Abstract
Despite the paradigm that carbohydrates are T cell-independent antigens, isotype-switched glycan-specific IgG antibodies and polysaccharide-specific T cells are found in humans. We employed a systems level approach combined with glycan array technology to decipher the repertoire of carbohydrate-specific IgG antibodies in intravenous and subcutaneous immunoglobulin (IVIG/SCIG) preparations. A strikingly universal architecture of this repertoire with modular organization among different donor populations revealed an association between immunogenicity or tolerance and particular structural features of glycans. Antibodies were identified with specificity not only for microbial antigens, but for a broad spectrum of host glycans that serve as attachment sites for viral and bacterial pathogens and/or exotoxins. Tumor-associated carbohydrate antigens were differentially detected by IgG antibodies, while non-IgG2 reactivity was predominantly absent. Our study highlights the power of systems biology approaches to analyze immune responses and reveals potential glycan antigen determinants that are relevant to vaccine design, diagnostic assays, and antibody-based therapies.
Keywords: IVIG, SCIG, humoral immunity, IgG subclasses, anti-carbohydrate antibodies, glycans, glycan array
One Sentence Summary
This broad analysis of intravenous and subcutaneous immunoglobulin preparations reveals a universal architecture of the IgG anti-carbohydrate repertoire, determinants of glycan immunogenicity, and reactivity to microbial attachment sites.
INTRODUCTION
The immune system is an integrated network of molecular and cellular players that together maintain host integrity and respond adequately and efficiently to a diverse range of exogenous or endogenous, autoimmune or damage-associated, challenges. The need for systems biology and interdisciplinary approaches to explore these complex immunological networks has been identified and resulted in the establishment of a number of consortia worldwide that aim to pursue this promising research strategy (1, 2). Indeed, the use of systems approaches, either alone or in combination with traditional reductionist strategies of molecular biology, have not only led to major advances in immunology and vaccinology (3), but also raised evidence challenging current concepts in these fields. Notably, contrary to the paradigm that carbohydrate-specific antibody responses are T cell-independent, data raised by a systems level approach using blood transcription modules (BTMs) analysis indicate that CD4+ T cells are involved in the generation of carbohydrate-specific antibodies following glycovaccination (4). This is consistent with our previously reported observation that the human repertoire of carbohydrate-specific antibodies contains a broader than expected spectrum of isotype-switched IgG molecules, that are not restricted to the IgG2 subclass (5).
In light of the abundance and diversity of microbial carbohydrate moieties on structural compounds, such as lipopolysaccharides or peptidoglycans, capsular antigens, or on secreted exopolysaccharides, increased focus has been placed on the glycomic aspects of humoral immunity by studies in microbiology and vaccinology. Protective immunity through antibodies specific for microbial carbohydrate epitopes can be achieved by immunization with polysaccharide or glycoconjugate vaccines (4, 6). Recent glycovaccines have played an important part in preventing infectious diseases caused by virulent pathogens such as Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumonia (4, 6, 7). While low immunogenicity and hyporesponsiveness represent one major limitation of pure polysaccharide vaccines, improved vaccine efficacy achieved by conjugation of polysaccharides to immunogenic carrier proteins, intended to augment T cell responses, comes at the cost of a lower number of serotypes that, for technical reasons, can be included (8, 9). Furthermore, conjugate vaccines have a number of other limitations, such as serotype replacement, increased frequency of colonization with other respiratory pathogens, serotype-specific immune hyporesponsiveness, and high costs of manufacture (8). Furthermore, the immunogenicity of glycovaccines has been variable depending on the structure of the particular polysaccharide in a given construct (10, 11). Decoding the structure-immunogenicity relationship of glycans might facilitate the design and development of more potent and immunogenic glycovaccines and promote predictions as to the immunogenicity of a given vaccine.
Host immunity also appears to be shaped by immunomodulatory effects of commensal-expressed polysaccharides of the microbiota (6). Pre-existing antibodies might limit disseminated infection following disruption or leakage of the epithelial barrier. However, because carbohydrate-epitopes are widely expressed on host tissues, effective mechanisms must be in place to protect against autoimmunity. In fact, aberrant antibody responses to carbohydrate antigens have been observed in autoimmune diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), and may play a pathogenetic role in certain autoimmune diseases (12).
Autoantibodies in healthy individuals are thought to promote tissue homeostasis and healing. This repertoire of autoantibodies is characterized by modular organization in healthy adults after ontogenetic maturation (13). It will be relevant to understand the repertoire of carbohydrate-specific antibodies, as glycans and glycan-protein interactions play a central role in multiple biological processes such as immunomodulation (14–17), infection (18, 19), and cancer (20–23). For instance, tumor-associated carbohydrate antigens (TACA) are essentially self-antigens that serve as well-known tumor markers and have been shown to influence tumor immunosurveillance by direct interaction with carbohydrate-binding receptors (lectins) on immune cells (22). Tissue-specific glycans of the host are specifically recognized by microbial lectins, including viral hemagglutinins or bacterial fimbriae and pili, which determines the tissue tropism of both commensals and pathogens (18). In fact, glycan-protein interactions serve as the most common means of microbial adhesion, a prerequisite of microbial colonization or infection (18). However, deciphering the repertoire of carbohydrate-specific autoantibodies might reveal unknown functions in human physiology, in disease or in host-microbial interactions, which might be diagnostically or therapeutically exploited (24).
Glycan array technology is a powerful tool to study protein-carbohydrate interactions on a large scale, and has been used by us and others to examine glycan-binding profiles of immunoglobulins from healthy donors (5, 24–26). However, in these studies only a limited number of either immunoglobulin preparations or glycans were analyzed, without use of systems level computational tools. Here, we performed a broad and comparative systems level analysis of intravenous and subcutaneous immunoglobulin (IVIG/SCIG) preparations from different manufacturers using glycan array version 5.1 of the Consortium for Functional Glycomics (CFG) with 610 immobilized glycans, and publicly available databases were used to deduce networks of immunoglobulins with specificity for biologically relevant glycans, including microbial antigens, microbial host attachment sites, tumor-associated carbohydrate antigens, blood group antigens, and known ligands of immune receptors. Given that IVIG/SCIG represent pools from plasma from thousands of healthy donors reflecting the antibody repertoire of the donor population, and these preparations are increasingly used as a high-dose therapy to treat inflammatory disorders even as off-label indications, the obtained information is not only important to clinicians, health institutions and plasma manufacturers, but reveals insights into aspects of humoral immunity that have biological significance and potential implications for diagnostics and the design of glycovaccines. However, the study design does not allow conclusions to be drawn on the quality of the analyzed commercial preparations in terms of clinical efficacy or specific manufacturing processes.
RESULTS
Broad carbohydrate reactivity in IVIG/SCIG
Commercial IVIG or SCIG immunoglobulin preparations (table S1) were evaluated by glycan array CFG version 5.1 analysis for specific IgG antibody binding to 610 distinct glycans. Consistent with our previously published work (5), a concentration of 180 µg/ml IVIG/SCIG was determined to be optimal and resulted in reproducible glycan-binding patterns with minimal background. Raw data are presented in table S2.
