Abstract
Chromosome segregation relies on coordinated activity of a large assembly of proteins, the “Kinetochore Interaction Network” (KIN). How conserved the underlying mechanisms driving the epigenetic phenomenon of centromere and kinetochore assembly and maintenance are remains unclear, even though various eukaryotic models have been studied. More than 50 different proteins, many in multiple copies, comprise the KIN or are associated with fungal centromeres and kinetochores. Proteins isolated from immune sera recognized centromeric regions on chromosomes and were thus named centromere proteins (“CENPs”). CENP-A, sometimes called “centromere-specific H3” (CenH3), is incorporated into nucleosomes within or near centromeres. The “constitutive centromere-associated network” (CCAN) assembles on this specialized chromatin, likely based on specific interactions with and requiring presence of CENP-C. The outer kinetochore comprises the Knl1-Mis12-Ndc80 (“KMN”) protein complexes that connect the CCAN to spindles, accomplished by binding and stabilizing microtubules (MTs) and in the process generating load-bearing assemblies for chromatid segregation. In most fungi the Dam1/DASH complex connects the KMN complexes to MTs. Fungi present a rich resource to investigate mechanistic commonalities but also differences in kinetochore architecture. While ascomycetes have sets of CCAN and KMN proteins that are conserved with those of either budding yeast or metazoans, searching other major branches of the fungal kingdom revealed that CCAN proteins are poorly conserved at the primary sequence level. Several conserved binding motifs or domains within KMN complexes have been described recently, and these features of ascomycete KIN proteins are shared with most metazoan proteins. In addition, several ascomycete-specific domains have been identified here.
Keywords: CCAN, centromere, Dam1, histones, kinetochore, KMN, Neurospora crassa, Zymoseptoria tritici
INTRODUCTION
The faithful transmission of chromosomes to daughter cells relies on a large assembly of proteins, the “Kinetochore Interaction Network” (KIN), yet after more than 30 years of intense research it is still unclear if the mechanisms driving this inherently epigenetic phenomenon in various eukaryotes are substantially different. Constricted, specialized sites of spindle attachment on chromosomes during mitosis were first described more than 130 years ago (Flemming 1882). In the 1930s two terms appeared, “centromere” and “kinetochore”, to describe these constrictions and attachment point of spindles (Cheeseman and Desai 2008; Fukagawa and Earnshaw 2014). Genetically, these regions are associated with reduced meiotic crossing over (Beadle 1932) and over time the centromere, as a genetic locus, became synonymous with a stretch of DNA. This perception intensified after identification and cloning of the budding and fission yeast centromeric DNA (Clarke and others 1986; Clarke and Carbon 1980) and the first proteins that associated with these specific DNA sequences (Earnshaw and Migeon 1985). Since then, “centromere” has regularly been used to describe DNA-associated features of chromatin, while “kinetochore” is now understood to mean a large protein complex that assembles on the centromere but does not contain DNA (Fukagawa and Earnshaw 2014). How exactly functional centromeres and kinetochores are maintained and inherited remains one of the fundamental questions in cell biology, biochemistry and biophysics.
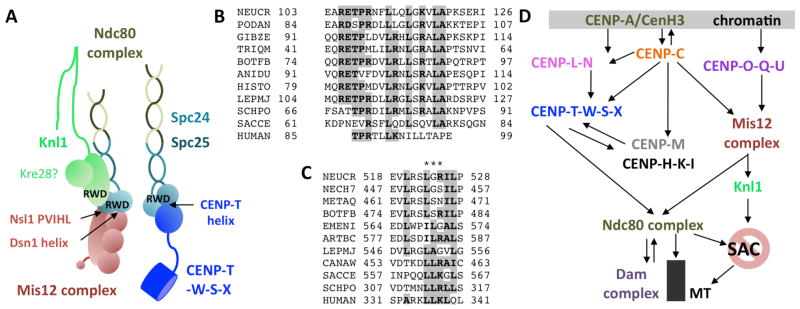
More than 50 different proteins that are associated more or less stably with fungal centromeres and kinetochores comprise the KIN (FIG. 1). The first centromere proteins (CENPs) were identified in immune serum from individuals with scleroderma because antibodies from these patients specifically recognized centromeric regions (Moroi and others 1980). Immunoprecipitation performed with these antibodies resulted in the identification of three proteins, CENP-A, CENP-B and CENP-C (Earnshaw and Migeon 1985). CENP-A is incorporated into nucleosomes within centromeres, and the “constitutive centromere-associated network” (CCAN) builds the foundation, or inner kinetochore layer, directly contacting centromeric DNA and the specialized CENP-A-containing nucleosomes. In yeasts, this network was initially defined by functional genetic studies and divided into several subcomplexes (Meraldi and others 2006). In metazoans, five homologous complexes were identified (CENP-C, CENP-H-I-K, CENP-L-M-N, CENP-O-P-Q-R-U, CENP-T-W-S-X) by extensive biochemistry (Foltz and others 2006; Nishino and others 2012; Okada and others 2006). The outer kinetochore of metazoans, the Knl1-Mis12-Ndc80 (KMN) network (Cheeseman and others 2006), contains at least three well-defined complexes that build the bridge from chromatin to spindles, by stabilizing microtubule (MT) attachment and generating load-bearing assemblies for chromatid segregation. The KMN network also controls the “spindle assembly checkpoint” (SAC), a signal transduction process that involves cell cycle regulators and prevents premature exit from mitosis when kinetochores are not under tension (Foley and Kapoor 2013; Lara-Gonzalez and others 2012). Chemical perturbation or mutation of components within this assembly results in chromosome segregation defects. Additional proteins contact KMN complexes in many fungi. The budding yeast Dam1/DASH complex, a large ring-shaped structure encircling the microtubule increases MT binding and processivity of KMN complexes (Cheeseman and others 2001a; Westermann and others 2005). Homologues for this complex are apparently absent from basal fungi (the phyla Microsporidia, Chytridiomycota, Blastocladiomycota, Neocallimastigomycota, Glomeromycota, and the unplaced subphyla Kickxellomycotina, Zoopagomycotina, Entomophthoromycotina and Mucoromycotina; Hibbett and others, 2007; FIG. 2A) and it is also not conserved in metazoans, though the three-subunit Ska complex may be a functional counterpart (Welburn and others 2009).
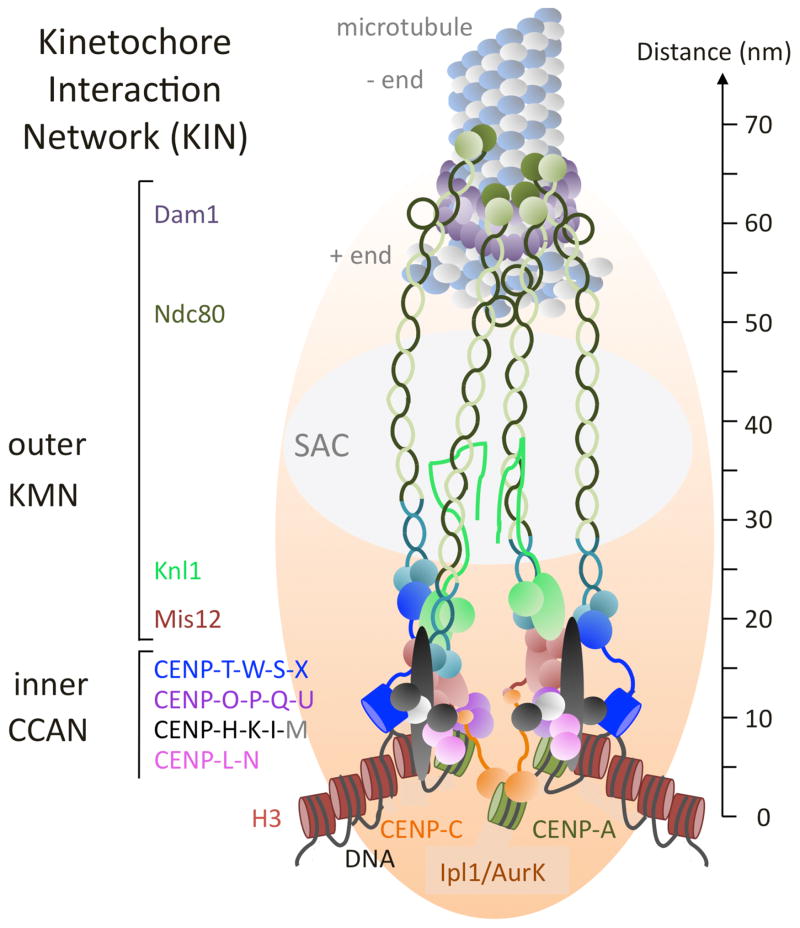
FIG. 1.
Working model of the Kinetochore Interaction Network (KIN). KIN components found in most fungi are shown and depicted roughly to scale (based on data compiled from the various sources cited in the text). Complexes are divided into the outer kinetochore (Knl1-Mis12-Ndc80, or “KMN” network and Dam1 complex) and the inner kinetochore (“constitutively centromere-associated network”; CCAN). Microtubule (MT) plus (+) ends are bound by the ten-component, fungal-specific Dam1 complex (purple). The Ndc80 and Nuf2 components of the Ndc80 complex (light and dark green) stretch across the Dam1 complex and bind to the MT. Four to 14 Ndc80 complexes are estimated to bind to one MT. Two Ndc80 subunits, Spc24 and Spc25 (light and dark teal) make connections to either the Mis12 or CENP-T complexes, respectively. Mis12 also binds to Knl1, a large protein that serves as an interaction and phosphorylation platform for the Spindle Assembly Checkpoint (SAC). It is unclear if filamentous ascomycetes have Kre28 or Zwint homologs that bind to Knl1. The rod-shaped Mis12 complex (light brown) integrates binding of CENP-C (orange) and the CENP-O-P-Q-U (purple) complex and thus connects centromeric chromatin, generated by assembly of CENP-A/CenH3 (green) nucleosomes and binding of CENP-L-N (pink), to the Ndc80 complex. CENP-H-K-I (black) form a complex when bound by CENP-M (grey); no homolog of CENP-M has been found in fungi by sequence similarity alone. CENP-H binds to CENP-C and helps to assemble CENP-T-W-S-X (blue) to form a second linkage to the Ndc80 complex by binding of CENP-T to Spc24/Spc25. Assembly of the CENP-C arm is dependent on the activity of Ipl1/Aurora B kinase and the gradient it generates from the inner chromatid faces towards the outer kinetochore. Several aspects of this model are speculative and based on testable hypotheses. It is still unclear if CENP-T-W-S-X assembles into a tetramer, let alone a nucleosome-like structure, but it is clear that the subunits contact DNA via their histone-fold domain. CENP-T-W binds preferentially to linker DNA. The precise shape of the CENP-H-K-I-M complex is unclear but the CENP-I subunit can stretch across long distances. Lastly, it is unclear how many CENP-A/CenH3 nucleosomes are involved in generating a MT attachment site (from one in S. cerevisiae and C. albicans to several in S. pombe), and how long these patches are, though all appear to be embedded in canonical H3 (brown) nucleosomes. This model shows three CENP-A/CenH3 nucleosomes and two CENP-T-W-S-X nucleosome-like structures to accommodate CENP-C and CENP-N binding to various domains of CENP-A/CenH3 nucleosomes. The five-nucleosome unit also fits with the phasing observed in ChIP-seq experiments with N. crassa CenH3 (Smith and others 2011, 2012).
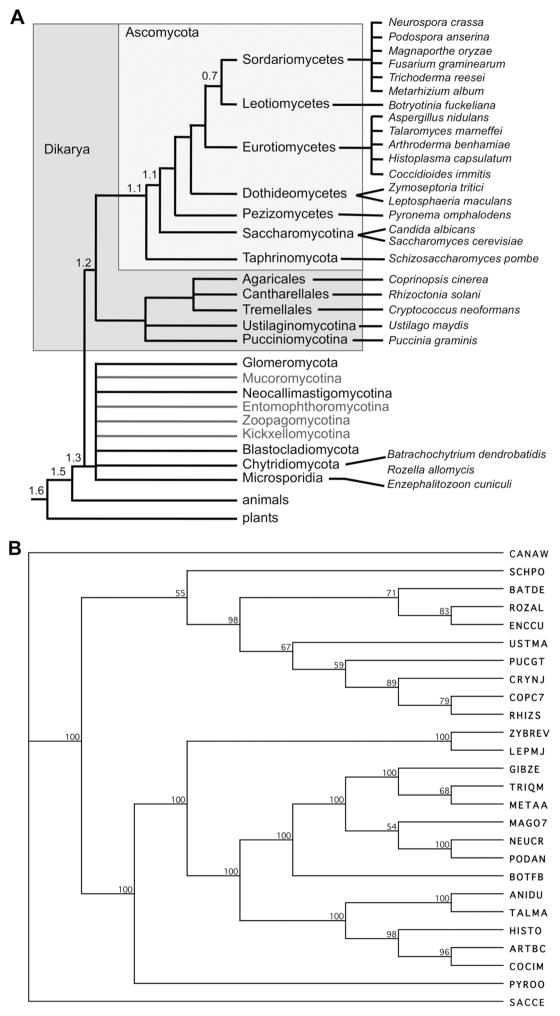
FIG. 2.
