Abstract
An association between mood disturbance, the inability to lose or to stop gaining weight, and a craving for carbohydrates is manifested by many people who are overweight or are becoming so. In a recent study, we observed that low-calorie weight loss diet lowered not only levels of leptin but also levels of essential amino acid tryptophan (TRP) significantly. The disturbed metabolism of TRP might affect biosynthesis of serotonin and could thereby increase the susceptibility for mood disturbances and carbohydrate craving, increasing the cessation probability of weight reduction programs. Alternatively, moderate physical exercise – a potent stimulus to modulate (reduce/normalize) proinflammatory cytokines, which may affect TRP levels – could be helpful in improving mood status and preventing uncontrolled weight gain. In contrast, excessive physical exercise may induce breakdown of TRP when proinflammatory cascades together with TRP-degrading enzyme indoleamine 2,3-dioxygenase-1 are stimulated, which may lead to neuropsychiatric symptoms such as fatigue and low mood.
Keywords: diet, exercise, mood, tryptophan, weight loss maintenance
Introduction
More than 2.1 billion people – close to 30% of the global population – today are overweight or obese.1 Obesity can therefore be seen as a global epidemic and thus as a major public health problem. Both overweight and obesity are characterized by the accumulation of excessive levels of body fat, and this creates an increased risk for cardiovascular diseases, some types of cancer, and overall mortality.2 Recent evidence has shown similar adverse effects of obesity on the brain.3 In particular, abdominal obesity is associated with significant metabolic changes that impinge upon the central nervous system in even younger, nonclinical (= general) populations at midlife.4 Furthermore, obesity has been implicated to play a role in cognitive deficits in patients with dementia5 and mental disorders.6 The underlying reasons for becoming obese are not well understood; however, it is likely that both genes and environmental factors, especially an age-related decrease in physical activity, contribute to this problem.7
Obesity is caused by perturbations of the balance between food intake and energy expenditure. A chronic positive energy balance induces expansion of adipose tissue and recruitment of macrophages. Enlarged adipocytes and activated macrophages secrete proteins and lipids, known as adipokines, that influence inflammation and overall carbohydrate and lipid metabolism.8 Moreover, an upregulation of indoleamine 2,3-dioxygenase-1 (IDO1) activity, caused by immune-mediated inflammation, which catalyzes the formation of kynurenine (KYN) and limits the availability of tryptophan (TRP), could be a key component in the initiation of obesity.9 Thereby, the KYN pathway is induced,10 and the elevation of the ratio of KYN to TRP concentrations that estimates the TRP breakdown rate is often linked with conditions of inflammation11 and neuropsychiatric symptoms.12 The accelerated TRP breakdown during inflammation is mainly fed by endogenously formed interferon-γ and is also influenced by other cytokines, such as tumor necrosis factor-α, interleukin (IL)-6, and lipopolysaccharide.13,14 As a precursor for 5-hydroxytryptamine (serotonin), the essential amino acid TRP is also a key player in caloric intake regulation by predominantly inhibiting carbohydrate intake.15 In the epiphysis, the sleep-regulating hormone melatonin is biosynthesized out from serotonin.
Lifestyle modification, specifically changes in diet, physical activity, and exercise are considered the cornerstone of obesity management.16 However, the adherence of victims to diet and exercise regimens is often limited and they may quit after the first six months before reaching their goals.17 Recently, we observed that low-calorie weight loss diet lowered not only levels of leptin but also levels of TRP significantly.18 The disturbed metabolism of TRP might affect the biosynthesis of serotonin and could thereby increase the susceptibility for mood disturbances and carbohydrate craving, increasing the cessation probability of weight reduction programs (Fig. 1). On the other side, moderate physical exercise – a potent stimulus to modulate proinflammatory cytokines, which may affect TRP levels – could be helpful in improving mood status and preventing uncontrolled weight gain.19
Figure 1.
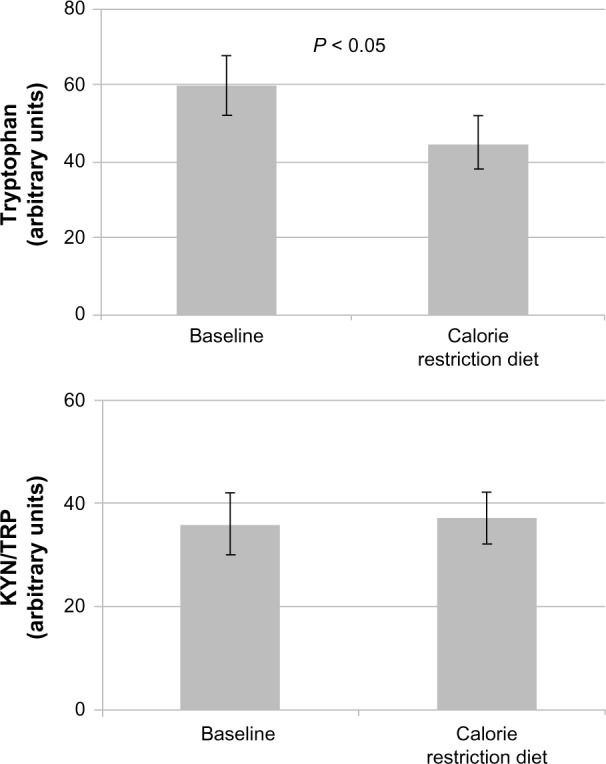
Low and very low calorie diet leads to a deficit of essential amino acid tryptophan because of insufficient dietary intake (upper graph), whereas the KYN-to-TRP ratio (lower graph) is not influenced.18
The purpose of this review is to provide a brief overview on the effects of diet and exercise on weight control and to describe the important role of TRP metabolism during dietetic interventions combined or not with physical exercise. A review of literature was conducted through PubMed database to identify relevant studies, especially published in the past 10 years. The following keywords were used alone or in various combinations: diet, exercise, mood, tryptophan, weight loss, and maintenance. Reference lists from original and review articles were also reviewed in order to identify additional relevant studies.
