Abstract
myo-Inositol, the precursor of all inositol compounds, has pivotal roles in cell metabolism and signaling pathways. Although physiological studies indicate a strong correlation between abnormal intracellular inositol levels and neurological disorders, very little is known about the regulation of inositol synthesis in mammalian cells. In this study, we report that IP6K1, an inositol hexakisphosphate kinase that catalyzes the synthesis of inositol pyrophosphate, regulates inositol synthesis in mammalian cells. Ip6k1 ablation led to profound changes in DNA methylation and expression of Isyna1 (designated mIno1), which encodes the rate-limiting enzyme inositol-3-phosphate synthase. Interestingly, IP6K1 preferentially bound to the phospholipid phosphatidic acid, and this binding was required for IP6K1 nuclear localization and the regulation of mIno1 transcription. This is the first demonstration of IP6K1 as a novel negative regulator of inositol synthesis in mammalian cells.
Keywords: genetics, inositol phosphate, lipid, metabolism, phosphatidic acid, ISYNA1, Opi1, inositol, inositol pyrophosphate
Introduction
Inositol is a ubiquitous six-carbon cyclitol that is essential for viability of eukaryotic cells. myo-Inositol, physiologically the most important stereoisomer of inositol, is the precursor of all inositol compounds, including phosphoinositides, inositol phosphates, inositol sphingolipids, and glycosylphosphatidylinositols. Inositol compounds are essential for gene expression (1), trafficking (2), signal transduction (3), and membrane biogenesis (4). The crucial role of inositol is underscored by the link between perturbation of inositol metabolism and human disorders (5). Therefore, elucidating the mechanisms underlying the control of inositol homeostasis is expected to have important implications for understanding cell function and the pathologies underlying many illnesses.
Although cellular inositol can be obtained from the extracellular environment or by recycling inositol lipids (6, 7), the de novo synthesis of inositol is essential for inositol homeostasis and cellular proliferation when environmental inositol is limiting (8, 9). Inositol synthesis is a two-step reaction in which glucose 6-phosphate (Glc-6-P),4 the branch point metabolite for glycolysis, the pentose phosphate pathway, and inositol synthesis, is converted to inositol 3-phosphate, which is dephosphorylated to inositol. The first and rate-limiting step of inositol synthesis is catalyzed by inositol-3-phosphate synthase (10–14). Significantly high levels of inositol are found in brain tissue, which has limited access to exogenous inositol (15), suggesting that de novo synthesis of inositol is critical for normal brain function. Importantly, alterations in inositol metabolism have been associated with bipolar disorder and Alzheimer disease (5, 16, 17).
Despite its importance, little is known about the regulation of inositol synthesis in mammals. In contrast, the regulation of inositol synthesis has been well characterized in the budding yeast Saccharomyces cerevisiae. Inositol synthesis in yeast is regulated at the level of transcription of INO1 (18, 19), the gene that codes for inositol-3-phosphate synthase, and by phosphorylation of the Ino1 protein (20). INO1 expression is controlled by the transcriptional repressor Opi1 in response to inositol and phosphatidic acid (PA) levels (21). Opi1 is stabilized by physically interacting with PA on the endoplasmic reticulum membrane. When inositol is limiting, PA levels are increased; Opi1 remains in the endoplasmic reticulum, and INO1 transcription is derepressed to increase inositol synthesis. When inositol levels are abundant, PA is rapidly consumed for phosphatidylinositol synthesis; Opi1 is released from the endoplasmic reticulum and translocates into the nucleus, where it represses INO1 transcription resulting in decreased inositol synthesis. We have recently demonstrated that transcription of INO1 in yeast is regulated by the synthesis of inositol pyrophosphates, as INO1 transcription requires the Kcs1-catalyzed synthesis of diphosphoinositol tetrakisphosphate (PP-IP4) (1).
In contrast to inositol synthesis in yeast, transcription of Isyna1 (the mammalian homolog of yeast INO1, which we refer as mIno1 in this report), is not regulated in response to inositol (9, 22). However, in this study, we demonstrate for the first time that inositol synthesis in mouse embryonic fibroblast (MEF) cells is regulated by inositol pyrophosphate. Knock-out of inositol hexakisphosphate kinase IP6K1 (which catalyzes the formation of pyrophosphate at position 5 of inositol pentakisphosphate/inositol hexakisphosphate to generate 5PP-IP4/5PP-IP5 (IP7), respectively (23)), led to increased transcription and altered DNA methylation of mIno1. This resulted in increased levels of mINO1 protein and increased intracellular inositol. Thus, in contrast to positive regulation of INO1 in yeast, inositol pyrophosphate negatively regulates mIno1 transcription. Intriguingly, similar to the yeast transcriptional repressor Opi1, IP6K1 protein bound with high affinity to PA. Deletion of the PA-binding sequence decreased both nuclear localization of IP6K1 and repression of mIno1 transcription. Our findings suggest a model whereby inositol pyrophosphate negatively regulates inositol synthesis by PA-facilitated entry into the nucleus and repression of mIno1 transcription.
Experimental Procedures
Cell Lines, Yeast Strains, and Growth Conditions
Wild type, IP6K1-KO, IP6K1-KO + pMX-IP6K1, and IP6K1-KO + pMX-EV MEF cell lines (kindly provided by Dr. Anutosh Chakraborty, Scripps Research Institute) were grown in DMEM (Gibco) containing 10% FBS (Hyclone) and penicillin (100 units/ml)/streptomycin (100 μg/ml) (Invitrogen) (24). IP6K1-KO + pMX-IP6K1 and IP6K1-KO + pMX-EV cells were grown in the media supplemented with 4 μg/ml blasticidin (Sigma). The yeast S. cerevisiae kcs1Δ strain was obtained from Yeast Deletion Clones (Invitrogen). Yeast cells were grown at 30 or 37 °C in synthetic complete (SC) medium, which contained glucose (2% w/v) and necessary supplements, including adenine (20 mg/liter), arginine (20 mg/liter), histidine (20 mg/liter), methionine (20 mg/liter), tryptophan (20 mg/liter), leucine (60 mg/liter), lysine (200 mg/liter), threonine (300 mg/liter), ammonium sulfate (0.2% w/v), inositol-free Difco vitamin mix, vitamin-free yeast base, plus agar (2% w/v) for solid medium. Inositol (75 μm) was added separately as indicated.
