitution with whole-grain foods for refined grains as part of a healthy eating pattern that also includes a variety of vegetables, whole fruits, low-fat or fat-free dairy foods, legumes, nuts, seeds, lean-protein foods, and nontropical oils (106). Healthful dietary patterns that have these characteristics have been associated with favorable health outcomes, including a reduced risk of type 2 diabetes (106).
Dietary Fiber at Breakfast
Dietary fiber may exert substantial benefits on carbohydrate metabolism through a variety of mechanisms. A prospective cohort study of 9702 men and 15,365 women aged 35–65 y showed that a higher cereal fiber intake was associated with a reduced risk of T2DM, independent of age, sex, and lifestyle risk factors (multivariate RR: 0.72; 95% CI: 0.56, 0.93 for the highest compared with the lowest quintile, P trend across quintiles = 0.02) (99). Regarding specific food sources of fiber, fruit fiber and vegetable fiber consumption have not been found to be significantly associated with a risk of diabetes (99). It should be emphasized that fruits and vegetables are important sources of essential nutrients that are underconsumed in the average US diet, and their consumption should be emphasized as part of a healthy dietary pattern (106). Nevertheless, the types of dietary fibers that show the most promise with regard to favorable effects on carbohydrate metabolism include those that create viscosity in the small intestine and those that are fermentable in the colon (e.g., certain cereal fibers such as those from oats, barley, and rye).
Consumption of some viscous fibers may slow gastric emptying and, in the small intestine, act as a barrier to access by digestive enzymes to starches, oligosaccharides, and disaccharides (107, 108). The viscous solution also acts as a physical barrier that slows the rate at which glucose molecules reach the intestinal brush border for absorption (109). The net result is that the ingestion of a sufficient quantity of viscous fiber with a meal will slow the rate of glucose absorption, thus lowering the GI of the meal and reducing the insulin response required to dispose of the absorbed glucose (25, 26, 105, 109, 110). Fibers with high viscosity include guar gum, pectin, psyllium, and β-glucan. In a randomized crossover study conducted by Kim et al. (44), obese women with an elevated risk of insulin resistance were given 5 breakfast cereal test meals containing wheat and/or barley to provide various levels of β-glucan. Consumption of 10 g β-glucan at breakfast significantly reduced peak postprandial glucose response compared with 0, 2.5, and 5 g doses, and increasing amounts of β-glucan reduced the postprandial insulin AUC in a dose-dependent manner (44). In a chronic feeding intervention (12 wk), men and women with elevated blood pressure who were randomly assigned to consume foods containing oat β-glucan demonstrated greater reductions from baseline in mean peak insulin concentration and postprandial incremental insulin AUC compared with the control group (111). Moreover, although the β-glucan intervention acutely lowered the postprandial incremental insulin AUC, further reduction was observed after the 12-wk intervention, suggesting that the effects of oat β-glucan were enhanced after chronic consumption. The potentiation of the acute effect of β-glucan to reduce postprandial insulinemia may be secondary to enhanced insulin sensitivity resulting from colonic fermentation (111–113).
Inclusion of fermentable fibers at breakfast may mitigate T2DM risk through the production of SCFAs that act on specific receptors (G protein–coupled receptor 43/free fatty acid receptor 2) in adipose tissue to lower the release of FFAs from adipose depots (114). In healthy subjects, experimentally increasing (with a lipid infusion) or reducing (with the drug acipimox) FFAs for several hours will reduce or increase insulin sensitivity, respectively (115, 116). Feeding studies in healthy humans consuming fermentable resistant starch have demonstrated enhanced insulin sensitivity in as little as 24 h (117), which appears to be maintained after longer periods of intake (118, 119).
Consuming breakfast, compared with skipping breakfast, appears to reduce postprandial glycemia without affecting insulinemia after a standard lunch meal (13–15, 73). Breakfast skipping is associated with a prolongation of the elevated concentration of FFAs observed during fasting. Thus, breakfast skippers have elevated concentrations of FFAs throughout the morning hours compared with breakfast consumers, and this is associated with relative insulin resistance, which manifests as an elevated glycemic response to a lunch meal.
