Abstract
The metabolic demand for methionine is great in neonates. Indeed, methionine is the only indispensable sulfur amino acid and is required not only for protein synthesis and growth but is also partitioned to a greater extent to transsulfuration for cysteine and taurine synthesis and to >50 transmethylation reactions that serve to methylate DNA and synthesize metabolites, including creatine and phosphatidylcholine. Therefore, the pediatric methionine requirement must accommodate the demands of rapid protein turnover as well as vast nonprotein demands. Because cysteine spares the methionine requirement, it is likely that the dietary provision of transmethylation products can also feasibly spare methionine. However, understanding the requirement of methionine is further complicated because demethylated methionine can be remethylated by the dietary methyl donors folate and betaine (derived from choline). Intakes of dietary methyl donors are highly variable, which is of particular concern for newborns. It has been demonstrated that many populations have enhanced requirements for these nutrients, and nutrient fortification may exacerbate this phenomenon by selecting phenotypes that increase methyl requirements. Moreover, higher transmethylation rates can limit methyl supply and affect other transmethylation reactions as well as protein synthesis. Therefore, careful investigations are needed to determine how remethylation and transmethylation contribute to the methionine requirement. The purpose of this review is to support our hypothesis that dietary methyl donors and consumers can drive methionine availability for protein synthesis and transmethylation reactions. We argue that nutritional strategies in neonates need to ensure that methionine is available to meet requirements for growth as well as for transmethylation products.
Keywords: infant nutrition, methyl donors, creatine, choline, betaine
Introduction
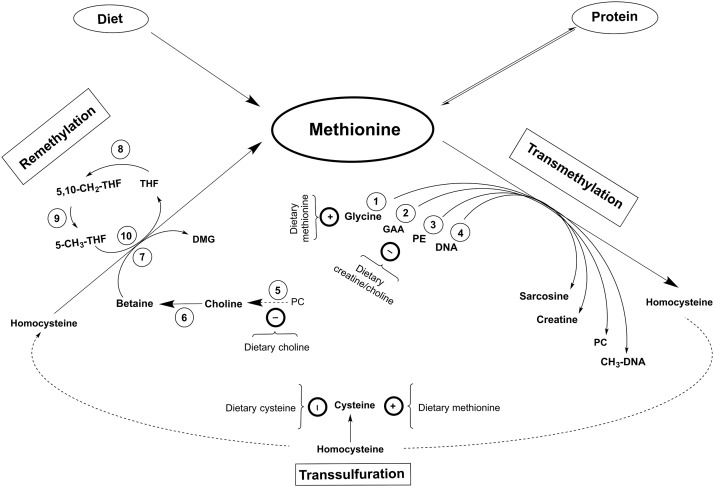
The dietary requirement of any amino acid must be adequate to fulfill the obligatory metabolic demand, which is a concept introduced by Millward (1). Briefly, the obligatory metabolic demand of a particular amino acid will fulfill growth demands, namely net protein synthesis, as well as nonprotein demands such as oxidation or the synthesis of other metabolic intermediates and end products such as nucleic acids, creatine, polyamines, phosphatidylcholine, glutathione, carnitine, and NO (1). Although the amino acid demands of protein synthesis are generally well described and relatively straightforward, there is a paucity of data on the nonprotein requirements of amino acids that are especially sensitive to intakes of the amino acid or its products/precursors (2–5). Because amino acids evolved primarily as substrates for protein incorporation, a neonate may exhibit normal growth at a particular amino acid intake at the expense of nonprotein requirements. Indeed, such a scenario would result in the transition of amino acid products from the status of dispensable, or conditionally indispensable, to indispensable. However, the obligatory metabolic demand for an amino acid is further complicated by the presence of amino acid products in the diet or, in some cases, metabolic precursors that support amino acid metabolism. It follows that the presence of amino acid products and precursors are integral to the amino acid requirement. Although the metabolism of each amino acid is unique, we draw attention to methionine because 1) it is indispensable and required for protein synthesis, 2) it has perhaps the greatest nonprotein requirement of all indispensable amino acids, and 3) methionine metabolism forms a cycle such that methionine can be reformed after it participates in a suite of transmethylation reactions that are critical during development (Figure 1).
FIGURE 1.
A summary of methionine partitioning. The most obvious site of methionine partitioning is into protein. It is expected that most dietary methionine is incorporated into proteins; however, methionine is also made available after protein breakdown, i.e., protein turnover. Methionine is partitioned into the methionine cycle upon irreversible adenosylation to SAM, which undergoes transmethylation. Most methyl groups are transferred to DNA and used to synthesize sarcosine, creatine, and PC during transmethylation, which is sensitive to intakes of transmethylation precursors/products as well as available methyl in the form of methionine. The common product of transmethylation is SAH, which is maintained in equilibrium with homocysteine. Homocysteine is either irreversibly oxidized toward cysteine during transsulfuration or methylated to reform methionine during remethylation. Transsulfuration is sensitive to dietary cysteine as well as available methionine, whereas remethylation represents an additional source of methionine for subsequent partitioning into protein or transmethylation. Circled numbers indicate the enzymes: 1) GNMT (EC 2.1.1.20); 2) GAMT (EC 2.1.1.2); 3) PEMT (EC 2.1.1.17); 4) DNMT (EC 2.1.1.204/37); 5) various phospholipases (mostly phospholipase D; EC 3.1.4.4); 6) both choline dehydrogenase (EC 1.2.1.8) and betaine aldehyde dehydrogenase (EC 1.1.3.17); 7) BHMT (EC 2.1.1.5); 8) SHMT (EC 2.1.2.1); 9) MTHFR (EC 1.5.1.15); and 10) MSyn (EC 2.1.1.13). BHMT, betaine homocysteine methyltransferase; DMG, dimethylglycine; DNMT, DNA methyltransferase; GAA, guanidinoacetic acid; GAMT, guanidinoacetate methyltransferase; GNMT, glycine N-methyltransferase; MSyn, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PEMT, phosphatidylethanolamine methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
The Methionine Cycle
Methionine is an indispensable sulfur-containing amino acid required for synthesizing protein as well as a number of other critical nutrients via the methionine cycle. The methionine cycle transfers the terminal methyl group from methionine to various methylated products (i.e., transmethylation) to form homocysteine, which can irreversibly transfer its sulfur atom for cysteine synthesis (i.e., transsulfuration). Therefore, a dietary methionine requirement must incorporate the demands of protein synthesis, transmethylation, and transsulfuration. In addition, a methionine requirement must also consider that methionine can be replenished by preformed dietary methyl groups that remethylate homocysteine (6). Indeed, intakes of methionine and preformed methyl groups affect the methionine cycle in adult humans (7), and rats can survive with homocysteine and preformed methyl groups in the absence of methionine (8–10). However, the extent to which dietary methyl groups contribute to the methionine requirement is unclear.
In the body, homocysteine is methylated to methionine by methyl groups that are either derived from choline, which acts via betaine, or by methylneogenesis and the formation of 5-methyltetrahydrofolate (folate). Therefore, the dietary supply of choline, betaine, and folate should govern the rate of homocysteine remethylation and affect the availability of methionine for partitioning into transmethylation and protein synthesis. These processes are critical during development, and thus it is important to determine how dietary methyl donors contribute to the methionine requirement of neonates, who have larger and more distinct demands of methionine metabolism relative to adults (Table 1). This review focuses on the metabolism of dietary methyl donors and rationalizes their contribution and importance to methionine partitioning in early life.
TABLE 1.
Requirements of methionine and related nutrients in human infants and adults
| Infants | Adults | Reference | |
| Methionine, mg ⋅ kg−1 ⋅ d−1 | 38 | 4.5 | 11, 12 |
| Choline,1 mg ⋅ kg−1 ⋅ d−1 | 62.5 | 7.8 men, 6.1 women | 13 |
| Creatine,1,2 mg ⋅ kg−1 ⋅ d−1 | 24.9 | 27.5 men, 20.5 women | 14, 15 |
| Cysteine + methionine, mg ⋅ kg−1 ⋅ d−1 | 80–88 | 12.6 | 16, 17 |
| Folate,1 μg/d | 20 | 5.7 | 13 |
| Betaine3 | — | — | |
| Protein, mg ⋅ kg−1 ⋅ d−1 | 8–11 | 3.5 | 18 |
Based on a 3.2-kg infant and 70-kg adult.
Based on creatine accretion rates.
Betaine accretion rates have not been reported.