RT-PCR
MEF cells were grown in DMEM containing 10% FBS. RNA was extracted from cells by using RNeasy Plus mini kit (Qiagen) and was converted to cDNA with a transcriptor first strand cDNA synthesis kit (Roche Applied Science). RT-PCR was performed using Brilliant III Ultra-Fast SYBR Green quantitative PCR master mix (Agilent) and MX3000p quantitative PCR system (Agilent). mRNA levels were normalized to GAPDH.
Immunoblotting
Cells were harvested, washed with ice-cold PBS, and lysed by vortexing in lysis buffer (50 mm Tris-HCl, 125 mm NaCl2, 1% Nonidet P-40, 2 mm EDTA, and 1× protease inhibitor) for 30 min at 4 °C. Protein concentration was determined using a Pierce BCA protein assay kit (Thermo). Cell extract corresponding to 30 μg of protein was analyzed by SDS-PAGE on a 10% gel. Immunoblotting was performed using primary antibodies against mINO1 (ISYNA1) (sc-134687, rabbit, Santa Cruz Biotechnology), IP6K1 (GTX103949, rabbit, Gene Tex), and β-actin (sc-69879, mouse, Santa Cruz Biotechnology) and corresponding secondary antibodies.
Measurement of Intracellular Inositol and Glc-6-P Levels in MEF Cells
Intracellular inositol levels were determined as described before using the method of Maslanski and Busa (25). In brief, MEF cells were lysed in lysis buffer (50 mm Tris-HCl, 125 mm NaCl2, 1% Nonidet P-40, and 2 mm EDTA) at 4 °C. Cell extracts were mixed with 7.5% perchloric acid and centrifuged at 10,000 × g for 10 min at 4 °C. Supernatants were collected and titrated with ice-cold KOH to pH 7. Samples were clarified by centrifugation and loaded onto columns containing 1 ml of AG 1-X8 resin/H2O (1:1) mixture. Inositol was eluted with 5 ml of distilled H2O; eluates were dried in an oven at 70 °C and stored at −80 °C. Prior to assay, samples were dissolved in distilled H2O. Inositol content in samples was measured as described previously (26).
To determine intracellular Glc-6-P levels, MEF cells were washed twice with ice-cold PBS. Cell pellets were mixed with 1 ml of ice-cold MeOH/CHCl3 (2:1), vortexed, and stored at −20 °C for 2 h. Samples were then mixed with extraction solution (50% MeOH, 4 mm Tricine, pH 5.4) and centrifuged at 18,000 × g for 10 min. The upper phase was collected and kept on ice. The lower chloroform phase was extracted again with extraction solution. Upper phases from both extractions were combined, dried with a SpeedVac, and stored at −80 °C. Prior to assay, samples were dissolved in distilled H2O. Glc-6-P content in samples was measured by the enzyme-coupled fluorescence assay developed by Zhu et al. (27) with modification.
Protein Lipid Overlay Assay
pGEX-6P-2 plasmids harboring Ip6k1 variants were constructed using a Q5 site-directed mutagenesis kit (New England Biolabs) with pGEX-6P-2-IP6K1 (from Dr. Anutosh Chakraborty) as template. pGEX-6P-2-IP6K1 and corresponding mutants were transformed into BL21 (DE3) pLysS cells for isopropyl 1-thio-β-d-galactopyranoside-induced overexpression. Escherichia coli cells were lysed by French press to obtain cell extracts. IP6K1 proteins were then purified from E. coli cell extracts using a GST spin purification kit (Life Technologies, Inc.). A protein lipid overlay assay was performed according to the protocol of Dowler et al. (28) with modification. In brief, lyophilized lipids were dissolved in a 1:1 solution of methanol and chloroform to make 1 mm stocks. Lipids were diluted to concentrations ranging from 500 to 5 μm in a 2:1:0.8 solution of methanol/chloroform/water. Then 1 μl lipid samples from each dilution were spotted on nitrocellulose membranes. After drying for 1 h at room temperature, membranes were incubated in blocking buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl2, 0.1% Tween 20, and 2 mg/ml BSA) with gentle rocking for 1 h at room temperature. Membranes were incubated overnight at 4 °C with gentle rocking in 10 ml of blocking buffer containing 25 μg of purified IP6K1 proteins. IP6K1 proteins bound to lipid drops on the membrane were detected by immunoblotting using primary antibodies against IP6K1 (GTX103949, rabbit, Gene Tex), GST-tag (2625, rabbit, Cell Signaling), and corresponding secondary antibodies.
Fluorescence Microscopy
pEGFP-C1 plasmids harboring Ip6k1 variants were constructed using a Q5 site-directed mutagenesis kit (New England Biolabs) with pEGFP-C1-IP6K1 (from Dr. Anutosh Chakraborty) as template. IP6K1-KO cells were transfected with pEGFP-C1-IP6K1 plasmid and corresponding mutants using Lipofectamine 2000 (Life Technologies, Inc.). Fluorescence microscopy was performed using an Olympus BX41 fluorescence microscope. Images were acquired using an Olympus Q-Color3 camera operated by QCapture2 software. All pictures were taken at ×400 at room temperature.