Predicted phylogenetic trees of the taxa discussed. A. Cartoon of currently accepted relationships of the taxa discussed here; no attempt has been made to resolve basal taxa (Hibbett and others, 2007). Branch lengths do not denote genetic distance. The approximate divergence times of major branches is indicated in billions of years (Hedges, 2002). Please note that the selection of taxa used for alignments was driven in part by general current research interests, not by taxonomic placement alone, thus some branches of the Ascomycota are underrepresented. B. Representative phylogenetic tree constructed by combined alignments of the Knl1 and Ndc80 complex (Ndc80, Nuf2, Spc24, Spc25) homologs of all 26 taxa discussed. Taxonomy and abbreviations are defined in SUPPLEMENTAL TABLE 1; accession numbers are listed in SUPPLEMENTAL TABLE 3. In this tree, budding yeasts in the Saccharomycotina (C. albicans and S. cerevisiae) and the fission yeast S. pombe are not placed according to accepted phylogeny, while all other major branches (basal fungi with microsporidia and “chytrids”, basidiomycetes and ascomycetes) are resolved according to expected relationships (see FIG. 1A). Tree was constructed by neighbor joining (bootstrap 1,000 replicates; tie breaking systematic; gaps were distributed proportionally).
Arguably, fungi present the richest resource to decipher overall similarities but also small mechanistic differences in kinetochore architecture and function within a single kingdom, as by now well over a thousand fungal genomes have been sequenced, thus yielding information about the kinetochore complement from closely related strains or species, as well as vastly different taxa. The first comprehensive appraisal of fungal kinetochore proteins was conducted just before proteomic screens of chicken and human cells substantially increased the number of known vertebrate kinetochore complexes (Meraldi and others 2006). Sequence similarity searches and hidden Markov model (HMM)-based modeling showed that fungi more closely related to S. cerevisiae contained 11 kinetochore proteins that had not yet been identified in vertebrates, and vertebrates appeared to contain proteins absent from budding yeasts (Meraldi and others 2006). For ascomycete fungi, however, large overlap between protein sets from yeasts and mammals was revealed, suggesting that structural features of kinetochores have been well conserved from yeast to humans, especially within the KMN network (FIG. 2B). More recent bioinformatics and functional studies showed that many yeast proteins are functionally homologous to the CCAN subunits isolated by proteomics approaches in vertebrates (Schleiffer and others 2012; Westermann and Schleiffer 2013). Most subunits have also been identified in many filamentous fungi (Smith and others 2012). Only three CCAN subunits (CENP-M, CENP-P, CENP-R) and one KMN protein (Zwint/Kre28) cannot be identified by sequence similarity to either yeasts or metazoan in most filamentous ascomycetes, though functionally similar proteins are expected to exist. Searching other branches of the fungal kingdom reveals that CCAN proteins are poorly conserved at the primary sequence level, as most of them cannot be identified by homology searches alone in basal fungi (microsporidia and the clades formerly called “chytrids” and “zygomycetes”) and basidiomycetes (SUPPLEMENTAL TABLE 1). Kinetochore proteins of ascomycetes share functionally important features with most metazoan proteins. Considering the large differences on the level of individual protein structure uncovered in early genetic and biochemical studies it is remarkable how similar the overall structures of CCAN and KMN complexes in metazoans and fungi are. The purpose of this review is to illustrate commonalities between metazoans and fungi and to identify proteins and interactions that should be further studied in the fungi.
MICROTUBULE-ASSOCIATED PROTEINS AT THE OUTER KINETOCHORE: THE DAM1/DASH COMPLEX
The most obvious difference between fungal and metazoan kinetochore complexes is the presence of the Dam1/DASH complex in fungi (FIG. 3A). The Saccharomyces cerevisiae Dam1 (in Schizosaccharomyces pombe DASH) complex is composed of ten subunits, all of which are essential for survival in budding yeast (Cheeseman and others 2001b; Janke and others 2002) and Candida albicans (Thakur and Sanyal, 2011) but not in fission yeast (Sanchez-Perez and others 2005). In Neurospora crassa single gene deletions have been generated for all Dam1 complex subunits and not all of them are essential (G. Ekberg and M. Freitag, unpublished data). These differences may be based on the different numbers of MTs attached per kinetochore (Burrack and others 2011). In budding yeast and the dimorphic Candida albicans a single MT attaches (Joglekar and others, 2006; Burrack and others 2011; Thakur and Sanyal, 2011), but in fission yeast two to four MT attach (Ding and others, 1993), which is likely also true for most filamentous fungi but has not been studied.
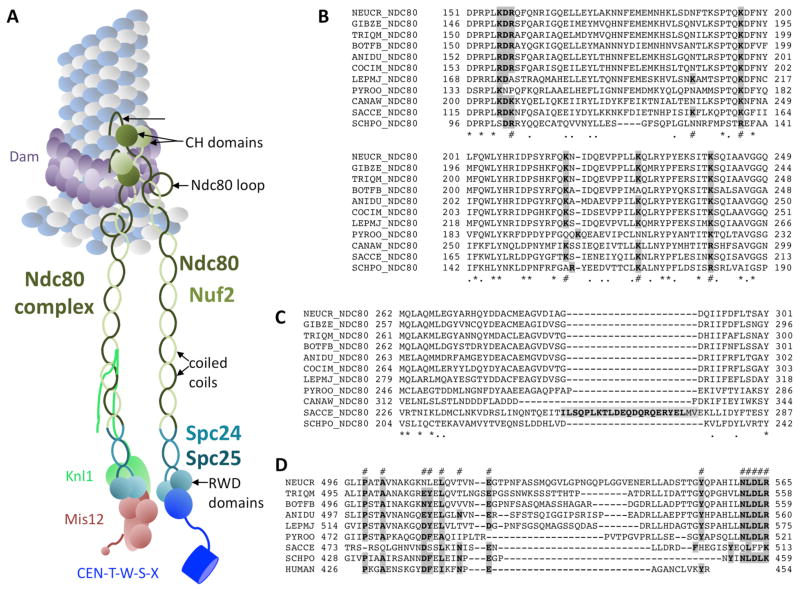
FIG. 3.
The Ndc80 complex connects microtubules (MT) to the Mis12 assembly and the CCAN CENP-T complex. A. Structural features of the Ndc80 complex. N-terminal, positively charged patches bind directly to the MT. Calponin-homology (CH) domains are well-conserved and essential for MT binding. The function of the exposed Ndc80 loop is still unclear. All four Ndc80 subunits (Ndc80, Nuf2, Spc24, Spc25) contain regions of coiled-coils that connect the two head units, the CH domains of Ndc80/Nuf2 and the heterodimeric RWD domain of Spc24/Spc25. Binding to the RWD domain by Mis12 (and Knl1) or CENP-T is exclusive – only one of these complexes can bind to one Spc24/Spc25 RWD domain. B. Alignment of a conserved regions within the Ndc80 CH domain, containing lysine (K) residues previously shown to be important for function (#). Completely conserved residues (*) or similar (.) residues are marked. Double mutants for the first (K122E) and last (K204E) lysine in yeast were unable to survive, and mutants with only K204E showed benomyl sensitivity. C. A short loop separating coiled-coil regions of Ndc80 in some yeast species is not found in filamentous ascomycetes. Instead these regions are highly conserved. D. The exposed Ndc80 loop varies in length and primary sequence but several residues are highly conserved (#). Mutational analyses of the features shown in B. – D. needs to be carried out for filamentous ascomycetes. For species abbreviations see SUPPLEMENTAL TABLE 1. Important residues are shown in bold and shaded in grey.
In vitro and with purified kinetochore particles, the budding yeast Dam1 complex forms heterodecamers that assemble into 16-unit rings encircling the MT plus end (Gonen and others 2012; Miranda and others 2005; Westermann and others 2005). In vivo, forming Dam1 complex rings around the MT may not be the rule fir all fungi, however, as Dam1 “speckles” or “patches” have been described in fission yeast (Gao and others, 2010). The function of this large assembly may be to couple force generated by depolymerization of MTs with chromatid movement (McIntosh and others 2013). Deletion of the Hsk3 subunit splits the complex into the oligomerization-deficient Dam1OD complex (Dam1, Duo1, Spc34, Spc19, Dad1, Dad3), which binds MTs in vitro, and a smaller Ask1/Dad2/Dad4 complex, which does not bind MTs (Miranda and others 2007). Oligomerization is required in vitro for the Dam1 complex to form microtubule attachments. Thus, the in vivo Dam1 complex may also form a ring that prevents detachment from the MT, especially under tension during mitosis. When Hsk3 was depleted in vivo the Dam1OD complex formed and bipolar centromere pairs collapsed to monopolar attachments (Umbreit and others 2014). Failure of MT attachment at one kinetochore was explained by the inability of the Dam1OD complex to mediate stable coupling to MTs under tension.
Thus, the Dam1 complex appears to be an integral part of the outer kinetochore in many fungi, though it only assembles when the kinetochore meets MTs head-on (Malvezzi and Westermann 2014) and in presence of Ndc80 (Janke and others 2002; Lampert and others 2013). Coordinated action of the Dam1 and Ndc80 complexes is required for correcting monopolar attachments by the Ipl1/Aurora B kinase in yeast. Also arguing for inclusion of the Dam complex as a bona fide kinetochore component are experiments showing that artificially tethering Dam1 complexes to DNA proved sufficient to segregate DNA in budding yeast (Kiermaier and others 2009; Lacefield and others 2009) and to assemble Cse4/CENP-A-containing kinetochores (Ho and others 2014). Outside of the yeasts no mechanistic work has been done on Dam1/DASH complexes in the fungi and it is still not clear if some basal taxa (FIG. 2A; SUPPLEMENTAL TABLE 2) have this complex.
NDC80 COMPLEX
The Ndc80 complex is required to connect the Mis12 assembly platform as well as the inner kinetochore CENP-T-W-S-X complex (FIG. 3A) to generate load-bearing MT attachments to centromeric nucleosomes (Powers and others 2009). Together with CENP-T it is the kinetochore component with the highest ability to stretch. The length of the Ndc80 complex was estimated by electron microscopy to be ~57 nm (Wei and others, 2005; Alushin and others 2010). In yeast strains in which the components of the Ndc80 complex were tagged with fluorescent proteins, the overall length of the complex changed from 55 to 34 nm, mostly due to changes within the Ndc80/Nuf2 dimer (Aravamudhan and others 2014). The dumbbell-shaped complex comprises two heterodimers, Ndc80-Nuf2 and Spc24-Spc25, respectively. Both dimers have globular head domains and interact via their long coiled-coil regions (Bharadwaj and others 2004; McCleland and others 2004; Wei and others 2005; Wigge and Kilmartin 2001). On average four Ndc80 complexes assemble on MTs in vitro (Alushin and others 2010) and 14 in vivo (Suzuki and others 2015), though the arrangement in fungi (attached ring-like around the MT and associated with the Dam1 complex) may be different from that in humans (as longitudinal oligomers). In vivo estimates from S. cerevisiae, S. pombe and C. albicans obtained by fluorescence microscopy also suggest that for each CENP-A about eight Ndc80 proteins are present, even though S. pombe has two to four MTs per kinetochore rather than the single MT of S. cerevisiae and C. albicans (Joglekar and others 2008). In yeast, the Ndc80 complex appears to stretch across the top surface of the Dam1 complex ring (Aravamudhan and others 2014).
A positively charged patch in the Ndc80 N-terminal tail is important for MT binding, potentially by interacting with acidic patches on tubulin and by oligomerizing Ndc80 (Guimaraes and others 2008; Miller and others 2008). In yeast this tail can be deleted as long as the Dam1 complex is present (Demirel and others 2012) but it is not yet known if this is true for other fungi as well. The N-terminal tail contains phosphorylation sites for Ipl1/Aurora B kinase, which is required to resolve erroneous kinetochore to MT connections (DeLuca and others 2011); there are kinase binding motifs in all fungal Ndc80 proteins in this region. This conserved pathway, the “spindle assembly checkpoint” (SAC), monitors kinetochore to MT attachment status, including presence of MTs and tension between sister kinetochores. In the absence of attachment or tension the SAC is activated, relaying the signal to master regulatory kinases that drive cell cycle progression (Musacchio and Ciliberto 2012). A phosphorylation gradient generated by Ipl1/Aurora B kinase that emanates from the centromere core has been suggested to reach outer kinetochore components when they are not under tension (Liu and others 2009; Welburn and others 2010). Phosphorylation of the very N-terminal tail may thus control clustering of Ndc80 and prevent interactions with tubulin.
