Abstract
Adenosine 5′-monophosphate activated protein kinase (AMPK) is a master sensor of cellular energy status that plays a key role in the regulation of whole-body energy homeostasis. AMPK is a serine/threonine kinase that is activated by upstream kinases LKB1, CaMKKβ and Tak1 among others. AMPK exists as αβγ trimeric complexes that are allosterically regulated by AMP, ADP and ATP. Dysregulation of AMPK has been implicated in a number of metabolic diseases including type 2 diabetes mellitus and obesity. Recent studies have associated roles of AMPK with the development of cancer and neurological conditions making it a potential therapeutic target to treat human diseases. This perspective focuses on the structure and function of AMPK, its role in human diseases and its direct substrates and provides a brief synopsis of key AMPK modulators and their relevance in human diseases.
Graphical Abstract
Introduction
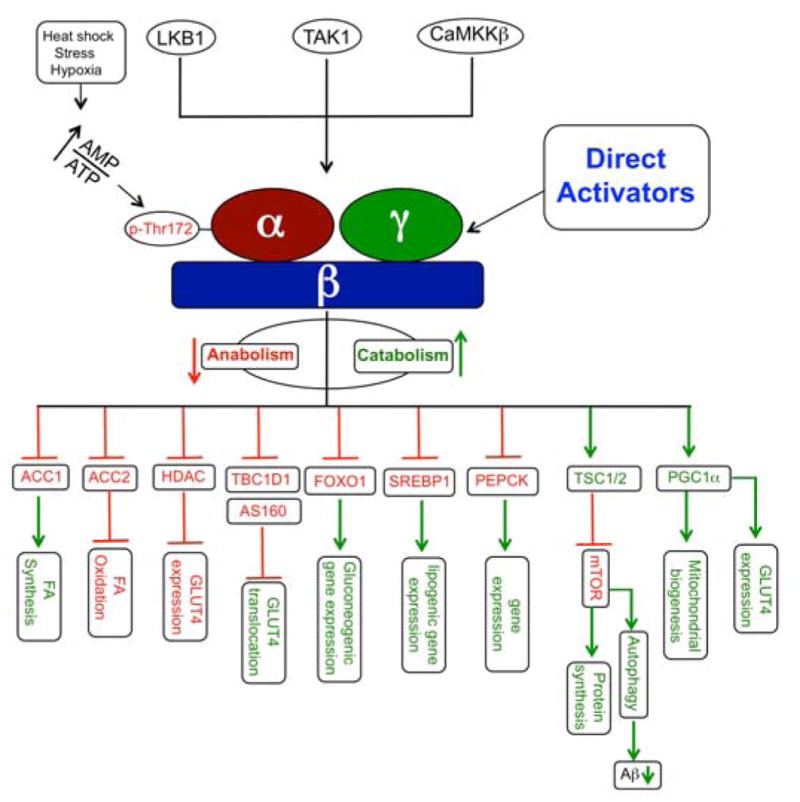
AMP-activated protein kinase (AMPK), which is present in all eukaryotes, is a master sensor of metabolic stress and exists as heterotrimeric αβγ complexes. AMPK is a nutrient and energy sensor that plays a key role in whole-body energy homeostasis.1,2 Its cellular functions are heavily dependent on ATP levels and alterations in the cellular AMP : ADP : ATP ratio lead to the activation or deactivation of AMPK. In response to energy needs (i.e., reduced ATP levels or increased AMP levels), AMPK is activated. Activated AMPK phosphorylates a plethora of substrates in metabolic pathways resulting in the inhibition of anabolic pathways and the activation of catabolic pathways.3–19
AMPK plays a central role in maintaining the energy and metabolic landscape of cells. An altered metabolic profile is often used as a biomarker in chronic human conditions such as diabetes, Alzheimer’s disease (AD), and cancer, and AMPK is implicated in these alterations. For example, AD is characterized by the accumulation of Amyloid-beta protein (Aβ). In cerebrospinal fluid, Aβ1–42 was identified as a potential biomarker for Alzheimer’s disease.20 Aβ peptide generation is increased in AMPKα2 knockout neurons and decreased in the presence of the AMPK stimulator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) suggesting AMPK is a key regulator of Aβ accumulation.21 AMPK dysfunction leads to altered cholesterol and sphingomyelin levels, which changes the distribution of amyloid precursor protein (APP), the source of Aβ, in lipid rafts. This is the current and well-accepted model for the role of AMPK in the accumulation of Aβ.21 This suggests that activation of AMPK by small molecules may be a viable therapeutic approach for restoring the energy and metabolic landscape and reversing the disease phenotype.
Regulation of AMPK by adenine nucleotides
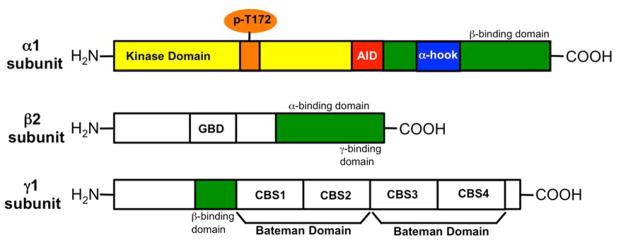
AMPK is a heterotrimeric kinase, composed of a highly conserved catalytic α subunit and a regulatory β and γ subunit. The catalytic α subunit and the regulatory β subunit exist as two isoforms (α1, α2 and β1, β2) respectively. The regulatory γ subunit exists as three isoforms (γ1, γ2 and γ3). These seven gene products lead to 12 possible heterotrimeric combinations: α1β1γ1, α1β1γ2, α1β1γ3, α1β2γ1, α1β2γ2, α1β2γ3, α2β1γ1, α2β1γ2, α2β1γ3, α2β2γ1, α2β2γ2, α2β2γ3. The domain architecture of the α1, β2 and γ1 subunits is summarized in Figure 1. The α subunit is composed of a serine/threonine kinase domain (KD), an autoinhibitory domain (AID), a α-hook domain and a C-terminal β subunit-binding domain. The β subunit is composed of a glycogen binding domain (GBD) and a C-terminal domain that has binding sites for α and γ subunits. The γ subunit has a β subunit-binding region and two Bateman domains that are assembled in a head-to-head manner. The Bateman domains are composed of two tandem cystathionine β-synthase (CBS) motifs.
Figure 1.
Domain architecture of α1, β2 and γ1 AMPK subunits.
The kinase domain of the α subunit is activated upon phosphorylation of Thr-172 of the activation loop.22 Upstream kinases, such as liver kinase B1 (LKB1)23,24 calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ),25,26 and mammalian transforming growth factor-β activated protein kinase-1 (TAK1),27 phosphorylate Thr-172. Phosphorylation of Thr-172 leads to a 2–3 orders of magnitude increase in AMPK activity.28 Deactivation of AMPK occurs through dephosphorylation of Thr-172.28,29 Studies by Voss et al. identified Mg2+/Mn2+-dependent protein serine/threonine phosphatase (Ppm) 1E as an AMPK phosphatase. Briefly, in HEK293 cells, depletion of Ppm1E by RNAi strategies increased Thr-172 phosphorylation.30 Allosteric effects such as binding of adenine nucleotides to the γ-domain, which will be discussed shortly, regulate the conformations around Thr-172 to allow or deny access to upstream kinases and phosphatases.
A truncated α subunit lacking the AID showed full kinase activity when compared to a α subunit containing both the KD and AID. Structural studies with the α subunit of AMPK from Schizosaccharomyces pombe and Saccharomyces cerevisiae reveal that hydrophobic residues drive the KD-AID interaction. Movement of the helix α-C in the KD is probably constrained upon AID binding, thus forcing the KD into a relatively open conformation. Point mutations of the hydrophobic residues in the AID to charged residues (L341D, L342D and M316E) increased the kinase activity by ten-fold. These studies support the regulation of the KD conformation by AID binding to the hydrophobic patch on the KD.31
The heterotrimeric AMPK complex is held together by the β subunit. It has a Cterminal α-subunit binding domain, which terminates in a short peptide sequence that interacts with the β subunit-binding domain of the γ subunit. The N-terminus of the β subunit is modified by myristoylation, which is suggested to facilitate shuttling of the AMPK complex between the cytoplasm and the nucleus.32 The GBD in the β subunit of AMPK is similar to carbohydrate-binding modules (CBM) found in proteins that are known to metabolize starch and glycogen. Glycogen particles are in complex with glycogen synthase (GS) and the GBD on the β subunit helps the AMPK complex to bind to the surface of glycogen particles.33 Isoforms of GS found in the liver and muscle are known substrates of AMPK and phosphorylation of GS could inhibit the anabolic process of glucose addition to glycogen.
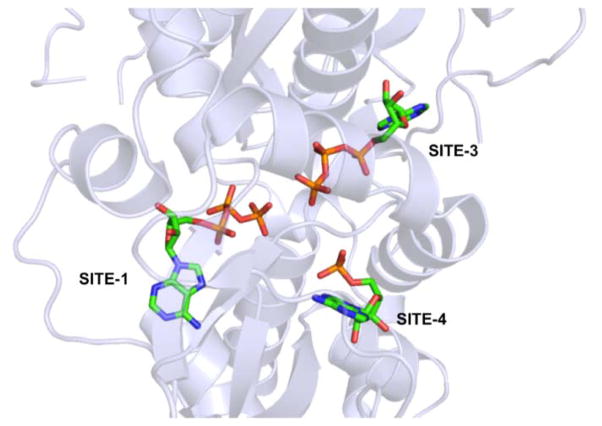
There are four CBS motifs present in γ subunit and three out of four CBS motifs recognize and bind adenine nucleotides (Figure 2).34 Adenine nucleotide binding SITE-1 and SITE-3 on the γ subunit lie on opposite faces and can exchangeably bind AMP, ADP or ATP with SITE-1 having a higher affinity for all three nucleotides than SITE-3. In Figure 2 we show ATP binding to SITE-1 and SITE-3. SITE-2 is empty because CBS2 lacks a critical aspartate residue, which is required to make hydrogen bonds with the hydroxyl groups of the pentose sugar in the adenine nucleotides, while a non-exchangeable AMP molecule permanently occupies SITE-4.35 Under physiological conditions, the concentration of ATP > ADP > AMP and most ATP molecules exist in complex with magnesium ion (Mg-ATP) while ADP and AMP do not.36 The relative binding affinities (Kd) of the various adenine nucleotides for the exchangeable binding sites (SITE-1 and SITE-3) on the γ subunit are ATP : ADP : AMP : Mg-ATP = 0.9 : 1.3 : 1.6 : 32. Additionally, myristoylation of residues in the N-terminus of the β subunit in the presence of AMP-bound γ subunit modestly increases AMPK activity.32 Changes in the cellular concentration of ATP, ADP or AMP will change occupancy of SITE-1 and SITE-3, which allows the γ subunit of AMPK to function as an energy sensor in cells.36 Changes to the occupancy of the adenine nucleotide-binding site lead to short- and long-range conformational effects transmitted through the β subunit-binding site on the γ subunit.
Figure 2.
Adenine nucleotide binding sites on the γ subunit of AMPK (generated using coordinates from PDB code 2V92 using pymol).
A decrease in ATP levels due to metabolic stress (i.e. decreased glucose levels) or rapid and increased consumption of ATP (e.g. during muscle contraction), leads to an increase in the ADP : ATP ratio. As ADP levels rise, a reverse adenylate kinase reaction (2ADP ➔ ATP + AMP) will drive the synthesis of ATP and AMP. This will alter the cellular ATP : ADP : AMP ratio. An increase in cellular ADP and AMP levels will drive the displacement of ATP, which is found in high levels when the cells are not stressed, from SITE-1 and SITE-3 of the γ subunit of AMPK.36
Xiao et al. proposed that the α-hook region on the catalytic α subunit interacts with exchangeable SITE-3 on the γ-domain when an AMP/ADP molecule occupies it. This α-hook interaction enhances the recruitment of the kinase domain to the regulatory subunits. The interaction between the CBM domain of the β subunit and the activation loop of the kinase domain stabilizes the activation loop structure. This maintains the activated state of AMPK. AMP binding to SITE-1 and SITE-3 of the γ subunit allosterically increases AMPK activity 2–5 fold.36 Recently, a full-length human α2β1γ1 AMPK crystal structure was reported, revealing that the phosphate group on Thr-172 is partially exposed to solvent and is not accessible to phosphatases.37 For dephosphorylation to occur, the activation loop must undergo a conformational change that enables the phosphate group to be solvent exposed. The interactions of the regulatory fragments with the activation loop block the dephosphorylation of Thr-172.36,37 Furthermore, AMP binding helps maintain AMPK in the activated state by decreasing the rate of Thr-172 dephosphorylation.36 In addition to the enzyme active site and nucleotide binding sites, the AMPK trimeric complex offers an array of protein-protein interfaces (PPI’s) that can be targeted to modulate AMPK function. We have used high throughput screening (HTS) and peptidomimetic approaches to develop chemical probes that target PPI’s.38–48 Similar strategies can be employed to develop inhibitors against the AMPK PPI’s.
The modes of activation listed above are driven by conformational changes that either allow or block access to Thr-172. Another mode of AMPK activation that is independent of cellular adenine nucleotide levels is Ca2+-mediated activation of the upstream kinase CaMKKβ.25,26,49,50 Intracellular Ca2+ levels are tightly controlled in cells with the endoplasmic reticulum (ER) serving as the Ca2+ store of the cell. Phospholipases activated by cell surface receptors lead to inositol triphosphate-induced Ca2+ release from the ER.
AMPK-mediated signaling and its effects on metabolic pathways
Depletion of ATP activates AMPK as ADP and AMP begin to displace ATP from the γ subunit. Depending on the severity of ATP depletion, different events such as increased glucose uptake, increased glycolysis and reduced glycogen synthesis will be triggered to restore ATP levels. Glucose transporters (GLUT) are a family of membrane proteins that play an integral part in responding to and assisting in glucose uptake by cells. Genetic and pharmacological manipulation of AMPK in adipocytes, muscle and neurons suggests indirect regulation of GLUT expression and translocation by AMPK.51–53 At this time the exact signal transduction pathway that leads to AMPK-driven GLUT-mediated glucose uptake is not fully understood. Given the diversity in the GLUTs and AMPK, it is highly likely that the signaling pathway that links these two proteins is tissue specific. Activation of glycolysis by AMPK is driven by isoform-specific phosphorylation and activation of phosphofructokinase (PFK).17,54 Inhibition of glycogen synthesis by the activation of AMPK is driven by the phosphorylation and inactivation of glycogen synthases.55 The severity of the metabolic stress will determine the level of activation as well as the number of processes activated to respond to the stress and restore normalcy.
Direct targets of AMPK
Since AMPK serves as the energy sensor in cells, it is not surprising that activation of AMPK leads to changes in a plethora of cellular functions. Acetyl-Co-Acarboxylase-1 (ACC1), Acetyl-Co-A-carboxylase-2 (ACC2), GS and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase are well-characterized direct targets of AMPK. In this section we will limit the discussions to lesser-known and recently discovered direct targets. A kinase assay using truncated GST-fusion kinase domain of AMPK α-subunit and a GST-fusion ACC was established. A structure activity relationship (SAR) with 25 mutant GST-ACC revealed a > 20 amino acid interaction region between AMPK and ACC.56 The study also predicted a consensus recognition sequence (xϕxxϕxxxϕxxβϕβxxxsxxxϕ where ϕ = hydrophobic residue, β = basic residue and s = phosphorylation residue).56,57
AMPK is a negative regulator of phosphoenolpyruvate carboxykinase (PEPCK) gene expression (Figure 4). An early step in hepatic gluconeogenesis is PEPCK-catalyzed conversion of oxaloacetate to phosphoenolpyruvate.61 AMPK modulators such as the small molecule AMPK activator AICAR, the hormone adiponectin and the antidiabetic drug metformin are known to reduce PEPCK gene expression.62–64 In a systematic study Inoue et al. reported the identification of AICAR response element binding protein (AREBP) as a zinc finger transcription factor that acts as a repressor of PEPCK gene expression. An in vitro AMPK phosphorylation assay using a series of AREBP point mutants identified Ser-470 as the molecular target of AMPK and gel electrophoresis mobility shift assay demonstrated this phosphorylation prevents AREBP binding to DNA.8 The model supports phosphorylation of Ser-470 by AMPK, which abolishes AREBP DNA-binding activity, as the molecular basis for the transcriptional repression of PEPCK gene expression by AREBP.
Figure 4.
AMPK signaling pathways and their downstream effects.