Determination of DNA Methylation Levels
Genomic DNA samples extracted from WT and IP6K1-KO cells using a Wizard Genomic DNA purification kit (Promega) were subjected to DNA bisulfite conversion using an EZ DNA Methylation-Gold kit (Zymo Research) and sequencing for the determination of methylation levels.
Results
IP6K1 Rescues Inositol Deficiency in the Yeast kcs1Δ Mutant
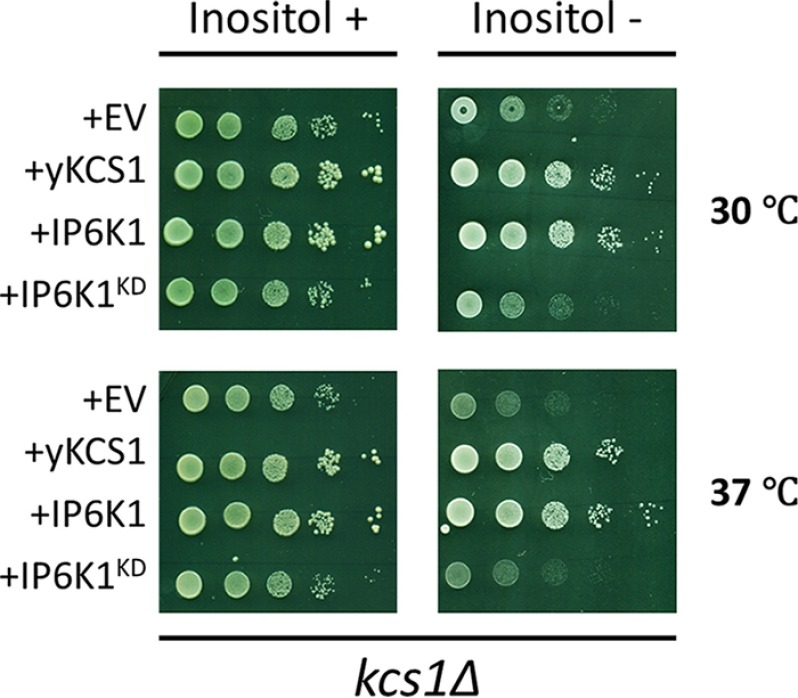
We have previously shown that Kcs1-catalyzed inositol pyrophosphate synthesis is required for optimal transcription of INO1 in yeast (1). To determine whether mouse IP6K1 can supply the function of yeast Kcs1, we expressed Ip6k1 in the yeast kcs1Δ mutant. As seen in Fig. 1, kcs1Δ mutant cells expressing the wild type Ip6k1 gene grew normally, whereas the kinase-dead Ip6k1 (Ip6k1KD) gene (29) failed to support growth of the kcs1Δ mutant. Therefore, mouse IP6K1 is the functional homolog of yeast Kcs1, which can rescue defective inositol synthesis in yeast by restoring inositol pyrophosphate synthesis.
FIGURE 1.
IP6K1 rescues kcs1Δ inositol auxotrophy. Serial dilutions of yeast kcs1Δ cells transformed with indicated plasmids were spotted on synthetic complete Ura− medium in the presence or absence or 75 μm inositol. Plates were incubated at the indicated temperatures for 3 days.
IP6K1 Is a Negative Regulator of Inositol Synthesis in MEF Cells
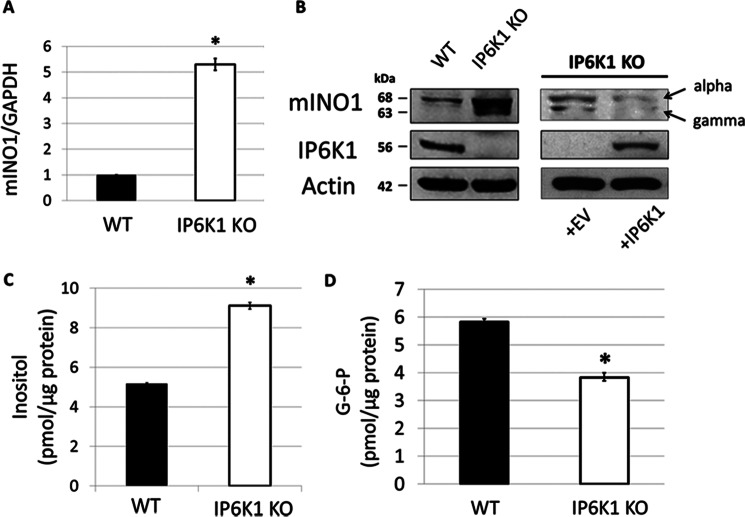
To ascertain whether IP6K1 regulates mammalian inositol synthesis, we determined the effects of knocking out Ip6k1 (IP6K1-KO). Surprisingly, IP6K1-KO cells exhibited a 5-fold increase in mIno1 mRNA levels (Fig. 2A). Consistent with increased transcription, mINO1 protein levels were also increased in IP6K1-KO cells (Fig. 2B), and inositol levels were 75% higher in the IP6K1-KO cells than in WT cells (Fig. 2C). Levels of intracellular Glc-6-P, the substrate for inositol synthesis, decreased correspondingly (Fig. 2D). Taken together, these findings indicate that IP6K1 is a negative regulator of inositol synthesis.
FIGURE 2.