The “second meal effect” refers to the ability of one meal to alter the glucose and/or insulin responses to carbohydrates consumed at the following meal. With regard to breakfast type, low-GI and high-fermentable–fiber breakfasts appear to provide the greatest potential for reducing glycemic and/or insulinemic responses after a lunch meal (27, 28, 45, 54–56, 74, 75). The second meal effect may be driven, at least in part, by the effects of consuming fermentable carbohydrates on FFA concentrations after a breakfast meal. Consumption of fermentable fibers at breakfast reduces FFA concentrations throughout the morning and produces relative insulin sensitivity, resulting in lower glycemia and/or insulinemia after a standard lunch meal (27, 28, 45, 56). It should be noted that one challenge of summarizing the available literature is that a wide range of dietary fiber types has been used across breakfast intervention studies, each having unique physical/chemical properties and thus potentially producing different metabolic effects.
Protein at Breakfast
Protein at breakfast may induce beneficial metabolic effects through several mechanisms, including the displacement of rapidly available carbohydrate and high GI/GL foods from the meal, increased satiety, and greater diet-induced thermogenesis compared with carbohydrates (120). Dietary protein, when partially substituted for rapidly available carbohydrate, has been demonstrated to produce favorable changes to blood lipids and blood pressure in controlled feeding trials in humans (121, 122). In a recent randomized controlled crossover trial of overweight premenopausal women conducted by Rains et al. (123), postprandial glucose and insulin excursions were lower, and visual analog scale ratings of fullness (satiety) were higher after the provision of breakfast meals that included 30 or 39 g of dietary protein than with a low-protein (3 g), higher-carbohydrate meal. Protein displaced carbohydrates in the test meals, markedly lowering glucose and insulin responses. Longer-term substitution of 10% of dietary carbohydrates with protein has also been shown to lower circulating concentrations of TGs, as well as blood pressure (121). Many dairy products have a high protein content, and data from observational studies show an inverse association between dairy intake and the risk of metabolic syndrome and T2DM (124–126). However, in a study completed by our group, increasing dairy product consumption had a neutral effect on determinants of glucose tolerance (insulin sensitivity index and pancreatic β cell function) relative to subjects’ habitual diets in subjects selected for having an elevated risk of the development of T2DM (127). An additional study by Turner et al. (128) showed lower insulin sensitivity after 4 wk of consuming a dairy diet than with a low-dairy control diet in women, but not in men. Thus, additional research is needed to assess whether dairy product consumption has advantages over other protein sources as a substitution for rapidly available sources of dietary carbohydrate.
Fats at Breakfast
Very little information is available regarding the role of dietary fats when consumed as part of the breakfast meal on carbohydrate metabolism. The majority of available randomized controlled trials, however, have demonstrated a favorable effect on the postprandial glucose response with higher- compared with lower-fat–containing breakfast meals (46, 67–69, 72). Findings from a meta-analysis of cohort studies (103) showed that increased vegetable fat consumption was associated with a significantly decreased risk of T2DM. Moreover, controlled feeding studies have shown that partial substitution of rapidly available carbohydrate with UFAs in the diet is associated with enhanced insulin sensitivity, as well as favorable changes in lipids and blood pressure (121, 122). The Mediterranean dietary pattern, characteristically rich in UFAs, has been associated with a decreased T2DM risk in both controlled feeding studies and observational investigations (129–131).
Conclusions
Epidemiologic research has shown associations between frequent breakfast consumption and a decreased risk of T2DM and metabolic syndrome compared with breakfast skipping or infrequent breakfast consumption (10). These associations have prompted interest in the assessment of the effects of breakfast consumption, as well as breakfast composition, on metabolic outcomes in randomized controlled trials. After a thorough review of the available literature, we concluded that breakfast consumption compared with breakfast skipping has favorable effects on indexes of carbohydrate metabolism and is associated with a lower T2DM risk. Results from a number of studies suggest that insulin sensitivity is higher in the morning than in the afternoon or evening, suggesting that consumption of carbohydrates at breakfast may produce lower demand on the pancreatic β cells than the same quantity and quality of carbohydrates consumed at other times of the day.
Excessive consumption of rapidly available carbohydrate may have adverse effects on carbohydrate homeostasis and T2DM risk. Potential options for partial replacement of rapidly available carbohydrate include slowly digested carbohydrates, dietary fibers, fats, and proteins. At this time, replacement of rapidly available carbohydrate with low-GI types of carbohydrates (especially whole grains rich in viscous and cereal fibers) at breakfast has the most evidence to support use as a dietary strategy for improving metabolic wellness. Displacement of rapidly available carbohydrate with UFAs and proteins also shows promise as a way to improve the metabolic profile.
