Summary
Recently, we demonstrated elevated numbers of CD4+ Foxp3+ regulatory T (Treg) cells in Plasmodium yoelii‐infected mice contributing to the regulation of anti‐malarial immune response. However, it remains unclear whether this increase in Treg cells is due to thymus‐derived Treg cell expansion or induction of Treg cells in the periphery. Here, we show that the frequency of Foxp3+ Treg cells expressing neuropilin‐1 (Nrp‐1) decreased at early time‐points during P. yoelii infection, whereas percentages of Helios+ Foxp3+ Treg cells remained unchanged. Both Foxp3+ Nrp‐1+ and Foxp3+ Nrp‐1− Treg cells from P. yoelii‐infected mice exhibited a similar T‐cell receptor Vβ chain usage and methylation pattern in the Treg‐specific demethylation region within the foxp3 locus. Strikingly, we did not observe induction of Foxp3 expression in Foxp3− T cells adoptively transferred to P. yoelii‐infected mice. Hence, our results suggest that P. yoelii infection triggered expansion of naturally occurring Treg cells rather than de novo induction of Foxp3+ Treg cells.
Keywords: parasitic protozoan, regulatory T cells, rodent
Abbreviations
- iTreg
induced Treg
- Nrp‐1
neuropilin‐1
- nTreg
natural Treg
- p.i.
post‐infection
- TCR
T‐cell receptor
- Treg cell
regulatory T cell
- TSDR
Treg‐specific demethylation region
Introduction
CD4+ Foxp3+ regulatory T (Treg) cells are well‐known key players in the maintenance of immunological homeostasis and there is an increasing body of evidence that Treg cells also control immune responses during infectious diseases. Elevated frequencies and/ or numbers of Treg cells were detected in mice infected with parasites1, 2 and viruses3 as well as in humans suffering from Helicobacter pylori infection.4 Likewise an increase in the number of CD4+ CD25+ Foxp3+ Treg cells was observed in the peripheral blood of patients infected with Plasmodium falciparum,5, 6 the parasite that causes severe malaria. Most recently, we detected elevated Treg numbers in spleens of BALB/c mice infected with Plasmodium yoelii,7 a well established experimental mouse model for studying Plasmodium infection at the blood stage. Strikingly, depletion of Treg cells from P. yoelii‐infected mice resulted in a more efficient T‐cell response accompanied by significantly reduced parasitaemia, suggesting that Foxp3+ Treg cells interfere with anti‐malarial immune responses.7
Besides naturally occurring thymus‐derived Foxp3+ Treg (nTreg) cells a heterogeneous population of induced Treg (iTreg) cells exists that arises from naive CD4+ CD25− T cells within the periphery in several experimental settings.8, 9, 10 Hence, the iTreg repertoire derives from conventional T cells, whereas nTreg cells were selected by high‐affinity interactions within the thymus.11, 12 It was assumed that nTreg cells mainly recognize self‐antigens, acting as important regulators of autoimmune responses. In contrast iTreg cells seem to respond to foreign antigens, so playing a crucial role in mucosal immune tolerance and chronic allergic reactions. However, they might also be generated in response to self‐antigens and have been suggested to collaborate with nTreg cells to achieve optimal regulation.13 Until now, it is difficult to study the phenotype and function of iTreg cells versus nTreg cells due to the lack of a single specific feature of either cell type in particular during ongoing immune responses. However, a better understanding of the origin of Treg cells would be helpful to develop specific therapeutics to modulate the number and function of Treg cells by interfering with pathways that are involved in their expansion or conversion from conventional T cells, respectively.
Helios, a member of the Ikaros family of transcription factors, was proposed for discriminating nTreg from iTreg cells,14 but its utility as an nTreg marker has been questioned due to its inconsistent expression on iTreg cells in distinct immune settings.15, 16 Most recently, an additional marker molecule for distinguishing thymus‐derived nTreg cells from iTreg cells has been described: the surface receptor neuropilin‐1 (Nrp‐1).17, 18 Whereas the majority of Foxp3+ nTreg cells expresses Nrp‐1 on their surface,19 Foxp3+ Treg cells converted under homeostatic conditions have been suggested to lack Nrp‐1 expression.18
Analysis of epigenetic modifications within the foxp3 locus has also been proposed to be helpful for discriminating thymus‐derived nTreg cells with stable Foxp3 expression from peripherally induced Treg cells, which often show an unstable Foxp3 expression. DNA demethylation at a conserved intronic CpG‐rich region, the Treg‐specific demethylated region (TSDR) was correlated with stable Foxp3 expression,20 but seems to be dispensable for initiation of Foxp3 expression.21 Accordingly, stable thymus‐derived nTreg cells display a fully demethylated TSDR, whereas the TSDR of CD4+ CD25− non‐Treg cells and in‐vitro‐induced Treg cells with unstable Foxp3 expression have been described as being heavily methylated.22
In our previous study we observed an increase in Foxp3+ Treg cells during P. yoelii infection of BALB/c mice7 that interferes with an effective anti‐parasitic immune response but the origin of these Treg cells is still unclear. For the development of therapeutic approaches that modulate Treg responses during malaria infection, it is critical to better understand the characteristics and origin of these immunosuppressive T cells. Here, we provide evidence that P. yoelii infection results in an expansion of thymus‐derived nTreg cells rather than peripheral induction of Foxp3+ Treg cells at least at early time‐points during infection.