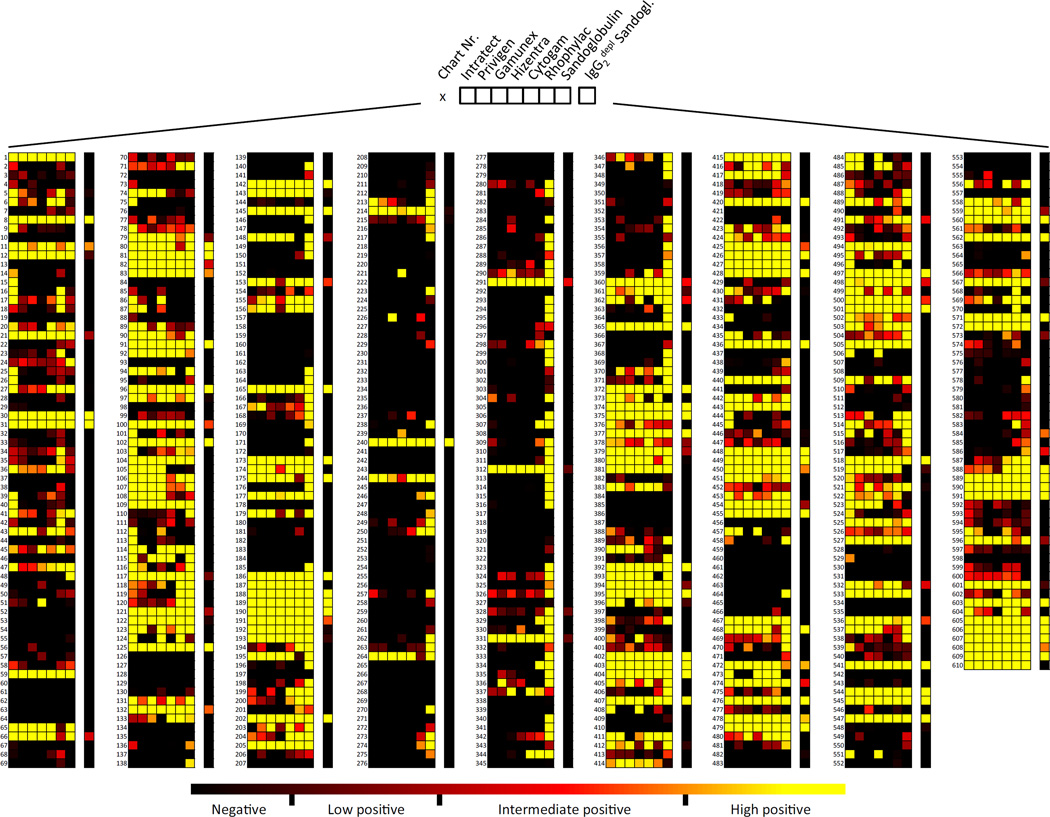
Recognition for the 610 glycans on the array was extensive for all IVIG/SCIG preparations (39.84–62.46%). The broadest range of antibody binding to 381 glycans (62.46%) was observed with Sandoglobulin, Rhophylac bound to 311 (50.98%), Intratect to 310 (50.82%), Privigen to 276 (45.25%), Cytogam to 268 (43.93%), Gamunex to 254 (41.64%), and Hizentra to 243 (39.84%) of the printed glycans (Fig. 1, and fig. S1). The maximal antibody binding levels were similar among most IVIG/SCIG preparations (95th-percentile value range: 2099–3508 relative fluorescent units [RFU]), except that Sandoglobulin exhibited slightly higher binding (95th-percentile value: 4942 RFU). Further analysis revealed that the broader range of glycans recognized by Sandoglobulin compared to other preparations is due to a larger proportion of glycans exhibiting high positive responses, but not a result of glycans with intermediate or low antibody binding reactivity (Fig. S2). The broader binding profile signature of Sandoglobulin might be linked to methodological differences during the fractionation process (27, 28). However, comparison of the different production processes identified no obvious reason that would account for these differences. Again, there was no impact on clinical efficacy.
Fig. 1. Comprehensive heat map depicting IgG antibody binding activities of IVIG/SCIG preparations to the 610 printed glycans on Consortium for Functional Glycomics (CFG) glycan array version 5.1.
Each column represents a specific IVIG/SCIG preparation, each row an individual glycan. Color code with computed categorization of binding intensity is indicated, as outlined in the Materials and methods section.
Although long-standing doctrine suggests that among IgG subclasses, IgG2 most commonly binds to carbohydrate epitopes (29, 30), we previously reported that the human IgG repertoire contains a broad range of anti-carbohydrate antibodies that are not restricted to the IgG2 subclass (5). To identify the relative contribution of IgG2 subclass antibodies to glycan binding we screened a well-characterized preparation on the microarray, i.e. Sandoglobulin that had been depleted of IgG2 by affinity chromatography (5). Only 104 glycans (17.05%) were recognized by IgG2-depleted Sandoglobulin compared to the 311 (50.98%) bound by the non-adsorbed preparation.
Universal architecture and modular organization of the carbohydrate-specific IgG repertoire
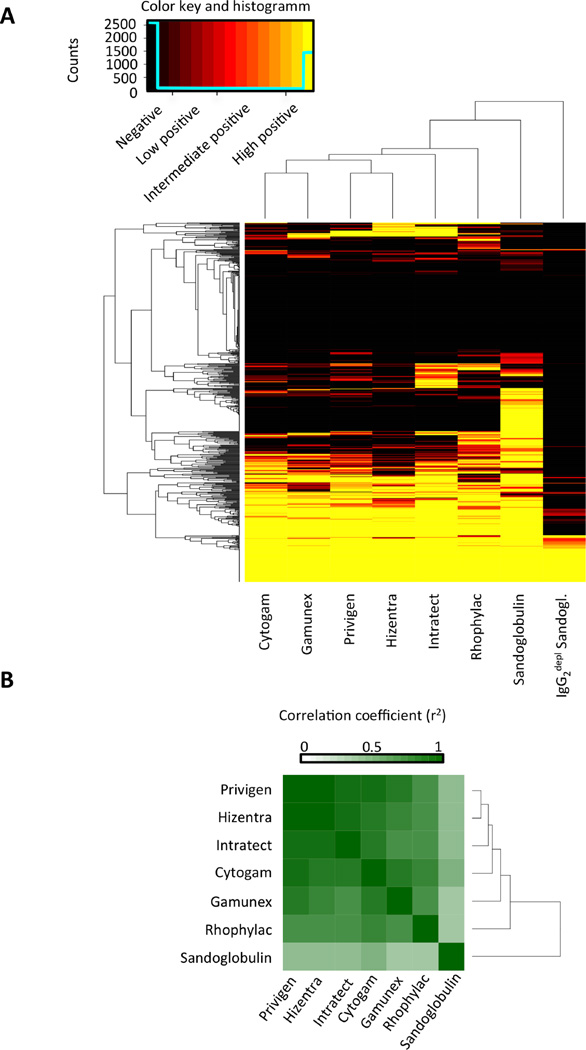
Hierarchical clustering analysis was performed to compute the reactivity matrix of the immunoglobulin preparations as depicted in Figure 2A. The rows in this matrix represent the antibody reactivity profiles (reactivities of one specific glycan antigen for the different immunoglobulin preparations) and the columns indicate the immune profiles of each IVIG/SCIG preparation. The matrix is reordered by a dendrogram clustering algorithm (13). The dendrogrammed reactivity matrix in Figure 2A indicates the reduced antibody-binding profile of IgG2-depleted Sandoglobulin when compared to the other preparations, thus illustrating the dominant, yet not exclusive, role of IgG2 class antibodies for carbohydrate epitope recognition. At the next level of the hierarchical tree, the glycan-binding profile of Sandoglobulin displayed a broader spectrum of glycan reactivity than the other preparations.
Fig. 2. Diverse commercial IVIG/SCIG preparations recognize carbohydrate structures on CFG glycan array version 5.1 with similar binding profiles.
(A) The ordered (by dendrogram algorithm) glycan-binding reactivity matrix for IgG antibodies in IVIG/SCIG. The color key and distribution histogram indicates reactivity levels as outlined in materials and methods. (B) The dendrogrammed correlation matrix for IVIG/SCIG preparations in color code representation.
Given that our IVIG/SCIG preparations differed in terms of manufacturer, purification technique, and donor population, we further compared the antibody glycan recognition patterns. Figure 2B shows the correlation matrix as computed by Pearson correlation analysis. High correlations of the glycan-binding profiles (r2= 0.7024 – 0.9419) were observed for all preparations, but lower for Sandoglobulin. The glycan-recognition patterns produced by Sandoglobulin, Privigen, Gamunex, Intratect and Hizentra revealed that most glycans were either recognized by IgG antibodies in all five preparations (30.00%) or by none (26.56%). 20.00% of glycans were bound only by antibodies in one specific IVIG preparation (Fig. S3). However, if Sandoglobulin was excluded, the percentage of single positive glycans was lower (8.36%), whereas the number of glycans recognized either by the total (34.75%) or by none (41.31%) of the preparations increased. These findings might be linked to methodological differences during the fractionation process. However, there is no indication that these differences impact on clinical efficacy. Furthermore, the highly correlated immune profiles of the five immunoglobulin preparations, point to a universal architecture of the IgG repertoire of carbohydrate-specific antibodies between the different donor populations.
