Abstract
Kangen-karyu, a prescription containing six herbs, has been shown to achieve its pharmacological effect through oxidative stress-dependent pathways in animal models. The aim of this study is to investigate the relationship between the antioxidative effect and pharmacological mechanisms of Kangen-karyu, specifically its body temperature elevating effect in humans. Healthy human volunteers, age 35 ± 15 years old, were enrolled in this study. Surface body temperature, serum nitrite, reactive oxygen species (ROS) scavenging activities, and inflammatory cytokines were investigated before and 120 min after Kangen-karyu oral intake. Kangen-karyu significantly increased the surface-body temperature of the entire body; this effect was more remarkable in the upper body and continued for more than 120 min. Accompanying this therapeutic effect, serum nitrite levels were increased 120 min after oral administration. Serum ROS scavenging activities were enhanced against singlet oxygen and were concomitantly decreased against the alkoxyl radical. Serum nitrite levels and superoxide scavenging activities were positively correlated, suggesting that Kangen-karyu affects the O2•−-NO balance in vivo. Kangen-karyu had no effect on IL-6, TNF-α and adiponectin levels. These results indicate that the therapeutic effect of Kangen-karyu is achieved through NO- and ROS-dependent mechanisms. Further, this mechanism is not limited to ROS production, but includes ROS-ROS or ROS-NO interactions.
Keywords: antioxidant capacity, hydroxyl radical, alkoxyl radical, nitric oxide, traditional herbal prescription
Introduction
Traditional herbal prescriptions have attracted much attention due to their beneficial effects, observed in the course of long-term clinical experience. Kangen-karyu, a prescription consisting of six herbs (Paeoniae Radix, Cnidii Rhizoma, Carthami Flos, Cyperi Rhizoma, Saussureae Radix and Salviae miltiorrhizae Radix) is known to have antihypertensive effects and has been clinically used for cardiovascular diseases such as angina pectoris and cerebrovascular disorders.(1) Recent studies demonstrated the therapeutic effects of Kangen-karyu including antihypertension, anti-platelet aggregation, neuroprotection and anti-dementia in animal models.(2–4) While the underlying biological reactions of these therapeutic effects are varied, an oxidative stress-controlling effect is currently suggested as a key mechanism in animal studies.(4–6) Although these reports revealed important results, little is known about the pharmacological mechanisms of Kangen-karyu in human subjects.
Currently, oxidative stress is recognized as an important pathological factor in many diseases. Ongoing investigations are revealing cellular reactions after oxidative stimuli, such as the NF-κB and Nrf2-Keap1 pathways.(7–9) However, upper stream reactions of oxidative stress that evoke the following responses, including the identification of reactive oxygen species (ROS) that act as the responsible stimulator of oxidative stress reactions, or interactions among ROS that generate oxidative stimulators, remain unclear.(10,11) Because ROS are not uniform and individual ROS have specific characteristics during in vivo reactions,(12) analysis of multiple ROS is necessary. Using electron spin resonance (ESR) technology, we have reported changes in the hydroxyl radical and superoxide scavenging activity in patients with various diseases.(13,14) Recently, we developed this ESR-based method for the analysis of multiple ROS scavenging activities in biological samples, allowing us to describe details of oxidative stress-related reactions.(15)
This study aimed to reveal the relationship between the pharmacological effect of Kangen-karyu and oxidative stress in human subjects. Changes in the body surface temperature induced by vasodilation were the targeted pharmacological effect. Oxidative stress was evaluated by measuring multiple free radical scavenging activities in serum and analyzing the evoked radical chain reactions. The results show that Kangen-karyu elevated the body surface temperature through alternations of singlet oxygen, alkoxyl radical, ROS interactions among specific ROS, and the ROS-nitric oxide (NO) balance. Our study provides the first evidence of the oxidative stress-controlling effect of Kangen-karyu in human subjects.
Materials and Methods
Subjects and informed consent
Eight healthy volunteers, four males and four females, mean age 35 ± 15 years old, were employed in the study. All human subject procedures were conducted with individual written consent and followed a protocol that had been approved by Tsukuba University of Technology Committee.
Study design
The study was conducted with a single oral administration of Kangen-karyu (5 g). Blood and urinary samples were obtained before and 120 min after oral administration of Kangen-karyu. Serum samples were separated by centrifugation and stored at –80°C until used. All subjects fasted at least 6 h before the oral administration of Kangen-karyu.
Thermography
Body surface temperature was measured using an infrared thermograph (JTG4310S; JEOL, Tokyo, Japan) placed in a temperature-controlled chamber. The room temperature and humidity of the chamber were set at 25 ± 1°C and 50%, respectively. The subjects entered into the chamber 30 min prior to the Kangen-karyu administration to void any outside effect. The thermographic measurements were performed before and 30, 60 and 120 min after Kangen-karyu administration. Body surface temperatures were measured on the face, frontal neck, abdomen, frontal lower limb, back of the neck, back, hand and dorsum of the hand. Female subjects were measured wearing undershirts.