Translational models have advanced our understanding of methionine metabolism considerably. Rodent and tissue culture studies have allowed for the identification of how individual tissues and enzymes contribute to the regulation of methionine metabolism, whereas the piglet represents a prime model for studying the clinical importance of methionine and its metabolic products in early life. Indeed, the piglet is an excellent model for the human infant with respect to amino acid requirements, digestive metabolism, and physiology (19). Furthermore, unlike rodents, nutrient requirements are well defined in pigs, which allows for strict control of intakes relative to requirements; a methionine requirement has been reported in the piglet that is sensitive to dietary cysteine (20) and total parenteral nutrition feeding (4). Moreover, because of their size at birth, piglets permit multiple catheterizations for extensive blood sampling and infusions for the measure of steady state kinetics. Existing kinetic models of methionine metabolism allow rates of remethylation, transmethylation, transsulfuration, and protein synthesis to be calculated in vivo (21, 22). Although these kinetic models are not without drawbacks, e.g., recycling and urinary excretion of label (23), they represent an unequivocal means of comparing methionine metabolism during various intakes of methionine and/or its precursors and products. Because of their rapid growth, piglets are an especially good model for studying early-life sulfur amino acid metabolism because metabolic changes can be observed in a relatively short period of time—without the long-term consequences that acute malnutrition has on human infants.
Current Status of Knowledge
Remethylation
Remethylation refers to the enzyme-mediated transfer of methyl groups to homocysteine and the subsequent appearance of methionine. Under normal dietary conditions in adults, it has been estimated that methionine proceeds through remethylation ∼1.5–2.0 times before being oxidized, but this estimation approximately doubled during methionine restriction (7). Indeed, remethylation is a major metabolic process that is regulated by methionine flux. For instance, remethylation in neonatal piglets contributed ∼20% of whole-body methionine flux regardless of whether dietary methionine and cysteine were provided (24). Similarly, children derive ∼25% of methionine flux from remethylation regardless of nutritional status (25). Therefore, although remethylation contributes greatly to methionine metabolism, it may be limited by the supply of dietary methionine; thus, the extent that remethylation contributes to the methionine requirement is not straightforward.
Remethylation has garnered considerable clinical interest because of the consequent removal of homocysteine. Indeed, an elevation of homocysteine concentrations in blood (i.e., hyperhomocysteinemia) is an independent risk factor of several chronic diseases (26); moreover, children that present with hyperhomocysteinemia are at a considerable risk of developing obesity and hypertension by adulthood (27). Although the sequelae of hyperhomocysteinemia are not within the scope of this review, a simple strategy to mitigate the risks associated with hyperhomocysteinemia is to eliminate homocysteine by remethylation. Therefore, supplements of the dietary methyl donors folate (28), betaine (29), and choline (30) have been administered to patients with hyperhomocysteinemia to try to increase the rate of homocysteine elimination. Although these supplements effectively lower homocysteine concentrations, trials have had mixed effectiveness at mitigating disease risk (28, 31). Nevertheless, the study of homocysteine reduction has furthered understanding of how dietary methyl donors contribute to remethylation.
Folate.
The generic term folate is used here to collectively describe the tetrahydrofolate (vitamin B-9) compounds that deliver a labile methyl group to homocysteine from the 1-carbon pool by methylneogenesis. Remethylation only represents a portion of folate biochemistry, and a broader review of folate metabolism is found elsewhere (32). Folate, as 5-methyltetrahydrofolate, provides a methyl group to homocysteine via the vitamin B-12-containing enzyme methionine synthase (MSyn)3 (EC 2.1.1.13), which is found in all tissues. The loss of a methyl group from 5-methyltetrahydrofolate yields tetrahydrofolate, which subsequently forms 5,10-methylenetetrahydrofolate upon gaining a hydroxymethyl group largely from serine by the vitamin B-6-dependent enzyme serine hydroxymethyltransferase (EC 2.1.2.1). To a lesser extent, 5,10-methylenetetrahydrofolate can also be formed from sarcosine (33, 34) by the riboflavin-dependent enzyme sarcosine dehydrogenase (EC 1.5.8.3). Finally, a methyl group becomes available for remethylation when 5,10-methylenetetrahydrofolate is irreversibly reduced to 5-methyltetrahydrofolate by methylenetetrahydrofolate reductase (MTHFR) (EC 1.5.1.15). Therefore, although serine is the predominant 1-carbon source for methylneogenesis (33), folate is required to deliver a labile methyl group to MSyn for remethylation.
The consumption of folate during development has long been a topical issue because of its association with neural tube defects, such as spina bifida and anencephaly. As a result, the CDC recommended that foodstuff fortification with folate become mandatory (35). In the United States and Canada, fortification was realized in 1998, after which cereal grain products were fortified with folic acid, a synthetic form of folate. Today, clinical folate deficiencies are rare among Canadians (36) and Americans (37) because folate intakes are considered to be adequate, yet highly variable (38), especially during pregnancy (39). Furthermore, it is expected that most neonates are folate-replete because of the folic acid fortification of infant formula (Table 2) and folic acid supplementation during pregnancy and periconception (39, 44). However, adequate folate status does not necessarily mean that remethylation demands are consistently fulfilled because it cannot be assumed that remethylation is an asymptotic function of folate intake. For example, Lamers et al. (45) demonstrated that homocysteine concentrations were unaffected by moderate vitamin B-6 restriction in adults, which should have affected methylneogenesis (via serine hydroxymethyltransferase) as well as the elimination of homocysteine by the conversion to cysteine (via cystathionine β-synthase). Furthermore, in an average adult, folate consumption provides ∼5–10 mmol methyl/d (46), which in a 70-kg person equates to ∼3–6 μmol/kg ⋅ h preformed methyl groups. That alone is inadequate to fulfill remethylation demands in pregnant women (17–27 μmol methionine/kg ⋅ h) (47), infants (19–26 μmol methionine/kg ⋅ h) (48), and piglets (10–49 μmol methionine/kg ⋅ h) (24). Whereas dietary folate is a source of labile methyl groups, remethylation by MSyn relies upon methylneogenesis, which involves no fewer than 4 vitamins and 5 enzymes.
TABLE 2.
Concentrations of methionine and related nutrients in breast milk and in bovine and soy-based infant formulas1
| Breast milk | Bovine formula | Soy-based formula | Reference | |
| Choline, μM | 116 ± 22 | 457 | 498 | 40 |
| Glycerophosphocholine, μM | 362 ± 70 | 499 | 68 | 40 |
| Phosphocholine, μM | 570 ± 136 | 120 | 27 | 40 |
| Phosphatidylcholine, μM | 82 ± 6 | 75 | 231 | 40 |
| Sphingomyelin, μM | 124 ± 9 | 82 | 24 | 40 |
| Betaine, μM | 26–52 | 34–55 | — | 41 |
| Creatine, μM | 60–70 | 114–334 | 11–13 | 14 |
| Guanidinoacetate, μM | 0.25 | 1.1–3.7 | 0.3–1.9 | 14 |
| Folate, nM | 365 ± 207 | 625 ± 128 | 1047 ± 183, 856 ± 372 | 42 |
| Methionine, mM | 1.0–1.4 | 2.0–2.3 | 1.3–2.9 | 43 |
| Cysteine, mM | 1.7–2.6 | 1.24–2.49 | 1.5–3.3 | 43 |
Choline and choline esters in breast milk are means ± SEMs (n = 33; 27–32 d postpartum). The listed values in bovine (n = 6) and soy-based formulas (n = 4) were interpreted from figures in the corresponding reference. Betaine is reported as a range in breast milk (n = 33; 0–7 d postpartum) and bovine formulas (n = 5). Creatine and guanidinoacetate are reported as a range in breast milk (n = 20; 1–42 d postpartum) and bovine (n = 6) and soy-based formulas (n = 2). Folate values are means ± SDs in breast milk (n = 17; 1–6 mo postpartum) and bovine formulas (n = 15). Soy-based formula values are from soy milk-based (n = 5) and soy-isolate (n = 11) formulas, respectively. Methionine and cysteine in breast milk are reported as a range (0–18 mo postpartum). Values are reported as a range in commercially available bovine (Mead-Johnson Enfamil A.R., Newborn, Reguline, Infant, Gentlease) and soy-based formulas (Mead-Johnson ProSobee; Abbott Similac Isomil).