Inositol synthesis is up-regulated in IP6K1-KO cells. A, mIno1 mRNA levels are increased in IP6K1-KO cells. WT and IP6K1-KO MEF cells were grown in DMEM with 10% FBS and harvested for mRNA extraction. mIno1 mRNA levels were determined by RT-PCR (two independent experiments with two replicates each) (*, p < 0.05). B, mINO1 protein levels were profoundly increased in IP6K1-KO cells (left). The expression of Ip6k1 in IP6K1-KO cells restored mINO1α and mINO1γ levels (right). MEF cells were grown in DMEM with 10% FBS in the presence or absence of 4 μg/ml blasticidin. Cells were harvested and lysed for protein extraction. 30 μg of total cell extract from each sample were subjected to Western blot analysis using 10% SDS-polyacrylamide gel. C, IP6K1-KO cells exhibited increased intracellular inositol levels. Intracellular inositol levels in MEF cells were determined as described under “Experimental Procedures” (three independent experiments with two replicates each) (*, p < 0.05). D, intracellular Glc-6-P levels were decreased in IP6K1-KO cells. Intracellular Glc-6-P levels in MEF cells were determined by enzyme-coupled fluorescence assay as described under “Experimental Procedures” (three independent experiments with two replicates each) (*, p < 0.05). Values are mean ± S.E. Statistical significance was determined by unpaired t test.
IP6K1 Regulates mIno1 DNA Methylation
As seen in Fig. 2B, two mINO1 proteins were expressed in IP6K1-KO cells. A previous study reported that alternative splicing of mIno1 results in α, β, and γ mRNA transcripts, which generate protein isoforms (30). The α transcript is the full-length mRNA; the β mRNA contains a truncated exon 2, and the γ mRNA is transcribed from a second ATG site within the unremoved intron 4. To determine which isoforms are expressed in IP6K1-KO cells, we compared cDNA from WT and IP6K1-KO cells by PCR analysis, using specific primers that distinguished among these mRNAs. Both the α and γ mRNAs were present in the IP6K1-KO cells, but only the α mRNA was detected in WT cells (data not shown). These findings indicated that isoforms observed in Fig. 2B are translated from the α (upper band) and γ (lower band) splicing forms.
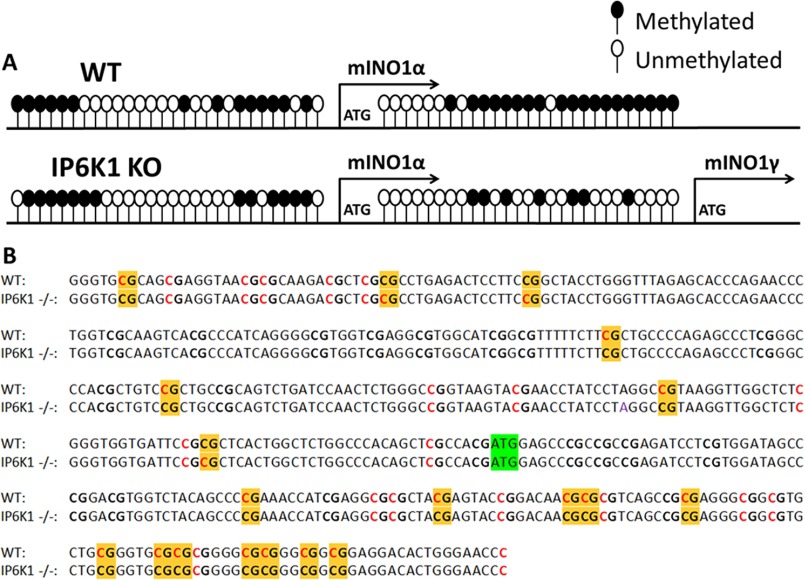
Previous studies indicated that in vitro methylation of the human INO1 promoter significantly decreased reporter gene expression (31). To determine whether altered DNA methylation could account for the mINO1 isoforms observed in IP6K1-KO cells, we analyzed methylation of mIno1 DNA using DNA bisulfite conversion of WT and IP6K1-KO genomic DNA followed by probing with primers targeting the sequence from −286 to +160, which is enriched in CpG islands (Fig. 3). In the mIno1 promoter region, most of the CpG sites exhibited a similar, but slightly decreased, pattern of methylation in IP6K1-KO cells compared with WT cells. However, the CpG sites between the first and second ATGs exhibited markedly less methylation in IP6K1-KO cells. These findings suggest that IP6K1 may negatively regulate mIno1 transcription by increasing the methylation of mIno1 DNA.
FIGURE 3.
Methylation pattern of mIno1 DNA is altered in IP6K1-KO cells. A, MEF cells were grown in DMEM with 10% FBS and harvested for DNA isolation. DNA methylation levels were determined as described under “Experimental Procedures.” CpG islands are depicted as balloons. Methylated cytosines are filled, and unmethylated cytosines are unfilled. B, raw data of mIno1 DNA methylation experiment. CpG islands are depicted in bold. Methylated cytosines are depicted in red, and unmethylated cytosines are depicted in black. CpG islands with altered methylation in IP6K1-KO cells are highlighted with yellow shading, and the start codon is highlighted with green shading.