It is not yet clear to what degree breakfast consumption per se is responsible for the observed relations between eating breakfast and favorable metabolic outcomes compared with other factors that may also influence metabolic risk, such as the effects of high-cereal-fiber foods consumed at breakfast. Additional research is needed to clarify these issues and to assess the impact of the types and quantities of fats and proteins consumed at breakfast. Finally, future investigation should provide greater insights on possible effect modification by variables such as sex, health status (e.g., insulin sensitivity and glucose tolerance), and behaviors (e.g., exercise/physical activity) on the relation between breakfast consumption, breakfast composition, and metabolic outcomes.
Acknowledgments
We thank Sylvia Poulos, Alexandra Palmisano, Mary Dicklin, Orsolya Palacios, and Theresa Tardi for their technical support. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: GI, glycemic index; GL, glycemic load; T2DM, type 2 diabetes mellitus; UFA, unsaturated fatty acid.
References
- 1.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 2009;13:1–7. [PubMed] [Google Scholar]
- 2. American Diabetes Association [Internet] [cited 2015 Jun 15]. Available from: http://www.diabetes.org/diabetes-basics/type-2/?loc=util-header_type2
- 3.Centers for Disease Control [Internet] [cited 2015 Jun 15]. Available from: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
- 4.Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groop L, Lyssenko V. Genes and type 2 diabetes mellitus. Curr Diab Rep 2008;8:192–7. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Skerrett P, Greenland P, VanItallie TB. The escalating pandemics of obesity and sedentary lifestyle: a call to action for clinicians. Arch Intern Med 2004;164:249–58. [DOI] [PubMed] [Google Scholar]
- 7.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9. [DOI] [PubMed] [Google Scholar]
- 9.Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, Tominaga M, Kawazu S, Sato Y, Usui T, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard AO, Jacobs DR Jr, Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care 2013;36:3100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr 2011;141:1381–9. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, Tokuyama K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes Res Clin Pract 2014;8:e201–98. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009;32:1199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MJ, Jovanovic A, Taylor R. Utilizing the second-meal effect in type 2 diabetes: practical use of a soya-yogurt snack. Diabetes Care 2010;33:2552–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovanovic A, Leverton E, Solanky B, Ravikumar B, Snaar JE, Morris PG, Taylor R. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin Sci 2009;117:119–27. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen A, Marckmann P, Sandstrom B. Postprandial lipoprotein, glucose and insulin responses after two consecutive meals containing rapeseed oil, sunflower oil or palm oil with or without glucose at the first meal. Br J Nutr 1999;82:97–104. [PubMed] [Google Scholar]
- 17.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005;81:388–96. [DOI] [PubMed] [Google Scholar]
- 18.Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 2014;100:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkner T, Nielsen JK, Sandahl TD, Bibby BM, Jensen BS, Christiansen JS. Do all patients with type 2 diabetes need breakfast? Eur J Clin Nutr 2011;65:761–3. [DOI] [PubMed] [Google Scholar]
- 20.König D, Muser K, Berg A, Deibert P. Fuel selection and appetite-regulating hormones after intake of a soy protein-based meal replacement. Nutrition 2012;28:35–9. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012;77:323–31. [DOI] [PubMed] [Google Scholar]
- 22.Boelsma E, Brink EJ, Stafleu A, Hendriks HF. Measures of postprandial wellness after single intake of two protein-carbohydrate meals. Appetite 2010;54:456–64. [DOI] [PubMed] [Google Scholar]