Relationship between structural features and immunogenicity of glycans
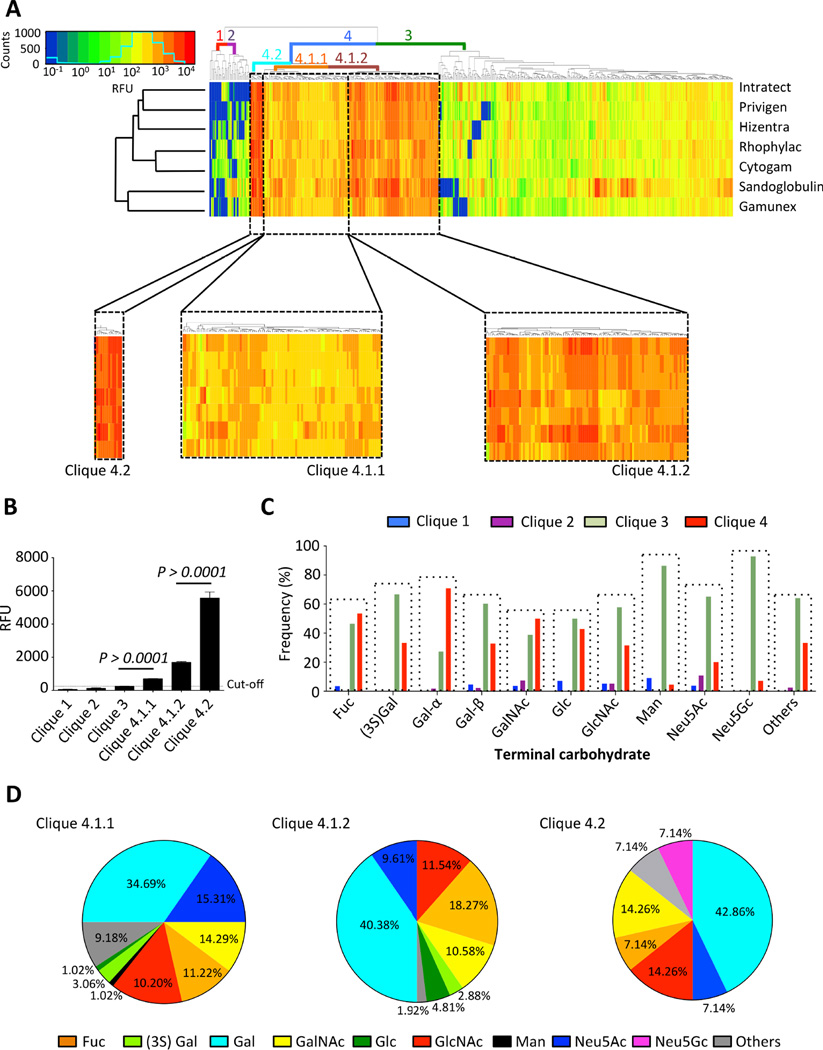
Performing hierarchical clustering analysis, 4 major subgroups of IgG antibodies with highly correlated reactivities (13), were identified in the repertoires of the IVIG/SCIG preparations (Fig. 3A). Only subgroup 4 and its three associated subordinate groups (4.1.1, 4.1.2, 4.2), which encompassed 35.41% of the printed glycan specificities, exhibited positive antibody results (Fig. 3B). Next, we explored to which extent the terminal carbohydrate moiety might correlate with the immunogenicity of the glycans. Structures with terminal mannose or Neu5Gc were only poorly represented in clique 4 (Fig. 3C) and they were absent or scarce in clique 4.2 that was associated with highest immune reactivity (Fig. 3D). Similarly, only a minority of carbohydrates containing terminal Neu5Ac, which is highly expressed at the terminal position of human glycoproteins and –lipids (14, 31), was found in clique 4 and only few distinct structures were present in clique 4.2. These findings suggest that tolerance mechanisms exist in healthy individuals that preclude generating adaptive immunity to glycan structures containing these terminal carbohydrate moieties. Indeed, Neu5Ac is a self-antigen that is ubiquitously expressed as the terminal moiety on many glycoproteins and glycolipids in human tissues, while Neu5Gc is a structurally related carbohydrate in non-human species (14). Diet-induced uptake of Neu5Gc and incorporation in human tissues as “xeno-autoantigens” may lead to an inflammatory condition called “xenosialitis” that has been associated with an increased risk of cancer (32). Anti-Neu5Gc antibodies in pooled human IgG have previously been described (33, 34). Indeed, we consistently observed intermediate signal intensity to one particular Neu5Gc-terminal structure (#556: Neu5Gcα2–8Neu5Gcα2–6Galβ1–4GlcNac) for most IVIG/SCIG preparations. In contrast, reactivity to other Neu5Gc-terminal glycans on CFG glycan array version 5.1 was low even for bacterial antigens (#282: Neu5Gcα2–3Galβ1–4(Fucα1–3)GlcNAc; and #555: Neu5Gcα2–8Neu5Gcα2–3Galβ1–4GlcNAcβ1–3Galβ1–4GlcNAc) (Fig. S4).
Fig. 3. The immunogenicity of glycans correlates with distinct terminal carbohydrate moieties, as revealed by systematic analysis of polyclonal IgG from healthy donor populations.
(A) The cliques of antibodies with highly correlated reactivities in the IgG repertoire of IVIG/SCIG preparations computed by the dendrogram clustering algorithm. Color coded lines indicate part of the dendrogram tree linked to cliques 1–4, and subordinate cliques 4.1.1, 4.1.2 and 4.2. The color key and distribution histogram is depicted. (B) Only cliques 4.1.1, 4.1.2 and 4.2 showed averaged RFU levels that were considerably higher than the isotype-controlled cut-off level, determined as outlined in the materials and methods section. Statistical analysis was performed using an unpaired t-test (n=7). Bars show mean ± SEM. (C) The clique distribution (clique 4: positive binding; cliques 1–3: no binding) of glycans with distinct terminal carbohydrate moieties. (D) Percentage of glycans with specific terminal carbohydrates represented in cliques 4.1.1, 4.1.2 and 4.2.
In contrast, glycans with terminal fucose, galactose, GalNAc, glucose and GlcNAc were abundantly represented in clique 4 (Fig. 3C). Most predominant in clique 4.2 were structures with terminal Gal (42.86%), its N-acetylated form GalNAc (14.26%) and GlcNAc (Fig. 3D), reflecting the high immunogenicity of carbohydrates with these terminal moieties. Together, these results suggest that the chemical structure of the terminal carbohydrate moiety has an impact on glycan immunogenicity and hence the IgG immune profile of healthy donors.
