Abstract
Morphological identification and molecular study on the COI gene were simultaneously conducted on Anagrus Haliday ‘atomus’ group individuals collected in the field in Italy or supplied from a UK biofactory. Females were morphologically identified as A. atomus L. and A. parvus Soyka sensu Viggiani (=A. ustulatus sensu Chiappini). Alignment of COI gene sequences from this study permitted recognition of a total of 34 haplotypes. Phylogenetic and network analyses of molecular data not only confirmed that A. atomus is a species distinct from A. parvus, but also suggested that two species may be included within morphologically identified A. parvus. Different geographical distribution and frequency of haplotypes were also evidenced. For males considered in this study, morphometric analyses revealed a character that could be useful to discriminate A. atomus from A. parvus. Both species were found in vineyards and surrounding vegetation, confirming the potential role of spontaneous vegetation as a source of parasitoids for leafhopper control in vineyards.
Keywords: mymaridae, identification, COI gene, molecular analysis, morphometry
Anagrus Haliday (Hymenoptera: Mymaridae) includes extremely small egg parasitoids mostly associated with leafhoppers (Hemiptera: Cicadellidae) (Huber 1986; Wallof and Jervis 1987; Arnò et al. 1988; Matteucig and Viggiani 2008; Triapitsyn et al. 2010). Within the genus, two species groups (i.e., ‘incarnatus’ and ‘atomus’) have been recognized morphologically (Chiappini et al. 1996). Until a short time ago, inside the ‘atomus’ group only Anagrus atomus (L.) and A. parvus Soyka sensu Viggiani (2014) (=A. ustulatus sensu Chiappini 1989) (hereafter A. parvus) had been described for Italy (Chiappini et al. 1996; Floreani et al. 2006; Viggiani et al. 2006), but recently also Anagrus lindberginae Nugnes and Viggiani was described in Nugnes and Viggiani (2014). The latter species is exclusively associated with Quercus ilex (L.) and eggs of the leafhopper Lindbergina aurovittata (Douglas), whereas A. atomus and A. parvus emerge from other plant species (e.g., grapevines and Rubus L. spp.). Females of these latter species were distinguished on the basis of a morphological character (Chiappini et al. 1996) which, in most cases, is combined with a morphometric one (Chiappini 1987; Floreani et al. 2006). However, morphological and morphometric identifications of mymarid species can fail because discriminant characters can be intermediate in some individuals (Chiappini et al. 1999) or be influenced both by the host parasitized (Huber and Rajakulendran 1988) and by the host plant from which individuals emerge (Nugnes and Viggiani 2014). In general, identification at species level of individuals belonging to Anagrus is often difficult due to the paucity of diagnostic characters and morphological variability within species (Triapitsyn et al. 2010). In the case of A. atomus and A. parvus males, are morphologically indistinguishable (Viggiani 1970, 1989; Chiappini and Mazzoni 2000). Regarding A. ustulatus there is also a misidentification problem, since Viggiani (2014) recently proposed to refer A. ustulatus sensu Chiappini et al. (1996) to A. parvus Soyka (Soyka 1955).
Sibling species are particularly frequent in extremely small Hymenoptera parasitoids (Masner 1975; Stouthamer et al. 1999; Borghuis et al. 2004). For example, Anagrus epos Girault has been sub-divided into several different species, grouped in the A. epos species complex (Triapitsyn 1998; Triapitsyn et al. 2010). In biological control, the characterization of natural enemies is essential because cryptic species may have different biological performances leading to a variable ability to control a specific pest (DeBach and Rosen 1991; Nugnes and Viggiani 2014). For example, both A. atomus and A. parvus can emerge from grapevine and bramble leaves (Chiappini 1987; Floreani et al. 2006; Viggiani et al. 2006), but in Northern Italy on grapevines only A. atomus was associated with the eggs of Empoasca vitis (Göthe) (Chiappini 1987; Zanolli and Pavan 2011, 2013), whereas A. parvus emerged only from the eggs of Zygina rhamni Ferrari (Zanolli and Pavan 2011). However, the number of potential hosts for Anagrus spp. in Europe is large (Huber 1986; Arnò et al. 1988; Matteucig and Viggiani 2008). In particular, on Rubus spp., considered an important source of Anagrus spp. for biological control of leafhoppers in vineyards (Arnò et al. 1988; Cerutti et al. 1991; Ponti et al. 2005; Matteucig and Viggiani 2008; Zanolli and Pavan 2011), the leafhoppers Zygina Fieber spp., Ribautiana (Zachvatkin) spp., Edwadrsiana rosae (L.), and Arboridia parvula (Boheman) were found to lay eggs.
Therefore, species limits based only on morphology and morphometry may be difficult to assess without supporting data from their biology, and from molecular and biochemical analyses. When two sibling species are identified with molecular analysis, the molecular results have a key role in making correct identifications, together with species identification based on morphological characters that may be of variable taxonomic value (Triapitsyn et al. 2010). In order to discriminate A. atomus and A. parvus, several enzymes were tested, but loci with alternative fixed alleles have not yet been detected (Cargnus and Pavan 2007). Taxonomic study of the two Anagrus species on the basis of cuticular hydrocarbons showed two distinct profiles that were largely, but not always, consistent with classification based on morphology (Floreani et al. 2006). Among the alternatives to morphological taxonomy, barcoding can help identify the amount of biodiversity (Hebert et al. 2003) and how to differentiate the species found (Kruse and Sperling 2002; Lin and Wood 2002, MacDonald and Loxdale 2004). As mitochondrial genes are weakly subject to recombination and are inherited maternally with independent replication (Saccone et al. 1999), primers for conserved regions of mitochondrial DNA (mtDNA) are valuable tools for molecular systematic studies (Folmer et al. 1994; Simon et al. 1994). For Chalcidoidea, the COI gene has been successfully used at the species level to distinguish closely related species because of its rapid rate of evolution (Barari et al. 2005; Monti et al. 2005; Bernardo et al. 2008). Chiappini et al. (1999) distinguished four species of Anagrus ‘incarnatus’ group using the Random amplified polymorphic DNA-Polymerase chain reaction (RAPD-PCR) technique. Inside Anagrus ‘atomus’ group, molecular analysis discriminated A. parvus from the North American A. erythroneurae Trjapitzin and Chiappini, which are not distinguishable based on morphological characters, but it does not discriminate A. parvus from A. atomus (de León et al. 2008), which can be separated morphologically (Chiappini et al. 1996).
The aims of this research were: 1) to study the phylogenetic relationships among A. atomus and A. parvus populations, on the basis of COI gene sequences; 2) to compare molecular results with discriminant morphological and morphometric characters, particularly in male individuals, that are not currently distinguishable morphologically.
Materials and Methods
Insect Collection
In total, 122 adult wasps, 101 females and 21 males, belonging to the Anagrus ‘atomus’ group were used for molecular study (Table 1). Most of them were also submitted to morphological and morphometric analyses. In total, 112 out of 122 individuals emerged in the laboratory from leaves of different woody plants collected in 12 open field Italian sites. The remaining 10 individuals were A. atomus that emerged in the laboratory from leaf portions of Primula L. sp. containing parasitized eggs of the leafhopper Hauptidia maroccana (Melichar), supplied by Biowise (Petworth, West Sussex, UK). Another 10 males that emerged from grapevine leaves collected in Friuli Venezia Giulia (FVG) were used exclusively for morphometric analysis. These individuals were identified as A. atomus on the basis of cuticular hydrocarbons (Floreani et al. 2006) or because they emerged from E. vitis eggs, known to be parasitized only by this species (Zanolli and Pavan 2013). All individuals were frozen as soon as they emerged and stored at −80°C until used. Once removed from the freezer, the parasitoids were soaked in ethanol at 95°C. Under a dissecting microscope, the head of females and the genitalia of males were dissected with fine pins from the rest of the body. All instruments used for dissection were disinfected in alcohol and flamed before processing each individual. Female head and male genitalia were mounted on slides in Berlese’s medium and used for morphological and morphometric analyses. The rest of the body was processed for DNA extraction.