IP6K1 Requires PA Binding for Nuclear Localization and Repression of mIno1 Transcription
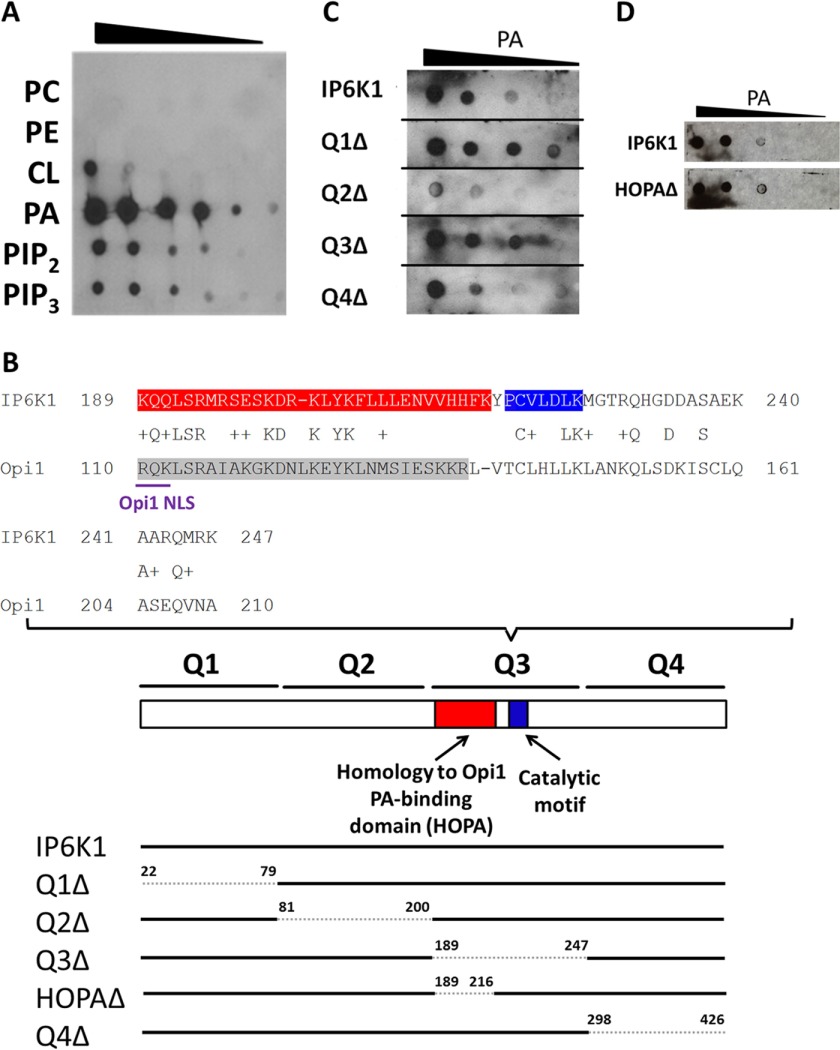
As discussed above, negative regulation of INO1 transcription in yeast is mediated by the Opi1 transcriptional repressor. Although homologs of Opi1 have not been identified in mammalian cells, we considered the possibility that IP6K1 may exhibit functional similarity to Opi1. Opi1 is retained in the cytoplasm by binding to PA and translocates to the nucleus when PA is limiting. As shown in the protein lipid overlay assay (Fig. 4A), purified recombinant IP6K1 displayed a markedly high affinity to PA and only weak binding to other phospholipids.
FIGURE 4.
IP6K1 binds preferentially to PA. A, IP6K1 protein was purified from E. coli cells expressing the Ip6k1 gene on the pGEX-6-P2 overexpression vector. Serial dilutions of the indicated lipids were spotted on a nitrocellulose membrane, which was incubated overnight in buffer containing 25 μg of IP6K1 protein. Interactions between IP6K1 and lipids were determined by immunoblotting, as described under “Experimental Procedures.” B, IP6K1 exhibits sequence homology to yeast Opi1 (upper panel). The IP6K1 HOPA domain, which exhibits homology to the PA-binding domain of yeast Opi1, is highlighted in red. The catalytic motif of IP6K1 is highlighted in blue. The PA-binding domain of yeast Opi1 is highlighted in gray. The NLS of yeast Opi1 is underlined in purple. IP6K1 deletion mutants were constructed by site-directed mutagenesis according to the schematic figure (lower panel). Residues deleted are indicated by numbers above the bar. C, deletion of the Q2 domain of IP6K1 decreased binding to PA. WT and mutant IP6K1 proteins were overexpressed and purified from E. coli. Interactions between protein and PA were determined as described previously. Figure represents three independent experiments. D, deletion of the HOPA domain of IP6K1 did not affect binding to PA. WT and HOPAΔ IP6K1 proteins were expressed and purified from E. coli. Interactions between protein and PA were determined as described previously.
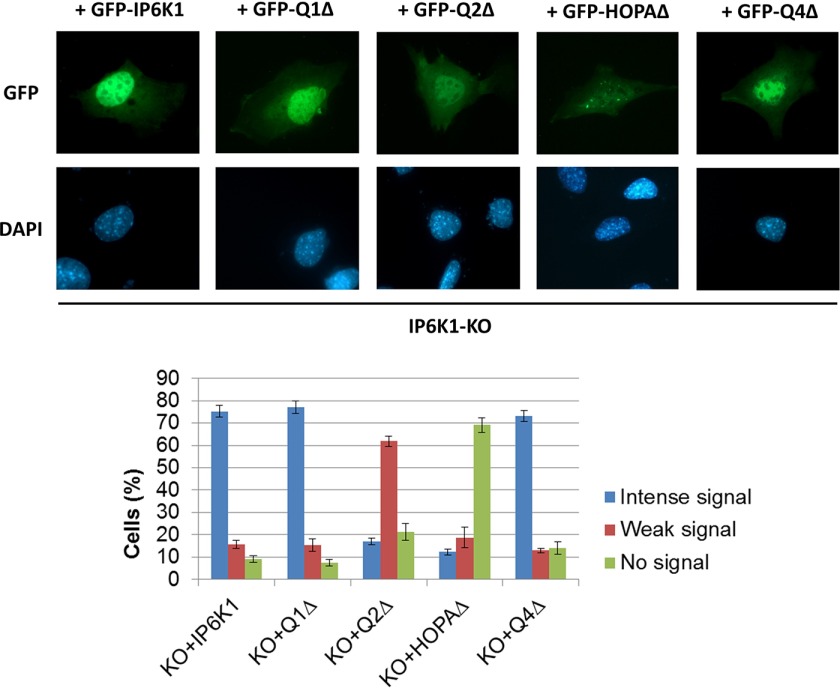
To determine the functional significance of PA binding, we constructed the IP6K1 deletions shown in Fig. 4B and determined the ability of truncated proteins to bind PA. Deletion of Q2 but not the other sequences greatly diminished PA binding activity of IP6K1 (Fig. 4C). We then determined the effect of Q2 deletion on localization of IP6K1. IP6K1-KO cells expressing GFP-tagged wild type IP6K1 exhibited intense fluorescence in the nucleus in about 75% of cells (Fig. 5). Deletion of the Q2 domain resulted in decreased nuclear localization in more than 60% of cells. Interestingly, IP6K1-KO cells expressing GFP-Q2Δ exhibited increased mIno1 mRNA levels compared with cells expressing wild type IP6K1 (Fig. 6). These results indicate that interaction with PA facilitates IP6K1 nuclear localization and is required by IP6K1 to repress mIno1 expression.