- 23.Makris AP, Borradaile KE, Oliver TL, Cassim NG, Rosenbaum DL, Boden GH, Homko CJ, Foster GD. The individual and combined effects of glycemic index and protein on glycemic response, hunger, and energy intake. Obesity (Silver Spring) 2011;19:2365–73. [DOI] [PubMed] [Google Scholar]
- 24.Maki KC, Reeves MS, Carson ML, Miller MP, Turowski M, Rains TM, Anderson K, Papanikolaou Y, Wilder DM. Dose-response characteristics of high-viscosity hydroxypropylmethylcellulose in subjects at risk for the development of type 2 diabetes mellitus. Diabetes Technol Ther 2009;11:119–25. [DOI] [PubMed] [Google Scholar]
- 25.Maki KC, Carson ML, Miller MP, Turowski M, Bell M, Wilder DM, Rains TM, Reeves MS. Hydroxypropylmethylcellulose and methylcellulose consumption reduce postprandial insulinemia in overweight and obese men and women. J Nutr 2008;138:292–6. [DOI] [PubMed] [Google Scholar]
- 26.Maki KC, Carson ML, Miller MP, Turowski M, Bell M, Wilder DM, Reeves MS. High-viscosity hydroxypropylmethylcellulose blunts postprandial glucose and insulin responses. Diabetes Care 2007;30:1039–43. [DOI] [PubMed] [Google Scholar]
- 27.Pastors JG, Blaisdell PW, Balm TK, Asplin CM, Pohl SL. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr 1991;53:1431–5. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson AC, Ostman EM, Granfeldt Y, Bjorck IM. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr 2008;87:645–54. [DOI] [PubMed] [Google Scholar]
- 29.Clark CA, Gardiner J, McBurney MI, Anderson S, Weatherspoon LJ, Henry DN, Hord NG. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr 2006;60:1122–9. [DOI] [PubMed] [Google Scholar]
- 30.Nazare JA, Sauvinet V, Normand S, Guerin-Deremaux L, Gabert L, Desage M, Wils D, Laville M. Impact of a resistant dextrin with a prolonged oxidation pattern on day-long ghrelin profile. J Am Coll Nutr 2011;30:63–72. [DOI] [PubMed] [Google Scholar]
- 31.Casiraghi MC, Garsetti M, Testolin G, Brighenti F. Post-prandial responses to cereal products enriched with barley beta-glucan. J Am Coll Nutr 2006;25:313–20. [DOI] [PubMed] [Google Scholar]
- 32.Barone Lumaga R, Azzali D, Fogliano V, Scalfi L, Vitaglione P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a beta-glucan-enriched beverage. Food Funct 2012;3:67–75. [DOI] [PubMed] [Google Scholar]
- 33.Vitaglione P, Lumaga RB, Stanzione A, Scalfi L, Fogliano V. beta-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite 2009;53:338–44. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins AL, Jenkins DJ, Zdravkovic U, Wursch P, Vuksan V. Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr 2002;56:622–8. [DOI] [PubMed] [Google Scholar]
- 35.Lu ZX, Gibson PR, Muir JG, Fielding M, O’Dea K. Arabinoxylan fiber from a by-product of wheat flour processing behaves physiologically like a soluble, fermentable fiber in the large bowel of rats. J Nutr 2000;130:1984–90. [DOI] [PubMed] [Google Scholar]
- 36.Fairchild RM, Ellis PR, Byrne AJ, Luzio SD, Mir MA. A new breakfast cereal containing guar gum reduces postprandial plasma glucose and insulin concentrations in normal-weight human subjects. Br J Nutr 1996;76:63–73. [DOI] [PubMed] [Google Scholar]
- 37.Gatenby SJ, Ellis PR, Morgan LM, Judd PA. Effect of partially depolymerized guar gum on acute metabolic variables in patients with non-insulin-dependent diabetes. Diabet Med 1996;13:358–64. [DOI] [PubMed] [Google Scholar]
- 38.Golay A, Koellreutter B, Bloise D, Assal JP, Wursch P. The effect of muesli or cornflakes at breakfast on carbohydrate metabolism in type 2 diabetic patients. Diabetes Res Clin Pract 1992;15:135–41. [DOI] [PubMed] [Google Scholar]
- 39.Behme MT, Dupre J. All bran vs corn flakes: plasma glucose and insulin responses in young females. Am J Clin Nutr 1989;50:1240–3. [DOI] [PubMed] [Google Scholar]
- 40.Asp NG, Agardh CD, Ahren B, Dencker I, Johansson CG, Lundquist I, Nyman M, Sartor G, Schersten B. Dietary fibre in type II diabetes. Acta Med Scand Suppl 1981;656:47–50. [DOI] [PubMed] [Google Scholar]