Material and methods
Mice and parasites
Foxp3/eGFP mice (BALB/c) (Jackson Laboratories, Bar Harbor, ME), Thy1.1 BALB/c mice (kindly provided by Jochen Hühn) and BALB/c mice (Harlan Laboratories, Borchen, Germany) were crossed and maintained under specific pathogen‐free conditions at the Animal Facility of the University Hospital Essen. Cryopreserved Plasmodium yoelii 17XNL (non‐lethal) infected red blood cells were passaged once through BALB/c mice before being used in experimental animals. For P. yoelii infection, mice were injected intravenously with 1 × 105 infected red blood cells. Parasitaemia levels were determined by microscopic examination of Giemsa‐stained blood films. All animal experiments were performed in strict accordance with the guidelines of the German Animal Protection Law, and were approved by the state authorities for Ethics in Animal Experiments of North‐Rhine Westphalia, Germany.
Cell separation and adoptive transfer
Spleens were rinsed with erythrocyte lysis buffer and washed with PBS supplemented with 2% fetal calf serum and 2 mm EDTA. For isolation of CD4+ Foxp3− T cells, CD4+ T cells were enriched from splenocytes isolated from Foxp3/eGFP mice by using the MACS CD4+ T‐cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations, stained with fluorochrome‐labelled CD4 antibody and sorted by using BD Aria II (BD Biosciences, Heidelberg, Germany). This process resulted in cells with a purity of 97–99%. For adoptive transfer experiments, 3 × 106 to 5 × 106 sorted CD4+ Foxp3+(eGFP+) or CD4+ Foxp3− (eGFP−) T cells from Foxp3/eGFP reporter mice were injected intravenously into Thy1.1 BALB/c mice and infected with 1 × 105 infected red blood cells at the same day.
Antibodies and flow cytometry
Anti‐CD4, anti‐CD25 (BD Biosciences, Heidelberg, Germany), anti‐T‐cell receptor‐Vβ (TCR‐Vβ) antibodies (Biolegend, London, UK), anti‐Helios, anti‐CD90.2 (eBioscience, Frankfurt, Germany) and anti‐Nrp‐1 antibodies (R&D Systems, Abingdon, UK) were used as Pacific Blue, phycoerythrin, eFluor450, allophycocyanin or peridinin‐chlorophyll protein conjugates. Dead cells were identified by staining with the fixable viability dye eFluor 780 (eBioscience). Flow cytometric expression analysis was performed with an LSR II instrument using DIVA software (BD Biosciences).
Methylation analysis
Quantification of methylation was performed as described recently.23 Briefly, DNA was extracted from sorted T‐cell subsets isolated from P. yoelii‐infected Foxp3/eGFP male mice by using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations and bisulphite DNA was generated using the BisulFlash DNA Modification kit (Epigentek, Farmingdale, NY). Quantitative real‐time PCR was performed by using specific primers (5′‐AAA TTT GTG GGG TAG ATT ATT TGT TTT TT‐3′ and 5′‐ATC ACA ACC TAA ACT TAA CCA AAT TTT TCT‐3′), specific VIC‐ or FAM‐labelled TaqMan probes detecting methylated progenitors (5′‐ATT CGG TCG TTA TGA CGT T‐3′) or demethylated progenitors (5′‐ATT TGG TTG TTA TGA TGT TAA T‐3′) and Roche TaqMan Probe Master 480 (Roche Diagnostics, Basel, Switzerland) on a Roche Light cycler 480 system.
Statistical analysis
Statistical analyses were performed with one‐way analysis of variance and two‐tailed Student's t‐test as indicated with significance set at the levels of *P < 0·05, **P < 0·01 and ***P < 0·001. All analyses were calculated with graph pad prism 5·0 software (Graph Pad Software, La Jolla, CA).
Results
Increase in Foxp3+ Treg cells with reduced frequencies of Nrp‐1 expressing cells at early time‐points during P. yoelii infection
We made use of the well established P. yoelii infection model of BALB/c mice to carefully characterize the CD4+ Foxp3+ Treg cell population during Plasmodium infection. Flow cytometric analysis revealed higher percentages and numbers of Foxp3+ Treg cells within spleen of P. yoelii‐infected mice at days 3 and 5 post‐infection (p.i.), respectively (Fig. 1a). These cells exhibited an activated phenotype as determined by the expression level of CD25 (Fig. 1b). However, it is unclear whether this increase in Treg cells is caused by the expansion of thymus‐derived nTreg cells or by the induction of iTreg cells within the periphery.
Figure 1.
