Abstract
Strategies for the total synthesis of complex natural products that contain two or more contiguous stereogenic quarternary carbon atoms in their intricate structures are reviewed with twelve representative examples. Emphasis has been put on the methods to create quarternary carbon stereocenters, including syntheses of the same natural product from different groups, thereby showcasing diversity of thought and individual creativity. A compendium of selected natural products containing two or more contiguous stereogenic quarternary carbon atoms and key reactions in their total or partial syntheses is provided in the Supporting Information section.
Keywords: contiguous quarternary carbon atoms, natural products, catalytic methods, biomimetic synthesis
Graphical Abstract
While biosynthetically easily accomplished by Nature’s evolutionary machinery with an arsenal of enzymatic processes among others, the incorporation of multiple quaternary carbon centers in natural products by total synthesis is one of the most daunting challenges that have faced synthetic chemists for decades. The period spanning the last 50 or more years has seen phenomenal progress in this area as evidenced by the first synthesis of thujopsene by Dauben in 1953, and the last synthesis of hyperforin by Maimone in 2015. In this review we highlight strategies used by different research groups toward the stereocontrolled total synthesis of a selected group of natural products that contain two or more contiguous quaternary carbon atoms
1. Introduction
Long before the accidental synthesis of urea by Friedrich Wöhler, which is historically considered to be the first recorded synthesis of a “natural product”,[1,2] humankind had already recognized the beneficial effects of plant extracts as remedies for its physical ills.[3] Anecdotal accounts of miraculous cures in the form of potions and the likes within some cultures, now known as non-traditional or alternative medicines, continue to perpetuate our fascination with natural products.[4] For millennia, Nature has been the provider, the healer, and the enticer.[4,5] The variety of known chemical structures today is indeed overwhelming. Of these, only a minuscule number has been studied for their biological properties,[6] while only a mere fraction has been synthesized or chemically manipulated.[7] The choice of target molecules to synthesize has been justified among other reasons, for their promise as potential therapeutic agents, a statement that is often found in the introductory paragraphs of published manuscripts. Already during the second half of the twentieth century the total synthesis of many highly complex natural products had been achieved, thanks to continually evolving methods of stereoselective reactions,[8] separation techniques, and spectroscopic analyses. The incentive to undertake the total synthesis of architecturally challenging natural products continues to prevail to this day, as our understanding of the biological effects at the molecular level becomes more and more evident thanks to ground-breaking advances in genetics and molecular biology. More than ever, synthetic organic chemists are engaging in fruitful collaborations with scientists in other disciplines based on newly discovered activities of known natural products. Thus, biology-inspired and chemistry-driven projects also relying on structure-based organic synthesis is becoming widely acknowledged.[6] In this regard, the “what” and “why” of total synthesis[4] may no longer need to be justified, especially when the target molecules are expected to provide important answers to biologically relevant questions. Having decided on a given target molecule to synthesize, we are confronted with the highly subjective task of “how”. [4] Past and present accomplishments have demonstrated the ingenuity, creativity and resolve of synthetic organic chemists to initiate and complete the total synthesis of a plethora of natural products.[4,9] Among these are a small number that encompass quaternary carbon atoms on one or more stereogenic centers, which when embedded within the core structure of an intended target molecule, presents a formidable challenge to a synthetic chemist. Creative solutions to such problems have been successfully addressed for natural products that only a few years ago would have been considered as beyond reach.[10]
In this review, we present a selection of such successful syntheses focusing on natural products that contain two and three quaternary carbon atoms of which at least two are contiguous. Nature’s ingenuity (and reasons) to create functionally and architecturally complex molecules that may contain up to four contiguous quaternary carbons atoms of which none are methyl bearing, such as in the neo-clerodane diterpenoid musabalbisiane A,[11] illicits awe and fascination. Whether or not, such molecules are biosynthesized for defense mechanisms against other natural predators, may forever remain a mystery. However, the apparent facility with which Nature constructs molecules of such high complexity is at first sight misleading. In fact, in many cases Nature uses highly functionalized precursors that are “pre-organized” to undergo intra- and intermolecular cyclizations as in enzyme (and non-enzyme)-catalyzed Diels–Alder reactions to achieve its objectives.[12] These late-stage transformations in biosynthetic pathways take place in a timeframe that greatly contrasts the often much slower reaction times when similar cyclizations are attempted in the laboratory. Nevertheless, elegant “biomimetic” total syntheses under extremely mild reaction conditions have been reported which also rely on pre-organization and proximity effects of reacting partners.
A brief introduction to each natural product is followed by a discussion of relevant and key reactions especially when quaternary carbon atoms are introduced. Syntheses of one and the same natural product by more than one research group are presented in order to demonstrate different methods and strategies in creating stereogenic quaternary carbon atoms embedded in the structures of highly complex natural products,. A particular challenge Spresents itself when the hindered quaternary steeocenters in a target molecule are adjacent.. We have limited our discussion to a total of twelve natural products, which in our opinion combine the criteria of diversity of strategies with individual creativity. A compendium of selected natural products containing two or more contiguous stereogenic quarternary carbon atoms and key reactions in their total or partial syntheses is provided in the Supporting Information section.
2. Sordaricin
Sordarin is a diterpene glycoside that was isolated from Sordaria araneosa and shown to possess antifungal properties with a unique mode of action.[13,14] It was established that sordaricin, the aglycone, is derived from the biosynthetic precursor cycloaraneosene, and it was proposed that the quaternary carbon centers arise from an intramolecular [4+2] cycloaddition.[15] Sordaricin is characterized by a complex and richly functionalized tetracyclic architecture featuring an unprecedented presence of three contiguous quaternary centers, one being a bridgehead carbon atom. Key steps in the three total syntheses of sordaricin are highlighted below.[16]
2.1. The Kato synthesis
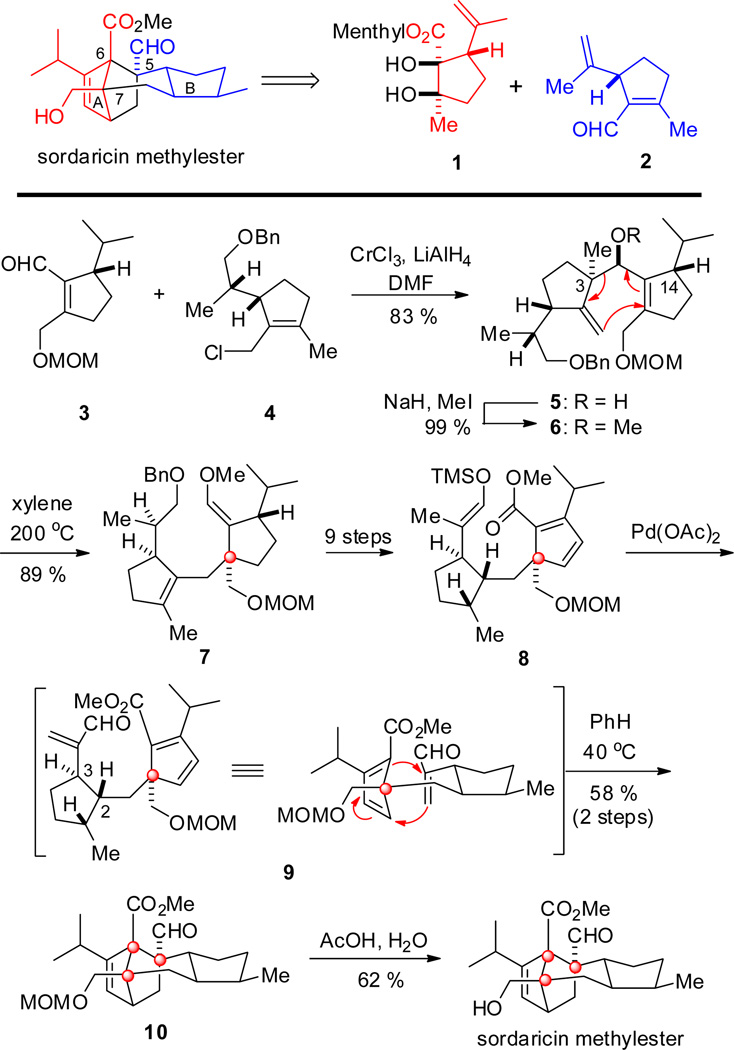
In 1993, Kato and co-workers completed the first total synthesis of optically pure sordaricin as its methyl ester (Scheme 1).[17] The key reactions for the synthesis of the polycyclic core harboring three contiguous quaternary centers were a Cope rearrangement and a bio-inspired intramolecular Diels–Alder cycloaddition. It was envisaged that two separate cyclopentane units, diol 1, and (3S)-1-iriden-7-al 2 obtained by chemical resolution of an iridoid menthyl ester originally synthesized from isoprene and methyl 2,4-dioxopentanoate,[18] may be used as chiral building blocks for the synthesis of the rings A and B (Scheme 1).
Scheme 1.
Key steps in the synthesis of sordaricin methyl ester by Kato and co-workers.
Thus, 3 and 4 (available from 1 and 2, respectively) were coupled through a Nozaki-Hiyama-Kishi reaction to generate secondary alcohol 5 which was O-methylated to 6. An irreversible Cope rearrangement then led to the formation of the first quaternary center at C-7 as in 7. It is noteworthy that, in developing a general strategy for the synthesis of related polyterpenes, Kato had previously established that such a Cope rearrangement proceeded via a boat-transition state in order to avoid a severe steric clash between the isopropyl group at C-14 and the methyl group at C-3.[18] Further functionalization of 7 afforded diene 8 which, under Saegusa conditions, yielded the Diels–Alder precursor 9. The intended biomimetic cycloaddition[19] then proceeded smoothly, albeit slowly, to generate the remaining two contiguous quaternary centers. Deprotection of the MOM ether 10 afforded sordaricin methyl ester.
The biomimetically-inspired total synthesis of sordaricin by Kato and co-workers features a late-stage intramolecular Diels–Alder cycloaddition that generates two contiguous quaternary carbon centers simultaneously next to an adjacent quaternary carbon. It is therefore impressive that this key cycloaddition could be achieved at a temperature as low as 40 °C.
2.2. The Mander synthesis
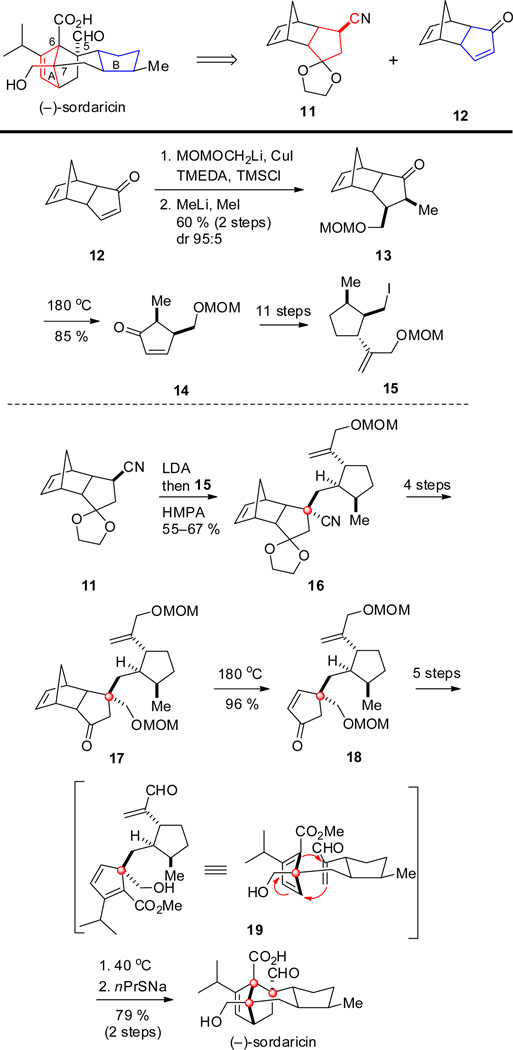
In a later independent study, Mander and co-workers completed the total synthesis of (−)-sordaricin in an approach that featured a late-stage intramolecular Diels–Alder cycloaddition reaction to generate the same two quaternary centers as in the Kato synthesis (Scheme 2).[20] Strategically, and considering the nature of the rings, Mander and co-workers chose norbornene intermediates 11 and 12 as starting materials whose cyclopentane and cyclopentene rings could be further functionalized to eventually converge with rings A and B respectively. Intermediate 12, which was obtained enantioselectively from cyclopentadiene,[21] was transformed to 13 in good yield and in excellent exo-diastereoselectivity due to the shape of the molecule. Thermal retro-Diels–Alder ring-opening of 13 led to the extrusion of the norbornene moiety and afforded cyclopentenone 14 in good yield, which was further elaborated to trisubstituted cyclopentane 15. Nitrile 11, which was prepared in an expedient and convergent fashion from intermediate 12,[22] was then combined with 15 upon alkylation to generate the first quaternary center at C-7 as a single diastereoisomer 16. Further transformations led to ketone 17, which was subjected to a thermally induced cycloreversion to give cyclopentenone 18. Subsequent transformations led to 19 which underwent intramolecular Diels–Alder cycloaddition smoothly at 40 °C, as in the Kato synthesis. In fact, cyclization proceeded slowly even at temperatures as low as 10 °C.
Scheme 2.
Key steps in the synthesis of sordaricin by Mander and co-workers.
It is noteworthy that the Diels–Alder cycloaddition of the allylic alcohol equivalent of 19 (instead of the unsaturated ester) also was successful in spite of its unfavorable HOMO–LUMO interactions, although the reaction in this case had to be carried out at 100 °C. Final saponification yielded (−)-sordaricin. A more extensive study of the Diels–Alder reaction in the preparation of simplified intermediates of sordaricin was reported by Liang, Ciufolini and co-workers.[16,23]
2.3. The Narasaka synthesis
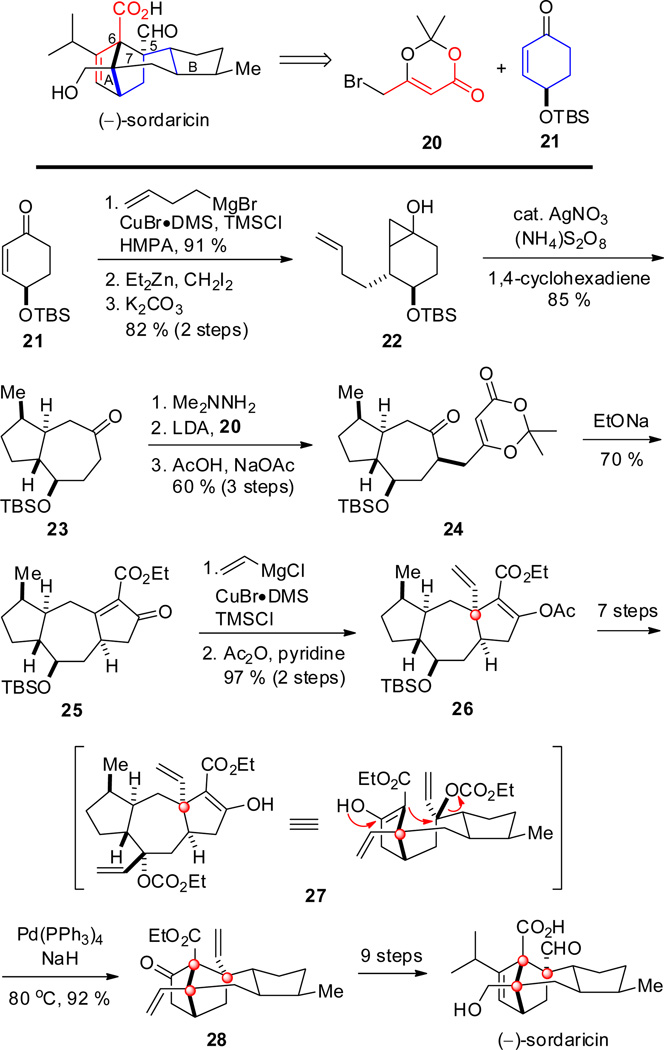
After having achieved a total synthesis of sordarin and sordaricin in racemic form in 2004,[24] Narasaka and co-workers completed an optimized, enantioselective total synthesis of both in 2006 (Scheme 3).[25]
Scheme 3.
Key steps in the synthesis of sordaricin by Narasaka and co-workers.
The key step in the synthesis was a Pd(0)-catalyzed intramolecular nucleophilic displacement of an allylcarbonate by a β-keto ester enolate to generate two of the three contiguous quaternary centers in one step. Enantiomerically pure chiron 21, prepared from quinic acid,[26] was utilized to generate an octahydroazulenone core 23 via a free-radical cyclization of cyclopropyl intermediate 22. Alkylation of the dimethylhydrazone derivative of 23 with bromide 20 gave ketone 24, which upon base-promoted cyclization, afforded tricyclic intermediate 25. A cuprate conjugate addition generated the first quaternary center of 26 at C-7 as a single epimer in good yield. After further transformations, the allylic carbonate intermediate 27 was subjected to a modified intramolecular Tsuji–Trost allylation,[27] to form the bicyclo[2.2.1]heptan-2-one core 28, the structure of which was corroborated by X-ray analysis. Considering the steric environment of the reacting partners, this transformation occurred in an astounding 92% yield. In studying this reaction as a strategy to form the tetracyclic core of sordaricin, it was found that the presence of stoichiometric NaH was critical, without which the diene formed by β-hydride elimination was the major product. A sequence of standard steps was then performed to transform the core structure 28 into (−)-sordaricin. The authors also completed the synthesis of sordarin by a glycosidation reaction.
The three total syntheses of sordaricin involved the use of enantiopure starting materials which in the case of Kato and Narasaka also provided a good portion of the carbon skeleton of sordaricin. Unlike Kato and Mander who used an intramolecular Diels-Alder reaction to set up the last two contiguous quaternary carbons, the Narasaka synthesis is conceptually different in the use of a Pd(0)-catalyzed intramolecular ring closure to stereoselectively establish the last two quaternary centers.
3. Vannusals A and B
Highly oxygenated marine natural products representing structurally unique triterpenes called vannusal A and vannusal B (differing only in the presence or absence of the C-25 acetyl group), were isolated in 1999 by Guella and co-workers[28] as secondary metabolites from tropical interstitial ciliate Euplotes vannus strains Si121 and BUN3. Structure determination using mass spectrometry, NMR spectroscopic data, and chemical transformations revealed a complex steroid-like structure possessing a 30-carbon backbone containing seven rings and 13 stereogenic centers that harbor three quaternary carbon centers of which two are contiguous.[29] The vannusals are biogenetically derived from farnesyl pyrophosphate with hemivannusal being the key intermediate leading to vannusal B by an intermolecular aldol reaction (dimerization) to set up the contiguous quaternary centers.[28,30] In spite of the intriguing and novel structure, no biological activity has been reported for the vannusals. The structurally most challenging feature is the presence of a spiroannulated cyclopentanol-norbornane subunit as part of an uniquely arranged triterpene architecture. Key steps in the total synthesis of vannusal B by Nicolaou and coworkers are highlighted below.