The enzymes of folate metabolism can have numerous genetic polymorphisms that predict the preponderance of hyperhomocysteinemia (49, 50), which presents an additional complexity to understanding the contribution of MSyn to methionine partitioning. Indeed, polymorphisms of the MTHFR gene are associated with fetal viability (51), and MTHFR knockout mice exhibit an 80% mortality rate (52). In humans, the frequency of various MTHFR polymorphisms can exceed 50% in some populations (53). Moreover, the frequency of MTHFR polymorphisms appears to be increasing in populations with high folic acid intakes (53, 54); whether the frequency of such polymorphisms is related to other dietary methyl donors is unknown. Regardless, any polymorphism within folate metabolism might hinder the capacity of MSyn to remethylate homocysteine, as would low intakes of folate, riboflavin, and vitamins B-6 and B-12. Of particular relevance is vitamin B-12, which forms the MSyn cofactor cyanocobalamin. Low vitamin B-12 status is a risk for vegetarians and is common in developing countries (55), and because maternal vitamin B-12 intake is predictive of fetal B12 status (56), it is important to study the early-life consequences of reduced MSyn activity. However, because vitamin B-12 deficiencies take months to develop and because serine is dispensable in neonates, the acute manipulation of dietary folate may represent the best means of understanding how MSyn contributes to methionine partitioning in early life.
Choline.
Choline (N,N,N-trimethylethanaminium) is an essential nutrient with a complex metabolism. It arises not only from methionine de novo (see Figure 1) but is also obtained in the diet as either free choline, glycerophosphocholine, phosphocholine, or phosphatidylcholine (57). The capacity of choline to contribute to remethylation was first recognized when du Vigneaud and colleagues (9, 58) discovered that feeding rats a methionine-deficient diet with homocysteine and methyl-labeled choline supported growth and, moreover, that the methyl moiety of choline appeared in methionine. Therefore, perinatal choline supplementation represents a potential nutritional strategy for accommodating acute methionine restriction or to improve methionine availability.
Endogenous choline synthesis in humans cannot fulfill the demand for this nutrient alone (59); thus, requirement levels have been determined for men (550 mg/d), women (425 mg/d), pregnant (450 mg/d) and lactating women (550 mg/d), and infants (200 mg/d) (13, 60). The importance of choline during infancy is underscored when expressed on a per-kilogram basis because the choline requirement of a 3.2-kg infant is ∼8 times that of a 70-kg adult (Table 2). Indeed, choline is critical during development, as evidenced by the massive transport of choline from placenta to fetus (61, 62), the constant hepatic release of maternal choline into circulation throughout gestation and lactation (60, 63, 64), and the high concentration of choline esters in breast milk, which is estimated to be ∼1500 μM (40). Furthermore, like folate, periconceptional choline intake is predictive of neural tube defects (65), and choline is critical for the developing rodent brain, as demonstrated by the permanent reduction in cognitive ability in response to early-life choline restriction (66, 67). However, the choline content of breast milk depends on maternal choline intakes (68, 69), which is of concern because two-thirds of lactating women have been reported as consuming choline well below requirement levels (68). Furthermore, in addition to containing adequate free choline, infant formulas provide a wide range of choline esters, with soy-based formulas providing less than half the amount of certain choline esters provided by breast milk (40) (Table 2).
Although choline intakes are generally considered adequate, they are highly variable in many populations. Indeed, as much as 25% of an otherwise choline-replete population was shown to consume less than half the choline requirement (67). Furthermore, when factoring in genetic polymorphisms, as much as 50% of the population may have choline demands that are greater than current requirements (46, 70, 71). Although choline restriction alone results in liver damage (59, 72), the consequences of low or variable choline status on methionine partitioning are difficult to interpret because of the numerous fates of choline. Indeed, choline is the limiting substrate for phosphatidylcholine synthesis by the cytidine diphosphate-choline pathway (73), the main component of the neurotransmitter acetylcholine (74) and a major precursor of the labile methyl donor betaine (75). Therefore, although the provision of choline has been shown to enhance remethylation in humans (7), rats (58, 76), and sheep (77), the capacity of choline to participate as a methyl donor relies upon its oxidation to betaine.
Betaine.
Betaine (N,N,N-trimethylglycine) is synthesized de novo in the mitochondria of liver and kidneys by the irreversible 2-step oxidation of choline, by choline dehydrogenase (EC 1.1.99.1), and betaine aldehyde dehydrogenase (EC 1.2.1.8) (78). Therefore, although choline can theoretically meet betaine demands, dietary betaine can only spare its own synthesis from choline, which in chicks amounts to 50% of the choline requirement (79). Although it was estimated that a substantial portion of ingested choline is converted to betaine in rats (75, 80), the primary source of betaine in the body is through betaine consumption (81, 82). Most betaine in the body is found within cells, where it contributes to osmotic balance (83), but the physiological importance of betaine metabolism has remained somewhat of an enigma in mammals. Clinical applications of betaine metabolism have emerged that associate betaine intakes with various diseases (84, 85). Indeed, in some cases of hyperhomocysteinemia, betaine supplementation was effective at reducing homocysteine when folate supplementation was ineffective (29, 52, 86).
Betaine donates a methyl group to homocysteine by the zinc-dependent cytosolic enzyme betaine homocysteine methyltransferase (BHMT) (EC 2.1.1.5), which yields methionine and dimethylglycine. BHMT is active mostly in the liver, but renal activity has also been reported in mammals (87–89). Polymorphisms of the BHMT gene have been described (90, 91) and are considered to occur at a high frequency in humans (70, 92). However, the utility of BHMT polymorphisms as an early-disease marker is currently unclear but will likely change as the importance of betaine metabolism is linked to numerous pathologies (93). BHMT knockout mice have been developed that exhibit a phenotype marked by the development of fatty liver and hepatocellular carcinoma. BHMT knockout mice are sexually viable but weigh less in early life than wild-type littermates (94), which is in agreement with the intrauterine growth-restricted piglet model of fetal programming that exhibits low hepatic BHMT capacity (95).
The developmental roles of betaine are not completely understood, but betaine metabolism changes drastically during pregnancy and early development. For example, in rats and humans, massive quantities of betaine are excreted in the urine after birth and continue until urinary betaine excretion rates gradually diminish to adult amounts by adolescence (85, 96). Although it is not clear whether betaine excretion is caused by immature kidney formation, rat pups also accumulate dietary betaine in the kidney and liver until well after weaning (85). Because BHMT is detected in mammals after 10 d of gestation (90, 97) and is critical for inner cell mass formation (98), it does not appear that urinary betaine excretion is caused by a lack of BHMT activity in early life. Indeed, BHMT knockout mice present with massive intracellular betaine concentrations compared with wild-type mice 5 wk after birth (94). Because BHMT is the only known mechanism of betaine catabolism, betaine excretion is only expected to occur once remethylation demands are fulfilled; however, the contribution of BHMT to remethylation in early life has not been confirmed in vivo, and the importance of betaine accretion and excretion in early life remains unknown.
During pregnancy, plasma betaine is a determinant of fasting homocysteine concentrations, and it has been suggested that betaine is a greater source of methyl groups in late pregnancy than is folate (63, 99). Indeed, maternal plasma choline concentrations increase continuously during pregnancy, whereas plasma betaine concentrations decrease initially and are maintained low until after birth in humans (63). Moreover, preimplantation mouse embryos rapidly accumulate betaine, and blastocyst formation depends on the presence of betaine and BHMT (98); however, unlike choline and folate, there is no association between maternal betaine intake and neural tube defects (97), and the importance of dietary betaine during gestation and early life is unknown. Regardless, dietary betaine should be considered both for its capacity to spare choline (76, 79, 81) and enhance methionine availability. Moreover, reduced intakes of choline, folate, and methionine are expected to enhance BHMT demands (87, 91), and it has been suggested that all polymorphisms of methyl metabolism, which occur regularly within the population (53, 67, 70), would also enhance the metabolic demand for betaine. Further investigations into the fate of dietary betaine during gestation and early life are warranted.
Dietary intakes of betaine are not well described, but estimates suggest that it is highly variable. In the United States and New Zealand, betaine intakes were estimated as 1–2.5 g/d (84) and ∼0.5 g/d (100), respectively. However, a more recent estimate of 131 mg betaine/d was reported based on the consumption of a Western diet (82). The situation in neonates is similar to adults because betaine intakes are estimated to be highly variable but low during suckling (30–60 mg/d). Indeed, the betaine contents of infant formulas were reported to be within the range of breast milk, which was largely dependent on maternal betaine intakes (41) (Table 2). Taken together, all of the reported estimates of betaine intake are well below supplemental concentrations that often provide 3–6 g betaine/d (29, 86, 101). However, because there is no dietary betaine requirement, it is unclear how variable intakes of this methyl donor affect methyl metabolism or whether there is a demand for dietary betaine in early life.