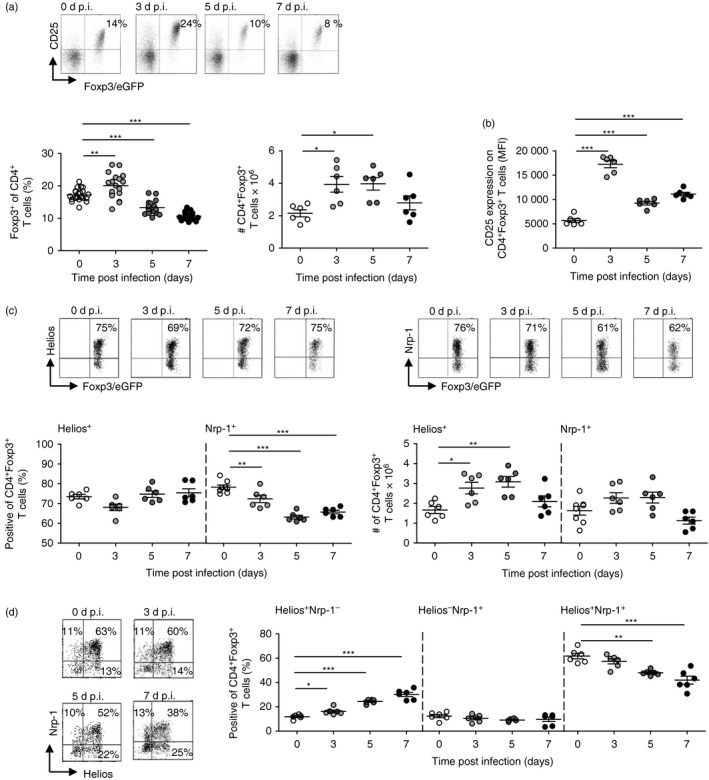
Plasmodium yoelii infection of BALB/c mice resulted in reduced percentages of neuropilin‐1 (Nrp‐1)‐expressing Foxp3+ regulatory T (Treg) cells, with unaffected Helios expression. Foxp3/eGFP reporter mice were infected with 1 × 105 infected red blood cells (iRBC) intravenously. Non‐infected [0 days post‐infection (d p.i.)] and P. yoelii‐infected Foxp3/eGFP mice were killed at 3, 5 and 7 d p.i. (a) The frequency (left panel) and absolute number (right panel) of Foxp3+ (eGFP+) Treg cells and (b) the mean fluorescence intensity (MFI) of CD25 expression on Foxp3+ Treg cells were determined on gated CD4+ T cells and CD4+ Foxp3+ Treg cells, respectively by flow cytometry. Representative dot plots are shown in the upper panel. (c) Helios and neuropilin‐1 (Nrp‐1) expression were analysed on CD4+ Foxp3+ Treg cells by flow cytometry. Representative dot plots are shown in the upper panel. Percentages and absolute numbers of Helios and Nrp‐1‐expressing Treg cells are summarized. (d) The frequencies of Helios+ Nrp‐1−, Helios− Nrp‐1+ and Helios+ Nrp‐1+ co‐expressing Foxp3+ Treg cells were determined by flow cytometry. Representative dot plots are shown in the left panel. Results from two independent experiments with n = 6 mice are summarized as mean ± SEM. Each dot represents one animal. One‐way analysis of variance with Dunett's post test was used for statistical analysis. *P < 0·05, **P < 0·01, ***P < 0·001.
To gain further insights into the origin of Treg cells at early time‐points during P. yoelii infection we analysed the expression of Helios and Nrp‐1, both molecules proposed to identify nTreg cells, on Foxp3+ Treg cells by flow cytometry. As depicted in Fig. 1(c), percentages of Helios‐expressing Foxp3+ Treg cells did not alter during P. yoelii infection, but we detected elevated numbers of Helios+ Foxp3+ Treg cells in infected mice compared with uninfected controls (Fig. 1c). In contrast, the frequency of Nrp‐1 expressing Foxp3+ Treg cells significantly decreased in spleen from P. yoelii‐infected mice at days 3, 5 and 7 p.i. in comparison to non‐infected mice, whereas the absolute numbers slightly increased at early time‐points during infection (Fig. 1c). Interestingly, analysis of Nrp‐1 and Helios co‐expression on Foxp3+ Treg cells revealed increasing percentages of Helios+ Nrp‐1− Foxp3+ Treg cells concomitant with decreasing frequencies of Helios+ Nrp‐1+ Foxp3+ Treg cells in the course of infection (Fig. 1d). These results suggest that analysing Nrp‐1 and Helios expression seems not to be sufficient to determine whether P. yoelii infection results in an expansion of thymus‐derived nTreg cells or peripheral Treg induction.
Similar T‐cell receptor‐Vβ chain repertoire of Nrp‐1+ and Nrp‐1− Foxp3+ Treg cells from P. yoelii‐infected mice
To get more information about the origin of Foxp3+ Treg cells in P. yoelii‐infected mice in particular with regard to Nrp‐1 expression, we next analysed the TCR‐Vβ chain repertoire on gated Nrp‐1+ and Nrp‐1− Foxp3+ Treg cells in comparison with Foxp3− T cells isolated from spleens of non‐infected and P. yoelii‐infected mice 3 and 7 days p.i. (Fig. 2a). Interestingly, similar to Foxp3+ Treg cells, the frequencies of most TCR‐Vβ chains were unaffected upon infection in Foxp3− T cells, suggesting a polyclonal T‐cell response to a mitogen released by the parasite, as already proposed.24 Nevertheless, at day 7 p.i. the frequency of Nrp‐1+ Foxp3+ Treg cells with TCR‐Vβ chain 8.1/8.2 usage was reduced, whereas the percentage of TCR‐Vβ11+ cells was increased (Fig. 2b). Similarly, we detected elevated levels of TCR‐Vβ + Nrp‐1− Foxp3+ Treg cells in mice infected with P. yoelii for 7 days compared with non‐infected mice (Fig. 2c), suggesting that both Foxp3+ Treg subsets originate from the same progenitor. Although the TCR‐Vβ usage by Foxp3− T cells was similar to Foxp3+ Treg cells, we detected lower frequencies of TCR‐Vβ7 and TCR‐Vβ9 and increased percentages of TCR‐Vβ6 and TCR‐Vβ11 expressing CD4+ Foxp3− T cells at day 7 p.i. (Fig. 2d).
Figure 2.