Diet- Versus Exercise-Induced Weight Loss
To lose weight, a negative energy balance must be evoked. Mechanistic influences on energy imbalance arise from changes in resting metabolic rate, nonexercise activity thermogenesis, fat-free mass, and energy intake. The impact of these changes on energy imbalance translates to changes in body weight.20 Dietary restriction and exercise are useful methods to create a negative energy balance. However, most of the exercise studies suggest that exercise activity alone has only a minor influence on body weight reduction21 and that mainly the combination of both diet and exercise training leads to significant reduction of body mass.22 The classic explanation for the secondary role of exercise is that exercise alone cannot generate enough energy expenditure to create a negative energy balance to the extent possible with caloric restriction. The frequency and intensity of exercise at public health recommendation levels may explain why exercise does not improve weight loss above that achieved with caloric restriction. Ross et al.23,24 showed that the energy expenditure of exercise has to be between 500 and 700 kcal/d to provide a body mass loss of approximately 6 kg in women and 8 kg in men within 12 weeks. This is true, but it is also true that, independent of the method of weight loss, a negative energy balance of 500–700 kcal/d always causes a fat loss of 6–8 kg within three months. When the energy deficit imposed by diet only and diet plus exercise interventions is similar, weight loss and/or percentage change in body weight are similar.25
Nevertheless, to be successful in losing weight, it takes a considerable amount of time and effort. Several studies have examined weight compensation after aerobic exercise training and concluded that the major factors limiting the expected weight loss from aerobic exercise were dietary compensation and a low exercise dose.20,26 Currently, fewer than 30% of European adults meet exercise guidelines with a lack of time, the major barrier to regular exercise participation. Accordingly, in contrast to traditional high-volume endurance training (at 3–5.9 metabolic equivalent tasks), vigorous intensity aerobic endurance training (at ≥6 metabolic equivalent tasks) or resistance training (muscular strength exercises using a resistance equivalent to 60%–80% of the individual’s one repetition maximal effort, including free weights, machines with stacked weights or pneumatic resistance, and resistance bands) can be a time-efficient strategy to reduce body fat and improve metabolic health.27 It has been shown that in terms of weight change, when different intensities of aerobic exercise are matched for caloric expenditure or dose, both vigorous and moderate intensity aerobic trainings result in similar amounts of weight loss.28 Furthermore, vigorous intensity aerobic endurance training can serve as an effective alternate to traditional endurance-based training, inducing even superior health benefits for important risk factors, including visceral fat,29 measures of glucose/insulin metabolism,30 and cardiorespiratory fitness.31 Although the addition of resistance training to dietary restriction has been shown to have limited effectiveness in reducing body weight – increases in muscle mass with resistance training may off-set loss of adipose tissue – the combination of diet with resistance training seems to be more effective than diet or diet with endurance training in the reduction of body mass and fat mass.32 Resistance training significantly increases resting metabolic rate after a training session33 and has the power to reduce low-grade inflammation independently from weight loss in overweight/obese adults.34 Muscle contractions lead to the production and release into the circulation of IL-6, which appears to have numerous biological effects, including effects on glucose and fat metabolism. In addition, IL-6 mediates anti-inflammatory effects. Acute elevations in IL-6 produced by contracting the skeletal muscle downregulate the production of tumor necrosis factor-α by monocytes and stimulate the release of IL-1 receptor antagonist from monocytes and macrophages, thus increasing the circulating concentrations of anti-inflammatory cytokines (such as adiponectin and IL-10) from adipose tissue.35
In addition to exercise interventions, many studies have manipulated the macronutrient content of energy-restricted diets and have reported favorable changes in body composition with many dietary approaches.36,37 Thus, reducing the calorie intake below expenditure results in a predictable initial rate of weight loss that is related to the energy deficit.38 Based on recent research and meta-analytic evidence, weight loss is achieved by adherence to any diet that successfully reduces calorie intake, but adherence rates are low with most diets.39 Although a low-carbohydrate diet may be associated with greater short-term weight loss, superior weight loss in the long-term has not been established.40 Furthermore, very-low–carbohydrate weight loss diets may be associated with more frequent side effects, such as mood swings.41 Carbohydrates are responsible for helping drive TRP across the blood–brain barrier and having an effect on the brain serotonin levels.42 Thus, by eating a low carbohydrate meal, the amount of TRP entering the brain will be substantially lower compared with the normal situation, contributing to symptoms of depression, such as mental fatigue and low mood. In addition, TRP competes with the other large neutral amino acids (LNAA) for transport across the blood–brain barrier. Since plasma amino acids change in obese persons on hypocaloric diet, a decrease in TRP–LNAA ratio may further influence serotonin synthesis.43 Previous work reported a decrease in plasma TRP and TRP/branched-chain amino acids (BCAAs) ratio in men and women by dieting.44
As a common consequence, caloric restriction weight loss diets, especially if based upon macronutrient content that is low in carbohydrate and high in protein, lead many dieters to revert to their old eating habits, eating more and using carbohydrate-rich foods, to feel better, which is a frequent cause of weight gain, the so-called yo-yo effect.45 In a recent study,18 concentrations of essential amino acid TRP decreased significantly with a caloric restriction weight loss diet, and lowest TRP concentrations were observed in the group of individuals with the lowest calorie intake. The decline of TRP levels can be referred to its reduced intake during caloric restriction diet as it was unrelated to the immune activation status of individuals, which remained unchanged. However, there may be gender differences in the response of plasma TRP to dieting. For example, in women, but not in men, dieting significantly lowered the plasma total and free TRP, indicating that dieting alters brain serotonin function in women, perhaps as a consequence of reducing the availability of plasma TRP.46
Calorie restriction decreases resting and total energy expenditure.47 Declines in energy expenditure favoring the regain of lost weight persist well beyond the period of dynamic weight loss.48 Findings from the POUNDS LOST Study confirmed a decrease in total energy expenditure, mainly contributed by a decrease in resting energy expenditure, in both men and women.49 The authors found, further, a decrease in energy expenditure from physical activity as dietary carbohydrate decreases followed by a decrease in lean body mass with a high fat diet.