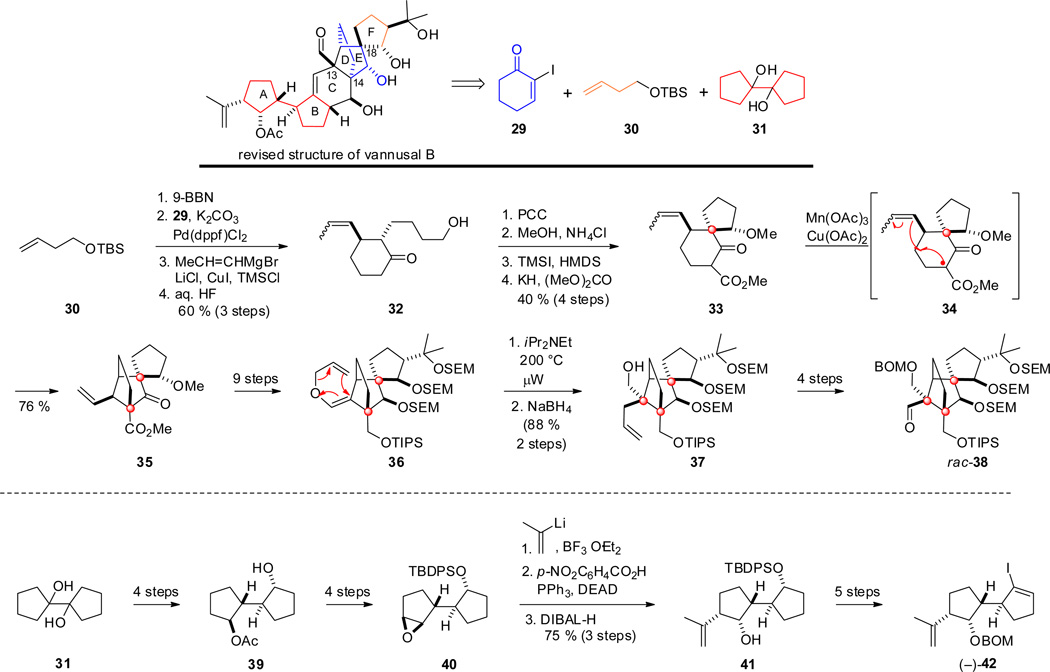
The enantioselective total synthesis of vannusal B in in 2010 also confirmed its absolute configuration.[29] Only after a series of elegant and careful studies involving the synthesis and characterization of no less than eight diastereomers would the correct structure and absolute configuration of vannusal B be secured beyond doubt.[31] The complex 30-carbon backbone structure containing six carbocyclic rings of which two are joined in a spirocyclic fashion was visually disconnected into two readily available starting materials ケ-iodocyclohexenone 29, and TBS protected 3-butenol 30 representing ring D (and the later to be formed ring F). Cyclopentanol dimer 31 would converge with rings A and B (Scheme 4).
Scheme 4.
Key steps in the synthesis of the intermediates of vannusal B by Nicolaou and co-workers.
For the preparation of the "northeastern" part, 29 and 30 were coupled under Suzuki conditions, followed by a Cu-mediated Michael addition and silyl ether deprotection to give the racemic alcohol 32, which was oxidized to the aldehyde, and the corresponding dimethylacetal was cyclized in the presence of TMSI, and the product further functionalized to the spirocycle 33.[32] Free-radical cyclization with Mn(OAc)3 led to tricycle 35 via a single electron oxidation by Mn(III) as shown in expression 34,[33] followed by Cu(II)-mediated single electron oxidation to give the exocyclic vinyl group in 35. This sequence led to the first quaternary center at C-14 in ring E with control of stereochemistry.[32] Further elaboration yielded allyl vinyl ether 36, which was subjected to a microwave-assisted Claisen rearrangement followed by reduction of the resulting aldehyde to give alcohol 37 in excellent yield, thus generating the second contiguous quaternary center at C-13.[31] This rearrangement was controlled by the spatial orientation of the ethano bridge. Intermediate 37 was further converted to racemic building block rac-38.[31c]
The preparation of the "southwestern" part of vannusal B commenced with a double dehydration of 31, followed by a double hydroboration, conversion to the diacetate and enzymatic desymmetrization to give the enantiomerically enriched acetate 39, which was further elaborated into epoxide 40 (Scheme 4).[31a] Regio- and diastereoselective epoxide opening with 2-propenyllithium, inversion of the resulting 'up'-alcohol under Mitsunobu conditions and ester cleavage yielded alcohol 41, which was converted into the enantiomerically enriched vinyl iodide building block (−)-42.[31a]
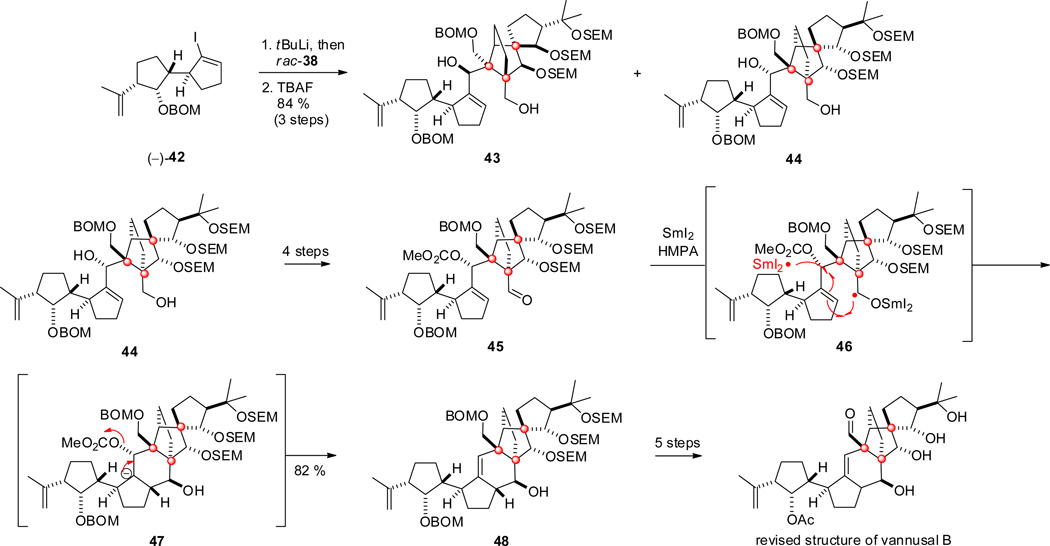
Transmetalation of vinyliodide (−)-42 and addition to racemic aldehyde rac-38, followed by chromatographic separation of the diastereomers, and TIPS deprotection gave the alcohols 43 and 44 in good yield and a ratio of 1:1 (Scheme 5).[31c] Based on the extensive comparisons of products arising from other diastereomers with natural vannusal B, only the data from 44 corresponded to the required spectral characteristics for the "northeastern" part; this isomer was further elaborated into 45. SmI2-mediated[34] cyclization via ketyl radical 46 and anionic hexacycle 47 by two single electron reductions and carbonate elimination furnished 48 as a single diastereomer in good yield. Further steps finally led to synthetic vannusal B, which was found to be identical to the proposed structure (Scheme 5).
Scheme 5.
Completion of the synthesis of the revised structure of vannusal B by Nicolaou and co-workers.
A noteworthy feature of the Nicolaou synthesis of vannusal B and other diastereomers thereof is a Suzuki coupling and cuprate addition giving selectively the tans-product 32, a strategy that was later developed into a catalytic asymmetric three-component 1,4-addition/aldol reaction.[35] Other remarkable features are the spirocyclization and the Mn- and Cu-mediated oxidative free-radical cyclization to generate the highly complex spirocyclopentanol-norbornane subunit. Finally, the SmI2-mediated cyclization and elimination completing the elaboration of the vannusal core structure is remarkable in its selectivity and efficiency.
4. Acutumine
A chlorine-containing alkaloid belonging to the hasubanan family named acutumine, was isolated in 1929 by Goto and Sudzuki,[36] as a minor component of the medicinal herb Sinomenium acutum. Its structure, determined nearly fifty years later by Tomita and co-workers[37] using X-ray crystallography, revealed a highly oxygenated tetracyclic skeleton containing four contiguous stereogenic carbon atoms of which two are quaternary. Other structurally intriguing features are the presence of a spiroannulated cyclopentenone-chlorocyclopentane subunit as part of an oxidized hexahydroindole ring and an azapropellane core. Acutumine is believed to biosynthetically arise from two units of tyrosine, which are further elaborated to a tetracyclic intermediate closely related to acutumine.[38] It is known for its analgesic properties and reported to selectively inhibit human T-cell growth with potential memory enhancing properties.[39] Two total syntheses of acutumine adopting entirely different strategies have been reported. Key steps are highlighted below.
4.1. The Castle synthesis
The first enantioselective synthesis of (−)-acutumine was reported by Castle and co-workers in 2009 (Scheme 6).[40,41] The key reaction leading to a highly stereoselective spiroannulation and the creation of the first of two quaternary centers at C-8 was based on a radical-polar crossover reaction.[42] Visual disconnection of the complex tetracyclic structure led Castle and co-workers to select the readily available, enzymatically desymmetrized chiron 49 and aryl iodide 50 as suitable starting materials to produce 51, which was converted to the allylic chloride 52 with inversion of configuration. Treatment with hexabutyldistannane under sunlamp photolysis conditions, and trapping the resulting α-keto radical intermediate with the Davis oxaziridine reagent 53[43] led to the spirocyclic product 54 as the major stereoisomer. Further steps afforded quinone 55, which was stereoselectively allylated with the Nakamura reagent 56[44] to give 57. Exposing allylic alcohol 57 to KOtBu and 18-crown-6 led to the oxy-Cope product 58, thus generating the second contiguous quaternary carbon center at C-9. Oxidative cleavage of the allylic double bond, followed by reductive amination and treatment with BCl3 triggered a Lewis-acid promoted cyclization to give 59. Further steps led to acutumine and its enol ether regioisomer 60.
Scheme 6.
Key steps in the synthesis of acutumine by Castle and co-workers.
Noteworthy features of the Castle synthesis of (−)-acutumine include an intramolecular tin radical-mediated conjugate addition of an aryl iodide onto a cyclopentenone and in-situ hydroxylation with high stereoselectivity, generating the unique spirocyclic motif. Another remarkable feature is the creation of a highly congested contiguous quaternary center based on an anion accelerated oxy-Cope rearrangement which was realized in 92% yield even at 0 °C. Since the direct 1,4-conjugate addition of an allyl group was not successful with model compounds, it was not attempted in the total synthesis. Thus, the successful implementation of the anionic oxy-Cope rearrangement to obtain 58, harboring the second quaternary carbon center was a most welcome alternative. A detailed account of the synthesis of acutumine by Castle and co-workers[41] provides further insights into the various strategies employed.[45]
4.2. The Herzon synthesis
Visual disconnection of acutumine into subunits representing rings A and B led Herzon and co-workers[46] to enantioenriched cyclopentenone 62, which is readily available from D-ribose in gram quantities, and the aromatic azide 61 as suitable starting materials (Scheme 7). Intermolecular Diels–Alder reaction between quinone 63 and trimethylsilyl cyclopentadiene 64 in the presence of the triflate salt of the Corey-Bakshi-Shibata (CBS) oxazaborolidine reagent,[47] led to endo-product 65 in excellent enantioselectivity.[48] Conversion to an amine via a Staudinger reaction, followed by N-methylation, gave iminium intermediate 66, which was allowed to react with lithium acetylide 67 (prepared from 61 in four steps) to give 1,2-adduct 68 in excellent yield as a single diastereomer. Thermal extrusion of silylcyclopentadiene provided the retro-Diels–Alder product 69 in a remarkable 98 % yield. Pd-catalyzed hydrostannylation led to the vinylstannane 70, which upon treatment with TBAF triggered an intramolecular conjugate addition, according to the Hosomi–Sakurai protocol,[49] to provide spirocyclic intermediate 71 as a single diastereomer, thereby securing the contiguous quaternary centers at C-8 and C-9 in a highly stereoselective manner in one synthetic operation. Exposure to cupric chloride promoted smooth destannylation and subsequent treatment with p-TsOH led to tetracyclic vinyl chloride 72. Adjusting its oxidation level over several steps led to dehydroacutumine 73. Rh-catalyzed homogeneous hydrogenation gave acutumine, whereas reduction with Pd/C and H2 gave (−)-dechloroacutumine 74.
Scheme 7.
Key steps in the synthesis of acutumine by Herzon and co-workers.
It is of interest that the Castle and Herzon approaches to acutumine started with enantiopure chirons which set up relative and absolute stereochemistries early in their respective syntheses. However, the strategies used for the crucial spiroannulation reaction are conceptually very different. Castle used a photochemically induced and highly stereoselective radical polar crossover reaction. Herzon, on the other hand, succeeded in generating the two contiguous quaternary carbon centers in acutumine via a cascade intramolecular conjugate reaction triggered by attack of fluoride on a distal trimethylsilyl group. Whereas D-ribose was the original source of the hydroxyl group in ring A of the Herzon synthesis, Castle resorted to a stereoselective hydroxylation. It is also of interest how and when the chlorine atom was introduced in the two syntheses
5. Bukittinggine
A lactone-containing alkaloid belonging to the Daphniphyllum family named bukittinggine was isolated in 1990 by Arbain and co-workers,[50] as the major component from the leaves and branches of Sapium baccatum. Its structure, as determined using X-ray crystallography, by Gibbons and Trotter,[51] revealed a heptacyclic skeleton containing nine contiguous stereogenic carbon atoms of which two are contiguous and quaternary. Another structurally intriguing feature within the azapolycyclic framework is the presence of a δ-lactone and an octahydroindolizidine subunit bearing a methyl substituent. Due to the close structural relationship with the daphniphyllum alkaloids,[52] bukittinggine is believed to biosynthetically arise from squalene dialdehyde which is produced from DL-mevalonic acid.[53,54] It is known for its anti-inflammatory activity with a presumed mechanism of action similar to that of acetylsalicylic acid.[55] The strategy toward the total synthesis of bukittinggine was inspired by a previously established biomimetic approach to the pentacyclic secodaphniphylline alkaloids pioneered by the Heathcock group.[54]
The first synthesis of racemic bukittinggine was reported by Heathcock and co-workers as part of synthetic investigations of several related daphniphyllum alkaloids.[56] The researchers selected amide 75 (part of lactone ring F), cyclopentenyl methyl ester 76 (ring H), and homogeranyl iodide 77 (a part of ring G) as readily available building blocks (Scheme 8). Sequential deprotonation of amide 75 with LDA, followed by Michael reaction with α,β-unsaturated ester 76, and trapping of the generated amide enolate with iodide 77 yielded racemic allylic ether 78 in an overall yield of 63 % in one operation. Other stereoisomers and unalkylated Michael product were obtained in 13% and 20% yields, respectively. Addition of DMPU as an additive was necessary in order to reduce the amount of additional byproducts resulting from reduction of iodide 77. Sequential reduction of the ester and amide functions in 78 led to diol 79 which set the stage for a one-pot tetracyclization reaction. Thus, Swern oxidation of 79 to the corresponding dialdehyde, and formation of the dihydropyridine 80 upon addition of ammonia, was followed by a biomimetic cascade reaction in the presence of acetic acid and heating at 75 °C. The first step is an intramolecular, inverse-electron-demand hetero Diels–Alder reaction involving the 2-aza diene and the internal double bond of the geranyl side chain resulting in the formation of the imine 81.[54]. In the second step of this cascade reaction sequence, an intramolecular formal ene or aza-Prins reaction of imine 81 resulted in ring closure to reveal ring B in the racemic pentacycle 82 in high yield as the only diastereomer (note: the hetero Diels–Alder and ene reactions might also take place from the protonated pyridinium or iminium species). Ring A was formed by Pd(II)-catalyzed oxidative cyclization of 82 to yield hexacycle 83 in good yield as a single diastereomer. According to the mechanism of this reaction developed independently by Trost and Hegedus,[57] a π-allyl complex is first formed which is attacked by the internal amine to give the fused octahydroindolizidine 83. Normally, non-aromatic amines are tightly coordinated to Pd and as a consequence are not sufficiently nucleophilic to participate in reactions of this type. Such coordination may be less important in the case of the secondary amine in 82, due to its sterically congested nature. A number of hydrogenation methods, including diimide reduction, proved to be problematic affording 84 in modest diastereoselectivity. Alternatively, a hydroboration/oxidation protocol with 83 led to the desired primary alcohol, which was converted to the tosylate, and the latter reduced with triethylborohydride to give 84 in very good yield and diastereoselectivity. Removal of the benzyl protecting groups, and oxidation of the resulting 1,4-diol with the Fétizon reagent,[58] afforded racemic bukittinggine as a single regioisomer.
Scheme 8.
Key steps in the synthesis of bukittinggine by Heathcock and co-workers.
Noteworthy features in the Heathcock synthesis include the Michael reaction/alkylation sequence to assemble all the necessary carbon atoms in one operation leading to a single diastereomer. Most remarkable is the one-pot biomimetic cascade reaction to give pentacycle 82 from 79, featuring a hetero Diels–Alder reaction to generate the contiguous quaternary stereocenters of bukittinggine. Adopting this key biomimetic strategy, Heathcock and co-workers were able to synthesize a large number of related daphniphyllum alkaloids.[54]
6. Daphmanidin E
A new class of hexacyclic daphniphyllum alkaloids represented by daphmanidin E was isolated by Kobayashi and co-workers from leaves of Daphniphyllum teijsmanni in 2006.[52e,59] Its structure, as determined by NOESY spectroscopy and a modified Mosher method, revealed a hexacyclic ring system containing six contiguous stereogenic carbon atoms of which three are quaternary including two that are contiguous. Other structurally intriguing features are the bicyclo[2.2.2]octane core surrounded by a fused dihydropyrrole and a decahydrocyclopentazulene ring. As a member of the daphniphyllum alkaloid family,[52] daphmanidin E is believed to biosynthetically arise from squalene dialdehyde.[53,54] Daphmanidin E showed moderate vasorelaxant activity.[53e,59] Key steps in the enantioselective total synthesis of daphmanidin E are highlighted below.
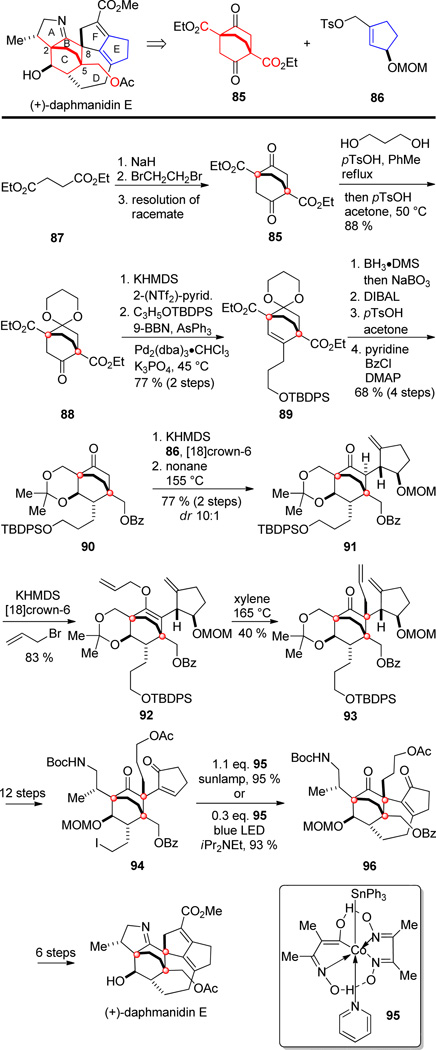
Weiss and Carreira reported the only total synthesis of (+)-daphmanidin E in 2011 (Scheme 9).[60] Visual disconnection led to the readily available starting materials bicyclo[2.2.2]octane 85, corresponding to rings B and C and containing two C2-symmetrically disposed quaternary centers, and cyclopentene 86 corresponding to ring E. Diethyl succinate 87 was converted via two Claisen condensations with dibromoethane to the corresponding racemic β-ketoester 85. Resolution by chromatographic separation of the corresponding diastereomeric hydrazones of 85, or enzymatic saponification of the esters[61] gave the enantioenriched variant of β-ketoester 85. Due to its symmetry, the carbonyl groups in 85 are homotopic and one could be selectively functionalized to give monoketone 88, which after formation of the enol triflatre and Suzuki coupling yielded olefin 89. Stereocontrolled hydroboration, reduction, acetalization and benzoyl protection afforded 90. Enolate O-alkylation of 90 with cyclopentene triflate 86, and Claisen rearrangement gave ketone 91 in good yield and high diastereoselectivity. Formation of the enolate with KHMDS and treatment with allyl bromide gave allyl vinyl ether 92. The second quaternary center at C-8 was then generated by another Claisen rearrangement to give 93 in moderate yield as a single diastereomer. Further functionalization of 93 gave 94 harboring a β-iodoethyl side chain, setting the stage for ring closure of the seven-membered ring D. The novel alkyl-Heck cyclization could be carried out using stoichiometric or catalytic (equimolar Hunig’s base necessary) cobaloxime 95[62] under irradiation with a sun lamp or blue LED to give tetracycle 96. In subsequent steps, an aldol reaction and a ketone-amine condensation constructed rings F and A respectively. Final protecting group manipulations completed the first total synthesis of (+)-daphmanidin E.