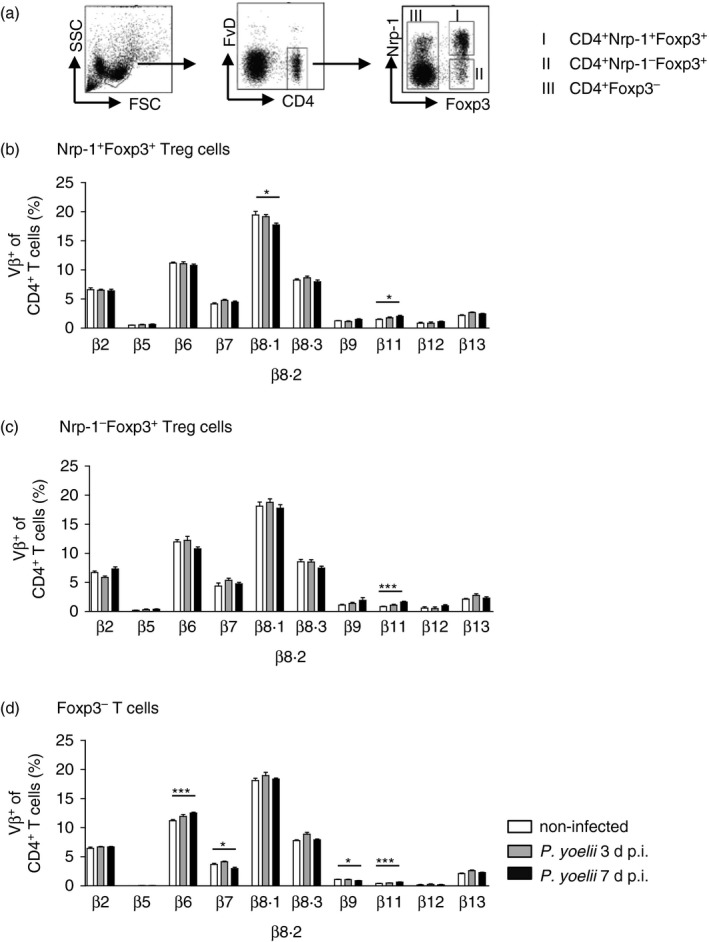
T‐cell receptor‐Vβ (TCR‐Vβ) chain usage of neuropilin‐1‐positive (Nrp‐1+) and Nrp‐1− Foxp3+ regulatory T (Treg) cells at day 3 and day 7 after Plasmodium yoelii infection. (a) Representative dot plots illustrating the gating strategy for analysis of TCR‐Vβ chain repertoire on different CD4+ T‐cell subsets by flow cytometry. Percentages of (b) Nrp‐1+ Foxp3+ Treg cells, (c) Nrp‐1− Foxp3+ Treg cells and (d) Foxp3− T cells using TCR‐Vβ2, TCR‐Vβ5, TCR‐Vβ6, TCR‐Vβ8.1/8.2, TCR‐Vβ8.3, TCR‐Vβ9, TCR‐Vβ11, TCR‐Vβ12 and TCR‐Vβ13 isolated from spleen of non‐infected [0 days post‐infection (d p.i.), white bars) and P. yoelii‐infected Foxp3/eGFP mice 3 days (grey bars) and 7 days p.i. (black bars). Results from two or three independent experiments with n = 6 to n = 10 mice in total are summarized as mean ± SEM. One‐way analysis of variance with Dunett's post test was used for statistical analysis. *P < 0·05, ***P < 0·001.
TSDR methylation analysis of Nrp‐1+ and Nrp‐1− Foxp3+ Treg cells from P. yoelii‐infected mice
Demethylation of CpGs within the TSDR located in the foxp3 locus has been correlated with a long‐term maintenance in Foxp3 expression, which is a specific feature of thymus‐derived nTreg cells.25 In contrast, some iTreg cell subpopulations might lose their Foxp3 expression upon activation and display a complete or at least intermediate TSDR methylation.26 To analyse whether Nrp‐1+ and Nrp‐1− Foxp3+ Treg subpopulations detected during P. yoelii infection differ in terms of stability in Foxp3 expression, we performed methylation analysis of the TSDR of sorted Nrp‐1+ and Nrp‐1− Foxp3+ Treg cells as well as Nrp‐1+ and Nrp‐1− Foxp3− T cells isolated from non‐infected and P. yoelii‐infected Foxp3/eGFP reporter mice.
Within the Foxp3− T‐cell subset we did not observe any significant difference in the TSDR methylation status between Nrp‐1+ Foxp3− and Nrp‐1− Foxp3− T cells isolated from P. yoelii‐infected and non‐infected mice (Fig. 3a). Nrp‐1+ Foxp3+ Treg cells from non‐infected, but also from P. yoelii‐infected mice exhibited 1–5% methylation within the TSDR (Fig. 3b) suggesting that these cells stably express Foxp3, a typical feature of nTreg cells. Similar results were also described by Weiss et al., who detected complete demethylation of the TSDR in Nrp‐1+ Foxp3+ Treg cells from WT mice.17 Importantly, the majority (> 90%) of Nrp‐1− Foxp3+ Treg cells isolated from P. yoelii‐infected mice also showed TSDR demethylation (Fig. 3b), indicating stable Foxp3 expression in most of these cells.
Figure 3.