The globular heads of Ndc80 and Nuf2 interact tightly through their calponin homology (CH) domains and have MT-binding activity (Alushin and others 2010; Wei and others 2007), which is reduced or lost in CH domain mutants (Ciferri and others 2008; Sundin and others 2011; Tooley and others 2011). In reconstitution experiments Dam1 enhanced Ndc80 binding to the MT plus end, suggesting that Dam1 might strengthen Ndc80-dependent MT attachment in vivo (Lampert and others 2010; Tien and others 2010). The CH domains of fungal Ndc80 have conserved lysine- or arginine-rich “toe” patches (FIG. 3B), which are predicted to bind to the MT surface, as shown with Ndc80 homologs (Alushin and others 2010). The toes are proposed to act as sensors for the depolymerization, thereby helping to track the MT ends (Alushin and others 2010). In yeast, positions K122, K152, K160, K181, K192 and K204 (indicated by # in FIG. 3B) have been mutated (Lampert and others 2013). The combination of K122E and K204E was lethal in yeast, matching mutation of human Ndc80 K89 and K166, which crippled Ndc80 to MT interactions in vitro (Ciferri and others 2008). Lysine 122 is not truly conserved in other ascomycetes, instead either arginines are found in the same position and/or there are lysines in the same context, changing an RDK into a KDR or RDR motif (FIG. 3B). By itself, mutation of yeast Ndc80 K204 yielded benomyl-sensitive strains. This position is conserved, though sometimes the lysine is replaced by arginine. Overall, MT binding by the Ndc80 CH domain is essential and conserved, based on the primary amino acid structure of all fungal Ndc80 proteins examined.
In yeast, the Ndc80 CH domain is connected via a short disordered segment to a helical region preceding the coiled-coil shaft of the protein (Lampert and others 2013); a shorter connector is also present in human Ndc80 (aa 203-211). While several Saccharomyces species contain large insertions in this region, all of which are enriched for polar residues that may yield an interface for protein–protein interactions, this region is missing from Ndc80 of other ascomycetes (FIG. 3C). In yeast, deletion of this stretch (aa 256–273) made Ndc80 insensitive to the presence of the Dam1 complex, though MT binding of the Ndc80 complex was not changed (Lampert and others 2013). Similar experiments on the Ndc80 tail of other ascomycetes need to be conducted to assess if the situation in yeast is conserved.
Much debate has centered on a loop (FIG. 3D) that breaks the coiled-coil region and contributes to the attachment of kinetochores to MTs, presumably by recruiting regulatory proteins (Ciferri and others 2008; Wang and others 2008). Whether this loop directly contacts Dam1 (Maure and others 2011) and human Ska 1 (Zhang and others 2012) or indirectly contributes to Ndc80-Dam1 interactions (Lampert and others 2013) is controversial. Yeast strains with short deletions (Ndc80 D490–510) or point mutations showed temperature-sensitive phenotypes (Maure and others 2011). Depletion of Ska complex activity resembles the phenotype obtained when the loop region is removed in Ndc80 (Guimaraes and Deluca 2009), and the loop is also required in fission yeast (Hsu and Toda 2011). Mutations within the loop may indirectly affect Dam1 binding in vivo by changing intramolecular Ndc80 flexibility (Ciferri and others 2008; Joglekar and others 2009; Maiolica and others 2007; Wang and others 2008). Separating defects in Ndc80 loop mutants caused by structural changes versus direct effects on binding of regulatory proteins will be important. Replacement of the loop region with alternative loop sequences from closely related species of ascomycetes followed by assays for sequence specificity may help to resolve this question. To address changes in Ndc80 flexibility mixed Ndc80 complexes could be assembled. Length requirements for Ndc80 loop mutants and other KMN network components need to be assessed, as shortened proteins may not be able to escape the Ipl1/Aurora B phosphorylation gradient. It is unclear if loop sizes are similar or vary significantly between taxa (FIG. 3D). It is also possible that loops themselves, just like the N-terminal tail, are phosphorylation targets.
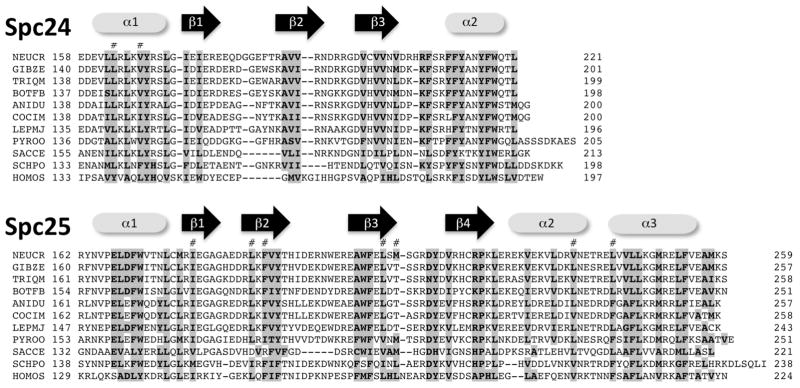
On the opposite end of the dumbbell-shaped Ndc80 complex, the globular domain of the conserved Spc24–Spc25 heterodimer connects to the Mis12 complex and to the CCAN by direct interaction with CENP-T (Gascoigne and others 2011; Petrovic and others 2010). Interactions between Spc24/Spc25 and the CENP-T Ndc binding motif or the Mis12/Nsl1 component are exclusive so that an Ndc80 complex bound to the Mis12 complex cannot interact with CENP-T at the same time (Bock and others 2012; Malvezzi and others 2013; Nishino and others 2013). Spc24/Spc25 contain N-terminal coiled-coil motifs and C-terminal RWD (RING finger, WD repeat, DEAD-box-like helicase) domains, used to form the heterodimer (Schmitzberger and Harrison 2012). Within the ascomycetes, Spc24 and Spc25 primary sequences are conserved, even in the N-terminal and central coiled-coil domain, which carry some hallmark motifs that can serve to differentiate SPC24 proteins from different fungal families into subclasses. RWD domains have emerged as hallmark interaction domains in kinetochores, forming numerous attachments, especially to the Mis12 complex (Petrovic 2014). Structurally highly conserved RWD domains are organized as α+β sandwich fold (FIG. 4). The Spc24/Spc25 heterodimeric RWD domain binds to sequence motifs at the C-termini of the Mis12 complex subunits Nsl1 (at PVIHL), and they can bind to conserved Dsn1 and CENP-T helical motifs (Malvezzi and others 2013; Petrovic and others 2010).
FIG. 4.
The heterodimeric RWD domain of Spc24/Spc25 is conserved at the primary sequence level. Each half of the RWD domain is at the very C-terminus of the proteins. Spc24 lacks the β4 and α3 feature, but SPC24/Spc25 form α + β sandwich RWD domain (Malvezzi and others 2013). Identical or conserved residues are shown in bold type. Residues that are predicted to contact the CENP-T N-terminal helix are indicated (#). Conserved residues are shown in bold and shaded in grey.
KNL1/SPC105/SPC7
Mammalian Knl1 (budding yeast Spc105, fission yeast Spc7) functions as scaffold during early mitosis, contributing to formation of proper attachment between kinetochore and microtubule (see Ghongane and others 2014, for a recent perspective and references therein). Knl1 is the largest single subunit of the KMN network, essential for accurate chromosome segregation during mitosis and required for both activation and inactivation of the SAC via Bub proteins.
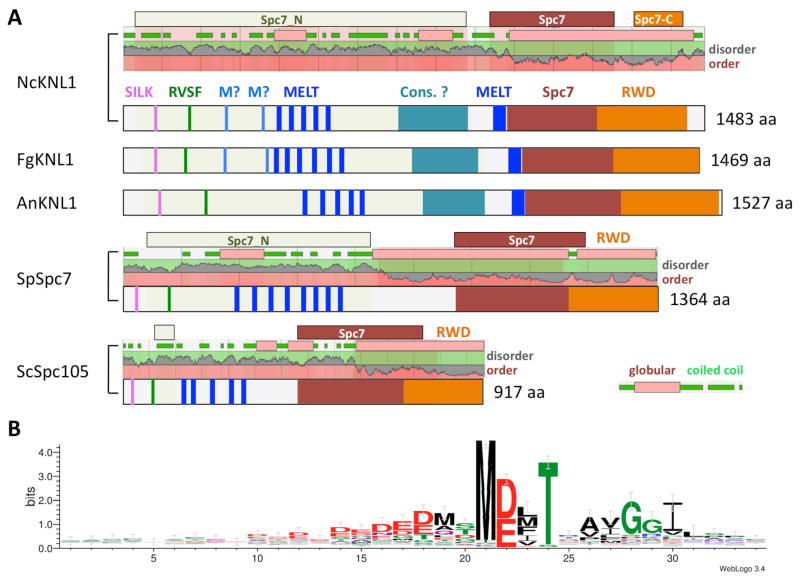
Overall, the primary sequence of Knl1 proteins is poorly conserved but a number of motifs and domains can be recognized in all ascomycetes. The N-terminal region contains patches of basic residues that are required for MT binding in Knl1 orthologs. Knl1 is also integrating the activity of Ipl1/Aurora B kinase and PP1 phosphatase via the highly conserved G/SILK and RVFS motifs (Welburn and others 2010), which are embedded in this basic N-terminus (Figure 5A). The RVFS motif is completely conserved in all fungal sequences examined, while the G/SILK sequence varies. In S. cerevisiae and S. pombe it is GILK, in Zymoseptoria and Leptosphaeria it is SILS and in all other taxa examined it is SILK. Aurora B inhibits the interaction between PP1 and Knl1 by phosphorylation of the RVSF serine, while PP1 requires KNL1 for its localization to kinetochores to inactivate the SAC. In mammals, PP1 antagonizes Aurora B phosphorylation of Ndc80, resulting in stabilization of the kinetochore to MT attachment. The mitotic checkpoint kinase Mps1 phosphorylates Knl1 of budding yeast, fission yeast and humans (Yamagishi and others 2014) at the threonine of the more centrally located MELT or MELT-like motifs. While the exact number of these motifs varies, their presence is conserved in the species that have Knl1. Phosphorylation by Mps1 is required for the recruitment of the SAC subunits Bub1 and Bub3, and the number and especially the type of MELT motifs available for phosphorylation may allow tuning of the SAC activity (Vleugel and others 2015). Examining predicted Knl1 sequences from selected species (SUPPLEMENTAL TABLE 3) showed that five motifs (MDMT, MDIT, MDVT, MEFT, MELT) made up two-thirds of all conserved MELT sites. Searching Knl1 sequences with all potential previously recognized motifs (i.e., M/I/L/V-E/D-L/M/I/V-T/S) showed that >95% of all motifs found begin with a methionine, followed by equal likelihood of aspartic and glutamic acid (FIG. 5B). In the third position, phenylalanine was found in a quarter of all motifs, somewhat unusual when compared to yeasts, where MELT and MD(L/I/V)T were most common. Based on activity assays the consensus sequence for human MELT sites has a DKT motif and an E/D-rich patch in the 15 residues preceding the MELT motif (M-E/D-L/M/I-T), followed by a K/S/R-S-H/Y-T motif. This region is quite different in ascomycetes, though the acidic patch is conserved with many E/D residues in the ten residues before the M-E/D-M/F/L/I/V-T motif. The immediately following residues are conserved in fungi (AxGGI motif, found by WebLogo; Crooks and others 2004). Specific patterns of motifs are conserved within taxa in respective orders, suggesting some adaptive role of specific MELT motifs, though this needs to be examined by deletion and swapping experiments.
FIG. 5.
Structural features of fungal Knl1/Spc7/Spc105 proteins. A. Cartoons showing important features of Knl1 homologs from fungi. Extent of Pfam domains (Spc7, Spc_N, Spc-C) are shown above plots depicting predicted disorder or order of proteins, and coiled-coil (green) or globular domains (pink). Knl1 homologs from N. crassa (NcKnl1), F. graminearum (FgKnl1) and A. nidulans (AnKnl1) are shown. Overall distribution of hallmark motifs (SILK, RVSF, MELT, Spc7 and tandem RWD domain) is similar in the three examples. The number and type of MELT motifs (MELT and M?) is different, however, and compared to the S. cerevisiae (ScSpc105) and S. pombe (SpSpc7), as well as the metazoan homologs, a potential additional MELT motif immediately before the Spc7 domain was identified. Also unique to filamentous ascomycetes is a conserved globular domain (“Cons.?”) of unknown function. The sequence of this motif is family or order-specific. B. Logo plot of 106 MELT motifs identified in 18 species (Neurospora crassa, Podospora anserina, Magnaporthe oryzae, Fusarium graminearum, Trichoderma reesei, Metarhizium album, Botryotinia fuckeliana, Aspergillus nidulans, Talaromyces marneffei, Arthroderma benhamiae, Histoplasma capsulatum, Coccidioides immitis, Zymoseptoria brevis, Leptosphaeria maculans, Pyronema omphalodens, Candida albicans, Saccharomyces cerevisiae, Schizosaccharomyces pombe) identified M-D/E-L/M/F-T as the preferred motif. Preceding residues are often D/E, the immediately following residues show preference for xAYGGI. Logo was generated by WebLogo 3.4 (Crooks and others, 2004).