Scheme 9.
Key steps in the synthesis of daphmanidin E by Carreira and Weiss.
Noteworthy features of the Carreira synthesis of daphmanidin E include the early installation of two quaternary centers within the bicyclo[2.2.2]octane core in one step with suitable handles for further functionalization. Furthermore, two Claisen rearrangements were conducted with very good diastereoselectivities, first to connect ring E to the core subunit B/C, and secondly to create the second quaternary center at C-8. Finally, the seven-membered ring D was obtained in a remarkably effective and mild Co-catalyzed alkyl Heck-cyclization under irradiation with visible light.
7. Calyciphylline N
Calyciphylline N, another member of the A-type daphmanidin family, was isolated in 2008 by Kobayashi and co-workers from leaves of Daphniphyllum calycinum and its structure was determined using NOESY spectroscopy.[63] Calyciphylline N showed the same skeletal features as discussed for daphmanidin E except for the absence of unsaturation in ring F, and the presence of a methyl substituent on the quaternary carbon atom at the junction of rings B/C and D instead of a CH2OAc in calyciphylline N. Among the eight stereogenic carbon atoms, six are contiguous, two are adjacent quaternary centers, and an additional quaternary center joining rings B/C and A is also present. Key steps in the enantioselective total synthesis of calyciphylline N are highlighted below.
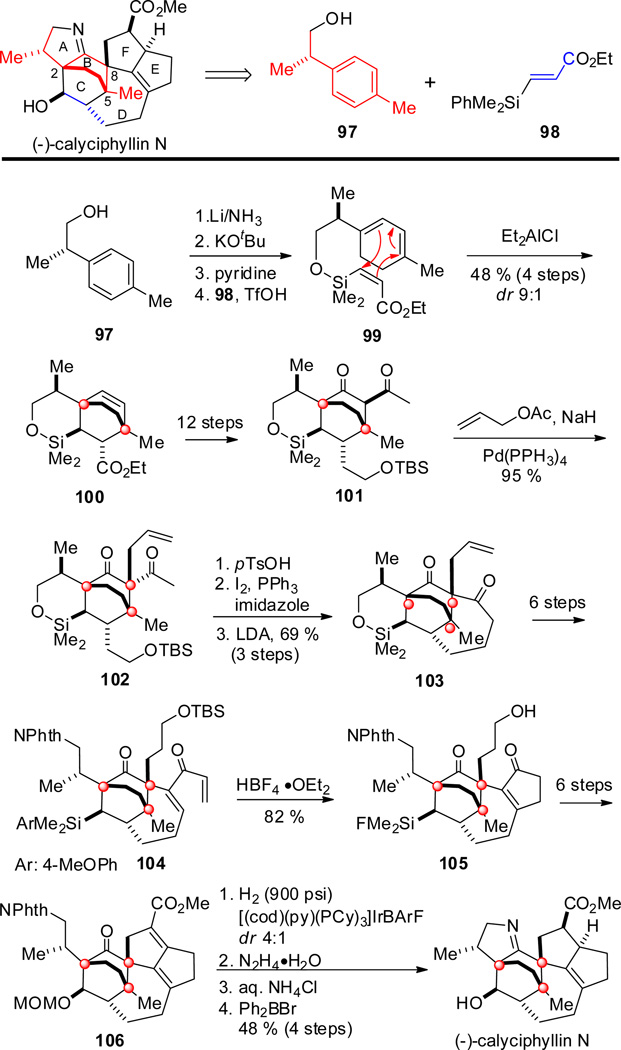
The first enantioselective total synthesis of (−)-calyciphylline N was reported by Smith and Shvartsbart in 2014 (Scheme 10).[64] In contrast to Carreira’s synthesis of daphmanidin E, which started with the bicycle[2.2.2]octane core, Smith and Shvartsbart disconnected the complex hexacyclic structure into the readily available enantioenriched alcohol 97, formally representing ring B and all carbon atoms including one stereogenic center bearing the methyl group in ring A. The other building block was α,β-unsaturated silyl ester 98 which would provide a three-carbon tether as part of ring B.
Scheme 10.
Key steps in the synthesis of calyciphylline N by Smith and Shvartsbart.
Birch reduction of 97 and alkylation of the major cyclohexadiene isomer with the silyl triflate derived from 98[65] led to the silicon-tethered triene 99. A thermal intramolecular Diels–Alder reaction gave all possible diastereoisomers of tricycle 100 bearing the methyl-containing quaternary center at C-5. In constrast, the Et2AlCl-promoted cyclization yielded 100 with good diastereoselectivity (9:1), presumably due to the spatial orientation of the methyl group enabling attack from the opposite side. Further functionalization yielded β-diketone 101, which could be C-allylated to give 102 using a Tsuji–Trost reaction[66] thereby generating the second quaternary center at C-8 in excellent yield and as a single stereoisomer. The seven-membered ring D was formed by transannular enolate alkylation using LDA to give tetracycle 103, which upon further elaboration gave dienone 104. Nazarov cyclization and protodesilylation to enable functionalization of the silicon moiety were achieved with HBF4•OEt2 in one operation to give tetracycle 105.[67,68] Cyclization to give pentacycle 106 was accomplished by an intramolecular aldol reaction in the same way as in Carreira’s synthesis of daphmanidin E. The remaining two stereogenic centers in rings E and F were generated by a selective 1,4-reduction using a cationic Ir-based modified Crabtree catalyst to give a 4:1 diastereomeric mixture of products in favor of the desired isomer.[69] Hydrazinolysis of the phthalimido group, formation of the imine, and cleavage of the MOM group completed the total synthesis of (−)-calyciphylline N.
Noteworthy features of the Smith synthesis of (−)-calyciphylline N include a diastereoselective (substrate-controlled) intramolecular Diels–Alder reaction with a silicon tethered dienophile generating the bicyclo[2.2.2]octane motif harboring two quaternary centers. Other remarkable features are a Tsuji-Trost C-allylation to generate the second contiguous quaternary center (in spite of the highly congested bridged core subunit), and a transannular enolate alkylation creating a seven-membered ring. Nazarov cyclization and a diastereo- and chemoselective hydrogenation of a conjugated diene ester are the added highlights.
8. Bilobalide
A tert-butyl-containing terpenoid belonging to the ginkgolide family named bilobalide, was isolated in 1971 by Nakanishi and co-workers,[70] from the ginkgo biloba extracts. The structure of the C-15 tetracyclic trilactone containing six stereogenic carbons, of which two are contiguous and quaternary, was determined spectroscopically. A structurally unique feature in this class of ginkgolides is the presence of a tert-butylcarbinol group. The bilobalides are biosynthesized by degradation of the related ginkgolides, which in turn are formed from geranylgeranyl pyrophosphate prepared by condensation of isopentenyl pyrophosphate and dimethylallyl pyrophosphate.[71,72] It is of interest that ginko plants have long been known in Chinese folk medicine for their therapeutic benefit as evidenced by their use in some European countries to treat cerebrovascular and peripheral circulatory problems in the elderly.[73] Ginkgolides act as a very effective anti-platelet-activating factor (PAF) antagonist to treat cardiovascular diseases.[71,72] Furthermore, bilobalide is known for its neuroprotective effects[74] and negative allosteric modulator of some GABA receptors.[75] Two total syntheses of bilobalide adopting entirely different strategies to create the contiguous quaternary centers have been reported. Key steps are highlighted below.
8.1. The Corey synthesis
The only enantioselective synthesis of (−)-bilobalide was reported by Corey and Su in 1988[76] based on a strategy used for the synthesis of the racemic natural product a year earlier.[77] Analysis of the tetracyclic structure led Corey and Su to alkyne 107 and (+)-trans-1,2-cyclohexene dicarboxylic acid dimenthoxy ester (chiron 108) as starting materials which would end up in a bicylic intermediate bearing all the requisite carbon atoms of the target compound (Scheme 11). It is of interest that chiron 108 shows no visually evident overlap with any of the rings in bilobalide. Alkyne 107[78] would eventually provide a segment of ring C, while the chiron 108 would be the source of the contiguous quaternary carbon atoms, albeit after some functional adjustments. In the enantioselective synthesis of (−)-bilobalide, chiron 108 was prepared by the Diels–Alder reaction of butadiene and (+)-menthol diester of fumaric acid catalyzed by diisobutylaluminum chloride according to Yamamoto and co-workers.[79] The first quaternary center at C-4 was installed by reaction of the mono lithium enolate of 108 with 107 to yield Claisen product 109 diastereoselectively. Remarkably, and in spite of severe steric congestion, intramolecular Michael reaction of the lithium enolate of 109 led to the bicyclic compound 112 now harboring the second quaternary center at C-5 in good yield and as a single diastereomer.
Scheme 11.
Key steps in the synthesis of bilobalide by Corey and Su.
According to the authors, after initial deprotonation to form enolate 110, an electron is transferred to the ynone moiety leading to the diradical 111, which undergoes ring closure to 112. This reaction can also be performed using 108 and 2 equivalents of LDA as was done in the original synthesis of racemic bilobalide.[77,80] Ketone 112 was reduced to the corresponding allylic alcohol (>10:1) by CBS reduction.
Sequential oxidative cleavage by ozonolysis and acetal formation yielded diester 113 in good yield. A series of oxidation-reduction and acetalization steps gave lactone 114. Functionalization of the A-ring ketal was achieved by base-catalyzed opening of the lactone ring and elimination via the mesylate ester to give diene 115. Stereoselective epoxidation using peroxy-3,5-dinitrobenzoic acid 116 yielded diepoxide 117. As a reductive epoxide opening was not feasible, an alternative approach was pursued involving acid-mediated formation of a diol from 117, acetylation and further conversion to α-acetoxy lactone mono epoxide 118. The intermediate was deoxygenated to give trilactone 119 in remarkably good yield, presumably via a free radical mechanism. Subsequently, 119 was dihydroxylated with osmium tetroxide, the less hindered secondary hydroxyl group was activated as the corresponding half oxalate ester, and deoxygenated utilizing free radical conditions. Acidic deprotection yielded (−)-bilobalide.
Noteworthy features in the 1988 Corey and Su synthesis of (−)-bilobalide include an early two-step generation of the contiguous quaternary centers by stereoselective Claisen and intramolecular Michael reactions, the latter probably proceeding via a diradical mechanism.
8.2. The Crimmins synthesis
In their approach toward the total synthesis of racemic bilobalide, Crimmins and co-workers chose 3-furaldehyde 120 representing ring A and 1,2-cyclopentanedione 121 (later to become a segment of ring D) as suitable starting materials[81] (Scheme 12). Addition of t-BuCeCl2 to 120,[82] oxidation to the ketone was followed by addition of lithioacetonitrile, and conversion to aldehyde 122. Aldol reaction with 2.2 equivalents of lithium enolate 123,[83] derived from 121, gave a 1:1 diastereomeric mixture of products 124, which was of no consequence due to the subsequent enolization of the ketone. Interestingly, the diastereomers showed the same desired anti relationship between the secondary and tertiary hydroxy groups, which can be explained by a chair-like six-membered Li-enolate transition state with the tert-butyl group occupying equatorial position and enolate attack from the preferred pseudoequatorial face. Protecting group manipulations and selective formation of enol ester 125 set the stage for the crucial intramolecular [2+2] photocycloaddition to establish the contiguous quaternary centers. In the event, photocyclization of 125 (uranium glass filter, > 350 nm) proceeded via transition state 126 having the tert-butyl and (trimethylsilyl)oxy groups of the newly formed ring C in pseudoequatorial position, to give the photocycloadduct 127 in 55 % yield as a 10:1 mixture of diastereomers favoring the ‘up’-disposed cyclopentyl isomer. A regioisomer of 127 was isolated in 25 % yield resulting from cycloaddition with the less substituted furan double bond to give 128 having one quaternary center. The photocycloadduct 127 was functionalized by hydroxylation with LDA and MoOPh,[84] oxidative degradation of the hydroxy ketone, and manipulation of the oxidation state to yield ketone 129. A regioselective Baeyer–Villiger oxidation was achieved utilizing mCPBA to give the desired lactone 130 in excellent yield after 5 min, whereas H2O2 or t-BuO2H in the presence of Triton B led exclusively to the undesired lactone. Finally, oxidation of the acetal, diastereoselective epoxidation with dimethyldioxirane,[85] and a second Jones oxidation gave racemic bilobalide.
Scheme 12.
Key steps in the synthesis of bilobalide by Crimmins and co-workers.
One of the key aspects of the Crimmins synthesis is the anti-stereoselective aldol reaction of lithium enolate of 123 with aldehyde 122 to secure the desired stereochemistry of the hydroxyl group in ring D, as well as its anti-relationship with the tert-butyl carbinol. Most noteworthy is the stereoselective intramolecular [2+2] photocycloaddition of 125 to generate the contiguous quaternary stereocenters at C-4 and C-5 in 127. Other remarkable features include the regioselective Baeyer–Villiger oxidation of 129 and other selective late-stage oxidations.
Adopting a different approach to generate the quaternary centers, Corey and co-workers reported total syntheses of ginkgolides A[89] and B[90] (Scheme 13). Thus, a titanium tetrachloride mediated Mukaiyama aldol reaction with silyl enolether 131 generated ketone 132 harboring the first quaternary center. Further functionalization led to 133 and set the stage for an intramolecular ketene-olefin cycloaddition with in situ generated ketene 134,[91] to give the contiguous quaternary centers in cyclobutanone 135, an intermediate that was converted to racemic ginkgolide A[89] and ginkgolide B.[90,92]
Scheme 13.
Key steps in the synthesis of ginkgolide B by Corey and Su.
The Corey and Crimmmins approaches toward the synthesis of bilobalide are conceptually different in their disconnection to simpler building blocks and in setting up the quaternary centers within the core cyclopentane structure. In the Corey and Su synthesis the unsaturated indanone already bearing two quaternary carbons was oxidatively cleaved to the corresponding diester thus providing a highly substituted cyclopentene core. Crimmins on the other hand capitalized of a photochemical [2+2] cyclization to introduce the two contiguous quaternary carbon centers within a core tetracyclic structure which was subjected to a series of steps to achieve the desired oxidation level present in bilobalide.
9. Maoecrystal V
Maoecrystal V is a pentacyclic ent-kaurenoid norditerpenoid showing cytotoxicity towards HeLa cells.[93] First isolated from Isodon eriocalyx in 1994,[93] its structure was not elucidated until 2004 by a combination of NMR and single-crystal X-ray analysis. Maoecrystal V is characterized structurally by a bicyclo[2.2.2]octan-2-one core fused to a δ-lactone and a strained bridging tetrahydrofuran ring, with two contiguous quarternary stereocenters, C-9 and C-10, common respectively to four and three separate rings. It is speculated that maoecrystal V arises biosynthetically from the tetracyclic diterpene epi-eriocalyxin A, which lacks the tetrahydrofuran ring of maoecrystal V.[93] Total syntheses of maoecrystal V have been completed by four research groups. Key steps in four of these syntheses are highlighted below.
9.1. The Yang synthesis
In 2010, Yang and co-workers reported the first total synthesis of racemic maoecrystal V.[94] It was envisioned that the complete ring system would be constructed by intramolecular Diels–Alder reaction of tetraene 136, which in turn would arise from the coupling of β-ketoester 137 and aryllead intermediate 138 (Scheme 14).[95]
Scheme 14.
Key steps in the synthesis of rac-maoecrystal V by Yang and co-workers.
After preparing 137 from 2,2-dimethyl-1,3-cyclohexandione,[96] it was α-arylated to give 139 in 88 % yield. Subsequent reduction of both the ketone and ester functionalities with LiAlH4 was low yielding and proved inferior to a two-step reduction using (Bu4N)BH4 followed by LiAlH4. Steglich esterification of the resulting primary alcohol using EDCI successfully appended the diethyl phosphonate. Upon reaction with tosyl azide under basic conditions the desired diazo ester 140 was isolated in 38 % yield from ketoester 139. Subjecting diazo ester 140 to catalytic rhodium(II) acetate generated the rhodium carbenoid, which underwent intramolecular O–H insertion to assemble the 3-oxa-ε-lactone. Horner-Wadsworth-Emmons reaction with paraformaldehyde and MOM deprotection gave enoic ester 141. To set the stage for the intramolecular Diels–Alder reaction, phenol 141 was subjected to Wessely-oxidative acetoxylation[97] to yield cyclohexadienone 136 as a mixture of C-16 epimers. Without purification, 136 was heated in toluene at 145 °C to afford a mixture of three cycloadducts 142–144, from which the desired endo-cycloadduct 144 was isolated in 36 % yield. After several additional steps, rac-maoecrystal V was obtained from Diels–Alder adduct 144. Although no stereoselectivity was realized in the pivotal intramolecular Diels–Alder step, this inaugural total synthesis of maoecrystal V was accomplished in a highly concise fashion.
9.2. The Danishefsky synthesis
Peng and Danishefsky also utilized an intramolecular Diels–Alder strategy to synthesize racemic maoecrystal V.[98] After initially examining an approach in which the stereocenters of ring A were present in the Diels–Alder precursor,[99] the authors developed a successful strategy in which the pivotal intramolecular Diels–Alder reaction was accomplished with an achiral substrate. In this analysis, maoecrystal V was disconnected to Diels–Alder precursor 145, which would arise from the coupling of dienoate 146, the product of Birch reduction of methyl benzoate, and 3-chlorocyclohexenone 147 (Scheme 15). Reaction of the lithium enolate of ester 146 with chloroenone 147 gave substitution product 148. Both the ester and ketone of 148 were reduced with DIBAL-H, and the allylic alcohol derived from the ketone was selectively oxidized with MnO2. Acylation of the primary alcohol with acid chloride 149, followed by enol silylation, rendered Diels–Alder precursor 145. Heating this intermediate at 166 °C gave cycloadduct intermediate 150, which subsequently lost phenylsulfinic acid. Desilylation of intermediate 151 furnished tetracyclic ketoester 152 in 62 % yield. Nucleophilic epoxidation of the enone double bond of 152 took place exclusively from the less-hindered β-face giving rise to alcohol 153 after selective iodide-mediated ring opening at the secondary carbon and successive dehalogenation with Bu3SnH. Differentiation of the two diastereotopically related double bonds in 153 to form the bridging tetrahydrofuran ring and generate the required relative configuration of the C-10 quaternary stereocenter was the result of hydroxyl-directed epoxidation with m-chloroperoxybenzoic acid to give epoxide 154, which cyclized when exposed to p-toluenesulfonic acid via intermediate 155 to deliver tetrahydrofuran 156. Elaboration of pentacyclic intermediate 156 to rac-maoecrystal V, which required isomerization of the A/B ring junction giving intermediate 157 and further functional group modification, was accomplished in a number of steps.