The effects of betaine supplementation are complicated and indicate that remethylation by BHMT is tightly regulated. For example, in men supplemented with 3 g betaine/d under otherwise normal dietary conditions, the whole-body rate of remethylation was unchanged despite increased transmethylation and decreased protein synthesis (101). In contrast, there is also convincing evidence that betaine is effective at enhancing remethylation in adults (7, 102) and protein deposition in growing swine (103). Furthermore, betaine supplements enhance remethylation in chickens (104) and diminish circulating homocysteine concentrations in humans (29). However, there are only a few studies to our knowledge that have described the in vivo effects of variable betaine intake in early life, especially in regard to its capacity to furnish a portion of the methionine requirement.
As a final remark regarding the contribution of BHMT to methionine availability, it is important to note that betaine can also provide a labile methyl group for MSyn. Indeed, a product of BHMT, dimethylglycine, inhibits BHMT activity (105) and participates in methylneogenesis upon conversion to sarcosine. This might explain why betaine (or choline) supplementation in chickens enhanced remethylation by MSyn and not BHMT (104). Therefore, studies focused on how dietary methyl donors furnish remethylation must also consider how folate, choline, and betaine work alone as well as in concert to replenish methionine.
Dietary methyl donors.
Remethylation is an intricate process that relies on the compensatory regulation of BHMT and MSyn in vivo. For example, studies in rats have demonstrated that a low choline diet depletes hepatic folate (106) and that a low folate intake depletes hepatic choline (107). Furthermore, lowering folate availability either by dietary restriction or folate antagonists resulted in the reduction of hepatic betaine and an increase in BHMT activity in rats (108, 109). In addition, when methylneogenesis was restricted in humans, BHMT flux increased to compensate and maintain remethylation (110), which is in agreement with others (111). However, BHMT activity plays an important role in remethylation, even during folate repletion. For example, in rats, the in vivo BHMT activity was conserved regardless of folate status (8, 10), and BHMT and MSyn were shown to contribute to remethylation equally (112). Furthermore, choline restriction reduced remethylation more than folate restriction in rats (76), and betaine was more effective than folate at mitigating an increase in homocysteine after a methionine load in chickens (104). It is clear that remethylation is a well-controlled process that is adaptive to substrate availability under normal conditions. However, neither the extent to which BHMT or MSyn compensates for whole-body remethylation nor the potential sparing capacity of remethylation on the methionine requirement is known. Because methionine is a precursor for creatine, phosphatidylcholine, carnitine, sarcosine, sphingomyelin, polyamines, and methylated DNA and proteins, it is critical that studies address how dietary methyl donors affect methionine partitioning for growth, metabolite synthesis, and gene regulation.
Transmethylation
Transmethylation represents the rate of methyl group transfer from methionine to various methylated products and can be considered as the nonprotein requirement of methionine (3). Transmethylation commences when the sulfur atom of methionine is irreversibly adenosylated to form S-adenosylmethionine (SAM) by the enzyme methionine adenosyltransferase (EC 2.5.1.6). SAM is considered to be the primary biological methyl donor (113), a feature partially attributed to the presence of a sulfonium ion that causes the terminal methyl group to become labile because of the electrophilic nature of carbon. This labile methyl group of SAM is transferred to a myriad of metabolic precursors by methyltransferase enzymes that yield the common intermediate S-adenosylhomocysteine (SAH), which is in equilibrium with homocysteine by S-adenosylhomocysteine hydrolase (EC 3.3.1.1).
The SAM molecule exerts allosteric control over methionine metabolism, and its concentration reflects the current supply of methyl groups. Indeed, an elevation in hepatic SAM occurs after a high methionine intake (114) and indicates that methyl groups are plentiful, which leads to enhanced methionine disposal (i.e., transsulfuration) and reduced remethylation (115). SAM and SAH concentrations are often expressed as a ratio to provide an index of methylation. Because SAH is an allosteric inhibitor of all known methyltransferase reactions (116), the SAM:SAH ratio provides an indication of the rate of overall transmethylation; i.e., a lower SAM:SAH ratio indicates that transmethylation flux is high. However, the SAM:SAH ratio must be interpreted with caution (117, 118) and cannot be assumed to reflect in vivo transmethylation. Because both transmethylation and the methionine cycle are governed by methyl group availability, the supply of dietary methyl donors is expected to make an important contribution to the transmethylation requirement.
Transmethylation represents a considerable metabolic burden on dietary methionine. Indeed, 75% of hepatic methionine in piglets participates in transmethylation (2), and a methionine molecule is estimated to proceed through transmethylation 2–4 times in human adults depending on the availability of dietary methyl groups (7). Within the scope of development, the plasticity of transmethylation is particularly notable. Indeed, in vivo transmethylation rates are responsive to a variety of dietary and physiological changes, including betaine supplementation in adults (101), progression of pregnancy (47), creatine supplementation in young pigs (119), and sulfur amino acid restriction in neonatal piglets (24). If dietary methyl intake is adequate, then it is assumed that remethylation can overcome enhanced transmethylation demands under normal conditions. However, if intakes of methyl groups in the form of methionine, folate, betaine, or choline are low or variable, can remethylation compensate for these deficiencies? Moreover, if methyl supply is low, are specific reactions of transmethylation prioritized?
There are ≥50 described reactions of transmethylation (120); however, it has been estimated that there may be >300 methyltransferase enzymes that use SAM as substrate (121, 122). Because of its vast nature, it is difficult to predict the effects of a global reduction in transmethylation during development. Therefore, it is of critical importance to quantify the obligatory portion of the pediatric methionine requirement that is required to furnish transmethylation in vivo as well as to identify potential sites of methyl partitioning during transmethylation. Like all synthetic reactions, transmethylation devotes a quantitatively greater amount of methyl groups to certain products and thus represents an opportunity to measure methyl partitioning. The key products of these reactions include but are not limited to sarcosine, creatine, phosphatidylcholine, and DNA (5, 123).
Sarcosine.
The formation of sarcosine during transmethylation is regarded as an overflow pathway when methyl groups are plentiful (102), and sarcosine is synthesized by methyl transfer to glycine via glycine N-methyltransferase (EC 2.1.1.20). However, quantifying the methyl burden of sarcosine synthesis during transmethylation is complicated by the fact that sarcosine’s methyl group is recycled to methionine because it is a methylneogenesis precursor. Furthermore, because sarcosine is also synthesized from dimethylglycine, the in vivo measure of sarcosine synthesis is even more ambiguous. Therefore, glycine N-methyltransferase activity is not considered to meaningfully contribute to the transmethylation requirement during infancy.
Creatine.
The quantitative importance of remethylation for creatine synthesis was first described in rats by tracing methyl-labeled choline into creatine and discovering that more methyl label was found in creatine than methionine (124). Creatine synthesis is a multistep process that occurs in the pancreas, kidney, and liver. In rats and piglets, the process commences in the pancreas and kidneys when l-arginine:glycine amidinotransferase (EC 2.1.4.1) catalyzes the formation of guanidinoacetic acid (GAA) and ornithine (125, 126). GAA is the precursor for creatine (127) when it is transported to the liver and methylated by guanidinoacetate N-methyltransferase (GAMT) (EC 2.1.1.2) (125, 126). It has been widely reported that creatine is a major product of transmethylation and that creatine synthesis is a critical process during development (5, 14, 128). Indeed, sow-fed neonatal piglets devote ∼35% of dietary methionine to creatine synthesis (125). Meanwhile, it has been estimated that only ∼20% of portally infused [3H-methyl]methionine flowed through GAMT (2), and thus remethylation meaningfully contributes to creatine synthesis in neonatal piglets. Furthermore, GAMT is sensitive to enhanced methyl demand, and its activity was increased after dietary GAA supplementation in adult rats (129) and portal GAA infusion in piglets (2). Therefore, enhanced GAMT activity was furnished either by enhanced remethylation, reduced protein synthesis, or the sacrifice of other transmethylation reactions. The rate of transmethylation was not measured in those studies, but clearly creatine relies heavily on dietary methyl donors for its synthesis, which is sensitive to enhanced methyl demand.