Table 1.
Individuals submitted to molecular, morphological and morphometric analyses differentiated for site, plant, and sex (M, male; F, female)
| Collection sites |
Host plant/leafhopper species | Sex | |||
|---|---|---|---|---|---|
| Region (State) | Locality | Coordinates | Acronym | ||
| West Sussex (UK) | Petworth (biofactory) | 50°56′N, 0°40′E | UK | Primula L. sp./H maroccana | 2F, 8M |
| FVG (IT) | Buttrio | 46°00′N, 13°21′E | FVG 1 | Rubus L. spp. | 11F |
| Vitis vinifera L. | 1F | ||||
| 46°00′N, 13°22′E | FVG 2 | Rosa canina L. | 4F | ||
| Rubus spp. | 4F | ||||
| V. vinifera | 1F | ||||
| 46°00′N, 13°22′E | FVG 3 | Rubus spp. | 3F | ||
| 46°29′N, 13°25′E | FVG 4 | R. canina | 8F | ||
| Rubus spp. | 22F, 4M | ||||
| V. vinifera | 3F | ||||
| 46°00′N, 13°25′E | FVG 5 | Rubus spp. | 5F | ||
| 45°59′N, 13°25′E | FVG 6 | Rubus spp. | 5F, 1M | ||
| Reana del Roiale | 46°08′N, 13°15′E | FVG 7 | V. vinifera | 5 M (*) | |
| Martignacco | 46°11′N, 13°17′E | FVG 8 | V. vinifera/E. vitis | 5 M (**) | |
| Lombardy (IT) | Morsone | 45°33′N, 10°28′E | LOM | V. vinifera | 4F |
| Tuscany (IT) | Greve in Chianti | 43°33′N, 11°18′E | TOS 1 | Rubus spp. | 10F, 1M |
| 43°34′N, 11°16′E | TOS 2 | Rubus spp. | 5F, 4M | ||
| 43°33′N, 11°18′E | TOS 3 | Rubus spp. | 8F, 2M | ||
| Umbria (IT) | Gualdo Tadino | 42°53′N, 12°34′E | UMB 1 | Rubus spp. | 2F, 1M |
| 42°48′N, 12°29′E | UMB 2 | Rubus spp. | 3F | ||
aIndividuals submitted to cuticular-hydrocarbon identification sensu Floreani et al. (2006).
bIndividuals emerged from E. vitis eggs from which only A. atomus was observed to emerge in north-eastern Italy.
Morphological and Morphometric Analyses
To establish with certainty that Anagrus females belonged to the ‘atomus’ group the presence of three multiporous plate sensilla (mps) (= sensory ridges, of authors) on the antennal club was checked (Chiappini et al. 1996). Females were also identified to species by the presence (A. atomus) or absence (A. parvus) of one mps on F4 (Chiappini et al. 1996). Anagrus ‘atomus’ group males were separated from ‘incarnatus’ group males according to Chiappini and Mazzoni (2000).
For individuals belonging to the ‘atomus’ group morphometric analyses were also conducted. For females, the length of club as well as length of funicle segments F3 and F4 was measured and the ratio between antennal club length and the combined length of F3 and F4 was calculated (Chiappini 1987; Floreani et al. 2006). For males, lengths of the entire genitalia (Fig. 1a), phallobase (Fig. 1b) and digitus (Fig. 1c) were measured (Gibson 1987; Chiappini and Mazzoni 2000; Floreani et al. 2006; Nugnes and Viggiani 2014).
Fig. 1.
Male genitalia of Anagrus ‘atomus’ group. a, genitalia length; b, phallobase length; c, digitus length.
Because of damage or loss of antennal segments during dissection only 91 out of 101 females were submitted to morphological and morphometric analyses in addition to molecular analysis. Only six out of eight UK males were submitted to morphometric measurements because the whole body of two individuals was processed for DNA extraction.
Data on measurements on female and male body-parts were compared with a t-test (two groups in comparison) or ANOVA and Tukey’s post-test (more than two groups in comparison). The statistical analysis was performed with GraphPad Instat 3.1a for Macintosh.
DNA Extraction
DNA extraction of 122 individuals was performed according to the salting out protocol (Patwary et al., 1994) from each individual adult wasp in 20 μl of lysis buffer (0.05 M Tris-HCl, 0.1 M EDTA). To avoid cross contamination among samples, one sterile plastic pestel for each insect was used. Each sample was crushed and then incubated with 17.5 μl of SDS solution 10% and 2 μl of proteinase-K (20 mg/μl) at 55°C overnight. The solution was treated with 2 μl of RNAase at 37°C for 5–10 min. Proteins were then precipitated out by adding 40 μl of NaCl saturated solution, hard shaking for 20 min and centrifuging for 30 min at 12,000 g, at 4°C. The DNA was precipitated with ice-cold isopropanol and washed with 70% ice-cold ethanol, then dried under vacuum and re-suspended in 20 μl TE (10 mM Tris pH 8.0, 0.1 mM EDTA). The extracted DNA was stored in two equal parts, one placed at −20°C and the other at −80° C until further use.
PCR Amplification, Sequencing, Phylogenetic, and Network Analyses
A fragment of about 650 base pairs (bps) of the barcoding region of the mtCOI gene was amplified using the primers forward (HCO-1490) 5′-GGTCAACAAATCATAAAGATATTGG-3′ and reverse (LCO-2198) 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ (Folmer et al. 1994). DNA amplifications (PCRs) were performed using 25 μl of total reaction volume containing: 1× PCR buffer, 1.5 mM MgCl2, 200 μM dNTPs, 1.25 U Go Taq Flexi DNA polymerase (Promega, Madison, WI), 0.4 μM of each primer and 1 μl template. PCR cycles were carried out in MJ Mini (Bio-Rad, Hercules, CA) thermalcycler using the following conditions: initial denaturation at 94°C for 2 min, 40 cycles consisting of initial denaturation at 94°C for 1 min, annealing at 49°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 5 min.
An aliquote (5 μl) of each PCR product was run on 1% (w/v) agarose gel (Conda, Madrid, Spain) in 1X TAE buffer at 100 V in horizontal electrophoresis cell Mini-Wide SubCell GT (Bio-Rad, Hercules, CA) with Gene Ruler 1 Kb DNA Ladder (Fermentas, Vilnius, Lithuania) and stained with the dye GelRed Nucleic Acid Gel Stain (10,000×, Biotium, Hayward, CA).
The purification was performed using the commercial kit Wizard SV Gel and PCR Clean-up System (Promega, Madison, WI), following the manufacturer’s instructions.