In mammalian Knl1, “KI” motifs help to recruit Bub proteins but these short motifs appear to be missing from all fungal Knl1 homologs, suggesting that the KI motif has evolved more recently (Ghongane and others 2014). In fungal Knl1 proteins the longest conserved domain is the “Spc7” region, located ~200 aa from the C-terminus (FIG. 5A); this domain is not conserved in human Knl1. This domain extends for ~300 aa in both yeasts and the filamentous ascomycetes, in which we found a highly conserved additional MELT-like motif at the N-terminal end that is not conserved in mammals or yeasts. In some filamentous ascomycetes a C-terminal Spc7-C2 domain is found, which is part of a conserved tandem RWD domain (FIG. 5A). Like in human Knl1 (Petrovic and others 2014), all fungi inspected have the standard arrangement of α+β sandwich folds (α-β-β-β-β-α-α) in both RWD domains, which have been strongly implicated in binding to the Mis12 complex subunit Nsl1.
Yeast Kre28 and mammalian Zwint together with the Mis12 complex are required for normal kinetochore assembly (Caldas and others 2013; Pagliuca and others 2009). Kre28 forms heterotrimers with Spc105, the budding yeast Knl1 homolog, and human Knl1 binds to Zwint likely via a coiled coil region N-terminal of the tandem RWD domains (Ghongane and others 2014). Homology searches failed to yield obvious Zwint or Kre28 counterparts in most ascomycete fungi, suggesting that the homolog is different in primary structure from both Kre28 and Zwint. We found a previously unrecognized globular domain of unknown function between the conserved MELT motifs and the tandem RWD domains of Knl1 that is highly conserved in Sordariomycetes, Leotiomycetes and Eurotiomycetes but not Dothideomycetes and Pezizomycetes (FIG. 5A). This domain may serve as interaction domain with a predicted Zwint homolog.
MIS12 COMPLEX
Four subunits (Mis12, Mis13/Dsn1, Mis14/Nsl1, Nnf1) constitute the rod-like Mis12 complex, which acts as a platform for the formation of the KMN network (Cheeseman and others 2006). In budding yeast, the centromere-specific H3, Cse4 (CENP-A), and Ndc10 help to recruit the Mis12 complex, while in fission yeast Knl1 is required for Mis12 complex recruitment (Kerres and others 2007). The Mis12 complex forms one connection between MTs/Ndc80 complex and the CCAN by direct binding to CENP-C (Przewloka and others 2011; Screpanti and others 2011) and CENP-U (Hornung and others 2014). Once recruited to kinetochores, the Mis12 complex serves as a platform for Ndc80 and Knl1/Zwint binding (Petrovic and others 2014).
Several key interactions of the Mis12 complex are made via RWD domains on the interacting proteins. These recent findings suggest that the RWD domain has evolved as an adaptor motif in kinetochore interactions (Petrovic and others 2014; Schmitzberger and Harrison 2012). The C-terminus of Mis13/Dsn1 interacts directly with the Spc24/Spc25 heterodimeric RWD domain, while the Knl1 tandem RWD domain directly interacts with the C-terminal PVIHL motif of Mis14/Nsl1, which also contacts Spc24/Spc25. The CCAN subcomplex containing homologs of CENP-P-Q-O-U in yeast, COMA (Ctf19, Okp1, Mcm21, Ame1), is also predicted to have a heterodimeric RWD domain (between Ctf19/CENP-P and Mcm21/CENP-O). How this subcomplex interacts with Mis12 has only recently been addressed (Hornung and others 2014). An additional yeast complex, monopolin, is recruited by Mis12/Dsn1 during meiosis via its Csm1 RWD domain (Sarkar and others 2013). It is unknown if the recruitment of CENP-P-Q-O-U and monopolin are conserved in other fungi.
CENTROMERE IDENTITY IS CONVEYED BY CENP-A-DEPENDENT ASSEMBLY OF THE CONSTITUTIVE CENTROMERE-ASSOCIATED NETWORK (CCAN)
The epigenetic nature of centromeres has been firmly established, as specific DNA sequences alone are insufficient, let alone essential, to generate normal centromeres or aberrant “neocentromeres” (Black and Cleveland 2011; Cleveland and others 2003), perhaps even in budding yeast (Ho and others 2014). Replacement of canonical H3 at centromeric nucleosomes with an H3 variant called CENP-A in mammals (Palmer and others 1987), Cid in Drosophila (James and Elgin 1986), Cnp1CenpA in S. pombe (Takahashi and others 2000), Cse4 in S. cerevisiae (Stoler and others 1995) and Candida albicans (Sanyal and Carbon 2002) and CenH3 in N. crassa (Smith and others 2011) has been found in most systems studied, though there are exceptions in insects and trypanosomes (Drinnenberg and others 2014).
How CENP-A is targeted and maintained in centromeric regions has been subject of intensive studies over the past decade (Fukagawa and Earnshaw 2014; Perpelescu and Fukagawa 2011; Scott and Sullivan 2014). CenH3 sequences of yeasts are highly variable both in length and sequence of the N-terminal tail and loop I region within the histone fold domain (Baker and Rogers 2006). The “CENP-A targeting domain” (CATD), embedded in the otherwise conserved “histone fold domain” (HFD), proved sufficient to target CenH3 (or heterologous H3 with a CATD) into centromeric nucleosomes (Black and others 2007), but not in Arabidopsis thaliana (Ravi and others 2010). The CATD is conserved in filamentous fungi, because CenH3 proteins from several filamentous fungi yield the expected chromocenter localization when the native Neurospora CenH3 gene is replaced with heterologous genes (P.A. Phatale, K.M. Smith, L.R. Connolly and M. Freitag, unpublished data).
CENP-A is deposited by its chaperone HJURP (Dunleavy and others 2009; Foltz and others 2009; Sanchez-Pulido and others 2009), called Scm3 in the fungi (Camahort and others 2007; Mizuguchi and others, 2007; Stoler and others 2007), which has now also been shown to interact with CENP-C (Tachiwana and others 2015). The dynamics of deposition of CENP-A is an intensely studied aspect of centromere biology but apart from trailblazing work in budding and fission yeast (Camahort and others 2007; Mizuguchi and others, 2007; Stoler and others 2007; Pidoux and others 2009; Mishra and others 2011) there is little known about these processes in fungi; thus these processes will not be further discussed here. Similarly, five proteins that are required for proper chromosomes segregation in fission yeast (named Mis14 to Mis18; Hayashi and others 2004; Shiroiwa and others 2011) have not been studied in filamentous fungi. Several of these proteins had known counterparts (Mis14 is CENP-I, Mis15 is CENP-N, and Mis16 is the small subunit of chromatin assembly factor 1, CAF1-3, or RbAp46/48 in mammals. A four-protein Mis18 complex was found in humans, though Mis18 showed little sequence identity (Fujita and others 2007; Barnhart and others 2011; Moree and others 2011). Among other fungi similar proteins can only be identified in the Ustilagomycotina and Mis17, which had previously been proposed to be a CENP-M homolog, remains unknown from other fungi.
Conserved mechanism for CENP-A recognition by CENP-C/Mif2 (Carroll and others 2010) and CENP-T (Fachinetti and others 2013; Folco and others 2015) have been proposed. Short motifs in the N-terminal tail and CATD were found to be required for recruitment of CENP-C and CENP-T by targeting chimeric proteins consisting of mammalian H3 and CENP-A that were tagged with a LacI domain to genomic LacO sequences (Logsdon and others 2015). The authors separated features of CENP-A necessary for centromere establishment from those required for centromere maintenance. After formation of centromeres the CENP-A N-terminal tail seems no longer necessary to recruit normal levels of CENP-C and CENP-T, and the CENP-A C-terminal tail is no longer essential to recruit normal levels of CENP-T (Logsdon and others 2015). The model emerging from these studies is that it is difficult to gain neocentromeres and that redundant connections to CCAN complexes must be broken to lose a functional centromere (Logsdon and others 2015). Even if CENP-A is mislocalized or available in concentrations higher than required for maintenance of centromeres (Heun and others 2006; Smith and others 2011), neocentromeres do not arise frequently by spreading of CENP-A. One approach to cast more light on the differences between assembly and maintenance pathways is by in vitro reconstitution experiments using CENP-A nucleosome arrays and purified CCAN complexes (Logsdon and others 2015). Another way would be to use the information available from ascomycete fungi to assemble centromeres and kinetochores in vivo in a heterologous system to determine which motif or domains of the CCAN are required for assembly or maintenance of centromeres. Zymoseptoria tritici may become a facile system for this approach, as its centromeric regions are short, comprised of unique, non-repetitive sequences, and resemble those of neocentromeres in other organisms (Schotanus and others 2015). At the same time, many CCAN components cannot be found by sequence homology alone (SUPPLEMENTAL TABLE 4).
CENP-C
While the identity of centromeres seems to be determined by the placement of CENP-A into nucleosomes, the way in which the CCAN assembles is topic of intense research and debate. Recent studies suggest that CENP-C acts as the major recruiter of CCAN complexes (Basilico and others 2014; Kim and Yu 2015; Klare and others 2015; Tachiwana and others 2015; Krizaic and others 2015; Nagpal and others 2015). CENP-C is the most widely conserved CCAN subunit, containing a central “CENP-C” motif and a C-terminal dimerization motif (Perpelescu and Fukagawa 2011). The N-terminal domain appears to be dispensable for centromere localization but is required for the localization of many other KIN proteins (Perpelescu and Fukagawa 2011) and interaction with the Mis12 subunit Nnf1 (Przewloka and others 2011). CENP-C binds directly to CENP-A in vivo (Carroll and others 2010; Logsdon and others 2015), and by this interaction reshapes and stabilizes the CENP-A nucleosome (Falk and others 2015). On synthetic centromeres, CENP-C recruitment requires the CENP-C binding region and the CATD of CENP-A (Tachiwana and others 2015). CENP-C also binds to the CENP-H-I-K complex by direct interaction of CENP-H with specific residues in the PEST domain of CENP-C (Klare and others 2015). Abrogating this interaction by mutation of conserved LFL residues also resulted in reduction of CENP-T-W recruitment via CENP-H interactions. CENP-C binding to CCAN proteins appears dependent on cell cycle stage, which has been studied in detail in chicken cells. During interphase, CENP-L-N bind to CENP-C but interactions with CENP-A nucleosomes may be weaker (Nagpal and others 2015). In contrast, during mitosis CENP-C binds CENP-A by its C-terminal dimerization domain but the middle region, which binds to CENP-N and CENP-H shows less association with centromeric nucleosomes (Nagpal and others 2015). If this is also true in mammals or fungi remains to be seen but the possibility of CENP-C shifting between CENP-L-N, CENP-H-I-K and CENP-A provides an excellent model for cell cycle regulation of CENP-A.
In fungi CENP-C is the only CCAN protein that can be detected in basidiomycetes by homology searches. In Z. tritici, only CENP-A, CENP-C and CENP-S-X were found by homology, all other CCAN members seem to be absent (SUPPLEMENTAL TABLE 4). Previous studies suggested that CENP-C has domains that are under negative or positive selection and that these domains vary depending on the taxa inspected (Talbert and others 2004). Limited phylogenetic analysis to define CENP-C regions under positive or negative selection has been carried out (Smith and others 2012). Signatures of positive selection were found in the central region (from 245-258 and 288-318 aa in Neurospora CENP-C) but overall, CENP-C of filamentous fungi show evidence of negative selection. Detailed experimental work on this is still lacking in fungi other than budding and fission yeast.
CENP-N-L COMPLEX
CENP-N-L and CENP-M were co-purified with CENP-H-I-K (Foltz and others 2006; Okada and others 2006) but detailed relationships between these subunits remained obscure (Perpelescu and Fukagawa 2011) until recent work revealed the importance of the mammalian CENP-M subunit (Basilico and others 2014). CENP-N interacts with CENP-A nucleosomes in vitro, independently of DNA sequence context, but dependent on the CENP-A CATD (Carroll and others 2009). The heterodimer assumes a structure that is reminiscent of TATA-binding protein and it contacts CENP-C directly. Deletion of the conserved C-terminus abrogated CENP-N targeting and interactions with CENP-A, CENP-H, CENP-K and CENP-L, suggesting that CENP-N may serve to integrate information carried by CENP-A to recruit the other CCAN components (Carroll and others, 2009; Klare and others, 2015). Indeed, recent findings suggest that CENP-N is recognized by the exposed RG loop in the CENP-A CATD. The RG loop helps to arrange CENP-A nucleosomes in more compact “ladder-like” structures in which the CATD loop is buried and thus unable to contact CENP-N (Fang and others 2015). During the transition to S phase, the chromatin changes to a more open conformation, exposing the RG residues and allowing recruitment of CENP-N. These results demonstrated how structural transitions of chromatin allow for stage-specific recruitment of CCAN components. Future studies will address how these interactions are integrated with the known direct CENP-C and CENP-H interactions with CENP-A (Carroll and others 2010; Fachinetti and others 2013; Folco and others 2015; Falk and others, 2015; Klare and others 2015; Logsdon and others 2015).