Protein synthesis involves three major steps, namely, initiation, elongation and termination. Among the three, >99% of the energy required for protein synthesis is used during elongation. In eukaryotic cells, peptide chain elongation requires two elongation factors, eEF1A and eEF2. A drop in cellular ATP levels leads to the phosphorylation of eEF2 by eEF2 kinase, which results in the inhibition of protein synthesis. AMPK serves as the link between cellular energy metabolism and regulation of protein synthesis, wherein AMPK directly phosphorylates eEF2 kinase at Ser-398. In vitro studies identified two additional sites, Ser-78 and Ser-366 that were phosphorylated by AMPK but to a lesser extent. However, only Ser-398 phosphorylation was observed in cells treated with AICAR, an AMPK activator.4
Nitric Oxide (NO) has been implicated as a modulator in different physiological processes such as mitochondrial respiration, glucose uptake, glycolysis and muscle contraction.65 Inhibition of nitric oxide synthase (NOS) reduces glucose uptake. As AMPK is involved in the regulation of glucose uptake in skeletal muscle during exercise, a possible link between AMPK and NOS may exist. Chen et al. showed that endothelial nitric-oxide synthase (eNOS) was phosphorylated and activated by AMPK and mass spectrometry studies revealed Ser-1177 as the phosphorylation site. Similarly, neuronal nitric-oxide synthase-μ (nNOS-μ) containing Ser-1451 at a similar position was phosphorylated by AMPK.14 A second site, Thr-495, on eNOS was also phosphorylated by AMPK in vitro in the absence of Ca+2/calmodulin resulting in inhibition of eNOS activity. However, during ischemia an increase in only Ser-1177 phosphorylation was observed.10
Using in vitro kinase assays, Hong et al. showed AMPK phosphorylates Ser-304 of the transcription factor hepatocyte nuclear factor 4alpha (HNF4α), increasing its degradation and reducing its ability to bind DNA. Introduction of a phosphoserine mimetic in HNF4α (S304D) resulted in decreased protein stability, dimerization, DNA binding and HNF4α-mediated transcription. A nonsense mutation in a single allele of HNF4α leads to maturity onset diabetes of the young (MODY). Patients with this inherited form of diabetes show defects in pancreatic function such as reduced insulin secretion.12
The insulin receptor substrate (IRS-1) contains multiple potential tyrosine phosphorylation sites with the consensus motif YMXM, which is recognized by the insulin receptor (IR) kinase. Proteins such as phosphatidylinositide 3-kinases (PI3K) bind to these sites via their Src homology 2 (SH2) domains, allowing IRS-1 to serve as a docking protein for SH2 domain-containing signal-transduction proteins.66,67 Furthermore, IRS-1 contains multiple serine and threonine phosphorylation sites. Chopra et al. demonstrated phosphorylation of IRS-1 Ser-789 in cardiac myocytes following glucose starvation and also showed that this phosphorylation was mimicked by AICAR treatment. However, phosphorylation at this site negatively regulates the insulin pathway. On the other hand, phosphorylation of Tyr-612 and Tyr-632 residues of IRS-1 also occurred following glucose starvation and was mimicked by AICAR treatment. Both glucose starvation and AICAR treatment resulted in levels of phosphorylated Tyr-612 and Tyr-632 similar to those seen upon insulin stimulation. Treatment with compound C, an AMPK inhibitor, blocked phosphorylation of Tyr-632.68 Furthermore, glucose starvation led to phosphorylation of insulin receptor (IR) Tyr-1162, which was inhibited by a dominant negative AMPK or compound C treatment. Overall, their studies suggest AMPK phosphorylates IR, which leads to allosteric activation of IR kinase and signal transduction through IRS-1 by direct binding of PI3K to Tyr-612/Tyr-632.69 However, studies by Jakobsen et al., suggest that phosphorylation of Ser-789 appears to potentiate the activity of PI3K associated with IRS-1 in C2C12 myotubes.13
p27Kip1 regulates a number of cellular functions and chief among them is induction of cell-cycle arrest by disruption of the cyclin E - cyclin dependent kinase - 2 (CDK2) complex. Transfection with the phosphomimetic T198D mutant allele of p27 modestly inhibited colony formation compared to wild-type and both wild-type and T198D p27 induced G1 arrest compared to the non-phosphorylatable T198A p27. Under serum or glucose deprivation of cells, a robust increase in phosphorylated p27 was observed. This increase in Thr-198 phosphorylation correlated with an increase in AMPK and ACC phosphorylation, suggesting Thr-198 phosphorylation of p27 is regulated by AMPK. Studies by Liang et al. showed purified AMPKα1 phosphorylates recombinant p27 and that mutation of Thr-198 to alanine resulted in altered p27 stability, indicating that Thr-198 of p27 is a direct target of AMPK in vitro. Their studies suggested that under stress, phosphorylation of Thr-198 promotes p27 stability. Accumulation of p27 in quiescent cells dictates whether cells enter the autophagy-mediated cell survival pathway or undergo apoptosis.15
The transcriptional coactivator p300 regulates transcription by recruiting transcription machinery to promoters and linking DNA-bound transcription factors to the basal transcription machinery. Furthermore, p300 may regulate transcription by acetylation of transcription factors or by modification of chromatin structure via acetylation of histones.70 Ser-89 on p300 is a target for phosphorylation by kinases including AMPK. Yang et al. demonstrated that Ser-89 on p300 is a direct substrate of AMPK. Using a mammalian two-hybrid system they showed that ligand-dependent peroxisome proliferator-activated receptor-γ (PPARγ)/p300 interaction-mediated transcriptional activity is reduced with a S89D p300 mutant. This is just one example that shows AMPK regulates gene expression in response to alterations in the energy and metabolic landscape.16
Marsin et al. demonstrated that under anaerobic conditions, such as ischemia, the AMP : ATP ratio increases, which lead to activation of AMPK and phosphorylation of heart phosphofructokinase-2 (PFK-2) at Ser-466. PFK-1, an enzyme that plays a key role in glycolysis, serves as a ubiquitous glycolytic signal and is directly stimulated by fructose 2,6-bisphosphate, which is synthesized by PFK-2. The net effect of PFK-2 phosphorylation by AMPK is the activation of glycolysis in the heart during ischemia. These studies show that phosphorylation of PFK-2 by AMPK is a secondary indirect mechanism of PFK-1 activation that leads to ATP production.17
Tuberous sclerosis complex (TSC) is an autosomal disorder caused by a mutation in either TSC1 or TSC2 tumor suppressor genes. TSC proteins negatively regulate translation through the mammalian target of rapamycin (mTOR) pathway. In cells, starvation activates TSC2, which leads to the phosphorylation of mTOR substrates ribosomal S6 kinase (S6K) and eukaryotic initiation factor 4E binding protein 1 (4EBP1). Two-dimensional phosphopeptide mapping and mutational studies showed that Thr-1227 and Ser-1345 on TSC2 are direct targets for phosphorylation and activation by the energy sensor AMPK.18
A chemical genetics screen was used to identify 28 new AMPK substrates. A subset of the substrates identified was validated using in vitro kinase assays. Follow up studies revealed that protein phosphatase 1 regulatory subunit 12C (PPP1R12C) and p21- activated protein kinase (PAK2) are direct targets of AMPK. Phosphorylation of Ser-20 on PAK2 and Ser-452 on PPP1R12C by AMPK promotes myosin regulatory light chain (MRLC)-mediated completion of mitosis (cytokinesis).19
A yeast two-hybrid screen of a human heart cDNA library using a truncated γ2 isoform of AMPK identified cTnI as a putative target for AMPK phosphorylation. In vitro studies identified Ser-150 as the site of phosphorylation by AMPK. AMPK activation of cTnI through Ser-150 phosphorylation was observed in whole hearts during ischemia.59
PIKfyve is a lipid kinase that phosphorylates phosphatidylinositides (PtdIns) to PtdIns5P and PtdIns3P to PtdIns(3,5)P2. Several studies have implicated PIKfyve in insulin-stimulated GLUT4 translocation and glucose uptake.71 Changes in the AMP : ADP : ATP ratios during muscle contraction activate AMPK and increases GLUT4 translocation and glucose uptake, suggesting a possible link between AMPK and PIKfyve in contraction-stimulated glucose uptake. In vitro studies using recombinant WT-PIKfyve, [γ-32]ATP and AMPK suggest direct phosphorylation of PIKfyve by AMPK. MS studies identified Ser-307 and Ser-48 as putative phosphorylation sites. Phosphorylation of PIKfyve Ser-307 did not affect its lipid kinase activity but did alter its subcellular localization, leading to its translocation to early endosomes and PtdIns(3,5)P2 production, which may increase GLUT4 translocation.60
Role of AMPK in Cancer
Patients with metabolic dysfunctions, such as insulin resistance, type 2 diabetes and obesity, have a higher risk of developing cancer.72 Under energetic stress, AMPK activates catabolic processes and inhibits anabolic processes, which results in the inhibition of cell proliferation. These observations led to speculation that AMPK is a tumor suppressor. In human cancer, however, AMPK mutations are rare, suggesting that loss of AMPK function is probably due to mutations in upstream kinases such as LKB1 or downstream targets like TSC2.23,24,73,74 As such, activation of AMPK has been proposed as a treatment option for cancer. Epidemiological data suggest metformin, the clinically used glucose-lowering agent, may reduce cancer risk. Buzzai et al. showed that metformin-treated, colon tumor-bearing (HC116 p53−/− xenografts) mice showed reduced tumor sizes when compared to vehicle treated mice. This suggests metformin-induced cytotoxicity of colon cancer cells with p53−/− phenotype. In vitro studies suggested that only cells grown in the absence of glucose were sensitive to metformin treatment, suggesting that p53 inactivation impairs cell survival under nutrient deprivation, which would occur in tumors that are hypoxic and have low vascularization.75 Germline mutations in the upstream kinase LKB1 prevent AMPK activation and cause Peutz-Jeghers syndrome (PJS), a risk factor for developing malignant tumors.76 Inactivation of LKB1 leads to hyperactivation of mammalian target of rapamycin complex-1 (mTORC1), a cell growth regulator, which promotes cell growth and cell proliferation.77 Dennis et al. reported that low nutrient conditions inhibit anabolic processes driven by mTOR signaling.78 AMPK is known to directly phosphorylate TSC2, which negatively regulates translation through the mTOR (Figure 4).18 These results suggest that LKB1 negatively regulates mTORC1 through phosphorylation of AMPK. Additionally, Tiainen et al. have demonstrated that LKB1 activation induces G1 arrest and up-regulation of p21Kip1, a p53 target gene, in a p53-dependent manner.79 Jones et al. later showed AMPK phosphorylates Ser-15 of p53 to induce cell-cycle arrest.80 AMPK, among others, phosphorylates raptor leading to inhibition of mTORC1 complex activity.18,81 TSC1 and TSC2 negatively regulate mTOR signaling and inactivation of either TSC1 or TSC2 is associated with elevated levels of mTOR activity and activation of the mTOR pathway is reported in TSC1 and TSC2-deficient tissues.82–85 Despite these reports, since both LKB1 and AMPK phosphorylate a plethora of cellular substrates and mTOR serves as a signaling node for a wide range of cellular functions, the exact mechanism of LKB1-AMPK-mediated mTOR activation is not fully understood.
AMPK inactivity is also implicated in the switch to aerobic glycolysis by cancer cells. During glycolysis cells metabolize glucose to pyruvate in the cytoplasm to generate 2 ATP molecules/glucose. In the Krebs cycle, also known as the tricarboxylic acid (TCA) cycle, pyruvate generated from glycolysis is oxidized to acetyl-CoA and used to generate Nicotinamide adenine dinucleotide (NADH). This NADH produced in the Krebs cycle is then used during oxidative phosphorylation in the mitochondria to generate 36 ATP/glucose. Under hypoxia lactate dehydrogenase (LDH) converts the pyruvate from glycolysis to lactic acid (anaerobic glycolysis). The ability of tumor slices to consume high levels of glucose and produce high levels of lactate ex vivo in the presence of adequate oxygen led Otto Warburg to postulate that a change in the metabolic landscape as the cause of cancer.86 The Warburg effect, also known as aerobic glycolysis, has since been confirmed, however, activation of oncogenes and loss of tumor suppressors are implicated as the cause.87 In the case of cancer, oncogene and tumor suppressor networks alter tumor cell metabolism to generate energy and biomass at higher rates to meet the demands of proliferation. AMPK serves as an ATP sensor in cells and AMPK signals to and from known tumor suppressors (TSC2 and LKB1). A recent study conducted by Faubert et al. explored the role of AMPK on the Warburg effect and tumorigenesis and concluded that inactivation of AMPK enhances aerobic glycolysis.88 They knocked out the α1 subunit of AMPK (α2 is not expressed in B lymphocytes) in Eμ-Myc transgenic mice.89 Both the homozygous Eμ-Myc/α1−/− and heterozygous Eμ-Myc/α1+/− mice displayed pre-B cell tumors with accelerated lymphomagenesis as opposed to mature B cell tumors found in Eμ-Myc/α1+/+ mice. These studies also found that HIF-1α is a key mediator of AMPK-dependent effects on cellular metabolism.88 The significant increase in lactate production observed in these studies suggests that down regulation of AMPK signaling is sufficient to enhance the Warburg effect in cancer cells. This data suggests that activation of AMPK may be a viable therapeutic option for cancer.
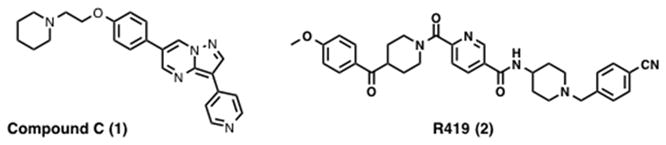
On the other hand, the use of AMPK inhibitors may also be a viable treatment option for cancer. For example, in a study by Shaw et al. LKB1 knock out led to increased mTOR activity and increased cell proliferation, however, Lkb1−/− Mouse embryonic fibroblasts (MEFs), unlike other tumor suppressor gene knockout MEFs, underwent rapid apoptosis under energy stress. This phenotype was rescued by the mTOR inhibitor rapamycin.76 Furthermore, in the study conducted by Faubert et al. knockdown of AMPKα1 using shRNAs in Eμ-Myc lymphoma cells resulted in sensitivity to metabolic stress induced by the glycolytic inhibitor 2-deoxyglucose (2-DG), suggesting that although AMPK favors the Warburg effect, it is also needed for metabolic checkpoints that allow cancer cells to adapt to stress.88 These studies suggest that certain AMPK inhibitors may provide a therapeutic advantage when used in combination with agents that induce energetic stress. Additionally, activation of AMPK is reported to induce autophagy via inhibition of mTOR, a negative regulator of autophagy.90,91 Hypoxia in prostate cancer activates AMPK, which functions to maintain cell survival. Using immunohistochemistry, Park et al. showed elevated levels of phosphorylated ACC, an AMPK substrate, in approximately 40% of human prostate cancer samples examined, thus implicating AMPK. Consistent with the above observation knockdown of the α1 and α2 subunits of AMPK in prostate cancer cell lines resulted in reduced proliferation. In the same study, prostate cancer cells treated with the AMPK inhibitor compound C (1, Figure 3) exhibited reduced cell growth and increased apoptosis.92 This cell survival-promoting role of AMPK may give cancer cells an advantage during selection pressure created by the tumor microenvironment. Since AMPK activation is reversed when the energy balance is restored, AMPK has been suggested as a conditional oncogene.93 AMPK activation may be essential for the survival of cancer cells early during tumorigenesis and studies support the notion that AMPK is required for anchorage-independent growth and survival during glucose deprivation.94 Though the seemingly paradoxical role of AMPK still remains partially unsolved, work by Jeon et al. demonstrates that AMPK promotes tumor cell survival by regulating NADPH homeostasis during energetic stress. Their study showed that AMPK functions to maintain levels of NADPH by inhibiting ACC1 and ACC2 and thereby inhibiting NADPH-consuming fatty acid synthesis and increasing fatty acid oxidation, during matrix detachment and when NADPH generation by the pentose phosphate pathway is decreased by glucose deprivation. They suggest that combinational treatment with AMPK activators and ACC activators may inhibit cancer cell survival, as this treatment strategy would drive AMPK inactivation of mTOR while blocking the regulation of NADPH homeostasis by AMPK.95 Information regarding tissue-specific expression of AMPK subunits and the roles of AMPK in early and late tumorigenesis is needed to determine if inhibitors or activators of AMPK are appropriate for the treatment of cancer.
Figure 3.
Structure of compound C and R419.
Role of AMPK in metabolic disease
Organs and tissues relevant to metabolic disorders include skeletal muscle, liver and adipose tissue. AMPK α2 knockout mice on a normal diet showed impaired glucose tolerance and reduced insulin-stimulated glucose metabolism. On a high-fat diet, these mice were glucose tolerant despite increased body weight and fat mass, highlighting the importance of AMPK in metabolic homeostasis.96 Since loss of glucose tolerance, increased body weight and increased fat mass are the hallmarks of type 2 diabetes and obesity, the above study suggests AMPK activation is a viable therapeutic approach for the treatment of metabolic disorders.97 Consistent with the above statement, pharmacological activation of AMPK by AICAR led to increased glucose uptake by muscles and inhibition of gluconeogenesis in the liver. The study concluded that AICAR treatment phenotypically mimics insulin-independent insulin action.62 Treatment with N-(1-(4-cyanobenzyl)piperidin-4-yl)-6-(4-(4-methoxybenzoyl)piperidine-1-carbonyl)nicotin amide (R419, 2, Figure 3), discussed later in the indirect activators section, leads to increased glucose uptake in myocytes and increased fatty acid oxidation in mouse primary hepatocytes.98 Another study conducted by Baltgalvis et al. at Rigel pharmaceutical suggested that activation of AMPK by pharmacological small molecule activators offers a suitable treatment for intermittent claudication associated with peripheral artery disease (PAD). High-fat fed mice demonstrated characteristics of PAD and treatment of these animals with AMPK activator, R118 (structure not disclosed), alleviated some of these characteristics.99 In a mouse model of diabetes, treatment with the indirect AMPK activator AdipoRon, also discussed later, leads to improved glucose tolerance and insulin resistance. The results from these studies suggest AMPK activation as a possible option for the treatment of diabetes.100
Role of AMPK in Lipogenesis
An emerging hallmark of cancer is increased rate of de novo fatty acid synthesis, which is a direct consequence of a tumor’s shift towards glycolytic metabolism. Glucose taken up by a cell is converted into glucose-6-phosphate by hexokinases and is used to generate ATP and pyruvate. This pyruvate is converted to acetyl CoA and enters the mitochondria and the citric acid cycle. If oxygen is available, this acetyl CoA is converted to citrate, which will enter oxidative phosphorylation. Under anaerobic conditions, however, this citrate is transported to the cytoplasm and reconverted by ATP citrate lyase to acetyl CoA, some of which is then converted into malonyl-CoA by ACC mediated carboxylation. Finally, fatty acid synthase condenses acetyl-CoA and malonyl-CoA to form saturated, long chain fatty acids, such as palmitate. These fatty acids can be further modified into phospholipids, triglycerides, and cholesterol esters, among others, which are primarily used to form the lipid bilayers of rapidly dividing tumor cells.101 AMPK activators may target de novo fatty acid synthesis through AMPK’s regulatory role in both the activation and expression of fatty acid synthesis proteins, such as ACC and fatty acid synthase. Winder et al. have shown that AMPK phosphorylates rat muscle ACC to increase its Km for ATP and acetyl-CoA.7 Swinnen et al. demonstrated that AICAR treatment of MDA-MB-231 breast cancer cells leads to AMPK activation, reduced lipogenesis, reduced DNA synthesis, and decreased protein synthesis.102 Overall, AICAR treatment led to decreased cancer cell proliferation, migration, and invasion with increased cancer cell death. Additionally, AMPK-mediated ACC phosphorylation has been shown to regulate mitotic exit. Inhibition of fatty acid synthesis arrests cells between metaphase and telophase, suggesting AMPK activation in dividing cancer cells may inhibit cell division.103 In fact, this phenomenon has been demonstrated in prostate cancer by Zadra et al.104 Additionally, Vazquez-Martin et al. have shown that metformin treatment leads to mitotic catastrophe in cancer cells.105 Overall, these studies suggest AMPK activation may indeed be beneficial for cancer treatment.