IgG-mediated reactivity to glycans associated with microbial antigens and host attachment sites
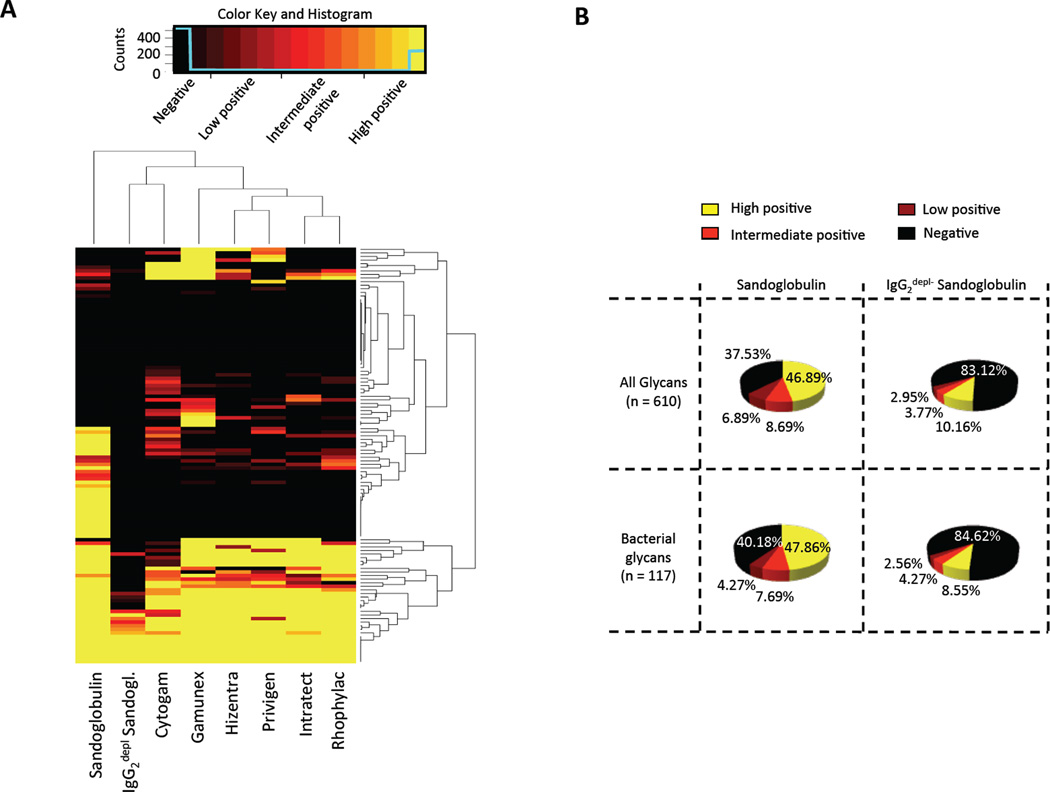
Next, we determined the identity, biological source and known function of the glycans recognized by the antibodies in the IVIG/SCIG. Out of the 610 glycans on the microarray 117 structures were defined as bacterial antigens by comparison to glycans in Bacterial Carbohydrate Structure Data Base (BCSDB). Figure 4A illustrates the computed reactivity matrix for the different immunoglobulin preparations. At least one third of these bacterial glycans were recognized by IgG antibodies in the IVIG/SCIG. Depletion of IgG2 resulted in a considerable loss of binding to bacterial and to all printed glycans (Fig. 4A) with distant reactivity signature as calculated by the dendrogram clustering algorithm (Fig. 4A). Sandoglobulin exhibited the widest spectrum of activity on glycans associated with bacteria (59.82% of bound bacterial glycans) forming an immune profile in the dendrogrammed matrix that is hierarchically distant from the other IVIG/SCIG preparations (Fig. 4A).
Fig. 4. Reactivity of IVIG/SCIG preparations with bacterial glycan antigens as identified using the Bacterial Carbohydrate Structure Data Base (BCSDB).
(A) Dendrogrammed glycan-reactivity matrix for identified bacterial antigens. The color key and distribution histogram indicates reactivity levels as outlined in materials and methods. (B) Comparison of IgG2-depleted Sandoglobulin and complete Sandoglobulin in terms of binding capacity to bacterial glycans and to the total of printed glycans on the array. Sectors of circle graphs indicate categories of signal intensity as defined in the Materials and methods section. Note: reactivity or non-reactivity to individual glycan epitopes on the array may not reflect overall reactivity to pathogens or functional properties of the individual preparations.
Bacterial glycans recognized by IVIG/SCIG included components of structural and capsular polysaccharides or exopolysaccharides of both commensal and pathogenic bacteria (fig. S5). Interestingly, these are composed of predominantly galactose (Gal)-terminated structures (60.00% of the top 20 recognized carbohydrate epitopes). In contrast, non-recognized bacterial structures frequently exhibited N-acetylneuraminic acid (Neu5Ac)-terminated glycans, a common feature of human glycoprotein and glycolipid structures and self-antigens (14, 31). The recognized bacterial structures included oligosaccharides of E. coli, lipopolysaccharides of Helicobater pylori, Acetinobacter baumanii, Haemophilus influenzae or E. coli, capsular polysaccharides of Streptococcus thermophilus and Streptococcus pneumonia as well as lipooligosaccharides and lipopolysaccharides of Neisseria gonorrhoeae and P. penneri or extrapolysaccharides of S. thermophilus and Lactobacillus reuteri. Besides bacterial glycans, antibodies in IVIG/SCIG also detected glycans expressed by other eukaryotic microorganisms, such as Leishmania major #100 (Galα1–2Galβ) and Trypanosoma cruzi #118 (Galα1–3Galβ) glycoconjugates (fig. S5).
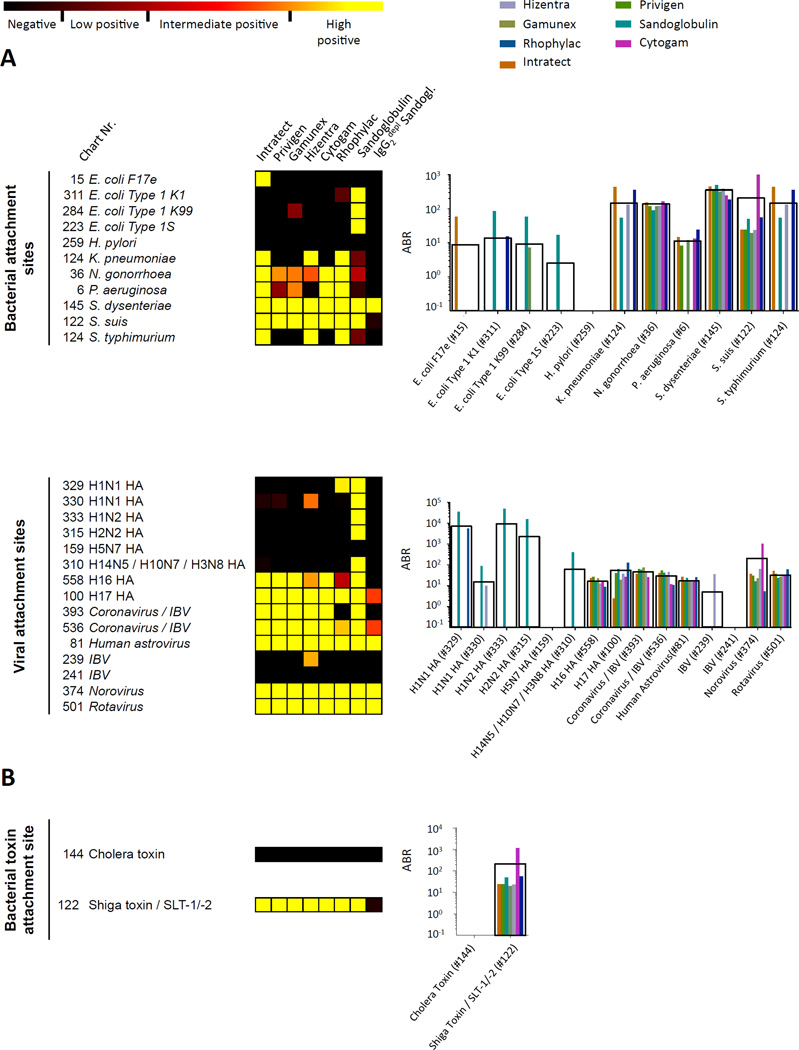
Epithelial mucosal surfaces, like all cells, are covered by a dense layer of polysaccharides or glycoproteins, the so-called glycocalyx. Adhesion to distinct host glycans is the prerequisite for microbial colonization or for most infectious diseases (18, 19). Specific carbohydrate attachment sites serve as ligands for viral and bacterial hemagglutinins, adhesins and lectins, which determine the tissue tropism of microbiota or infectious agents (18, 19). Given that IgG, similar to secretory IgA or IgM (35), has been shown to reach mucosal surfaces, such as within the gut, oral cavity and urinary tract (36), we set out to analyze binding capacities of IVIG/SCIG preparations to specific carbohydrate structures that represent well-known microbial attachment sites. The majority of these attachment sites were similarly bound by the various IVIG/SCIG preparations (Fig. 5A), as seen for attachment site glycan #36 in the genital tract for Neisseria gonorrhoeae, the attachment site in the respiratory tract for Streptococcus Suis #122, the intestinal attachment site for Shigella dysenteriae #145 or attachment sites for Corona viruses #393, Rotaviruses #501 and for the Norovirus #374, latter of which are the most common cause of gastroenteritis outbreaks and causing challenging infections in immunocompromised patients (37, 38). Most, but not all antibodies binding to viral or bacterial attachments sites appear to be IgG2 antibodies.