Creatine synthesis has also been shown to be sensitive to reduced methylation demands in rodents, as evidenced by creatine supplementation studies. Creatine supplementation effectively reduced plasma homocysteine concentrations (129, 130), and in a rat model of high-fat feeding, creatine supplementation also increased hepatic SAM and reduced the SAM:SAH ratio (131). However, it is not yet clear what the consequences of variable creatine supply are on in vivo transmethylation partitioning, but creatine intakes vary greatly in neonates (Table 2). Breastfed infants are estimated to synthesize ∼90% of their creatine needs, whereas formula-fed infants are only required to synthesize ∼65% of creatine de novo (14). Therefore, it is assumed that creatine synthesis exhibits plasticity during development depending on the supply of dietary creatine and that enhanced creatine demands are furnished either by dietary methyl donors or the sacrifice of other transmethylation reactions. However, the priority of creatine synthesis among other transmethylation reactions is currently unknown, and it is interesting that creatine demands of the neonate are essentially the same as an adult, but the intakes are expected to be much lower (Tables 1 and 2) (14, 15).
Phosphatidylcholine.
Phosphatidylcholine synthesis is especially complex because 2 different pathways contribute to its production under normal conditions. If preformed choline is available, then phosphatidylcholine can be formed by the cytidine diphosphate-choline pathway, which is also known as the Kennedy pathway in honor of the pathway’s discoverer Eugene Kennedy (132). The Kennedy pathway occurs in all tissues, but because it does not require labile methyl groups to synthesize phosphatidylcholine, the biochemistry of this synthetic pathway does not pertain to this discussion and is reviewed elsewhere (73, 133). When dietary choline is limited, phosphatidylcholine synthesis occurs by the sequential addition of 3 methyl groups from SAM to phosphatidylethanolamine by the hepatic enzyme phosphatidylethanolamine methyltransferase (PEMT) (EC 2.1.1.17) (134). Indeed, PEMT has been postulated as an evolutionary mechanism for overcoming acute choline restriction (135). Because PEMT requires 3 M SAM to form 1 M phosphatidylcholine, the methyl demand of the PEMT pathway is considered to be quantitatively greater than creatine and potentially all other transmethylation reactions (2, 5).
Although both PEMT and the Kennedy pathway synthesize phosphatidylcholine, the 2 pathways work in concert under normal conditions to meet the demands of phosphatidylcholine synthesis. For example, mice lacking the PEMT gene exhibit normal growth when choline is provided, but feeding a choline-deficient diet to PEMT knockout mice is lethal (135). However, PEMT knockout mice exhibit hepatosteatosis and lower hepatic phosphatidylcholine concentrations despite the provision of dietary choline (136). Indeed, the Kennedy pathway can only furnish ∼70% of phosphatidylcholine demands, and thus obligatory flux through PEMT accounts for a considerable portion of the total transmethylation flux under all conditions (137).
The metabolic consequences of choline restriction represent an interesting point of metabolic control. PEMT activity is increased during choline restriction in rats (138), and presumably the rate of phosphatidylcholine oxidation to choline was also enhanced in those animals to supply the Kennedy pathway. However, the interplay between PEMT and the Kennedy pathway is extremely complex and is discussed elsewhere (139). Because phosphatidylcholine is a major transmethylation product (5) that is sensitive to choline supply (138), whole-body transmethylation should be responsive to the presence or absence of choline.
As previously mentioned, two-thirds of lactating women do not consume adequate choline (68), and as much as 50% of the population is predicted to have a genetic preponderance for enhanced choline demands (46, 70, 71). Included among those genetic abnormalities are PEMT polymorphisms that occur at a relatively high frequency, such as in 1 study population in which 20% of women exhibited a specific PEMT mutation (140). Therefore, we consider it likely that neonates commonly experience choline restriction, but it is unclear how much of the neonatal choline requirement can be fulfilled by PEMT, especially if there is an inherent PEMT mutation. Furthermore, it is also unclear whether global transmethylation or remethylation is increased to accommodate enhanced phosphatidylcholine synthesis during choline restriction or whether other transmethylation reactions, such as creatine synthesis or DNA methylation, are killed to furnish PEMT demands.
DNA.
One of the most intriguing sites of early-life methylation is of the cytosine-guanine dinucleotides located in the promoter regions of DNA. The methylation of DNA occurs in most tissues by DNA methyltransferase enzymes 1, 2, and 3 (EC 2.1.1.204/37). In general, DNA methylation represses gene expression (46, 141) and persists throughout the lifetime of an organism (142). Indeed, DNA methylation has become an important focus of study for the fetal origins of adult disease hypothesis (143). The hypothesis establishes that early-life exposure to nutritional insults will permanently affect gene expression and thus “program” an organism to be more or less susceptible to certain diseases later in life (144). Because dietary reductions in methionine, choline, and/or folate have been shown to reduce DNA methylation (46, 142, 145, 146), it is likely that dietary methyl donors and consumers can affect methyl group partitioning to DNA.
Transsulfuration.
Transsulfuration represents the sulfur transfer from homocysteine to cysteine and provides an irreversible mechanism of homocysteine elimination that occurs in a variety of tissues. Transsulfuration commences upon the condensation of homocysteine with serine by the vitamin B-6-dependent enzyme cystathionine β-synthase (EC 4.2.1.22) to form cystathionine, which is converted to cysteine by an additional vitamin B-6-dependent enzyme, cystathionine γ-lyase (EC 4.4.1.1). Indeed, transsulfuration accounts for the nutritional observation that dietary cysteine spares the methionine requirement by 40% in piglets (4, 20) and 55% in children (147).
The capacity of cysteine to spare the methionine requirement provides a rationale that dietary methyl donors (or consumers) can also spare the methionine requirement. Furthermore, it is possible that dietary methyl donors will enhance transsulfuration (i.e., methionine disposal) once the requirements of transmethylation and protein synthesis are met. Indeed, transsulfuration was killed for transmethylation and protein synthesis in piglets when sulfur amino acids were removed from the diet (24). Therefore, the dietary methyl supply is not expected to affect partitioning toward transsulfuration until SAM concentrations accumulate (87); thus, dietary methyl donors should only enhance the partitioning of methionine toward transsulfuration after the requirements of transmethylation and protein synthesis are met.
Protein.
Perhaps the most critical site of methionine partitioning during development is into protein, which can be considered as a surrogate measure of growth. Indeed, like all amino acids, methionine was evolutionarily selected for a role in protein incorporation before its nonprotein role was exploited (i.e., the methionine cycle) (148, 149). In proteins, methionine plays an important structural role because of its highly hydrophobic nature (121), and, interestingly, methionine occupies a highly conserved position in the substrate recognition pocket of most protein kinases (150) and is thought to play a major role in mitigating oxidative stress in bacterial cells (151). It is not known how dietary methyl donors contribute to methionine availability for protein incorporation, but because methionine is cycled ∼2 times before disposal in adults and because the provision of methyl groups affects methionine cycling (7), it is likely that if the methyl supply is restricted then so is the availability of methionine for protein incorporation. Because early-life protein demands are estimated to be as much as 3 times that of adults (Table 1), further studies are required to discern the plasticity of methionine partitioning into protein compared with transmethylation and the potential for remethylation to contribute available methionine for growth in early life. Such studies should include whole-body methionine kinetics combined with growth outcomes under varying clinically relevant dietary conditions.