AMPK activation leads to fatty acid oxidation while blocking fatty acid and triglyceride synthesis, therefore novel activators of AMPK may prove useful for the treatment of metabolic disorders. Ruderman’s group showed that AMPK activity is reduced in severely obese patients with insulin resistance and that AMPK activity is lower in visceral abdominal adipose tissue than in subcutaneous abdominal adipose tissue.106 Furthermore, they showed decreased AMPK activity in a variety of animal models including the fa/fa (leptin-receptor-deficient, non-diabetic) and Zucker diabetic fatty (leptin-receptor-deficient, diabetes-prone) rats and ob/ob (leptin-deficient)107 and Interleukin 6 (IL-6)-knockout mice.108 Overall, these studies suggest that reduced AMPK activation may contribute to metabolic disease. Activation of AMPK with various small molecules for the treatment of metabolic disorders has led to modest success. For example, AICAR treatment of Zucker diabetic fatty rats prevents the development of diabetes and ectopic lipid accumulation.107 Treatment of Dahl-S rats (a Sprague-Dawley strain with hypertriglyceridemia and high malonyl-CoA levels) with pioglitazone led to restoration of hepatic phosphorylated AMPK and phosphorylated ACC. Furthermore, treatment with pioglitazone led to decreased plasma triglyceride levels in Dahl-S rats.109 The Erion group at Metabasis Therapeutics, Inc. reported the development of an AMPK activator (EC50 = 6.3 nM) that is > 900-fold more potent activator of AMPK than AMP (EC50 = 5.9 μM) and unlike 5-amino-4-imidazolecarboxamide ribotide (ZMP), it is inactive against glycogen phosphorylase (GPPase) and fructose-1,6-bisphosphatase (FBPase). Furthermore, ester and carbonate prodrugs of this AMPK activator inhibit de novo lipogenesis in rat hepatocytes with EC50 values < 1 μM, presumably through increased phosphorylation of ACC. In mice, these AMPK activators inhibited de novo lipogenesis by more than 30%.110 However, the use of AMPK activators for the treatment of metabolic disease needs further investigation. Long-term (8 days) treatment of ob/ob mice with 0.25 – 0.5 mg/g AICAR improves glucose sensitivity of these mice, however, an increase in circulating triglycerides was also observed.111
Role of AMPK in Alzheimer’s Disease
AD is a neurological protein misfolding disease, characterized by progressive dementia that leads to incapacitation and death. It includes synaptic loss and neuronal death, which over time are responsible for the loss of memory, personality changes and eventual death. There are two characteristic neuropathological lesions that define AD, namely, extracellular plaques and intracellular tangles.112
Extracellular plaques, also called amyloid plaques, are mainly composed of Aβ peptides, which consist of 39–43 amino acids that are proteolytic cleavage products of APP. The Aβ peptide segment is generated by the endoproteolysis of the transmembrane protein APP by beta (β) and gamma (γ) secretase enzymes. The conversion of Aβ from a soluble monomeric form to soluble aggregated forms appears to be the initial process of amyloid neurotoxicity.113 Several studies demonstrated that AMPK plays an important role in the pathogenesis of Aβ generation.114–116 Won et al. showed elevated levels of Aβ peptide in AMPKα2 knockout mice and activation of AMPK decreased Aβ production by regulating APP processing in lipid rafts. Their studies also showed that neurons from AMPKα2 knockout mice have elevated levels of cholesterol and sphingomyelin. Since cholesterol and sphingomyelin are associated with APP processing, which leads to Aβ production, AMPK may play a role in cholesterol and sphingomyelin regulation and APP processing in lipid rafts.21 AICAR and other AMPK activators induced the opposite phenotype, wherein they show reduced accumulation of Aβ117 whereas compound 1, an AMPK inhibitor, treatment had the opposite effect.118 On the other hand, treatment of N2a695 cells (N2a neuroblastoma cells) with the AMPK activator metformin increased Aβ generation, which was inhibited by compound 1 treatment. Also β-secretase (BACE1) promoter activity is upregulated by metformin treatment in these cells, suggesting a link between AMPK and BACE1.119
Intracellular tangles, also called neurofibrillary tangles (NFTs), are aggregates of Aβ and the microtubule associated protein tau.120 Phosphorylation of tau protein in neuronal microtubules regulates its binding to tubulin. Phosphorylation of tau is required for neurite growth and axonal transport, however, hyperphosphorylation of tau leads to its self-aggregation into NFTs, the formation of which is another causative factor for AD.121 Phosphorylation of tau is regulated by a series of kinases, such as stress-activated protein kinase,122 CaMKKβ,123 Glycogen synthase kinase-3-beta (GSK-3β),124 cyclin-dependent kinase 5124, and Src family tyrosine kinases.125 Recent studies have identified recombinant AMPK as a tau kinase that is activated in response to amyloid Aβ peptide exposure.126 AMPK phosphorylation of tau has been shown to prevent tau binding to microtubules, implicating tau in the formation of NFTs. Additionally, a link between AMPK, adenylate kinase-1 (AK1), and Aβ accumulation appears to exist in Alzheimer’s disease.127 Phosphorylation of AMPK Thr-172 appears to be reduced in patients with AD. Treatment of primary cortical neurons with Aβ42 leads to a reduction of AMPK Thr-172 phosphorylation and impairment of AMPK activity. Aβ42 also increases AK1 expression. Additionally, overexpression of WT AK1 leads to reduced AMPK Thr-172 phosphorylation while overexpression of a mutant AK1 does not. Down regulation of the AMP/ATP ratio by AK1 may impact AMPK activity. As AMPK activation with AICAR leads to increased inhibitory phosphorylation of GSK3β and a decrease in tau phosphorylation, it is possible that AK1 drives tau phosphorylation through inhibition AMPK and as a consequence activation of GSK3β.127 Following Ca+2-dependent stimulation by Aβ(1–42) AMPK is phosphorylated by CaMKKβ.126 Ca+2 homeostasis is critical for maintenance of synaptic plasticity, learning and memory, and disruption of Ca2+ homeostasis has been implicated in AD pathogenesis.128
On the other hand, studies have implicated tau acetylation, which results in inhibition of its degradation, in AD. Hyperphosphorylation of tau and the formation of NFTs occurred after tau acetylation in AD. NAD-dependent deacetylase sirtuin-1 (SIRT1), which is activated by AMPK, plays an important role in the reduction of tau acetylation and thus decreased tau hyperphosphorylation.129 Additionally, studies have shown that the AMPK activator AICAR inhibits tau phosphorylation whereas, AMPK inhibition increases tau phosphorylation, further implicating AMPK activation in blocking tau phosphorylation.130,131 Pharmacological targeting of AMPK may also be beneficial in other brain-related injuries and diseases. Several studies showed a direct correlation between AMPK activation and detrimental outcomes of experimental stroke probably due to ischemia-induced metabolic changes.132–134 Hypothermia inhibited activation of AMPK in the brain, which resulted in neuroprotection following stroke in mice. Pharmacological inhibition of AMPK by 1 and AMPKα2 knockout prevented hypothermia-induced neuroprotection during experimental stroke, suggesting the protection provided by hypothermia is due to inhibition of AMPKα2.135 In Huntington’s disease, over activation of AMPKα1 potentiated striatal neurodegeneration.136 Additional investigations into the brain specific role of AMPK are essential to determine if it is a viable target for AD and other neurodegenerative diseases.
Direct Activators
AICAR
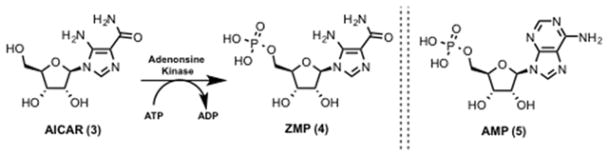
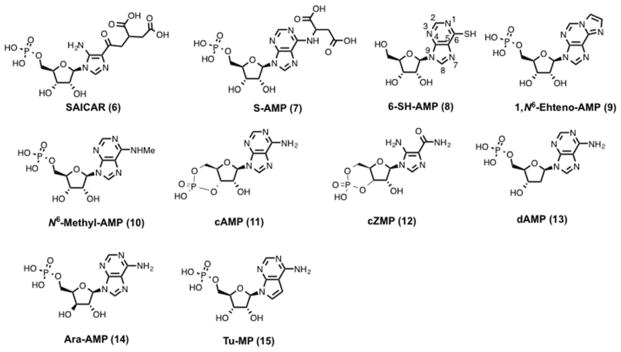
AICAR (3, Scheme 1) was isolated as a crystalline compound from a sulfonamide-inhibited Escherichia coli culture in 1956.137–139 Compound 3 is an inosine precursor and an adenosine analog that is transported across the cell membrane by adenosine transporters.140 Compound 3 is phosphorylated in cells by adenosine kinase on the 5′-hydroxyl to generate 5-aminoimidazole-4-carboxamide ribonucleotide monophosphate (ZMP) (4, Scheme 1).141 Initial reports argued that 3 treatment led to the accumulation of 4, which inhibited adenylosuccinate AMP lyase (5, Scheme 1) and led to muscle dysfunction.140,142,143 Sullivan et al. in 1994 showed that 4 stimulates human and rat AMPK and had a 20-fold better Km than 5. They concluded saying, “These novel activators of AMPK should prove useful in studying the role of the kinase in the regulation of cell metabolism.”144 Compound 4 binds to site 1 and/or site 3 of the regulatory γ subunit of AMPK and activates AMPK.145 4 and 5 bind to the same allosteric site on AMPK and have similar kinetic effects on AMPK activation isolated from rat liver.146 Unlike other AMPK activators such as fructose, heat shock, and arsenite treatment, AMPK activation by 3 is independent of the AMP : ATP ratio making it an ideal probe to study the role of AMPK.147 Structural studies with AMP analogs (Figure 5) suggest that the free amino group at position 6 is important for stimulation of AMPK. Compared to parent 5 and 4, 6-substituted AMP analogs such as 5-amino-4-imidazole-N-succinocarboxamide ribonucleotide (SAICAR) (6, Figure 5), adenyl-succinic acid (S-AMP) (7, Figure 5), 6-mercaptopurine riboside (6-SH-AMP) (8, Figure 5), 1, N6-Ethenoadenosine- 5′-O- monophosphate (1,N6-Etheno-AMP) (9, Figure 5) and N6-Methyl-AMP (10, Figure 5) displayed no AMPK activation. Also, c-AMP (11, Figure 5) and c-ZMP (12, Figure 5) have no AMPK stimulating activity suggesting the importance of the monophosphate group. On the other hand, changes in the ribose moiety of 2′-deoxyadenosine-5′-monophosphate (dAMP) (13, Figure 5) and adenine-9-beta-D-arabinofuranoside 5′-monophosphate (Ara-AMP) (14, Figure 5) yielded partial activation. Changes in the adenine functionality in tubercidin 5′-monophosphate (Tu-MP) (15, Figure 5) also resulted in diminished enzyme activity.146
Scheme 1.
Phosphorylation of AICAR and structure of AMP.
Figure 5.
Structure of AMP analogs.
Compound 3 treatment results in the activation of AMPK through the phosphorylation of Thr-172 on its activation loop. Once activated, AMPK phosphorylates its downstream targets ACC, HMG-CoA reductase and fructose-1,6-bisphosphatase, among others (Table 1). These signaling cascades regulate numerous cellular functions that include fatty acid synthesis, cholesterol synthesis, gluconeogenesis and glucose uptake in skeletal muscle.148–152 AICAR-stimulated glucose uptake was abolished in mouse muscle that expressed a kinase dead mutant (K46R) of AMPK establishing the link between 3 activation of AMPK and downstream cellular function (glucose uptake in muscle).153 In the same study, glucose transport was only partially blocked in response to contraction suggesting the presence of parallel pathways that alter glucose flux into muscle. Rac1, the actin cytoskeleton-regulating GTPase, drives GLUT4 translocation in an insulin-dependent manner and this is an alternate pathway for contraction-stimulated glucose uptake.154 Studies in mice and rat models consistently show that 3 treatment activates AMPK in different tissues and leads to the translocation of GLUT to the plasma membrane.155 The observation that 3-stimulated glucose transport is insulin independent led to preclinical studies that showed 3 treatment lowered blood glucose and improved glucose tolerance in the ob/ob mouse model.149,156
Table 1.
AMPK direct substrates and their phosphorylation sites (those reviewed here are highlighted in bold).
| S. No | Substrate | Residue |
|---|---|---|
| 1 | Acetyl-CoA carboxylase 15 | Ser-80, Ser-1201 and Ser-1216 |
| 2 | Acetyl-CoA carboxylase 27 | Ser-221 and other minor phosphorylation site |
| 3 | Glycogen synthase (muscle)3 | Ser-8 |
| 4 | HMG-CoA reductase3 | Ser-872 |
| 5 | Zinc finger transcription factor (AREBP)8 | Ser-470 |
| 6 | Eukaryotic elongation factor (eEF2) kinase4 | Ser-398 |
| 7 | Endothelial NO synthase (eNOS)10 | Ser-1177 (in presence of Ca+2/calmodulin) and Thr-495 (in absence of Ca+2/calmodulin) |
| 8 | GLUT4 enhancer factor (GEF)58 | - |
| 9 | Hepatic nuclear factor (HNF4α)12 | Ser-304 and other minor phosphorylation site |
| 10 | Insulin receptor substrate-113 | Ser-789 |
| 11 | p27Kip115 | Thr-198 |
| 12 | Transcriptional coactivator p30016 | Ser-89 |
| 13 | 6-phosphofructo-2-kinase (PFK-2)17 | Ser-466 and Ser-483 |
| 14 | Tuberous sclerosis 2 (TSC2)18 | Thr-1227 and Ser-1345 |
| 15 | Protein phosphatase 1 regulatory subunit 12C (PPP1R12C)19 | Ser-452 |
| 16 | p21-activated protein kinase (PAK2)19 | Ser-20 |
| 17 | Cardiac troponin I (cTnI)59 | Ser-150 |
| 18 | PIKfyve (FYVE domain-containing phosphatidylinositol 3-phosphate 5-kinase)60 | Ser-307 |
There are numerous studies that show 3 treatment leads to decreased cancer cell growth. Here we will limit the discussions to the studies that describe a mechanism of action. Among the many targets of AMPK, Ser-15 of p53 was identified to be present within a consensus recognition motif for AMPK.80 Cells treated with 3 showed sustained p53-Ser-15 phosphorylation along with increased levels of CDK inhibitors p21WAF/Cip1 and p27. In a panel of cancer cell lines, 3 induced cytostatic effects by arresting cells in the S-phase.157,158 It is known that 3-induced activation of AMPK leads to TSC2 phosphorylation-mediated mTOR inhibition.81,159 In glioblastoma patients with mutant epidermal growth factor receptor (EGFR), transformation and signaling is driven by the mTOR/S6K pathway.160,161 The use of rapamycin to block mTOR signaling failed in the clinics probably due to an protein kinase B (Akt) feedback loop.162 An elegant preclinical study showed that despite only partial inhibition of mTOR signaling, compound 3 ability to block glucose uptake and lipogenesis made 3 a more effective therapeutic than rapamycin for EGFR mutant glioblastoma.163
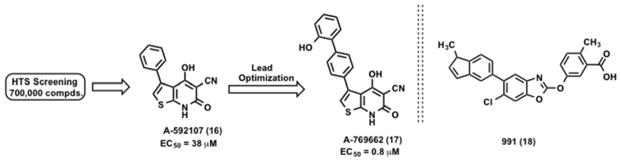
Thienopyridone (A-769662 Abbott’s compound)
The critical role of AMPK in the regulation of glucose and lipid metabolism led pharmaceutical companies to focus on the development of AMPK activators for the treatment of obesity and other metabolic diseases. Cool et al. conducted a HTS campaign to identify AMPK activators with a library of 700,000 compounds and a partially purified rat liver AMPK enzyme.164 The agonist-induced phosphorylation of SAMS peptide (HMRSAMSGLHLVKRR) by AMPK was monitored. Non-nucleoside thienopyridone compound A-592107 (16, Scheme 2) was identified as a direct AMPK activator.164 A lead optimization program starting with the original hit 16 (EC50 = 38 μM) led to the identification of a submicromolar compound A-769662 (17, Scheme 2) (EC50 = 0.8 μM).164 Validation studies confirmed 17 is a reversible AMPK activator. The EC50 values for 17 were determined using partially purified AMPK extracts from rat heart, rat muscle and in human HEK cells.164
Scheme 2.