Fig. 5. Differential recognition of attachment sites for distinct viruses, bacteria (A) and bacterial toxins (B) by commercial IVIG/SCIG preparations.
On the left, heat map presentation, on the right, corresponding antigen binding ratio (ABR) values based on isotype controls, as outlined in the Materials and methods section, black boxes indicate average ABR values.
Several bacterial toxins are lectins that bind to host carbohydrate structures, including cholera toxin of Vibrio cholerae, Shiga toxin produced by Shigella dysenteriae type 1, and the homologous Shiga-like toxins (SLTs) of E. coli, also called Verotoxins (VTs) (39, 40). Interestingly, specific IgG antibodies to the carbohydrate-binding site #122 (Galα1–4Galβ1–4Glcβ) of Shiga toxin, and the E. coli SLT-I and SLT-II toxins were detected in all preparations with the exception of IgG2-depleted Sandoglobulin (Fig. 5B). In contrast, none of the preparations contained IgG antibodies to the glycan-binding site #144 (Galβ1–3GalNAcβ1–4(Neu5Acα2–3)Galβ1–4Glcβ) of cholera toxin. These findings show that IVIG/SCIG preparations contain a broad panel of IgG antibodies with specificity for carbohydrate-binding sites of microorganisms and distinct clinically relevant microbial toxins.
Reactivity of IVIG/SCIG to endogenous glycan epitopes and immune receptor ligands
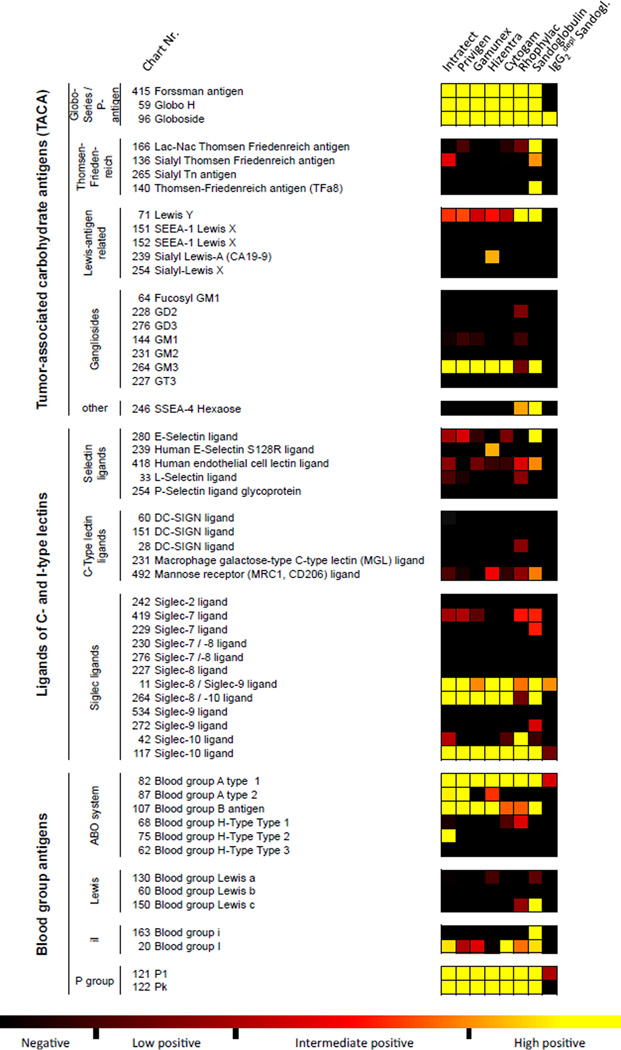
Endogenous glycans (e.g. blood group antigens, selectin and siglec ligands, tumor antigens, host attachment sites for pathogens) have important biological functions that may influence pathogenic and clinically relevant processes. Figure 6 depicts IVIG/SCIG binding profile to distinct biologically relevant glycans on the CFG glycan array version 5.1. IVIG/SCIG are known to contain IgG antibodies against blood group antigens and regulatory requirements are in place to control levels of these hemagglutinins to avoid adverse reactions in recipients (41). In our analysis, variable binding intensities of antibodies to blood group antigens were detected among the different preparations. The RFU levels of antibody binding for Privigen, Intratect and Hizentra against blood group A1 (glycan #82; RFU = 1793–2290) and A2 (glycan #87; RFU = 516–624) antigens were considerably higher than RFU levels of Sandoglobulin and Gamunex (A1: RFU = 984 or 1095; A2: RFU = 64 or 28, respectively). Although higher anti-A antibodies have been reported as a class effect for some IVIG preparations (42), the RFU values reported here do not necessarily reflect the actual anti-A reactivity of preparations, as certain determinants, such as binding affinity, were not assessed. Moreover, binding levels against blood group B (glycan #107) did not considerably differ between the preparations (RFU 748–1068). Reactivity to ligands of selectins, C-type lectins or siglecs was low or absent, which was not unexpected because of the self-antigenic nature of these endogenous molecules. One important exception was the binding of antibodies to the siglec ligands #11 (Neu5Acβ), #117 (Galα1–3Galβ1–4Glc), and #264 (Neu5Acα2–3Galβ1–4Glcβ). For glycans #11 and #264 BCSDB entries were found suggesting that these glycans represent exogenous siglec ligands.
Fig. 6. Differential recognition of known, biologically relevant, carbohydrate-structures by IVIG/SCIG preparations.
Color code with computed categorization of binding intensity is indicated, as outlined in the Materials and methods section.
Presence of IgG, but low frequency of non-IgG2 antibodies to tumor-associated carbohydrate antigens in healthy individuals
Cancer cells frequently display altered surface glycosylation and a number of known tumor-associated carbohydrate antigens (20, 21). Highly positive IgG antibody binding to the TACA Forssman antigen, Globo H, Globoside and GM3, and to a lesser extent to Lewis Y, was found in the analyzed IVIG/SCIG preparations (Fig. 6). The recognition of TACA, with the exception of Globoside, was predominantly dependent on the presence of IgG2 antibodies. Highly positive binding to the T or TF (Thomsen-Friedenreich) antigen (Galβ1–3GalNAcα) was exclusively detected for Sandoglobulin. In contrast, IgG binding to the majority of Lewis-antigen related TACA and gangliosides was low or absent. Together, these data demonstrate that IVIG/SCIG contains a broad range of anti-carbohydrate antibodies to biologically relevant glycans. Conversely, certain glycans, such as inflammation-associated carbohydrates and some TACA, are relatively non-immunogenic or are not expressed in sufficient quantity by healthy donors to induce detectable antibody responses. Interestingly, IgG reactivity (ABR; mean +/− S.E.M; assessed for glycans recognized with at least intermediate binding intensity by at least 6 out of 7 preparations) to bacterial glycans (3019.2 +/− 866.2) exhibited higher responses as compared to endogenous glycans, such as TACAs (19.39 +/− 2.25), ligands of C- and I-type lectins (23.3 +/− 5.08) or blood group antigens (1763 +/− 549.0), or endogenous attachment sites for viruses (53.5 +/− 20.8), bacteria (224.2 +/− 55.1) and bacterial toxins (193.5 +/− 140.0). This probably reflects the pathophysiological and immunological circumstances of their induction.