Measurements of multiple free radical scavenging activity
Multiple free radical scavenging activities (MULTIS method) were measured by an ESR based method developed by one of our co-authors.(15) The ROS employed in this system were five free radicals including superoxide (O2•−), hydroxyl radical (•OH), alkoxyl radical (RO•, t-BuO•), peroxyl radical (ROO•, t-BuOO•), alkyl radical (R•, CH3•), and singlet oxygen (1O2). Each ROS was produced via in situ illumination with UV/visible light from an illuminator (RUVF-203SR UV illuminator; Radical Research Inc., Tokyo, Japan). 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) was used as an ESR-spin trapping reagent. Light sources, illumination times, precursors and photo-sensitizers used to produce ROS are summarized in Table 1. The UV light source was a 200 W medium-pressure mercury/xenon arc lamp, where UV-visible light was guided through a quartz light-guide into the resonator cavity. The ESR spectrometer employed was a JEOL FR-80 equipped with 100 kHz field modulation and WIN-RAD operation software (Radical Research Inc.). Typical spectrometer settings were: field modulation width 0.1 mT; microwave power 10 mW; field scan width and rate ±7.5 mT/2 min; time constant 0.1 s. ROS scavenging activities were calculated according to the previously described method,(15) and expressed as the unit equivalent to known pure scavengers; GSH for •OH and 1ΔO2, superoxide dismutase (SOD) for O2•−, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (TROLOX) for RO•, and α-lipoic acid (αLA) for ROO•. Because the alkyl radical has no specific scavenger, scavenging activity against R• was expressed as CYPMPO equivalents.
Table 1.
Photolytic production methods of multiple reactive oxygen species
| Free radical | Spin trap | Precursor/Sensitizer | UV/VL | Irradiation period | Antioxiidant equivalent |
|---|---|---|---|---|---|
| •OH | CYPMPO | H2O2 10 mM | UV | 5 s | GSH |
| O2•− | CYPMPO | Riboflavin 20 µM | VL | 60 s | SOD |
| RO• | CYPMPO | AAPH 10 mM | UV | 5 s | Trolox |
| ROO• | CYPMPO | tBHP 10 mM | UV | 5 s | α-lipoic acid |
| R• | CYPMPO | H2O2 100 mM | UV | 30 s | CYPMPO |
| DMSO 10 mM | |||||
| 1O2 | TMPO | Rosebengal 200 µM | VL | 30 s | GSH |
UV, ultraviolet (300–400 nm); VL, visual light (500–600 nm); AAPH, 2,2'-azobis-2-methyl-propanimidamide, dihydrochloride, tBHP; tert-butyl hydroperoxide; GSH, glutathione; SOD, superoxide dismutase; CYPMPO, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide; TMPO, 4-hydroxy-2,2,6,6-tetramethylpiperidine.
The MULTIS method was applied to the measurement of the in vitro profiles of prescriptions and the changes of antioxidative activities after the administration of Kangen-karyu. The in vitro profiles were obtained for Kangen-karyu and Tsudosan, a Kampo formula also reported to possess high antioxidative activity.(16) In vitro scavenging activities of the prescriptions were measured using 10% aqueous solutions and expressed in terms of their daily doses. Serum scavenging activities were determined in 10% buffer solution of serum or pure water and the total volume of the sample solution was approximately 0.2 ml.
Measurements of NO metabolites and inflammatory cytokines
Serum NO metabolites were measured as NO2 by colorimetric measurement based on the Griess reaction described elsewhere.(17) IL-6 and adiponectin were measured using a chemiluminescent enzyme immunoassay, TNF-α was measured using an enzyme-linked immunoassay, both at SRL laboratory (Tokyo, Japan).
Materials
CYPMPO was obtained from Radical Research Inc.; hydrogen peroxide, riboflavin, 2,2'-azobis (2-amidinopropane) hydrochrolide (AAPH), tert-butyl hydroperoxide, dimethyl sulfoxide (DMSO), rosebengal, and 4-hydroxy-2,2,6,6-tetramethylpiperidine (4-OH-TEMP) were purchased from Tokyo Chemical Industry (Tokyo, Japan) and used without modification. Buffers and biochemical reagents were obtained from Wako Chemical Co. (Osaka, Japan).
Statistical analysis
Statistical analysis was performed using computer software (Prism 6; GraphPad Software Inc., La Jolla). Comparison of data from two groups was analyzed with a Student’s paired t test. Multiple data comparisons were performed using the one-way factorial analysis of valiance (ANOVA). Repeated measures of ANOVA with the Dunnett’s test were used for the time-course measurements of body surface temperature. Multiple regression analysis results are expressed as the mean ± SEM.
Results
Kangen-karyu raises body surface temperature
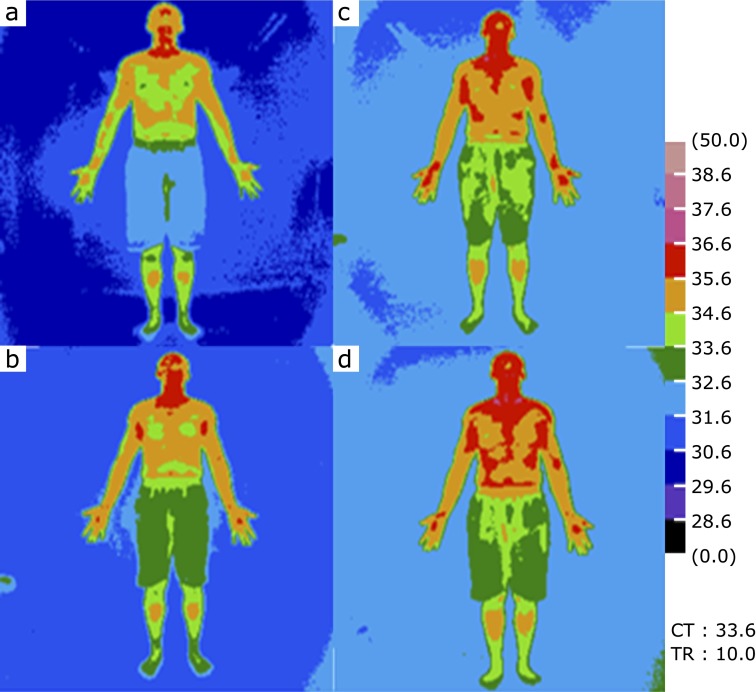
Before Kangen-karyu administration, the body surface temperature of subjects was 35.59 ± 0.06°C in the facial area. Thirty, 60 and 120 min after Kangen-karyu administration, the corresponding temperature rose to 35.90 ± 0.09, 36.01 ± 0.12 and 36.08 ± 0.14°C, respectively (Table 2). These significant elevations in surface body temperature were also observed in the areas of the shoulder, forehead, abdomen, occiput, posterior shoulder and back, and continued until 90 to 120 min after Kangen-karyu administration. A typical sequential change in body temperature is shown in Fig. 1. This effect on temperature was predominantly observed in the upper-half of the subjects, and was not significant in the area of hands, palms and lower legs. No remarkable changes in blood pressure were observed during the study period (data not shown).