Identification and optimization of thienopyridone compounds.
To determine if 17 acts as an AMP mimic in vitro studies were carried out using the enzymes glycogen phosphorylase (GPPase) and fructose-1,6-bisphosphatase (FBPase). 5 activated GPPase and inhibited FBPase.165–167 However 17 had no effect on FBPase and GPPase suggesting that the mechanism of activation of AMPK by 17 is different from that of 5. Consistent with the above observation, combination studies showed that 17 increased AMPK activity in the presence of saturating concentrations of 5. Conversely, 5 stimulated AMPK in the presence of saturating concentrations of 17. Although, like 5, compound 17 activates AMPK by allosterically inhibiting Thr-172 dephosphorylation, the mode of activation by 17 is distinct from that of 5. A systematic study with 17 showed that it activated only AMPK heterotrimers containing a β1 subunit.168 Mutation R298G in the γ subunit, which abolished 5 activation, had no effect on compound 17 activation. On the other hand, mutation S108A in the regulatory β subunit completely abolished allosteric activation of 17 while sparing 5 activation.169 A speculative model for activation of AMPK by 17 suggests that it binds to the glycogen-binding domain in the β subunit and stabilizes the conformation of AMPK that is resistant to Thr-172 dephosphorylation.169 The model does not show direct interaction with the catalytic α subunit or the autoinhibitory domain. Together these suggest that 17 is a cell permeable AMPK activator and the mechanism of AMPK activation by 17 is different from that of 5. Recently, Xiao et al. solved the structure of full-length human α2β1γ1 AMPK bound to small molecules 17 and 991 (18).37 As anticipated, activator 17 sits at the interface between the N-terminal kinase domain and the CBM (Figure 6).
Figure 6.
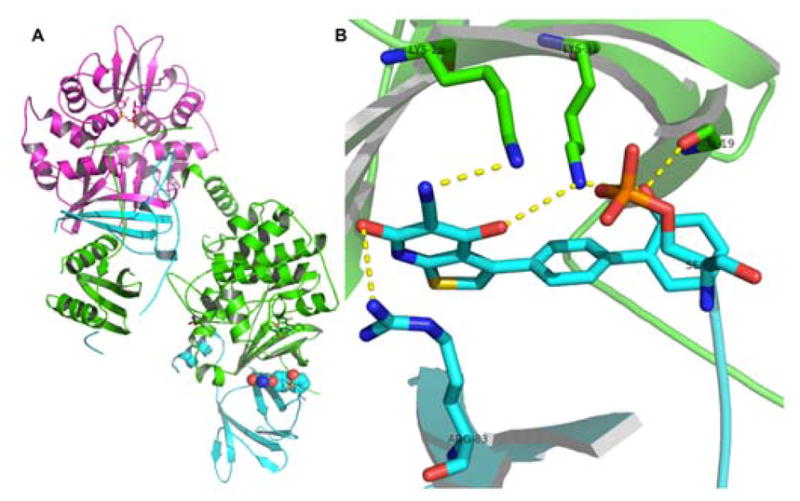
(A) Cartoon representation of full-length α2β1γ1 in complex with 17, represented in spheres. (B) Polar interactions that contribute to activator binding.
In vitro treatment of primary rat hepatocytes with 17 increased ACC phosphorylation and inhibited fatty acid synthesis.164 Additionally, Sprague-Dawley (SD) rats treated with 17 resulted in increased fatty acid utilization and partially reduced malonyl CoA levels. Chronic treatment of ob/ob mice with 17 led to decreased plasma glucose and triglycerides, decreased expression of gluconeogenic enzymes and decreased weight gain compared to vehicle control.164
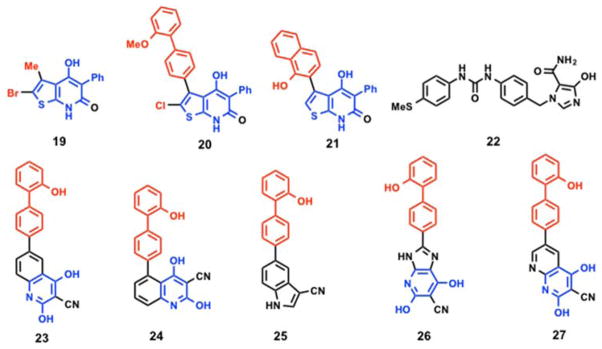
Following the work of Abbott laboratories, Merck GmbH identified thienopyridone compounds as AMPK activators for the treatment of diabetes, metabolic syndrome related disorders and obesity.170 Initial screening with two different fluorescent based technologies, AlphaScreen and Delfia, using the synthetic peptide substrate (AMARAASAAALARRR) yielded compound 19 (Figure 7) as an AMPK activator that increased basal AMPK activity by > 300%. Substitution of the methyl group at 5-position with a methoxy biphenyl 20 (Figure 7) showed a marginal increase in AMPK basal activity (111% at 30 μM) whereas analog 21 (Figure 7) with a hydroxy naphthalene substitution displayed better AMPK activation (625% at 30 μM).171,172 Additional scaffolds reported by Merck GmbH include compound 22 (Figure 7), a 4-hydroxyimidazole-5-carboxamide (like 3) substituted diphenyl urea, which showed > 310% increase in basal AMPK (purified from rat liver) activation at 200 μM concentration.173 With the availability of the crystal structures and the limited SAR presented above, the thienopyridone core could be revisited as a viable starting point for a structure guided optimization of AMPK activators.
Figure 7.
Direct AMPK activators.
Mercury Therapeutics reported the synthesis and screening of hydroxybiphenyl compounds as AMPK modulators for the treatment of cancer, diabetes, and neurological diseases.174 AMPK activity was measured by phosphorylation of an N-terminal fragment of human ACC1. This resulted in the identification of five biphenyl compounds (23 – 27) (Figure 7) with ED50 values < 10 μM.
Pyrrolopyridones
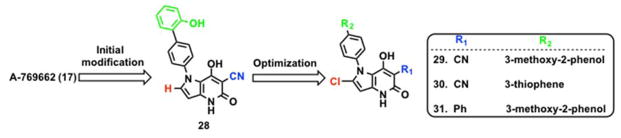
Using the Abbott compound 17 as a template, Mirguet et al. at GlaxoSmithKline (GSK) identified pyrrolopyridone analogs as a novel class of AMPK activators.175 The major goal of the GSK group was to improve oral absorption and the selectivity profile. Compound 17 is an AMPK activator that is selective for β1-containing heterotrimeric complexes with poor oral bioavailability. The thiophene ring was targeted for replacement to improve oral bioavailability. A bioisosteric replacement of the thiophene ring in 17 with a pyrrole yielded compound pyrrolo[3,2-b]pyridine-5(4H)one (28, Scheme 3) with better oral bioavailability. However, 28 also showed higher blood clearance. A series of analogs with varying functional groups at the R1, and R2 positions were synthesized to reduce blood clearance and increase potency. A 3-methoxy-2-phenol substituent at the R2 position resulted in a potent compound with reduced blood clearance but poor oral bioavailability. Substitution of hydrogen with chlorine (29, Scheme 3) in pyrrole ring did not improve the permeability or blood clearance but improved oral bioavailability. Substitution of the phenol with thiophene at R2 position yielded compound 30 (Scheme 3) with good oral exposure and bioavailability.175 The presence of the cyano group at the R1 position on the pyridone ring was associated with poor permeability due to the acidic nature of the 3-cyanopyridone ring. Replacing the cyano group at R1 with phenyl substitution improved permeability with good oral bioavailability (31, Scheme 3). Several analogs in this series showed improved blood exposure when co-dosed with a P-glycoprotein (P-gp) inhibitor suggesting that they may susceptible to Pgp-mediated efflux.175
Scheme 3.
Initial modification and optimization of pyrrolopyridone analogs
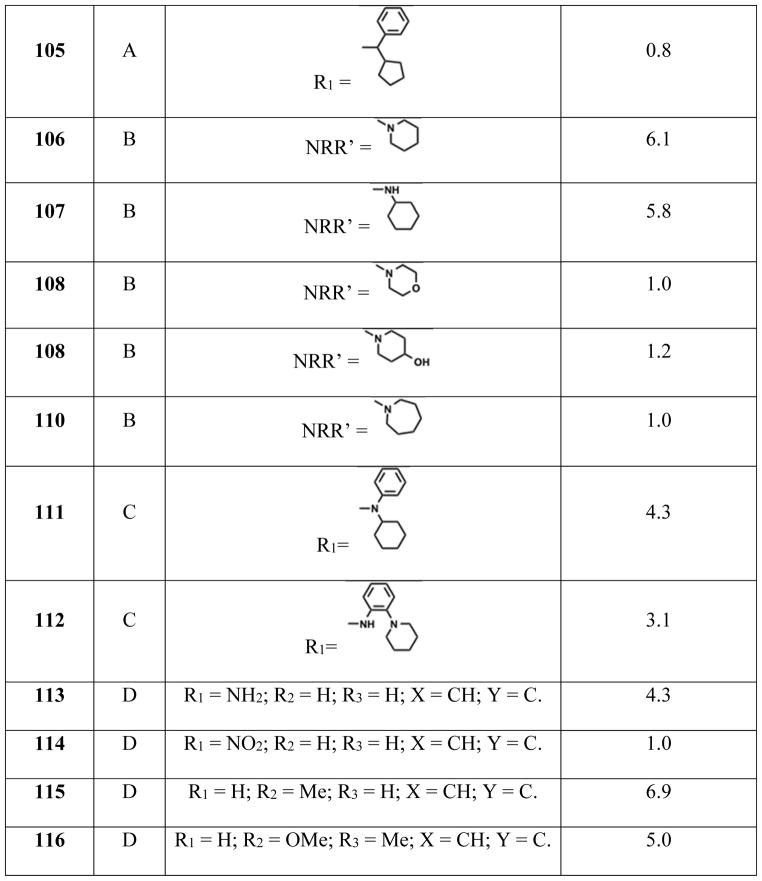
Abbott compound 17 is selective for β1-containing AMPK heterotrimers.168 On the other hand, compound 29 activates seven (α1β1γ1, α1β1γ2, α1β1γ3, α2β1γ1, α1β2γ1, α2β2γ1, α2β2γ3) of the twelve possible AMPK heterotrimers with pEC200s in the lower μm range (Table 2). Oral treatment of ob/ob mice with compound 31 (30 mg/kg for 5 days, bid) showed a 17% drop in blood glucose levels.175
Table 2.
AMPK heterotrimers activity profile with compound 29.
| AMPK Isoforms | Activity (pEC200) | AMPK Isoforms | Activity (pEC200) |
|---|---|---|---|
| α1β1γ1 | 9.2 | α1β2γ1 | 6.3 |
| α1β1γ2 | 9.4 | α2β2γ1 | 73 |
| α1β1γ3 | 8.5 | α2β2γ3 | 7.3 |
| α2β1γ1 | 8.4 |
pEC200 = −log(compound concentration leading to a 2-fold AMPK activity increase)
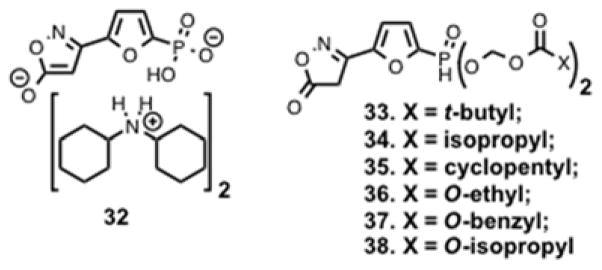
Using phosphorylation of SAMS peptide as a readout for AMPK activation, a research group at Metabasis Therapeutics screened a focused library of 1,200 AMP mimetics and discovered compound 32 (Figure 8) as a potent and selective AMPK activator.110 Compound 32 activated human AMPK with an EC50 of 6.3 nM, which is > 900 fold better than the endogenous activator 5 (EC50 = 5.9 μM). To overcome the poor cellular permeability, due to the charged nature of compound 32, the Erion group designed different esterase-sensitive phosphonate prodrugs (33 – 38, Figure 8).110 ACC is a direct substrate of AMPK and activation of AMPK leads to phosphorylation-mediated inactivation of ACC. Inactivation of ACC resulted in reduction of malonyl Co-A, and inhibition of de novo lipogenesis (DNL). Therefore compounds 33 – 38 were evaluated in vitro and in vivo for inhibition of DNL, and the results from this study are summarized in Table 3. Compounds were dosed (30 mg/kg) to C57BL/6 mice one hour prior to intraperitoneal administration of 14C-acetate in saline. After one hour, newly synthesized lipids and sterols in liver and plasma were quantified and compared to vehicle control.110 The limited SAR led to the identification of prodrugs with nM EC50 values and > 70% inhibition of DNL with a high correlation (R2 > 0.95).
Figure 8.
AMPK activators that inhibit DNL.
Table 3.
In vitro and in vivo inhibition of DNL.
| Compound no | Rat EC50 (nM) | In vivo DNL inhibition (%) |
|---|---|---|
| 32 | > 10000 | Not determined |
| 33 | 100 | 65 |
| 34 | 20 | 78 |
| 35 | 30 | Not determined |
| 36 | 42 | 73 |
| 37 | 609 | 34 |
| 38 | 27 | 73 |
Benzimidazoles
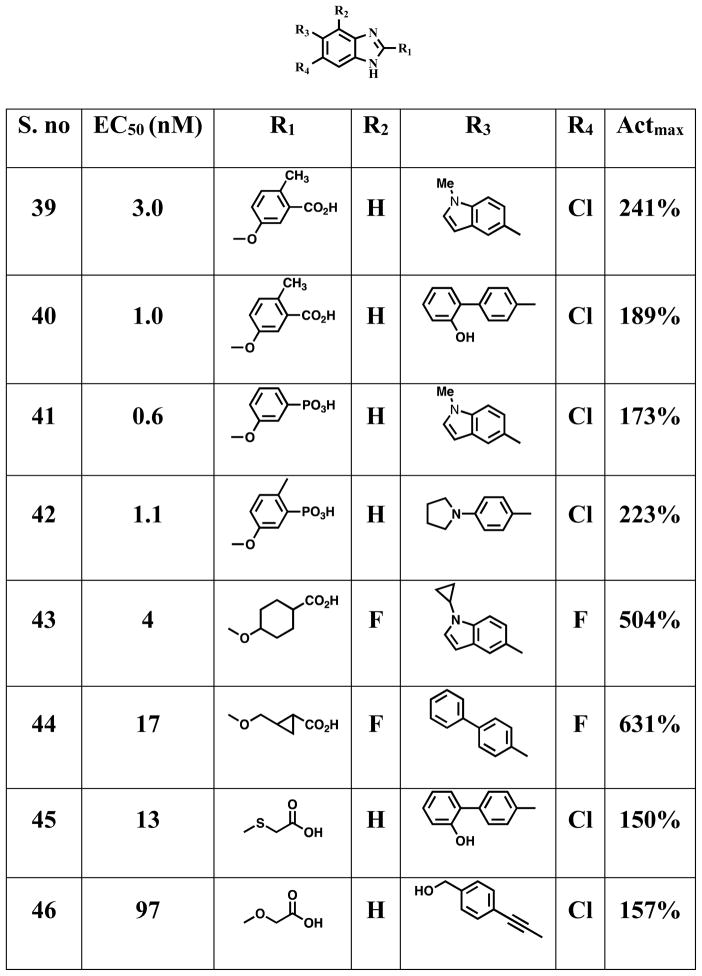
A series of patent applications were filed claiming benzimidazoles 39 – 48 (Figure 9) are therapeutically relevant AMPK activators for the treatment, prevention, and suppression of diseases susceptible to AMPK activation.176–180 AMPK activity was measured using α1β1γ1 recombinant human AMPK as half-maximal effective concentrations (EC50 relative to max activation by AMP) and activation effect relative to maximal activation by AMP (Actmax) respectively. A crystal structure of full-length α2β1γ1 AMPK complex with a small molecule activator 18, which has a benzoxazole core is reported.37 Compound 18 binds at the interface of α-kinase domain and the CBM of the β-subunit and preferentially activates AMPK complexes containing β1-subunits and binds with 10-fold higher affinity than 17.37
Figure 9.
General core structures of benzimidazole AMPK activators.
PT1
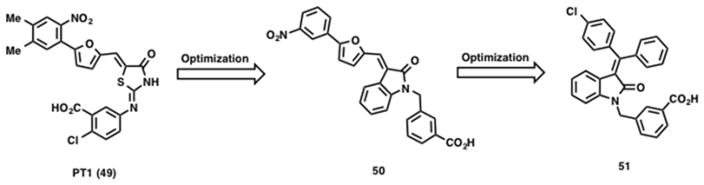
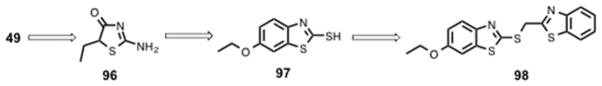
Using an inactive form of truncated AMPK α subunit, Pang et al. screened a library of 3,600 compounds for their ability to activate this inactive form of the catalytic α subunit (α1(1–394) containing the autoinhibitory domain). This led to the discovery of a small molecule activator (PT1) (49, Scheme 4) with an EC50 ~ 8 μM.181 Follow up studies showed that 49 did not increase AMPK activity of the truncation mutant α1(1–312) lacking the autoinhibitory domain. A plausible mechanism for AMPK activation by 49 is conformational change-induced dissociation of the AID domain from the catalytic domain of the α subunit.181 Subsequent studies confirmed that 49 interacts with the AMPK heterotrimeric complex α1β1γ1 in a dose-dependent manner (EC50 ~ 0.3 μM). A similar activation effect was observed when inactive AMPK α2 was treated with 49 with an EC50 ~ 12 μM. Docking studies suggest electrostatic interactions of 49 with Glu-96 and Lys-156 near the autoinhibitory domain in α1 subunit, relieve the autoinhibitory conformation and activate AMPK. When treated with 49, other AMPK-related protein kinases such as human MAP/microtubule affinity-regulating kinase 2 (MARK2), BR serine/threonine kinase 1 (BRSK1), NUAK family SNF1-like kinase 2 (NUAK2) and maternal embryonic leucine zipper kinase (MELK) showed no change in activity, suggesting 49 is selective for the α subunit of AMPK. Treatment of L6 myotubes with 49 activated AMPK in a dose-dependent and time-dependent manner without changing the AMP : ATP ratio suggesting 49 is a direct AMPK activator. However, due to poor bioavailability and/or insufficient potency, 49 was inactive in vivo.182
Scheme 4.