Fungal homologs for CENP-N and CENP-L have only been studied in budding and fission yeast so far, where they are called Chl4 and Iml3, and Mis15 and Fta1, respectively (Meraldi and others 2006). Chl4 and Iml3 form a heterodimer and interact with Sgo1, a protein involved in sister chromatid cohesion (Hinshaw and Harrison 2013). All three proteins were found necessary in a screen for genes required for centromeric cohesion (Marston and others 2004) but their roles in chromosome segregation were known before (Pot and others 2003; Mythreye and Bloom 2003). De novo centromere assembly required Chl4 both on introduced plasmids and conditionally active centromeric DNA on chromosomes but Chl4 was not required for the maintenance of centromeric chromatin (Mythreye and Bloom 2003). Fission yeast CENP-N (Mis15) forms a complex with CENP-I (Mis6) and a proposed functional equivalent of CENP-M (Mis17; Hayashi and others 2004). Overall both CENP-L and CENP-N from filamentous ascomycetes are quite conserved; phylogenetic trees constructed by several different methods reveal relationships that mirror generally accepted phylogeny (data not shown).
CENP-H-K-I-M COMPLEX
This complex co-purified as a CCAN unit (Foltz and others 2006; Okada and others 2006), and previous extensive genetic and biochemical studies had established all subunits as CCAN components (Perpelescu and Fukagawa 2011). Mutation or depletion results in severe kinetochore defects and CENP-H overexpression has become a diagnostic tool in many forms of cancers (Liao and others 2007; Tomonaga and others 2005). CENP-I/Mis6 affects centromeric localization of CENP-A in fission but not in budding yeast and vertebrates (Perpelescu and Fukagawa 2011), and it can directly interact with the Ndc80 complex (Kim and Yu 2015). CENP-H-K-I are conserved in budding yeast and interact (Measday and others 2002). All subunits are present in most ascomycetes, and Aspergillus CENP-H has been shown to interact with the CENP-E kinesin (Herrero and others 2011). Depletion of CENP-H coupled to Aurora B kinase inhibition resulted in SAC defects (Kim and Yu 2015; Matson and others 2012), and CENP-T attachment to the Ndc80 complex depends on CENP-H-K-I. CENP-H-K-I alone, however, is insufficient to establish KMN-MT interactions (Basilico and others 2014; Kim and Yu 2015).
CENP-H-K-I and CENP-T-W-S-X complexes mutually recruit each other in a CENP-M-dependent manner to mediate one of two partially redundant pathways to fulfill the SAC function of the KMN network (Basilico and others 2014; Kim and Yu 2015). So far CENP-M homologues have only been studied in detail in animals. CENP-I bridges the CENP-H-K heterodimer and CENP-M, CENP-I forming a fold similar to that of importin-β and CENP-M fitting into the fold similar to Ran proteins (Basilico and others 2014). CENP-M was originally isolated as “proliferation associated nuclear element 1” (PANE1) as it is highly expressed in proliferating cells but was later also found by proteomic approaches (Foltz and others 2006; Okada and others 2006) and it is required for chromosome alignment and mitotic progression (Basilico and others 2014). It influences localization of CENP-N-L, as well as CENP-H-K-I and CENP-O-P-Q-U, and has been shown to be a non-catalytic GTPase (Basilico and others 2014). Similar “pseudo GTPase” have not been found in most fungal genomes by homology searches. A recent study confirmed this (Basilico and others 2014), yet in the CENP-M phylogenetic tree presented a potential homolog from Mucor was included. Indeed, this protein has some similarity to proteins from other zygomycetes and seems to contain a CENP-M domain with a conserved arrangement of β sheets and α helices (FIG. 6). Whether this is a true homolog of mammalian CENP-M, however, remains to be determined. It is possible that fungal CENP-M-like proteins are very different in structure or that the Mucor pseudo GTPase is involved in other processes. Fission yeast Mis17, for example, was identified as potential regulator of the CENP-I/Mis6 complex (Perpelescu and Fukagawa 2011), yet based on structural studies it seems unlikely that this is a true functional homolog of CENP-M (Basilico and others 2014); it also lacks homologs in other fungi. It is also possible that that a functional GTPase has taken the place of CENP-M in fungi – this needs to be investigated.
FIG. 6.
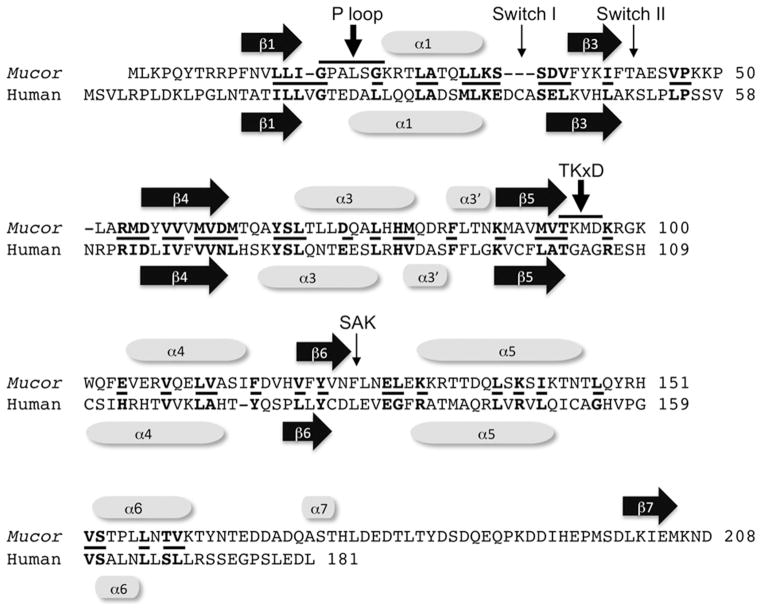
Alignment of human CENP-M and a potential fungal CENP-M homolog. CENP-M from human (Q9NSP4.1) and Mucor circinelloides f. circinelloides (EPB82532.1) share overall similar domain structure. Similar proteins can be found in several other Mucoromycotina but not in other fungi. CENP-M is predicted to be a nonfunctional “pseudo GTPase” (Basilico and others, 2014). Several features of functional GTPases are no longer present in CENP-M, for example the P-loop (GxxxxGKS/T) involved in GTP binding, Switch I and Switch II motifs required for conformational changes, the TKxD and the SAK motifs. A conserved catalytic glutamine residue right after Switch II is present in GTPases and is changed into a leucine in human and glutamic acid in Mucor CENP-M. Based on structural similarity to CENP-M and small GTPases the Mucor protein is predicted to assume a pseudo-GTPase fold but if it acts as a true CENP-M homolog will need to be addressed experimentally. Conserved residues are shown in bold and short lines between the sequences.
CENP-T-W-S-X COMPLEX
Homologs for all four CENP-T-W-S-X subunits have been found in most ascomycetes (Schleiffer and others 2012; Smith and others 2011; Smith and others 2012; Westermann and Schleiffer 2013) but they are more difficult to identify in basidiomycetes. Relationships of all four proteins across taxa resemble established phylogenies (Smith and others 2012). Studies with the Neurospora proteins revealed that only CENP-T and CENP-W are essential, while deletion of CENP-S and CENP-X resulted in only minor growth defects (J. Galaska and M. Freitag, unpublished data). The fission yeast CENP-T, Cnp20, is also essential (Tanaka and others 2009), while the budding yeast CENP-T, Cnn1, is not (Bock and others 2012).
CENP-T has a C-terminal HFD, which can bind DNA and is necessary for CENP-T centromere localization, while CENP-W is a small protein comprised entirely by a HFD (Hori and others 2008a). CENP-T and CENP-W are tightly associated and form a heterodimer (Nishino and others 2012), which has also been found in MS analyses of Neurospora extracts (J. Galazka and M. Freitag, unpublished results). CENP-S was identified by proteomic approaches (Foltz and others 2006) and CENP-X was subsequently described as its binding partner (Amano and others 2009). Both proteins have potential HFDs that are conserved within the ascomycetes. Localization of CENP-S and CENP-X is interdependent and also dependent on CENP-H-K-I (Amano and others 2009). CENP-S-X are identical to MHF1/MHF2, binding partners of FANCM (a member of the Fanconi anemia nuclear core complex), but centromeric localization of FANCM has not been reported (Perpelescu and Fukagawa 2011). Studies with metazoan CENP-T-W-S-X suggested that these proteins can form a tetrameric complex that may mimic canonical nucleosomes (Nishino and others 2012). In both yeast (Schleiffer and others 2012) and in Neurospora (J. Galazka and M. Freitag, unpublished results), however, direct interactions of all four proteins were not found by in vivo proteomic approaches with tagged CENP-S or CENP-X, while analyses with tagged CENP-T at low stringency revealed interactions with most CCAN subunits (Schleiffer and others 2012).
Tethering experiments suggested that the CENP-T-W-S-X complex, in the presence of CENP-C, is sufficient to nucleate at least partially functional kinetochores, even in the absence of CENP-A (Gascoigne and others 2011). These interactions rely on direct binding of the CENP-T N-terminal region to the Ndc80 complex via a conserved motif (Schleiffer and others 2012). The conserved Ndc80 “receptor motif” of CENP-T/Cnn1 (FIG. 7) is bound in a hydrophobic cleft between Spc24/Spc25 (Malvezzi and others 2013). Mutations in Spc24/Spc25 disrupting this binding are lethal in yeast. Residues that are important for the interaction in yeast are mostly conserved in other fungi, though some critical residues are different in several species. The precise interactions should therefore be tested by mutational analyses. A similar motif in the Mis12 complex subunit Mis12/Dsn1/Mtw1 (FIG. 7) was also found to be necessary for Ndc80 binding and mutation was lethal in yeast, but replacement with the motif from CENP-T/Cnn1 restored growth, suggesting another modular motif in CCAN and KMN interactions, similar to RWD domains (Malvezzi and others 2013).
FIG. 7.
Interactions between Ndc80, Mis12 and CENP-T and assembly of the KIN. A. The Spc24/Spc25 heterodimeric RWD domain interacts either with a helical patch on the CENP-T N-terminus (CENP-T helix) or a similar region on the Mis12 subunit Dsn1 (Dsn1 helix). This latter interaction also involves the Mis12 subunit Nsl1 PVIHL motif, which brings the Knl1 tandem RWD domain closer for binding to Spc24/Spc25. Kre28/Zwint homologs have not been identified in filamentous fungi by homology searches. B. Conservation of the CENP-T helix motif from human and ten different fungi (abbreviations as shown in SUPPLEMENTAL TABLE 1). Conserved residues are shown in bold and shaded in grey. C. Conservation of the Dsn1 helix motif from human and ten different fungi (abbreviations as shown in SUPPLEMENTAL TABLE 1) and aligned with the motif shown in B. Conserved residues are shown in bold and shaded in grey and residues that resulted in loss of function in yeast (Malvezzi and others, 2013) are indicated (***). D. Proposed hierarchy of CCAN and KMN assembly in fungi and mammals. See text for details. SAC, spindle assembly checkpoint.
The CENP-T N-terminal domain is phosphorylated by CDK and Mps1 for mitotic progression, coordinating binding of the different partners to the Mis12 complex (Malvezzi and others 2013). Yeast CENP-T/Cnn1 localizes to the kinetochore from G1 through metaphase and mutation of either the HFD or Spc24/Spc25 interaction domain results in abnormal CENP-T levels at the kinetochore (Thapa and others 2015). When Mps1 activity decreaes (at the onset of anaphase) CENP-T is enriched mostly through its N-terminal Spc24/Spc25 interaction sequence; thus, this study provided in vivo evidence of CENP-T enrichment during anaphase.
That CENP-T/Cnn1 relied on CENP-A and CENP-C for targeting to the kinetochore had been established in budding yeast (Bock and others 2012) and in mammals (Basilico and others 2014). Studies in fission yeast dissected the importance of the CENP-A N-terminal tail for centromere functions (Folco and others 2015). An altered CENP-A tail reduced recruitment of CENP-T and CENP-I but not CENP-C. Overexpression of CENP-T suppressed defects caused by mutation of the CENP-A N-terminal tail mutant. Ndc80 complex localization was, however, not altered, perhaps because CENP-T mutants also do not change Ndc80 recruitment (Tanaka and others 2009). Thus effects of mutation, deletion and overexpression of CENP-T in different fungi result in different phenotypes. In Neurospora, recruitment of CENP-T in CENP-A point mutants or strains with heterologous CENP-A was not altered by cytology and ChIP-seq (P. Phatale, J. Galazka, S. Friedman and M. Freitag, unpublished data) but this must be addressed further by examining the situation in additional species.
CENP-P-Q-O-U-(R) COMPLEX
This complex was identified as interacting with CENP-H-K-I and as part of the inner centromere complexes (Foltz and others 2006; Izuta and others 2006; Okada and others 2006), and is important in the recovery from spindle damage (Hori and others 2008b). CENP-R appears to be located most downstream in all assays and is not conserved in fungi, thus may not be a bona fide member of this complex. All other components are interdependent on each other for centromere localization and require the CENP-H-K-I complex (Hori and others 2008b; Okada and others 2006). The homologous complex, called “COMA”, had been isolated earlier from budding yeast, where the CENP-U/Ame1 and CENP-Q/Okp1 subunits are essential (Ortiz and others 1999). CENP-U mutants are viable in certain mammalian cell lines but essential in mouse embryos and embryonic stem cells (Kagawa and others 2014). CENP-U is required for recovery from spindle damage, preventing premature sister chromatid separation, a process controlled by CENP-U phosphorylation (Foltz and others 2006; Hori and others 2008b). The leucine-zipper domain of CENP-U and the coiled-coil domain of CENP-Q are necessary for interaction (Kang and others 2011).