Table 2.
Effect of Kangen-karyu administration on changes in body surface temperatures
| Before | 30 min | 60 min | 120 min | |
|---|---|---|---|---|
| Face | 35.59 ± 0.06 | 35.9 ± 0.09* | 36.01 ± 0.12* | 36.08 ± 0.15* |
| Shoulder | 35.2 ± 0.12 | 35.58 ± 0.1* | 35.89 ± 0.1* | 36.01 ± 0.09* |
| Forehead | 35.58 ± 0.13 | 35.85 ± 0.13* | 36.13 ± 0.08* | 36.29 ± 0.08* |
| Abdomen | 34.14 ± 0.26 | 34.55 ± 0.21* | 34.96 ± 0.22* | 35.3 ± 0.21* |
| Occiput | 34.76 ± 0.1 | 35.15 ± 0.1* | 35.54 ± 0.13* | 35.73 ± 0.15* |
| Posterior shoulder | 34.53 ± 0.17 | 34.84 ± 0.21 | 35.06 ± 0.24* | 35.31 ± 0.18* |
| Back | 34.81 ± 0.17 | 35.34 ± 0.27* | 35.55 ± 0.29* | 35.74 ± 0.24* |
| Hand | 34.73 ± 0.11 | 35.19 ± 0.14* | 35.25 ± 0.19 | 34.85 ± 0.2 |
| Palm | 35.3 ± 0.08 | 35.44 ± 0.15 | 35.5 ± 0.18 | 34.81 ± 0.24 |
| Lower thigh | 33.99 ± 0.1 | 34.14 ± 0.06 | 34.38 ± 0.11* | 34.45 ± 0.07* |
Body surface temperatures were measured before and 30, 60, and 120 min after Kangen-karyu administration. Data represent means ± SEM. *p<0.05 vs before administration.
Fig. 1.
Image of a representative whole body thermograph. (a) Before Kangen-karyu administration. (b), (c) and (d) 30, 60 and 120 after Kangen-karyu administration, respectively.
In vitro ROS scavenging activities of Kangen-karyu
The ESR spectra of the spin adducts for •OH, O2•−, RO•, ROO•, R• and 1O2 agreed with the previously reported spectra of corresponding radical adducts, confirmed by hyperfine coupling constants (Supplemental Fig. 1*).(15) In the presence of the Kangen-karyu solution, the signal amplitude decreased from that from which the scavenging activity was calculated.
The in vitro profiles of scavenging activity converted into equivalent units of known pure scavengers are summarized in Table 3. The obtained scavenging activities were compared to those of Tsudosan, which was reported to possess a high oxygen radical absorbance capacity (ORAC) value.(16) The reported ORAC value of Tsudosan is 5913.27 ORAC unit (formula/day), and the RO• scavenging activity in our method, which may relate to ORAC in principle, was 8665.62 µM-TROLOX equivalent (formula/day). The scavenging activities of Kangen-karyu were higher against O2•− and lower against ROO• and R• than those of Tsudosan. The scavenging activities against •OH, RO• and 1O2 were of the same level in the two formulae.
Table 3.
In vitro scavenging activities of Kangen-karyu and Tsudosan as measured for multiple ROS converted into the equivalent units to specific scavengers
| Free radical |
||||||
|---|---|---|---|---|---|---|
| •OH | O2•− | RO• | ROO• | R• | 1ΔO2 | |
| mM-GSHeq | U/ml-SODeq | mM-TROLOXeq | mM-aLAeq | mM-CYPMPOeq | mM-GSHeq | |
| Kangen-karyu | 275.1 ± 8.3 | 2789.9 ± 88.5 | 9.39 ± 1.13 | 42.4 ± 5.6 | 212.7 ± 27.1 | 392.0 ± 26.8 |
| Tsudosan | 308.5 ± 10.9 | 833.5 ± 52.8 | 8.67 ± 0.76 | 53.4 ± 5.2 | 308.6 ± 37.9 | 350.5 ± 22.4 |
Both values of Kangen-karyu and Tsudosan are expressed as those of daily doses.
Effect of Kangen-karyu oral administration on serum ROS scavenging activities
Decreases in signal amplitudes in the presence of serum and scavenging activities were calculated. Table 4 and Fig. 2 show the serum radical scavenging activities before and 120 min after Kangen-karyu administration. Before Kangen-karyu administration, the serum ROS scavenging activities were 10.0 ± 2.63 mM-GSHeq for •OH, 12.6 ± 1.2 U/ml-SODeq for O2•−, 1.7 ± 0.3 mM-TROLOXeq for RO•, 1.05 ± 0.05 mM-αLAeq for ROO•, 0.24 ± 0.02 mM-CYPMPOeq for R• and 22.2 ± 2.5 µM-GSHeq for 1O2.
Table 4.