DISCUSSION
Immunoglobulin preparations that are clinically used as IVIG or SCIG formulations contain the immunological repertoire of IgG antibodies of the donor population with trace amounts of IgM or, IgA or IgE. Although most glycans are considered to be T cell-independent antigens (43), the present broad glycan analysis, using CFG glycan array version 5.1, revealed strong reactivity of IgG for at least one third of 610 different glycan, including both IgG2 or non-IgG2 isotype-switched antibodies. This observation complements recent evidence provided by blood transcription modules (BTMs) analysis which points to the involvement of CD4+ T cells in the generation of carbohydrate-specific antibody responses to polysaccharide vaccines (4). Indeed, the existence of polysaccharide-specific T cells has recently been reported in relation to αβTCR-mediated recognition of the carbohydrate portion of glycoconjugates in the context of major histocompatibility complex class II (MHC-II), these T cells produce cytokines such as IL-4 and IL-2 which lead to consequent B cell maturation and production of carbohydrate-specific IgG antibodies (44). Conversion of glycans into T cell-dependent antigens may occur if the specific carbohydrate is coupled to a carrier (glycoconjugates) and is presented as a glycopeptide or glycolipid antigen to T cells (45–47). However, in analogy to proteins, zwitterionic capsular polysaccharides from some bacteria can be processed and presented to CD4+ T cells by antigen presenting cells (APCs) in a MHC-II dependent fashion (48, 49). Alternatively, binding to certain glycan epitopes might result from cross-reactive antibodies originally raised to protein antigens. Indeed, use of peptide mimotopes as surrogates of carbohydrate antigens has been proposed as a promising strategy for vaccination (50). Our analysis supports the hypothesis that the IgG2 subclass is more commonly, but not exclusively associated with anti-carbohydrate immune responses (5), and contradicts the notion that the humoral glycan-binding capacity of IgG is restricted to the IgG2 subclass (51).
Strikingly, high correlations of glycan reactivities were identified for immunoglobulin preparations that are derived from different donor populations. Hierarchical clustering analysis revealed a modular organization of these immunoprofiles. Together, these observations point to a universal architecture of the human repertoire of carbohydrate-specific IgG. As many printed glycans represent self-antigens, such uniform signature of the repertoire matches the concepts of a functional system-level network organization of the autoantibody repertoire and the immunological homunculus hypothesis, suggesting that different individuals in similar physiological states share common features of their autoimmune repertoires (13, 52). Not surprisingly, healthy individuals had low or absent titers of antibodies against endogenous ligands for the immune receptors including selectins, C-type lectins, and siglecs. Important exceptions were some distinct siglec ligands that do not represent exclusive autoantigens, but are also expressed in bacteria. Notably, it has been reported that molecular mimicry of host sialylated glycans allows bacterial pathogens to engage inhibitory siglec receptors and dampen the innate immune response (53, 54). On the other hand, the similarities of reactivity to non-self-antigens, eventually induced by prior infection or vaccination, implies that humoral immune responses to carbohydrate antigens resulting in class-switched IgG antibodies must follow relatively uniform modes of events that finally determine the immunogenicity of a specific glycan.
Whether there are universal predictors of vaccine-induced immunity remains a central question in vaccinology (4). It has been realized that responses to pure polysaccharide vaccines are difficult to induce, and hyporesponsiveness or immune tolerance to repeated vaccination with polysaccharide antigens was already described by Felton and Bailey in 1926 (55). To date, hyporesponsiveness remains a limitation of clinically used polysaccharide vaccines, including the pneumococcal polysaccharide vaccine (PPV) PPV23, and the non-conjugated polysaccharide meningococcal and Haemophilus vaccines (8, 9, 56). Chemical conjugation of polysaccharide antigens to a carrier protein, such as for pneumococcal conjugate vaccines (PCV), is thought to improve vaccine efficacy due to commitment of supportive T-cell responses. In light of the prolonged persistence of polysaccharide antigens in vivo, it has been postulated that the high content of polysaccharide required for vaccination with PPV23, at least 575 µg as compared to <20 µg in PCV7, may be responsible for the phenomenon of hyporesponsiveness, by a mechanism that involves depletion or exhaustion of the B-cell pool, i.e. distinct memory and B1b–cell subsets, due to chronic antigen stimulation (56). Our analysis revealed modular organization and a non-random association of specific glycan-structures with antigen reactivity that are dependent on structural features, i.e. the terminal carbohydrate-moiety. The absent or low representation of glycans with terminal Neu5Ac, Neu5Gc and mannose in clique 4 (positive binding) of the hierarchical clustering analysis suggests that tolerance mechanisms must exist that limit the generation of IgG antibodies to these structures. Yet, in agreement with previous reports we found binding activity to certain Neu5Gc-xenoglycan structures, which may result from antibodies induced via dietary-Neu5Gc uptake by commensal bacteria (33, 34). In contrast, high immunogenicity was observed for glycans with terminal Fuc, Gal, GalNAc and GlcNAc, the latter three forming the majority of glycans found in clique 4.2 (highest antigen reactivity). Given the uniform and modular organization of the IgG repertoire of carbohydrate-specific antibodies, it is tempting to speculate that the recognition of an association between structural characteristics and immunogenicity of glycans has critical implications for the prediction of vaccine-induced immunity, and for the rational design of new-generation vaccines against emerging infections. Our findings suggest, that modification of glycan substructures of vaccine antigens by enrichment with immunogenic or avoidance of tolerogenic carbohydrate moieties might enhance the efficacy and potency of wide-spectrum polysaccharide vaccines such as PPV23, and reduce the frequency of patient hyporesponsivenss induced by excessive amounts of polysaccharides (56).
In an era of antibiotic resistance, viral pandemics and insufficient control of certain infectious diseases, alternative anti-infectious strategies, such as therapies interfering with pathogen adhesion might provide a valuable option for prevention or adjuvant therapy of local or systemic infectious diseases (18, 19). Glycan-based anti-adhesive strategies are being tested in preclinical and clinical studies for the prophylaxis of infection, or as combination therapy for antimicrobial treatment (18, 19, 57). Inhibition of cell adhesion due to the presence of anti-adhesive IgG antibodies to protein antigens in IVIG has been reported, and may contribute to its immunomodulatory effects (58). Our glycan array analysis revealed that IVIG/SCIG preparations contain antibodies not only to structural components of commensals or pathogenic microorganisms, but also to known mammalian glycan attachment sites for bacterial and viral pathogens. Multivalent antibody-mediated targeting of attachment sites by IVIG/SCIG might overcome limitations of current anti-adhesive strategies that have been hampered by the multiplicity and redundancy of microbial adhesion mechanisms, as well as the low-affinity of glycan-protein interactions (18, 19). Strikingly, we also detected IgG antibodies binding to the glycan moiety (#122) of glycolipid Gb3 that acts as mammalian cell binding-site of Shiga toxin secreted by Shigella dysenteriae type 1, as well as of the Verotoxins SLT-I and SLT-II of E. coli (39). The origin of these anti-adhesive IgG antibodies remains unclear, but they might consist of ‘natural antibodies’ (13, 59), anti-idiotypic antibodies (60), or cross-reactive antibodies. As IgG can be detected on mucosal surfaces (36), and further IgG molecules might reach body surfaces following leakage of the epithelial barrier, anti-adhesive IgG may act in concert with antibodies to microbial epitopes in order to maintain host defense against infection or to shape the microbiota on body surfaces, e.g. by glycan-selective and anti-adhesive pressure.
Distinctly glycosylated tumor antigens are diagnostically exploited as tumor markers (e.g., CA125, MUC1, CEA, and CA19-9). TACA on the array were recognized with different reactivity by IVIG/SCIG, but specific glycans were bound with similar reactivity by most preparations. TACA-specific antibodies in IVIG preparations have previously been reported, and a GD2 peptide mimotope was used for affinity chromatography to enrich specific antibodies in Gammagard more than 10 times (26). Given that most TACA represent autoantigens, the low level of reactivity to certain TACA was not surprising and reflects their potential as biomarkers for the detection of cancer. With the exception of Globoside, the antibody reactivity to TACA was mediated by IgG2 antibodies. This suggests that the search for diagnostic tumor markers should also include non-IgG2 antibodies of the IgG class.