FIGURE 5.
PA binding is required for nuclear localization of IP6K1. IP6K1-KO cells were transfected with plasmids harboring GFP-tagged WT or mutant IP6K1. Intracellular localization of IP6K1 (upper panel) was determined by fluorescence microscopy. The number of cells showing each phenotype (lower panel) was determined in three independent experiments (n > 150). Values are mean ± S.E.
FIGURE 6.
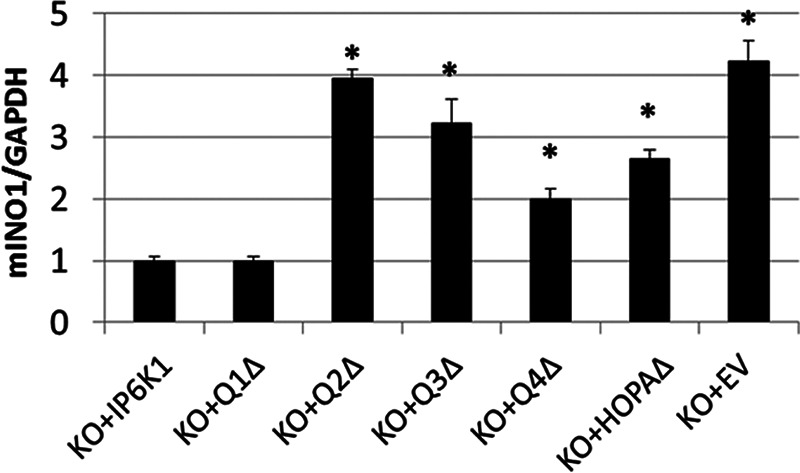
PA binding to IP6K1 is required for repression of mIno1 transcription. IP6K1-KO cells were transfected with plasmids harboring the indicated GFP-tagged IP6K1 mutants. The mIno1 mRNA levels were determined by RT-PCR (two independent experiments with two replicates) (*, p < 0.05). Values are mean ± S.E. Statistical significance comparing transcription levels to KO + IP6K1 control was determined by unpaired t test.
Protein BLAST sequence alignment identified a small region of sequence homology between the Q3 domain of IP6K1 and yeast Opi1, especially in the region of positively charged residues that are critical for the PA binding activity of the yeast protein (Fig. 4B). The IP6K1 region containing these sequences was designated HOPA (Homology to Opi1 PA binding). To determine the function of the HOPA domain, we constructed an IP6K1 mutant in which this region is deleted (HOPAΔ). The HOPAΔ protein exhibited normal PA binding activity (Fig. 4D) as expected, as PA binding is conferred by the Q2 domain. Strikingly, however, deletion of the HOPA domain resulted in exclusion of IP6K1 from the nucleus in more than 70% of cells (Fig. 5). Consistent with this finding, cNLS Mapper predicts a potential nuclear localization signal (NLS) in the HOPA domain. Interestingly, the NLS of yeast Opi1 is in the homologous region (Fig. 4B). Expression of HOPAΔ in IP6K1-KO cells resulted in increased mIno1 mRNA levels relative to cells expressing WT IP6K1 (Fig. 6). The catalytic motif of IP6K1 also lies in the Q3 domain (32, 33). Expression of Q3Δ, which lacks both the HOPA domain and the catalytic motif, also led to increased mIno1 mRNA levels in IP6K1-KO cells (Fig. 6).
The Q1Δ deletion did not affect nuclear localization (Fig. 5) or mIno1 mRNA levels. Although the Q4Δ deletion did not affect nuclear localization (Fig. 5), it resulted in increased mIno1 transcription. The Q4 domain contains the ATP-binding motif (32, 33), which is critical for IP6K1 catalytic activity (29, 34, 35). Increased mIno1 transcription in IP6K1-KO cells expressing Q4Δ indicates that IP6K1 requires catalytic activity to suppress mIno1 expression. Together, these findings indicate that binding of PA is required for localization of IP6K1 to the nucleus, where the ATP binding-dependent synthesis of inositol pyrophosphate represses mIno1 transcription.
Discussion
Despite the essential role of inositol in cell function, the mechanisms that regulate inositol synthesis in mammalian cells have not been characterized. This study demonstrates for the first time that de novo synthesis of inositol in MEF cells is regulated by IP6K1, which mediates transcriptional control of the gene (mIno1) coding for the rate-limiting enzyme of inositol synthesis. Our findings indicate that IP6K1 alters methylation and negatively regulates transcription of mIno1 and that binding of IP6K1 to PA is essential for nuclear localization of IP6K1 and repression of transcription.
IP6K1-KO cells exhibited increased expression of mIno1 (Fig. 2A), which was accompanied by increased mINO1 protein and inositol levels (Fig. 2, B and C). These findings indicate that IP6K1 is a negative regulator of mIno1 transcription. Increased mIno1 expression in IP6K1-KO cells is most likely due to decreased DNA methylation (Fig. 3), which is associated with silencing of gene expression (36). Seelan et al. (31) investigated the effects of methylation of the INO1 promoter on transcription of INO1 in human neuroblastoma cells transfected with plasmids carrying in vitro methylated INO1. They determined that the methylated INO1 promoter led to significantly less transcription than the non-methylated control. Our finding that IP6K1-KO cells exhibited both increased mIno1 transcription and decreased DNA methylation is the first in vivo demonstration of regulation of mIno1 by in vivo methylation.