Scheme 15.
Key steps in the synthesis of rac-maoecrystal V by Danishefsky and Peng.
9.3. The Davies–Zakarian synthesis
Zakarian and co-workers reported the third total synthesis of rac-maoecrystal V in 2013.[100]
One year later, in a collaboration with the Davies group, a related strategy was employed to complete the first enantioselective synthesis of (−)-maoecrystal V.[101] With the challenge of forming the strained tetrahydrofuran ring in mind, this ring was constructed early. Thus, maoecrystal V was disconnected to Diels–Alder precursor 158 (Scheme 16). Intermediate 158 was designed with a removable silyl tether to control facial selectivity and exploit intramolecularity in the cycloaddition. Dienone 158 was seen arising from a dihydrobenzofuran intermediate that would be constructed using catalytic enantioselective C–H insertion chemistry developed earlier in the Davies group,[102] with sesamol (160) being the ultimate starting material. To achieve high enantioselectivity in the Rh-catalyzed C–H insertion step, it was ultimately found that an auxilliary-based approach was preferable to enantioselective catalysis. Sesamol (160) was O-alkylated with primary alcohol 159 under Mitsunobu conditions to afford phenolic ether 161. Directed ortho-metallation of 161 and addition of methyl chloroxoacetate led to pyruvic acid 162 in high yield after saponification. After esterification with mandelamide derivative 163, conversion to the tosylhydrazone and reaction with Et3N to eliminate tosylsulfinate provided diazoester 164. Intramolecular C–H insertion of 164 catalyzed by Rh2(OAc)4 gave, after base-promoted isomerization of the initially produced mixture of ester epimers, dihydrobenzofuran 165 in 84 % yield and 84 % ee. Installation of the C-10 quaternary stereocenter by alkylation of the zinc enolate of ester 165 took place in 95 % yield and >20:1 diastereoselectivity from the face opposite the adjacent side chain. Reduction of the ester and ring opening of the dioxolane gave dihydrobenzofuran 166. Oxidative dearomatization of 166 provided dienenone 167, which was coupled with chlorodimethylvinylsilane to give Diels–Alder precursor 158. Heating in refluxing toluene promoted intramolecular cycloaddition to provide tetracyclic product 168 and form the second of the contiguous quaternary stereocenters in near quantitative yield. Reductive removal of the diethoxyacetal upon reaction with SmI2 and discharge of the silicon tether delivered tricycle 169, which was advanced in several steps to intermediate 170. Ring-closing metathesis using Hoveyda-Grubbs II catalyst and oxidation with Dess-Martin periodinane yielded (−)-maoecrystal V.
Scheme 16.
Key steps in the synthesis of (−)-maoecrystal V by Davies, Zakarian and co-workers.
9.4. The Thomson synthesis
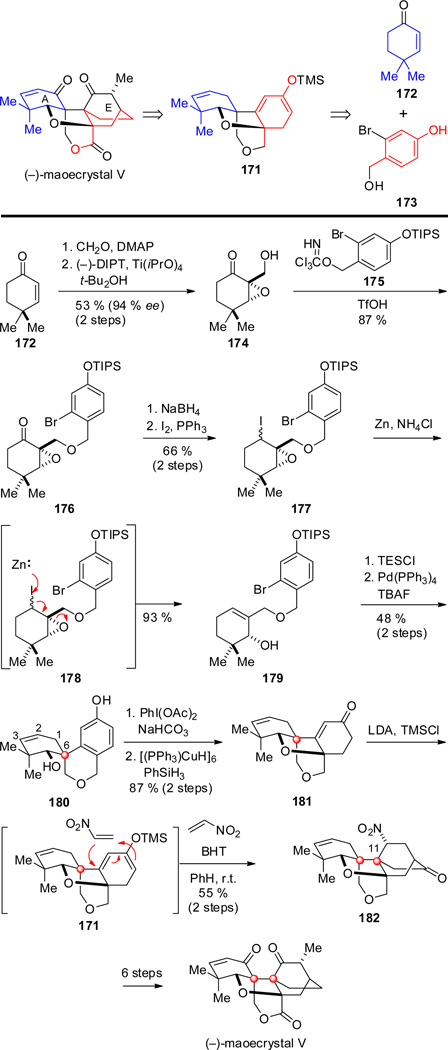
Shortly after the Davies–Zakarian synthesis appeared, a synthesis of (−)-maoecrystal V was disclosed by Thomson and co-workers.[103] In their approach, maoecrystal V was disconnected to dioxatetracyclic intermediate 171, with the aim of fashioning the bicyclo[2.2.2]octan-2-one fragment using a bimolecular Diels–Alder (Scheme 17). Disconnection of tetracyclic intermediate 171 led Thomson and co-workers to select 4,4-dimethylcyclohexenone 172 and bromophenol 173 as the precursors to rings A and E. The synthesis began with the Baylis–Hillman reaction of cyclohexenone 172 with formaldehyde, followed by Sharpless asymmetric epoxidation of the resulting allylic alcohol to give 174 in moderate yield and high enantiopurity.[104] Reaction with benzyl trichloroacetimidate 175 in the presence of TfOH produced benzylic ether 176.
Scheme 17.
Key steps in the synthesis of (−)-maoecrystal V by Thomson and co-workers.
Following reduction of the carbonyl group of 176 with NaBH4, the resulting alcohol was converted under Appel conditions into alkyl iodide 177 as an inconsequential mixture of epimers. β-iodoepoxide 177, was reduced with elemental zinc as depicted in 178 to supply allylic alcohol 179. The first quaternary stereocenter at C-6 was installed by a diastereoselective intramolecular Heck reaction of 179 from the face opposite the silyloxy substituent to give oxaspirotricyclic product 180 after removal of the silyl protecting groups. Predominant formation of the 2,3-double-bond isomer resulted from isomerization of the intermediate Pd-hydride complex formed upon initial spirocyclization. Hypervalent iodide-mediated cyclode-aromatization of 180 formed the dienone, which was selectively reduced with Stryker’s reagent to yield tetracyclic enone 181. After formation of dienoxysilane 171, the Diels–Alder reaction with nitroethylene took place at room temperature exclusively from the less-hindered β-face of the diene to give cycloadduct 182 harboring the second quaternary center at C-11 in 55 % yield after acidic workup. With the pentacyclic ring system constructed, Thomson and co-workers carried cycloadduct 182 forward to complete (−)-maoecrystal V.
From a strategic point of view, it is not surprising that a Diels–Alder reaction was employed to construct the bicyclo[2.2.2]octan-2-one fragment of maeocrystal V in each synthesis. In all but the Thompson synthesis, an intramolecular Diels–Alder reaction was used. Only in the Davis–Zakarian synthesis is this reaction performed on an enatioenriched precursor that allows the stereorelationship of the C-9 and C-10 contiguous quaternary stereocenters to be established with high selectivity in this step. The Thompson synthesis is distinctive in utilizing a bimolecular Diels–Alder reaction to construct the bicyclo[2.2.2]octan-2-one fragment and establish the stereorelationship of the contiguous quaternary stereocenters. The highly reactive cycloaddends used in this step (Danishefsky-type diene and nitroethylene) and the fact that the strained tetrahydrofuran ring was already in place are undoubtedly responsible for the cycloaddition of 171 taking place at room temperature.
10. Hyperforin
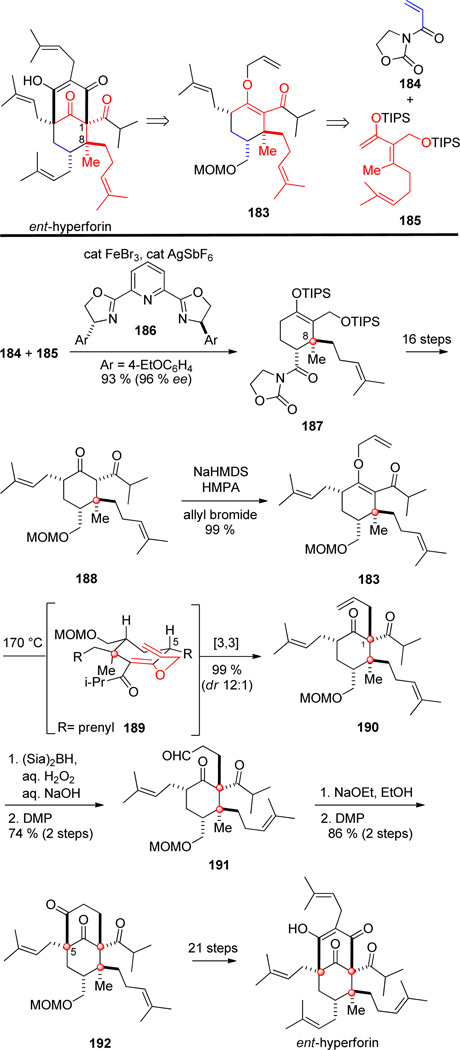
A member of the polycyclic polyprenylated acylphloroglucinols (PPAPs), hyperforin, was isolated in 1971 from St. John’s wort (Hypericum perforatum).[105] Its structure and absolute configuration were confirmed by X-ray crystallography in 1983.[106] Like many PPAPs, hyperforin contains a heavily substituted bicyclo[3.3.1]nonane unit. Embedded on this skeleton are four prenyl side chains and three quaternary stereocenters, two of which are contiguous. The C-8 stereocenter incorporates a prenyl unit, a feature unique from other PPAPs that contain a geminal dimethyl functionality at this site. Hyperforin is proposed to arise biosynthetically from alkylation of a substituted acylphloroglucinol with geranyl pyrophosphate and prenyl pyrophosphate.[107] Hyperforin, the primary active ingredient in the popular nutraceutical St. John’s wort, is responsible for the plant’s well-known antidepressant property[108] along with other biological activities.[109] Herein, we present three of the five total syntheses of hyperforin to date that employ different strategies. In the 2104 total synthesis of rac-hyperforin by Bellavance and Barriault,[110] the quaternary centers were introduced through a series of enolate alkylation and conjugate addition reactons. In the shortest total synthesis of racemic hyperforin to date, Maimone and Ting.[111] started with 2-methyl-cyclopentenone, and effected a series of stereocontroled C-C functionalizations to reach the target in 10 steps.
10.1. The Shibasaki synthesis
The first enantioselective synthesis was reported by Shibasaki and co-workers in 2010 and delivered ent-hyperforin.[112] Central to the synthesis was a catalytic enantioselective Diels–Alder reaction previously developed by the Shibasaki group.[113]
Shibasaki and co-workers envisioned ent-hyperforin being constructed by late-stage functionalization and Claisen rearrangement of allyl vinyl ether 183, with the cyclohexanone core of 183 being formed from a [4+2] cycloaddition between acryloyl oxazolidinone 184 and triene 185 (Scheme 18). The Shibasaki synthesis began with an enantioselective Diels–Alder reaction between dienophile 184 and triene 185, catalyzed by FeBr3, AgSbF6, and (R,R)-pybox ligand 186, to provide exo-cycloadduct 187 in high yield and enantioselectivity. The geometry and steric bulk of the tetrasubstituted double bond of 185 dictates the facial approach of dienophile 184 to favor the exo cycloaddition mode, setting the initial quaternary stereocenter at C-8.[114] Following acylation and prenylation of 187, β-diketone 188 was selectively O-allylated to provide allyl vinyl ether 183. Shibasaki and co-workers had previously examined diastereoselectivity of the Claisen rearrangement in related systems and discovered strong directing effects by allylic substituents at C-5.[114] Rearrangement of allyl vinyl ether 183, via chair transition structure 189 having the C-5-prenyl substituent pseudo equatorial, delivered β-diketone 190 in quantitative yield thereby securing the second of the contiguous quaternary stereocenters with 12:1 diastereoselectivity. β-Diketone 190 was elaborated to aldehyde 191 in two steps, and the aldehyde smoothly underwent intramolecular aldol cyclization to form the bicyclo[3.3.1]nonane ring system as a single diastereomer now harboring the final quaternary stereocenter at C-5. Following oxidation of the resulting alcohol, bicyclo[3.3.1]nonatrione 192 was carried forward to complete the total synthesis of ent-hyperforin.
Scheme 18.
Key steps in the synthesis of ent-hyperforin by Shibasaki and co-workers.
10.2. The Shair synthesis
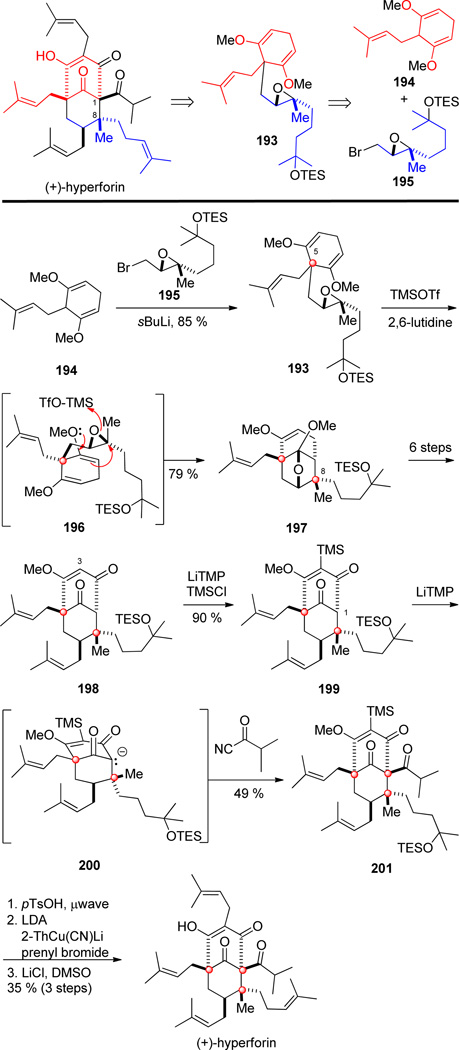
The second enantioselective synthesis of hyperforin by Shair and co-workers took inspiration from its proposed biosynthesis.[115] Whereas nature constructs the C-1/C-8 linkage between the contiguous quaternary stereocenters by alkylation of an acyl phoroglucinol with geranyl pyrophosphate, Shair and co-workers planned to form this bond by an epoxide-opening cyclization of 193. This functionalized intermediate would then be accessible from coupling diene 194 and geraniol-derived bromide 195 (Scheme 19).
Scheme 19.
Key steps in the synthesis of (+)-hyperforin by Shair and co-workers.
The synthesis commenced with construction of diene 194 from Birch reduction and alkylation of 1,3-dimethoxybenzene. The other coupling partner, enantioenriched bromide 195, was synthesized in four steps from geraniol, using Sharpless epoxidation to access the desired enantiomer. Base-mediated coupling of diene 194 with bromoepoxide 195 lead to epoxide 193 harboring a prochiral quaternary center at C-5. Exposure of this intermediate at low temperature to trimethylsilyl triflate promoted diastereoselective cyclization to form exclusively bicyclo[3.3.1]nonane ketal 197 by way of the proposed chair transition structure 196. This step both desymmetrizes the prochiral quaternary carbon of 193 and incorporates the C-8 quaternary stereocenter. Oxatricyclic ketal 197 was elaborated to diketone 198 with the aim of forming the second of the contiguous quaternary stereocenters by acylation of a bridgehead carbanion intermediate.
To accomplish this transformation, the kinetically more acidic vinylic hydrogen (H-3) of 198 required masking with a trimethylsilyl group. Subsequent lithiation of 1,3-diketone 199 with lithium tetramethylpiperidide (LiTMP) and trapping with isobutyryl cyanide delivered triketone 201 in 49 % yield via anion 200.
As a result of poor overlap of the adjacent carbonyl groups and steric congestion at the bridgehead carbon, bridgehead functionalizations to construct contiguous quaternary carbons of other PPAPs have been notoriously difficult.[110,116] Removal of protecting groups and installation of the last prenyl unit completed this synthesis of (+)-hyperforin.
10.3. The Nakada synthesis
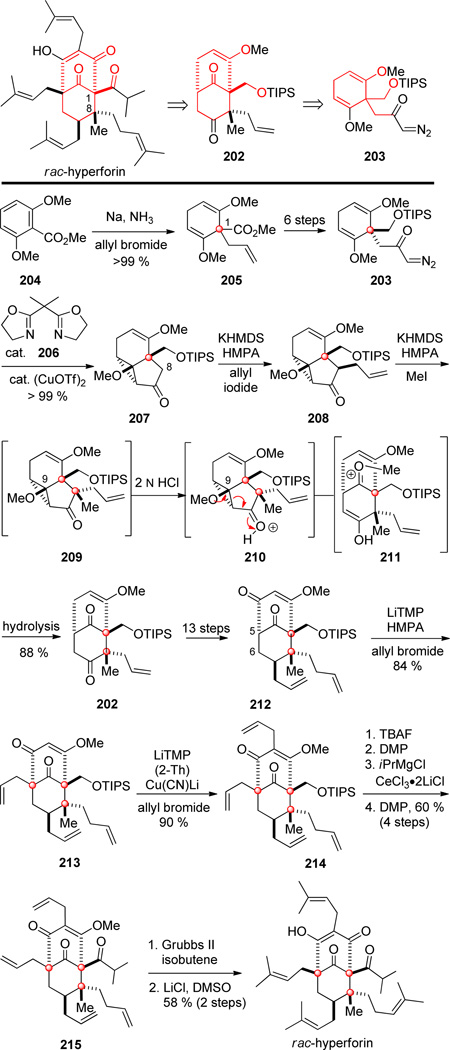
Nakada and co-workers described the synthesis of rac-hyperforin[117] shortly after Shair and co-workers published their enantioselective synthesis. This synthesis featured a cyclopropane ring-opening reaction previously developed in the Nakada group for the total synthesis of nemorosone.[118] In targeting hyperforin, they aimed for late-stage installation of four prenyl groups on bicyclo[3.3.1]nonendione intermediate 202 (Scheme 20). The bicyclic scaffold of 202 would then be constructed from cyclopropanation and ring-opening of their nemorosone intermediate 203.
Scheme 20.
Key steps in the synthesis of rac-hyperforin by Nakada and co-workers.
The synthesis of rac-hyperforin began by accessing α-diazoketone 203 from dimethoxybenzoate 204.[118] In this sequence, the first quaternary center at C-1 was installed by Birch reduction of 204 and alkylation with allyl bromide to give cyclohexadiene 205. Following functionalization to α-diazoketone 203, copper-catalyzed intramolecular cyclopropanation with ligand 206 formed tricyclic cyclopropane 207. The synthetic sequence to hyperforin then diverged from the nemorosone route by resorting to a stepwise alkylation strategy to introduce the C-8 quaternary stereocenter. Relying on facial bias, intermediate 207 was alkylated first with allyl iodide to give ketone 208 and then methyl iodide to form product 209 containing the second of the contiguous quaternary stereocenters of hyperforin in high yield and excellent stereoselectivity. Quenching the second alkylation reaction with strong acid induced cyclopropane ring-opening (210) to give 211, which upon hydrolysis provided bicyclo[3.3.1]nonane 202 in 88 % yield from cyclopropane precursor 208. Elaboration of 202 to diketone 212 and bridgehead alkylation upon reaction with LiTMP and allyl bromide installed the third quaternary stereocenter at C-5 of 213. This bridgehead alkylation proceeded in high yield (84 %), presumably because of the absence of an adjacent quaternary stereocenter. Subsequent C-3 allylation required (2-Th)Cu(CN)Li and LTMP to obtain a good yield of diallyl ketone 214. A four step sequence to install the isopropyl ketone then afforded triketone 215, and the four allyl fragments were then simultaneously converted to prenyl units using cross metathesis with isobutene. Deprotection of resulting methyl ether completed the synthesis of rac-hyperforin.