Our findings highlight the utility of systems biology approaches to decipher the complexity of humoral immunity networks. The use of a high-throughput glycan array technology however does not overcome the need of subsequent functional experiments in order to confirm certain results. Use of the enlarged V5.1 glycan array allows for an efficient first screening for glycan reactivity patterns which then serve to direct future experimental validation of immunogenic versus tolerogenic terminal glycans. Glycans in vivo may exhibit additional three-dimensional configurations and certain protein-glycan interactions might be underestimated in glycan array screening assays. Furthermore, minor differences between various commercially available IVIG/SCIG products in this analysis may not translate into clinical effects.
Given the broad repertoire of carbohydrate-specific antibodies to biologically relevant glycans, and the aberrant glycan-specific humoral immunoprofile in autoimmune disease (12) and eventually in immunodeficiency, it is conceivable that the reconstitution of a normal repertoire using SCIG/IVIG might contribute to their beneficial effects (61). The identification of universal correlates of immunogenicity has implications for the prediction of immune responses and for a rational design of future vaccines. The reported findings might pave the way to improved and broader glycan-based diagnostic and therapeutic applications and to a better understanding of humoral immunity.
MATERIALS AND METHODS
Study design
This non-randomized study was designed to investigate the human IgG anti-carbohydrate repertoire using glycan array technology combined with a computational system level approach. The commercial IVIG/SCIG products were blinded and screened using CFG glycan array version 5.1, whereby glycan binding was assessed at least six times for each sample. The computation and statistical analysis of the data was performed as described.
Reagents
The IgG preparations used in this study included Sandoglobulin, Privigen, Hizentra, Cytogam and Rhophylac (CSL Behring AG), Gamunex (Talecris Biotherapeutics) and Intratect (Biotest). Sandoglobulin and Privigen (IgG content ≥98%) contained ≤2% and ≤0.01% IgA, respectively, and only traces of IgM, IgD, and IgE (as reported by CSL Behring). The IgG subclass composition of Sandoglobulin was analyzed by nephelometry (Quest Diagnostics) and contained principally IgG1 (61%) and IgG2 (32%), with small quantities of IgG3 (5%) and IgG4 (2%). Privigen contained 66% IgG1, 29% IgG2, 3% IgG3, and 3% IgG4 (as reported by CSL Behring). To remove the stabilizing sucrose component of Sandoglobulin, dialysis was performed as previously described (62). Further information on the immunoglobulin preparations is provided in table S2.
A control IgG preparation (IgG control mix) was made by mixing 2 monoclonal human myeloma proteins, IgG1λ (67%) and IgG2κ (33%), purchased from Sigma-Aldrich. This process resulted in a κ/λ ratio of 0.5, which is well within the range found in normal serum (0.26–1.65). Anti-human IgG monoclonal antibody (clone HP-6043-Biot), recognizing all IgG subtypes, and Streptavidin-Alexa633 were purchased from Invitrogen (Invitrogen, Life Technologies).
Glycan array analysis
The glycan microarrays from the CFG (http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml) were prepared from amine functionalized glycan structures covalently coupled in microarrays to N-hydroxysuccinimide-derivatized microscope slides as previously described (63). The IVIG preparations and mixed human myeloma IgG1 and IgG2 control were screened at 180 ug/ml for binding to glycans on CFG glycan array version 5.1 (610 different glycans) using anti-human IgG mAb at 5 ug/ml. Myeloma IgG (IgG control mix, see above) was used to determine the background level (95% percentile) (64). The computed antibody binding ratio (ABR) represents the quotient of the respective sample relative fluorescence unit (RFU) and the corresponding IgG control mix RFU (12). Data are expressed as the mean of RFU or ABR values from six repeated experiments, if not indicated otherwise. Antibody binding levels were arbitrarily defined based on RFU levels above the 95% percentile control mix cut-off (RFU > 10 SD: high positive; RFU 5–10 SD: intermediate positive; RFU 2–5 SD: low positive; RFU < 2 SD: negative).
IgG2 depletion of IVIG
IgG2 depletion was performed by column affinity chromatography as previously described (5). Greater than 99% of the IgG2 was removed from the anti-IgG2 affinity column-adsorbed Sandoglobulin IVIG fraction as assessed by surface plasmon resonance (5).
Database search
The identity or characteristics of glycans was investigated by consulting the databases of the Consortium of Functional Glycomics (http://www.functionalglycomics.org/fg/) or PubMed (http://www.ncbi.nlm.nih.gov/pubmed/guide/). The online Bacterial Carbohydrate Structure Data Base (BCSDB) was consulted to identify the bacterial origin of the glycans (http://csdb.glycoscience.ru/bacterial/).
Statistical analysis
Heatmap and hierarchical clustering were performed using “R” (The R Foundation for Statistical Computing, Version 3.0.2), statistical analysis and other illustrations were performed using Microsoft Excel (Microsoft Corporation, 2011, Version 14.0.0) and GraphPad PRISM (Graphpad Software, Inc., Version 6.0c). For clique distribution analysis, only groups with common terminal carbohydrate moiety were considered that are represented at least with 12 glycans (2% of total) on CFG glycan array version 5.1. For statistical analysis, a two-tailed unpaired t-test was used.
Supplementary Material
Acknowledgments
The authors thank Dr. Adrian W. Zuercher, Research and Development, CSL Behring AG, Bern, Switzerland, for his expert assistance. Several immunoglobulin preparations were kindly provided by CSL Behring AG, Bern, Switzerland.
Funding: This work was supported in part by CSL Behring AG, Bern, Switzerland, the Swiss National Science Foundation (SNSF) grant No. 310030_135734 and Research Scholarship Award (RS 01/2009) to S. von Gunten; the Bulgarian-Swiss Research Program (BSRP) No. IZEBZ0_142967 to S. von Gunten and T. Vassilev; and grant number GM62116 to the Consortium for Functional Glycomics, P41GM103694 and GM098791 to R.D. Cummings.
Footnotes
Author contributions: S.V.G., B.S.B., and D.F.S. designed the study. C.S. and S.V.G. analyzed the data. Glycan array experiments at the CFG were conducted under supervision of D.F.S. and R.D.C. Database searches and computational analysis of the data set were performed by C.S., K.F.B., and S.V.G. All authors had full access to the data, helped draft the report or critically revised the draft, contributed to data interpretation, and reviewed and approved the final version of the report.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Glycan array data (CFG projects 2324 and 2694) are deposited in the GFG database (http://www.functionalglycomics.org/).
SUPPLEMENTARY MATERIALS
Fig. S1. Recognition of distinct carbohydrate-structures (glycans) by IgG antibodies in Privigen IVIG
Fig. S2. Relative distribution of negative, high-, low and intermediate positive bound glycans.
Fig. S3. Similarities in glycan recognition among different IVIG preparations
Fig. S4. Immunogenicity of Neu5Gc-terminated glycans.
Fig. S5. IVIG binding to microorganism-associated glycans.
