Abstract
OBJECTIVES--To evaluate whether a triple dose of gadolinium-DTPA (Gd-DTPA) or delayed MRI increase the number, size, and conspicuousness of enhancing lesions in patients with benign multiple sclerosis. METHODS--T1 weighted brain MRI was carried out on 20 patients with benign multiple sclerosis (expanded disability status scale < 3 with a disease duration > 10 years) in two sessions. In the first session, one scan was obtained before and two scans five to seven minutes and 20-30 minutes after the injection of 0.1 mmol/kg Gd-DTPA (standard dose). In the second session, six to 24 hours later, the same procedure was repeated with 0.3 mmol/kg Gd-DTPA (triple dose). RESULTS--Nine enhancing lesions were found in seven patients (35%) using the standard dose of Gd-DTPA. The numbers of enhancing lesions increased to 13 (P = 0.03) and the number of patients with such lesions to eight (40%) on the delayed standard dose scans. On the early triple dose scans, we found 19 enhancing lesions in 10 patients (50%). The number of enhancing lesions was significantly higher (P = 0.01) than that obtained with the early standard dose. The number of enhancing lesions was 18 and the number of "active" patients 11 (55%) on the delayed triple dose scans. The enhancing areas increased progressively from the early standard dose scans to the delayed triple dose scans. The contrast ratios of the lesions detected in early standard dose scans was lower than those of lesions present in the early (P = 0.01) and delayed (P = 0.04) triple dose scans. CONCLUSIONS--More enhancing lesions were detected in patients with benign multiple sclerosis with both delay of MRI and the use of triple dose of Gd-DTPA suggesting that the amount of inflammation in the lesions of such patients is mild and heterogeneous.
Full text
PDF

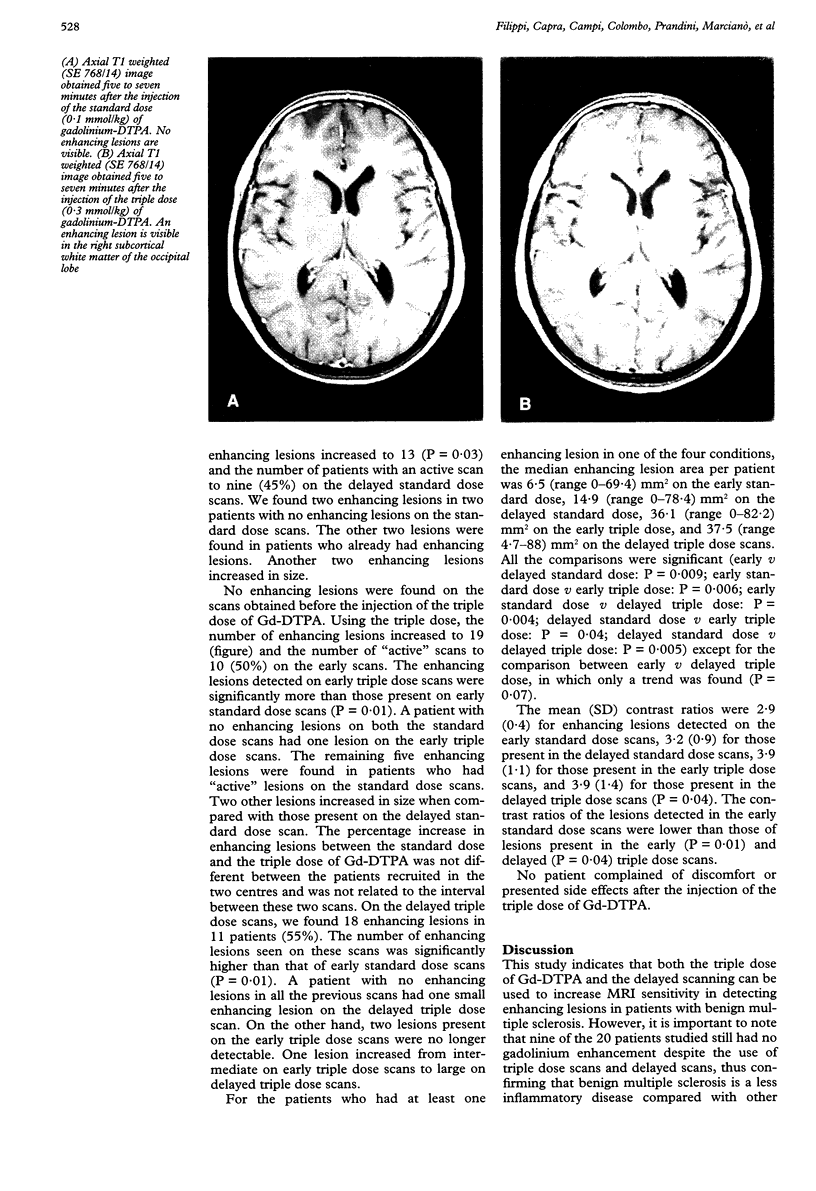


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Filippi M., Barker G. J., Horsfield M. A., Sacares P. R., MacManus D. G., Thompson A. J., Tofts P. S., McDonald W. I., Miller D. H. Benign and secondary progressive multiple sclerosis: a preliminary quantitative MRI study. J Neurol. 1994 Feb;241(4):246–251. doi: 10.1007/BF00863776. [DOI] [PubMed] [Google Scholar]
- Filippi M., Campi A., Dousset V., Baratti C., Martinelli V., Canal N., Scotti G., Comi G. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology. 1995 Mar;45(3 Pt 1):478–482. doi: 10.1212/wnl.45.3.478. [DOI] [PubMed] [Google Scholar]
- Filippi M., Campi A., Martinelli V., Colombo B., Yousry T., Canal N., Scotti G., Comi G. Comparison of triple dose versus standard dose gadolinium-DTPA for detection of MRI enhancing lesions in patients with primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995 Nov;59(5):540–544. doi: 10.1136/jnnp.59.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Horsfield M. A., Morrissey S. P., MacManus D. G., Rudge P., McDonald W. I., Miller D. H. Quantitative brain MRI lesion load predicts the course of clinically isolated syndromes suggestive of multiple sclerosis. Neurology. 1994 Apr;44(4):635–641. doi: 10.1212/wnl.44.4.635. [DOI] [PubMed] [Google Scholar]
- Gass A., Barker G. J., Kidd D., Thorpe J. W., MacManus D., Brennan A., Tofts P. S., Thompson A. J., McDonald W. I., Miller D. H. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol. 1994 Jul;36(1):62–67. doi: 10.1002/ana.410360113. [DOI] [PubMed] [Google Scholar]
- Gerard G., Weisberg L. A. MRI periventricular lesions in adults. Neurology. 1986 Jul;36(7):998–1001. doi: 10.1212/wnl.36.7.998. [DOI] [PubMed] [Google Scholar]
- Kermode A. G., Tofts P. S., Thompson A. J., MacManus D. G., Rudge P., Kendall B. E., Kingsley D. P., Moseley I. F., du Boulay E. P., McDonald W. I. Heterogeneity of blood-brain barrier changes in multiple sclerosis: an MRI study with gadolinium-DTPA enhancement. Neurology. 1990 Feb;40(2):229–235. doi: 10.1212/wnl.40.2.229. [DOI] [PubMed] [Google Scholar]