Structure of initial hit PT1 (49) and other optimized AMPK activators.
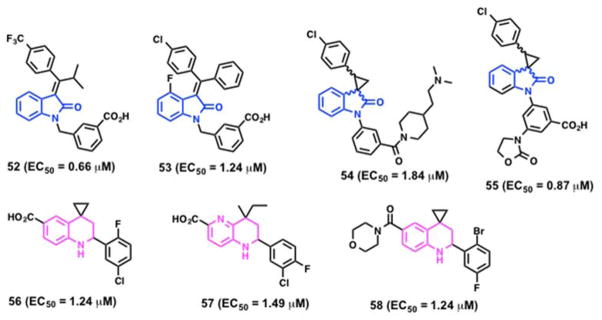
In an effort to improve potency, a series of 49 analogs were synthesized and screened for AMPK activation.182 Replacement of the central 2-imino-4-thiazolidone with a 3-alkylideneoxindole ring system yielded 50 (Scheme 4). Compared to 49, compound 50 displayed a 3-fold increase in AMPK activity and a 5-fold increase in potency (EC50 = 2.1 μM). Further structural modifications resulted in the potent analog 51 (Scheme 4), which showed > 4-fold improvement in EC50 value over 49 in an AMPK activation assay and demonstrated beneficial metabolic effects in a diet-induced obesity model. A 4-week oral administration of 51 in db/db mice showed reduction of plasma triglycerides and improved glucose tolerance when compared with metformin.183 A series of alkene-oxindole compounds (52 – 55, Figure 10) and 1,2,3,4-tetrahydroquinoline compounds (56 – 58, Figure 10) as AMPK activators were also reported by Hoffmann-La Roche AG for the treatment of diet-induced obesity and type 2 diabetes.184–187
Figure 10.
AMPK activators reported by Hoffmann-La Roche AG.
Salicylate
Salicylate, an active component of willow bark, is a hormone produced by plants to fight infection.188 Its synthetic derivatives such as aspirin and salsalate have been used towards the treatment of headache, lower back pain, osteoarthritis, and type 2-diabetes. Activation of AMPK was observed when HEK293 cells were treated with 1 mM of salicylate.189 To test whether effects of salicylate are due to changes in ATP, ADP and AMP levels, isogenic cells expressing wild-type (WT) AMPK or mutated AMPK (R531G γ2 subunit, a mutation which renders AMPK insensitive to AMP or ADP) were treated with salicylate, which activated AMPK to the same extent without changing the ADP : ATP ratio suggesting an AMP-independent mechanism.34 Concentration dependent studies suggest that at lower concentrations, AMPK phosphorylation and activation is independent of AMP and ADP levels, however at higher concentrations partial AMP and ADP dependent effects were observed.189 Under physiological concentrations of ATP, salicylate caused 1.6-fold activation of AMPK with half-maximal effect at 1.0 ± 0.2 mM. Competition studies suggest that salicylate binds to the same site as activator 17 and similar protection against dephosphorylation and inactivation by phosphatases was observed. Increased fatty acid oxidation that was associated with phosphorylation and activation of AMPK was observed in isolated WT hepatocytes of salicylate-treated WT mice. Furthermore, phosphorylation of liver AMPK, soleus muscle AMPK and adipose tissue AMPK was observed in salicylate-treated WT mice but not in β1-KO mice.189 Aspirin, a synthetic derivative of salicylate reduced mTOR signaling in colorectal cancer cells by inhibiting mTOR effectors S6K1 and 4E-BP1 and increased AMPK and ACC phosphorylation.190
Sanguinarine
In search of therapeutically relevant AMPK activators, Choi et al. screened a diverse library of 1,200 compounds using an in vitro fluorescence resonance energy transfer (FRET) assay.191 Sanguinarine (59, Figure 11), a benzophenanthridine alkaloid, was identified as a validated hit.191 Follow up studies with recombinant AMPK heterotrimers showed that 59 activated only AMPK heterotrimers that contained both the α1 and γ1 subunits and was ~5–10 fold less potent than AMP.191
Figure 11.
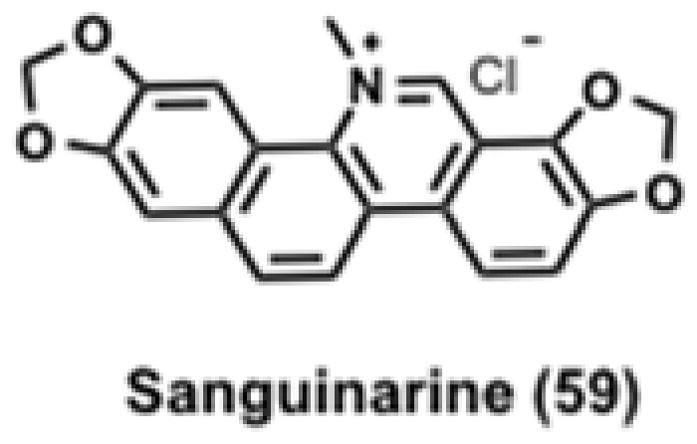
Structure of Sanguinarine, an AMPK activator.
Indirect Activators
AMPK serves as a signaling hub that can be activated by different modes: (i) allosteric activation by AMP and ADP, (ii) activation by upstream kinases, (iii) deactivation by phosphatases, (iv) conformational change to deactivate the autoinhibitory domain and (v) compounds that increases the AMP : ATP ratios within the cells are termed indirect AMPK activators. Well-characterized upstream kinases of AMPK include LKB1, CaMKKβ and Tak1. However additional yet-to-be-defined kinases may activate AMPK. Furthermore, the phosphatases that deactivate AMPK and all the AMPK complexes found in cells are not yet fully defined. Consequently, the mechanisms of action of many of the indirect activators listed below are not known. Nevertheless, treatment with these compounds ultimately leads to indirect activation of AMPK and alteration of the energy or metabolic landscape.
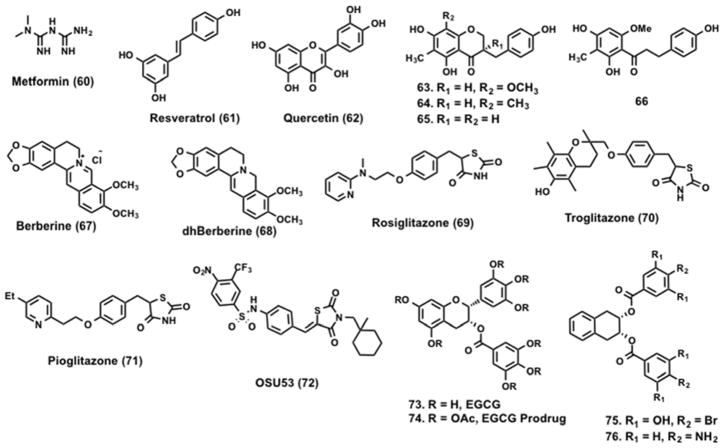
Metformin (60, Figure 12) a biguanide derivative of guanidine, is an antihyperglycemic agent. It is widely used for the treatment of type 2 diabetes and acts by suppressing hepatic glucose production.192 Some studies suggest that 60 reduces the rate of gluconeogenesis,193,194 while others suggest its mechanism of action is increased glucose uptake by skeletal muscle.195,196 Studies by Zhou et al. established a connection between AMPK and 60.68 In rat hepatocytes, 60 treatment activated AMPK, which phosphorylated and reduced ACC activity. Inactivation of ACC reduced expression of sterol regulatory element binding protein-1 (SREBP-1), a lipogenic transcription factor, which led to the suppression of lipogenesis. Elevated levels of SREBP-1 are associated with insulin resistance in type 2 diabetes.197 AMPK activation by 60 is dependent on the presence of LKB1. Shaw et al. showed 60 treatment lowered blood glucose by 40% in LKB1+/+ ob/ob mice and no such effect was observed in the LKB1 null mice.198 60 is also a substrate of organic cation transporters (OCT). OCT1 plays a critical role in hepatic uptake of metformin and genetic (OCT1−/−) and chemical (ethynylestradiol-induced cholestasis) disruption of OCT1 (expressed abundantly in the liver) function leads to reduced 60 distribution and consequently lowers its efficacy in reducing blood glucose levels.199,200 OCT1 is highly polymorphic, in clinical studies individuals carrying OCT1 polymorphisms that resulted in reduced function had a poor response to 60 effects in glucose tolerant tests.200
Figure 12.
Indirect AMPK activators.
Resveratrol (61, Figure 12) is a polyhydroxy-substituted stilbene found in several natural sources including the skin of red grapes. Studies by Vingtdeux et al. suggest 61 increases intracellular Ca+2 levels, which activates the kinase CaMKKβ that in turn phosphorylates and activates AMPK, although additional studies are needed to validate this mechanism of action.201 Activation of AMPK by 61 resulted in mTOR inhibition, Aβ clearance in mice and potentiation of autophagy. 61-mediated activation of AMPK has thus been suggested as a therapeutic strategy to combat AD. A library of 158 compounds, structurally similar to 61, were screened at 10 μM in APP-transfected HEK293 cells for their ability to reduce amyloid Aβ levels.202 Like 61, its analogs are not direct activators of AMPK but instead perturb upstream effectors, which lead to the activation of AMPK. Unlike 60, compound 61 analogs were able to induce phosphorylation of AMPK and ACC in LKB1-deficient HeLa cells, suggesting that activation of AMPK by 61 is LKB1 independent. On the other hand, AMPK activation and ACC phosphorylation by the 61 analogs was dampened by co-treatment with CaMKKβ inhibitor. 61 has been reported to activate sirtuins and increase cell survival by stimulating SIRT1-dependent deacetylation of p53.203 61 increased human SIRT1 activity, but not the activity of other human Sir2 homologs (SIRT2), in fluorophore-labeled acetylated p53 derived peptide substrates.204,205 Subsequent studies with full-length substrates in cell-free assays suggest indirect activation of SIRT1 by 61.206,207 Desquiret-Dumas et al. showed that 61 activates SIRT3 through an increase in NADH oxidation by complex 1.208 Although the exact mechanism of action of 61 is not clear, 61 analogs have been shown to inhibit the mTOR pathway, induce autophagy and promote Aβ degradation by the lysosomal system in cells to lower Aβ accumulation/deposition in mice.201
Quercetin (62, Figure 12) is a flavanoid that is commonly found in a variety of fruits and vegetables. In isolated rat adipocytes, 62 inhibited methylglucose uptake with a Ki of 16 μM.209 3T3-L1 preadipocytes treated with 62 showed induction of AMPK phosphorylation in a dose-dependent manner. ACC is a known substrate of AMPK and phosphorylation of ACC inhibits adipogenesis. 62-treated 3T3-L1 adipocytes showed decreased extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinases (JNK) phosphorylation and increased apoptosis.210 Recent reports demonstrate that treatment with 62 decreases the expression levels of transcription regulators such as CCAAT/enhancer binding protein (C/EBP), alpha (C/EPBα) and PPARγ thereby suppressing the differentiation of preadipocytes to adipocytes.211 However, the exact molecular mechanism underlying 62-induced effects on adipocytes remain unclear.
The rhizomes of Polygonatum odaratum have been used as a traditional medicine and are commercially sold as food supplements. Guo et al. isolated homoisoflavanoids and dihydrochalcone from the rhizomes of Polygonatum odaratum (Mill.) Druce.212 Compounds 63 – 66 (Figure 12) showed a significant increase in the phosphorylation of AMPK as well as the downstream substrate ACC. Pharmacological studies have demonstrated hypoglycemic effects with P. odoratum in diabetic animal models.213,214
Berberine (67, Figure 12) is a botanical alkaloid found in the roots and bark of several plants such as Berberis vulgaris, Berberis asitata and Coptis chinensis, among others. 67 is reported to have antihyperglycemic properties, antifungal, antiviral and antimicrobial activites.215–221 In adipocytes, 67 treatment alters the AMP : ATP ratio, which leads to LKB1 and CaMKKβ independent activation of AMPK.28, 222 Treatment with 67 reduced oxygen consumption in isolated muscle mitochondria containing complex I.223 Other studies also suggest that 67 targets respiratory complex I.219,224–227 3T3-L1 adipocytes and L6 myotubes subjected to 67 treatment showed significant reduction in oxygen consumption suggesting a switch to anaerobic respiration in cells.223 Like other AMPK activators, 67 treatment resulted in increased glucose uptake in an insulin pathway independent manner. In a diet-induced obesity model, five weeks of 67 treatment significantly reduced fasting blood glucose and fasting insulin levels and improved insulin sensitivity.228 A 67 derivative dihydroberberine (dhBBR) (68, Figure 12) showed improved oral bioavailability while phencopying berberine-induced effects.223
Thiazolidinediones (TZDs) such as rosiglitazone (69, Figure 12), troglitazone (70, Figure 12), and pioglitazone (71, Figure 12) are insulin-sensitizing agents commonly used for the treatment of type 2 diabetes. TZD compounds are high affinity ligands of the transcription factor PPAR-γ, which belongs to the nuclear hormone receptor superfamily.229,230 Through PPAR-γ, TZDs modulate the transcription of critical genes involved in preadipocytes differentiation and fatty acid synthesis and storage.231 TZD and their analogs also possess anticancer effects that are independent of PPAR-γ.232–234 Compound 69 is a member of the thiazolidinedione class of oral antidiabetic drugs and improves insulin sensitivity and glucose homeostasis in type 2 diabetes patients.235,236 The exact mechanism by which 69 improves insulin sensitivity and alters lipid and glucose metabolism remains poorly understood. Studies from the Carling group suggest that 69 activates AMPK in muscle by alterating the AMP : ATP ratio.237 Treatment of H-2Kb muscle cells with 69 leads to activation of AMPK as inferred by phosphorylation of its substrate, ACC.
Guh et al. screened a focused library of in-house thiazolidinedione-based compounds and identified 72 (Figure 12) as a novel AMPK activator. Compound 72 activates AMPK in an LKB1 independent manner and inhibits lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) production in human THP-1 cells.238 Cytokine IL-6 plays an important role in the production of signal transducer and activator of transcription 3 (Stat3), which is constitutively active in 50% of primary breast tumors and is associated with poor prognosis.239 In vitro and in vivo studies demonstrated that 72 is a potent antitumor agent that downregulates mTOR signaling through AMPK activation. The exact mechanism of AMPK activation by 72 is unclear, however, electrostatic potential map suggests that 72 might mediate AMPK activation via allosteric binding.238 Direct AMPK activators that do not rely on LKB1 function have the potential to alleviate pathological conditions induced by LKB1 dysfunction.240
Epigallocatechin-3-gallate (EGCG) (73, Figure 12) is a natural compound found in green tea and has been suggested as a food supplement for the treatment of diet-induced obesity and type 2 diabetes.241–243 Cellular studies show 73 treatment leads to inhibition of hepatic gluconeogenesis244 and apoptosis in cancer cells.245 However, limitations such as stability under physiological conditions, poor bioavailability and lower potency hinder its usage.246,247 Compound 73 is unstable under physiological pH and tends to undergo methylation.246 In order to improve its stability, the reactive hydroxyl groups of 73 were acetylated and prodrug (74, Figure 12) of EGCG with improved bioavailability was developed.248 A focused library of epigallocatechin analogs was synthesized by replacing the reactive hydroxyl groups with H, OH, OAc, NH2, alkyl, and halogens, among others. Evaluation of these analogs led to the identification of two new analogs (75 and 76, Figure 12) with improved AMPK activity.249
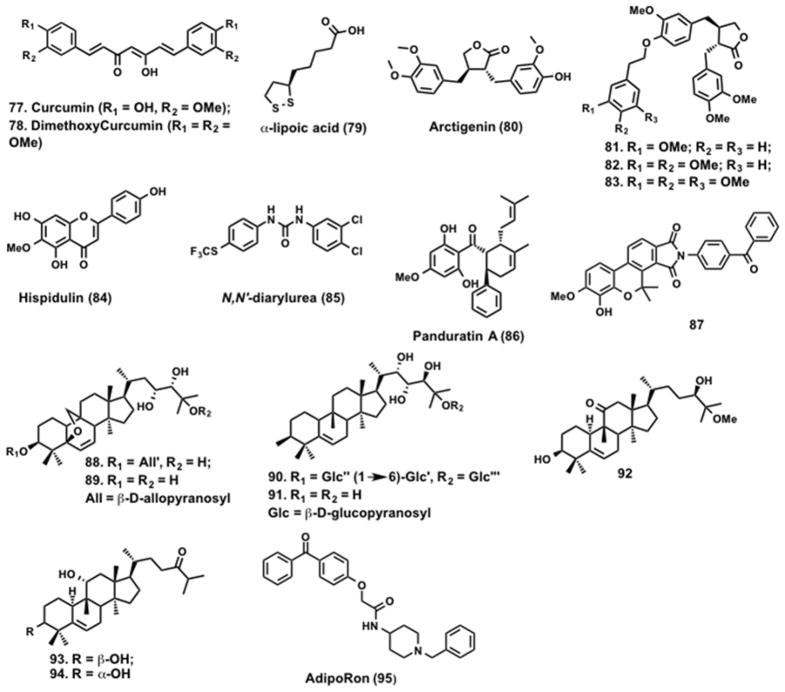
Curcumin (77, Figure 13) is a natural polyphenolic compound found in the rhizomes of turmeric and has a broad range of biological activities including anticancer activity. Studies by Pan et al. showed that 77 treatment of CaOV3 ovarian cancer cells increased phosphorylation of LKB1, ACC, p38 and p53.250 Studies suggest that 77 activates LKB1-AMPK pathway, which results in cytotoxic effects in ovarian cancer cells. The α,β unsaturated ketones in curcumin makes it susceptible to nucleophiles in the cellular matrix.251 The curcumin derivative, dimethoxycurcumin (DMC) (78, Figure 13) is a more stable and a potent activator of AMPK.252
Figure 13.