The concentration of the purified products was determined using the NanoDrop 1,000 Spectrophotometer (Thermo Scientific, Wilmington, DE). Products were sequenced with a BigDye Terminator v3.1 Cycle Sequencing Kit and POP-7 Polymer (Applied Biosystems, Foster City, CA) on an AB 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA) (IGA laboratory, Udine, Italy). Sequences were trimmed to the final length of 445 bp. All sequences were verified by NCBI Basic Local Alignment Search Tool (BLAST) and Barcode of Life Database species identification tools. All the identified haplotype sequences were also submitted to GenBank. Based on results of the BLAST search in GenBank database 10 sequences of A. parvus, 5 of A. atomus, and 5 of A. erythroneurae were found and successively used as reference taxa for the sequence and phylogenetic analyses. The mtCOI gene sequences of the 122 individuals processed in this study and those obtained from GenBank were aligned using BioEdit program (Hall 1999). The alignment permitted identification of the different haplotypes. The genetic distances between and within phylogenetic groups (clades) and pairwise genetic distances between and among species were estimated under the Kimura 2-parameter (K2P) distance model (Kimura 1980) with pairwise deletion in MEGA version 5.1 (Tamura et al. 2011). Phylogenetic analyses were performed with PAUP 4.0 for Power Mac G4 (Swofford 2003) using the distance method with the neighbor joining (NJ) algorithm and the maximum parsimony (MP) method (replicated 1,000 times). For both methods a bootstrap analyses (500 replications) was used to estimate the stability of the inferred phylogenetic groups (Felsenstein 1985). Phylogenetic analyses were performed on 87 of 122 sequences obtained in this study, eliminating identical sequences of some of the individuals belonging to the most represented haplotypes (13 individuals of haplotype No. 1, 8 individuals of haplotype No. 3, 8 individuals of haplotype No. 4, 3 individuals of haplotype No. 15, and 2 individuals of haplotype No. 32), together with the 20 sequences obtained from GenBank (de León et al. 2008). Gonatocerus triguttatus Girault and Gonatocerus ashmeadi Girault (Mymaridae) were used as outgroup in order to generate a rooted phylogenetic tree.
Sequences were also used to construct phylogenetic networks, which are more appropriate to display close genetic relationships (Clement et al. 2000). The mitochondrial haplotype network was constructed using TCS 1.21 program (Clement et al. 2000). This creates a haplotype network using statistical parsimony (SP), which outputs the 95% plausible set of the most parsimonious linkages among haplotypes (Templeton et al. 1992).
Results
Morphological and Morphometric Analyses of Females
On the basis of female morphology 72 females belonged to A. parvus and 17 to A. atomus. Two individuals did not belong with certainty to either of the two species considered because they had the mps on F4 only on one of the two antennae (7 h A. sp. FVG 4v and 9e 31 09 A. parvus FVG 5rv). Both these individuals were collected in FVG, one from grapevine and one from bramble. All the females from Central Italy (N. 28) were morphologically identified as A. parvus, whereas among the females from FVG and Lombardy both species were found.
Morphometric analysis showed significant differences between A. parvus and A. atomus females for all the four characters considered, but only for two of these: F4 length and the ratio of club/(F3+F4), was there no overlap between the value ranges (Table 2). The two intermediate females showed morphometric characters one of A. atomus (7 h A sp. FVG 4v) and one of A. parvus (9e 31 09 A. parvus FVG 5rv).
Table 2.
Morphometric analyses on morphological identified females of A. atomus and A. parvus
| Characters |
A. atomus |
A. parvus |
t–test | ||
|---|---|---|---|---|---|
| No. 17 |
No. 72 |
||||
| Average ± SD | min, max | Average ± SD | min, max | ||
| club length (μm) | 104.15 ± 6.22 | 95.0, 117.5 | 100.53 ± 4.63 | 90.0, 115.62 | t87 = 2.71, P = 0.0081 |
| F3 length (μm) | 47.68 ± 4.78 | 38.75, 55.0 | 36.03 ± 3.23 | 27.5, 42.5 | t87 = 12.11, P < 0.0001 |
| F4 length (μm) | 57.39 ± 3.17 | 51.88, 62.5 | 40.73 ± 3.36 | 33.13, 50.0 | t87 = 18.06, P < 0.0001 |
| ratio club/(F3 + F4) | 0.99 ± 0.06 | 0.89, 1.08 | 1.32 ± 0.09 | 1.17, 1.58 | t87 = 13.73, P < 0.0001 |
Sequencing and Phylogenetic Analyses
Sequencing of the mtCOI partial gene generated 445-bp sequence fragments from all the individuals tested, after trimming a portion of 3′ end due to high background signals. The mean frequency of each nucleotide in the mtCOI partial gene sequences was the following [T (U) 45.4%, C 11.5%, A 30.5%, and G 12.7%] showing a bias of A+T. The A+T content at the third, second, and first codon positions were 97.8, 59.3, and 70.5%, respectively. The nucleotide C was the lowest (0.7%) and the T the highest (53%) at the third codon position. The 445-bp COI sequences were 70.1–80.9% A+T rich and 23.6–26.1% C+G rich.
MP and NJ phylogenetic analyses conducted on COI partial gene sequences obtained from this study and from GenBank allowing us to distinguish four clades (Figs. 2 and 3). All individuals of this study belonged to clades 1, 2, and 4. Therefore, no individuals clustered together with those of clade 3 in which all the individuals of A. erythroneurae from GenBank clustered. In clades 1 and 2 all females that we identified morphologically as A. parvus in our study (N. 72) clustered together. In clade 4 all individuals of A. atomus identified morphologically from our study clustered together with A. atomus sequences retrieved from GenBank. Among the morphologically identified A. ustulatus (= parvus) from GenBank, the majority clustered in clade 4, whereas only two haplotypes clustered in clade 1. In the correspondence between morphological and genetic identifications of individuals from this study, there were three exceptions for clade 1, in which also three individuals morphologically identified as A. atomus (8g A. parvus FVG1rv haplotype No. 27, 9a A. parvus FVG1rv apl No. 27 and 9e A. parvus FVG2rv apl No. 2) clustered. The three individuals morphologically identified as A. atomus disagreed with molecular results, even when considering the morphometric identification. The two individuals with intermediate characters (7 h A spp FVG4v apl No. 2 and 9e 31 09 A. parvus FVG5rv apl No. 13) clustered in clade 1 and one of the two individuals disagreed with molecular results even when considering the morphometric analysis (7 h A spp FVG4v apl No. 2).
Fig. 2.
Most parsimonious phylogram out of 172 trees of relationships among Anagrus spp. populations inferred from ribosomal COI partial sequences [A. parvus sensu Viggiani (2014) ( = A. ustulatus sensu Chiappini 1989]. Bootstrap values are shown above respective branches for nodes with >50% bootstrap support (500 replicates).
Fig. 3.
NJ tree among Anagrus spp. populations inferred from ribosomal COI partial sequences (A. parvus sensu Viggiani (2014) (=A. ustulatus sensu Chiappini 1989). Bootstrap values are shown above respective branches for nodes with >50% bootstrap support (500 replicates).
Alignment of the sequences obtained from this study demonstrated that a total of 34 haplotypes were recognized out of 122 Anagrus spp. individuals. In particular, 5, 15, and 14 haplotypes were identified for clades 1, 2, and 4, respectively (Supp Table 1 [online only]). The mtCOI partial gene sequences from one representative individual of each haplotype have been submitted to GenBank; the accession numbers have been reported in Supp Table 1 [online only].