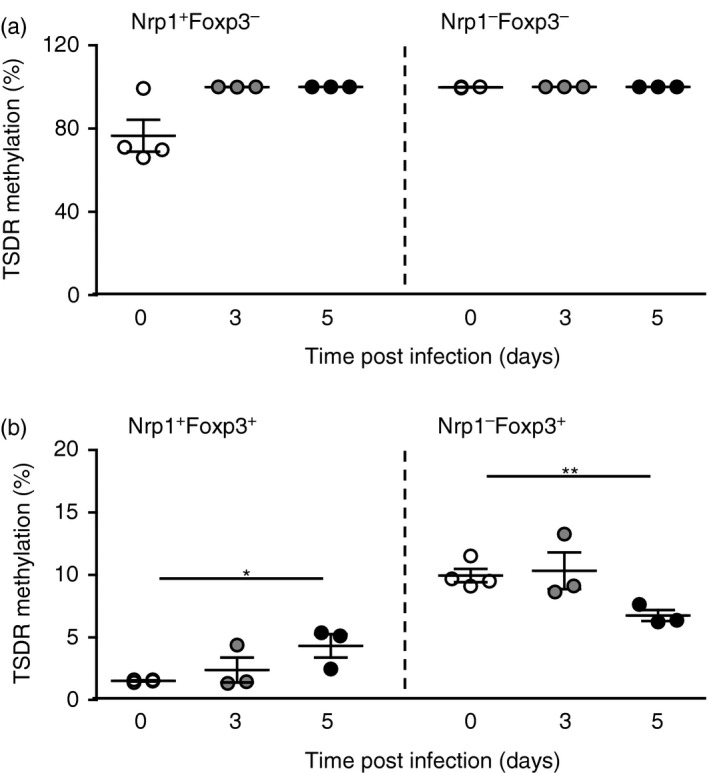
The majority of neuropilin‐1‐positive (Nrp‐1+) and Nrp‐1− Foxp3+ regulatory T (Treg) cells from Plasmodium yoelii‐infected mice are highly demethylated in their Treg‐specific demethylation region (TSDR). (a) Nrp‐1+ Foxp3− and Nrp‐1− Foxp3− T cells as well as (b) Nrp‐1+ Foxp3+ and Nrp‐1− Foxp3+ Treg cells were isolated from spleen of non‐infected Foxp3/eGFP reporter mice and P. yoelii‐infected Foxp3/eGFP mice at days 3 and 5 post‐infection (d p.i.). DNA was isolated and bisulphate treated before methylation analysis by real‐time PCR. Results from three or four independent experiments with n = 4 to n = 6 mice each were summarized as mean ± SEM. One‐way analysis of variance with Dunett's post test was used for statistical analysis. *P < 0·05, **P < 0·01.
Adoptively transferred Foxp3− T cells did not acquire Foxp3 expression upon P. yoelii infection
Results from our Helios expression analysis, TCR‐Vβ chain usage and TSDR methylation analysis suggest that P. yoelii infection resulted in an expansion of nTreg cells with stable Foxp3 expression rather than induction of Foxp3+ Treg cells from Foxp3− precursors within the periphery. To further corroborate these findings we adoptively transferred sorted Thy1.2+ CD4+ Foxp3− T cells or Thy1.2+ CD4+ Foxp3+ T cells into Thy1.1+ BALB/c mice before P. yoelii infection (Fig. 4a). At days 3 and 5 p.i. Foxp3/eGFP expression was analysed in Thy1.2+ splenocytes by flow cytometry. As depicted in Fig. 4 we did not detect induction of Foxp3 expression in adoptively transferred Foxp3− T cells upon P. yoelii infection. Only 1% of transferred Thy1.2+ CD4+ T cells expressed Foxp3, whereas 16–19% of endogenous Thy1.1+ CD4+ T cells in non‐infected and P. yoelii‐infected mice exhibited Foxp3 expression, respectively (Fig. 4b,c). These results indicate that P. yoelii infection of BALB/c mice was not sufficient to confer Foxp3 expression to peripheral CD4+ Foxp3− T cells.
Figure 4.
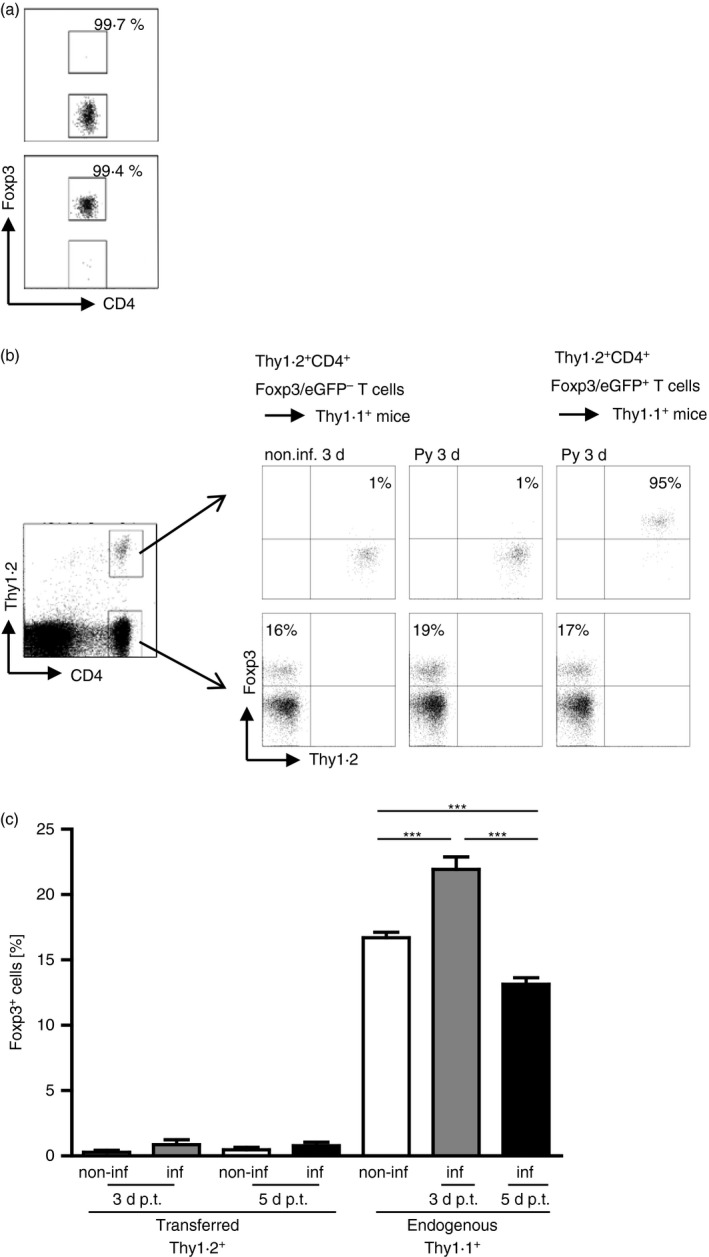
Adoptively transferred CD4+ Foxp3− T cells do not acquire Foxp3 expression in Plasmodium yoelii‐infected mice. From 3 × 106 to 5 × 106 sorted Thy1.2+ CD4+ Foxp3− T cells or Thy1.2+ CD4+ Foxp3+ T cells from naive Foxp3/eGFP mice were injected intravenously into Thy1.1+ BALB/c mice before infection with 1 × 105 infected red blood cells (iRBC) or in naive, non‐infected mice. At 3 and 5 days post‐infection (d p.i.) mice were killed and Foxp3 expression was analysed in gated CD4+ Thy1.2+ and CD4+ Thy1.1+ T cells, respectively by flow cytometry. (a) Purity of sorted CD4+ Foxp3/eGFP− T cells and CD4+ Foxp3/eGFP+ T cells is shown in representative dot plots. (b) Representative dot plots for either non‐infected or P. yoelii‐infected Thy1.1 mice receiving either Thy1.2+ CD4+Foxp3− T cells or Thy1.2+ CD4+ Foxp3+ T cells 3 d p.i. / cell transfer. (c) Data from two or three independent experiments with n = 4 to n = 11 mice in total are summarized as mean ± SEM. Student's t‐test was used for statistical analysis. ***P < 0·001.
Discusssion
In our previous study, we demonstrated that P. yoelii infection of BALB/c mice resulted in a significant increase in Treg cell numbers in the spleens of infected mice interfering with effective clearance of the parasite.7 Hence, Treg cells might represent a promising target to modulate the anti‐parasitic immune response for the development of immune‐based therapeutics and improvement of vaccination strategies that target the blood stage of Plasmodium infection. However, for this purpose careful phenotypic characterization of these Treg cells is necessary. Here, we provide evidence that P. yoelii infection of BALB/c mice triggers expansion of thymus‐derived Foxp3+ nTreg cells rather than de novo induction in the periphery.