The three total syntheses of hyperforin are conceptually different in the manner with which the quaternary centers were introduced. Noteworthy features of the Shibasaki synthesis are the use of a catalytic enantioselective Diels–Alder reaction to construct the cyclohexanone ring and two stereocenters, one being the C-8 quaternary stereocenter, and the reliable Claisen rearrangement to form the adjacent C-1 quaternary stereocenter. Whereas the Shibasaki synthesis constructs the bicyclo[3.3.1]nonane framework at a late stage, the Shair synthesis constructs this scaffold early on. Of particular note in Shair’s short synthesis of hyperforin is an epoxide-initiated alkene cyclization that directly constructs the C-8 quaternary stereocenter while desymmetrizing the C-5 quaternary stereocenter in the process. The Nakada synthesis is noteworthy for its use of an intramolecular catalytic enantioselective cyclopropanation reaction to form a tricyclic intermediate, whose pronounced facial bias allows the C-8 quaternary stereocenter to be formed by reliable enolate alkylation reactions, with the cyclopropane ring ultimately cleaving to reveal the bicyclo[3.3.1]nonendione ring system of hyperforin.
11. Scopadulcic Acids A and B
Two structurally unique tetracyclic diterpenoid acids, scopadulcic acids A and B, were reported by Hayashi in 1987 from Scoparia dulcis L.[119] This flowering plant is widely distributed in the tropics and subtropics and is considered by several native populations to possess various medicinal properties.[120] Subsequent studies showed that scopadulcic acid B is a powerful inhibitor of H+,K+-ATPase, the proton pump for gastric acid secretion, as well as an inhibitor of herpes simplex virus type 1 and of bone resorption by osteoclast cells.[121] The structure of scopadulcic acid A was established by X-ray crystallography,[122] whereas the absolute configuration of these diterpenoid acids was suggested on the basis of CD measurements and later confirmed by enantioselective synthesis.[123] The bicyclo[3.2.1]octane subunit of scopadulcic acids A and B is also found in tetracyclic diterpenes such as aphidicolin and stemarin. The structural feature unique to the scopadulcic acids is the presentation of this fragment on a tricyclic hydrophenanthrene backbone.
11.1. The Overman synthesis of rac-scopadulcic acid B
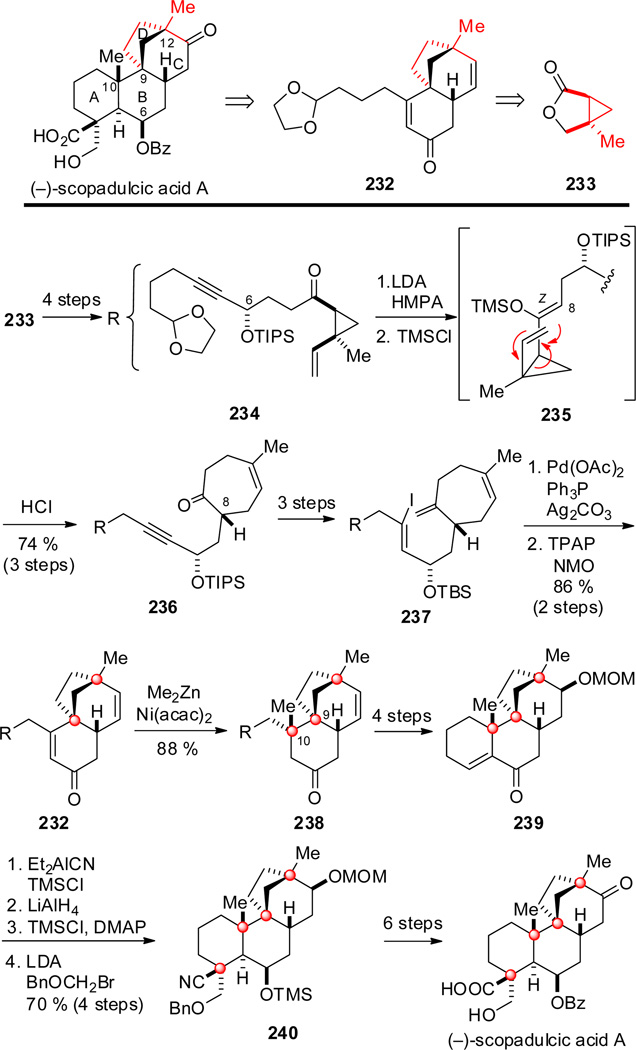
The synthesis of rac-scopadulcic acid B, the first total synthesis of a scopadulan diterpene, was reported by Overman and coworkers in 1993 (Scheme 21).[124] The plan deferred construction of the contiguous C-10 quaternary center to a late stage, hence scopadulcic acid was disconnected to tetracyclic intermediate 216. A cascade intramolecular Heck reaction was envisaged to construct rings B, C and D of the scopadulan ring system. As sequential Heck cyclizations of structurally simple dienes had been described only earlier that year,[125] a simple benzene ring was chosen for the ring A fragment to allow the pivotal cascade cyclization to be quickly evaluated. The cycloheptene ring of the Heck cyclization precursor was seen arising from a divinylcyclopropane rearrangement, thus 2-iodobenzaldehyde (217) and vinylcyclopropyl bromide 218 were identified as the starting materials. Bromide 218 was available in two steps from isoprene as a 3:2 mixture of epimers favoring the required cis stereoisomer. Coupling of aldehyde 219, available in four steps from 217, with the cyclopropyl Grignard reagent generated from 218 and oxidation of the alcohol product delivered cyclopropyl ketone 220. Enol silylation of 220 and subsequent heating to 80 °C promoted divinylcyclopropane rearrangement to give, after desilylation, cycloheptenone 221. After advancing this product to iodide 222, the cascade Heck cyclization was accomplished in 80–85 % yield to form a mixture of tetracyclic alkene products 225 bearing two quaternary centers via carbopalladium intermediates 223 and 224, which converged to dienone 226 after dehydrogenation of the crude Heck product with DDQ. The required oxidation in the C ring and the trans-C/D ring fusion was then installed by way of the epoxide formed by electrophilic epoxidation of the distal double bond of dienone 226. The pseudoequatorial alcohol of the resulting tetracyclic intermediate 227 was then exploited to regioselectvely carboxylate ring A at C-4, allowing the aromatic ring to be dearomatized and the C-4 quaternary center introduced by Birch reduction and methylation of the resulting 3-carboxypentadienyl anion intermediate. After oxidation state modification, enone 229 was formed in 56 % overall yield from hydroxy acid 228. At this point, the severe challenge of constructing the second of the contiguous quaternary stereocenters at C-10 was encountered. Although Me2CuLi added in high yield to a structurally related tetrasubstituted decalin enone, this reagent, and many other regents commonly used for conjugate addition, were surveyed without success in introducing a methyl substituent at C-10 of enone 229.
Scheme 21.
Key steps in the synthesis of rac-scopadulcic acid B by Overman and co-workers.
The only carbon nucleophile that could be incorporated at C-10 was cyanide, which was selectively introduced from the face opposite the larger ethano bridge upon reaction of 229 with the Nagata reagent, Et2AlCN.[126] The yield of 230 was raised to 85 % by two recycles of unreacted enone 229. Transformation of the nitrile to the desired methyl group was simplified by the unexpected formation of an intramolecular aminal upon reaction of 230 with excess LiAlH4 at 75 °C, which allowed this intermediate to be transformed to product 231 by Wolff–Kishner reduction under forcing conditions. In three additional steps, intermediate 231 gave rac-scopadulcic acid B.
11.2. The Overman synthesis of (−)-scopadulcic acid A
Overman and co-workers also reported in 1993 a second-generation approach to the scopadulcic acids, culminating in the synthesis of rac-scopadulcic acid A.[127] In 1999, this strategy was adapted to synthesize both natural (−)-scopadulcic acid A and its enantiomer.[123] To allow the contiguous quaternary center relationship to be formed directly while avoiding the multistep sequence required in their earlier approach to functionalize ring A, (−)-scopadulcic acid A was disconnected to tricyclic dienone 232 (Scheme 22). As in their inaugural synthesis, rings B, C and D would be formed by a cascade intramolecular Heck cyclization. The cycloheptene ring of the bicyclization precursor would again be constructed by a divinylcyclopropane rearrangement, thus (1S,5R)-5-methyloxabicyclo[3.1.0]hexanone (233), whose synthesis by catalytic enantioselective intramolecular cyclopropanation had been reported previously,[128] was identified as the starting material. The synthesis began by converting enantiomerically pure lactone 233 to cyclopropyl ketone 234. The cycloheptenone ring and critical C-8 stereocenter were formed by stereoselective enolization of ketone 234 and O-silylation to form (Z)-enoxysilane intermediate 235, which rearranged upon warming to give cycloheptenone 236 in high yield. After elaborating 236 to trienyl iodide 237, Pd-catalyzed cascade cyclization and oxidation of the tricyclic alcohol product gave dienone 232 in 86 % yield. In stark contrast to tetracyclic enone 229 (Scheme 21), 232 reacted successfully with Me2CuLi to install the C-10 methyl group. However, this procedure was found to be less reliable than Ni-catalyzed conjugate addition of Me2Zn,[129] which reliably gave tricycle 238 harboring the contiguous C-9 and C-10 quaternary stereocenters in 88 % yield. The success realized in the addition of methyl to enone 232, and failure of identical reactions with the tetrasubstituted enone 229, likely reflects the more facile coordination of the less-hindered enone double bond of 232 with transition metals. After introduction of oxygen functionality in ring D, ring A was formed by intramolecular aldolization/dehydration to provide tetracyclic intermediate 239. The final quaternary center was then incorporated by conjugate addition of cyanide, again using the Nagata reagent, followed by selective equatorial-face reduction of the ring B ketone. After protection of the resulting alcohol, the hydroxymethyl substituent was installed by alkylation of the α-cyanolithium intermediate from the face opposite the angular methyl group to give 240 in good overall yield. The enantioselective total synthesis of (−)-scopadulcic acid A was completed from this intermediate by routine functional group modifications.
Scheme 22.
Key steps in the synthesis of (−)-scopadulcic acid A by Overman and co-workers.
In addition to the efficient intramolecular Heck cyclization cascade of this second-generation approach, the strategic choice of chiron 233 is noteworthy, as neither of the stereogenic centers of this precursor are retained in the natural product target, although two carbons of this fragment become the C-9 and C-12 quaternary stereocenters of the bicyclo[3.2.1]octane unit. The stereocenters of cyclopropane 234 regulate formation of the C-8 stereocenter, which subsequently controls the evolution of the four quaternary stereocenters of scopadulcic acid A. A noteworthy feature of the strategy employed in this synthesis was the opportunity to also fashion the unnatural enantiomer of scopadulcic acid A by divinylcyclopropane rearrangement of the (E)-enoxysilane intermediate formed upon soft enolization of the C-6 epimer of cyclopropyl ketone 235 with trimethylsilyl triflate and triethylamine.[123]
11.3. The Ziegler synthesis of rac-scopadulcic acid A
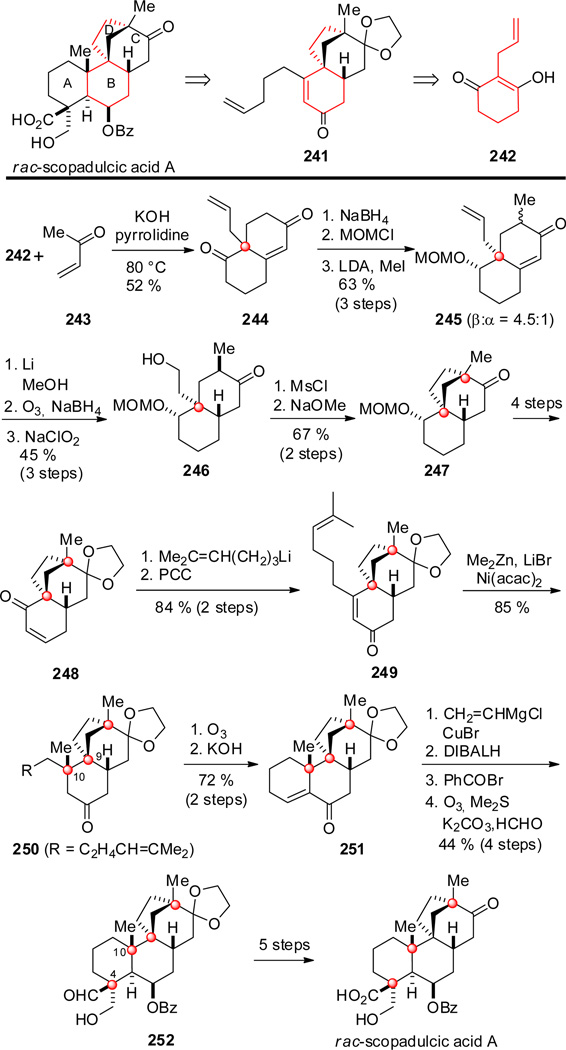
Ziegler and Wallace reported their total syntheses of rac-scopadulcic acids A and B two years after Overman and coworkers published the inaugural total syntheses of racemic scopadulcic acids A and B.[130] As in the Overman synthesis, these authors disconnected scopadulcic acid A to an enone intermediate harboring rings B, C and D. This intermediate, 241, was further disconnected to 2-allylcyclohexan-1,3-dione 242 (Scheme 23). Robinson annulation of dione 242 with methyl vinyl ketone (243) gave decalin dione 244,[131] whose saturated ketone carbonyl group was masked by reduction and protection of the resulting alcohol as a methoxymethyl ether. Methylation of the kinetic enolate of this product gave bicyclic intermediate 245 as a mixture of methyl epimers. The major isomer harboring a pseudoequatorial β-methyl substituent underwent stereoselective conjugate reduction to install the trans C/D ring fusion. Three steps terminating with selective oxidation of the secondary alcohol advanced 245 to decalone 246. Intermediate 247, containing rings BCD of scopadulcic acid A, was then formed in 74 % yield by intramolecular alkylation of the keto mesylate generated from 246 upon reaction with sodium methoxide and methanol. The tricyclic product resulting from cyclization of the alternate, less substituted, enolate intermediate was produced as a minor product only. In four routine steps, 247 was advanced to enone 248. Addition of 4-pentenyllithium and oxidative transposition of the allylic alcohol gave tetracycle 241 (top line Scheme 23). The authors were surprised to find that the C-10 quaternary stereocenter was not incorporated upon reaction of 241 with Me2CuLi, Me2CuCNLi2/BF3 · Et2O or Me3Al/Ni(acac)2. As this result stood in stark contrast to a closely related success in the Overman synthesis, the authors conjectured that the failure of the Ni-mediated methylation was the result of coordination of the terminal vinyl group of 241 with a Ni intermediate. To minimize such an interaction, the related enone 249 containing a pendant trisubstituted double bond was prepared in a similar fashion and 84 % yield from 248. As hoped, enone 249 underwent conjugate addition of a methyl nucleophile in high yield upon reaction with Me3Al and Ni(acac)2. The resulting product 250 now harboring the contiguous quaternary centers at C-9 and C-10 was elaborated to rac-scopadulcic acid A following the general strategy of the earlier Overman synthesis. In this case, tetracyclic enone 251 was fashioned from 250 by oxidative cleavage of the alkene, intramolecular aldol cyclization and dehydration. Introduction of the final quaternary stereocenter at C-4 was accomplished by conjugate addition of vinylcuprate to enone 251, stereoselective reduction of the ring B carbonyl group, and benzoylation. In a noteworthy one-pot process, the vinyl group of the resulting intermediate was cleaved with ozone to the corresponding aldehyde, which, after purging with nitrogen to remove excess ozone, was exposed to K2CO3 and formaldehyde to give aldol product 252 in 44 % yield from 251. Stereoselection in introducing this final quaternary stereocenter was the result of the aldol condensation occurring from the enolate face opposite the angular C-10 methyl group.
Scheme 23.
Key steps in the synthesis of rac-scopadulcic acid A by Ziegler and Wallace.
The β-hydroxyladehyde intermediate 252 served as a precursor of rac-scopadulcic acid A (as depicted in Scheme 23), and also of rac-scopadulcic acid B and rac-scopadulciol.
All three syntheses postponed installing the C-10 stereocenter until after construction of a BCD tricyclic fragment and relied on conjugate addition to enones to fashion the C-4 and C-10 quaternary stereocenters. A noteworthy aspect of the Ziegler synthesis is the rapid construction of the BCD tricyclic fragment harboring two quaternary stereocenters, with the first arising from a classical Robinson annulation reaction. Overman, on the other hand, used a cascade Heck cyclization to construct a related BCD tricyclic fragment. Also notable in Overman’s second-generation approach and Ziegler’s synthesis is the success realized in introducing the C-10 contiguous quaternary methyl substituent by conjugate addition of a methyl nucleophile to an enone intermediate harboring a trisubstituted double bond, whereas related conjugate addition reactions to enone intermediates having tetrasubstituted double bonds failed.
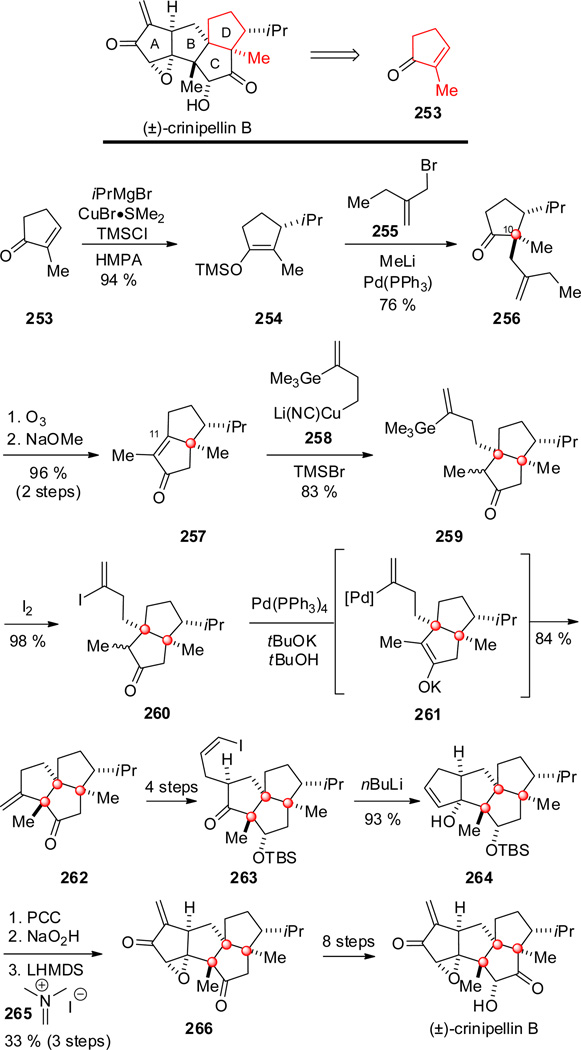
12. Crinipellins A and B
The first natural products containing a tetraquinane skeleton, crinipellins A and B, were described by Steglich in 1985 from cultures of the fungus Crinipellis stipitaria.[132] The potent antibiotic activity of what turned out to be the O-acetyl derivative of crinipellin B had been described six-years earlier.[133] The relative configuration of crinipellin B was defined by X-ray crystallography, although the absolute configuration of the crinipellins had to await elucidation by total synthesis nearly 30 years later.[134] Crinipellins A and B feature four rings containing both linear and angular triquinane motifs and eight contiguous stereocenters, three of which are contiguous quaternary stereocenters at the junctures of rings B, C, and D. The crinipellins also contain a variety of oxygenation states: epoxide, alcohol, and two ketone carbonyl groups. Only one total synthesis of rac-crinipellin A and one of (−)-crinipellin B have been reported. Key steps of these syntheses are highlighted below.