Thus, weight loss leads to both physiological and psychological changes that promote subsequent weight regain. The path to overcome this tendency for weight regain may involve exercise and dietary strategies that improve adherence, counter the physiological and behavioral adaptations, and reestablish the balance between intake and expenditure.50
Improving Weight Loss Maintenance
Although many individuals have success in losing weight with diet, weight maintenance is a challenge, regardless of the initial modality used for weight loss. Long-term adherence to restrictive diets is made difficult because of the reduction in energy expenditure that is induced by weight loss but also because of changes in the peripheral hormone signals that increase appetite.51 Hence, most dieters subsequently regain much or all of the lost weight. Exercise and behavioral interventions may help individuals maintain weight loss since exercise programs modify responses in a direction expected to enhance satiety and permit weight loss and/or maintenance.50
Mechanisms of weight regain after weight loss
Weight loss leads to compensatory changes in the homeostatic processes, including alterations in energy expenditure, substrate metabolism, and hormone pathways involved in appetite regulation that result in increased hunger and energy storage, favoring weight regain.51 In a recent review, MacLean et al.52 summarized the adaptations to energy-restricted weight loss that are thought to promote weight regain. During weight loss, metabolic requirements decline as a function of (i) lost body mass, (ii) reduced consumption of food, and (iii) increased metabolic efficiency of peripheral tissues. Neuroendocrine signals from the periphery transfer a message of energy depletion and low nutrient availability – favoring signals of hunger – to the hypothalamus and hindbrain that serve as the primary control centers for energy balance regulation. The response is that appetite increases and the expenditure of energy declines, named as the energy gap.52 To maintain the reduced weight, food intake must be restricted to the level that expended energy is suppressed or adherence to exercise must be enhanced in order to reduce the gap between appetite and expenditure.
Another reason why it is so hard to maintain weight loss is the evidence that weight loss may be associated with increased depressive symptoms.53 Mood improvements often occur early in treatment, prior to achieving significant weight loss; however, in the long term, personal costs of losing weight exceed the benefits. Lowered TRP availability during weight loss may limit the production of neurotransmitter serotonin, and this may result in mood disturbances and can, further, diminish serotonin functions ultimately leading to satiety dysregulation and increased food intake.54 It is also apparent that disturbed mood can affect the self-rewarding mechanisms of food consumption, which is likely to involve foods that are high, both in carbohydrates and saturated fats, and which in turn may promote weight gain, which could further depress mood and self-esteem over the long term.55 Notably, calorie restriction diet is not only associated with a decline in the amino acid TRP, the precursor of the neurotransmitter serotonin, but also with a reduction in phenylalanine, the precursor of the tyrosine–dopamine pathway.18 Furthermore, in obese individuals, increased activity of the immunomodulatory enzyme IDO1 during immune activation results in TRP depletion, and this persists in spite of significant weight reduction,56 which may slow-down the production of serotonin in the brain and further lower mood.57
Countering biology with physical exercise
Following weight loss, compensatory changes include changes in the levels of circulating appetite-related hormones, such as increases in orexigenic hormones (eg, ghrelin), which stimulate appetite, and decreases in anorexigenic hormones (eg, leptin), which inhibit appetite, with the net result that appetite is increased.51 Currently, there is a disparity in the literature concerning the influence of exercise on appetite. Some researchers suggest that chronic exercise training does not induce substantial weight loss due to compensatory responses, ie, increase in appetite and food intake,58,59 while some others consistently document that exercise has no influence on appetite or ad libitum food intake.60,61 Based on a recent meta-analysis, acute exercise has a trivial effect on subsequent energy intake.62 Differences in lean body mass, fat mass loss, volume and duration of exercise interventions, and inclusion of different genders are likely to be factors contributing to the discrepancy. Staten noted a gender difference in compensatory food intake after exercise, with an increased caloric intake in men but not in women.63 One possible reason for this is that men exhibit greater amounts of fat-free mass.64 Indeed, resting metabolic rate (largely determined by fat-free mass) is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite.65 On the contrary, the appetite and energy intake response to exercise did not differ between men and women in a recent study.66 However, women exhibit compensatory appetite, gut hormone, and food intake responses to acute food restriction-induced energy deficit but not in response to an acute bout of exercise. Furthermore, there may be variability in weight-reduced subjects according to individual physiological characteristics, with clear responders and nonresponders.67
Since serotonergic mechanisms may reduce body weight by accelerating the onset of satiety68 besides suppressing excessive snacking of carbohydrate-rich foods, nonpharmacological methods of raising brain serotonin during weight loss and/or maintenance could be highly useful in preventing uncontrolled weight gain. It has been shown that physical exercise (mild stress) normalizes levels of proinflammatory cytokines and may counteract the activation of inflammation/IDO1 pathways, which may decrease the susceptibility for mood disturbances and carbohydrate craving.69 Animal and human studies have shown that aerobic exercise can stimulate brain serotonin activity and trigger parallel elevations in plasma-free TRP and brain TRP.70–72 In the primary study by Chaouloff et al.70, nonesterified fatty acid elevation increased free TRP, hence its entry in the brain for serotonin synthesis and also in the liver. Acute exercise seems to elevate the activity of TRP 5-monooxygenase, the enzyme involved