Both Helios and Neuropilin‐1 have been proposed as reliable marker molecules to discriminate nTreg cells from iTreg cells.14, 17, 18 Interestingly, the frequency of Nrp‐1 expressing Foxp3+ Treg cells declined in P. yoelii‐infected mice whereas the percentage of Helios+ Foxp3+ Treg cells remained unchanged, suggesting that at least one of these molecules is not suitable for identifying nTreg cells during Plasmodium infection.
Helios expression was originally identified to be restricted to Foxp3+ nTreg cells as the majority of Foxp3+ Treg cells in naive mice expresses the transcription factor Helios.14 However, contradictory results have been described for the expression of Helios in in vivo induced Treg cells. Whereas Foxp3+ Treg cells induced by administration of antigen through the oral route exhibited no Helios expression,14 intravenous injection of low‐dose antigen resulted in elevated Helios expression in Foxp3+ iTreg cells16 suggesting that the use of Helios as a specific marker for nTreg cells seems to be dependent on the in vivo environment.
Interestingly, we detected significantly lower percentages of Nrp‐1‐expressing Foxp3+ Treg cells in P. yoelii‐infected mice. This might suggest that increased Foxp3+ Treg cells represent induced Treg cells because Nrp‐1 was described as highly expressed by thymus‐derived nTreg cells.17, 18 However, similar to Helios, the usage of Nrp‐1 as an nTreg marker seems to be dependent on the in vivo environment. Weiss et al. detected elevated Nrp‐1 expression on Foxp3+ iTreg cells under inflammatory conditions17 and, most recently Petzold and colleagues described an up‐regulation of Nrp‐1 on Nrp‐1− iTreg progenitors upon TCR engagement in the presence of interleukin‐2.27 Hence, the reliability of Nrp‐1 and Helios as marker molecules to discriminate nTreg cells from iTreg cells is still discussed controversially and seems to be dependent on the site and mode of Treg cell induction. Moreover it is unclear, whether these marker molecules define specific subpopulations of Foxp3+ Treg cells exhibiting different functions or whether the expression of Helios and Nrp‐1 on Foxp3+ Treg cells is regulated based on their activation and/ or maturation. This issue has to be clarified in future experiments in the context of different types of immune responses. Interestingly, we detected elevated percentages of Helios+ Nrp‐1− Foxp3+ Treg cells in the course of infection in contrast to decreasing frequencies of Helios+ Nrp‐1+ Foxp3+ Treg cells. This observation might reflect either down‐regulation of Nrp‐1 on Helios+ Foxp3+ Treg cells or expansion of Helios+ Nrp‐1− Foxp3+ Treg cells. Since Helios expression was also correlated with the activation status of CD4+ T cells independent of Foxp3 expression,15 the significant increase in the number of Helios‐expressing cells within Foxp3+ Treg cells in P. yoelii‐infected mice, might reflect the activated phenotype of Treg cells at early time‐points during P. yoelii infection underpinned by an up‐regulation of CD25 expression on Foxp3+ Treg cells upon P. yoelii infection. We also observed an up‐regulation of Helios expression on Foxp3− T cells in the course of P. yoelii infection (data not shown).