- 41.Würsch P, Acheson K, Koellreutter B, Jequier E. Metabolic effects of instant bean and potato over 6 hours. Am J Clin Nutr 1988;48:1418–23. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins AL, Kacinik V, Lyon M, Wolever TM. Effect of adding the novel fiber, PGX(R), to commonly consumed foods on glycemic response, glycemic index and GRIP: a simple and effective strategy for reducing post prandial blood glucose levels–a randomized, controlled trial. Nutr J 2010;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H, Jenkins AL. Effect of guar crispbread with cereal products and leguminous seeds on blood glucose concentrations of diabetics. BMJ 1980;281:1248–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H, Stote KS, Behall KM, Spears K, Vinyard B, Conway JM. Glucose and insulin responses to whole grain breakfasts varying in soluble fiber, beta-glucan: a dose response study in obese women with increased risk for insulin resistance. Eur J Nutr 2009;48:170–5. [DOI] [PubMed] [Google Scholar]
- 45.Trinick TR, Laker MF, Johnston DG, Keir M, Buchanan KD, Alberti KG. Effect of guar on second-meal glucose tolerance in normal man. Clin Sci 1986;71:49–55. [DOI] [PubMed] [Google Scholar]
- 46.Burton-Freeman B, Davis PA, Schneeman BO. Plasma cholecystokinin is associated with subjective measures of satiety in women. Am J Clin Nutr 2002;76:659–67. [DOI] [PubMed] [Google Scholar]
- 47.Shaheen SM, Fleming SE. High-fiber foods at breakfast: influence on plasma glucose and insulin responses to lunch. Am J Clin Nutr 1987;46:804–11. [DOI] [PubMed] [Google Scholar]
- 48.Hlebowicz J, Wickenberg J, Fahlstrom R, Bjorgell O, Almer LO, Darwiche G. Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized blinded crossover trial. Nutr J 2007;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willis HJ, Thomas W, Eldridge AL, Harkness L, Green H, Slavin JL. Glucose and insulin do not decrease in a dose-dependent manner after increasing doses of mixed fibers that are consumed in muffins for breakfast. Nutr Res 2011;31:42–7. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins DJ, Kendall CW, Augustin LS, Martini MC, Axelsen M, Faulkner D, Vidgen E, Parker T, Lau H, Connelly PW, et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002;25:1522–8. [DOI] [PubMed] [Google Scholar]
- 51.Bell LP, Hectorn KJ, Reynolds H, Hunninghake DB. Cholesterol-lowering effects of soluble-fiber cereals as part of a prudent diet for patients with mild to moderate hypercholesterolemia. Am J Clin Nutr 1990;52:1020–6. [DOI] [PubMed] [Google Scholar]
- 52.Wolever TM, Tosh SM, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Thomson BA, Duss R, Wood PJ. Physicochemical properties of oat beta-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr 2010;92:723–32. [DOI] [PubMed] [Google Scholar]
- 53.Adamsson V, Reumark A, Marklund M, Larsson A, Riserus U. Role of a prudent breakfast in improving cardiometabolic risk factors in subjects with hypercholesterolemia: a randomized controlled trial. Clin Nutr 2015;34:20–6. [DOI] [PubMed] [Google Scholar]
- 54.Arai H, Mizuno A, Sakuma M, Fukaya M, Matsuo K, Muto K, Sasaki H, Matsuura M, Okumura H, Yamamoto H, et al. Effects of a palatinose-based liquid diet (Inslow) on glycemic control and the second-meal effect in healthy men. Metabolism 2007;56:115–21. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA, Bennett CM, Goff DV, Sarson DL, Bloom SR. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr 1982;35:1339–46. [DOI] [PubMed] [Google Scholar]
- 56.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr 2006;83:817–22. [DOI] [PubMed] [Google Scholar]
- 57.Kabir M, Oppert JM, Vidal H, Bruzzo F, Fiquet C, Wursch P, Slama G, Rizkalla SW. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002;51:819–26. [DOI] [PubMed] [Google Scholar]
- 58.Chandler-Laney PC, Morrison SA, Goree LL, Ellis AC, Casazza K, Desmond R, Gower BA. Return of hunger following a relatively high carbohydrate breakfast is associated with earlier recorded glucose peak and nadir. Appetite 2014;80:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pal S, Lim S, Egger G. The effect of a low glycaemic index breakfast on blood glucose, insulin, lipid profiles, blood pressure, body weight, body composition and satiety in obese and overweight individuals: a pilot study. J Am Coll Nutr 2008;27:387–93. [DOI] [PubMed] [Google Scholar]