12.1. The Piers synthesis
Piers and Renaud reported in 1993 the synthesis of rac-crinipellin B, the first total synthesis of a crinipellin natural product.[135] Piers constructed rings C, B, and A in sequential fashion starting with 2-methyl-cyclopent-2-one 253 (Scheme 24). The synthesis began with copper-mediated conjugate addition of isopropylmagnesium bromide to 253. The enoxysilane product 254 was converted to the corresponding lithium enolate, which underwent stereoselective Pd-mediated coupling with allylic bromide 255 to give cyclopentanone 256 harboring the first of the three contiguous quaternary stereocenters at C-10. Ozonolysis to reveal the 1,4-diketone and cyclization with NaOMe in MeOH delivered pentalenone 257 as a prelude to a second conjugate addition to form the C-11 quaternary stereocenter. Despite the presence of the adjacent C-10 quaternary carbon and the β-disubstitution of the enone, Lewis acid-mediated addition of cuprate 258[136] to enone 257 proceeded in excellent yield to form ketone 259. As previously discovered by Piers, this conjugate addition occurred exclusively from the convex face to form the cis-pentalene product.[136b] Following germanium-iodide exchange, vinyl iodide 260 underwent Pd-catalyzed enolate vinylation[136b] to yield triquinane 262 by way of vinylpalladium enolate intermediate 261. Subsequent functionalization of triquinane 262 gave vinyl iodide 263. Lithium-iodide exchange of 263 and intramolecular addition of the vinyllithium intermediate to the carbonyl group fashioned ring A, giving tetracyclic alcohol 264. Exposure of tertiary-allylic alcohol 264 to excess pyridinium chlorochromate formed the rearranged enone and unexpectedly transformed the ring B siloxy ether to a carbonyl group. Epoxidation of this product under basic conditions and introduction of the a-methylene group with Eschenmoser’s salt (265) gave epoxide 266, which upon additional oxidation and functional group modification yielded rac-crinipellin B.
Scheme 24.
Key steps in the synthesis of rac-crinipellin B by Piers and Renaud.
The most noteworthy feature of the Piers synthesis is the construction of triquinane 262 harboring three contiguous quaternary stereocenters in only 7 steps and 47 % overall yield from 2-methyl-2-cyclopentenone (253). Key to this remarkable sequence was the facility of cuprate conjugate additions to 5-membered enones, which even took place in high yield to construct contiguous quaternary stereocenters with an enone substrate 257 harboring a tetrasubstituted double bond. Also noteworthy was this early use of palladium-catalyzed coupling of a basic enolate and a vinylhalide to fashion the third ring and third contiguous quaternary carbon center.
12.2. The Lee synthesis
Lee and co-workers reported the enantioselective total synthesis of (−)-crinipellin A in 2014.[134] In their approach (Scheme 25), the diterpenoid’s tetraquinane framework would be constructed from monocyclic tosylhydrazone 267 by a cascade sequence involving the intermediacy of a trimethylenemethane diyl.[137] Synthesis of tosylhydrazone 267 was foreseen arising from cyclopentanone 253 in a fashion similar to the opening steps of the Piers synthesis. The Lee synthesis commenced by converting cyclopentenone 253 to enantioenriched cyclopentanol 269 by a known seven-step sequence featuring CBS reduction for enantioenrichment.[138,139] Thereby, the first quaternary center at C-10 was installed by cuprate addition to cyclopentenone 253, enolization and alkylation with allylbromide to yield cyclopentanone 268. Further functionalization and chemical racemate resolution of ketone 268 via alcohol 269 led to allylic alcohol 270. Sharpless asymmetric epoxidation of allylic alcohol 270 followed by oxidation and Seyferth-Gilbert homologation with the Ohira-Bestmann reagent (271) provided alkynyl epoxide 272.
Scheme 25.
Key steps in the synthesis of (−)-crinipellin A by Lee and co-workers.
The allene was introduced as a 1:1 mixture of stereoisomers by Fe-promoted reaction of 272 with Grignard reagent 273. Subsequent functional group modification led to tosyl hydrazone 267, which upon heating with NaH in refluxing toluene gave tetraquinane 277 in 87 % yield. This remarkable stereoselective transformation is believed to occur by base-promoted elimination to generate alkyl diazo intermediate 274, which cycloadds to the distal allene double bond to yield dihydropyrazole 275. Loss of nitrogen from 275 generates a diyl intermediate, which undergoes formal [3+2]-cycloaddition via favored chair conformer 276 to yield tetraquinane 277, forming three C–C bonds as well as the A, B, and C rings. The last step of this sequence constructs the two final quaternary stereocenters of the crinipellin skeleton (C-7 and C-11). Although diastereoselectivity in forming the C-11 stereocenter is influenced by the adjacent C-10 stereocenter, Lee notes that the relative configuration of the oxygen substituent and its bulky TBDPS-protecting group are necessary to realize high stereoselection in the formation of tetraquinane 277. Introduction of the three remaining oxygen substituents and the exomethylene group completed the enantioselective synthesis of (−)-crinipellin A.
The strategy of the Lee synthesis provides a striking contrast to the linear approach to ring formation utilized by Piers. In Lee’s approach, three of the four 5-membered rings are fashioned in the one-step conversion of a tosylhydrazone to a tetraquinane. In this remarkable transformation, the ultimate 1,3-diyl-alkene cycloaddition step forms two rings and two of the three contiguous quaternary carbons of the tetraquinane target.
13. Chimonanthines
The chimonanthines have a distinctive hexacyclic structure in which two pyrrolidino[2,3-b]indoline (cyclotryptamine) subunits are linked at the benzylic quaternary carbons. They were first isolated from plants,[140] and subsequently identified in fungi and Colombian poison dart frogs.[141] All three possible stereoisomers—meso and a pair of C2-symmetric enantiomers—are found in nature. Hexacyclic chimonanthine fragments are core units in many larger polyindoline alkaloids such as hodgkinsine and the quadrigemines. The structure of (−)-chimonanthine was settled in 1962 by an early application of X-ray crystallography.[142] The chimonanthines are undoubtedly biosynthesized from two tryptophan units, although details of the biosynthetic pathway, in particular how the contiguous quaternary carbons are linked, are not known. The challenges involved in linking two cyclotryptophan fragments to form the weak C-3a/C-3a’ bond linking the two contiguous quaternary carbons has been discussed in some detail.[143] Although non-stereocontrolled syntheses of chimonanthines were reported as early as 1964,[144] the first stereocontrolled synthesis, that of meso-chimonanthine, was not reported until 1996.[145] Soon thereafter, stereo- and enantiocontrolled syntheses of the C2-symmetric enantiomers were developed. Key steps in four of these enantioselective syntheses are discussed.
13.1. The Overman syntheses of (–)- and (+)-chimonanthine
The first enantioselective syntheses of a C2-symmetric chimonanthine was published in 1999 by Overman and coworkers.[146] In this approach, (−)-chimonanthine was disconnected to hexacyclic precursor 278, with the aim of elaborating the oxindole rings to cyclotryptamine fragments in a classical fashion[147] after cleavage of the central six-membered ring (Scheme 26). It was visualized that 278 would then arise from a diastereoselective double Heck cyclization, and that the Heck cascade precursor could be constructred from tartrate-derived diiodide 279, dimethyl succinate (280), and 2-iodoaniline (281). Early exploration of this approach showed that the desired anti arrangement of the spirooxindole units in Heck product 278 was obtained when the tans-cyclohexene diol was masked as an acetonide. The synthesis began with diethyl L-tartrate 282, which after transformation to the acetonide variant of diiodide 279, was dialkylated with diester 280 and the resulting cyclohexane product advanced to diamide 283. Cascade Heck cyclization of this diiodide gave a single pentacyclic product 278 in 90 % yield via tricyclic palladium intermediate 284 thereby constructing both contiguous quaternary stereocenters in a single step. Removal of the acetonide group of 278, cleavage of the resulting six-membered ring diol with Pb(OAc)4, and hydride reduction gave diol 285 in high yield. In five additional steps, 285 was advanced to (−)-chimonanthine.
Scheme 26.
Key steps in the synthesis of of (−)-chimonanthine by Overman using a diasteroselective Heck cyclization cascade.
The subtle factors that control stereoselection in the cascade Heck cyclization of diiodide 283 were subsequently studied in detail.[148] The acetonide plays an essential role, as protection of the oxygen substituents as benzyl ethers yields a major cyclization product having syn-oriented spirooxindole units, with this product being a precursor of meso-chimonanthine.[146] One year later, Overman and co-workers reported a second construction of C2-symmetric chimonanthines.[149] The strategy was similar to that depicted in Scheme 26, but in this approach a cyclohexane intermediate having anti-oriented spirooxindole units was fashioned by dialkylation of dioxindole 286 with L-tartrate-derived dielectrophile 287 (Scheme 27).
Scheme 27.
Key steps in the synthesis of of (+)-chimonanthine by Overman using a diasteroselective dialkylation strategy.
This synthesis began with the preparation of dioxindole 286 in 3 steps and 71 % yield from oxindole and isatin. Choice of the counterion and reaction conditions was critical to the success of the diastereoselective dialkylation reaction. To favor the second alkylation proceeding as depicted in representation 288, chelation of the enolate counterion with the carbonyl group of the initially formed 3,3-dialkylspirooxindole had to be prevented. Use of a lithium counter ion and the additive 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU) known to tightly bind lithium cation was found to be essential. Under the optimized conditions depicted in Scheme 27, C2-symmetric product 289 and its meso stereoisomer were formed in a 2.8:1 ratio. Factors that govern diastereoselection in the first alkylation to generate intermediate 288 with high selectivity remain poorly understood.
13.2. The Movassaghi synthesis of (+)-chimonanthine
In 2007, Movassaghi and Schmidt disclosed a convergent, efficient strategy to synthesize (+)-chimonanthine.[150] A variation of this strategy was subsequently employed by Ma and coworkers to prepare (−)-chimonanthine.[151] In Movassaghi’s approach, the bond connecting the contiguous quaternary stereocenters is disconnected—in a fashion mimicking a plausible biosynthetic dimerization of cyclotryptamine tertiary radicals—to give tertiary bromide 290 (Scheme 28). To allow rapid access to the cyclotryptamine unit of 290 in enantiomerically enriched form, it would be assembled from a derivative of natural tryptophan 291. The synthesis commenced by a known diastereoselective isomerization of commercially available tryptophan derivative 291 to its cyclotryptophan isomer, followed by protection of the indoline nitrogen to give 292 in high yield.[152] Stereoselective benzylic halogenation using dibromohydantoin 293[153] then delivered bromide 290 in 3 steps and 65 % overall yield from 291.
Scheme 28.
Key steps in the synthesis of (+)-chimonanthine by Movassaghi and Schmidt.
In the key step, reaction of bromide 290 with CoCl(PPh3)3 generated tertiary radical 294, which upon dimerization gave bis-pyrrolidinoindoline product 295 in a remarkable 60 % yield. The high diastereoselectivity observed in forming the contiguous quaternary stereocenters is a result of dimerization occurring from the more accessible convex face of each cyclotryptophan fragment. In four additional steps, the ester group was removed and the methyl groups were installed to yield (+)-chimonanthine in a notably concise sequence.
13.3. The Kanai-Matsunaga synthesis of (+)-chimonanthine
Kanai and Matsunaga reported the synthesis of (+)-chimonanthine in 2012 by a strategy quite different from those discussed thusfar in which enantioselective catalysis plays a key role.[154] In this approach, chimonanthine was disconnected to dioxindole 296, which would be formed from dioxindole precursor 297 by two Michael additions to nitroethylene 298 (Scheme 29).[155] In this key step, the first Michael reaction would dictate enantioselectivity and the second Michael reaction would dictate diastereoselectivity. With access to dioxindole 297 in two steps from commercial materials, initial studies focused on accomplishing the double Michael addition in a single step to form C2-symmetric product 296. Using a manganese co-catalyst and Schiff base 299, double-Michael product 296 was obtained in both modest yield (44 %) and diastereoselectivity (5:1).
Scheme 29.
Key steps in the synthesis of (+)-chimonanthine by Kanai, Matsunaga and co-workers.
The authors proposed that steric congestion created by the first quaternary stereocenter limits the efficacy of the second Michael addition. A two-step sequence was then developed in which product 300 of the first enantioselective Michael addition, after filtration to remove the catalysts, a more reactive catalyst was introduced to facilitate the second highly diastereoselective Michael addition giving dioxindole product 296 in 69 % yield and 95 % ee with >20:1 diastereoselectivity. Dioxindole 296 was subsequently advanced to complete an especially short synthesis of (+)-chimonanthine.
Although both contiguous quaternary stereocenters are formed in a single step (or a one-pot sequence in the Kanai-Matsunaga synthesis) in these four syntheses, the strategies employed by the three research groups are notably different. In the Overman syntheses, the contiguous quaternary carbons are formed by diastereoselective reactions in which stereocontrol in the critical first C–C bond-forming step is dictated by two oxygen-containing stereocenters that are not found in the chimonanthine products. The Movassaghi synthesis, in which the key step is dimerization of a tricyclic persistent tertiary-benzylic radical intermediate,[156] illustrates the ultimate in convergent synthesis strategy. The notably short Kanai-Matsunaga synthesis exploits enantioselective catalysis to incorporate the contiguous quaternary stereocenters on a readily available dioxindole precursor. The fact that the second catalytic enantioselective Michael reaction of intermediate 300 required more forcing conditions than the first, provides yet another example of the challenge in forming contiguous quaternary carbons.
14. Epilogue
When the target molecule of a chemical synthesis endeavor contains multiple quaternary carbon stereocenters, how to construct such highly functionalized segments becomes a central focus of synthesis planning.[10] When these quaternary carbons are contiguous and stereogenic, the hindered environment surrounding them renders such constructions in stereoselective manner unusually difficult.[10e] The syntheses of natural products of extraordinary structural complexity highlighted in this review reveal how this challenge can be successfully met. The remarkable creativity of synthetic chemists to address such challenges in various ways over the past six decades is striking.[4,9] A compendium of selected natural products harboring two or more contiguous stereogenic carbons that have been previously synthesized, as well as key reaction types, is provided in the Supporting Information section.
Several insights emerge from examining how the second or third quaternary center of a set of contiguous quaternary stereocenters within a molecule was formed in the syntheses discussed in this review. Considering the serious steric challenge, it is not surprising that intramolecuar C-C bond constructions were utilized in two thirds of the syntheses. In fact, pericyclic reactions exemplified by cycloadditions and sigmatropic rearrangements account for half of the C-C bond forming reactions in the syntheses, with enolate alkylations and conjugate additions being utilized in just over a third. Completing the list are other creative C-C bond forming methods involving free radical chemistry and photochemical transformations. Considering the remarkable progress recorded during the past decade in forming quaternary carbons using enantioselective catalysis,[10a] it seems assured that these methods will begin to play a larger role in the chemical synthesis of structurally more intricate molecules.
Numerous future opportunities and challenges in this area will emerge through creative thinking and being inspired by biogenetic principles. In particular, we envisage the invention of C-C bond forming methods in which multiple stereogenic centers, including quaternary ones, can be created in a single operation via cascade or sequential bond forming steps. Complex molecules containing three and four contiguous quaternary stereocenters such as musabalbisiane[11], waihoensene[157], and the taxane-derived propellane[158], which are depicted in the frontispiece and yet to be synthesized, are excellent incentives to test the feasibility of newly developed synthetic methods. Associating such complex molecules with interesting biological activities would further heighten interest in their total synthesis thus heralding numerous opportunities for future innovation.
Supplementary Material
Biographies

Martin Büschleb, born in Leinefelde (Germany), obtained his diploma (2008) and PhD in organic chemistry from Georg-August University in Göttingen in 2012 under Professor Christian Ducho, where he studied the synthesis and application of epicapreomycidine in natural product synthesis. Martin pursued postdoctoral studies at Université de Montréal in Professor Stephen Hanessian’s laboratory in the field of natural product synthesis. Currently, he is working in process development at Qiagen in Hilden, Germany.

Stéphane Dorich was born in Montréal and raised in Chertsey, Québec, Canada. He obtained a B. Sc. with First-Class Honors at McGill University in 2006. He then joined the Hanessian group at the Université de Montréal and worked on diverse projects involving synthetic methodology and natural products harboring quaternary carbon centers. Stéphane completed his Ph. D. in 2013 with Exceptional Honors. Since then, he has been working as a Research Scientist at OmegaChem.

Stephen Hanessian was born in Alexandria, Egypt in 1935. After attending the American Mission School, he graduated from the University of Alexandria with First Class Honors in 1956. Following a year as research chemist at the Starch Products Co., he obtained his Ph.D. degree in 1960 under the guidance of the late Professor Melville L. Wolfrom at the Ohio State University working on early examples of chelation controlled organometallic additions to sugar aldehydes. From 1961–1968, he was employed as research scientist at Parke-Davis Research Laboratories in Ann Arbor, Michigan. In 1969, he joined the faculty at the Université de Montréal as Associate Professor and became Full Professor in 1970. He currently holds the Isis Pharmaceuticals Research Chair at the Université de Montréal, and is also on the Faculty in the Departments of Pharmaceutical Sciences, Chemistry, and Pharmacology at the University of California, Irvine where he is the Director of the Medicinal Chemistry and Pharmacology Graduate Program. His research interests cover a broad cross-section of areas related to synthetic organic, bioorganic, and medicinal chemistry published in over 500 Journal articles

Daniel Tao was born and raised in Lafayette, Indiana. He obtained a B.S. degree in chemistry from Calvin College in 2010, during which he was awarded NSF fellowships under Professor Stefan France at Georgia Institute of Technology and later Professor John Montgomery at University of Michigan. Daniel is currently a graduate student at University of California, Irvine working in Professor Larry Overman’s laboratory on applying photoredox catalysis in natural product total synthesis.

Kyle Schenthal was born in New Orleans, Louisiana in 1990 and raised in Destin, Florida. He obtained his A.B. degree from Princeton University in 2012, working in the lab of Professor Erik J. Sorensen on the synthesis of complex natural products. He began his graduate studies at the University of California, Irvine where he is currently a doctoral candidate in Professor Larry Overman’s laboratory investigating the photoredox-catalyzed construction of quaternary carbons in biologically active systems.