Sequences of the 34 haplotypes found in this study and the 20 sequences retrieved from GenBank for the Anagrus ‘atomus’ group were aligned and compared in Supp Table 2 [online only], reporting the individuated 22 nucleotide positions which allowed discrimination among Anagrus spp. haplotypes belonging to different clades. Most of the substitutions were silent. Fifteen positions of nucleotides substitutions were identified and allowed to discriminate all the individuals of one clade from those of the other clades (clade-specific nucleotides). These substitutions were identified as 13 transitions (Nos. 063, 120, 138, 144, 192, 204, 213, 237, 243, 420, 435, 438, and 444) and 2 transversions (Nos. 054 and 381). In particular, four positions (Nos. 120, 192, 243, and 381) allowed discrimination of haplotypes from A. parvus (clade 2), two positions (Nos. 054 and 237) are specific for haplotypes of A. parvus (clade 1), eight positions (Nos. 063, 144, 204, 213, 420, 435, 438, and 444) for A. atomus (clade 4), and one position (No. 138) for A. erythroneurae (clade 3). Among the remaining seven positions, one position (No. 147) is shared between haplotypes from A. parvus-clade 1 and A. erythroneurae-clade 3. Three positions (Nos. 051, 252, and 375) were identified as specific for all Italian A. atomus haplotypes from this study, for the Italian individuals from the literature (de León et al. 2008), except for haplotype EU015025 A. atomus hapl 3, and for one individual from the UK (8UK). For the same three positions 3 UK haplotypes showed the presence of the nucleotide ‘A’ instead of the nucleotide ‘G’, typical of the Italian A. atomus individuals. This was also verified for all the Italian haplotypes of A. ustulatus (= parvus) from literature clustering in clade 4 (de León et al. 2008). Finally, the three positions Nos. 159, 165, and 318 represented quite conserved sites at least among haplotypes of A. parvus (clades 1 and 2) and A. erythroneurae (clade 3), together with other 11 nucleotide positions (Nos. 051, 063, 144, 204, 213, 252, 375, 420, 435, 438, and 444), demonstrating their high genetic similarity. Considering the Italian Anagrus spp. individuals from this study 11 nucleotides substitutions were able to differentiate A. parvus individuals from A. atomus ones. In particular, eight were transitions (Nos. 063, 144, 204, 213, 420, 435, 438, and 444) and three transversions (Nos. 051, 252, and 375).
Levels of genetic divergence in the mtCOI partial gene among clades as percentage sequence divergence (%) are shown in Table 3. The intra-clade distance was on average <1% for clades 1–3, but close to 3% for clade 4 demonstrating that the highest genetic distance occurred among individuals of this latter clade. For inter-clade distance, clades 1 and 3 showed the lowest genetic distance whereas clades 3 and 4 demonstrated the highest genetic distance. The inter-clade genetic distance was higher between clades 1 and 2, than both the latter and clade 3.
Table 3.
Pairwise percent nucleotide differences in a 445 bp fragment of COI mtDNA sequences calculated by the K2P model (min, max, average) within and between the four individuated clades of the atomus group individuals
| Species | Clade | Clade percentage nucleotide difference: min–max (average) |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| A. parvus | 1 | 0–1.59% (0.79) | |||
| A. parvus | 2 | 2.28–4.16% (3.22) | 0–1.60% (0.8) | ||
| A. erythroneurae | 3 | 1.59–3.21% (2.4) | 1.82–3.22% (2.52) | 0.23–1.37% (0.8) | |
| A. atomus | 4 | 4.17–6.82% (5.49) | 3.69–6.57% (5.13) | 3.59-7.08% (5.33) | 0–5.62% (2.81) |
MP phylogenetic analysis generated 172 parsimonious trees. Among all analyzed sequences, 327 nucleotide positions were conserved sites (73.5%) and 118 nucleotide positions were variable (26.5%): 76 were parsimony-informative (17.1%) and 42 were not informative (singleton positions) (9.4%). One of the MP trees is shown in Figure 2 and the NJ tree in Figure 3. Overall, the MP and NJ phylogenetic trees showed similar topologies with two major branches supported by relatively high values (>70%) and four main phylogenetic groups or clades supported by moderate to high bootstrap values (Figs. 2 and 3). In particular branch 1 included the clades 1–3 (bootstrap values of 73, 89, and 80% respectively in MP tree and 60, 100, and 99%, respectively in NJ tree). In branch 2, especially of the MP tree, two monophyletic groups were observed but not supported by high bootstrap values (Fig. 2).
Haplotype Distribution in Relation to Geographic Area and Plant Host
A different geographical distribution of individuals and haplotypes was found between and within the two clades (1 and 2) associated with morphologically identified A. parvus (Fig. 2; Supp Table 1 [online only]). Clade 1 grouped most of the individuals (49 out of 62) and haplotypes (5 out of 8) from FVG and only 1 of 30 individuals from Tuscany. All five haplotypes of clade 1 were detected in FVG. Clade 2 grouped almost all the individuals from central Italy (22 from Tuscany and 6 from Umbria), 13 individuals from FVG and one individual from Lombardy. In clade 2, 12 of 15 haplotypes were obtained from individuals sampled in central Italy.
Anagrus atomus (clade 4) was detected in FVG, Lombardy and UK, but none of the 14 haplotypes was shared between the two Italian areas (Lombardy and FVG), and between these and UK. One of the two monophyletic groups of this clade included all the individuals of the Italian A. atomus from this study (N. 8) and from GenBank (N. 5), and one A. atomus from UK. The other group included several Italian individuals of A. ustulatus from GenBank (N. 8) and the majority of A. atomus individuals from UK (N. 7).
A different frequency of haplotypes was also observed in the 13 sites (Supp Table 1 [online only]). For clade 1, the most representative haplotypes were haplotype No. 1 and No. 3. The five haplotypes of this clade were presented at least in two sites and haplotype No. 1 was detected in all FVG sites and in Tuscany. For clade 2, the most frequent haplotype was haplotype No. 4 present in all three sites of Tuscany but also in five out of six sites of FVG. In this clade 9 haplotypes out of 15 were present in only one site. All the other haplotypes in clade 2 included especially individuals from central Italy; among them, haplotype No. 15 was one of the most frequent. All haplotypes of clade 4 (A. atomus) including Italian individuals were little represented, whereas haplotype No. 32 was the most frequent among UK haplotypes.
Concerning association of the haplotypes with the plant hosts collected we observed that in clade 1, haplotypes Nos. 1 and 3 were the most frequent both on Rubus sp. and Rosa canina L. and haplotype No. 2 was shared by all three host plants sampled (Supp Table 1 [online only]). In clade 2, haplotype No. 4 was the most frequent both in FVG, where it was shared by all three the sampled host plants, and in Tuscany. In clade 4, Italian haplotype No. 10 was shared by all three host plants sampled (Supp Table 1 [online only]).
Network Analysis of Haplotypes
For A. parvus, applying SP criterion to the mitochondrial (COI) dataset resulted in two distinct and unconnected sub-networks, separated by 10 mutational steps which were beyond the 95% probability limit for connecting haplotypes with the TCS program. The two distinct networks could only be joined at the 93% probability level (Fig. 4). Sub-networks I and II corresponded respectively to clades 1 and 2, resolved in the phylogenetic trees (Fig. 2). For sub-network I the analysis showed five closely related haplotypes all from FVG. Only the most frequent haplotype No. 1 was also shared by an individual from Tuscany. In sub-network II, it was found that some haplotypes grouped in a star-like shape with the haplotypes coalescing to haplotype No. 4 (from FVG and Tuscany) and in most cases they were connected by a single mutational step. This network included one haplotype from Lombardy and all the four haplotypes retrieved from Umbria, two of which were shared with Tuscany (haplotypes Nos. 18 and 22).