in the rate-limiting step in the synthesis of serotonin, and so leads to an increase in the concentration of serotonin in some areas of the brain, ie, the brain stem and hypothalamus.73 Chronic exercise (30 min/d, six days per week for four weeks) causes neural adaptations by activating not only the synthesis but also the metabolism of serotonin in the cerebral cortex.74 Furthermore, salivary and serum cortisol levels in humans and corticoster-one in rats are increased by exercise,75,76 and this could induce liver TRP 2,3-dioxygenase, as demonstrated in rats. The combined effects of increased liver TRP and TRP 2,3-dioxygenase induction can elevate serum KYN in humans77 and in rats.78 In both of these latter studies, increases in serum KYN after exercise have been shown. Although exercise increased the IDO1 activity of macrophages in rats,78 until now, there is no direct information on the effect of exercise on IDO1 activity in humans. Furthermore, the effects of elevations in pro- and anti-inflammatory cytokines by exercise on IDO1 activity still remain to be examined. However, a recent study in healthy human volunteers showed a significant induction of TRP breakdown as indicated by an increase in KYN/TRP, which could be referred to an enhanced activity of IDO1 because a significant association of KYN/TRP with the immune activation marker neopterin was observed.79
TRP is not only a precursor of the serotonin biochemical pathway but also the key element for the formation of nicotinamide adenine dinucleotides, NAD and NADH, via the KYN pathway.80 Potential signals during exercise include increased NAD+/NADH. With increasing exercise intensity, the cytosolic NAD+/NADH ratio declines as lactate accumulates.81 NAD+ regulates the expression of sirtuins (SIRT) and peroxisome proliferator-activated receptor-γ coactivator (PGC-1α). Enhanced SIRT1 and PGC-1α activities are associated with improved mitochondrial function and exercise performance82 and protection against obesogenic feeding.83 Recently, a mechanism was discovered by which overexpression of PGC-1α1 in muscle mimics antidepressant effects of exercise by promoting KYN aminotransferase expression. By this way, crossing of KYN through the blood brain barrier and the disruption of neural plasticity are prevented.84
During exercise, the entry of TRP into the brain through the blood–brain barrier is favored by increased muscle use of BCAAs that inhibit TRP transport into the brain and elevated plasma fatty acids, as this elevates the ratio of unbound TRP to BCAA. Because of the increase in plasma TRP and decrease in BCAA, there is a substantial increase in TRP availability to the brain, consequently leading to higher serotonin concentrations in some areas of the brain.85 Serotonin plays a key role in signal transduction between neurons, and an exercise-induced increase in the concentrations of serotonin has been linked to central fatigue.86,87 Although there is some evidence that BCAA supplementation may increase exercise intensity at lactate threshold,88 upper body muscle power,89 and the rate of perceived recovery after strenuous exercise,90 the association between the ergogenic effect of the BCAA intake and the reduction in serotonin production in the brain has not been tested in humans. In a recent study,91 during a half-ironman triathlon, serum amino acids’ concentrations were reduced by >20%. However, neither the changes in serum-free amino acids nor the TRP/BCAA ratio was related to muscle fatigue or muscle damage during the race. Paradoxically, TRP supplementation can decrease fatigue perception during an aerobic exercise and likewise may improve physical performance, possibly by acting via endogenous opioids.92,93 Taken together, exercise increases TRP availability to the brain, and these findings provide support to the hypothesis that increases in serotonin synthesis and activity might be involved in the antidepressant effect of exercise.94 However, too high serotonin levels may impair mood and increase the sensitivity to fatigue, a situation similar to the development of a transient serotonin syndrome.95 Given that there is a reciprocal link in mood disorders and obesity,48 physical exercise could mitigate the biological changes that occur with weight loss (ie, decreased metabolic mass, increased metabolic efficiency, and increased hunger and depression) via both an increase in energy expenditure (with the potential to generate an energy deficit) and the induction of an anti-inflammatory environment (with a subsequent increased release of serotonin).
However, one has to distinguish recreational physical activity (activity that people engage in during their free time, that people enjoy, and that people recognize as having socially redeeming values) from repeated high level training sessions or intense endurance sports. Although the recreational physical activity performed every other day maximum will exert the above-mentioned positive impact on physiological pathways and parameters, repeated high-level activities could easily turn toward a negative net effect. Exhaustive physical activity significantly impacts on inflammation cascades that involve several proinflammatory cytokines, such as interferon-γ and down-stream biochemical pathways.96,97 This includes also alterations of amino acid profiles, eg, during and after a half iron man triathlon.91 Such data fit well with the observation that intense training was associated with an accelerated TRP breakdown and an increased KYN/TRP.79
Interestingly, intense versus moderate physical activities might also exert contrasting effects on neuropsychiatric circuits (Fig. 2). Acute and moderate physical exercises, such as noncompetitive physical exercise such as jogging, increase the activity of GTP-cyclohydrolase-1 (GCH1) and contribute to increased productions of pteridine derivatives.98 By contrast, in our recent study, a drop in phenylalanine and PHE/TYR concentrations by exhaustive exercise was noted,79 which indicates a decreased PAH activity that could be due to a decline in tetrahydrobiopterin (BH4).99 In the same population, nitrite levels dropped (unpublished data) that could represent another consequence of lowered NO• production related to diminished BH4. Unfortunately, the direct measurements of BH4 are almost impossible because only very stringent preanalytical measures can limit the oxidative loss of this compound and practically limit its monitoring to laboratory animal or in vitro studies.
Figure 2.