From our TCR‐Vβ chain usage analysis one might speculate about the same origin of both Nrp‐1+ and Nrp‐1− Treg cells because we detected an increase in TCR‐Vβ11 usage in both populations upon P. yoelii infection. Within the Foxp3− T‐cell population we also observed elevated percentages of TCR‐Vβ11+ cells in P. yoelii‐infected mice, but additionally decreased frequencies of TCR‐Vβ7 and TCR‐Vβ9 expressing cells. Preferential deletion of T cells expressing TCR‐Vβ9 was already described in P. yoelii‐infected C57BL/6 mice and proposed to be a result of super‐antigenic activity during acute infection.28 However, Swardson and colleagues observed no differences in the course of P. yoelii infection between BALB/c mice and BALB/c.D2 mice, which express a super‐antigen encoded by an endogenous retroviral gene resulting in deletion of TCR‐Vβ9+ T cells.29 Moreover, TCR‐Vβ repertoire studies in African children suffering from malaria argue against the idea of dominant super‐antigenic activities.30 Therefore, further studies with regard to the TCR‐Vβ profile and also TCR‐Vα chain usage by T cells during Plasmodium infection would be helpful to better understand elicitation and regulation of parasite‐specific adaptive immune response.
Analysis of TSDR methylation of Nrp‐1+ and Nrp‐1− Foxp3+ Treg cells as well as Nrp‐1+ and Nrp‐1− Foxp3+ T cells from P. yoelii‐infected mice revealed an almost complete TSDR demethylation in Foxp3+ Treg cells independent of Nrp‐1 expression, whereas Foxp3− T cells exhibited 100% TSDR methylation upon P. yoelii infection. Interestingly, at day 5 p.i. the methylation status of the TSDR in Nrp‐1− Foxp3+ Treg cells significantly decreased, which might suggest that stable Foxp3‐expressing nTreg cells lose their Nrp‐1 expression in the course of infection. This would argue for an expansion of thymus‐derived nTreg cells upon P. yoelii infection in line with our TCR‐Vβ usage analysis. However, as we have not analysed all TCR‐Vβ chains, we could not exclude that T‐cell clones with distinct specificities are also affected during P. yoelii infection.
We showed that the level of CD4+ Foxp3+ Treg cells was elevated post P. yoelii infection, whereas the expression of Foxp3 was not induced in adoptively transferred Foxp3− T cells in mice previously infected with P. yoelii. These results argue against de novo induction of Foxp3+ Treg cells during P. yoelii infection. However, we and others detected adaptive interleukin‐10 producing CD4+ Foxp3− T cells that were generated during P. yoelii infection.7, 31 These cells exhibited immunosuppressive function in vitro and T‐cell‐specific interleukin‐10 deletion resulted in enhanced T‐cell activation in P. yoelii‐infected mice.7 Hence, our data provide evidence that P. yoelii infection of BALB/c mice triggers induction of interleukin‐10‐producing Foxp3− Treg cells and expansion of pre‐existing Foxp3+ thymus‐derived nTreg cells. Similar results were also described for viral infections. Foxp3− T cells did not acquire Foxp3 expression upon adoptive transfer to Friend virus‐infected32 or lymphocytic choriomeningitis virus‐infected mice,33 suggesting expansion of nTreg cells rather than conversion of non‐Treg cells into Foxp3+ iTreg cells during these viral infections.
With regard to future therapeutic interventions one might think of blocking molecules highly expressed by nTreg cells using small interfering RNA approaches or application of specific antibodies. Since we observed elevated frequencies of Helios‐expressing Foxp3+ Treg cells and Helios+ Foxp3+ Treg cells have been described to have a high suppressive activity,34 probably due to their more activated phenotype, Helios might be an attractive target to interfere with Foxp3+ Treg cell function during Plasmodium infection. However, one has to keep in mind that Helios is also up‐regulated on Foxp3− T cells during infection and modulating the intrinsic suppressive function of Foxp3+ Treg cells might also result in immunopathological side effects. Therefore, approaches to prevent expansion of nTreg cells would be a more reasonable therapeutic strategy. Our findings should foster further experiments to identify specific factors that support nTreg expansion in Plasmodium infection because they represent promising targets for therapeutic regulation of anti‐parasitic immune responses.
Disclosure
The authors declare no conflict of interest.
Acknowledgements
We are thankful to Carolin Wevers and Sina Luppus for excellent technical assistance and Patrick Juszczak and Witold Bartosik for cell sorting. This work was supported by Deutsche Forschungsgemeinschaft (GRK1949) to W.H.