In vivo scavenging activity of serum before and 120 min after Kangen-karyu administration, as measured for multiple ROS converted into the equivalent units to specific scavengers
| Free radical |
||||||
|---|---|---|---|---|---|---|
| •OH | O2•− | RO• | ROO• | R• | 1ΔO2 | |
| mM-GSHeq | U/ml-SODeq | mM-TROLOXeq | mM-aLAeq | mM-CYPMPOeq | mM-GSHeq | |
| Before | 10.0 ± 2.63 | 12.6 ± 1.2 | 1.7 ± 0.3 | 1.05 ± 0.05 | 0.24 ± 0.02 | 22.2 ± 2.5 |
| After | 8.32 ± 2.22 | 11.9 ± 2.5 | 1.0 ± 0.2* | 1.06 ± 0.07 | 0.21 ± 0.03 | 30.1 ± 4.0* |
Data represent means ± SEM. *p<0.05 vs before administration.
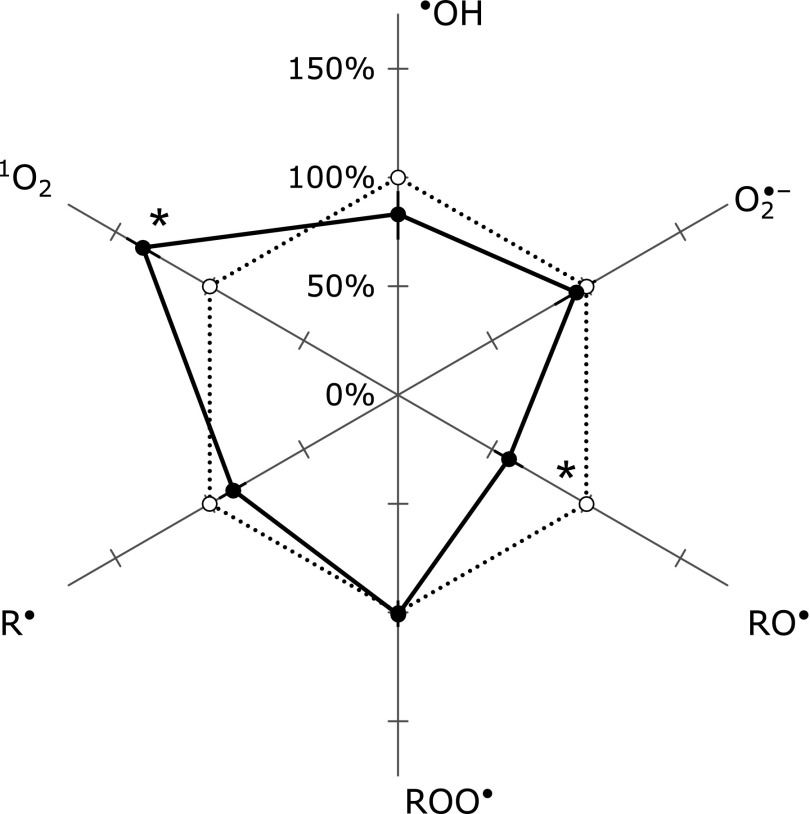
Fig. 2.
A radar chart of serum scavenging activity before and after Kangen-karyu administration. Data are listed in Table 3. Percent changes in the scavenging activity after Kangen-karyu administration (dashed lines) are shown with respect to that of before the administration (solid line). Error bars shown for each free radical species are SEM. *p<0.05 vs before administration.
The serum ROS scavenging activities before administration generally agreed with the previously reported values of healthy adults.(15) After Kangen-karyu administration, the serum ROS scavenging activities were 8.32 ± 2.22 mM-GSHeq for •OH, 11.9 ± 2.5 U/ml-SODeq for O2•−, 1.0 ± 0.2 mM-TROLOXeq for RO•, 1.06 ± 0.07 mM-αLAeq for ROO•, 0.21 ± 0.03 mM-CYPMPOeq for R• and 30.1 ± 4.0 µM-GSHeq for 1O2. Serum scavenging activity against 1O2 was significantly increased and that against RO• was significantly decreased. Scavenging activities against O2•−, in which the in vitro activity of Kangen-karyu was remarkable, showed no significant increase. The scavenging activities against •OH, ROO• and R• also showed no remarkable changes.