Recent evidence from studies with yeast strongly suggests that the COMA (Ctf19, Okp1, Mcm21, Ame1) complex, equivalent to CENP-P-Q-O-U in metazoans, respectively, constitutes a third independent linkage between centromeric chromatin and the KMN network, acting via the Mis12 complex (Corbett and Desai 2014; Hornung and others 2014). Detailed mass spectrometry showed that CENP-U/Ame1 associates with other CCAN proteins as well as KMN complexes, and reconstituted CENP-U-Q/Ame1–Okp1 binds directly to a reconstituted Mis12 complex in vitro. Binding is mediated by a short motif in the Ame1 amino terminus; mutations in this motif abolish Mis12 interactions and are lethal in vivo, causing chromosome missegregation. Thus, Ame1 to Mis12 complex interactions are critical for inner–outer kinetochore linkage in budding yeast. Deletion of the Ame1 N-terminal motif reduced both Mis12 and COMA complex localization, suggesting interdependence for assembly, but as Ame1-Okp1 can bind directly to CENP-C/Mif2 and to DNA, at least in vitro, and it was suggested that it forms a link between the CDEIII-binding CBF3 complex and the KMN (Ortiz and others 1999) it remains possible that there are secondary effects. These interactions have not been studied in any great detail, however. The Mis12-binding motif of Ame1 seems conserved in the CENP-U/Ame1 proteins of other ascomycetes but cannot be found by homology searches in basidiomycetes, where CENP-U is difficult to identify, or in the metazoan CENP-U sequences. In the region of the Mis12-binding motif of Ame1, however, mammalian CENP-U shows similar predicted secondary structure (Hornung and others 2014).
Identification of the RWD domain, important for many interactions between KMN proteins and the Mis12 complex in particular suggest that there may be another way that the CENP-U complex can interact with Mis12, as CENP-P/Ctf19 and CENP-O/Mcm21 can form a heterodimeric RWD domain (Petrovic and others 2014). While putative CENP-O homologs can be identified from many filamentous ascomycetes CENP-P or Ctf19 homologs have not been identified by homology searches.
ASSEMBLY OF THE INNER KINETOCHORE: WHO’S ON FIRST?
Much recent work has been dedicated to understanding the interactions between CENP-A and the innermost CCAN subunits, including CENP-C, CENP-T (and its potential binding partners, CENP-W-S-X) and the yeast COMA or mammalian CENP-O-P-Q-U complexes (FIG. 7). While ChIP-seq showed that CENP-T and CENP-C are localized to the same regions as CENP-A (Smith and others 2011; Thakur and others 2015), they appeared to interact primarily with centromeric H3 oligonucleosomes within CENP-A chromatin (Hori and others 2008a), which was also confirmed by super-resolution microscopy for CENP-T (Ribeiro and others 2010). Ordering the recruitment pathway in chicken DT40 cells by analyses of mutants placed CENP-T-W upstream of CENP-H-K-I and the CENP-U complex but parallel with CENP-C (Hori and Fukagawa 2012; Nishino and others 2012). Thus, one model proposes that CENP-C, together with the CENP-U and CENP-T forms a three-legged platform on which the Mis12 and Ndc80 complexes assemble (Gascoigne and others 2011; Hori and Fukagawa 2012; Hornung and others 2014; Screpanti and others 2011). This view is not unchallenged, as recent studies showed dependence of CENP-T assembly on CENP-C (Basilico and others 2014; Klare and others; Suzuki and others 2015; Tachiwana and others 2015;), thus placing CENP-C at the root of CCAN assembly as previously proposed based on RNAi experiments in human cells (Carroll and others 2010). Depletion of CENP-C reduces both CENP-T and CENP-H-K-I levels (Carroll and others 2010; Gascoigne and others 2011), but depletion of CENP-M did not affect CENP-C or Nsl1 levels, yet resulted in reduced CENP-T and Ndc80 levels, where interactions between CENP-M and CENP-T-W seem to be mediated by the HFD (Basilico and others 2014). Recruitment of Ndc80 complexes by CENP-C and CENP-T was tested by quantitative immunofluorescence of kinetochore proteins in HeLa cells (Suzuki and others 2015). In these studies, a chimeric N-terminal CENP-T with CENP-C DNA-binding domain under CENP-C depletion conditions was used to measure “CENP-T only” assemblies. On average there were 244 Ndc80 complexes, 215 CENP-C, 72 CENP-T and 151 Mis12 at a HeLa kinetochore (Susuki and others 2015). This translates to 13 CENP-C and four CENP-T molecules per MT (nine Mis12 and 14 Ndc80 molecules). Each CENP-T recruits one Mis12/KMN network and one Ndc80 but only ~38% of CENP-C proteins recruit a KMN. The “CENP-T only” chimeric linkage to the KMN resulted in functional kinetochores with the expected numbers of Ndc80 and Mis12 proteins. It is still unclear why both CENP-C and CENP-T are needed but the authors suggested that it is the total number of Ndc80 complexes recruited that determines functionality of the kinetochore (Suzuki and others 2015).
What emerges from the studies discussed above is a partial feedback loop of assembly (FIG. 7D) that is subject to cell cycle control. CENP-C and CENP-L-N interact directly with the CENP-A nucleosome; CENP-L-N requires CENP-C for recruitment. CENP-C also appears to be essential for the recruitment of both CENP-T-W-S-X and CENP-H-K-I bound to CENP-M, and these two complexes are interdependently recruiting each other (Basilico and others 2014). There are, however, conflicting studies available on this point, as recruitment pathways to artificially tethered subcomplexes were in parallel and not hierarchical. CENP-C-driven KMN assembly controlled by Aurora B and CENP-T-driven KMN assembly controlled by CDK (Rago and others 2015) and multiple depletion studies also suggested parallel pathways (Kim and Yu 2015; Susuki and others 2015). In parallel to the CENP-T and CENP-C axes, CENP-U binds to the Mis12 complex, thus connecting DNA- or chromatin binding activities of CBF3 or the other CENP-O-P-Q-U subunits (Hornung and others 2014) but this needs to be established in other organisms as well. Thus, while CENP-T-W-S-X directly interacts with the Ndc80 complex, Mis12 integrates the binding of the other CCAN complexes. It remains to be seen if CENP-T, CENP-U and CENP-C (the latter via Mis12) are connected to Ndc80 complexes at the same time. It is already clear that binding to one heterodimeric Spc24/Spc25 RWD domain is exclusive: either Mis12 or CENP-T are bound. It is likely that Ndc80 complexes in one kinetochore use both CENP-T and Mis12 as binding partners, however, and that CENP-T binding is dependent on its phosphorylation by CDK during mitosis (Malvezzi and others 2013; Nishino and others 2013).
CONCLUDING REMARKS
Centromeres and kinetochores of filamentous fungi appear to span the gap between those of S. cerevisiae and S. pombe to those of animals. Filamentous fungi offer now almost the same experimental advantages for the analyses of kinetochore proteins and their interactions as the yeasts. The availability of hundreds of genome sequences allows in-depth studies on the relatedness of centromere and kinetochore proteins and will help to decipher truly conserved versus specialized mechanisms of kinetochore function. That some specialization has occurred and that KIN components may be useful to study adaptive evolution is exemplified by CCAN complements of Dothideomycetes. While homologs can be found for most known components in Leptosphaeria maculans and Pyrenophora species these proteins (save for CENP-A, and CENP-C), cannot be found in all known Zymoseptoria species when relying on homology searches.
Thus, the composition of CCAN and even some KMN complexes within all fungi is still unresolved (SUPPLEMENTAL TABLES 1 - 4). This is not a unique situation as some well-studied model organisms, e.g. Drosophila melanogaster and Caenorhabditis elegans, also have “incomplete” sets of kinetochore proteins, for example most CCAN subunits conserved in ascomycetes and mammals cannot be found in either species by homology searches. For the fungi it seems logical to use proteomic approaches that have been applied with such success in yeast, chicken, and human cells to identify more components of centromeres and kinetochores in the basal lineages and the basidiomycetes. As there are well known and intensely studied pathogens present in all these lineages there should be sufficient impetus to conduct these studies. Related to this, because filamentous fungi are exceedingly successful pathogens and few dedicated antifungals are currently available there seems to be ample opportunity to screen for or design small-molecule drugs that will interfere with CCAN and KMN function in specific taxa only. That competition between proteins can occur has been shown in viruses, where a HPV18 protein E7 can compete with CENP-C binding (Yaginuma and others 2015).
This application-oriented goal will need to rely on a complete understanding of interactions within KIN subcomplexes. Over the past four years interaction domains between the Ndc80, Mis12 and CENP-T complexes have emerged and these will need to be characterized further. RWD domains are clearly conserved in the ascomcyetes but inspection of 26 taxa from various groups of fungi also revealed that multiple additional conserved regions are present, most of which are lacking in yeasts or animals. Swapping domains of CCAN or KMN proteins among closely or distantly related fungi may help decipher mechanistic details that will not be uncovered when the budding yeast protein is introduced into human cells or vice versa. Thus, comparative biology on single domains will help to distinguish between generally applicable mechanisms and interactions that are taxon-specific. Specific examples of interactions that need to be examined in more detail are those between Dam1 and Ndc80, the function of the exposed Ndc80 loop, interactions between Knl1, Mis12 and the Spc24/Spc25 heterodimeric RWD domain, the true nature of the CENP-T complex and potential adaptive nature of all CCAN complexes.
Supplementary Material
Acknowledgments
I thank all members of my laboratory for fruitful discussions, and Jason Stajich and Kaustuv Sanyal for input about fungal Dam1 and CCAN complexes, respectively. I would like to thank one anonymous reviewer for helpful comments and corrections that made this a better manuscript. I apologize to those authors whose work was not cited because of space concerns; this is a fast-moving field, and only literature published by August 2015 was included here. This manuscript was prepared during a sabbatical in the laboratory of Eva H. Stukenbrock at the Max-Planck-Institut für Evolutionsbiologie in Plön, and I would like to thank her and the members of the MPI for discussions and their hospitality during my stay. Financial support through a grant from the NIH (R01GM097637) is gratefully acknowledged.