Two lines of evidence support a role for regulation of methylation by inositol pyrophosphate. First, IP7 (the product of IP6K1) was shown to inhibit Akt (37), which negatively regulates methylation of imprinted genes (38). Therefore, the deficiency of IP7 in IP6K1-KO cells may lead to decreased mIno1 methylation as a result of increased Akt signaling. Second, IP6K1 associates with chromatin and controls histone demethylation by regulating the demethylase Jumonji domain-containing 2C (JMJD2C), which catalyzes the removal of trimethyl groups from lysines 9 and 36 of histone H3 (39). IP6K1-KO cells exhibited decreased levels of histone H3 lysine 9 trimethylation (H3K9me3) and increased transcription of JMJD2C-regulated genes. Interestingly, DNA regions associated with H3K9me3 exhibited increased methylation (40), whereas mutation of H3K3 led to decreased DNA methylation (41). In this light, IP6K1 may regulate mIno1 methylation indirectly by regulating histone modification.
IP6K1 exhibits dual localization in the cytoplasm and nucleus (Fig. 5). The probable NLS of IP6K1 was localized to the Q3 domain, the deletion of which led to exclusion of IP6K1 from the nucleus (Fig. 5). Binding of the IP6K1 Q2 domain to PA is essential for translocation of the protein to the nucleus and repression of mIno1 transcription (Figs. 5 and 6). Binding to PA has been shown to promote nuclear localization of transcription factor WER in Arabidopsis (42, 43). Inhibition of phospholipase D, resulting in decreased conversion of phosphatidylcholine to PA, suppresses nuclear localization of WER (43). Nuclear association of phospholipase D has been reported both in mammals and plants (44, 45). Furthermore, PA synthesis has been demonstrated in the nuclear envelope of mammalian cells (46, 47), and PA has been detected in the nucleus (48). To our knowledge, this study is the first to report the dependence on binding to PA for nuclear localization of a mammalian protein.
The catalytic function of IP6K1 is necessary for repression of mIno1 transcription, as deletion of the Q4 domain resulted in increased mIno1 expression (Fig. 6). The “SLL” ATP-binding motif, which is highly conserved in the inositol polyphosphate kinase family, is localized in the Q4 domain (32, 33), and mutation of the ATP-binding site impairs catalysis (29, 34). Therefore, the synthesis of inositol pyrophosphate is required for repression of mIno1 transcription.
Surprisingly, although Kcs1 and IP6K1 carry out the same enzymatic function, they regulate INO1 expression in an opposite manner in yeast and mouse cells. INO1 expression is positively regulated in yeast by Kcs1 (1) but negatively regulated by IP6K1 in mouse cells (Fig. 2C). In fact, the observed negative regulation of mIno1 expression by IP6K1 more closely resembles that of the yeast transcriptional repressor Opi1. Both Opi1 and IP6K1 bind to PA, translocate from the cytoplasm to the nucleus, and repress INO1 transcription. However, although PA binding retains Opi1 in the cytoplasm, PA binding mediates IP6K1 nuclear translocation (Fig. 5). In yeast, PA levels indirectly reflect inositol synthesis, which regulates INO1 expression. As inositol does not regulate INO1 expression in mammalian cells (9, 22), we speculate that PA levels may reflect the state of cellular energy metabolism, which would affect inositol synthesis as a result of competition for the common substrate Glc-6-P.
Inositol pyrophosphate regulation is an intriguing area of signaling research. Shears (49) suggested that inositol pyrophosphates are metabolic messengers that respond to the cellular energy demand. In support of this concept, perturbing the energy balance in mammalian cells leads to decreased synthesis of inositol pyrophosphates (50). Interestingly, inositol pyrophosphate deficiency in yeast kcs1Δ cells leads to dysfunctional mitochondria with greatly reduced respiratory capacity (51). To compensate, glycolysis is increased to generate ATP. Glc-6-P is the branch point of inositol de novo synthesis and glycolysis. We observed decreased intracellular Glc-6-P levels in IP6K1-KO cells (Fig. 2D), which may be caused, at least in part, by increased inositol synthesis. Therefore, IP6K1 may repress mIno1 expression to maintain inositol synthesis at a relatively low level, reserving Glc-6-P for glycolysis.
Our findings suggest the following model (Fig. 7). Translocation of IP6K1 to the nucleus is facilitated by interaction with PA. In the nucleus, IP6K1 associates with chromatin and synthesizes IP7/PP-IP4. IP7/PP-IP4 inhibits transcription of mIno1 by increasing methylation of mIno1 DNA, inhibiting the recruitment of transcription factors to the promoter region of mIno1 or perturbing assembly of the transcription complex. This study is the first to describe the molecular control of de novo synthesis of inositol in mammalian cells and suggests that inositol synthesis and cellular energy demand are coordinately controlled. These findings have important implications for understanding essential cellular functions that are dependent on inositol and human disorders that result from perturbation of inositol homeostasis.
FIGURE 7.