Kangen-karyu effects on NO metabolites and ROS-NO balance
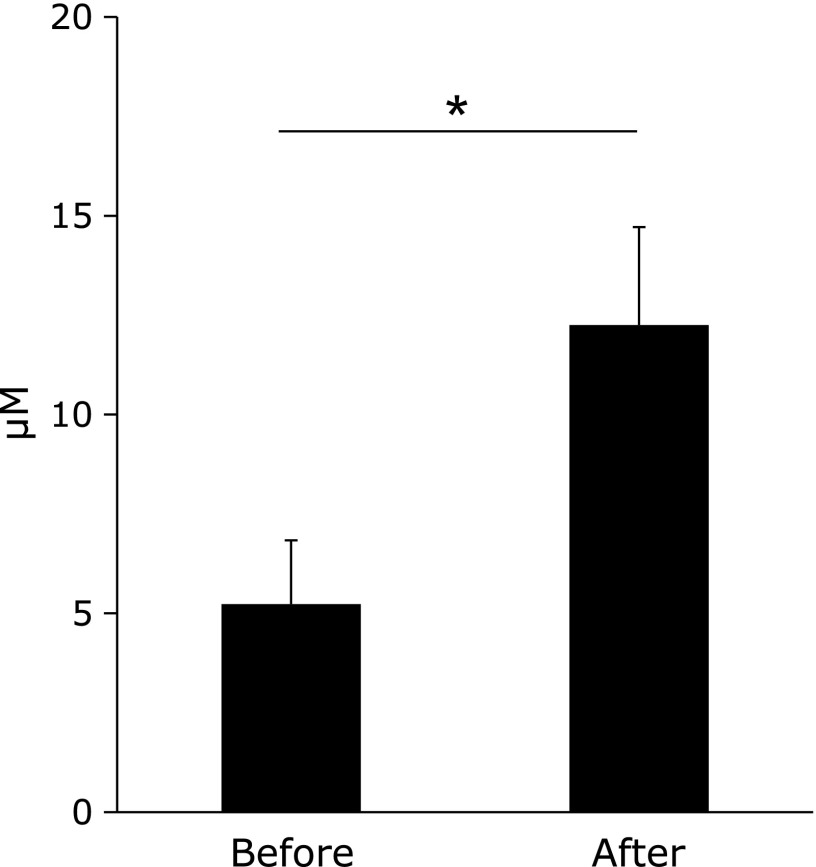
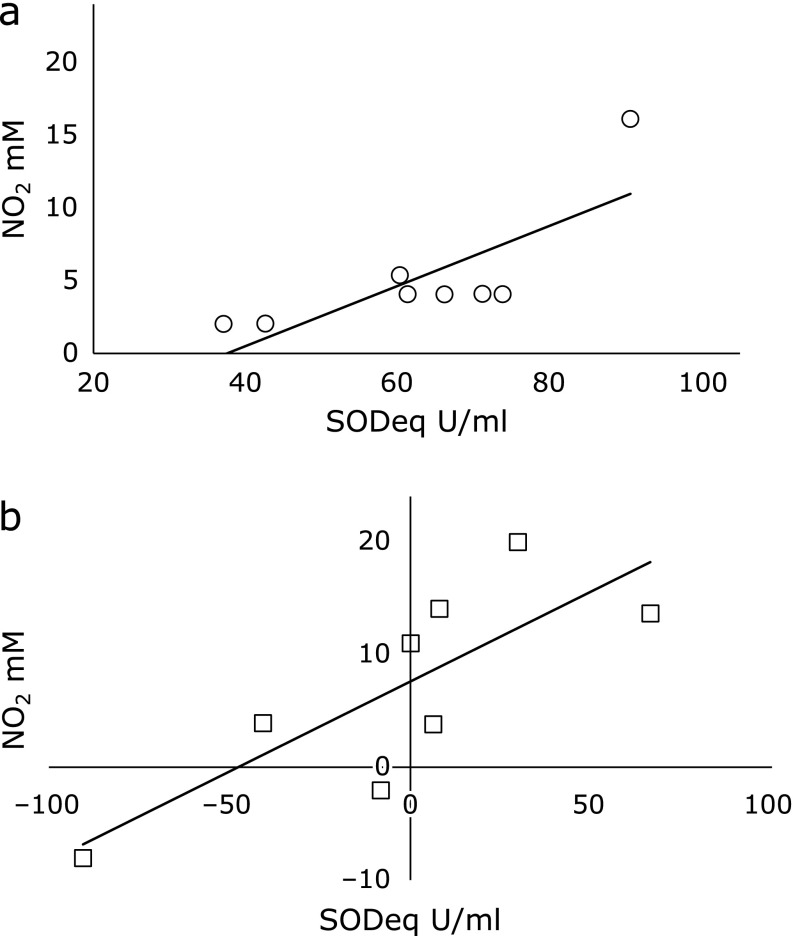
The serum NO2 concentration was 5.22 ± 1.60 µM before the test. Two hours after Kangen-karyu administration, serum NO2 value was significantly increased to 12.2 ± 2.46 µM (Fig. 3). The effects of Kangen-karyu on ROS-ROS or ROS-NO interactions were evaluated by measuring ROS scavenging activities. Before Kangen-karyu administration, the serum O2•− scavenging activity significantly correlated with serum NO2 concentrations, suggesting the existence of an O2•−-NO balance that regulates the redox status in serum (Fig. 4a). After Kangen-karyu administration, the O2•−-NO balance became indefinite, while a significant correlation between O2•− scavenging activity and NO2 concentration appeared (Fig. 4b).
Fig. 3.
Serum NOx concentration before and 120 min after Kangen-karyu administration. Data represent means ± SEM. *p<0.05.
Fig. 4.
Correlation between serum superoxide scavenging activity and NO2 concentration (a) before Kangen-karyu administration, (b) variation between before and after Kangen-karyu administration. Significant correlations were observed in (a) (R = 0.78, p<0.05) and (b) (R = 0.80, p<0.05).