Table S1: Characteristics of immunoglobulin products used in the study
Table S2: Comprehensive list of glycans bound by antibodies contained in IVIG
REFERENCES
- 1.Brusic V, Gottardo R, Kleinstein SH, Davis MM, committee Hs. Computational resources for high-dimensional immune analysis from the Human Immunology Project Consortium. Nat Biotech. 2014;32:146–148. doi: 10.1038/nbt.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9:741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Gunten S, Smith D, Cummings R, Riedel S, Miescher S, Schaub A, Hamilton R, Bochner BS. Intravenous immunoglobulin contains a broad repertoire of anti-carbohydrate antibodies that is no restricted to the IgG2 subclass. J Allergy Clin Immunol. 2009;123:1268–1276. doi: 10.1016/j.jaci.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 8.Feldman C, Anderson R. Review: Current and new generation pneumococcal vaccines. J Infect. 2014;69:309–325. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. doi: 10.1016/j.ijantimicag.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Kamboj KK, Kirchner HL, Kimmel R, Greenspan NS, Schreiber JR. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J Infect Dis. 2003;187:1629–1638. doi: 10.1086/374785. [DOI] [PubMed] [Google Scholar]
- 11.Leonard EG, Canaday DH, Harding CV, Schreiber JR. Antigen processing of the heptavalent pneumococcal conjugate vaccine carrier protein CRM(197) differs depending on the serotype of the attached polysaccharide. Infect Immun. 2003;71:4186–4189. doi: 10.1128/IAI.71.7.4186-4189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grader-Beck T, Boin F, von Gunten S, Smith D, Rosen A, Bochner BS. Antibodies recognising sulfated carbohydrates are prevalent in systemic sclerosis and associated with pulmonary vascular disease. Ann Rheum Dis. 2011;70:2218–2224. doi: 10.1136/ard.2011.153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madi A, Hecht I, Bransburg-Zabary S, Merbl Y, Pick A, Zucker-Toledano M, Quintana FJ, Tauber AI, Cohen IR, Ben-Jacob E. Organization of the autoantibody repertoire in healthy newborns and adults revealed by system level informatics of antigen microarray data. Proc Natl Acad Sci U S A. 2009;106:14484–14489. doi: 10.1073/pnas.0901528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 15.Jandus C, Simon HU, von Gunten S. Targeting Siglecs--a novel pharmacological strategy for immuno- and glycotherapy. Biochem Pharmacol. 2011;82:323–332. doi: 10.1016/j.bcp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 16.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, Takala J, Villiger PM, Simon HU. Siglec-9 transduces apoptotic and non-apoptotic death signals into neutrophils depending on the pro-inflammatory cytokine environment. Blood. 2005;106:1423–1431. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 18.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Cozens D, Read RC. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev Anti Infect Ther. 2012;10:1457–1468. doi: 10.1586/eri.12.145. [DOI] [PubMed] [Google Scholar]
- 20.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 21.Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Démoulins T, Schneider C, Wehrli M, Hunger RE, Baerlocher GM, Simon H-U, Romero P, Münz C, von Gunten S. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124:1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onodera Y, Nam J-M, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124:367–384. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, Bovin N. Bovin, Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, Rodrigues LC, Gourdine J-P, Noll AJ, von Gunten S, Smith DF, Knirel YA, Paulson JC, Cummings DR. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol. 2014;10:470–476. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pashov A, Monzavi-Karbassi B, Kieber-Emmons T. Immune surveillance and immunotherapy: Lessons from carbohydrate mimotopes. Vaccine. 2009;27:3405–3415. doi: 10.1016/j.vaccine.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 27.Djoumerska I, Tchorbanov A, Pashov A, Vassilev T. The autoreactivity of therapeutic intravenous immunoglobulin (IVIG) preparations depends on the fractionation methods used. Scand J Immunol. 2005;61:357–363. doi: 10.1111/j.1365-3083.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 28.Bouvet J-P, Stahl D, Rose S, Quan CP, Kazatchkine MD, Kaveri SV. Induction of natural autoantibody activity following treatment of human immunoglobulin with dissociating agents. J Autoimmun. 2001;16:163–172. doi: 10.1006/jaut.2000.0472. [DOI] [PubMed] [Google Scholar]
- 29.Riesen WF, Skvaril F, Braun DG. Natural infection of man with group A streptococci Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5:383–390. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 30.Yount WJ, Dorner MM, Kunkel HG, Kabat EA. Studies on human VI antibodies Selective variations in subgroup composition and genetic markers. J Exp Med. 1968;127:633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen M, Varki A. The sialome--far more than the sum of its parts. OMICS. 2010;14:455–464. doi: 10.1089/omi.2009.0148. [DOI] [PubMed] [Google Scholar]
- 32.Samraj A, Läubli H, Varki N, Varki A. Involvement of a non-human sialic acid in human cancer. Front Oncol. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amon R, Reuven EM, Leviatan Ben-Arye S, Padler-Karavani V. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115–122. doi: 10.1016/j.carres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–S15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 37.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frange P, Touzot F, Debré M, Héritier S, Leruez-Ville M, Cros G, Rouzioux C, Blanche S, Fischer A, Avettand-Fenoël V. Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J Infect Dis. 2012;206:1269–1274. doi: 10.1093/infdis/jis498. [DOI] [PubMed] [Google Scholar]
- 39.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 40.Merritt EA, Sarfaty S, Akker FVD, L'Hoir C, Martial JA, Hol WGJ. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorpe SJ, Fox B, Sharp G, Heath AB, Behr-Gross ME, Terao E, Virata-Theimer ML, Yu MW. International collaborative study to evaluate candidate reference reagents to standardize haemagglutination testing for anti-A and anti-B in normal intravenous immunoglobulin products. Vox Sang. 2009;97:160–168. doi: 10.1111/j.1423-0410.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- 42.Dhainaut F, Guillaumat PO, Dib H, Perret G, Sauger A, de Coupade C, Beaudet M, Elzaabi M, Mouthon L. In vitro and in vivo properties differ among liquid intravenous immunoglobulin preparations. Vox Sang. 2013;104:115–126. doi: 10.1111/j.1423-0410.2012.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snapper MC. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr Protein Pept Sci. 2006;7:295–305. doi: 10.2174/138920306778017972. [DOI] [PubMed] [Google Scholar]
- 44.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor CE, Cobb BA, Rittenhouse-Olson K, Paulson JC, Schreiber JR. Carbohydrate moieties as vaccine candidates: Targeting the sweet spot in the immune response. Vaccine. 2012;30:4409–4413. doi: 10.1016/j.vaccine.2012.04.090. [DOI] [PubMed] [Google Scholar]
- 47.Lucas AH, Rittenhouse-Olson K, Kronenberg M, Apicella MA, Wang D, Schreiber JR, Taylor CE. Carbohydrate moieties as vaccine candidates: Meeting summary. Vaccine. 2010;28:1121–1131. doi: 10.1016/j.vaccine.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 48.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 50.Cunto-Amesty G, Luo P, Monzavi-Karbassi B, Lees A, Alexander J, del Guercio MF, Nahm MH, Artaud C, Stanley J, Kieber-Emmons T. Peptide mimotopes as prototypic templates of broad-spectrum surrogates of carbohydrate antigens. Cell Mol Biol. 2003;49:245–254. [PubMed] [Google Scholar]
- 51.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–134. [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992;13:490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 53.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang YC, Olson J, Beasley FC, Tung C, Zhang J, Crocker PR, Varki A, Nizet V. Group B streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014;10:e1003846. doi: 10.1371/journal.ppat.1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felton LD, Bailey G. Biological significance of the soluble specific substances of pneumococci. J Infect Dis. 1926;38:131–144. [Google Scholar]
- 56.Clutterbuck EA, Lazarus R, Yu L-M, Bowman J, Bateman EAL, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, Pollard AJ. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–1416. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. 1996;347:1017–1021. doi: 10.1016/s0140-6736(96)90150-6. [DOI] [PubMed] [Google Scholar]
- 58.Vassilev T, Kazatchkine M, Van Huyen J, Mekrache M, Bonnin E, Mani J, Lecroubier C, Korinth D, Baruch D, Schriever F, Kaveri S. Inhibition of cell adhesion by antibodies to Arg-Gly-Asp (RGD) in normal immunoglobulin for therapeutic use (intravenous immunoglobulin, IVIg) Blood. 1999;93:3624–3631. [PubMed] [Google Scholar]
- 59.Kaveri SV. Intravenous immunoglobulin: Exploiting the potential of natural antibodies. Autoimmun Rev. 2012;11:792–794. doi: 10.1016/j.autrev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Schaub A, von Gunten S, Vogel M, Wymann S, Rüegsegger M, Stadler BM, Spycher M, Simon HU, Miescher S. Dimeric IVIG contains natural anti-Siglec-9 autoantibodies and their anti-idiotypes. Allergy. 2011;66:1030–1037. doi: 10.1111/j.1398-9995.2011.02579.x. [DOI] [PubMed] [Google Scholar]
- 61.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 62.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 63.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong C-H, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, Fiscella M, Gorovits B, Kirschner S, Moxness M, Parish T, Quarmby V, Smith H, Smith W, Zuckerman LA, Koren E. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–1281. doi: 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.