Conclusions
The recommended methionine intake for infants is currently based on breast milk, which is 28 mg ⋅ kg−1 ⋅ d−1 (152); however, a minimum estimate of 38 mg ⋅ kg−1 ⋅ d−1 was determined by feeding graded levels of methionine to infants (11). Although these values are similar and widespread methionine deficiencies are not expected in the population, this discrepancy demonstrates the complexity in determining the methionine requirement because breast milk contains different quantities of methyl donors or transmethylation products than do commercial infant formulations (Table 2). Therefore, the application of the methionine requirement must consider that methionine services protein synthesis, transmethylation, and transsulfuration and that transmethylation and transsulfuration are affected by the supply of their precursors and/or products (129, 138, 153) (Figure 1). Furthermore, it stands to reason that remethylation also contributes to the methionine requirement and thus is important in quantifying the contribution of remethylation to methionine partitioning in early life when transmethylation and protein demands are greater (Table 1). Methionine requirement estimates should eventually consider all of these dietary factors, but more data are necessary before current recommendations can be refined.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: BHMT, betaine homocysteine methyltransferase; GAA, guanidinoacetic acid; GAMT, guanidinoacetate N-methyltransferase; MSyn, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; PEMT, phosphatidylethanolamine methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
References
- 1.Millward DJ. Metabolic demands for amino acids and the human dietary requirement: Millward and Rivers (1988) revisited. J Nutr 1998;128:2563S–76S. [DOI] [PubMed] [Google Scholar]
- 2.McBreairty LE, McGowan RA, Brunton JA, Bertolo RF. Partitioning of [methyl-3H]methionine to methylated products and protein is altered during high methyl demand conditions in young Yucatan miniature pigs. J Nutr 2013;143:804–9. [DOI] [PubMed] [Google Scholar]
- 3.Bertolo RF, McBreairty LE. The nutritional burden of methylation reactions. Curr Opin Clin Nutr Metab Care 2013;16:102–8. [DOI] [PubMed] [Google Scholar]
- 4.Shoveller AK, Brunton JA, House JD, Pencharz PB, Ball RO. Dietary cysteine reduces the methionine requirement by an equal proportion in both parenterally and enterally fed piglets. J Nutr 2003;133:4215–24. [DOI] [PubMed] [Google Scholar]
- 5.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 2006;83:5–10. [DOI] [PubMed] [Google Scholar]
- 6.Keller EB, Rachele JR, Vigneaud Du V. A study of transmethylation with methionine containing deuterium and C14 in the methyl group. J Biol Chem 1949;177:733–8. [PubMed] [Google Scholar]
- 7.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism 1975;24:721–35. [DOI] [PubMed] [Google Scholar]
- 8.Du Vigneaud V, Ressler C, Rachele JR, Reyniers JA, Luckey TD. The synthesis of “biologically labile” methyl groups in the germ-free rat. J Nutr 1951;45:361–76. [DOI] [PubMed] [Google Scholar]
- 9.Du Vigneaud V, Chandler JP, Moyer AW. The effect of choline on the ability of homocystine to replace methionine in the diet. J Biol Chem; 1939;131:57–76. [Google Scholar]
- 10.Vigneaud Du V. Trail of research in sulfur chemistry and metabolism and related fields. Ithaca (NY): Cornell University Press; 1952. [Google Scholar]
- 11.Huang L, Hogewind-Schoonenboom JE, van Dongen MJA, de Groof F, Voortman GJ, Schierbeek H, Twisk JWR, Vermes A, Chen C, Huang Y, et al. Methionine requirement of the enterally fed term infant in the first month of life in the presence of cysteine. Am J Clin Nutr 2012;95:1048–54. [DOI] [PubMed] [Google Scholar]
- 12.Di Buono M, Wykes LJ, Ball RO, Pencharz PB. Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr 2001;74:761–6. [DOI] [PubMed] [Google Scholar]
- 13.Pitkin RM, Allen LH, Bernfield M, De Wals P, McCormick DB, Green R, Russel RM, Shane B, Zeisel SH, editors. Other B vitamins, and choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 14.Edison EE, Brosnan ME, Aziz K, Brosnan JT. Creatine and guanidinoacetate content of human milk and infant formulas: implications for creatine deficiency syndromes and amino acid metabolism. Br J Nutr 2013;110:1075–8. [DOI] [PubMed] [Google Scholar]
- 15.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007;27:241–61. [DOI] [PubMed] [Google Scholar]
- 16.Courtney-Martin G, Ball RO, Pencharz PB. Sulfur amino acid metabolism and requirements. Nutr Rev 2012;70:170–5. [DOI] [PubMed] [Google Scholar]
- 17.Di Buono M, Wykes LJ, Ball RO, Pencharz PB. Total sulfur amino acid requirement in young men as determined by indicator amino acid oxidation with L-[1-13C]phenylalanine. Am J Clin Nutr 2001;74:756–60. [DOI] [PubMed] [Google Scholar]
- 18.Reeds PJ, Burrin DG, Davis TA, Fiorotto ML, Stoll B, van Goudoever JB. Protein nutrition of the neonate. Proc Nutr Soc 2000;59:87–97. [DOI] [PubMed] [Google Scholar]
- 19.Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care 2008;11:601–6. [DOI] [PubMed] [Google Scholar]
- 20.Shoveller AK, Brunton JA, Pencharz PB, Ball RO. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J Nutr 2003;133:1390–7. [DOI] [PubMed] [Google Scholar]
- 21.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol 1988;255:E322–31. [DOI] [PubMed] [Google Scholar]
- 22.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab 2001;280:E947–55. [DOI] [PubMed] [Google Scholar]
- 23.Hoffer LJ. Homocysteine remethylation and trans-sulfuration. Metabolism 2004;53:1480–3. [DOI] [PubMed] [Google Scholar]
- 24.Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 2009;296:E1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: methionine kinetics. Am J Clin Nutr 2006;84:1400–5. [DOI] [PubMed] [Google Scholar]
- 26.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res 2007;4:143–50. [DOI] [PubMed] [Google Scholar]
- 27.Osganian SK, Stampfer MJ, Spiegelman D, Rimm E, Cutler JA, Feldman HA, Montgomery DH, Webber LS, Lytle LA, Bausserman L, et al. Distribution of and factors associated with serum homocysteine levels in children: Child and Adolescent Trial for Cardiovascular Health. JAMA 1999;281:1189–96. [DOI] [PubMed] [Google Scholar]
- 28.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bønaa KH, Spence JD, Nygård O, Jamison R, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 2010;170:1622–31. [DOI] [PubMed] [Google Scholar]
- 29.Olthof MR, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab 2005;6:15–22. [DOI] [PubMed] [Google Scholar]
- 30.Olthof MR, Brink EJ, Katan MB, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am J Clin Nutr 2005;82:111–7. [DOI] [PubMed] [Google Scholar]
- 31.Strain JJ, Dowey L, Ward M, Pentieva K, McNulty H. B-vitamins, homocysteine metabolism and CVD. Proc Nutr Soc 2004;63:597–603. [DOI] [PubMed] [Google Scholar]
- 32.Bailey LB, Gregory JF. Folate metabolism and requirements. J Nutr 1999;129:779–82. [DOI] [PubMed] [Google Scholar]
- 33.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 2004;286:E272–9. [DOI] [PubMed] [Google Scholar]
- 34.Du Vigneaud V, Simmonds S, Cohn M. A further investigation of the ability of sarcosine to serve as a labile methyl donor. J Biol Chem 1946;166:47–52. [PubMed] [Google Scholar]
- 35.Houk VM, Oakley GP Jr, Erickson JD. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR 1992;41:1–7. [PubMed] [Google Scholar]
- 36.Gudgeon P, Cavalcanti R. Folate testing in hospital inpatients. Am J Med 2015;128:56–9. [DOI] [PubMed] [Google Scholar]
- 37.McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, Yetley EA, Kennedy-Stephenson J, Johnson CL. Blood folate levels: the latest NHANES results. Atlanta (GA):Centers for Disease Control and Prevention; 2008. [PubMed] [Google Scholar]
- 38.Shakur YA, Garriguet D, Corey P, O’Connor DL. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am J Clin Nutr 2010;92:818–25. [DOI] [PubMed] [Google Scholar]
- 39.Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr 2013;143:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr 1996;64:572–6. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto A, Ono H, Mizoguchi N, Sakura N. Betaine and homocysteine concentrations in infant formulae and breast milk. Pediatr Int 2001;43:637–40. [DOI] [PubMed] [Google Scholar]
- 42.Han Y-H, Yon M, Han H-S, Kim K-Y, Tamura T, Hyun TH. Folate contents in human milk and casein-based and soya-based formulas, and folate status in Korean infants. Br J Nutr 2009;101:1769–74. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Adelman AS, Rai D, Boettcher J, Lőnnerdal B. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients 2013;5:4800–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houghton LA, Yang J, O’Connor DL. Unmetabolized folic acid and total folate concentrations in breast milk are unaffected by low-dose folate supplements. Am J Clin Nutr 2009;89:216–20. [DOI] [PubMed] [Google Scholar]