Kangen-karyu does not alter inflammatory cytokines
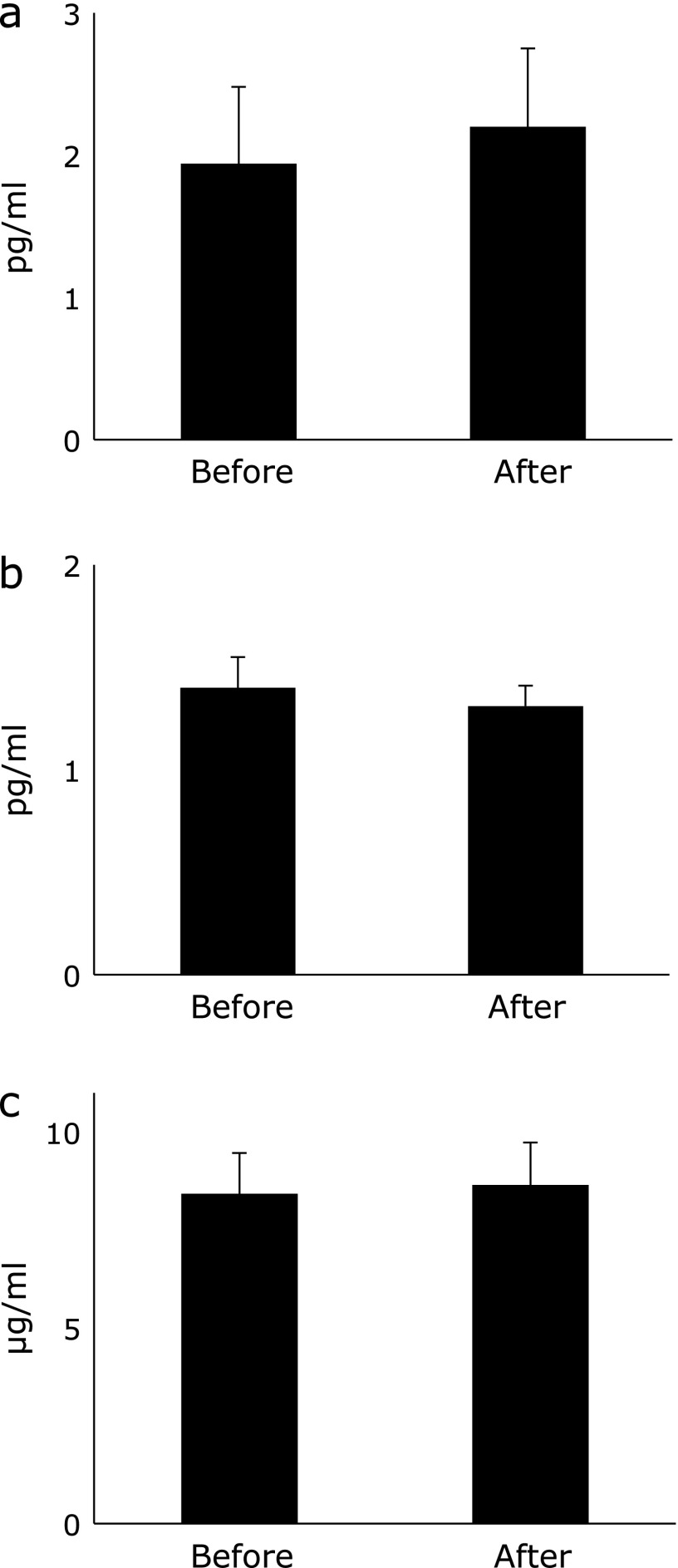
Effects of Kangen-karyu on non-radical pathways were evaluated by measuring adiponectin, IL-6 and TNF-α. Before Kangen-karyu administration, adiponectin, IL-6 and TNF-α levels were 8.42 ± 1.04 µg/ml, 1.95 ± 0.54 pg/ml and 1.40 ± 0.15 pg/ml, respectively (Fig. 5). After administration, the values were 8.65 ± 1.08 µg/ml, 2.20 ± 0.55 pg/ml and 1.31 ± 0.10 pg/ml, respectively, not significantly different. No remarkable correlations were observed between inflammatory cytokine levels and ROS scavenging activities.
Fig. 5.
Serum concentrations of (a) interleukin-6 and (b) tumor necrosis factor-α and (c) adiponectin before and 120 min after Kangen-karyu administration. Data represent means ± SEM. No significant differences were observed.
Discussion
Oxidative stress is one of the most critical and universal pathological factors impacting various diseases. A series of studies revealed the roles of oxidative stress during Kangen-karyu treatment in fructose-induced metabolic syndrome, diet-induced hypercholesterolemic rats, hyperlipidemia in streptozotocin-induced type 1 diabetic rats and type 2 diabetic db/db mice.(1,4,18,19) Though these reports revealed important results, little is known about the pharmacological mechanisms of Kangen-karyu in humans. Here, we reveal details of Kangen-karyu induced oxidative stress in human subjects.
First, we evaluated the antioxidative profile of Kangen-karyu in vitro. Many Kampo medicines are known to possess beneficial effects against oxidative stress. Prescriptions containing Rhei rhizoma, such as Tsudosan or Dajoukito, exhibit high ORAC values.(16) ORAC values are measured using TROLOX as the antioxidant, and mainly relate to reactions toward RO•.(20) Differences were noted between the reported ORAC value and the RO• scavenging activity of Tsudosan, due in part to methodological differences. A previous study applying MULTIS for food chemistry found correlations between ORAC and MULTIS for O2•− and RO•, but not •OH.(21) Since the United States Department of Agriculture withdrew ORAC data from their website, alternative methods that are more reliable for oxidative stress measurements are required.(22) Consequently we employed an ESR-based MULTIS method for this study. Although ESR has methodological demerits, such as dielectric loss, ESR is able to specify the target ROS and has high sensitivity for the detection of oxidative stress.(13,14,23,24)
Currently, only some reports specify the target radical(s) in the measurement of antioxidative activity. A series of reports from Kohno and colleagues made an extensive screening of O2•− scavenging activity of herbal extracts.(25,26) They found that extracts such as Rheum palmatum, Ephedra sinica, Punica granatum and Caesalpinia sappan have high O2•− scavenging activity. However, the O2•− scavenging activity of the main contents of Kangen-karyu, including Paeoniae Radix, Cnidii Rhizoma, Carthami Flos, Cyperi Rhizoma, Saussureae Radix and Salviae miltiorrhizae Radix, are relatively low.(26) In contrast, the superoxide scavenging activity of Kangen-karyu measured using our method was remarkable, and was higher than that of Tsudosan, which contains Rheum palmatum.