References
- 1. Maizels R. Regulation of the immune system in metazoan parasite infections. Novartis Found Symp 2007; 281:192–204. [DOI] [PubMed] [Google Scholar]
- 2. McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi . J Immunol 2008; 181:6456–66. [DOI] [PubMed] [Google Scholar]
- 3. Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci U S A 2001; 98:9226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kandulski A, Wex T, Kuester D, Peitz U, Gebert I, Roessner A, Malfertheiner P. Naturally occurring regulatory T cells (CD4+, CD25high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF‐β1. Helicobacter 2008; 13:295–303. [DOI] [PubMed] [Google Scholar]
- 5. Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF et al Upregulation of TGF‐β, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 2005; 23:287–96. [DOI] [PubMed] [Google Scholar]
- 6. Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN et al Parasite‐dependent expansion of TNF receptor II‐positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 2009; 5:e1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abel S, Luckheide N, Westendorf AM, Geffers R, Roers A, Muller W, Sparwasser T et al Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell‐derived IL‐10 on pathogen clearance during Plasmodium yoelii infection. J Immunol 2012; 188:5467–77. [DOI] [PubMed] [Google Scholar]
- 8. Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med 2004; 199:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H et al On the edge of autoimmunity: T‐cell stimulation by steady‐state dendritic cells prevents autoimmune diabetes. Diabetes 2005; 54:3395–401. [DOI] [PubMed] [Google Scholar]
- 10. Hansen W, Westendorf AM, Reinwald S, Bruder D, Deppenmeier S, Groebe L, Probst‐Kepper M et al Chronic antigen stimulation in vivo induces a distinct population of antigen‐specific Foxp3 CD25 regulatory T cells. J Immunol 2007; 179:8059–68. [DOI] [PubMed] [Google Scholar]
- 11. Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 2002; 3:756–63. [DOI] [PubMed] [Google Scholar]
- 12. Larkin J III, Rankin AL, Picca CC, Riley MP, Jenks SA, Sant AJ, Caton AJ. CD4+CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self‐antigen. J Immunol 2008; 180:2149–57. [DOI] [PubMed] [Google Scholar]
- 13. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009; 30:626–35. [DOI] [PubMed] [Google Scholar]
- 14. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184:3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS ONE 2011; 6:e24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 2012; 188:976–80. [DOI] [PubMed] [Google Scholar]
- 17. Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H et al Neuropilin 1 is expressed on thymus‐derived natural regulatory T cells, but not mucosa‐generated induced Foxp3+ T reg cells. J Exp Med 2012; 209:1723–42, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yadav M, Louvet C, Davini D, Gardner JM, Martinez‐Llordella M, Bailey‐Bucktrout S, Anthony BA et al Neuropilin‐1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo . J Exp Med 2012; 209:1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruder D, Probst‐Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H et al Neuropilin‐1: a surface marker of regulatory T cells. Eur J Immunol 2004; 34:623–30. [DOI] [PubMed] [Google Scholar]
- 20. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K et al Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T‐cell lineage? Nat Rev Immunol 2009; 9:83–9. [DOI] [PubMed] [Google Scholar]
- 22. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S et al DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008; 38:1654–63. [DOI] [PubMed] [Google Scholar]
- 23. Tatura R, Zeschnigk M, Hansen W, Steinmann J, Goncales Vidigal P, Pastille E, Buer J et al Relevance of Foxp3+ regulatory T cells for early and late phase of murine sepsis. Immunology 2015; 146:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenwood BM, Oduloju AJ, Platts‐Mills TA. Partial characterization of a malaria mitogen. Trans R Soc Trop Med Hyg 1979; 73:178–82. [DOI] [PubMed] [Google Scholar]
- 25. Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, Baron U et al Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol 2013; 190:3180–8. [DOI] [PubMed] [Google Scholar]
- 26. Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J et al Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012; 36:262–75. [DOI] [PubMed] [Google Scholar]
- 27. Petzold C, Steinbronn N, Gereke M, Strasser RH, Sparwasser T, Bruder D, Geffers R et al Fluorochrome‐based definition of naturally occurring Foxp3 regulatory T cells of intra‐ and extrathymic origin. Eur J Immunol 2014; 44:3632–45. [DOI] [PubMed] [Google Scholar]
- 28. Pied S, Voegtle D, Marussig M, Renia L, Miltgen F, Mazier D, Cazenave PA. Evidence for superantigenic activity during murine malaria infection. Int Immunol 1997; 9:17–25. [DOI] [PubMed] [Google Scholar]
- 29. Swardson CJ, Wassom DL, Avery AC. Plasmodium yoelii: resistance to disease is linked to the mtv‐7 locus in BALB/c mice. Exp Parasitol 1997; 86:102–9. [DOI] [PubMed] [Google Scholar]
- 30. Loizon S, Boeuf P, Tetteh JK, Goka B, Obeng‐Adjei G, Kurtzhals JA, Rogier C et al Vβ profiles in African children with acute cerebral or uncomplicated malaria: very focused changes among a remarkable global stability. Microbes Infect 2007; 9:1252–9. [DOI] [PubMed] [Google Scholar]
- 31. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA et al IL‐10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 2008; 4:e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Myers L, Joedicke JJ, Carmody AB, Messer RJ, Kassiotis G, Dudley JP, Dittmer U et al IL‐2‐independent and TNF‐α‐dependent expansion of Vβ5+ natural regulatory T cells during retrovirus infection. J Immunol 2013; 190:5485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Punkosdy GA, Blain M, Glass DD, Lozano MM, O'Mara L, Dudley JP, Ahmed R et al Regulatory T‐cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci U S A 2011; 108:3677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh K, Hjort M, Thorvaldson L, Sandler S. Concomitant analysis of Helios and Neuropilin‐1 as a marker to detect thymic derived regulatory T cells in naive mice. Sci Rep 2015; 5:7767. [DOI] [PMC free article] [PubMed] [Google Scholar]