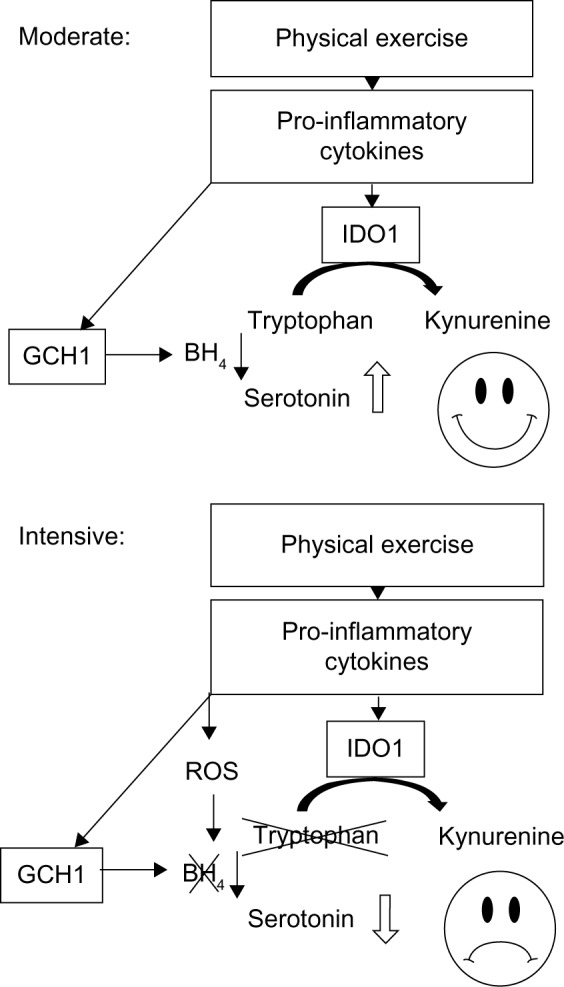
Hypothesis of the impact of moderate (upper graph) versus intensive (lower graph) physical exercise on the breakdown of tryptophan and the production of 5-hydroxytryptamine (serotonin) in a healthy individual: Physical exercise evokes a proinflammatory immune response, which is associated with induction of IDO1. In parallel, GCH1 is activated, which leads to the production of BH4, the necessary cofactor of several amino acid hydroxylases, including tryptophan 5-hydroxylase. In the moderate situation (upper graph), the increase in BH4 is able to compensate for a possible loss of tryptophan due to IDO1 activity, increased serotonin availability will enhance mood. However, in the situation after prolonged or heavy (= intensive) exercise (lower graph), this is no longer true. High level production of ROS can reduce the life span of BH4, and athletes may be faced to insufficient supply with serotonin and low mood.
Still, data fit well with existing literature, which shows that acute endurance exercise may increase serotonin availability as was reflected in the periphery by increased concentration of free TRP.100 The pathogenesis of negative mood is certainly multifactorial, TRP and serotonin metabolism can be of some greater relevance, and implicating reactive oxygen species (ROS) and BH4 is currently still rather speculative, although successful therapy of depression with BH4 has been reported already several years earlier.101
One may conclude that moderate physical exercise enhances the production of neurotransmitters, such as serotonin, noradrenaline, adrenaline, and dopamine, and can thereby contribute to a heightening of mood.102 However, only sports performed occasionally with two or three days interval when performed as recreational activity seems to exert this beneficial effect on general well-being, whereas intense training will rather adversely affect both mood103 and immune system function.104
Other factors such as psychological stress, lack of sleep, and malnutrition can increase the risk of infection when immunity becomes suppressed. This relationship will become especially important when sports activity is performed together with a reduction in food intake as a part of a weight-loss program. After short time, calorie restriction diet was associated with a decline in essential amino acid TRP in serum/plasma followed by a significant drop in phenylalanine.18 TRP would further decline when the weight loss diet is combined with heavy physical exercise that induces TRP degradation. It could result in deficiency of the essential amino acids that – because of their relevance for neurotransmitter biosynthesis – may affect the adherence to weight loss programs. To avoid this, one might consider supplementation with TRP or 5-hydroxy-TRP during such periods to improve weight loss maintenance. Indeed, it has been shown that acute TRP supplementation while dieting could be helpful in improving mood status and preventing uncontrolled weight gain or neuropsychiatric symptoms.13 However, in healthy subjects, only large increases in brain TRP levels are able to improve mood significantly, whereas relatively small increases in brain TRP result in an improved mood in vulnerable subjects. So eating foods with relatively high TRP content, such as turkey meat, cacao, nuts, and other seeds, is considered to increase TRP availability to the brain, albeit slightly, and contributes to some mood enhancement.105 This conclusion is, however, weakened by the fact that such food usually also contains high concentrations of competing LNAA that reduces the TRP:LNAA ratio and thus again limits the transport of TRP to brain via the leucine-preferring L1 system.106 By contrast, in vitro observations show that antioxidant compounds are able to suppress IDO1, which supports the conclusion that healthy food rich in antioxidants could exert some additional positive effect on TRP availability when TRP breakdown is slowed down.107
Conclusion
The obesity epidemic that we are facing today may relate to various aspects, such as increased general availability of energy-rich foods, increased sedentary activity, and decreased occupational physical activity (traveling to or from work or school by a means involving physical activity, such as walking and riding a bicycle).108 In theory, eating less and increasing physical activity will be able to counteract this development. However, obesity is often associated with disturbed mood, which is closely linked with the inability to lose or to stop gaining weight, and a craving for carbohydrates is manifested by many people who are overweight or are becoming so. The disturbed metabolism of TRP in overweight victims might affect the biosynthesis of serotonin and could thereby increase the susceptibility for mood disturbances and carbohydrate craving, increasing the cessation probability of weight reduction programs. Physical exercise could be helpful in improving mood status and preventing uncontrolled weight gain. However, breakdown of TRP triggered by proinflammatory cascades that are elicited during excessive physical exercise may again relate to the development of neuropsychiatric symptoms, such as fatigue and low mood. Thus, a balance between eating habits and the optimal dose of physical activity needs to be reached.