Fig. 4.
A. parvus haplotypes network realized by TCS 1.21. Two unconnected sub-networks (I and II) were obtained (95%, connection limit = 9). Each haplotype is represented by a circle, with the area of the circle proportional to its frequency. Numbers denote haplotype reported in Supp Table 1 [online only]. Each line represents a single mutation while small white circle symbolize intermediate missing or unsampled haplotypes.
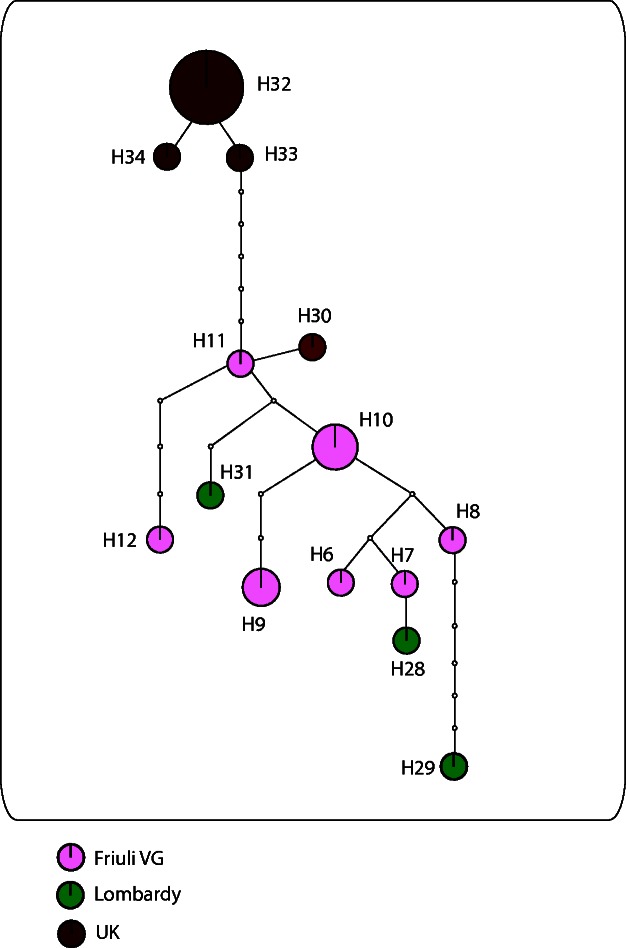
For A. atomus the network obtained showed presence of two distinct genetic groups corresponding to precise geographic areas and separated from each other by five mutational steps (Fig. 5). The first group included only haplotypes from the UK whereas the second group included all the Italian haplotypes (from FVG and Lombardy) and one haplotype from the UK.
Fig. 5.
A. atomus haplotypes realized by TCS 1.21. Each haplotype is represented by a circle, with the area of the circle proportional to its frequency. Numbers denote haplotype identifier presented in Supp Table 1 [online only]. Each line represents a single mutation while small white circle symbolize intermediate missing or unsampled haplotypes.
Morphometric Analysis of Males
Morphometric analysis of the aedeagus of individuals belonging to A. atomus or A. parvus identified on the basis of the molecular study showed highly significant differences for both phallobase and digitus lengths (Fig. 6). However, only for the digitus length was no overlapping between the measured ranges observed. Individuals identified as A. atomus, on the basis of cuticular hydrocarbons or because they emerged from E. vitis eggs, showed the same range in digitus length as for molecular-identified A. atomus individuals. The ratios of genitalia/digitus, pallobase/digitus, and genitalia/phallobase were not discriminant values (data not reported).
Fig. 6.
Genitalia, phallobase and digitus lengths (average ± SD) of A. parvus and A. atomus male genitalia. For A. atomus three different identification criteria are considered (molecular, cuticular hydrocarbons and specific leafhopper host). Inside each column the number of individuals measured is reported. ANOVA genitalia (F3,31 = 7.68, P < 0.001); ANOVA phallobase (F3,31 = 12.35, P < 0.0001); ANOVA digitus (F3,31 = 35.90, P < 0.0001). Capital letters indicate difference at 0.01 levels for the three characters at Tukey test.
Discussion
Morphological Identification of Females and Its Limits
Morphological identification of the Anagrus spp. females considered in this study confirmed the presence of two distinct taxa within the ‘atomus’ group (Chiappini et al. 1996; Floreani et al. 2006; de León et al. 2008). The two taxa were clearly separated using morphometric characteristics of the antenna. For both A. atomus and A. parvus, the average lengths of F4 and F3 and the values of the ratio of club/(F3+F4) were similar to that reported in Chiappini (1987) and Nugnes and Viggiani (2014).
COI phylogenetic analysis clustered our Anagrus individuals in two major branches (A. parvus branch and A. atomus branch) supported by high bootstrap values, in most but not all cases in agreement with morphological and morphometric identifications. An overall correct identification rate of only 96.3% was observed because 3 of 89 females showed morphological and morphometric characters of A. atomus but clustered in the A. parvus branch. An inverse problem of identification was observed for individuals from southern Italy morphologically identified as A. ustulatus (= parvus) by de León et al. (2008). Only a few of these individuals clustered in the A. parvus branch in our study whereas most of them clustered in the A. atomus branch. Another identification problem was represented by the two individuals that showed an mps on F4 in only one antenna and they clustered in the A. parvus branch. These hybrid-like individuals showing intermediate taxonomic characters were observed also by Chiappini et al. (1999). The presence of these individuals could, e.g., indicate that we are in presence of recently diverged sister species (Montgomery et al. 2011), but in this study we were not able to detect hybrid individuals because the COI gene is maternally inherited. Probably these individuals showed variable characters within intraspecific variations. Therefore, the morphological and morphometric tools used for Anagrus species identification do not always solve with certainty the problem of species separation for female individuals.
The same identification problem was found with Anagrus spp. when comparing hydrocarbon profiles with morphological and morphometric data (Floreani et al. 2006). Some contradictions were observed in particular for female individuals emerged from Rubus spp. Comparing cuticular hydrocarbons could be a more efficient method than genetic methods (saving time and money) to discriminate species, but it is necessary to verify if cuticular hydrocarbons analysis is coherent with genetic analysis. For this purpose, the same individuals should be submitted to both hydrocarbon analysis and genetic analysis.
Morphometric Identification of Males
For male individuals considered in this study, morphometric analysis permitted recognition of a distinct character (i.e. length of digitus) able to discriminate between individuals belonging, respectively; to A. atomus and A. parvus identified using molecular analyses. Moreover, this study also highlighted for males a perfect correspondence between cuticular hydrocarbons analyses and the morphometric character. Comparing the measures recorded in this study with those of Nugnes and Viggiani (2014), our genitalia length was greater for A. atomus and slightly greater for A. parvus, and our phallobase length was slightly greater for A. atomus. However, we have to consider that, since the digitus length is a morphometric character, it cannot be excluded that some big A. parvus individuals might have a big digitus as well as some tiny A. atomus individuals might have tiny digitus comparable to those of A. parvus. Unfortunately, characters based on ratios do not allow discrimination between the two species. In favor of the goodness of discriminant character, there's the fact that the individuals measured came from many localities. However, since it is know that morphometric characters can be influenced by the host parasitized (Huber and Rajakulendran 1988), further investigations are necessary to make sure that this character allows to discriminate with certainty the males of the two species. Moreover, it must be considered that for females morphological and morphometric identification also gives a low margin of error.