- Kidd D., Thompson A. J., Kendall B. E., Miller D. H., McDonald W. I. Benign form of multiple sclerosis: MRI evidence for less frequent and less inflammatory disease activity. J Neurol Neurosurg Psychiatry. 1994 Sep;57(9):1070–1072. doi: 10.1136/jnnp.57.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd D., Thorpe J. W., Thompson A. J., Kendall B. E., Moseley I. F., MacManus D. G., McDonald W. I., Miller D. H. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993 Dec;43(12):2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Miller D. H., Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992 Aug;18(4):319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Barkhof F., Berry I., Kappos L., Scotti G., Thompson A. J. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry. 1991 Aug;54(8):683–688. doi: 10.1136/jnnp.54.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz T., Kidd D., Thompson A. J., Barnard R. O., McDonald W. I. A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain. 1994 Aug;117(Pt 4):759–765. doi: 10.1093/brain/117.4.759. [DOI] [PubMed] [Google Scholar]
- Sears E. S., McCammon A., Bigelow R., Hayman L. A. Maximizing the harvest of contrast enhancing lesions in multiple sclerosis. Neurology. 1982 Aug;32(8):815–820. doi: 10.1212/wnl.32.8.815. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Stone L. A., Albert P. S., Frank J. A., Martin R., Armstrong M., Maloni H., McFarlin D. E., McFarland H. F. Clinical worsening in multiple sclerosis is associated with increased frequency and area of gadopentetate dimeglumine-enhancing magnetic resonance imaging lesions. Ann Neurol. 1993 May;33(5):480–489. doi: 10.1002/ana.410330511. [DOI] [PubMed] [Google Scholar]
- Spiegel S. M., Viñuela F., Fox A. J., Pelz D. M. CT of multiple sclerosis: reassessment of delayed scanning with high doses of contrast material. AJR Am J Roentgenol. 1985 Sep;145(3):497–500. doi: 10.2214/ajr.145.3.497. [DOI] [PubMed] [Google Scholar]
- Stone L. A., Frank J. A., Albert P. S., Bash C., Smith M. E., Maloni H., McFarland H. F. The effect of interferon-beta on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing-remitting multiple sclerosis. Ann Neurol. 1995 May;37(5):611–619. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., MacManus D. G., Kendall B. E., Kingsley D. P., Moseley I. F., McDonald W. I. Patterns of disease activity in multiple sclerosis: clinical and magnetic resonance imaging study. BMJ. 1990 Mar 10;300(6725):631–634. doi: 10.1136/bmj.300.6725.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. J., Miller D., Youl B., MacManus D., Moore S., Kingsley D., Kendall B., Feinstein A., McDonald W. I. Serial gadolinium-enhanced MRI in relapsing/remitting multiple sclerosis of varying disease duration. Neurology. 1992 Jan;42(1):60–63. doi: 10.1212/wnl.42.1.60. [DOI] [PubMed] [Google Scholar]
- Tofts P. S., Kermode A. G. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991 Feb;17(2):357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- Weinshenker B. G. The natural history of multiple sclerosis. Neurol Clin. 1995 Feb;13(1):119–146. [PubMed] [Google Scholar]
- Wicks D. A., Tofts P. S., Miller D. H., du Boulay G. H., Feinstein A., Sacares R. P., Harvey I., Brenner R., McDonald W. I. Volume measurement of multiple sclerosis lesions with magnetic resonance images. A preliminary study. Neuroradiology. 1992;34(6):475–479. doi: 10.1007/BF00598953. [DOI] [PubMed] [Google Scholar]
- van Walderveen M. A., Barkhof F., Hommes O. R., Polman C. H., Tobi H., Frequin S. T., Valk J. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology. 1995 Sep;45(9):1684–1690. doi: 10.1212/wnl.45.9.1684. [DOI] [PubMed] [Google Scholar]