LITERATURE CITED
- Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–10. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Suzuki A, Hori T, Backer C, Okawa K, Cheeseman IM, Fukagawa T. The CENP-S complex is essential for the stable assembly of outer kinetochore structure. J Cell Biol. 2009;186:173–82. doi: 10.1083/jcb.200903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudhan P, Felzer-Kim I, Gurunathan K, Joglekar AP. Assembling the protein architecture of the budding yeast kinetochore-microtubule attachment using FRET. Curr Biol. 2014;24:1437–46. doi: 10.1016/j.cub.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RE, Rogers K. Phylogenetic analysis of fungal centromere H3 proteins. Genetics. 2006;174:1481–92. doi: 10.1534/genetics.106.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart MC, Kuich PH, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol. 2011;194:229–43. doi: 10.1083/jcb.201012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico F, Maffini S, Weir JR, Prumbaum D, Rojas AM, Zimniak T, De Antoni A, Jeganathan S, Voss B, van Gerwen S, et al. The pseudo GTPase CENP-M drives human kinetochore assembly. elife. 2014;3:e02978. doi: 10.7554/eLife.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle GW. A Possible Influence of the Spindle Fibre on Crossing-Over in Drosophila. Proc Natl Acad Sci U S A. 1932;18:160–5. doi: 10.1073/pnas.18.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj R, Qi W, Yu H. Identification of two novel components of the human NDC80 kinetochore complex. J Biol Chem. 2004;279:13076–85. doi: 10.1074/jbc.M310224200. [DOI] [PubMed] [Google Scholar]
- Black BE, Cleveland DW. Epigenetic centromere propagation and the nature of CENP-a nucleosomes. Cell. 2011;144:471–9. doi: 10.1016/j.cell.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Jansen LE, Maddox PS, Foltz DR, Desai AB, Shah JV, Cleveland DW. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol Cell. 2007;25(2):309–22. doi: 10.1016/j.molcel.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Bock LJ, Pagliuca C, Kobayashi N, Grove RA, Oku Y, Shrestha K, Alfieri C, Golfieri C, Oldani A, Dal Maschio M, et al. Cnn1 inhibits the interactions between the KMN complexes of the yeast kinetochore. Nat Cell Biol. 2012;14(6):614–24. doi: 10.1038/ncb2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack LS, Applen SE, Berman J. The requirement for the Dam1 complex is dependent upon the number of kinetochore proteins and microtubules. Curr Biol. 2011;21(10):889–96. doi: 10.1016/j.cub.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas GV, DeLuca KF, DeLuca JG. KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity. J Cell Biol. 2013;203(6):957–69. doi: 10.1083/jcb.201306054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camahort R, Li B, Florens L, Swanson SK, Washburn MP, Gerton JL. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol Cell. 2007;26:853–65. doi: 10.1016/j.molcel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Milks KJ, Straight AF. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol. 2010;189(7):1143–55. doi: 10.1083/jcb.201001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Silva MC, Godek KM, Jansen LE, Straight AF. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11(7):896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Brew C, Wolyniak M, Desai A, Anderson S, Muster N, Yates JR, Huffaker TC, Drubin DG, Barnes G. Implication of a novel multiprotein Dam1p complex in outer kinetochore function. J Cell Biol. 2001a;155(7):1137–45. doi: 10.1083/jcb.200109063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127(5):983–97. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9(1):33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Enquist-Newman M, Muller-Reichert T, Drubin DG, Barnes G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J Cell Biol. 2001b;152(1):197–212. doi: 10.1083/jcb.152.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133(3):427–39. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Amstutz H, Fishel B, Carbon J. Analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1986;83(21):8253–7. doi: 10.1073/pnas.83.21.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287:504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112(4):407–21. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Desai A. A new piece in the kinetochore jigsaw puzzle. J Cell Biol. 2014;206(4):457–9. doi: 10.1083/jcb.201407048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188–90. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124(Pt 4):622–34. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel PB, Keyes BE, Chaterjee M, Remington CE, Burke DJ. A redundant function for the N-terminal tail of Ndc80 in kinetochore-microtubule interaction in Saccharomyces cerevisiae. Genetics. 2012;192(2):753–6. doi: 10.1534/genetics.112.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, McDonald KL, McIntosh JR. Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J Cell Biol. 1993;120:141–51. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg IA, deYoung D, Henikoff S, Malik HS. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife. 2014:3. doi: 10.7554/eLife.03676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–97. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92(4):290–6. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Fachinetti D, Folco HD, Nechemia-Arbely Y, Valente LP, Nguyen K, Wong AJ, Zhu Q, Holland AJ, Desai A, Jansen LE, et al. A two-step mechanism for epigenetic specification of centromere identity and function. Nat Cell Biol. 2013;15(9):1056–66. doi: 10.1038/ncb2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LE, Van Duyne GD, Vinogradov SA, et al. Chromosomes. CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science. 2015;348(6235):699–703. doi: 10.1126/science.1259308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Liu Y, Wei Y, Deng W, Yu Z, Huang L, Teng Y, Yao T, You Q, Ruan H, et al. Structural transitions of centromeric chromatin regulate the cell cycle-dependent recruitment of CENP-N. Genes Dev. 2015;29:1058–73. doi: 10.1101/gad.259432.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming W. Zellsubstanz, Kern und Zellteilung. Leipzig: Vogel; 1882. [Google Scholar]
- Folco HD, Campbell CS, May KM, Espinoza CA, Oegema K, Hardwick KG, Grewal SI, Desai A. The CENP-A N-tail confers epigenetic stability to centromeres via the CENP-T branch of the CCAN in fission yeast. Curr Biol. 2015;25(3):348–56. doi: 10.1016/j.cub.2014.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14(1):25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LE, Black BE, Bailey AO, Yates JR, 3rd, Cleveland DW. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8(5):458–69. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–84. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell. 2007;12:17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Fukagawa T, Earnshaw WC. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell. 2014;30(5):496–508. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Courtheoux T, Gachet Y, Tournier S, He X. A non-ring-like form of the Dam1 complex modulates microtubule dynamics in fission yeast. Proc Natl Acad Sci U S A. 2010;107:13330–5. doi: 10.1073/pnas.1004887107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne KE, Takeuchi K, Suzuki A, Hori T, Fukagawa T, Cheeseman IM. Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell. 2011;145(3):410–22. doi: 10.1016/j.cell.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghongane P, Kapanidou M, Asghar A, Elowe S, Bolanos-Garcia VM. The dynamic protein Knl1 - a kinetochore rendezvous. J Cell Sci. 2014;127(Pt 16):3415–23. doi: 10.1242/jcs.149922. [DOI] [PubMed] [Google Scholar]
- Gonen S, Akiyoshi B, Iadanza MG, Shi D, Duggan N, Biggins S, Gonen T. The structure of purified kinetochores reveals multiple microtubule-attachment sites. Nat Struct Mol Biol. 2012;19(9):925–9. doi: 10.1038/nsmb.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes GJ, Deluca JG. Connecting with Ska, a key complex at the kinetochore-microtubule interface. EMBO J. 2009;28(10):1375–7. doi: 10.1038/emboj.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18(22):1778–84. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3:838–49. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118(6):715–29. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Herrero S, Takeshita N, Fischer R. The Aspergillus nidulans CENP-E kinesin motor KipA interacts with the fungal homologue of the centromere-associated protein CENP-H at the kinetochore. Mol Microbiol. 2011;80(4):981–94. doi: 10.1111/j.1365-2958.2011.07624.x. [DOI] [PubMed] [Google Scholar]
- Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell. 2006;10(3):303–15. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111:509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hinshaw SM, Harrison SC. An Iml3-Chl4 heterodimer links the core centromere to factors required for accurate chromosome segregation. Cell Rep. 2013;5:29–36. doi: 10.1016/j.celrep.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KH, Tsuchiya D, Oliger AC, Lacefield S. Localization and function of budding yeast CENP-A depends upon kinetochore protein interactions and is independent of canonical centromere sequence. Cell Rep. 2014;9(6):2027–33. doi: 10.1016/j.celrep.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang WH, Suzuki E, Okawa K, et al. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008a;135(6):1039–52. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hori T, Fukagawa T. Establishment of the vertebrate kinetochores. Chromosome Res. 2012;20(5):547–61. doi: 10.1007/s10577-012-9289-9. [DOI] [PubMed] [Google Scholar]
- Hori T, Okada M, Maenaka K, Fukagawa T. CENP-O class proteins form a stable complex and are required for proper kinetochore function. Mol Biol Cell. 2008b;19(3):843–54. doi: 10.1091/mbc.E07-06-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung P, Troc P, Malvezzi F, Maier M, Demianova Z, Zimniak T, Litos G, Lampert F, Schleiffer A, Brunner M, et al. A cooperative mechanism drives budding yeast kinetochore assembly downstream of CENP-A. J Cell Biol. 2014;206(4):509–24. doi: 10.1083/jcb.201403081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Toda T. Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore-spindle attachment in fission yeast. Curr Biol. 2011;21(3):214–20. doi: 10.1016/j.cub.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuta H, Ikeno M, Suzuki N, Tomonaga T, Nozaki N, Obuse C, Kisu Y, Goshima N, Nomura F, Nomura N, et al. Comprehensive analysis of the ICEN (Interphase Centromere Complex) components enriched in the CENP-A chromatin of human cells. Genes Cells. 2006;11(6):673–84. doi: 10.1111/j.1365-2443.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- James TC, Elgin SC. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986;6(11):3862–3872. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Ortiz J, Tanaka TU, Lechner J, Schiebel E. Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. Embo J. 2002;21(1–2):181–93. doi: 10.1093/emboj/21.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED. Molecular architecture of a kinetochore-microtubule attachment site. Nat Cell Biol. 2006;8:581–5. doi: 10.1038/ncb1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bouck D, Finley K, Liu X, Wan Y, Berman J, He X, Salmon ED, Bloom KS. Molecular architecture of the kinetochore-microtubule attachment site is conserved between point and regional centromeres. J Cell Biol. 2008;181:587–94. doi: 10.1083/jcb.200803027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bloom K, Salmon ED. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr Biol. 2009;19(8):694–9. doi: 10.1016/j.cub.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa N, Hori T, Hoki Y, Hosoya O, Tsutsui K, Saga Y, Sado T, Fukagawa T. The CENP-O complex requirement varies among different cell types. Chromosome Res. 2014;22(3):293–303. doi: 10.1007/s10577-014-9404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Park CH, Kim TS, Soung NK, Bang JK, Kim BY, Park JE, Lee KS. Mammalian polo-like kinase 1-dependent regulation of the PBIP1-CENP-Q complex at kinetochores. J Biol Chem. 2011;286(22):19744–57. doi: 10.1074/jbc.M111.224105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerres A, Jakopec V, Fleig U. The conserved Spc7 protein is required for spindle integrity and links kinetochore complexes in fission yeast. Mol Biol Cell. 2007;18(7):2441–54. doi: 10.1091/mbc.E06-08-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermaier E, Woehrer S, Peng Y, Mechtler K, Westermann S. A Dam1-based artificial kinetochore is sufficient to promote chromosome segregation in budding yeast. Nat Cell Biol. 2009;11(9):1109–15. doi: 10.1038/ncb1924. [DOI] [PubMed] [Google Scholar]
- Kim S, Yu H. Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J Cell Biol. 2015;208(2):181–96. doi: 10.1083/jcb.201407074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare K, Weir JR, Basilico F, Zimniak T, Massimiliano L, Ludwigs N, Herzog F, Musacchio A. CENP-C is a blueprint for constitutive centromere-associated network assembly within human kinetochores. J Cell Biol. 2015;210:11–22. doi: 10.1083/jcb.201412028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaic I, Williams SJ, Sanchez P, Rodriguez-Corsino M, Stukenberg PT, Losada A. The distinct functions of CENP-C and CENP-T/W in centromere propagation and function in Xenopus egg extracts. Nucleus. 2015;6:133–43. doi: 10.1080/19491034.2014.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield S, Lau DT, Murray AW. Recruiting a microtubule-binding complex to DNA directs chromosome segregation in budding yeast. Nat Cell Biol. 2009;11(9):1116–20. doi: 10.1038/ncb1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F, Hornung P, Westermann S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol. 2010;189(4):641–9. doi: 10.1083/jcb.200912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F, Mieck C, Alushin GM, Nogales E, Westermann S. Molecular requirements for the formation of a kinetochore-microtubule interface by Dam1 and Ndc80 complexes. J Cell Biol. 2013;200(1):21–30. doi: 10.1083/jcb.201210091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22(22):R966–80. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Liao WT, Song LB, Zhang HZ, Zhang X, Zhang L, Liu WL, Feng Y, Guo BH, Mai HQ, Cao SM, et al. Centromere protein H is a novel prognostic marker for nasopharyngeal carcinoma progression and overall patient survival. Clin Cancer Res. 2007;13(2 Pt 1):508–14. doi: 10.1158/1078-0432.CCR-06-1512. [DOI] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323(5919):1350–3. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon GA, Barrey EJ, Bassett EA, DeNizio JE, Guo LY, Panchenko T, Dawicki-McKenna JM, Heun P, Black BE. Both tails and the centromere targeting domain of CENP-A are required for centromere establishment. J Cell Biol. 2015;208(5):521–31. doi: 10.1083/jcb.201412011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolica A, Cittaro D, Borsotti D, Sennels L, Ciferri C, Tarricone C, Musacchio A, Rappsilber J. Structural analysis of multiprotein complexes by cross-linking, mass spectrometry, and database searching. Mol Cell Proteomics. 2007;6(12):2200–11. doi: 10.1074/mcp.M700274-MCP200. [DOI] [PubMed] [Google Scholar]
- Malvezzi F, Litos G, Schleiffer A, Heuck A, Mechtler K, Clausen T, Westermann S. A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors. EMBO J. 2013;32(3):409–23. doi: 10.1038/emboj.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvezzi F, Westermann S. “Uno, nessuno e centomila”: the different faces of the budding yeast kinetochore. Chromosoma. 2014;123(5):447–57. doi: 10.1007/s00412-014-0472-y. [DOI] [PubMed] [Google Scholar]