Major Clades Inferred from Phylogenetic Analysis on COI Gene
Phylogenetic analyses on COI partial gene sequence allowed description of a higher diversity among all our individuals than morphological and morphometric analyses. Within the A. parvus branch three different clades supported by high bootstrap values were distinguished: two of them (clades 1 and 2) corresponding to the morphologically identified A. parvus individuals and the third (clade 3) corresponding to A. erythroneurae individuals from GenBank. Also within the A. atomus branch, two different clusters were distinguished especially in the MP tree but not supported by high bootstrap values; therefore in this case the whole branch corresponded to clade 4. Overall, on the basis of these results we conclude that the two A. parvus clades and A. erythroneurae clade are phylogenetically closely related, and quite distinct from A. atomus.
The mean intraspecific COI differences of Anagrus spp. individuals in this report showed lower intra-clade variations, ranging from 0.79 to 2.81%, than that reported for other hymenopteran insects, which generally ranges from 0.60 to 5.50% (Danforth et al. 1998, Cognato 2006).
Comparing the mean genetic difference among the three clades in the A. parvus branch, the results showed the distance between clades 1 and 2 is greater than that between the A. erythroneurae clade 3 and each of clades 1 and 2. These results suggested the possibility that clades 1 and 2 represent two distinct species, in agreement with the criteria of Cognato (2006). Moreover, the presence of two unconnected networks, obtained from the network analyses with 95% parsimony connection limit which has been proposed for designating operational species based on DNA sequences data (Hart and Sunday 2007) supported the two-species hypothesis. However, the morphometric analysis of flagellar characters (club length, F3 length, F4 length, and ratio of club/F3+F4) carried out separately on individuals from clades 1 and 2 did not show any statistical significant differences (P ≥ 0.13; data not reported); therefore, these characters are not useful for discriminating individuals belonging to the two clades, if the two-species hypothesis was true. Further morphological and molecular analyses of other genomic regions (e.g. ITS2) may allow validate this hypothesis (de León et al. 2008).
In Italy, research by Nugnes and Viggiani (2014) had revealed that within the morphologically identified A. parvus there were two species distinguishable on the basis of morphometric characters. This confirms that within morphologically identified A. parvus more species could be included. The genetic differences between the A. parvus clades 1 and 2 cannot be attributed to different collection localities since haplotypes belonging to the both clades were detected in the same sites. The two clades cannot even be associated with different host plants from which the parasitoid wasps emerged, as reported in other studies Nugnes and Viggiani (2014), since individuals of both clades emerged from Rubus spp. It is very likely that these individuals clustered in two different clades because they emerged from eggs of different leafhopper species. The fact that only one individual from Tuscany clustered in clade 1 supports this latter hypothesis. Because the morphometric characters of the antennae considered in this study did not allow us to distinguish the individuals belonging to the two clades, in the future it would be interesting to investigate other characters (e.g., ovipositor length/fore tibia length ratio) reported in literature for Anagrus spp. (Triapitsyn et al. 2010; Nugnes and Viggiani 2014).
Considering the A. atomus branch, the high intra-clade genetic distance (on average 2.81%) is due to the presence of the two clusters that can be distinguished especially in the MP tree even if they are not supported by high bootstrap values (Fig. 2). One cluster grouped all Italian individuals from this study, from the study by de León et al. (2008) and one UK individual. Regarding the individuals from the study by de León et al. (2008) one haplotype (EU15025 A. atomus haplotype No. 3) showed a high divergence underlined by the different nucleotides in the three positions (Nos. 051, 252, and 375). The other cluster grouped UK A. atomus individuals (‘UK’ cluster) and morphologically identified A. ustulatus (= parvus) individuals in the study by de León et al. (2008). UK A. atomus individuals presented a low genetic divergence and formed a monophyletic group with high bootstrap values within this cluster. UK A. atomus individuals were reared on the leafhopper H. maroccana in a biofactory. However, in the UK o one haplotype was recorded clustering with the ‘Italian’ haplotypes. Probably, in the biofactory, selective pressure favors individuals belonging to the ‘UK’ cluster, but the periodical introduction of wild strains of the parasitoid in the rearing has determined that one individual clustered with the ‘Italian’ haplotypes. The parsimony network analysis of the sequences belonging to A. atomus (Fig. 5) showed a unique network distinct from the A. parvus sub-networks (Fig. 4). Nevertheless, five mutational steps separated the closely related UK haplotypes from the rest of the ‘Italian’ haplotypes. The Italian portion of the network was highly reticulated with all haplotypes connected to each other, frequently by up to three mutational steps, except for one haplotype from Lombardy (No. 29) which was separated by five mutational steps.
The possibility that both morphological species, A. parvus and A. atomus, represent a complex of species, each one associated with different leafhopper species, is reflected not only in the new species identified by Nugnes and Viggiani (2014) within the ‘atomus’ group but also in the complex of species recognized in North America within A. epos (Triapitsyn et al. 2010).
Variability in Nucleotide Composition Within ‘atomus’ Group
COI partial gene sequence analysis showed that, in regards to nucleotide composition, the collected populations had a high percentage of A+T content which is characteristic of Hymenoptera and similar to other values reported (Crozier et al. 1989; Jermiin and Crozier 1994; Dowton and Austin 1997; Whitfield and Cameron 1998; Baer et al. 2004; Wei et al. 2010). Moreover, the strongest A/T bias was found in the third position (Danforth et al. 1998).
Although the geographical coverage of our sampling of Anagrus individuals of ‘atomus’ group was not widespread, the mtDNA results showed diverse haplotypes. In particular, 34 haplotypes were recognized among the 122 individuals analyzed. This suggests the presence of a high level of molecular polymorphism, in agreement with that reported for Anagrus spp. by Chiappini et al. (1999) and for other Hymenopteran parasitoids belonging to Anaphes Haliday (Landry et al. 1993) and Trichogramma Westwood (Vanlerberghe-Masutti 1994). From our study, most of the polymorphisms in the populations were shown to be neutral mutations.
Sequence analyses permitted us also to determine that each distinct clade is characterized by a series of clade-specific nucleotides (or diagnostic nucleotides). Clade-specific nucleotides are useful for molecular identification of the different species and can be used to corroborate morphological identification of field-collected individuals. Molecular identification is recommended especially when limitations of a morphological based identification have been recognized for members of a certain species complex.
In conclusion, our results from the inferred phylogenetic trees, genetic networks and the sequence analysis based on partial COI gene showed that this sequence can successfully elucidate the relationships of closely related species and also potentially discriminate new ones. Therefore, we confirm the validity of COI as a genetic marker for discrimination of closely related species (Monti et al. 2005; Sha et al. 2006) and also for molecular identification of field-collected specimens on the bases of diagnostic nucleotides.
Implication of this Study on Grapevine Leafhopper Control
In each clade, there is one haplotype whose individuals emerged from both grapevines and the two plants in the hedgerows (haplotype No. 2 for clade 1, haplotype No. 4 for clade 2, and haplotype No. 10 for clade 4). This confirms the role of vegetation surrounding vineyards in the biological control of grapevine leafhoppers. Parasitoid individuals emerged from Rubus sp. or R. canina can colonize grapevines in spring (Cerutti et al. 1991; Ponti et al. 2005). In early autumn, the same plants can be sites where Anagrus females emerging from grapevines can lay over-wintering eggs (Zanolli and Pavan 2011).