The changes of in vivo ROS scavenging activities produced by Kangen-karyu were completely different from its in vitro antioxidative profile. In contrast, with its high O2•− scavenging activity in vitro, the remarkable in vivo changes showed an increase against 1O2 and a decrease against RO•. Compared to O2•− or •OH, the detailed roles of RO•, ROO•, R•, 1O2 and other oxygen radicals in human diseases remains unknown.(10) A previous report from one of our co-authors revealed remarkable shifts in MULTIS-measured ROS scavenging activities in patients with chronic kidney disease.(15) However, the in vivo role of RO• was not clear in the study. On the other hand, 1O2 is reportedly produced through myeloperoxidase, prostaglandin hydroperoxidase and the interaction between O2•− and H2O2 during the Haber–Weiss reaction.(27,28) Among ROS, 1O2 has relatively high toxicity in vivo, provokes lipid peroxidation, inducing further RO• and ROO•, and diminishes radical chain reactions.(29) In ischemia-reperfusion injury, 1O2 is thought to play a crucial role and may relate to the protective effects of Kangen-karyu.(30)
On the other hand, Kangen-karyu also affected the internal NO and NO-ROS balance. Kangen-karyu remarkably increased the surface body temperature in the face and trunk and was accompanied by an increase in serum NO2, suggesting that Kangen-karyu causes direct and/or indirect stimulation of NO production. A previous animal study detected an inhibition of inducible nitric oxide synthase (iNOS) after Kangen-karyu treatment in a diabetic model, a result that is not in agreement with our present study.(31) The cause of this disagreement is not clear, although our finding of increasing 1O2 scavenging activity may be related. A previous report revealed that quenching of 1O2 increased NO synthase (NOS) activity in synaptosomes isolated from rabbit brain.(32) Also, 1O2 deactivating activity was reduced in serum from patients with diabetes mellitus, in which a major complication was endothelial injury.(33) Considering these results, the increased scavenging activity of 1O2 by Kangen-karyu may increase NO production. Moreover, we also observed the relation between alterations in serum O2•− scavenging activity and NO2 concentration, suggesting Kangen-karyu modifies the O2•−-NO balance. The pathophysiological role of in vivo O2•−-NO balance is investigated mainly in hypoxia, though the detailed mechanism remains unclear.(34–36) Further studies analyzing the roles of specific ROS are required.
In conclusion, our results show that Kangen-karyu raises surface-body temperature and is accompanied by a significant increase in serum 1O2 scavenging activity and serum NO and amelioration of O2•−-NO balance. Therefore, Kangen-karyu may alter the in vivo oxidative balance.
Acknowledgments
This work is financially supported by grants from Tsukuba University of Technology. Kangen-karyu was provided by Iskra Co., Ltd. We thank Dr. Burton D. Cohen, Professor Emeritus, Albert Einstein Medical College for his support.
Supplementary Materials
References
- 1.Yokozawa T, Kim HJ, Yamabe N, Okamoto T, Cho EJ. The protective role of Kangen-karyu against fructose-induced metabolic syndrome in a rat model. J Pharm Pharmacol. 2007;59:1271–1278. doi: 10.1211/jpp.59.9.0012. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Q, Yokozawa T, Yamabe N, Tsuneyama K, Li X, Matsumoto K. Kangen-karyu improves memory deficit caused by aging through normalization of neuro-plasticity-related signaling system and VEGF system in the brain. J Ethnopharmacol. 2010;131:377–385. doi: 10.1016/j.jep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Yamabe N, Kim HY, Kang KS, Zhao Q, Matsumoto K, Yokozawa T. Effect of Chinese prescription Kangen-karyu on lipid metabolism in type 2 diabetic db/db mice. J Ethnopharmacol. 2010;129:299–305. doi: 10.1016/j.jep.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Kim HY, Okamoto T, Yokozawa T. Beneficial effects of Chinese prescription Kangen-karyu on diabetes associated with hyperlipidemia, advanced glycation endproducts, and oxidative stress in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2009;124:263–269. doi: 10.1016/j.jep.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Noh JS, Park CH, Kim HY, et al. Chinese prescription Kangen-karyu prevents dyslipidaemia and oxidative stress in mouse model of type 2 diabetes. J Pharm Pharmacol. 2011;63:111–119. doi: 10.1111/j.2042-7158.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- 6.Thent ZC, Das S. Involvement of liver in diabetes mellitus: herbal remedies. Clin Ter. 2014;165:223–230. doi: 10.7417/CT.2014.1738. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama A, Yoh K, Nagase S, et al. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic Biol Med. 2003;34:1236–1242. doi: 10.1016/s0891-5849(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 8.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 9.Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. 2013;75:637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirayama A, Nagase S, Gotoh M, et al. Reduced serum hydroxyl radical scavenging activity in erythropoietin therapy resistant renal anemia. Free Radic Res. 2002;36:1155–1161. doi: 10.1080/1071576021000016418. [DOI] [PubMed] [Google Scholar]
- 14.Nagase S, Aoyagi K, Hirayama A, et al. Favorable effect of hemodialysis on decreased serum antioxidant activity in hemodialysis patients demonstrated by electron spin resonance. J Am Soc Nephrol. 1997;8:1157–1163. doi: 10.1681/ASN.V871157. [DOI] [PubMed] [Google Scholar]