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1896 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the review: BS, DF. Wrote the first draft of the article: BS. Contributed to the writing of the article: BS, DF. Jointly developed the structure and arguments for the article: BS, DF. Made critical revisions and approved the final version: BS, DF. Both authors reviewed and approved the final article.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Obesity and overweight. Fact Sheet No. 311. 2014. [Accessed January 2016]. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- 3.Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year followup of body mass index and cerebral atrophy. Neurology. 2004;63:1876–81. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 4.Kaur S, Gonzales MM, Strasser B, et al. Central adiposity and cortical thickness in midlife. Psychosom Med. 2015;77:671–8. doi: 10.1097/PSY.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 5.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 6.Lackner N, Bengesser SA, Birner A, et al. Abdominal obesity is associated with impaired cognitive function in euthymic bipolar individuals. World J Biol Psychiatry. 2015;12:1–12. doi: 10.3109/15622975.2015.1046917. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Zhao JH, Luan J, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 2010 Aug 31;7(8) doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Brandacher G, Hoeller E, Fuchs D, Weiss HG. Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab. 2007;8:289–95. doi: 10.2174/138920007780362590. [DOI] [PubMed] [Google Scholar]
- 10.Mangge H, Summers KL, Meinitzer A, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity. 2014;22:195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Schroecksnadel S, Féart C, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–82. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Myint AM, Kim YK. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:304–13. doi: 10.1016/j.pnpbp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Brzezinski A, Shalitin N, Ever-Hadani P, Schenker JG. Plasma concentrations of tryptophan and dieting. Br Med J. 1990;301:183. doi: 10.1136/bmj.301.6744.183-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Neopterin formation and tryptophan degradation by a human myelomonocytic cell line (THP-1) Cancer Res. 1990;50:2863–7. [PubMed] [Google Scholar]
- 15.Steinert RE, Luscombe-Marsh ND, Little TJ, et al. Effects of intraduodenal infusion of L-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J Clin Endocrinol Metab. 2014;99:3275–84. doi: 10.1210/jc.2014-1943. [DOI] [PubMed] [Google Scholar]
- 16.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the Obesity Expert Panel, 2013. Obesity. 2014;22(suppl 2):S5–39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA, Wadden TA. Improving long-term weight loss maintenance: can we do it? Obesity. 2015;23:2–3. doi: 10.1002/oby.20964. [DOI] [PubMed] [Google Scholar]
- 18.Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr. 2015;54:101–7. doi: 10.1007/s00394-014-0690-3. [DOI] [PubMed] [Google Scholar]
- 19.Strasser B, Fuchs D. Role of physical activity and diet on mood, behavior, and cognition. Neurol Psychiatry Brain Res. 2015;21:118–26. [Google Scholar]
- 20.Thomas DM, Bouchard C, Church T, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13:835–47. doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorogood A, Mottillo S, Shimony A, et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med. 2011;124:747–55. doi: 10.1016/j.amjmed.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 24.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 25.Strasser B, Spreitzer A, Haber P. Fat loss depends on energy deficit only, independently of the method for weight loss. Ann Nutr Metab. 2007;51:428–32. doi: 10.1159/000111162. [DOI] [PubMed] [Google Scholar]
- 26.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32:177–84. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 27.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 28.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–7. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863–72. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–9. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- 31.O’Donovan G, Owen A, Bird SR, et al. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol. 2005;98:1619–25. doi: 10.1152/japplphysiol.01310.2004. [DOI] [PubMed] [Google Scholar]
- 32.Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord. 2015;14:31. doi: 10.1186/s40200-015-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heden T, Lox C, Rose P, Reid S, Kirk EP. One-set resistance training elevates energy expenditure for 72 h similar to three sets. Eur J Appl Physiol. 2011;111:477–84. doi: 10.1007/s00421-010-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasser B, Arvandi M, Siebert U. Resistance training, visceral obesity and inflammatory response: a review of the evidence. Obes Rev. 2012;13:578–91. doi: 10.1111/j.1467-789X.2012.00988.x. [DOI] [PubMed] [Google Scholar]
- 35.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 36.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312:923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 37.de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95:614–25. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–54. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 39.Van Horn LA. Diet by any other name is still about energy. JAMA. 2014;312:900–1. doi: 10.1001/jama.2014.10837. [DOI] [PubMed] [Google Scholar]
- 40.Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 41.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 42.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–52. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 43.Gatti E, Porrini M, Noe D, Crovetti R, Testolin G. Plasma amino acids changes in obese patients on very low-calorie diets. Int J Vitam Nutr Res. 1994;64:81–5. [PubMed] [Google Scholar]