- 60.Wu H, Xia FZ, Xu H, Zhai HL, Zhang MF, Zhang HX, Li YX, Li Y, Gu T, Ma LM, et al. Acute effects of different glycemic index diets on serum motilin, orexin and neuropeptide Y concentrations in healthy individuals. Neuropeptides 2012;46:113–8. [DOI] [PubMed] [Google Scholar]
- 61.Cohen C, Wylie-Rosett J, Shamoon H. Insulin response and glycemic effects of meals in non-insulin-dependent diabetes. Am J Clin Nutr 1990;52:519–23. [DOI] [PubMed] [Google Scholar]
- 62.Tsihlias EB, Gibbs AL, McBurney MI, Wolever TM. Comparison of high- and low-glycemic-index breakfast cereals with monounsaturated fat in the long-term dietary management of type 2 diabetes. Am J Clin Nutr 2000;72:439–49. [DOI] [PubMed] [Google Scholar]
- 63.Díaz EO, Galgani JE, Aguirre CA, Atwater IJ, Burrows R. Effect of glycemic index on whole-body substrate oxidation in obese women. Int J Obes (Lond) 2005;29:108–14. [DOI] [PubMed] [Google Scholar]
- 64.Stenvers DJ, Schouten LJ, Jurgens J, Endert E, Kalsbeek A, Fliers E, Bisschop PH. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 2014;112:504–12. [DOI] [PubMed] [Google Scholar]
- 65.Wolever TM, Bentum-Williams A, Jenkins DJ. Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care 1995;18:962–70. [DOI] [PubMed] [Google Scholar]
- 66.Pasman WJ, Blokdijk VM, Bertina FM, Hopman WP, Hendriks HF. Effect of two breakfasts, different in carbohydrate composition, on hunger and satiety and mood in healthy men. Int J Obes Relat Metab Disord 2003;27:663–8. [DOI] [PubMed] [Google Scholar]
- 67.Ercan N, Gannon MC, Nuttall FQ. Effect of added fat on the plasma glucose and insulin response to ingested potato given in various combinations as two meals in normal individuals. Diabetes Care 1994;17:1453–9. [DOI] [PubMed] [Google Scholar]
- 68.Reis CE, Ribeiro DN, Costa NM, Bressan J, Alfenas RC, Mattes RD. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: a randomised cross-over clinical trial. Br J Nutr 2013;109:2015–23. [DOI] [PubMed] [Google Scholar]
- 69.Collier GR, Wolever TM, Jenkins DJ. Concurrent ingestion of fat and reduction in starch content impairs carbohydrate tolerance to subsequent meals. Am J Clin Nutr 1987;45:963–9. [DOI] [PubMed] [Google Scholar]
- 70.Frape DL, Williams NR, Scriven AJ, Palmer CR, O’Sullivan K, Fletcher RJ. Diurnal trends in responses of blood plasma concentrations of glucose, insulin, and C-peptide following high- and low-fat meals and their relation to fat metabolism in healthy middle-aged volunteers. Br J Nutr 1997;77:523–35. [DOI] [PubMed] [Google Scholar]
- 71.Khoury DE, Hwalla N, Frochot V, Lacorte JM, Chabert M, Kalopissis AD. Postprandial metabolic and hormonal responses of obese dyslipidemic subjects with metabolic syndrome to test meals, rich in carbohydrate, fat or protein. Atherosclerosis 2010;210:307–13. [DOI] [PubMed] [Google Scholar]
- 72.Frape DL, Williams NR, Rajput-Williams J, Maitland BW, Scriven AJ, Palmer CR, Fletcher RJ. Effect of breakfast fat content on glucose tolerance and risk factors of atherosclerosis and thrombosis. Br J Nutr 1998;80:323–31. [DOI] [PubMed] [Google Scholar]
- 73.Lee SH, Tura A, Mari A, Ko SH, Kwon HS, Song KH, Yoon KH, Lee KW, Ahn YB. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am J Physiol Endocrinol Metab 2011;301:E984–90. [DOI] [PubMed] [Google Scholar]
- 74.Liljeberg HG, Akerberg AK, Bjorck IM. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr 1999;69:647–55. [DOI] [PubMed] [Google Scholar]
- 75.Liljeberg H, Bjorck I. Effects of a low-glycaemic index spaghetti meal on glucose tolerance and lipaemia at a subsequent meal in healthy subjects. Eur J Clin Nutr 2000;54:24–8. [DOI] [PubMed] [Google Scholar]
- 76.The Academy of Nutrition and Dietetics Evidence Analysis Manual [Internet] [cited 2015 Nov 19]. Available from: https://www.andeal.org/files/Docs/2012_Jan_EA_Manual.pdf.