Indirect AMPK activators.
α-lipoic acid (ALA) (79, Figure 13) is a naturally occurring plant-derived antioxidant that increases glucose uptake in skeletal muscle, increases insulin sensitivity in type 2 diabetes patients and reduces blood glucose levels. Recent studies have suggested that the increase in insulin sensitivity upon 79 treatment is due to activation of AMPK.253 In 79-treated C2C12 myotubes increased intracellular Ca2+ was observed. This led to the activation of CaMKKβ, an AMPK upstream kinase. Co-treatment with STO-609, a calcium chelator, blocked 79 induced AMPK activation.254 Preclinical studies in animals showed that treatment with 79 reduced lipid accumulation.255,256 Studies by Park et al. demonstrated that hepatic steatosis induced by high fat diet or liver X receptors (LXRs) agonist was blocked by 79 treatment. Compound 79 treatment also decreased the expression of SREBP-1c expression in these animals. Although 79 prevent fatty liver diseases its mechanism of action is yet to be fully defined.257
Arctigenin (80, Figure 13) is a phenylpropanoid dibenzylbutyrolactone isolated from the seeds of Arctium lappa L. Screening of an in-house natural product library by Tang et al. identified 80, which activates AMPK both in vitro and in vivo.258 A cell-based assay revealed that 80 promoted AMPK phosphorylation selectively at Thr-172 through the upstream kinases LKB1 and CaMKKβ. In an effort to improve potency and build a SAR, 80 analogs were synthesized and screened for AMPK phosphorylation in L6 myoblasts incubated with the analogs (40 μM) for 30 min. This led to the identification of additional analogs (81 – 83, Figure 12) with improved activity.259
5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one (Hispid ulin) (84, Figure 13) is a naturally occurring flavone found in Saussurea involucrate, a traditional Chinese herb with a range of biological activities.260 Treatment of SKOV3 ovarian cancer cells with 84 resulted in activation of AMPK, inhibition of mTOR and significantly reduced Mcl-1 levels. Interestingly, 84 enhanced tumor necrosis factor-related apoptotic-inducing ligand (TRAIL)-induced apoptosis in SKOV3 cells providing a rationale for the combined use of AMPK activators and death receptor (DR) ligands for cancer therapy.261
Using HTS, Sviripa et al. identified N,N′-diarylurea (85, Figure 13), as an AMPK activator.262 Several N,N′-diarylureas such as regorafenib, and sorafenib are used for the treatment of metastatic colorectal cancer, kidney cancer, and advanced liver cancer.263,264 Treatment of LS147T colon cancer cells with 85 increased the phosphorylation of AMPK without changing the overall AMPK levels.
In search of natural AMPK activators, Kim et al. screened a natural product library and found panduratin (PAN) A (86, Figure 13), a compound isolated from the rhizomes of Boesenbergia pandurata.265 Compound 86 treatment increased phosphorylation of AMPK and ACC in 3T3-L1 adipocytes, HepG2 liver carcinoma cells and L6 skeletal muscle cells. This effect was abolished by treatment with a AMPK inhibitor 1.68 Furthermore, activation of AMPK by 86 was completely abolished in LKB1-deficient cells suggesting LKB1-dependent AMPK activation. In addition, 86 altered AMPK subcellular localization and activated PPARα/γ. Oral administration of 86 to C57BL/6J mice on a high fat diet reduced triglycerides, total cholesterol and low-density lipoprotein cholesterol.265
Seeing the beneficial effects of AMPK activation towards the treatment of type-2 diabetes and obesity, Oh et al. screened a library of 2500 compounds and identified a small molecule AMPK activator, ampkinone (87, Figure 13).266 In vitro studies suggested compound 87 activated AMPK and subsequent phosphorylation of ACC substrate. Immunocomplex kinase assays with 87 (10μM) led to a 2.7 fold increase in AMPK activity towards the SAMS peptide whereas 1mM 3 treatment induced 3.2 fold AMPK activation. Follow up studies suggests 87 as an indirect AMPK activator. Consistent with in vitro studies, 87 increased pAMPK and pACC levels in liver cells of diet-induced obese (DIO) mice. Lower glucose levels were observed in 87-treated mice, which indicate that 87 is an AMPK activator with anti-diabetic effect.266
Tan et al. isolated two different classes of curcubitane triterpenoids from Momordica charantia (bitter melon).267 Compounds 88 and 90 (Figure 13) and their aglycones 89 and 91 (Figure 13) activated AMPK in L6 muscle cells and 3T3L1 adipocytes in a dose-dependent manner and stimulated translocation of GLUT4 to the plasma membrane. Concentrations required for 88 – 91 are 10,000 times lower than compound 3 suggesting curcubitane triterpenoids are highly efficacious stimulants of GLUT4 translocation. No activation of AMPK in HeLa cells lacking LKB1 was observed suggesting that the mechanism of action of these curcubitane might be similar to metformin.267 Recently, Chen et al. isolated new curcubitane triterpenoids (92 – 94, Figure 13) and reported them as potent AMPK activators in HepG2 cells.268
Adipocytes secrete adiponectin, which bind to AdipoR1 and AdipoR2 to activate AMPK and exert antidiabetic effects. In a screen of the chemical library, Chen et al. identified a small molecule activator of AdipoR, termed AdipoRon (95, Figure 13) that binds AdipoR1 and AdipoR2.100 Surface plasmon resonance studies showed 95 binds both AdipoR1 and AdipoR2 with a Kd 1.8 and 3.1_μM, respectively. Increased AMPK Thr-172 phosphorylation was seen in C2C12 myocytes treated with 95 and was almost completely lost by treatment with AdipoR1 siRNA. In db/db mice on normal chow diet, treatment with 95 improved glucose intolerance and insulin resistance, suggesting AdipoR stimulation may be a viable therapeutic option for diabetes treatment.100
Small molecule R419 (2, Figure 1) was identified by Jenkins et al. as an AMPK activator that, like metformin, inhibits complex I. The small molecule activates liver, muscle, and adipose AMPK. Using ACC Ser-79 phosphorylation as a readout, the EC50 for the compound in HepG2 and C2C12 myotubes was estimated at ~ 0.03 and 0.23 μM, respectively. Compound 2 treatment resulted in increased glucose uptake in myocytes, possibly through increased GLUT4 expression, and increased fatty acid oxidation in mouse primary hepatocytes, highlighting the potential therapeutic value of AMPK activation and regulation of mitochondrial function for the treatment of diabetes.98
Meltzer-Mats et al. used 49 as a starting compound and truncated it sequentially to identify benzothiazole (96, Scheme 5) as the minimally active moiety (100 μM treatment leads to 1.3 fold increase in glucose uptake) that is required to induce AMPK phosphorylation and glucose uptake in L6 myotubes.269 A second fragment capable of activating AMPK was also identified (97, 1.8 fold at 100 μM, Scheme 5). A series of benzothiazole derivatives that combined the two fragment cores were synthesized and screened for glucose uptake and AMPK phosphorylation in L6 myotubes. Compound 98 (Scheme 5) was identified as an efficacious and potent AMPK activator that induced a 2.5 fold increase in glucose uptake at 100 μM. In vivo efficacy of compound 98 was evaluated in the KKAy mice model, which had previously been reported to develop diabetic traits similar to human patients.270 In this model, compound 98 treatment improved total blood glucose clearance by ~50%.
Scheme 5.
Optimization of benzothiazole AMPK activators.
Charton et al. screened a library that led to the identification of S27847 (99, Scheme 6) as an AMPK activator.271 In primary hepatocytes culture and in H-2K muscle cells, 99 was found to be more effective in activating AMPK than the control compound 3. A focused library of 74 benzimidazole analogs with different modifications on the benzimidazole ring was synthesized and subjected to an AMPK kinase assay using the SAMS peptide as the substrate. This exercise yielded a well-defined SAR around the benzimidazole core and identified analogs with a greater potency.271 In series A modifications, replacement of 1-phenylcyclohexyl with 1-phenylheptyl (100), trans-2-phenylcyclopropyl (101), biphenyl-2-yl (102) showed improved potency compared to the initial hit whereas substitution of cyclohexyl with phenyl (103), or phenyl with cyclohexyl (104), cyclohexyl with cyclopentyl (105), led to a drop in activity. In the second series (B), the phenyl group was conserved and the cyclohexyl group was substituted. Substitution of cyclohexyl with piperidine (106) and cyclohexylamine (107) resulted in gain of activity whereas substitutions with morpholine (108), 4-hydroxypiperidine (109), homopiperidine (110) resulted in complete loss of activity. In series C, the carbon atom between phenyl and cyclohexyl of 99 was substituted with nitrogen atom (111) and no change in activity was observed. On the other hand, substitution of phenylcyclohexylmethyl with 2-(1-piperidino)aniline (112) resulted in modest loss of activity. In series D, the phenylcyclohexyl core was kept constant and different substitutions were made at the benzimidazole rings. Substitution of amino at R1 position (113) resulted in similar activity, whereas the nitro group at R1 (114) resulted in complete loss of activity. At R2 position, substitution of the methyl group (115) or methoxy group (116) resulted in a gain of activity whereas any other electron withdrawing substitution resulted in a loss of activity. At the present time the exact mechanism of AMPK activation by the benzimidazoles is not known.
Scheme 6.
Identification of S27847 and its novel series.
ETC-1002 (8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid) is a novel small molecule currently in clinical trials for the treatment of dyslipidemia and other cardio-metabolic risk factors.272 ETC-1002 was shown to reduce LDL-cholesterol levels in preclinical models of dyslipidemia and improve glucose homeostasis in mouse models.147,273 ETC-1002 has a unique dual mechanism of action. In liver, ETC-1002 inhibits ATP citrate lyase (ACL), a key enzyme in the cholesterol biosynthesis pathway and activates AMPK. HepG2 cells treated with ETC-1002 showed concentration dependent activation of AMPK and ACC phosphorylation that was comparable to metformin. CaMKKβ inhibitor treatment has no effect on ETC-1002-induced AMPK activation suggesting intracellular Ca+2-independent AMPK activation. Also, treatment of ETC-1002 did not alter AMP, ADP and ATP levels suggesting AMPK activation by ETC-1002 is independent of adenine nucleotides. HepG2 cell studies using siRNA showed ETC-1002 activates AMPK in LKB1-dependent fashion.272
AMPK Inhibitor
Zhou et al. screened a large library and identified a cell permeable pyrazolopyrimidine compound C (1, Figure 1) that inhibits the phosphorylation of the SAMS peptide by partially purified AMPK from the liver of SD rats.68 Kinetic studies using variable ATP concentrations showed compound 1 is a reversible and ATP-competitive inhibitor of AMPK (Ki = 109 ±16 nM) in the absence of 5. In vitro assays using structurally related kinases such as spleen tyrosine kinase (SYK), protein kinase A (PKA), and janus kinase 3 (JAK3) suggested 1 is a selective AMPK inhibitor. Compounds 3 and 60 treatment induces activation of AMPK and inactivation of ACC in primary hepatocytes. This ACC inactivation is inhibited by 1 treatment, which suggests that compound 1 block the stimulation of AMPK activation by pharmacological AMPK activators.
Concluding Remarks
AMPK is a master regulator that controls the energy and metabolic landscape in cells and tissues. There are twelve possible AMPK heterotrimeric complexes that are expressed in cells and tissues. The relative distribution as well as the tissue distribution of AMPK subunits is poorly defined at the present time. Although challenging but development of isoform specific AMPK activators will help elucidate the functional role of AMPK trimeric isoforms. Significant progress has been made towards identification of isoform selective AMPK activators. For instance, Abbott compound 17 is selective for β1-containing AMPK heterotrimers whereas 59 is selective towards α1 and γ1 subunits. AMPK activation can be achieved in different ways such as (i) compounds 17 and 18 bind to the interface between the N-terminal kinase domain and the CBM domain to induce AMPK activation; (ii) 49 binds to charged residues to alter the conformation of the AID to induce AMPK activation; (iii) inhibition of phosphatases etc. There are at least twenty well-characterized substrates of AMPK currently known and additional substrates are being identified through proteomic approaches. Based on available data, it is clear that AMPK is a major signaling hub, however its composition in various cells and tissues is yet to be fully defined. A systematic combination study using different AMPK activators will not only provide useful information regarding the composition of the complexes but also offer opportunities for combination therapy using AMPK activators targeting different subunits/pathways. Additionally studies in various tissues using inhibitors that target upstream kinase and phosphatases to modulate AMPK function irrespective of the composition of the complex could lead to validation of additional targets. A third and more challenging option is to develop inhibitors against protein-protein interfaces (PPIs) in the AMPK trimeric complex. For example, the structural basis for the regulation of the kinase function by AID has been established. Since the interaction is driven by hydrophobic residues lessons learnt from the development of inhibitors of p53-MDM2/X could used to accelerate this process.274 In theory, these PPI inhibitors should phenocopy the effects of 49 that binds to charged residues near the AID in the α subunit to activate AMPK. Since the N-terminus of the β subunit undergoes myristoylation that drives the nuclear-cytoplasm shuttling of the AMPK complex, chemical probes against this PPI could help understand the effects of AMPK mislocalization. HTS and peptidomimetic approaches can be used to develop chemical probes that target these PPI’s. The availability of AMPK crystal structures offers the possibility of structure-based design of AMPK modulators. In silico methods can be used to revisit core structures that have SAR data, which could lead to the identification of suitable compounds for structure-guided optimization. Although most preclinical models suggest that AMPK activators will be useful for the treatment of metabolic diseases, cancer and AD, there are conflicting reports that suggest that AMPK is a contextual oncogene and AMPK inhibition as opposed to activation is beneficial for AD therapy. The importance of AMPK mediated signaling in a plethora of diseases and its complexity suggests that there is an urgent need for additional AMPK modulators that can be used to not only dissect the mechanism of action but also as lead compounds for therapeutic development.
Acknowledgments
The authors would like to acknowledge all the lab members and the reviewers for their valuable suggestions and critiques. The authors are grateful for support from the Nebraska Research Initiative and Fred and Pamela Buffet Cancer Center.