- 44.Goodwin GM, Cowen PJ, Fairburn CG, Parry-Billings M, Calder PC, Newsholme EA. Plasma concentrations of tryptophan and dieting. Br Med J. 1990;300:1499–500. doi: 10.1136/bmj.300.6738.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amigo I, Fernández C. Effects of diets and their role in weight control. Psychol Health Med. 2007;12:321–7. doi: 10.1080/13548500600621545. [DOI] [PubMed] [Google Scholar]
- 46.Walsh AE, Oldman AD, Franklin M, Fairburn CG, Cowen PJ. Dieting decreases plasma tryptophan and increases the prolactin response to d-fenfluramine in women but not men. J Affect Disord. 1995;33:89–97. doi: 10.1016/0165-0327(94)00078-n. [DOI] [PubMed] [Google Scholar]
- 47.Redman LM, Heilbronn LK, Martin CK, et al. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–12. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 49.Bray GA, Smith SR, DeJonge L, et al. Effect of diet composition on energy expenditure during weight loss: the POUNDS LOST Study. Int J Obes. 2012;36:448–55. doi: 10.1038/ijo.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci. 2013;124:231–41. doi: 10.1042/CS20120223. [DOI] [PubMed] [Google Scholar]
- 52.MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16(suppl 1):45–54. doi: 10.1111/obr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson SE, Steptoe A, Beeken RJ, Kivimaki M, Wardle J. Psychological changes following weight loss in overweight and obese adults: a prospective cohort study. PLoS One. 2014;9(8):e104552. doi: 10.1371/journal.pone.0104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangge H, Summers K, Almer G, et al. Antioxidant food supplements and obesity-related inflammation. Curr Med Chem. 2013;20:2330–7. doi: 10.2174/0929867311320180004. [DOI] [PubMed] [Google Scholar]
- 55.Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925. doi: 10.3389/fpsyg.2014.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandacher G, Winkler C, Aigner F, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–8. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 57.Gostner J, Becker K, Sperner-Unterweger B, Überall F, Fuchs D, Strasser B. Role of tryptophan metabolism in mood, behavior, and cognition. In: Mittal S, editor. Targeting the Broadly Pathogenic Kynurenine Pathway. Berlin, Heidelberg, New York, Tokyo: Springer; 2015. pp. 75–89. [Google Scholar]
- 58.Hopkins M, Gibbons C, Caudwell P, et al. The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. Eur J Clin Nutr. 2014;68:581–6. doi: 10.1038/ejcn.2013.277. [DOI] [PubMed] [Google Scholar]
- 59.Hopkins M, King NA, Blundell JE. Acute and long-term effects of exercise on appetite control: is there any benefit for weight control? Curr Opin Clin Nutr Metab Care. 2010;13:635–40. doi: 10.1097/MCO.0b013e32833e343b. [DOI] [PubMed] [Google Scholar]
- 60.Douglas JA, King JA, McFarlane E, et al. Appetite, appetite hormone and energy intake responses to two consecutive days of aerobic exercise in healthy young men. Appetite. 2015;92:57–65. doi: 10.1016/j.appet.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Deighton K, Karra E, Batterham RL, Stensel DJ. Appetite, energy intake, and PYY3–36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl Physiol Nutr Metab. 2013;38:947–52. doi: 10.1139/apnm-2012-0484. [DOI] [PubMed] [Google Scholar]
- 62.Schubert MM, Desbrow B, Sabapathy S, Leveritt M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite. 2013;63:92–104. doi: 10.1016/j.appet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Staten MA. The effect of exercise on food intake in men and women. Am J Clin Nutr. 1991;53:27–31. doi: 10.1093/ajcn/53.1.27. [DOI] [PubMed] [Google Scholar]
- 64.Blundell JE, Caudwell P, Gibbons C, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–9. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 65.Caudwell P, Finlayson G, Gibbons C, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97:7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 66.Alajmi N, Deighton K, King JA, et al. Appetite and energy intake responses to acute energy deficits in females versus males. Med Sci Sports Exerc. 2016;48:412–20. doi: 10.1249/MSS.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16(suppl 1):67–76. doi: 10.1111/obr.12257. [DOI] [PubMed] [Google Scholar]
- 68.Blundell JE. Serotonin manipulations and the structure of feeding behaviour. Appetite. 1986;7(suppl):39–56. doi: 10.1016/s0195-6663(86)80051-4. [DOI] [PubMed] [Google Scholar]
- 69.Liu W, Sheng H, Xu Y, Liu Y, Lu J, Ni X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res. 2013;242:110–6. doi: 10.1016/j.bbr.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 70.Chaouloff F, Elghozi JL, Guezennec Y, Laude D. Effects of conditioned running on plasma, liver and brain tryptophan and on brain 5-hydroxytryptamine metabolism of the rat. Br J Pharmacol. 1985;86:33–41. doi: 10.1111/j.1476-5381.1985.tb09432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–88. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- 72.Melancon MO, Lorrain D, Dionne IJ. Exercise increases tryptophan availability to the brain in older men age 57–70 years. Med Sci Sports Exerc. 2012;44:881–7. doi: 10.1249/MSS.0b013e31823ede8e. [DOI] [PubMed] [Google Scholar]
- 73.Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand. 1989;136:473–81. doi: 10.1111/j.1748-1716.1989.tb08689.x. [DOI] [PubMed] [Google Scholar]
- 74.Dey S, Singh RH, Dey PK. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992;52:1095–9. doi: 10.1016/0031-9384(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 75.O’Connor PJ, Corrigan DL. Influence of short-term cycling on salivary cortisol levels. Med Sci Sports Exerc. 1987;19:224–8. [PubMed] [Google Scholar]
- 76.Viru A, Janson T, Viru M. Effect of prolonged exercise on liver tryptophan oxygenase activity in rat. Biol Sport. 2005;22:29–33. [Google Scholar]
- 77.Ito Y, Yonekura R, Kobayashi C, et al. Relationship between serum kynurenine concentration and exercise performance. Int Congr Ser. 2007;1304:167–70. [Google Scholar]
- 78.Ito Y, Yonekura R, Maruta K, et al. Tryptophan metabolism was accelerated by exercise in rat. Adv Exp Med Biol. 2003;527:531–5. doi: 10.1007/978-1-4615-0135-0_61. [DOI] [PubMed] [Google Scholar]