As E. vitis is the only leafhopper capable of causing economic damage to grapevines in Europe, and is parasitized only by A. atomus, so molecular identification of the parasitoid might be conducted on leafhopper eggs laid in plant species surrounding vineyards. If a given plant species is host to many leafhopper species, it would be desirable to conduct molecular identification of both leafhopper and parasitoid. In this way we can know not only the plants but also the leafhopper species as potential sources of A. atomus for E. vitis biocontrol in vineyards. It is also possible to know what leafhopper species is parasitized by an Anagrus species by marking and exposing to parasitization leafhopper eggs laid on a plant by identified females (Zanolli and Pavan 2013). This knowledge is crucial to set up conservation biological control strategies based on habitat management.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
This research was partially supported from a PhD grant from the University of Udine (Italy). We would like to thank S.V. Triapitsyn for the critical and accurate revision of the article. The authors would like to thank the reviewers of the article for their useful comments and suggestions.
References Cited
- Arnò C., Alma A., Arzone A. 1988. Anagrus atomus as egg parasite of Typhlocybinae (Rhynchota Auchenorrhyncha), pp. 611–615. In Proceedings, 6th symposium Auchenorrhyncha Meeting, Turin, Italy. [Google Scholar]
- Baer C. F., Tripp D. W., Bjorksten T. A., Antolin M. F. 2004. Phylogeography of a parasitoid wasp (Diaeretiella rapae): no evidence of host-associated lineages. Mol. Ecol. 13: 1859–1869. [DOI] [PubMed] [Google Scholar]
- Barari H., Ferguson A. W., Piper R. W., Smith E., Quicke D. L. J., Williams I. H. 2005. The separation of two hymenopteran parasitoids, Tersilochus obscurator and Tersilochus microgaster (Ichneumonidae), of stem-mining pests of winter oilseed rape sing DNA, morphometric and ecological data. Bull. Entomol. Res. 95: 299–307. [DOI] [PubMed] [Google Scholar]
- Bernardo U., Monti M. M., Nappo A. G., Gebiola M., Russo A., Pedata P. A., Viggiani G. 2008. Species status of two populations of Pnigalio soemius (Hymenoptera: Eulophidae) reared from two different hosts: an integrative approach. Biol. Control. 46: 293–303. [Google Scholar]
- Borghuis A., Pinto J. D., Platner G. R., Stouthamer R. 2004. Partial cytochrome oxidase II sequences distinguish the sibling species Trichogramma minutum Riley and Trichogramma platneri Nagarkatti. Biol. Control. 30: 90–94. [Google Scholar]
- Cargnus E., Pavan F. 2007. Potenzialità dell’elettroforesi enzimatica nello studio tassonomico di Anagrus gruppo atomus (Hymenoptera: Mymaridae), p. 348. In Proceedings, XXI Congresso Nazionale Italiano di Entomologia Agraria, Campobasso, Italy. [Google Scholar]
- Cerutti F., Baumgärtner J., Delucchi V. 1991. The dynamics of grape leafhopper Empoasca vitis Göthe populations in Southern Switzerland and the implications for habitat management. Biocontrol Sc. Techn. 1: 177–194. [Google Scholar]
- Chiappini E. 1987. Ricerche sulla variabilità di Anagrus atomus (L.) (Hymenoptera Mymaridae) e di una specie affine presente su rovo. Boll. Zool. Agr. Bachic. Ser. II. 19: 71–97. [Google Scholar]
- Chiappini E., Mazzoni E. 2000. Differing morphology and ultrastructure of the male copulatory apparatus in species-groups of Anagrus Haliday (Hymenoptera: Mymaridae). J. Nat. Hist. 34: 1661–1676. [Google Scholar]
- Chiappini E., Triapitsyn S. V., Donev A. 1996. Key to Holarctic species of Anagrus Haliday (Hymenoptera: Mymaridae) with a review of the Nearctic and Palearctic (other than European) species and description of new taxa. J. Nat. Hist. 30: 551–595. [Google Scholar]
- Chiappini E., Soressi L., Fogher C., Zanirato M. 1999. Genetic identity and relationship between four Anagrus species (Hymenoptera: Mymaridae) using RAPD analysis. Eur. J. Entomol. 96: 393–400. [Google Scholar]
- Clement M., Posada D., Crandall K. 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9: 1657–1660. [DOI] [PubMed] [Google Scholar]
- Cognato A. I. 2006. Standard percent DNA sequence difference for insects does not predict species boundaries . J. Econ. Entomol. 99: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Crozier R. H., Crozier Y. C., Mackinlay A. G. 1989. The CO-I and CO-II region of honeybee mitochondrial DNA: evidence for variation in insect mitochondrial evolutionary rates. Mol. Biol. Evol. 6: 399–411. [DOI] [PubMed] [Google Scholar]
- Danforth B. N., Mitchel P. L., Packer L. 1998. Mitochondrial DNA differentiation between two cryptic Halyctus (Hymenoptera: Halyctidae) species. Ann. Entomol. Soc. Am. 91: 387–391. [Google Scholar]
- DeBach P., Rosen D. 1991. Biological control by natural enemies, 2nd ed. Cambridge University Press, Cambridge, UK. [Google Scholar]
- de León J. H., Triapitsyn S. V., Matteucig G., Viggiani G. 2008. Molecular and morphometric analyses of Anagrus erythroneurae Triapitsyn et Chiappini and Anagrus ustulatus Haliday (Hymenoptera: Mymaridae). Boll. Lab. Entomol. Agr. Filippo Silvestri. 62: 19–32. [Google Scholar]
- Dowton M., Austin A. D. 1997. Evidence of AT-transversion bias in wasp (Hymenoptera: Symphyta) mitochondrial genes and its implications for the origin of parasitism. J. Mol. Evol. 44: 398–405. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Floreani C., Pavan F., Nazzi F. 2006. Analysis of cuticular hydrocarbons in two Anagrus species (Hymenoptera: Mymaridae) as a tool to improve their correct identification. Can. Entomol. 138: 348–356. [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijehoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3: 294–299. [PubMed] [Google Scholar]
- Gibson G. A. P. 1997. Morphology and terminology, pp. 16–44. In Gibson G. A. P., Huber J. T., Woolley J. B. (eds.), Annotated keys to the genera of Nearctic Chalcidoidea (Hymenoptera), NRC Research Press, Ottawa, Ontario, Canada. [Google Scholar]
- Hall T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41: 95–98. [Google Scholar]
- Hart M. W., Sunday J. 2007. Things fall apart: biological species form unconnected parsimony networks. Biol. Lett. 3: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P. D., Cywinska A., Ball S. L., de Waard J. R. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B. 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. T. 1986. Systematics, biology, and hosts of the Mymaridae and Mymarommatidae (Insecta: Hymenoptera): 1758–1984. Entomography 4: 185–243. [Google Scholar]