Abbreviations Used
- AD
Alzheimer’s disease
- ADP
Adenosine diphosphate
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide
- AID
Autoinhibitory domain
- APP
Amyloid precursor protein
- AKT
Protein kinase B
- ALA
α-lipoic acid
- AMPK
AMP-activated protein kinase
- AMP
Adenosine monophosphate
- Ara-AMP
Adenine-9-beta-D-arabinofuranoside 5′-monophosphate
- Aβ
Amyloid-beta protein
- AREBP
AICAR response element binding protein
- ATP
Adenosine-5′-triphosphate
- BRSK1
BR serine/threonine kinase 1
- CaMKKβ
Calcium/calmodulin-dependent protein kinase kinase β
- CBM
Carbohydrate binding module
- CBS
Cystathionine β-synthase
- C/EPBα
CCAAT/enhancer binding protein α
- dAMP
2′-Deoxy-AMP, 2′-deoxyadenosine-5′-monophosphate
- DNL
De novo lipogenesis
- 2-DG
2-deoxyglucose
- DIO
Diet-induced obese
- DMC
Dimethoxycurcumin
- dhBBR
dihydroberberine
- DR
Death receptor
- eEF
Elongation factors
- EGCG
Epigallocatechin-3-gallate
- EGFR
Epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinases
- ER
Endoplasmic reticulum
- eNOS
Endothelial nitric-oxide synthase
- 4EBP1
Eukaryotic initiation factor 4E binding protein 1
- FBPase
Fructose-1,6-bisphosphatase
- FRET
Fluorescence resonance energy transfer
- GBD
Glycogen binding domain
- GPPase
Glycogen phosphorylase
- GSK-3β
Glycogen synthase kinase-3-beta
- GLUT
Glucose transporters
- GS
Glycogen synthase
- GSK
GlaxoSmithKline
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- HNF4α
Hepatocyte nuclear factor 4-alpha
- HTS
High throughput screening
- IL-6
Interleukin 6
- IR
insulin receptor
- IRS-1
Insulin receptor substrate
- JAK3
Janus kinase 3
- JNK
c-Jun N-terminal kinases
- KD
Kinase domain
- LDH
Lactate dehydrogenase
- LKB1
Liver Kinase B1
- LPS
Lipopolysaccharide
- LXR
Liver X receptors
- MEFs
Mouse embryonic fibroblasts
- MARK2
MAP/microtubule affinity-regulating kinase 2
- MELK
Maternal embryonic leucine zipper kinase
- mTOR
Mammalian target of rapamycin
- mTORC1
Mammalian target of rapamycin complex-1
- MODY
Maturity onset diabetes of the young
- MRLC
Myosin regulatory light chain
- NADH
Nicotinamide adenine dinucleotide
- NFTs
Neurofibrillary tangles
- NO
Nitric Oxide
- NOS
Nitric oxide synthase
- NUAK2
NUAK family SNF1-like kinase 2
- OCT
Organic cation transporters
- PAN
Panduratin
- PKA
Protein kinase A
- PPARγ
Peroxisome proliferator-activated receptor-γ
- PFK
Phosphofructokinase
- PEPCK
Phosphoenolpyruvate carboxykinase
- PI3K
Phosphatidylinositide 3-kinases
- PFK-2
phosphofructokinase-2
- Ppm1E
Protein serine/threonine phosphatase 1E
- PPP1R12C
protein phosphatase 1 regulatory subunit 12C
- PAK2
p21-activated protein kinase
- P-gp
P-glycoprotein
- PIKfyve
FYVE finger-containing phosphoinositide kinase
- PtdIns
phosphatidylinositides
- PJS
Peutz-Jeghers syndrome
- PPI
Protein-protein interfaces
- SAR
Structure activity relationship
- S-AMP
adenyl-succinic acid
- SAICAR
5-Amino-4-imidazole-N-succinocarboxamide ribonucleotide
- 6-SH-PMP
6-mercaptopurine riboside 5′-monophosphate
- S6K
Ribosomal S6 kinase
- SD
Sprague-Dawley
- SH2
Src homology 2
- SREBP
Sterol regulatory element binding protein
- SIRT1
Silent mating type information regulation 2 homolog 1
- SYK
Spleen tyrosine kinase
- TAK1
Transforming growth factor-β activated protein kinase-1
- TCA
Tricarboxylic acid
- TSC
Tuberous sclerosis complex
- TZDs
Thiazolidinediones
- TMPA
Ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl]acetate
- TRAIL
Tumor necrosis factor-related apoptotic-inducing ligand
- Tu-MP
tubercidin 5′-monophosphate
- ZMP
5-amino-4-imidazolecarboxamide ribotide
Biographies
Sandeep Rana is a Research Assistant Professor at the Eppley Institute for Cancer Research and Allied Diseases, University of Nebraska Medical Center. Prior to joining UNMC, he completed a two-year postdoctoral research position at Mayo Clinic, MN. He received his Master’s degree in chemistry from University of Delhi, India. After finishing his master’s, he was awarded a research fellowship from the Council of Scientific and Industrial research, New Delhi to pursue his graduate studies. He moved to the United States, attended Kansas State University and received his Ph.D. in organic chemistry under the guidance of Dr. Duy H Hua in 2009. His research interests lie in medicinal chemistry and drug discovery in the area of cancer and neurodegenerative diseases.
Elizabeth C. Blowers is a graduate student at the Eppley Institute for Cancer Research and Allied Diseases, University of Nebraska Medical Center. She completed her bachelor’s degree in biology at the University of Tennessee, where she studied in the laboratory of Dr. Erik Zinser. Her research at UNMC focuses on the characterization of small molecule inhibitors that target posttranslationally modified forms of proteins implicated in disease.
Amarnath Natarajan, Professor, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center. Dr. Natarajan hails from the south Indian state of Tamil Nadu. He completed his bachelors and masters in chemistry from Madras Christian College and Indian Institute of Technology, Chennai, respectively. He obtained his PhD in organic chemistry from the University of Vermont and conducted postdoctoral research at Harvard Medical School. He started his independent career as an Assistant Professor at the University of Texas Medical Branch. His lab is focused on developing chemical probes to dissect signaling pathways relevant to human diseases.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186:129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 3.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 4.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 5.Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187:183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 6.Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 7.Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol (1985) 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- 8.Inoue E, Yamauchi J. AMP-activated protein kinase regulates PEPCK gene expression by direct phosphorylation of a novel zinc finger transcription factor. Biochem Biophys Res Commun. 2006;351:793–799. doi: 10.1016/j.bbrc.2006.10.124. [DOI] [PubMed] [Google Scholar]
- 9.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 11.Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2005;289:E1071–E1076. doi: 10.1152/ajpendo.00606.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 16.Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 17.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 19.Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, Villen J, Wang B, Kim SR, Sakamoto K, Gygi SP, Cantley LC, Yaffe MB, Shokat KM, Brunet A. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–892. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Won JS, Im YB, Kim J, Singh AK, Singh I. Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochem Biophys Res Commun. 2010;399:487–491. doi: 10.1016/j.bbrc.2010.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 28.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 29.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 30.Voss M, Paterson J, Kelsall IR, Martin-Granados C, Hastie CJ, Peggie MW, Cohen PT. Ppm1E is an in cellulo AMP-activated protein kinase phosphatase. Cell Signal. 2011;23:114–124. doi: 10.1016/j.cellsig.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- 32.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci U S A. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 35.Kemp BE, Oakhill JS, Scott JW. AMPK structure and regulation from three angles. Structure. 2007;15:1161–1163. doi: 10.1016/j.str.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar EA, Chen Q, Kizhake S, Kolar C, Kang M, Chang CE, Borgstahl GE, Natarajan A. The paradox of conformational constraint in the design of Cbl(TKB)-binding peptides. Sci Rep. 2013;3:1639. doi: 10.1038/srep01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pessetto ZY, Yan Y, Bessho T, Natarajan A. Inhibition of BRCT(BRCA1)-phosphoprotein interaction enhances the cytotoxic effect of olaparib in breast cancer cells: a proof of concept study for synthetic lethal therapeutic option. Breast Cancer Res Treat. 2012;134:511–517. doi: 10.1007/s10549-012-2079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar EA, Yuan Z, Palermo NY, Dong L, Ahmad G, Lokesh GL, Kolar C, Kizhake S, Borgstahl GE, Band H, Natarajan A. Peptide truncation leads to a twist and an unusual increase in affinity for casitas B-lineage lymphoma tyrosine kinase binding domain. J Med Chem. 2012;55:3583–3587. doi: 10.1021/jm300078z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan Z, Kumar EA, Kizhake S, Natarajan A. Structure-activity relationship studies to probe the phosphoprotein binding site on the carboxy terminal domains of the breast cancer susceptibility gene 1. J Med Chem. 2011;54:4264–4268. doi: 10.1021/jm1016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Z, Kumar EA, Campbell SJ, Palermo NY, Kizhake S, Mark Glover JN, Natarajan A. Exploiting the P-1 pocket of BRCT domains toward a structure guided inhibitor design. ACS Med Chem Lett. 2011;2:764–767. doi: 10.1021/ml200147a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar EA, Charvet CD, Lokesh GL, Natarajan A. High-throughput fluorescence polarization assay to identify inhibitors of Cbl(TKB)-protein tyrosine kinase interactions. Anal Biochem. 2011;411:254–260. doi: 10.1016/j.ab.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anisimov VM, Ziemys A, Kizhake S, Yuan Z, Natarajan A, Cavasotto CN. Computational and experimental studies of the interaction between phospho-peptides and the C-terminal domain of BRCA1. J Comput Aided Mol Des. 2011;25:1071–1084. doi: 10.1007/s10822-011-9484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph PR, Yuan Z, Kumar EA, Lokesh GL, Kizhake S, Rajarathnam K, Natarajan A. Structural characterization of BRCT-tetrapeptide binding interactions. Biochem Biophys Res Commun. 2010;393:207–210. doi: 10.1016/j.bbrc.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simeonov A, Yasgar A, Jadhav A, Lokesh GL, Klumpp C, Michael S, Austin CP, Natarajan A, Inglese J. Dual-fluorophore quantitative high-throughput screen for inhibitors of BRCT-phosphoprotein interaction. Anal Biochem. 2008;375:60–70. doi: 10.1016/j.ab.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lokesh GL, Muralidhara BK, Negi SS, Natarajan A. Thermodynamics of phosphopeptide tethering to BRCT: the structural minima for inhibitor design. J Am Chem Soc. 2007;129:10658–10659. doi: 10.1021/ja0739178. [DOI] [PubMed] [Google Scholar]
- 48.Lokesh GL, Rachamallu A, Kumar GD, Natarajan A. High-throughput fluorescence polarization assay to identify small molecule inhibitors of BRCT domains of breast cancer gene 1. Anal Biochem. 2006;352:135–141. doi: 10.1016/j.ab.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 50.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 51.Grisouard J, Timper K, Radimerski TM, Frey DM, Peterli R, Kola B, Korbonits M, Herrmann P, Krahenbuhl S, Zulewski H, Keller U, Muller B, Christ-Crain M. Mechanisms of metformin action on glucose transport and metabolism in human adipocytes. Biochem Pharmacol. 2010;80:1736–1745. doi: 10.1016/j.bcp.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Ong KW, Hsu A, Tan BK. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 55.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012;443:193–203. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 56.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol. 2002;317:309–323. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- 57.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2005;289:E1071–1076. doi: 10.1152/ajpendo.00606.2004. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira SM, Zhang YH, Solis RS, Isackson H, Bellahcene M, Yavari A, Pinter K, Davies JK, Ge Y, Ashrafian H, Walker JW, Carling D, Watkins H, Casadei B, Redwood C. AMP-activated protein kinase phosphorylates cardiac troponin I and alters contractility of murine ventricular myocytes. Circ Res. 2012;110:1192–1201. doi: 10.1161/CIRCRESAHA.111.259952. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Lai YC, Hill EV, Tyteca D, Carpentier S, Ingvaldsen A, Vertommen D, Lantier L, Foretz M, Dequiedt F, Courtoy PJ, Erneux C, Viollet B, Shepherd PR, Tavare JM, Jensen J, Rider MH. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem J. 2013;455:195–206. doi: 10.1042/BJ20130644. [DOI] [PubMed] [Google Scholar]
- 61.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol. 2000;20:6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 63.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 64.Yuan L, Ziegler R, Hamann A. Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J (Engl) 2002;115:1843–1848. [PubMed] [Google Scholar]
- 65.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- 66.Shoelson SE, Chatterjee S, Chaudhuri M, White MF. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci U S A. 1992;89:2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yenush L, White MF. The IRS-signalling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 68.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012;55:783–794. doi: 10.1007/s00125-011-2407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F, Marshall CB, Ikura M. Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition. Cell Mol Life Sci. 2013;70:3989–4008. doi: 10.1007/s00018-012-1254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikonomov OC, Sbrissa D, Dondapati R, Shisheva A. ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp Cell Res. 2007;313:2404–2416. doi: 10.1016/j.yexcr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dossus L, Kaaks R. Nutrition, metabolic factors and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22:551–571. doi: 10.1016/j.beem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemminki A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell Mol Life Sci. 1999;55:735–750. doi: 10.1007/s000180050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 76.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell. 2003;12:271–280. doi: 10.1016/j.molcel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 79.Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1) Hum Mol Genet. 2002;11:1497–1504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- 80.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 81.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 85.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 87.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 88.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 90.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 91.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 92.Park HU, Suy S, Danner M, Dailey V, Zhang Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, Kumar D, Brown ML, Fornace A, Dritschilo A, Collins SP. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–741. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeon SM, Hay N. The dark face of AMPK as an essential tumor promoter. Cell Logist. 2012;2:197–202. doi: 10.4161/cl.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- 97.Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins Y, Sun TQ, Markovtsov V, Foretz M, Li W, Nguyen H, Li Y, Pan A, Uy G, Gross L, Baltgalvis K, Yung SL, Gururaja T, Kinoshita T, Owyang A, Smith IJ, McCaughey K, White K, Godinez G, Alcantara R, Choy C, Ren H, Basile R, Sweeny DJ, Xu X, Issakani SD, Carroll DC, Goff DA, Shaw SJ, Singh R, Boros LG, Laplante MA, Marcotte B, Kohen R, Viollet B, Marette A, Payan DG, Kinsella TM, Hitoshi Y. AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS One. 2013;8:e81870. doi: 10.1371/journal.pone.0081870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baltgalvis KA, White K, Li W, Claypool MD, Lang W, Alcantara R, Singh BK, Friera AM, McLaughlin J, Hansen D, McCaughey K, Nguyen H, Smith IJ, Godinez G, Shaw SJ, Goff D, Singh R, Markovtsov V, Sun TQ, Jenkins Y, Uy G, Li Y, Pan A, Gururaja T, Lau D, Park G, Hitoshi Y, Payan DG, Kinsella TM. Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice. Am J Physiol Heart Circ Physiol. 2014;306:H1128–1145. doi: 10.1152/ajpheart.00839.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 101.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 102.Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, De Schrijver E, Van de Sande T, Noel A, Foufelle F, Verhoeven G. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 103.Scaglia N, Tyekucheva S, Zadra G, Photopoulos C, Loda M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle. 2014;13 doi: 10.4161/cc.27767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zadra G, Photopoulos C, Tyekucheva S, Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska E, Mahmood U, Signoretti S, Birnberg N, Loda M. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol Med. 2014 doi: 10.1002/emmm.201302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle. 2009;8:3679–3683. doi: 10.4161/cc.8.22.9905. [DOI] [PubMed] [Google Scholar]
- 106.Gauthier MS, O’Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, Gokce N, Apovian C, Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 108.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 109.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 110.Gómez-Galeno Jorge E, QD, Nguyen Thanh H, Boyer Serge H, Grote Matthew P, Sun Zhili, Chen Mingwei, Craigo William A, van Poelje Paul D, MacKenna Deidre A, Cable Edward E, Rolzin Paul A, Finn Patricia D, Chi Bert, Linemeyer David L, Hecker Scott J, Erion Mark D. A Potent and Selective AMPK Activator That Inhibits de Novo Lipogenesis. ACS Med Chem Lett. 2010;1:478–482. doi: 10.1021/ml100143q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem Biophys Res Commun. 2002;294:798–805. doi: 10.1016/S0006-291X(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 112.Crowther RA. Structural aspects of pathology in Alzheimer’s disease. Biochim Biophys Acta. 1990;1096:1–9. doi: 10.1016/0925-4439(90)90004-9. [DOI] [PubMed] [Google Scholar]
- 113.Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide--the first step of a fatal cascade. J Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 114.Erol A. An integrated and unifying hypothesis for the metabolic basis of sporadic Alzheimer’s disease. J Alzheimers Dis. 2008;13:241–253. doi: 10.3233/jad-2008-13302. [DOI] [PubMed] [Google Scholar]
- 115.Poels J, Spasic MR, Callaerts P, Norga KK. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009;31:944–952. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- 116.Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2011;121:337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. Ab: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 121.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 122.Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997;409:57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- 123.Sengupta A, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by protein kinases in rat brain. Neurochem Res. 2006;31:1473–1480. doi: 10.1007/s11064-006-9205-9. [DOI] [PubMed] [Google Scholar]
- 124.Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000;62:463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 125.Scales TM, Derkinderen P, Leung KY, Byers HL, Ward MA, Price C, Bird IN, Perera T, Kellie S, Williamson R, Anderton BH, Reynolds CH. Tyrosine phosphorylation of tau by the SRC family kinases lck and fyn. Mol Neurodegener. 2011;6:12. doi: 10.1186/1750-1326-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J. 2011;434:503–512. doi: 10.1042/BJ20101485. [DOI] [PubMed] [Google Scholar]
- 127.Park H, Kam TI, Kim Y, Choi H, Gwon Y, Kim C, Koh JY, Jung YK. Neuropathogenic role of adenylate kinase-1 in Abeta-mediated tau phosphorylation via AMPK and GSK3beta. Hum Mol Genet. 2012;21:2725–2737. doi: 10.1093/hmg/dds100. [DOI] [PubMed] [Google Scholar]
- 128.Yu JT, Chang RC, Tan L. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol. 2009;89:240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 129.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009;455:191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim J, Park YJ, Jang Y, Kwon YH. AMPK activation inhibits apoptosis and tau hyperphosphorylation mediated by palmitate in SH-SY5Y cells. Brain Res. 2011;1418:42–51. doi: 10.1016/j.brainres.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 132.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li J, Benashski S, McCullough LD. Post-stroke hypothermia provides neuroprotection through inhibition of AMP-activated protein kinase. J Neurotrauma. 2011;28:1281–1218. doi: 10.1089/neu.2011.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, Sun CP, Tao MH, Tu PH, Chang C, Dickson DW, Chern Y. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J Cell Biol. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goldthwait DA, Greenberg GR, Peabody RA. The structure of glycinamide ribotide. J Biol Chem. 1956;221:1071–1081. [PubMed] [Google Scholar]