- 45.Lamers Y, Coats B, Ralat M, Quinlivan EP, Stacpoole PW, Gregory JF. Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women. J Nutr 2011;141:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr 2002;132:2333S–5S. [DOI] [PubMed] [Google Scholar]
- 47.Dasarathy J, Gruca LL, Bennett C, Parimi PS, Duenas C, Marczewski S, Fierro JL, Kalhan SC. Methionine metabolism in human pregnancy. Am J Clin Nutr 2010;91:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC. Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients. Pediatr Res 2008;64:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ. Heijer den M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111–3. [DOI] [PubMed] [Google Scholar]
- 50.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996;93:7–9. [DOI] [PubMed] [Google Scholar]
- 51.Isotalo PA, Wells GA, Donnelly JG. Neonatal and fetal methylenetetrahydrofolate reductase genetic polymorphisms: an examination of C677T and A1298C mutations. Am J Hum Genet 2000;67:986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwahn BC, Laryea MD, Chen Z, Melnyk S, Pogribny I, Garrow T, James SJ, Rozen R. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem J 2004;382:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guéant-Rodriguez R-M, Guéant J-L, Debard R, Thirion S, Hong LX, Bronowicki J-P, Namour F, Chabi NW, Sanni A, Anello G, et al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr 2006;83:701–7. [DOI] [PubMed] [Google Scholar]
- 54.Lucock M, Yates Z. Folic acid - vitamin and panacea or genetic time bomb? Nat Rev Genet 2005;6:235–40. [DOI] [PubMed] [Google Scholar]
- 55.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89:693S–6S. [DOI] [PubMed] [Google Scholar]
- 56.Hussein L, Abdel Aziz S, Tapouzada S, Boehles H. Serum vitamin B(12) concentrations among mothers and newborns and follow-up study to assess implication on the growth velocity and the urinary methylmalonic acid excretion. Int J Vitam Nutr Res 2009;79:297–307. [DOI] [PubMed] [Google Scholar]
- 57.Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7. [DOI] [PubMed] [Google Scholar]
- 58.Simmonds S, Cohn M, Chandler JP, du Vigneaud V. The utilization of the methyl groups of choline in the biological synthesis of methionine. J Biol Chem 1943;149:519–25. [Google Scholar]
- 59.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J 1991;5:2093–8. [PubMed] [Google Scholar]
- 60.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta 1985;149:1–12. [DOI] [PubMed] [Google Scholar]
- 62.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Dev Physiol 1986;8:435–45. [PubMed] [Google Scholar]
- 63.Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr 2005;81:1383–9. [DOI] [PubMed] [Google Scholar]
- 64.Zeisel SH, Mar MH, Zhou Z, Da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr 1995;125:3049–54. [DOI] [PubMed] [Google Scholar]
- 65.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9. [DOI] [PubMed] [Google Scholar]
- 66.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev 2003;27:385–99. [DOI] [PubMed] [Google Scholar]
- 67.Zeisel SH, da Costa K-A. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer LM, da Costa K-A, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 2010;92:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 2005;16:489–99. [DOI] [PubMed] [Google Scholar]
- 70.da Costa K-A, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohlmeier M, da Costa K-A, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA 2005;102:16025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995;22:1399–403. [PubMed] [Google Scholar]
- 73.Gibellini F, Smith TK. The Kennedy pathway—de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010;62:414–28. [DOI] [PubMed] [Google Scholar]
- 74.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science 1983;221:614–20. [DOI] [PubMed] [Google Scholar]
- 75.Weinhold PA, Sanders R. The oxidation of choline by liver slices and mitochondria during liver development in the rat. Life Sci 1973;13:621–9. [Google Scholar]
- 76.Shinohara Y, Hasegawa H, Ogawa K, Tagoku K, Hashimoto T. Distinct effects of folate and choline deficiency on plasma kinetics of methionine and homocysteine in rats. Metabolism 2006;55:899–906. [DOI] [PubMed] [Google Scholar]
- 77.Lobley GE, Connell A. The importance of transmethylation reactions to methionine metabolism in sheep: effects of supplementation with creatine and choline. Br J Nutr 1996;75:47–56. [DOI] [PubMed] [Google Scholar]
- 78.Chern MK, Pietruszko R. Evidence for mitochondrial localization of betaine aldehyde dehydrogenase in rat liver: purification, characterization, and comparison with human cytoplasmic E3 isozyme. Biochem Cell Biol 1999;77:179–87. [PubMed] [Google Scholar]
- 79.Dilger RN, Garrow TA, Baker DH. Betaine can partially spare choline in chicks but only when added to diets containing a minimal level of choline. J Nutr 2007;137:2224–8. [DOI] [PubMed] [Google Scholar]
- 80.Cheng WL, Holmes-McNary MQ, Mar MH, Lien EL, Zeisel SH. Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 1996;7:457–64. [Google Scholar]
- 81.Clow KA, Treberg JR, Brosnan ME, Brosnan JT. Elevated tissue betaine contents in developing rats are due to dietary betaine, not to synthesis. J Nutr 2008;138:1641–6. [DOI] [PubMed] [Google Scholar]
- 82.Ross AB, Zangger A, Guiraud SP. Cereal foods are the major source of betaine in the Western diet—analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem 2014;145:859–65. [DOI] [PubMed] [Google Scholar]
- 83.Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr 2007;26:613S–23S. [DOI] [PubMed] [Google Scholar]
- 84.Craig SAS. Betaine in human nutrition. Am J Clin Nutr 2004;80:539–49. [DOI] [PubMed] [Google Scholar]
- 85.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 2010;43:732–44. [DOI] [PubMed] [Google Scholar]
- 86.Ronge E, Kjellman B. Long term treatment with betaine in methylenetetrahydrofolate reductase deficiency. Arch Dis Child 1996;74:239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finkelstein JD, Kyle W, Harris BJ. Methionine metabolism in mammals. Regulation of homocysteine methyltransferases in rat tissue. Arch Biochem Biophys 1971;146:84–92. [DOI] [PubMed] [Google Scholar]
- 88.McKeever MP, Weir DG, Molloy A, Scott JM. Betaine-homocysteine methyltransferase: organ distribution in man, pig and rat and subcellular distribution in the rat. Clin Sci 1991;81:551–6. [DOI] [PubMed] [Google Scholar]
- 89.Sunden SL, Renduchintala MS, Park EI, Miklasz SD, Garrow TA. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys 1997;345:171–4. [DOI] [PubMed] [Google Scholar]
- 90.Feng Q, Kalari K, Fridley BL, Jenkins G, Ji Y, Abo R, Hebbring S, Zhang J, Nye MD, Leeder SL, et al. Betaine-homocysteine methyltransferase: human liver genotype-phenotype correlation. Mol Genet Metab 2011;102:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem 1999;274:7816–24. [DOI] [PubMed] [Google Scholar]
- 92.Morin I, Platt R, Weisberg I, Sabbaghian N, Wu Q, Garrow TA, Rozen R. Common variant in betaine-homocysteine methyltransferase (BHMT) and risk for spina bifida. Am J Med Genet A 2003;119A:172–6. [DOI] [PubMed] [Google Scholar]
- 93.Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis 2011;34:3–15. [DOI] [PubMed] [Google Scholar]
- 94.Teng Y-W, Mehedint MG, Garrow TA, Zeisel SH. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem 2011;286:36258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacKay DS, Brophy JD, McBreairty LE, McGowan RA, Bertolo RF. Intrauterine growth restriction leads to changes in sulfur amino acid metabolism, but not global DNA methylation, in Yucatan miniature piglets. J Nutr Biochem 2012;23:1121–7. [DOI] [PubMed] [Google Scholar]
- 96.Davies SE, Chalmers RA, Randall EW, Iles RA. Betaine metabolism in human neonates and developing rats. Clin Chim Acta 1988;178:241–9. [DOI] [PubMed] [Google Scholar]
- 97.Fisher MC, Zeisel SH, Mar M-H, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J 2002;16:619–21. [DOI] [PubMed] [Google Scholar]
- 98.Lee MB, Kooistra M, Zhang B, Slow S, Fortier AL, Garrow TA, Lever M, Trasler JM, Baltz JM. Betaine homocysteine methyltransferase is active in the mouse blastocyst and promotes inner cell mass development. J Biol Chem 2012;287:33094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 2005;43:1069–75. [DOI] [PubMed] [Google Scholar]