- Huber J. T., Rajakulendran S. V. 1988. Redescription of and host-induced antennal variation in Anaphes iole Girault (Hymenoptera: Mymaridae), an egg parasite of Miridae (Hemiptera) in North America. Can. Entomol. 120: 893–901. [Google Scholar]
- Jermiin L. S., Crozier R. H. 1994. The cytochrome b region in the mitochondrial DNA of the ant Tetraponera rufoniger: sequence divergence in Hymenoptera may be associated with nucleotide content. J. Mol. Evol. 38: 282–294. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 116: 11–120. [DOI] [PubMed] [Google Scholar]
- Kruse J. J., Sperling F. A. H. 2002. Molecular phylogeny within and between species of the Archips argyrospila complex (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 94: 166–173. [Google Scholar]
- Landry B. S., Dextraze L., Boivin G. 1993. Random polymorphic DNA markers for DNA fingerprinting and genetic variability assessment of minute parasitic wasp species (Hymenoptera: Mymaridae and Trichogrammatidae) used in biological control programs of phytophagous insects. Genome. 36: 580–586. [DOI] [PubMed] [Google Scholar]
- Lin C. P., Wood T. K. 2002. Molecular phylogeny of the North American Enchopa binotata (Homoptera: Membracidae) species complex. Ann. Entomol. Soc. Am. 95: 162–171. [Google Scholar]
- MacDonald C., Loxdale H. 2004. Molecular markers to study population structure and dynamics in beneficial insects (predators and parasitoids). Int. J. Pest Manag. 50: 215–224. [Google Scholar]
- Masner L. 1975. Two new sibling species of Gryon Haliday (Hymenoptera, Scelionidae) egg parasites of blood-sucking Reduviidae (Heteroptera). Bull. Entomol. Res. 65: 209–213. [Google Scholar]
- Matteucig G., Viggiani G. 2008. Fenologia e ospiti di Anagrus gruppo atomus (Linnaeus) (Hymenoptera: Mymaridae) in Campania. Boll. Lab. Entomol. Agr. Filippo Silvestri. 62: 45–50. [Google Scholar]
- Montgomery M. E., Shiyake S., Havill N. P., Leschen R. A. B. 2011. A new species of Laricobius (Coleoptera: Derodontidae) from Japan with phylogeny and a key for native and introduced congeners in North America. Ann. Entomol. Soc. Am. 104: 389–401. [Google Scholar]
- Monti M. M., Nappo A. G., Giorgini M. 2005. Molecular characterization of closely related species in the parasitic genus Encarsia (Hymenoptera: Aphelinidae) based on the mitochondrial cytochrome oxidase subunit I gene. Bull. Entomol. Res. 95: 401–408. [DOI] [PubMed] [Google Scholar]
- Nugnes F., Viggiani G. 2014. Description and biological features of a new species of Anagrus Haliday (Hymenoptera, Mymaridae). Entomologia. 2: 176. [Google Scholar]
- Patwary M. U., Kenchington E. L., Bird C. J., Zouros E. 1994. The use of random amplified polymorphic DNA markers in genetic studies of the sea scallop Plactopecten magellanicus (Gmelin, 1791). J. Shellfish Res. 13: 547–553. [Google Scholar]
- Ponti L., Ricci C., Veronesi F., Torricelli R. 2005. Natural hedges as an element of functional biodiversity in agroecosystem: the case of a central Italy vineyard. Bull. Insectol. 58: 19–23. [Google Scholar]
- Saccone C., De Giorgi C., Gissi C., Pesole G., Reyes A. 1999. Evolutionary genomics in Metazoa: the mitochondrial DNA as a model system. Gene 238: 195–209. [DOI] [PubMed] [Google Scholar]
- Sha Z. L., Zhu C. D., Murphy R. W., La Salle J., Huang D. W. 2006. Mitochondrial phylogeography of a leafminer parasitoid, Diglyphus isaea (Hymenoptera: Eulophidae) in China. Biol. Control 38: 380–389. [Google Scholar]
- Simon C., Frati F., Beckenbach A., Crespi B., Liu H., Flook P. 1994. Evolution, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701. [Google Scholar]
- Soyka W. 1955. Ueberblick das Genus Anagrus Holiday (Alaptidae, Mymaridae, Chalcidoidea, Hymenoptera). Entomol. Nachr., 7: 23–26. [Google Scholar]
- Stouthamer R., Hu J., Van Kan F. J. P. M., Platner G. R., Pinto J. D. 1999. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 43: 421–440. [Google Scholar]
- Swofford D. L. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton A. R., Crandall K. A., Sing C. F. 1992. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics. 132: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triapitsyn S. V. 1998. Anagrus (Hymenoptera: Mymaridae) egg parasitoids of Erythroneura spp. and other leafhoppers (Homoptera: Cicadellidae) in North American vineyards and orchards: a taxonomic review. Trans. Am. Entomol. Soc. 124: 77–112. [Google Scholar]
- Triapitsyn S. V., Rugman-Jones P. F., Jeong G., Morse J. G., Stouthamer R. 2010. Morphological and molecular differentiation of the Anagrus epos species complex (Hymenoptera: Mymaridae), egg parasitoids of leafhoppers (Hemiptera: Cicadellidae) in North America. Zootaxa. 2428: 1–21. [Google Scholar]
- Vanlerberghe-Masutti F. 1994. Molecular identification and phylogeny of parasitic wasp species (Hymenoptera: Trichogrammatidae) by mitochondrial DNA RFLP and RAPD markers. Insect Mol. Biol. 3: 229–237. [DOI] [PubMed] [Google Scholar]
- Viggiani G. 1970. Ricerche sugli Hymenoptera Chalcidoidea. XXIV. Sul valore tassonomico dell'organo copulatore nei Mimaridi del genere Anagrus Hal. Boll. Lab. Entomol. agr. Filippo Silvestri. 28: 11–18. [Google Scholar]
- Viggiani G. 1989. A preliminary classification of the Mymaridae (Hymenoptera: Chalcidoidea) based on the external male genitalia characters. Boll. Lab. Entomol. Agr. Filippo Silvestri. 45: 141–148. [Google Scholar]
- Viggiani G. 2014. On the misidentification of Anagrus ustulatus Haliday (Hymenoptera: Mymaridae). Zootaxa 3826: 397–400. [DOI] [PubMed] [Google Scholar]
- Viggiani G., Di Luca A., Matteucig G. 2006. The egg parasitoids of the genus Anagrus (Hymenoptera:Mymaridae) as functional biodiversity of the vineyard agroecosystem. IOBC/WPRS Bull 29: 157–160. [Google Scholar]
- Wallof N., Jervis M. A. 1987. Communities of parasitoids associated with leafhoppers and planthoppers in Europe. Adv. Ecol. Res. 17: 281–402. [Google Scholar]
- Wei S.-J., Shi M., Sharkey M. J., van Achterberg C., Chen X.-X. 2010. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to holometabolous insetcs. BMC Genomics 11: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. B., Cameron S. A. 1998. Hierarchical analysis of variation in the mitochondrial 16S rRNA gene among Hymenoptera. Mol. Biol. Evol. 42: 737–754. [DOI] [PubMed] [Google Scholar]
- Zanolli P., Pavan F. 2011. Autumnal emergence of Anagrus wasps, egg parasitoids of Empoasca vitis, from grapevine leaves and their migration towards brambles. Agric. Forest Entomol. 13: 423–433. [Google Scholar]
- Zanolli P., Pavan F. 2013. Occurrence of different development time patterns induced by photoperiod in Anagrus atomus (Hymenoptera: Mymaridae), an egg parasitoid of Empoasca vitis (Homoptera: Cicadellidae). Physiol. Entomol. 38: 269–278. [Google Scholar]