- 138.Goldthwait DA, Greenberg GR, Peabody RA. On the mechanism of synthesis of glycinamide ribotide and its formyl derivative. J Biol Chem. 1956;221:569–577. [PubMed] [Google Scholar]
- 139.Goldthwait DA, Greenberg GR, Peabody RA. On the occurrence of glycinamide ribotide and its formyl derivative. J Biol Chem. 1956;221:555–567. [PubMed] [Google Scholar]
- 140.Sabina RL, Patterson D, Holmes EW. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J Biol Chem. 1985;260:6107–6714. [PubMed] [Google Scholar]
- 141.Schnebli HP, Hill DL, Bennett LL., Jr Purification and properties of adenosine kinase from human tumor cells of type H. Ep. No. 2. J Biol Chem. 1967;242:1997–2004. [PubMed] [Google Scholar]
- 142.Sabina RL, Kernstine KH, Boyd RL, Holmes EW, Swain JL. Metabolism of 5-amino-4-imidazolecarboxamide riboside in cardiac and skeletal muscle. Effects on purine nucleotide synthesis. J Biol Chem. 1982;257:10178–10183. [PubMed] [Google Scholar]
- 143.Swain JL, Hines JJ, Sabina RL, Harbury OL, Holmes EW. Disruption of the purine nucleotide cycle by inhibition of adenylosuccinate lyase produces skeletal muscle dysfunction. J Clin Invest. 1984;74:1422–1427. doi: 10.1172/JCI111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sullivan JE, Carey F, Carling D, Beri RK. Characterisation of 5′-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5′-AMP analogues on enzyme activity. Biochem Biophys Res Commun. 1994;200:1551–1556. doi: 10.1006/bbrc.1994.1627. [DOI] [PubMed] [Google Scholar]
- 145.Day P, Sharff A, Parra L, Cleasby A, Williams M, Horer S, Nar H, Redemann N, Tickle I, Yon J. Structure of a CBS-domain pair from the regulatory gamma1 subunit of human AMPK in complex with AMP and ZMP. Acta Crystallogr D Biol Crystallogr. 2007;63:587–596. doi: 10.1107/S0907444907009110. [DOI] [PubMed] [Google Scholar]
- 146.Henin N, Vincent MF, Van den Berghe G. Stimulation of rat liver AMPactivated protein kinase by AMP analogues. Biochim Biophys Acta. 1996;1290:197–203. doi: 10.1016/0304-4165(96)00021-9. [DOI] [PubMed] [Google Scholar]
- 147.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 148.Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 150.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- 151.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 152.Vincent MF, Erion MD, Gruber HE, Van den Berghe G. Hypoglycaemic effect of AICAriboside in mice. Diabetologia. 1996;39:1148–1155. doi: 10.1007/BF02658500. [DOI] [PubMed] [Google Scholar]
- 153.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 154.Sylow L, Jensen TE, Kleinert M, Hojlund K, Kiens B, Wojtaszewski J, Prats C, Schjerling P, Richter EA. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes. 2013;62:1865–1875. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 156.Bergeron R, Russell RR, 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276:E938–944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 157.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 158.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 159.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 160.Nakamura JL, Garcia E, Pieper RO. S6K1 plays a key role in glial transformation. Cancer Res. 2008;68:6516–6523. doi: 10.1158/0008-5472.CAN-07-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 162.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, Radu CG, Cloughesy TF, Mischel PS. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A. 2009;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 165.Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF. 5-Aminoimidazole-4-carboxamide 1-beta -D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003;284:R936–944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- 166.Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- 167.Young ME, Radda GK, Leighton B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR--an activator of AMP-activated protein kinase. FEBS Lett. 1996;382:43–47. doi: 10.1016/0014-5793(96)00129-9. [DOI] [PubMed] [Google Scholar]
- 168.Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ, Koay A, Gooley PR, Stapleton D, Kemp BE. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 169.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662; a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 170.GMBH MP. WO2007019914 Use of thienopyridone derivatives as AMPK activators and pharmaceutical compositions containing them. 2007
- 171.GMBH MP. WO2009124636. Thienopyridone derivatives as AMP-activated protein kinase (AMPK) activators and their preparation, pharmaceutical compositions, and use in the treatment of diseases. 2009
- 172.GMBH MP. WO2009135580 Preparation of thieno[2,3-b]pyridin-6-one derivatives as AMP-activated protein kinase (AMPK) activators. 2009
- 173.GMBH MP. WO2008006432 Imidazole derivatives as AMPK activators, their preparation, pharmaceutical compositions, and use in therapy. 2008
- 174.Mercury Therapeutics, I. WO2009100130 AMP kinase modulators. 2009
- 175.Olivier Mirguet SS, Clément Catherine-Anne, Toum Jérôme, Donche Frédéric, Marques Celine, Rondet Emilie, Pizzonero Mathieu, Beaufils Benjamin, Dudit Yann, Huet Pascal, Trottet Lionel, Grondin Pascal, Brusq Jean-Marie, Boursier Eric, Saintillan Yannick, Edwige Nicodeme. Discovery of Pyridones As Oral AMPK Direct Activators. ACS Med Chem Lett. 2013;4:632–636. doi: 10.1021/ml400157g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Merck Sharp & Dohme Corp., M. T., Inc. WO2010036613. Novel cyclic benzimidazole derivatives useful anti-diabetic agents. 2010
- 177.Merck Sharp & Dohme Corp., M. T., Inc. WO2010047982. Novel cyclic benzimidazole derivatives useful anti-diabetic agents. 2010
- 178.Merck Sharp & Dohme Corp., M. T., Inc. WO2010051176. Novel cyclic benzimidazole derivatives useful anti-diabetic agents. 2010
- 179.Merck Sharp & Dohme Corp., M. T., Inc. WO2010051206 Novel cyclic benzimidazole derivatives as AMP-activated protein kinase activators and anti-diabetic agents and their preparation. 2010
- 180.Corp., M. S. D. WO2011106273. Novel cyclic benzimidazole derivatives useful anti-diabetic agents. 2011
- 181.Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, Li J. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li-Fang Yu YYL, Su Ming-Bo, Zhang Mei, Zhang Wei, Zhang Li-Na, Pang Tao, Zhang Run-Tao, Liu Bing, Li Jing-Ya, Li Jia, Nan Fa-Jun. Development of Novel Alkene Oxindole Derivatives As Orally Efficacious AMP-Activated Protein Kinase Activators. ACS Med Chem Lett. 2013;4:475–480. doi: 10.1021/ml400028q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li YY, Yu LF, Zhang LN, Qiu BY, Su MB, Wu F, Chen DK, Pang T, Gu M, Zhang W, Ma WP, Jiang HW, Li JY, Nan FJ, Li J. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol Appl Pharmacol. 2013;273:325–334. doi: 10.1016/j.taap.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 184.AGFH-LR WO2011032320. Preparation of alkene oxindole derivatives as activators of AMP activated protein kinase. 2011
- 185.AGFH-LR WO2011033099 Preparation of alkene oxindole derivatives as activators of AMP activated protein kinase. 2011
- 186.AGFH-LR WO2011069298 Spiro-cyclopropane-indolinone derivatives as AMPK modulators and their preparation, pharmaceutical compositions and use in the treatment of diseases. 2011
- 187.AGFH-LR WO2011070039 Sprio indole--cyclopropane indolinones useful as AMPK modulators. 2011
- 188.Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- 189.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. e1503. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Choi J, He N, Sung MK, Yang Y, Yoon S. Sanguinarine is an allosteric activator of AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;413:259–263. doi: 10.1016/j.bbrc.2011.08.081. [DOI] [PubMed] [Google Scholar]
- 192.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 193.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 194.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- 196.Galuska D, Nolte LA, Zierath JR, Wallberg-Henriksson H. Effect of metformin on insulin-stimulated glucose transport in isolated skeletal muscle obtained from patients with NIDDM. Diabetologia. 1994;37:826–832. doi: 10.1007/BF00404340. [DOI] [PubMed] [Google Scholar]
- 197.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 198.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jin HE, Hong SS, Choi MK, Maeng HJ, Kim DD, Chung SJ, Shim CK. Reduced antidiabetic effect of metformin and down-regulation of hepatic Oct1 in rats with ethynylestradiol-induced cholestasis. Pharm Res. 2009;26:549–559. doi: 10.1007/s11095-008-9770-5. [DOI] [PubMed] [Google Scholar]
- 200.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J. 2011;25:219–231. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 204.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 205.Gu XS, Wang ZB, Ye Z, Lei JP, Li L, Su DF, Zheng X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet Mol Res. 2014;13:323–335. doi: 10.4238/2014.January.17.17. [DOI] [PubMed] [Google Scholar]
- 206.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720; SRT2183; SRT1460; and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 208.Desquiret-Dumas V, Gueguen N, Leman G, Baron S, Nivet-Antoine V, Chupin S, Chevrollier A, Vessieres E, Ayer A, Ferre M, Bonneau D, Henrion D, Reynier P, Procaccio V. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem. 2013;288:36662–36675. doi: 10.1074/jbc.M113.466490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem J. 2005;386:471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–549. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 211.Lee SM, Moon J, Cho Y, Chung JH, Shin MJ. Quercetin up-regulates expressions of peroxisome proliferator-activated receptor gamma, liver X receptor alpha, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr Res. 2013;33:136–143. doi: 10.1016/j.nutres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 212.Guo H, Zhao H, Kanno Y, Li W, Mu Y, Kuang X, Inouye Y, Koike K, Jiang H, Bai H. A dihydrochalcone and several homoisoflavonoids from Polygonatum odoratum are activators of adenosine monophosphate-activated protein kinase. Bioorg Med Chem Lett. 2013;23:3137–3139. doi: 10.1016/j.bmcl.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 213.Deng Y, He K, Ye X, Chen X, Huang J, Li X, Yuan L, Jin Y, Jin Q, Li P. Saponin rich fractions from Polygonatum odoratum (Mill.) Druce with more potential hypoglycemic effects. J Ethnopharmacol. 2012;141:228–233. doi: 10.1016/j.jep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 214.Choi SB, Park S. A steroidal glycoside from Polygonatum odoratum (Mill.) Druce improves insulin resistance but does not alter insulin secretion in 90% pancreatectomized rats. Biosci Biotechnol Biochem. 2002;66:2036–2043. doi: 10.1271/bbb.66.2036. [DOI] [PubMed] [Google Scholar]
- 215.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 216.Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 217.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2564. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 218.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 219.Mikes V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. J Bioenerg Biomembr. 1985;17:23–32. doi: 10.1007/BF00744986. [DOI] [PubMed] [Google Scholar]
- 220.Ni YX. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Zhong Xi Yi Jie He Za Zhi. 1988;8:711–713. 707. [PubMed] [Google Scholar]
- 221.Sack RB, Froehlich JL. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun. 1982;35:471–475. doi: 10.1128/iai.35.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 224.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 226.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 227.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 228.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 230.Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 231.Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, Dugail I, Morin J, Auwerx J, Ferre P. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–1399. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 232.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferatoractivated receptor gamma and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213–6218. [PubMed] [Google Scholar]
- 233.Fan YH, Chen H, Natarajan A, Guo Y, Harbinski F, Iyasere J, Christ W, Aktas H, Halperin JA. Structure-activity requirements for the antiproliferative effect of troglitazone derivatives mediated by depletion of intracellular calcium. Bioorg Med Chem Lett. 2004;14:2547–2550. doi: 10.1016/j.bmcl.2004.02.087. [DOI] [PubMed] [Google Scholar]
- 234.Chen H, Fan YH, Natarajan A, Guo Y, Iyasere J, Harbinski F, Luus L, Christ W, Aktas H, Halperin JA. Synthesis and biological evaluation of thiazolidine-2,4-dione and 2,4-thione derivatives as inhibitors of translation initiation. Bioorg Med Chem Lett. 2004;14:5401–5405. doi: 10.1016/j.bmcl.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 235.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 236.Balfour JA, Plosker GL. Rosiglitazone. Drugs. 1999;57:921–930. doi: 10.2165/00003495-199957060-00007. discussion 931–932. [DOI] [PubMed] [Google Scholar]
- 237.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 238.Guh JH, Chang WL, Yang J, Lee SL, Wei S, Wang D, Kulp SK, Chen CS. Development of novel adenosine monophosphate-activated protein kinase activators. J Med Chem. 2010;53:2552–2561. doi: 10.1021/jm901773d. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 239.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 240.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002;8:2085–2090. [PubMed] [Google Scholar]
- 241.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 242.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 243.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 244.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 246.Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- 247.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 248.Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–4210. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 249.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pan W, Yang H, Cao C, Song X, Wallin B, Kivlin R, Lu S, Hu G, Di W, Wan Y. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep. 2008;20:1553–1559. [PubMed] [Google Scholar]
- 251.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 252.Hung CM, Su YH, Lin HY, Lin JN, Liu LC, Ho CT, Way TD. Demethoxycurcumin Modulates Prostate Cancer Cell Proliferation via AMPK-Induced Down-regulation of HSP70 and EGFR. J Agric Food Chem. 2012;60:8427–8434. doi: 10.1021/jf302754w. [DOI] [PubMed] [Google Scholar]
- 253.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 254.Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–C1403. doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- 255.Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem Biophys Res Commun. 2006;344:446–452. doi: 10.1016/j.bbrc.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 256.Targonsky ED, Dai F, Koshkin V, Karaman GT, Gyulkhandanyan AV, Zhang Y, Chan CB, Wheeler MB. alpha-lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia. 2006;49:1587–1598. doi: 10.1007/s00125-006-0265-9. [DOI] [PubMed] [Google Scholar]
- 257.Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, Kim JB, Choi HS, Park JY, Lee IK, Lee KU. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48:1477–1486. doi: 10.1002/hep.22496. [DOI] [PubMed] [Google Scholar]
- 258.Tang X, Zhuang J, Chen J, Yu L, Hu L, Jiang H, Shen X. Arctigenin efficiently enhanced sedentary mice treadmill endurance. PLoS One. 2011;6:e24224. doi: 10.1371/journal.pone.0024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shen S, Zhuang J, Chen Y, Lei M, Chen J, Shen X, Hu L. Synthesis and biological evaluation of arctigenin ester and ether derivatives as activators of AMPK. Bioorg Med Chem. 2013;21:3882–3893. doi: 10.1016/j.bmc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 260.Way TD, Lee JC, Kuo DH, Fan LL, Huang CH, Lin HY, Shieh PC, Kuo PT, Liao CF, Liu H, Kao JY. Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. J Agric Food Chem. 2010;58:3356–3365. doi: 10.1021/jf903793p. [DOI] [PubMed] [Google Scholar]
- 261.Yang JM, Hung CM, Fu CN, Lee JC, Huang CH, Yang MH, Lin CL, Kao JY, Way TD. Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. J Agric Food Chem. 2010;58:10020–10026. doi: 10.1021/jf102304g. [DOI] [PubMed] [Google Scholar]
- 262.Sviripa V, Zhang W, Conroy MD, Schmidt ES, Liu AX, Truong J, Liu C, Watt DS. Fluorinated N,N′-diarylureas as AMPK activators. Bioorg Med Chem Lett. 2013;23:1600–1603. doi: 10.1016/j.bmcl.2013.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sartore-Bianchi A, Zeppellini A, Amatu A, Ricotta R, Bencardino K, Siena S. Regorafenib in metastatic colorectal cancer. Expert Rev Anticancer Ther. 2014;14:255–265. doi: 10.1586/14737140.2014.894887. [DOI] [PubMed] [Google Scholar]
- 264.Procopio G, Derosa L, Gernone A, Morelli F, Sava T, Zustovich F, De Giorgi U, Ferrari V, Sabbatini R, Gasparro D, Felici A, Burattini L, Calvani N, Lo Re G, Banna G, Brizzi MP, Rizzo M, Ciuffreda L, Iacovelli R, Ferrau F, Taibi E, Bracarda S, Porta C, Galligioni E, Contu A. Sorafenib as first- or second-line therapy in patients with metastatic renal cell carcinoma in a community setting. Future Oncol. 2014 doi: 10.2217/fon.14.48. [DOI] [PubMed] [Google Scholar]
- 265.Kim D, Lee MS, Jo K, Lee KE, Hwang JK. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARalpha/delta for the treatment of obesity. Diabetes Obes Metab. 2011;13:584–593. doi: 10.1111/j.1463-1326.2011.01379.x. [DOI] [PubMed] [Google Scholar]
- 266.Oh S, Kim SJ, Hwang JH, Lee HY, Ryu MJ, Park J, Jo YS, Kim YK, Lee CH, Kweon KR, Shong M, Park SB. Antidiabetic and antiobesity effects of Ampkinone (6f), a novel small molecule activator of AMP-activated protein kinase. J Med Chem. 2010;53:7405–7413. doi: 10.1021/jm100565d. [DOI] [PubMed] [Google Scholar]
- 267.Tan MJ, Ye JM, Turner N, Hohnen-Behrens C, Ke CQ, Tang CP, Chen T, Weiss HC, Gesing ER, Rowland A, James DE, Ye Y. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol. 2008;15:263–273. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 268.Chen XB, Zhuang JJ, Liu JH, Lei M, Ma L, Chen J, Shen X, Hu LH. Potential AMPK activators of cucurbitane triterpenoids from Siraitia grosvenorii Swingle. Bioorg Med Chem. 2011;19:5776–5781. doi: 10.1016/j.bmc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 269.Meltzer-Mats E, Babai-Shani G, Pasternak L, Uritsky N, Getter T, Viskind O, Eckel J, Cerasi E, Senderowitz H, Sasson S, Gruzman A. Synthesis and Mechanism of Hypoglycemic Activity of Benzothiazole Derivatives. J Med Chem. 2013;56:5335–5350. doi: 10.1021/jm4001488. [DOI] [PubMed] [Google Scholar]
- 270.Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970;17:23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 271.Charton J, Girault-Mizzi S, Debreu-Fontaine MA, Foufelle F, Hainault I, Bizot-Espiard JG, Caignard DH, Sergheraert C. Synthesis and biological evaluation of benzimidazole derivatives as potent AMP-activated protein kinase activators. Bioorg Med Chem. 2006;14:4490–4518. doi: 10.1016/j.bmc.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 272.Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, Cramer CT, Spahr MA, Brant AF, Houghton JL, Baker C, Naples M, Adeli K, Newton RS. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002; a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54:134–151. doi: 10.1194/jlr.M030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ballantyne CM, Davidson M, MacDougall D, Margulies J, DiCarlo L. ETC-1002 lowers LDL-C and beneficially modulates other cardio-metabolic risk factors in hypercholesterolemic subjects with either normal or elevated triglycerides. J Am Coll Cardiol. 2012;59:E1625. [Google Scholar]
- 274.Chene P. Inhibition of the p53-MDM2 interaction: targeting a protein-protein interface. Molecular cancer research: MCR. 2004;2:20–28. [PubMed] [Google Scholar]