- 100.Slow S, Donaggio M, Cressey PJ, Lever M. The betaine content of New Zealand foods and estimated intake in the New Zealand diet. J Food Compos Anal 2005;18:473–85. [Google Scholar]
- 101.Storch KJ, Wagner DA, Young VR. Methionine kinetics in adult men: effects of dietary betaine on L-[2H3-methyl-1-13C]methionine. Am J Clin Nutr 1991;54:386–94. [DOI] [PubMed] [Google Scholar]
- 102.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism 1980;29:707–20. [DOI] [PubMed] [Google Scholar]
- 103.Fernández-Fígares I, Wray-Cahen D, Steele NC, Campbell RG, Hall DD, Virtanen E, Caperna TJ. Effect of dietary betaine on nutrient utilization and partitioning in the young growing feed-restricted pig. J Anim Sci 2002;80:421–8. [DOI] [PubMed] [Google Scholar]
- 104.Pillai PB, Fanatico AC, Beers KW, Blair ME, Emmert JL. Homocysteine remethylation in young broilers fed varying levels of methionine, choline, and betaine. Poult Sci 2006;85:90–5. [DOI] [PubMed] [Google Scholar]
- 105.Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem 1996;271:22831–8. [DOI] [PubMed] [Google Scholar]
- 106.Varela-Moreiras G, Selhub J, Da Costa KA. Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem 1992;3:519–22. [Google Scholar]
- 107.Kim YI, Miller JW, Da Costa KA, Nadeau M, Smith D, Selhub J, Zeisel SH, Mason JB. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr 1994;124:2197–203. [DOI] [PubMed] [Google Scholar]
- 108.Barak AJ, Beckenhauer HC, Tuma DJ, Donohue TM. Adaptive increase in betaine-homocysteine methyltransferase activity maintains hepatic S-adenosylmethionine levels in ethanol-treated rats. IRCS Med Sci Bioc. 1984;12:866–7. [Google Scholar]
- 109.Barak AJ, Kemmy RJ, Tuma DJ. The effect of methotrexate on homocysteine methylating agents in rat liver. Drug Nutr Interact 1982;1:303–6. [PubMed] [Google Scholar]
- 110.Davis SR, Quinlivan EP, Shelnutt KP, Ghandour H, Capdevila A, Coats BS, Wagner C, Shane B, Selhub J, Bailey LB, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C->T polymorphism and by dietary folate restriction in young women. J Nutr 2005;135:1045–50. [DOI] [PubMed] [Google Scholar]
- 111.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr 1999;129:712–7. [DOI] [PubMed] [Google Scholar]
- 112.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem 1984;259:9508–13. [PubMed] [Google Scholar]
- 113.Catoni GL. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem 1953;204:403–16. [PubMed] [Google Scholar]
- 114.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Adaptation to methionine excess. J Biol Chem 1986;261:1582–7. [PubMed] [Google Scholar]
- 115.Finkelstein JD. Inborn errors of sulfur-containing amino acid metabolism. J Nutr 2006;136:1750S–4S. [DOI] [PubMed] [Google Scholar]
- 116.Brosnan JT, Brosnan ME, Bertolo RF, Brunton JA. Methionine: a metabolically unique amino acid. Livest Sci 2010;112:2–7. [Google Scholar]
- 117.Uthus EO, Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol 2003;38:491–8. [DOI] [PubMed] [Google Scholar]
- 118.Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr 2007;137:311–4. [DOI] [PubMed] [Google Scholar]
- 119.McBreairty LE, Robinson JL, Furlong KR, Brunton JA. Guanidinoacetate is more effective than creatine at enhancing tissue creatine stores while consequently limiting methionine availability in Yucatan miniature pigs. PLoS One 2015;10:e0131563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci 2003;28:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr 2006;136:1636S–40S. [DOI] [PubMed] [Google Scholar]
- 122.Katz JE, Dlakić M, Clarke S. Automated identification of putative methyltransferases from genomic open reading frames. Mol Cell Proteomics 2003;2:525–40. [DOI] [PubMed] [Google Scholar]
- 123.Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, Wagner C. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr 2007;85:19–25. [DOI] [PubMed] [Google Scholar]
- 124.Vigneaud Du V, Cohn M, Chandler JP. The utilization of the methyl group of methionine in the biological synthesis of choline and creatine. J Biol Chem 1941;140:625–41. [DOI] [PubMed] [Google Scholar]
- 125.Brosnan JT, Wijekoon EP, Warford-Woolgar L, Trottier NL, Brosnan ME, Brunton JA, Bertolo RFP. Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J Nutr 2009;139:1292–7. [DOI] [PubMed] [Google Scholar]
- 126.da Silva RP, Nissim I, Brosnan ME, Brosnan JT. Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab 2009;296:E256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bloch K, Schoenheimer R. The biological precursors of creatine. J Biol Chem 1941;138:167–94. [Google Scholar]
- 128.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids 2011;40:1325–31. [DOI] [PubMed] [Google Scholar]
- 129.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab 2001;281:E1095–100. [DOI] [PubMed] [Google Scholar]
- 130.Deminice R, Portari GV, Vannucchi H, Jordao AA. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr 2009;102:110–6. [DOI] [PubMed] [Google Scholar]
- 131.Deminice R, da Silva RP, Lamarre SG, Brown C, Furey GN, McCarter SA, Jordao AA, Kelly KB, King-Jones K, Jacobs RL, et al. Creatine supplementation prevents the accumulation of fat in the livers of rats fed a high-fat diet. J Nutr 2011;141:1799–804. [DOI] [PubMed] [Google Scholar]
- 132.Kresge N, Simoni RD, Hill RL. The Kennedy pathway for phospholipid synthesis: the work of Eugene Kennedy. J Biol Chem 2005;280:e22–4. [Google Scholar]
- 133.Fagone P, Jackowski S.. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta 2013;1831:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schneider WJ, Vance DE. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat liver. Partial purification and characterization of the enzymatic activities. J Biol Chem 1979;254:3886–91. [PubMed] [Google Scholar]
- 135.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem 1998;273:27043–6. [DOI] [PubMed] [Google Scholar]
- 136.Zhu X, Song J, Mar M-H, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J 2003;370:987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vance DE, Li Z, Jacobs RL. Hepatic phosphatidylethanolamine N-methyltransferase, unexpected roles in animal biochemistry and physiology. J Biol Chem 2007;282:33237–41. [DOI] [PubMed] [Google Scholar]
- 138.Schneider WJ, Vance DE. Effect of choline deficiency on the enzymes that synthesize phosphatidylcholine and phosphatidylethanolamine in rat liver. Eur J Biochem 1978;85:181–7. [DOI] [PubMed] [Google Scholar]
- 139.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta 1997;1348:142–50. [DOI] [PubMed] [Google Scholar]
- 140.Zeisel SH. Diet-gene interactions underlie metabolic individuality and influence brain development: implications for clinical practice derived from studies on choline metabolism. Ann Nutr Metab 2012;60 Suppl 3:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science 2001;293:1068–70. [DOI] [PubMed] [Google Scholar]
- 142.Kotsopoulos J, Sohn K-J, Kim Y-I. Postweaning dietary folate deficiency provided through childhood to puberty permanently increases genomic DNA methylation in adult rat liver. J Nutr 2008;138:703–9. [DOI] [PubMed] [Google Scholar]
- 143.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005;85:571–633. [DOI] [PubMed] [Google Scholar]
- 144.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 2000;71:1344S–52S. [DOI] [PubMed] [Google Scholar]
- 145.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 2009;139:1054–60. [DOI] [PubMed] [Google Scholar]
- 146.Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr 2013;8:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Humayun MA, Turner JM, Elango R, Rafii M, Langos V, Ball RO, Pencharz PB. Minimum methionine requirement and cysteine sparing of methionine in healthy school-age children. Am J Clin Nutr 2006;84:1080–5. [DOI] [PubMed] [Google Scholar]
- 148.Teichmann SA, Rison SC, Thornton JM, Riley M, Gough J, Chothia C. The evolution and structural anatomy of the small molecule metabolic pathways in Escherichia coli. J Mol Biol 2001;311:693–708. [DOI] [PubMed] [Google Scholar]
- 149.Horowitz NH. On the evolution of biochemical syntheses. Proc Natl Acad Sci U S A 1945;31:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Torkamani A. Accurate prediction of causative protein kinase polymorphisms in inherited disease and cancer [dissertation]. San Diego (CA): UC San Diego; 2008. [Google Scholar]
- 151.Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J 2009;23:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.FAO/WHO/UNU. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007;935:1–284. [PubMed] [Google Scholar]
- 153.Finkelstein JD, Mudd SH. Trans-sulfuration in mammals. The methionine-sparing effect of cystine. J Biol Chem 1967;242:873–80. [PubMed] [Google Scholar]