- 15.Oowada S, Endo N, Kameya H, Shimmei M, Kotake Y. Multiple free-radical scavenging capacity in serum. J Clin Biochem Nutr. 2012;51:117–121. doi: 10.3164/jcbn.11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura K, Osawa T, Watanabe K. Evaluation of oxygen radical absorbance capacity in kampo medicine. Evid Based Complement Alternat Med. 2011;2011:812163. doi: 10.1093/ecam/nen082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagase S, Hirayama A, Ueda A, et al. Light-shielded hemodialysis prevents hypotension and lipid peroxidation by inhibiting nitric oxide production. Clin Chem. 2005;51:2397–2398. doi: 10.1373/clinchem.2005.058669. [DOI] [PubMed] [Google Scholar]
- 18.Yokozawa T, Cho EJ, Sasaki S, Satoh A, Okamoto T, Sei Y. The protective role of Chinese prescription Kangen-karyu extract on diet-induced hypercholesterolemia in rats. Biol Pharm Bull. 2006;29:760–765. doi: 10.1248/bpb.29.760. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Noh JS, Okamoto T, Park JC, Yokozawa T. Evaluation of effects of Chinese prescription Kangen-karyu on diabetes-induced alterations such as oxidative stress and apoptosis in the liver of type 2 diabetic db/db mice. Evid Based Complement Alternat Med. 2012;2012:143489. doi: 10.1155/2012/143489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima A, Matsuda E, Masuda Y, Sameshima H, Ikenoue T. Characteristics of the spin-trapping reaction of a free radical derived from AAPH: further development of the ORAC-ESR assay. Anal Bioanal Chem. 2012;403:1961–1970. doi: 10.1007/s00216-012-6021-8. [DOI] [PubMed] [Google Scholar]
- 21.Kameya H, Watanabe J, Takano-Ishikawa Y, Todoriki S. Comparison of scavenging capacities of vegetables by ORAC and EPR. Food Chem. 2014;145:866–873. doi: 10.1016/j.foodchem.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010). http://www.ars.usda.gov/services/docs.htm?docid=15866 [Google Scholar]
- 23.Hirayama A, Nagase S. Electron paramagnetic resonance imaging of oxidative stress in renal disease. Nephron Clin Pract. 2006;103:c71–c76. doi: 10.1159/000090612. [DOI] [PubMed] [Google Scholar]
- 24.Owada S, Maeba T, Sugano Y, et al. Spherical carbon adsorbent (AST-120) protects deterioration of renal function in chronic kidney disease rats through inhibition of reactive oxygen species production from mitochondria and reduction of serum lipid peroxidation. Nephron Exp Nephrol. 2010;115:e101–e111. doi: 10.1159/000313491. [DOI] [PubMed] [Google Scholar]
- 25.Saito K, Kohno M, Yoshizaki F, Niwano Y. Extensive screening for edible herbal extracts with potent scavenging activity against superoxide anions. Plant Foods Hum Nutr. 2008;63:65–70. doi: 10.1007/s11130-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 26.Niwano Y, Saito K, Yoshizaki F, Kohno M, Ozawa T. Extensive screening for herbal extracts with potent antioxidant properties. J Clin Biochem Nutr. 2011;48:78–84. doi: 10.3164/jcbn.11-013FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toufektsian MC, Boucher FR, Tanguy S, Morel S, de Leiris JG. Cardiac toxicity of singlet oxygen: implication in reperfusion injury. Antioxid Redox Signal. 2001;3:63–69. doi: 10.1089/152308601750100506. [DOI] [PubMed] [Google Scholar]
- 28.Noronha-Dutra AA, Epperlein MM, Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett. 1993;321:59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- 29.Kalyanaraman B, Feix JB, Sieber F, Thomas JP, Girotti AW. Photodynamic action of merocyanine 540 on artificial and natural cell membranes: involvement of singlet molecular oxygen. Proc Natl Acad Sci U S A. 1987;84:2999–3003. doi: 10.1073/pnas.84.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM. Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol. 1999;31:113–121. doi: 10.1006/jmcc.1998.0850. [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Yamabe N, Okamoto T, Toriizuka K, Yokozawa T. Chinese prescription kangen-karyu ameliorates the development of diabetic hepatic damages via regulating oxidative stress and inflammation in the liver of db/db mice. Biol Pharm Bull. 2011;34:383–388. doi: 10.1248/bpb.34.383. [DOI] [PubMed] [Google Scholar]
- 32.Fotiou S, Fotiou D, Alamanou A, Deliconstantinos G. Resveratrol activation of nitric oxide synthase in rabbit brain synaptosomes: singlet oxygen (1O2) formation as a causative factor of neurotoxicity. In vivo. 2010;24:49–53. [PubMed] [Google Scholar]
- 33.Lhommeau I, Douillard S, Bigot E, Benoit I, Krempf M, Patrice T. Serum resistance to singlet oxygen in patients with diabetes mellitus in comparison to healthy donors. Metabolism. 2011;60:1340–1348. doi: 10.1016/j.metabol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Steiner DR, Gonzalez NC, Wood JG. Interaction between reactive oxygen species and nitric oxide in the microvascular response to systemic hypoxia. J Appl Physiol. 2002;93:1411–1418. doi: 10.1152/japplphysiol.00251.2002. [DOI] [PubMed] [Google Scholar]
- 35.Araujo AS, Diniz GP, Seibel FE, et al. Reactive oxygen and nitrogen species balance in the determination of thyroid hormones-induced cardiac hypertrophy mediated by renin-angiotensin system. Mol Cell Endocrinol. 2011;333:78–84. doi: 10.1016/j.mce.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Singh M, Thomas P, Shukla D, Tulsawani R, Saxena S, Bansal A. Effect of subchronic hypobaric hypoxia on oxidative stress in rat heart. Appl Biochem Biotechnol. 2013;169:2405–2419. doi: 10.1007/s12010-013-0141-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.