- 77.Haines PS, Guilkey DK, Popkin BM. Trends in breakfast consumption of US adults between 1965 and 1991. J Am Diet Assoc 1996;96:464–70. [DOI] [PubMed] [Google Scholar]
- 78.Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 2012;95:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mekary RA, Giovannucci E, Cahill L, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in older women: breakfast consumption and eating frequency. Am J Clin Nutr 2013;98:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica (Cairo) 2014;2014:253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shafiee G, Kelishadi R, Qorbani M, Motlagh ME, Taheri M, Ardalan G, Taslimi M, Poursafa P, Heshmat R, Larijani B. Association of breakfast intake with cardiometabolic risk factors. J Pediatr (Rio J) 2013;89:575–82. [DOI] [PubMed] [Google Scholar]
- 82.Trivedi T, Liu J, Probst JC, Martin AB. The metabolic syndrome: are rural residents at increased risk? J Rural Health 2013;29:188–97. [DOI] [PubMed] [Google Scholar]
- 83.Yoo KB, Suh HJ, Lee M, Kim JH, Kwon JA, Park EC. Breakfast eating patterns and the metabolic syndrome: the Korea National Health and Nutrition Examination Survey (KNHANES) 2007–2009. Asia Pac J Clin Nutr 2014;23:128–37. [DOI] [PubMed] [Google Scholar]
- 84.di Giuseppe R, Di Castelnuovo A, Melegari C, De Lucia F, Santimone I, Sciarretta A, Barisciano P, Persichillo M, De Curtis A, Zito F, et al. Typical breakfast food consumption and risk factors for cardiovascular disease in a large sample of Italian adults. Nutr Metab Cardiovasc Dis 2012;22:347–54. [DOI] [PubMed] [Google Scholar]
- 85.Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int 2009;26:544–59. [DOI] [PubMed] [Google Scholar]
- 86.Kochar J, Djousse L, Gaziano JM. Breakfast cereals and risk of type 2 diabetes in the Physicians’ Health Study I. Obesity (Silver Spring) 2007;15:3039–44. [DOI] [PubMed] [Google Scholar]
- 87.Jakubowicz D, Wainstein J, Ahren B, Bar-Dayan Y, Landau Z, Rabinovitz HR, Froy O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia 2015;58:912–9. [DOI] [PubMed] [Google Scholar]
- 88.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, et al. Durnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgan LM, Aspostolakou R, Wright J, Gama R. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: a possible link? Ann Clin Biochem 1999;36:447–50. [DOI] [PubMed] [Google Scholar]
- 90.Yoshino J, Almeda-Valdes P, Patterson BW, Okunade AL, Imai S, Mittendorfer B, Klein S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolsim in metabolically normal women. J Clin Endocrinol Metab 2014;99:E1666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Neil CE, Nicklas TA, Fulgoni VL 3rd. Nutrient intake, diet quality, and weight/adiposity parameters in breakfast patterns compared with no breakfast in adults: National Health and Nutrition Examination Survey 2001–2008. J Acad Nutr Diet 2014;114:S27–43. [DOI] [PubMed] [Google Scholar]
- 92.Barr SI, DiFrancesco L, Fulgoni VL 3rd. Breakfast consumption is positively associated with nutrient adequacy in Canadian children and adolescents. Br J Nutr 2014;112:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barr SI, DiFrancesco L, Fulgoni VL 3rd. Consumption of breakfast and the type of breakfast consumed are positively associated with nutrient intakes and adequacy of Canadian adults. J Nutr 2013;143:86–92. [DOI] [PubMed] [Google Scholar]
- 94.Huang YL, Hoerr SL, Song WO. Breakfast is the lowest fat meal for young adult women. J Nutr Educ 1997;29:184–8. [Google Scholar]
- 95.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5:1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- 97.Gross LS, Li L, Ford ES, Liu L. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 2004;79:774–9. [DOI] [PubMed] [Google Scholar]
- 98.McEvoy CT, Cardwell CR, Woodslide JV, Young IS, Hunter SJ, McKinley MC. A posteriori dietary patterns are related to risk of type 2 diabetes: findings from a systematic review and meta-analysis. J Acad Nutr Diet 2014;114:1759–75.e4. [DOI] [PubMed] [Google Scholar]
- 99.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956–65. [DOI] [PubMed] [Google Scholar]
- 100.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013;28:845–58. [DOI] [PubMed] [Google Scholar]