- 79.Strasser B, Geiger D, Schauer M, Gatterer H, Burtscher M, Fuchs D. Effects of exhaustive aerobic exercise on tryptophan-kynurenine metabolism in trained athletes. PlosOne. 2016 Apr 28;11(4):e0153617. doi: 10.1371/journal.pone.0153617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badawy AA. Tryptophan availability for kynurenine pathway metabolism across the life span: control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 81.Sahlin K, Katz A, Henriksson J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J. 1987;245:551–6. doi: 10.1042/bj2450551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agudelo LZ, Femenía T, Orhan F, et al. Skeletal muscle PGC-1?1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 85.Meeusen R. Exercise, nutrition and the brain. Sports Med. 2014;44(suppl 1):S47–56. doi: 10.1007/s40279-014-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newsholme EA, Blomstrand E, Hassmen P, Ekblom B. Physical and mental fatigue: do changes in plasma amino acids play a role? Biochem Soc Trans. 1991;19:358–62. doi: 10.1042/bst0190358. [DOI] [PubMed] [Google Scholar]
- 87.Newsholme EA, Blomstrand E. Branched-chain amino acids and central fatigue. J Nutr. 2006;136:274S–6S. doi: 10.1093/jn/136.1.274S. [DOI] [PubMed] [Google Scholar]
- 88.Matsumoto K, Koba T, Hamada K, Tsujimoto H, Mitsuzono R. Branched-chain amino acid supplementation increases the lactate threshold during an incremental exercise test in trained individuals. J Nutr Sci Vitaminol (Tokyo) 2009;55:52–8. doi: 10.3177/jnsv.55.52. [DOI] [PubMed] [Google Scholar]
- 89.Crowe MJ, Weatherson JN, Bowden BF. Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol. 2006;97:664–72. doi: 10.1007/s00421-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 90.Hsu MC, Chien KY, Hsu CC, Chung CJ, Chan KH, Su B. Effects of BCAA, arginine and carbohydrate combined drink on post-exercise biochemical response and psychological condition. Chin J Physiol. 2011;54:71–8. doi: 10.4077/cjp.2011.amk075. [DOI] [PubMed] [Google Scholar]
- 91.Areces F, González-Millán C, Salinero JJ, et al. Changes in serum free amino acids and muscle fatigue experienced during a half-ironman triathlon. PLoS One. 2015;10(9):e0138376. doi: 10.1371/journal.pone.0138376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segura R, Ventura JL. Effect of L-tryptophan supplementation on exercise performance. Int J Sports Med. 1988;9:301–5. doi: 10.1055/s-2007-1025027. [DOI] [PubMed] [Google Scholar]
- 93.Javierre C, Segura R, Ventura JL, Suárez A, Rosés JM. L-tryptophan supplementation can decrease fatigue perception during an aerobic exercise with supramaximal intercalated anaerobic bouts in young healthy men. Int J Neurosci. 2010;120:319–27. doi: 10.3109/00207450903389404. [DOI] [PubMed] [Google Scholar]
- 94.Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta-analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13:1002–14. doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- 95.Alusik S, Kalatova D, Paluch Z. Serotonin syndrome. Neuro Endocrinol Lett. 2014;35:265–73. [PubMed] [Google Scholar]
- 96.Sprenger H, Jacobs C, Nain M, et al. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin Immunol Immunopathol. 1992;63:188–95. doi: 10.1016/0090-1229(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 97.Tilz GP, Domej W, Diez-Ruiz A, et al. Increased immune activation during and after physical exercise. Immunobiology. 1993;188:194–202. doi: 10.1016/s0171-2985(11)80497-3. [DOI] [PubMed] [Google Scholar]
- 98.Rokos H, Rokos K, Kunzee ROF. Variations of neopterin, dihydroneopterin and immunological parameters during physical exercise (jogging) In: Curtius HCh, Blau N, Levine RA., editors. Unconjugated Pterins and Related Biogenic Amines. Berlin, New York: Walter deGruyter Publishers; 1987. pp. 187–96. [Google Scholar]
- 99.Anderson DN, Wilkinson AM, Abou-Saleh MT, Blair JA. Recovery from depression after electroconvulsive therapy is accompanied by evidence of increased tetrahydrobiopterin-dependent hydroxylation. Acta Psychiatr Scand. 1994;90:10–3. doi: 10.1111/j.1600-0447.1994.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 100.Strüder HK, Hollmann W, Platen P, Wöstmann R, Weicker H, Molderings GJ. Effect of acute and chronic exercise on plasma amino acids and prolactin concentrations and on [3H]ketanserin binding to serotonin2 A receptors on human platelets. Eur J Appl Physiol Occup Physiol. 1999;79(4):318–24. doi: 10.1007/s004210050514. [DOI] [PubMed] [Google Scholar]
- 101.Curtius HC, Niederwieser A, Levine RA, Lovenberg W, Woggon B, Angst J. Successful treatment of depression with tetrahydrobiopterin. Lancet. 1983;1(8325):657–8. doi: 10.1016/s0140-6736(83)91837-8. [DOI] [PubMed] [Google Scholar]
- 102.Strasser B, Sperner-Unterweger B, Fuchs D, Gostner JM. Immune activation-induced alterations of tryptophan and phenylalanine metabolisms and their association with depression. In: Capuron L, Dantzer R, editors. Inflammation-Associated Depression: Evidence, Mechanisms and Implication. Current Topics in Behavioral Neuroscience. Berlin, Heidelberg, New York, Tokyo: Springer; In press. [Google Scholar]
- 103.Saanijoki T, Nummenmaa L, Eskelinen JJ, et al. Affective responses to repeated sessions of high-intensity interval training. Med Sci Sports Exerc. 2015;47:2604–11. doi: 10.1249/MSS.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 104.Gleeson M, Bishop NC. URI in athletes: are mucosal immunity and cytokine responses key risk factors? Exerc Sport Sci Rev. 2013;41:148–53. doi: 10.1097/JES.0b013e3182956ead. [DOI] [PubMed] [Google Scholar]
- 105.Hulsken S, Märtin A, Mohajeri MH, Homberg JR. Food-derived serotonergic modulators: effects on mood and cognition. Nutr Res Rev. 2013;26:223–34. doi: 10.1017/S0954422413000164. [DOI] [PubMed] [Google Scholar]
- 106.Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:1211–27. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strasser B, Gostner JM, Fuchs D. Mood, food and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 108.Bassett DR, Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42:1819–25. doi: 10.1249/MSS.0b013e3181dc2e54. [DOI] [PMC free article] [PubMed] [Google Scholar]