Larry Overman was born in Chicago, Illinois in 1943 and raised in Hammond, Indiana. He obtained a B.A. degree from Earlham College in 1965 and completed his doctoral dissertation in 1969 with Professor Howard W. Whitlock, Jr. at the University of Wisconsin. After a NIH postdoctoral fellowship with Professor Ronald Breslow at Columbia University, he joined the faculty at the University of California, Irvine in 1971 where he is now Distinguished Professor of Chemistry. Professor Overman’s research interests center on the invention of new transformations and strategies in organic synthesis and the total synthesis of natural products and their congeners. Using synthesis strategies developed largely in his laboratory, Professor Overman’s group has completed total syntheses of more than 100 structurally complex natural products.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Wöhler F. Ann. Physik. Chem. 1828;88:253–256. [Google Scholar]
- 2.a) Balandrin MF, Kinghorn AD, R N. Farnsworth in Plant derived Natural Products in Drug Discovery and Development: an Overview. In: Kinghorn AD, Balandrin MF, editors. Human Medicinal Agents from Plants, American Chemical Society Symposium No.534. Washington, DC: American Chemical Society; 1993. pp. 2–12. [Google Scholar]; b) Brahmanchari G. Handbook of Pharmaceutical Natural Products. 1,2. Weinheim: Wiley-VCH; 2010. [Google Scholar]
- 3.For selected examples, see: Offit P. Do You Believe in Magic? The Sense and Nonsense of Alternative Medicine. New York: Harper Collins; 2013. Fontanarosa PB, Lundberg. GD. JAMA. 1998;280:1618–1619. doi: 10.1001/jama.280.18.1618. Bivins R. Alternative medicine? A History. Oxford UK: Oxford University Press; 2007.
- 4.Hanessian S, Giroux S, Merner BL. Design and Strategy in Organic Synthesis: From the Chiron Approach to Catalysis. Weinheim: Wiley-VCH; 2013. Chapters 2–4. [Google Scholar]
- 5.For selected examples, see: Newman DJ, Cragg GM. In: Natural Products as Drugs and Leads to Drugs: An Introduction and Perspective as of end of 2012, in Natural Products in Medicinal Chemistrysource. Hanessian S, editor. Weinheim: Wiley-VCH; 2014.
- 6.For selected examples, see: Hanessian S. Chem. Med. Chem. 2006;1:1300–1330. for a historical perspective, see: Nicolaou KC, Montagnon T. Molecules that Changed the World. Weinheim: Wiley-VCH; 2008. Corey EJ, Kurti L, Czako B. Molecules and Medicine. New York: Wiley-Interscience; 2007.
- 7.Nicolaou KC, Vourloumis D, Winssinger N, Baran PS. Angew. Chem. Int. Ed. 2000;39:44–122. [PubMed] [Google Scholar]; Angew. Chem. 2000;112:46–126. [Google Scholar]
- 8.Corey EJ, Kurti L. Enantioselective Chemical Synthesis: Methods, Logic and Practice. Dallas, TX: 2010. [Google Scholar]
- 9.For selected recent monographs, see: Nicolaou KC, Chen JS. Classics in Total Synthesis III: Further Targets, Strategies, Methods. Weinheim: Wiley-VCH; 2011. Carreira EM, Kvaerno L. Classics in Stereoselective Synthesis. Weinheim: Wiley-VCH; 2009. Harmata M, editor. Strategies and Tactics in Organic Synthesis. 1–7. New York: Academic Press; 2008. Taber DF. Organic Synthesis: State of the Art. 2005–2007 New Jersey: Wiley, Hoboken; 2003–2005. Hudlicky T, Reed JW. The Way of Synthesis. Weinheim: Wiley-VCH; 2007.
- 10.For representative reviews and monographs discussing enantioselective construction of stereogenic quaternary carbons, see: Quasdorf KW, Overman LE. Nature. 2014;516:181–191. doi: 10.1038/nature14007. Hong AY, Stoltz BM. Eur. J. Org. Chem. 2013:2745–2759. doi: 10.1002/ejoc.201201761. Das JP, Marek I. Chem. Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a. Christoffers J, Baro A, editors. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Weinheim: Wiley-VCH; 2005. Douglas CJ, Overman LE. Proc. Natl. Acad Sci. USA. 2004;101:5663–5367. doi: 10.1073/pnas.0307113101. Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146. Christoffers J, Mann A. Angew. Chem. Int. Ed. 2001;40:4591–4597. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v. Angew. Chem. 2001;113:4725–4732. Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. Angew. Chem. 1998;110:402–415. Fuji K. Chem. Rev. 1993;93:2037–2066. Martin S. Tetrahedron. 1980;36:419–460.
- 11.Ali M. Phytochemistry. 1992;31:2173–2175. [Google Scholar]
- 12.For selected reviews, see: Oikawa H, Tokiwano T. Nat. Prod. Rev. 2004:321–352. doi: 10.1039/b305068h. Stocking EM, Williams RM. Angew. Chem. Int. Ed. 2003;42:3078–3115. doi: 10.1002/anie.200200534. Stocking EM, Williams RM. Angew. Chem. 2003;115:3186–3223. doi: 10.1002/anie.200200534.
- 13.a) Hauser D, Sigg HP. Helv. Chim. Acta. 1971;54:1178–1190. doi: 10.1002/hlca.19710540427. [DOI] [PubMed] [Google Scholar]; b) Vasella A. PhD Dissertation. Zurich, Switzerland: ETH; 1972. [Google Scholar]
- 14.a) Gargallo-Viola D. Curr. Opin. Anti-Infect. Invest. Drugs. 1999;1:297–305. [Google Scholar]; b) Justice MC, Hsu M-J, Tse B, Ku T, Balkovec J, Schmatz D, Nielsen J. J. Biol. Chem. 1998;273:3148–3151. doi: 10.1074/jbc.273.6.3148. [DOI] [PubMed] [Google Scholar]
- 15.Borschberg HJ. PhD Dissertation. Zurich, Switzerland: ETH; 1975. [Google Scholar]
- 16.Liang H. Beilstein J. Org. Chem. 2008;4:31. doi: 10.3762/bjoc.4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato N, Kusakabe S, Wu X, Kamitamari M, Takeshita H. J. Chem. Soc., Chem. Commun. 1993:1002–1004. [Google Scholar]
- 18.a) Kato N, Nakanishi K, Takeshita H. Bull. Chem. Soc. Jpn. 1986;59:1109–1123. [Google Scholar]; b) Kato N, Wu X, Takeshita H. Chem. Express. 1991;6:687–690. [Google Scholar]
- 19.The use of Diels–Alder reactions had already been proven useful in the synthesis of related core structures. See: Mander LN, Robinson RP. J. Org. Chem. 1991;56:3595–3601. See also reference 12.
- 20.a) Mander LN, Thomson RJ. Org. Lett. 2003;5:1321–1324. doi: 10.1021/ol0342599. [DOI] [PubMed] [Google Scholar]; b) Mander LN, Thomson RJ. J. Org. Chem. 2005;70:1654–1670. doi: 10.1021/jo048199b. [DOI] [PubMed] [Google Scholar]
- 21.a) Takano S, Inomata K, Takahashi M, Ogasawara K. Synlett. 1991:636–639. [Google Scholar]; b) Takano S, Moriya M, Tanaka K, Ogasawara K. Synthesis. 1994:687–689. [Google Scholar]
- 22.Bugel JP, Ducos P, Gringore O, Rouessac F. Bull. Soc. Chim. Fr. 1972:4371–4374. [Google Scholar]
- 23.a) Liang H, Schulé A, Vors J-P, Ciufolini MA. Org. Lett. 2007;9:4119–4122. doi: 10.1021/ol701547r. [DOI] [PubMed] [Google Scholar]; b) Liang H, Ciufolini MA. Org. Prep. Proc Int. 2010;42:111–132. [Google Scholar]
- 24.Kitamura M, Chiba S, Narasaka K. Chem. Lett. 2004;33:942–943. [Google Scholar]
- 25.Chiba S, Kitamura M, Narasaka K. J. Am. Chem. Soc. 2006;128:6931–6937. doi: 10.1021/ja060408h. [DOI] [PubMed] [Google Scholar]
- 26.Audia JE, Boisvert L, Patten AD, Villalobos A, Danishefsky SJ. J. Org. Chem. 1989;54:3738–3740. [Google Scholar]
- 27.For some reviews on Tsuji-Trost reactions, see: Tsuji J, Minami I. Acc. Chem. Res. 1987;20:140–145. Tsuji. J. Tetrahedron. 1986;42:4361–4401. Tsuji J. Acc. Chem. Res. 1969;2:144–152.
- 28.a) Guella G, Callone E, Di Giuseppe G, Frassanito R, Frontini FP, Mancini I, Dini F. Eur. J. Org. Chem. 2007:5226–5234. [Google Scholar]; b) Guella G, Dini F, Pietra F. Angew. Chem. Int. Ed. 1999;38:1134–1136. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1134::AID-ANIE1134>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1999;111:1217–1220. [Google Scholar]
- 29.a) Nicolaou KC, Ortiz A, Zhang H, Dagneau P, Lanver A, Jennings MP, Arseniyadis S, Faraoni R, Lizos DE. J. Am. Chem. Soc. 2010;132:7138–7152. doi: 10.1021/ja100740t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nicolaou KC, Ortiz A, Zhang H, Guella G. J. Am. Chem. Soc. 2010;132:7153–7176. doi: 10.1021/ja100742b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guella G, Skropeta D, Di Giuseppe G, Dini F. Mar. Drugs. 2010;8:2080–2116. doi: 10.3390/md8072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.a) Nicolaou KC, Zhang H, Ortiz A, Dagneau P. Angew. Chem. Int. Ed. 2008;47:8605–8610. doi: 10.1002/anie.200804228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2008;120:8733–8738. [Google Scholar]; b) Nicolaou KC, Zhang H, Ortiz A. Angew. Chem. Int. Ed. 2009;48:5642–5647. doi: 10.1002/anie.200902028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009;121:5752–5757. [Google Scholar]; c) Nicolaou KC, Ortiz A, Zhang H. Angew. Chem. Int. Ed. 2009;48:5648–5652. doi: 10.1002/anie.200902029. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009;121:5758–5762. [Google Scholar]
- 32.Nicolaou KC, Jennings MP, Dagneau P. Chem. Commun. 2002:2480–2481. [Google Scholar]
- 33.Snider BB. Chem. Rev. 1996;96:339–363. doi: 10.1021/cr950026m. [DOI] [PubMed] [Google Scholar]
- 34.For some reviews on SmI2-mediated cyclizations, see: Nicolaou KC, Ellery SP, S J. Chen., Angew. Chem. Int. Ed. 2009;48:7140–7165. doi: 10.1002/anie.200902151. Angew. Chem. 2009;121:7276–7301. Edmonds DJ, Johnston D, Procter DJ. Chem. Rev. 2004;104:3371–3403. doi: 10.1021/cr030017a. Kagan HB. Tetrahedron. 2003;59:10351–10372. Molander GA, Harris CR. Chem. Rev. 1996;96:307–338. doi: 10.1021/cr950019y.
- 35.Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew. Chem. Int. Ed. 2005;44:3874–3879. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005;117:3942–3947. [Google Scholar]
- 36.Goto K, Sudzuki H. Bull. Chem. Soc. Jpn. 1929;4:220–224. [Google Scholar]
- 37.a) Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Chem. Pharm. Bull. 1971;19:770–791. [Google Scholar]; b) Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967;8:2421–2424. [Google Scholar]; c) Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967;8:2425–2430. [Google Scholar]
- 38.a) Babiker HAA, Sugimoto Y, Saisho T, Inanaga S, Hashimoto M, Isogai A. Biosci. Biotechnol. Biochem. 1999;63:515–518. doi: 10.1271/bbb.63.515. [DOI] [PubMed] [Google Scholar]; b) Sugimoto Y, Uchida S, Inanaga S, Kimura Y, Hashimoto M, Isogai A. Biosci. Biotechnol. Biochem. 1996;60:503–505. doi: 10.1271/bbb.60.503. [DOI] [PubMed] [Google Scholar]; c) Sugimoto Y, Matsui M, Babiker HAA. Phytochemistry. 2007;68:493–498. doi: 10.1016/j.phytochem.2006.10.029. [DOI] [PubMed] [Google Scholar]; d) Barton DHR, Kirby AJ, Kirby GW. J. Chem. Soc. C. 1968:929–936. doi: 10.1039/j39680000929. [DOI] [PubMed] [Google Scholar]; e) Matoba K, Karibe N, Yamazaki T. Chem. Pharm. Bull. 1984;32:2639–2645. [Google Scholar]; f) Waller DL, Stephenson CRJ, Wipf P. Org. Biomol. Chem. 2007;5:58–60. doi: 10.1039/b612992g. [DOI] [PubMed] [Google Scholar]
- 39.a) Yu B-W, Chen J-Y, Wang Y-P, Cheng K-F, Li X-Y, Qin G-W. Phytochemistry. 2002;61:439–442. doi: 10.1016/s0031-9422(02)00162-0. [DOI] [PubMed] [Google Scholar]; b) Qin G-W, Tang X-C, Lestage P, Caignard D-H, Renard P. 2004000815. PCT Int. Appl. WO. 2003
- 40.a) Li F, Castle SL. Org. Lett. 2007;9:4033–4036. doi: 10.1021/ol701757f. [DOI] [PubMed] [Google Scholar]; b) Li F, Tartakoff SS, Castle SL. J. Am. Chem. Soc. 2009;131:6674–6675. doi: 10.1021/ja9024403. [DOI] [PubMed] [Google Scholar]
- 41.Li F, Tartakoff SS, Castle SL. J. Org. Chem. 2009;74:9082–9093. doi: 10.1021/jo902006q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy JA. In: Radicals in Organic Synthesis. Renaus P, Sibi MP, editors. Vol. 1. Weinheim: Wiley-VCH; 2001. pp. 298–315. For a review, see: Godineau E, Landais Y. Chem. Eur. J. 2009;15:3044–3055. doi: 10.1002/chem.200802415.
- 43.Davis FA, Vishwakarma LC, Billmers JG, Finn J. J. Org. Chem. 1984;49:3241–3243. [Google Scholar]
- 44.Nakamura M, Hirai A, Sogi M, Nakamura E. J. Am. Chem. Soc. 1998;120:5846–5847. [Google Scholar]
- 45.For studies toward the synthesis of the core structures of acutumine, see: Moreau RJ, Sorensen EJ. Tetrahedron. 2007;63:6446–6453. Navarro R, Reisman SE. Org. Lett. 2012;14:4354–4357. doi: 10.1021/ol3017963.
- 46.a) King SM, Calandra NA, Herzon SB. Angew. Chem. Int. Ed. 2013;52:3642–3645. doi: 10.1002/anie.201210076. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125:3730–3733. [Google Scholar]
- 47.Corey EJ, Shibata T, Lee TW. J. Am. Chem. Soc. 2009;124:3808–3809. doi: 10.1021/ja025848x. [DOI] [PubMed] [Google Scholar]
- 48.a) Herzon SB, Calandra NA, King SM. Angew. Chem. Int. Ed. 2011;50:8863–8866. doi: 10.1002/anie.201102226. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011;123:9025–9028. [Google Scholar]
- 49.For an example of the Hosomi-Sakurai reaction, see: Hosomi A, Sakurai H. J. Am. Chem. Soc. 1977;99:1673–1675. For a review, see: Schinzer D. Synthesis. 1988:263–273.
- 50.Arbain D, Byme LT, Cannon JR, Patrick VA, White AH. Aust. J. Chem. 1990;43:185–190. [Google Scholar]
- 51.Gibbons CS, Trotter J. J. Chem. Soc. B. 1969:840–843. [Google Scholar]
- 52.For reviews on daphniphyllum alkaloids, see: Yamamura S, Hirata Y. In: The Alkaloids. Manske RHF, editor. Vol. 15. New York: Academic Press; 1975. pp. 41–81. Yamamura S. In: The Alkaloids. Brossi A, editor. Vol. 29. New York: Academic Press; 1986. pp. 265–286. Kobayashi J, Morita H. In: The Alkaloids. Cordell GA, editor. Vol. 60. New York: Academic Press; 2003. pp. 165–205. Morita H, Kobayashi J. In: Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Fattorusso E, Taglialatela-Scafati O, editors. Weinheim: Wiley-VCH; 2008. pp. 541–589. Kobayashi J, Kubota T. Nat. Prod. Rep. 2009;26:936–962. doi: 10.1039/b813006j. Wu H, Zhang X, Ding L, Chen S, Yang X, Xu J. Planta Med. 2013;79:1589–1598. doi: 10.1055/s-0033-1351024. Kang B, Jakubec P, Dixon DJ. Nat. Prod. Rep. 2014;31:550–562. doi: 10.1039/c3np70115h.
- 53.a) Suzuki KT, Okuda S, Niwa H, Toda M, Hirata Y, Yamamura S. Tetrahedron Lett. 1973;14:799–802. [Google Scholar]; b) Niwa H, Hirata Y, Suzuki KT, Yamamura S. Tetrahedron Lett. 1973;14:2129–2132. [Google Scholar]
- 54.Heathcock CH. Proc Natl. Acad. Sci. USA. 1996;93:14323–14327. doi: 10.1073/pnas.93.25.14323. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panthong A, Kanjanapothi D, Thitiponpunt Y, Taesotikul T, Arbain D. Planta Med. 1998;64:530–535. doi: 10.1055/s-2006-957508. [DOI] [PubMed] [Google Scholar]
- 56.Heathcock CH, Stafford JA, Clark DL. J. Org. Chem. 1992;57:2575–2585. [Google Scholar]
- 57.a) Trost BM, Genet JP. J. Am. Chem. Soc. 1976;98:8516–8517. [Google Scholar]; b) Trost BM, Cossy JP. J. Soc. Am. Chem. 1982;104:6881–6882. [Google Scholar]; c) Hegedus LS, Allen GF, Bozell JJ, Waterman EL. J. Am. Chem. Soc. 1978;100:5800–5807. [Google Scholar]; d) Trost BM, Metzner PJ. J. Am. Chem. Soc. 1980;102:3572–3577. [Google Scholar]
- 58. Fétizon M, Golfier M, Louis J-M. J. Chem. Soc. D., Chem. Commun. 1969:1118–1119. Fétizon M, Golfier M, Louis J-M. Tetrahedron. 1975;31:171–176. for a review, see: McKillop A, Young DW. Synthesis. 1979:401–422.
- 59.Morita H, Ishioka N, Takatsu H, Iizuka T, Kobayashi J. J. Nat. Prod. 2006;69:418–420. doi: 10.1021/np0503799. [DOI] [PubMed] [Google Scholar]
- 60.Weiss ME, Carreira EM. Angew. Chem. Int. Ed. 2011;50:11501–11505. doi: 10.1002/anie.201104681. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011;123:11703–11707. [Google Scholar]
- 61.a) Guha PC. Ber Dtsch. Chem. Ges. 1939;72:1359–1373. [Google Scholar]; b) Luo YF, Carnell AJ. Angew. Chem. Int. Ed. 2010;49:2750–2754. doi: 10.1002/anie.200907033. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010;122:2810–2814. [Google Scholar]
- 62.a) Schrauzer GN, Kratel G. Chem. Ber. 1969;102:2392–2407. [Google Scholar]; b) Tada M, Kaneko K. J. Org. Chem. 1995;60:6635–6636. [Google Scholar]
- 63.Yahata H, Kubota T, Kobayashi J. J. Nat. Prod. 2008;72:148–151. doi: 10.1021/np800515s. [DOI] [PubMed] [Google Scholar]
- 64.a) Shvartsbart A, Smith AB., III J. Am. Chem. Soc. 2014;136:870–873. doi: 10.1021/ja411539w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shvartsbart A, Smith AB., III J. Am. Chem. Soc. 2015;137:3510–3519. doi: 10.1021/ja503899t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sieburth SM, Lang J. J. Org. Chem. 1999;64:1780–1781. doi: 10.1021/jo990082d. [DOI] [PubMed] [Google Scholar]
- 66.a) Trost BM, Fullerton TJ. J. Am. Chem. Soc. 1973;95:292–294. [Google Scholar]; b) Tsuji J, Takahashi H, Morikawa M. Tetrahedron Lett. 1965;6:4387–4388. [Google Scholar]
- 67. Nazarov IN, Zaretskaya II, Sorkina TI. Zh. Obshch. Khim. 1960;30:746–754. For a review, see: Frontier AJ, Collison C. Tetrahedron. 2005;61:7577–7606.