- Marston AL, Tham WH, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science. 2004;303:1367–70. doi: 10.1126/science.1094220. [DOI] [PubMed] [Google Scholar]
- Matson DR, Demirel PB, Stukenberg PT, Burke DJ. A conserved role for COMA/CENP-H/I/N kinetochore proteins in the spindle checkpoint. Genes Dev. 2012;26(6):542–7. doi: 10.1101/gad.184184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maure JF, Komoto S, Oku Y, Mino A, Pasqualato S, Natsume K, Clayton L, Musacchio A, Tanaka TU. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr Biol. 2011;21(3):207–13. doi: 10.1016/j.cub.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleland ML, Kallio MJ, Barrett-Wilt GA, Kestner CA, Shabanowitz J, Hunt DF, Gorbsky GJ, Stukenberg PT. The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr Biol. 2004;14(2):131–7. doi: 10.1016/j.cub.2003.12.058. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, O’Toole E, Zhudenkov K, Morphew M, Schwartz C, Ataullakhanov FI, Grishchuk EL. Conserved and divergent features of kinetochores and spindle microtubule ends from five species. J Cell Biol. 2013;200(4):459–74. doi: 10.1083/jcb.201209154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measday V, Hailey DW, Pot I, Givan SA, Hyland KM, Cagney G, Fields S, Davis TN, Hieter P. Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore. Genes Dev. 2002;16(1):101–13. doi: 10.1101/gad.949302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, McAinsh AD, Rheinbay E, Sorger PK. Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol. 2006;7(3):R23. doi: 10.1186/gb-2006-7-3-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Johnson ML, Stukenberg PT. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1) Curr Biol. 2008;18(22):1785–91. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12(2):138–43. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- Miranda JJ, King DS, Harrison SC. Protein arms in the kinetochore-microtubule interface of the yeast DASH complex. Mol Biol Cell. 2007;18(7):2503–10. doi: 10.1091/mbc.E07-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Au WC, Choy JS, Kuich PH, Baker RE, Foltz DR, Basrai MA. Misregulation of Scm3p/HJURP causes chromosome instability in Saccharomyces cerevisiae and human cells. PLoS Genet. 2011;7:e1002303. doi: 10.1371/journal.pgen.1002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C. Nonhistone Scm3 and Histones CenH3-H4 Assemble the Core of Centromere-Specific Nucleosomes. Cell. 2007;129:1153–64. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Moree B, Meyer CB, Fuller CJ, Straight AF. CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J Cell Biol. 2011;194:855–71. doi: 10.1083/jcb.201106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM. Autoantibody to Centromere (Kinetochore) in Scleroderma Sera. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1980;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Ciliberto A. The spindle-assembly checkpoint and the beauty of self-destruction. Nat Struct Mol Biol. 2012;19(11):1059–61. doi: 10.1038/nsmb.2429. [DOI] [PubMed] [Google Scholar]
- Mythreye K, Bloom KS. Differential kinetochore protein requirements for establishment versus propagation of centromere activity in Saccharomyces cerevisiae. J Cell Biol. 2003;160:833–43. doi: 10.1083/jcb.200211116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal H, Hori T, Furukawa A, Sugase K, Osakabe A, Kurumizaka H, Fukagawa T. Dynamic changes in the CCAN organization through CENP-C during cell-cycle progression. Mol Biol Cell. 2015 doi: 10.1091/mbc.E15-07-0531. E pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Rago F, Hori T, Tomii K, Cheeseman IM, Fukagawa T. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 2013;32(3):424–36. doi: 10.1038/emboj.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Takeuchi K, Gascoigne KE, Suzuki A, Hori T, Oyama T, Morikawa K, Cheeseman IM, Fukagawa T. CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell. 2012;148(3):487–501. doi: 10.1016/j.cell.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, 3rd, Desai A, Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8(5):446–57. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Stemmann O, Rank S, Lechner J. A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev. 1999;13(9):1140–55. doi: 10.1101/gad.13.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca C, Draviam VM, Marco E, Sorger PK, De Wulf P. Roles for the conserved spc105p/kre28p complex in kinetochore-microtubule binding and the spindle assembly checkpoint. PLoS One. 2009;4(10):e7640. doi: 10.1371/journal.pone.0007640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987;104(4):805–15. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpelescu M, Fukagawa T. The ABCs of CENPs. Chromosoma. 2011;120(5):425–46. doi: 10.1007/s00412-011-0330-0. [DOI] [PubMed] [Google Scholar]
- Petrovic A, Mosalaganti S, Keller J, Mattiuzzo M, Overlack K, Krenn V, De Antoni A, Wohlgemuth S, Cecatiello V, Pasqualato S, et al. Modular assembly of RWD domains on the Mis12 complex underlies outer kinetochore organization. Mol Cell. 2014;53(4):591–605. doi: 10.1016/j.molcel.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Petrovic A, Pasqualato S, Dube P, Krenn V, Santaguida S, Cittaro D, Monzani S, Massimiliano L, Keller J, Tarricone A, et al. The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J Cell Biol. 2010;190(5):835–52. doi: 10.1083/jcb.201002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, Wordeman L, Davis TN, Asbury CL. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136(5):865–75. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Choi ES, Abbott JK, Liu X, Kagansky A, Castillo AG, Hamilton GL, Richardson W, Rappsilber J, He X, et al. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell. 2009;33:299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot I, Measday V, Snydsman B, Cagney G, Fields S, Davis TN, Muller EG, Hieter P. Chl4p and iml3p are two new members of the budding yeast outer kinetochore. Mol Biol Cell. 2003;14:460–76. doi: 10.1091/mbc.E02-08-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewloka MR, Venkei Z, Bolanos-Garcia VM, Debski J, Dadlez M, Glover DM. CENP-C is a structural platform for kinetochore assembly. Curr Biol. 2011;21(5):399–405. doi: 10.1016/j.cub.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Rago F, Gascoigne KE, Cheeseman IM. Distinct organization and regulation of the outer kinetochore KMN network downstream of CENP-C and CENP-T. Curr Biol. 2015;25(5):671–7. doi: 10.1016/j.cub.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi M, Kwong PN, Menorca RM, Valencia JT, Ramahi JS, Stewart JL, Tran RK, Sundaresan V, Comai L, Chan SW. The rapidly evolving centromere-specific histone has stringent functional requirements in Arabidopsis thaliana. Genetics. 2010;186(2):461–71. doi: 10.1534/genetics.110.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SA, Vagnarelli P, Dong Y, Hori T, McEwen BF, Fukagawa T, Flors C, Earnshaw WC. A super-resolution map of the vertebrate kinetochore. Proc Natl Acad Sci U S A. 2010;107:10484–10489. doi: 10.1073/pnas.1002325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez I, Renwick SJ, Crawley K, Karig I, Buck V, Meadows JC, Franco-Sanchez A, Fleig U, Toda T, Millar JB. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 2005;24(16):2931–43. doi: 10.1038/sj.emboj.7600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pulido L, Pidoux AL, Ponting CP, Allshire RC. Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell. 2009;137:1173–4. doi: 10.1016/j.cell.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal K, Carbon J. The CENP-A homolog CaCse4p in the pathogenic yeast Candida albicans is a centromere protein essential for chromosome transmission. Proc Natl Acad Sci U S A. 2002;99(20):12969–74. doi: 10.1073/pnas.162488299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Shenoy RT, Dalgaard JZ, Newnham L, Hoffmann E, Millar JB, Arumugam P. Monopolin subunit Csm1 associates with MIND complex to establish monopolar attachment of sister kinetochores at meiosis I. PLoS Genet. 2013;9(7):e1003610. doi: 10.1371/journal.pgen.1003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffer A, Maier M, Litos G, Lampert F, Hornung P, Mechtler K, Westermann S. CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol. 2012;14(6):604–13. doi: 10.1038/ncb2493. [DOI] [PubMed] [Google Scholar]
- Schmitzberger F, Harrison SC. RWD domain: a recurring module in kinetochore architecture shown by a Ctf19-Mcm21 complex structure. EMBO Rep. 2012;13:216–22. doi: 10.1038/embor.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus K, Soyer JL, Connolly LR, Grandaubert J, Happel P, Smith KM, Freitag M, Stukenbrock EH. Histone modifications rather than the novel regional centromeres of Zymoseptoria tritici distinguish core and accessory chromosomes. Epigenetics Chromatin. 2015;8:41. doi: 10.1186/s13072-015-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KC, Sullivan BA. Neocentromeres: a place for everything and everything in its place. Trends Genet. 2014;30(2):66–74. doi: 10.1016/j.tig.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti E, De Antoni A, Alushin GM, Petrovic A, Melis T, Nogales E, Musacchio A. Direct Binding of Cenp-C to the Mis12 Complex Joins the Inner and Outer Kinetochore. Current Biology. 2011;21(5):391–398. doi: 10.1016/j.cub.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Galazka JM, Phatale PA, Connolly LR, Freitag M. Centromeres of filamentous fungi. Chromosome Res. 2012;20(5):635–56. doi: 10.1007/s10577-012-9290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroiwa Y, Hayashi T, Fujita Y, Villar-Briones A, Ikai N, Takeda K, Ebe M, Yanagida M. Mis17 is a regulatory module of the Mis6-Mal2-Sim4 centromere complex that is required for the recruitment of CenH3/CENP-A in fission yeast. PLoS One. 2011;6:e17761. doi: 10.1371/journal.pone.0017761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Phatale PA, Sullivan CM, Pomraning KR, Freitag M. Heterochromatin is required for normal distribution of Neurospora crassa CenH3. Mol Cell Biol. 2011;31(12):2528–2542. doi: 10.1128/MCB.01285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev. 1995;9:573–86. doi: 10.1101/gad.9.5.573. [DOI] [PubMed] [Google Scholar]
- Stoler S, Rogers K, Weitze S, Morey L, Fitzgerald-Hayes M, Baker RE. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci U S A. 2007;104:10571–6. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin LJ, Guimaraes GJ, Deluca JG. The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis. Mol Biol Cell. 2011;22(6):759–68. doi: 10.1091/mbc.E10-08-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Badger BL, Salmon ED. A quantitative description of Ndc80 complex linkage to human kinetochores. Nat Commun. 2015;6:8161. doi: 10.1038/ncomms9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiwana H, Muller S, Blumer J, Klare K, Musacchio A, Almouzni G. HJURP involvement in de novo CenH3(CENP-A) and CENP-C recruitment. Cell Rep. 2015;11:22–32. doi: 10.1016/j.celrep.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Chen ES, Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288(5474):2215–9. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Bryson TD, Henikoff S. Adaptive evolution of centromere proteins in plants and animals. J Biol. 2004;3:18. doi: 10.1186/jbiol11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Chang HL, Kagami A, Watanabe Y. CENP-C functions as a scaffold for effectors with essential kinetochore functions in mitosis and meiosis. Dev Cell. 2009;17(3):334–43. doi: 10.1016/j.devcel.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Thakur J, Sanyal K. The Essentiality of the Fungus-Specific Dam1 Complex Is Correlated with a One-Kinetochore–One-Microtubule Interaction Present throughout the Cell Cycle, Independent of the Nature of a Centromere. Euk Cell. 2011;10:1295–1305. doi: 10.1128/EC.05093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur J, Talbert PB, Henikoff S. Inner Kinetochore Protein Interactions with Regional Centromeres of Fission Yeast. Genetics. 2015;201:543–61. doi: 10.1534/genetics.115.179788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa KS, Oldani A, Pagliuca C, De Wulf P, Hazbun TR. The Mps1 Kinase Modulates the Recruitment and Activity of Cnn1CENP-T at Saccharomyces cerevisiae Kinetochores. Genetics. 2015;200(1):79–90. doi: 10.1534/genetics.115.175786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien JF, Umbreit NT, Gestaut DR, Franck AD, Cooper J, Wordeman L, Gonen T, Asbury CL, Davis TN. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J Cell Biol. 2010;189(4):713–23. doi: 10.1083/jcb.200910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga T, Matsushita K, Ishibashi M, Nezu M, Shimada H, Ochiai T, Yoda K, Nomura F. Centromere protein H is up-regulated in primary human colorectal cancer and its overexpression induces aneuploidy. Cancer Res. 2005;65(11):4683–9. doi: 10.1158/0008-5472.CAN-04-3613. [DOI] [PubMed] [Google Scholar]
- Tooley JG, Miller SA, Stukenberg PT. The Ndc80 complex uses a tripartite attachment point to couple microtubule depolymerization to chromosome movement. Mol Biol Cell. 2011;22(8):1217–26. doi: 10.1091/mbc.E10-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit NT, Miller MP, Tien JF, Ortola JC, Gui L, Lee KK, Biggins S, Asbury CL, Davis TN. Kinetochores require oligomerization of Dam1 complex to maintain microtubule attachments against tension and promote biorientation. Nat Commun. 2014;5:4951. doi: 10.1038/ncomms5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugel M, Omerzu M, Groenewold V, Hadders MA, Lens SM, Kops GJ. Sequential multisite phospho-regulation of KNL1-BUB3 interfaces at mitotic kinetochores. Mol Cell. 2015;57(5):824–35. doi: 10.1016/j.molcel.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Wang HW, Long S, Ciferri C, Westermann S, Drubin D, Barnes G, Nogales E. Architecture and flexibility of the yeast Ndc80 kinetochore complex. J Mol Biol. 2008;383(4):894–903. doi: 10.1016/j.jmb.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol. 2007;14(1):54–9. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- Wei RR, Sorger PK, Harrison SC. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci U S A. 2005;102(15):5363–7. doi: 10.1073/pnas.0501168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16(3):374–85. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38(3):383–92. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17(2):277–90. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Westermann S, Schleiffer A. Family matters: structural and functional conservation of centromere-associated proteins from yeast to humans. Trends Cell Biol. 2013;23(6):260–9. doi: 10.1016/j.tcb.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kilmartin JV. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol. 2001;152(2):349–60. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma Y, Yoshimoto M, Eguchi A, Tokuda A, Takahashi S. The human papillomavirus18 E7 protein inhibits CENP-C binding to alpha-satellite DNA. Virus Res. 2015;205:27–32. doi: 10.1016/j.virusres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Yamagishi Y, Sakuno T, Goto Y, Watanabe Y. Kinetochore composition and its function: lessons from yeasts. FEMS Microbiol Rev. 2014;38(2):185–200. doi: 10.1111/1574-6976.12049. [DOI] [PubMed] [Google Scholar]
- Zhang G, Kelstrup CD, Hu XW, Kaas Hansen MJ, Singleton MR, Olsen JV, Nilsson J. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J Cell Sci. 2012;125(Pt 13):3243–53. doi: 10.1242/jcs.104208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.