- 101.Parker ED, Liu S, Van Horn L, Tinker LF, Shikany JM, Eaton CB, Margolis KL. The association of whole grain consumption with incident type 2 diabetes: the Women’s Health Initiative Observational Study. Ann Epidemiol 2013;23:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alhazmi A, Stojanovski E, McEvoy M, Garg ML. Macronutrient intakes and development of type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr 2012;31:243–58. [DOI] [PubMed] [Google Scholar]
- 103.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 2002;76:274S–80S. [DOI] [PubMed] [Google Scholar]
- 104.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 105.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 106.US Department of Agriculture and US Department of Health and Human Services [Internet]. 2015–2020 Dietary guidelines for Americans. 8th Edition. December 2015. [cited 2016 Mar 10]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 107.Jenkins DJ, Kendall CW, Axelsen M, Augustin LS, Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol 2000;11:49–56. [DOI] [PubMed] [Google Scholar]
- 108.Leclère CJ, Champ M, Boillot J, Guille G, Lecannu G, Molis C, Bornet F, Krempf M, Delort-Laval J, Galmiche JP. Role of viscous guar gums in lowering the glycemic response after a solid meal. Am J Clin Nutr 1994;59:914–21. [DOI] [PubMed] [Google Scholar]
- 109.Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KG. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. BMJ 1978;1:1392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolever TM, Vuksan V, Eshuis H, Spadafora P, Peterson RD, Chao ES, Storey ML, Jenkins DJ. Effect of method of administration of psyllium on glycemic response and carbohydrate digestibility. J Am Coll Nutr 1991;10:364–71. [DOI] [PubMed] [Google Scholar]
- 111.Maki KC, Galant R, Samuel P, Tesser J, Witchger MS, Ribaya-Mercado JD, Blumberg JB, Geohas J. Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur J Clin Nutr 2007;61:786–95. [DOI] [PubMed] [Google Scholar]
- 112.Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact 2011;10:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol 2014;20:16079–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sleeth ML, Thompson EL, Ford HE, Zac-Varghese SE, Frost G. Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev 2010;23:135–45. [DOI] [PubMed] [Google Scholar]
- 115.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol 2010;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E1775–81. [DOI] [PubMed] [Google Scholar]
- 117.Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia 2003;46:659–65. [DOI] [PubMed] [Google Scholar]
- 118.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005;82:559–67. [DOI] [PubMed] [Google Scholar]
- 119.Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, Rains TM. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 2012;142:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu FB. Protein, body weight, and cardiovascular health. Am J Clin Nutr 2005;82:242S–7S. [DOI] [PubMed] [Google Scholar]
- 121.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 122.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER 3rd. The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care 2013;36:1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rains TM, Leidy HJ, Sanoshy KD, Lawless AL, Maki KC. A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. Nutr J 2015;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism 2014;63:618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turner KM, Keogh JB, Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies. Nutr Metab Cardiovasc Dis 2015;25:3–8. [DOI] [PubMed] [Google Scholar]
- 126.Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y, Li Q. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One 2013;8:e73965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maki KC, Nieman KM, Schild AL, Kaden VN, Lawless AL, Kelley KM, Rains TM. Sugar-sweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr 2015;145:459–66. [DOI] [PubMed] [Google Scholar]
- 128.Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr 2015;101:1173–9. [DOI] [PubMed] [Google Scholar]
- 129.Salas-Salvadó J, Bullo M, Babio N, Martinez-Gonzalez MA, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Aros F, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, Benito S, Tortosa A, Bes-Rastrollo M. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ 2008;336:1348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Esposito K, Maiorino MI, Di Palo C, Giugliano D, Campanian Postprandial Hyperglycemia Study Group. Adherence to a Mediterranean diet and glycaemic control in Type 2 diabetes mellitus. Diabet Med 2009;26:900–7. [DOI] [PubMed] [Google Scholar]