- 68.Fleming I, Henning R, Plaut H. J. Chem. Soc. Chem. Commun. 1984:29–31. [Google Scholar]
- 69.a) Crabtree R. Acc. Chem. Res. 1979;12:331–337. [Google Scholar]; b) Wuestenberg B, Pfaltz A. Adv. Synth. Catal. 2008;350:174–178. [Google Scholar]
- 70.Nakanishi K, Habaguchi K, Woods Y, Nakadaira MC, Maruyama M, Major RT, Alauddin M, Patel AR, Weinges K, Baehr W. J. Am. Chem. Soc. 1971;93:3544–3546. [Google Scholar]
- 71.Zeng Z, Zhu J, Chen L, Wen W, Yu R. Pharmacogn Rev. 2013;7:47–52. doi: 10.4103/0973-7847.112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strømgaard K, Nakanishi K. Angew. Chem. Int. Ed. 2004;43:1640–1658. doi: 10.1002/anie.200300601. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004;116:1670–1689. [Google Scholar]
- 73.Braquet P. Drugs Future. 1987;12:643–699. [Google Scholar]
- 74.a) Defeudis FV. Pharmacological Research. 2002;46:565–568. doi: 10.1016/s1043-6618(02)00233-5. [DOI] [PubMed] [Google Scholar]; b) Kiewert C, Kumar V, Hildmann O, Hartmann J, Hillert M, Klein J. Brain. Research. 2008;1201:143–150. doi: 10.1016/j.brainres.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 75.Chebib M, Hanrahan JR, Mewett KN, K Duke R, Johnston GAR. Annu. Rep. Med. Chem. 2004;39:13–23. [Google Scholar]
- 76.Corey EJ, Su W-g. Tetrahedron Lett. 1988;29:3423–3426. doi: 10.1016/0040-4039(88)85122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corey EJ, Su W-g. J. Am. Chem. Soc. 1987;109:7534–7536. [Google Scholar]
- 78.Taschner MJ, Rose T, Heathcock CH. Org. Synth. 1985;64:108–113. [Google Scholar]
- 79.Furuta K, Iwanaga K, Yamamoto H. Tetrahedron Lett. 1986;27:4507–4510. [Google Scholar]
- 80.Corey EJ, Su W-g. Tetrahedron Lett. 1987;28:5241–5244. doi: 10.1016/S0040-4039(00)95679-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crimmins MT, K Jung D, Gray JL. J. Am. Chem. Soc. 1992;114:445–5447. [Google Scholar]
- 82.Imamoto T, Takiyama N, Nakamura K, Hatajima T, Kamiya Y. J. Am. Chem. Soc. 1989;111:4392–4398. [Google Scholar]
- 83.Tomari K, Machiya K, Ichimoto I, Ueda H. Agric. Biol. Chem. 1980;44:2135–2138. [PubMed] [Google Scholar]
- 84.Vedejs E, Engler DA, Telschow JE. J. Org. Chem. 1978;43:188–196. [Google Scholar]
- 85.a) Adam W, Curci R, Edwards JO. Acc. Chem. Res. 1989;22:205–211. [Google Scholar]; b) Murray RW. Chem. Rev. 1989;89:1187–1201. [Google Scholar]
- 86.Crimmins MT, Jung DK, Gray JL. J. Am. Chem. Soc. 1993;115:3146–3155. [Google Scholar]
- 87.For a review on the synthesis of the ginkgolides, see: Busch T, Kirschning A. Nat. Prod. Rep. 2008;25:318–341. doi: 10.1039/b705652b. For total synthesis, see: Crimmins MT, Pace JM, Nantermet PG, Kim-Meade AS, Thomas JB, Watterson SH, Wagman AS. J. Am. Chem. Soc. 1999;121:10249–10250. Crimmins MT, Pace JM, Nantermet PG, Kim-Meade AS, Thomas JB, Watterson SH, Wagman AS. J. Am. Chem. Soc. 2000;122:8453–8463.
- 88.Crimmins MT, Thomas JB. Tetrahedron Lett. 1989;30:5997–6000. [Google Scholar]
- 89.Corey EJ, Ghosh AK. Tetrahedron Lett. 1988;29:3205–3206. doi: 10.1016/0040-4039(88)85122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.a) Corey EJ, Gavai AV. Tetrahedron Lett. 1988;29:3201–3204. [Google Scholar]; b) Corey EJ, Kang MC, Desai MC, Ghosh AK, Houpis IN. J. Am. Chem. Soc. 1988;110:649–651. doi: 10.1021/ja00210a083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.a) Corey EJ, Desai MC, Engler TA. J. Am. Chem. Soc. 1985;107:4339–4340. [Google Scholar]; b) Corey EJ, Desai MC. Tetrahedron Lett. 1985;26:3535–3538. [Google Scholar]
- 92.A concise approach to the synthesis of a core structure containing the central tetrahydrofuran and the two lactones of the ginkgolides has been reported, see: Villhauer EB, Anderson RC. J. Org. Chem. 1987;52:1186–1189.
- 93.Li S-H, Wang J, Niu X-M, Shen Y-H, Zhang H-J, Sun H-D, Li M-L, Tian Q-E, Lu Y, Cao P, Zheng Q-T. Org. Lett. 2004;6:4327–4330. doi: 10.1021/ol0481535. [DOI] [PubMed] [Google Scholar]
- 94.Gong J, Lin G, Sun W, Li C-C, Yang Z. J. Am. Chem. Soc. 2010;132:16745–16746. doi: 10.1021/ja108907x. [DOI] [PubMed] [Google Scholar]
- 95.Elliott GI, Konopelski JP, Olmstead MM. Org. Lett. 1999;1:1867–1870. [Google Scholar]
- 96.Krief A, Lorvelec G, Jeanmart S. Tetrahedron Lett. 2000;41:3871–3874. [Google Scholar]
- 97.a) Wessely F, Lauterbach-Keil G, Sinwel F. Monatsch. Chem. 1950;81:811–818. [Google Scholar]; b) Metlesics W, Wessely F. Monatsch. Chem. 1957;88:108–117. [Google Scholar]
- 98.Peng F, Danishefsky SJ. J. Am. Chem. Soc. 2012;134:18860–18867. doi: 10.1021/ja309905j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng F, Yu M, Danishefsky SJ. Tetrahedron Lett. 2009;50:6586–6587. doi: 10.1016/j.tetlet.2009.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu P, Gu Z, Zakarian A. J. Am. Chem. Soc. 2013;135:14552–14555. doi: 10.1021/ja408231t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu P, Mailyan A, Gu Z, Guptill DM, Wang H, Davies HML, Zakarian A. J. Am. Chem. Soc. 2014;136:17738–17749. doi: 10.1021/ja510573v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.a) Davies HML, Grazini MVA, Aouad E. Org. Lett. 2001;3:1475–1477. doi: 10.1021/ol0157858. [DOI] [PubMed] [Google Scholar]; b) Saito H, Oishi H, Kitagaki S, Nakamura S, Anada M, Hashimoto S. Org. Lett. 2002;4:3887–3890. doi: 10.1021/ol0267127. [DOI] [PubMed] [Google Scholar]
- 103.Zheng C, Dubovyk I, Lazarski KE, Thomson RJ. J. Am. Chem. Soc. 2014;136:17750–17756. doi: 10.1021/ja5109694. [DOI] [PubMed] [Google Scholar]
- 104.Nagasawa T, Shimada N, Torihata M, Kuwahara S. Tetrahedron. 2010;66:4965–4969. [Google Scholar]
- 105.Gurevich AI, Dobrynin VN, Kolosov MN, Popravko SA, Ryabova ID, Chernov BK, Derbentseva NA, Aizenman BE, Garagulya AD. Antibiotiki. 1971;16:510–513. [PubMed] [Google Scholar]
- 106.Brondz I, Greibrokk T, Groth PA, Aasen AJ. Acta Chem. Scand. 1983;37A:263–265. [Google Scholar]
- 107.Adam P, Arigoni D, Bacher A, Eisenreich W. J. Med. Chem. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- 108.Zanoli P. CNS Drug Rev. 2004;10:203–218. doi: 10.1111/j.1527-3458.2004.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.a) Schempp CM, Kirkin V, Simon-Haarhaus B, Kersten A, Kiss J, Termeer CC, Gilb B, Kaufmann T, Borner C, Sleeman JP, Simon JC. Oncogene. 2002;21:1242–1250. doi: 10.1038/sj.onc.1205190. [DOI] [PubMed] [Google Scholar]; b) Schempp CM, Pelz K, Wittmer A, Schöpf E, Simon JC. Lancet. 1999;353:2129–2132. doi: 10.1016/S0140-6736(99)00214-7. [DOI] [PubMed] [Google Scholar]; c) Medina MA, Martínez-Poveda B, Amores-Sánchez MI, Quesada AR. Life Sci. 2006;79:105–111. doi: 10.1016/j.lfs.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 110.Bellavance G, Barriault L. Angew. Chem. Int. Ed. 2014;53:6701–6704. doi: 10.1002/anie.201403939. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014;126:6819–6822. [Google Scholar]
- 111.Ting CP, Maimone TJ. J. Am. Chem. Soc. 2015;13:10516–10519. doi: 10.1021/jacs.5b06939. [DOI] [PubMed] [Google Scholar]
- 112.Shimizu Y, Shi S-L, Usuda H, Kanai M, Shibasaki M. Angew. Chem. Int. Ed. 2010;49:1103–1106. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010;122:1121–1124. [Google Scholar]
- 113.Usuda H, Kuramochi A, Nanai M, Shibasaki M. Org. Lett. 2004;6:4387–4390. doi: 10.1021/ol048018s. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu Y, Kuramochi A, Usuda H, Kanai M, Shibasaki M. Tetrahedron Lett. 2007;48:4173–4177. [Google Scholar]
- 115.Sparling BA, Moebius DC, Shair MD. J. Am. Chem. Soc. 2013;135:644–647. doi: 10.1021/ja312150d. [DOI] [PubMed] [Google Scholar]
- 116.a) Siegel DR, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:1048–1049. doi: 10.1021/ja057418n. [DOI] [PubMed] [Google Scholar]; b) Simpkins NS, Taylor JD, Weller MD, Hayes CJ. Synlett. 2010:639–643. [Google Scholar]
- 117.Uwamori M, Nakada M. Tetrahedron Lett. 2013;54:2022–2025. [Google Scholar]
- 118.Uwamori M, Saito A, Abe M, Nakada M. J. Org. Chem. 2012;77:5098–5107. doi: 10.1021/jo300646j. [DOI] [PubMed] [Google Scholar]
- 119.a) Hayashi T, Kishi M, Kawasaki M, Arisawa M, Shimizu M, Suzuki S, Yoshizaki M, Morita N, Tezuka Y, Kikuchi T, Berganza LH, Ferro E, Basualdo I. Tetrahedron Lett. 1987;28:3693–3696. [Google Scholar]; b) Hayashi T, Kishi M, Kawasaki M, Arisawa M, Morita N. J. Nat. Prod. 1988;51:360–363. doi: 10.1021/np50056a030. [DOI] [PubMed] [Google Scholar]
- 120.Hostettmann K. Phytochemistry of Plants Used in Traditional Medicine. New York: Oxford University Press; 1995. [Google Scholar]
- 121.Asano S, Mizutani M, Hayashi T, Morita N, Takeguchi N. Biol. Chem. 1990;265:22167–22173. [PubMed] [Google Scholar]
- 122.Hayashi T, Kishi M, Kawasaki M, Arisawa M, Morita N, Berganza LH. J. Nat. Prod. 1988;51:360–363. doi: 10.1021/np50056a030. [DOI] [PubMed] [Google Scholar]
- 123.Fox ME, Li C, Marino JP, Jr, Overman LE. J. Am. Chem. Soc. 1999;121:5467–5480. [Google Scholar]
- 124.a) Overman LE, Ricca DJ, Tran VD. J. Am. Chem. Soc. 1993;115:2042–2044. [Google Scholar]; b) Overman LE, Ricca DJ, Tran VD. J. Am. Chem. Soc. 1997;119:12031–12040. [Google Scholar]
- 125.Abelman MM, Overman LE. J. Am. Chem. Soc. 1988;110:2328–2329. [Google Scholar]
- 126.Nagata W, Yoshioka M. Org. React. (New York) 1977;25:255–476. [Google Scholar]
- 127.Kucera DJ, O’Connor SJ, Overman LE. J. Org. Chem. 1993;58:5304–5306. [Google Scholar]
- 128.Doyle MP, Austin RE, Bailey AS, Dwyer MP, Dyatkin AB, Kalinin AV, Kwan MMY, Liras S, Oalmann CJ, Pieters RJ, Protopopova MN, Raab CE, Roos GHP, Zhou Q-L, Martin SF. J. Am. Chem. Soc. 1995;117:5763–5775. [Google Scholar]
- 129.Luche JL, Petrier C, Lansard JP, Greene AE. J. Org. Chem. 1983;48:3837–3839. [Google Scholar]
- 130.Ziegler FE, Wallace OB. J. Org. Chem. 1995;60:3626–3636. [Google Scholar]
- 131.Reusch W, Grimm K, Karoglan JE, Martin J, Subrahamanian KP, Toong Y-C, Venkataramani PS, Yorky JD, Zoutendam P. J. Am [Google Scholar]; Chem. Soc. 1977;99:1953–1958. [Google Scholar]
- 132.Anke T, Heim J, Knoch F, Mocek U, Steffan B, Steglich W. Angew. Chem. Int. Ed. 1985;24:709–711. [Google Scholar]; Angew. Chem. 1985;97:714–716. [Google Scholar]
- 133.Kupka J, Anke T, Oberwinkler F, Schramm G, Steglich W. Antibiot. 1979;32:130–135. doi: 10.7164/antibiotics.32.130. [DOI] [PubMed] [Google Scholar]
- 134.Kang T, Song SB, Kim W-Y, Kim BG, Lee H-Y. J. Am. Chem. Soc. 2014;136:10274–10276. doi: 10.1021/ja5054412. [DOI] [PubMed] [Google Scholar]
- 135.a) Piers E, Renaud J. J. Org. Chem. 1993;58:11–13. [Google Scholar]; b) Piers E. Synthesis. 1998:590–602. [Google Scholar]
- 136.a) Piers E, Marais PC. J. Chem. Soc, Chem. Commun. 1989:1222–1223. [Google Scholar]; b) Piers E, Marais PC. J. Org. Chem. 1990;55:3454–3455. [Google Scholar]
- 137.Kang T, Kim W-Y, Yoon Y, Kim BG, Lee H-Y. J. Am. Chem. Soc. 2011;133:18050–18053. doi: 10.1021/ja207591e. and references therein. [DOI] [PubMed] [Google Scholar]
- 138.a) Williams DR, Heidebrecht RW. J. Am. Chem. Soc. 2003;125:1843–1850. doi: 10.1021/ja0279803. [DOI] [PubMed] [Google Scholar]; b) Williams DR, Pinchman JR. Can. J. Chem. 2013;91:21–37. [Google Scholar]
- 139.For a review of chiral oxazaborolidine reductions, see: Corey EJ, Helal CJ. Angew. Chem. Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. Angew. Chem. 1998;110:2092–2118.
- 140.a) Anthoni U, Christophersen C, Nielsen PH. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 13. New York: Pergamon; 1999. pp. 163–236. [Google Scholar]; b) Cordell GA, Saxton JE. In: The Alkaloids: Chemistry and Physiology. Manske RHF, Rodrigo RGA, editors. Vol. 20. New York: Academic Press; 1981. pp. 3–295. [Google Scholar]
- 141.Tokuyama T, Daly JW. Tetrahedron. 1983;39:41–47. [Google Scholar]
- 142.a) Grant IJ, Hamor TA, Robertson JM, Sim GA. Proc. Chem. Soc. 1962:148–149. [Google Scholar]; b) Grant IJ, Hamor TA, Robertson JM, Sim GA. J. Chem. Soc. 1965:5678–5696. [Google Scholar]
- 143.Steven A, Overman LE. Angew. Chem. Int. Ed. 2007;46:5488–5508. doi: 10.1002/anie.200700612. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007;119:5584–5605. [Google Scholar]
- 144.Hendrickson JB, Göschke R, Rees R. Tetrahedron. 1964;20:565–579. [Google Scholar]
- 145.Link JT, Overman LE. J. Am. Chem. Soc. 1996;118:8166–8167. [Google Scholar]
- 146.Overman LE, Paone DV, Stearns BA. J. Am. Chem. Soc. 1999;121:7702–7703. [Google Scholar]
- 147.Julian PL, Pikl J. J. Am. Chem. Soc. 1935;57:539–544. [Google Scholar]
- 148.a) Overman LE, Watson DA. J. Org. Chem. 2006;71:2587–2599. doi: 10.1021/jo052335a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Overman LE, Watson DA. J. Org. Chem. 2006;71:2600–2608. doi: 10.1021/jo0523363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Overman LE, Larrow JF, Stearns BA, Vance JM. Angew. Chem. Int. Ed. 2000;39:213–215. doi: 10.1002/(sici)1521-3773(20000103)39:1<213::aid-anie213>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000;112:219–221. [Google Scholar]
- 150.Movassaghi M, Schmidt MA. Angew. Chem. Int. Ed. 2007;46:3725–3728. doi: 10.1002/anie.200700705. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007;119:3799–3802. [Google Scholar]
- 151.Xie W, Jiang G, Liu H, Hu J, Pan X, Zhang H, Wan X, Lai Y, Ma D. Angew. Chem. Int. Ed. 2013;52:12924–12927. doi: 10.1002/anie.201306774. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125:13162–13165. [Google Scholar]
- 152.Crich D, Banerjee A. Acc. Chem. Res. 2007;40:151–161. doi: 10.1021/ar050175j. [DOI] [PubMed] [Google Scholar]
- 153.Taniguchi M, Gonsho A, Nakagawa M, Hino T. Chem. Pharm. Bull. 1983;31:1856–1865. [Google Scholar]
- 154.Mitsunuma H, Shibasaki M, Kanai M, Matsunaga S. Angew. Chem. Int. Ed. 2012;51:5217–5221. doi: 10.1002/anie.201201132. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012;124:5307–5311. [Google Scholar]
- 155.Kato Y, Furutachi M, Chen Z, Mitsunuma H, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2009;131:9168–9169. doi: 10.1021/ja903566u. [DOI] [PubMed] [Google Scholar]
- 156.Fischer H. Chem. Rev. 2001;101:3581–3610. doi: 10.1021/cr990124y. [DOI] [PubMed] [Google Scholar]
- 157.Clark DB, Hinley SFR, Weavers RT. Tetrahedron Lett. 1997;38:4297. [Google Scholar]
- 158.Shi QW, Sauriol F, Mamer O, Zamir LO. Chem. Comm. 2003:68–69. doi: 10.1039/b209312j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.