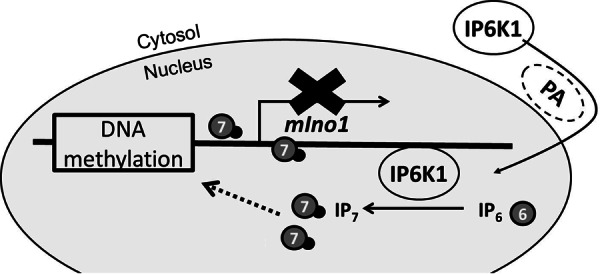
Model of regulation of mIno1 transcription by IP6K1. PA binding facilitates localization of IP6K1 to the nucleus, where it associates with chromatin and synthesizes IP7/PP-IP4. IP7/PP-IP4 represses mIno1 expression by promoting methylation of mIno1 DNA and/or inhibiting the transcription apparatus.
Author Contributions
W. Y., C. Y., and M. L. G. designed the research; W. Y. and C. Y. performed the experiments; W. Y., C. Y., and M. L. G. analyzed the data; and W. Y. and M. L. G. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We are grateful to Dr. Anutosh Chakraborty for kindly supplying MEF cell lines and plasmids. We thank Dr. Xiang-Dong Zhang for sharing cell culture facilities.
This work was supported by National Institutes of Health Grant DK081367 (to M. L. G.) and the Graduate School of Wayne State University (to W. Y.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was selected as a Paper of the Week.
- Glc-6-P
- glucose 6-phosphate
- IP6K1
- inositol hexakisphosphate kinase 1
- PA
- phosphatidic acid
- PP-IP4
- diphosphoinositol tetrakisphosphate
- IP7
- diphosphoinositol pentakisphosphate
- MEF
- mouse embryonic fibroblast
- NLS
- nuclear localization signal
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
References
- 1. Ye C., Bandara W. M., and Greenberg M. L. (2013) Regulation of inositol metabolism is fine-tuned by inositol pyrophosphates in Saccharomyces cerevisiae. J. Biol. Chem. 288, 24898–24908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saiardi A., Sciambi C., McCaffery J. M., Wendland B., and Snyder S. H. (2002) Inositol pyrophosphates regulate endocytic trafficking. Proc. Natl. Acad. Sci. U.S.A. 99, 14206–14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Streb H., Irvine R. F., Berridge M. J., and Schulz I. (1983) Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 [DOI] [PubMed] [Google Scholar]
- 4. Bankaitis V. A., Garcia-Mata R., and Mousley C. J. (2012) Golgi membrane dynamics and lipid metabolism. Curr. Biol. 22, R414–R424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Y., Azab A. N., Thompson M. N., and Greenberg M. L. (2006) Inositol phosphates and phosphoinositides in health and disease. Subcell. Biochem. 39, 265–292 [DOI] [PubMed] [Google Scholar]
- 6. Nikawa J., Tsukagoshi Y., and Yamashita S. (1991) Isolation and characterization of two distinct myo-inositol transporter genes of Saccharomyces cerevisiae. J. Biol. Chem. 266, 11184–11191 [PubMed] [Google Scholar]
- 7. Berridge M. J., and Irvine R. F. (1989) Inositol phosphates and cell signalling. Nature 341, 197–205 [DOI] [PubMed] [Google Scholar]
- 8. Henry S. A., Atkinson K. D., Kolat A. I., and Culbertson M. R. (1977) Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J. Bacteriol. 130, 472–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye C., and Greenberg M. L. (2015) Inositol synthesis regulates the activation of GSK-3α in neuronal cells. J. Neurochem. 133, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stein A. J., and Geiger J. H. (2002) The crystal structure and mechanism of 1-l-myo-inositol-1-phosphate synthase. J. Biol. Chem. 277, 9484–9491 [DOI] [PubMed] [Google Scholar]
- 11. Parthasarathy L. K., Seelan R. S., Tobias C., Casanova M. F., and Parthasarathy R. N. (2006) Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent. Subcell. Biochem. 39, 293–314 [DOI] [PubMed] [Google Scholar]
- 12. Eisenberg F., Jr. (1967) d-Myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J. Biol. Chem. 242, 1375–1382 [PubMed] [Google Scholar]
- 13. Kindl H., and Hoffmann-Ostenhof O. (1964) Studies on the biosynthesis of cyclitols. Ii. Formation of Meso-Inositol from C14–1-glucose in sinapis alba and selective degradation of the resulting product. Biochemische Zeitschrift 339, 374–381 [PubMed] [Google Scholar]
- 14. Loewus F. A., and Kelly S. (1962) Conversion of glucose to inositol in parsley leaves. Biochem. Biophys. Res. Commun. 7, 204–208 [DOI] [PubMed] [Google Scholar]
- 15. Wong Y. H., Kalmbach S. J., Hartman B. K., and Sherman W. R. (1987) Immunohistochemical staining and enzyme activity measurements show myo-inositol-1-phosphate synthase to be localized in the vasculature of brain. J. Neurochem. 48, 1434–1442 [DOI] [PubMed] [Google Scholar]
- 16. Shimon H., Agam G., Belmaker R. H., Hyde T. M., and Kleinman J. E. (1997) Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am. J. Psychiatry 154, 1148–1150 [DOI] [PubMed] [Google Scholar]
- 17. Croze M. L., and Soulage C. O. (2013) Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 95, 1811–1827 [DOI] [PubMed] [Google Scholar]
- 18. Henry S. A., Kohlwein S. D., and Carman G. M. (2012) Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 190, 317–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White M. J., Lopes J. M., and Henry S. A. (1991) Inositol metabolism in yeasts. Adv. Microb. Physiol. 32, 1–51 [DOI] [PubMed] [Google Scholar]
- 20. Deranieh R. M., He Q., Caruso J. A., and Greenberg M. L. (2013) Phosphorylation regulates myo-inositol-3-phosphate synthase: a novel regulatory mechanism of inositol biosynthesis. J. Biol. Chem. 288, 26822–26833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loewen C. J., Gaspar M. L., Jesch S. A., Delon C., Ktistakis N. T., Henry S. A., and Levine T. P. (2004) Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science 304, 1644–1647 [DOI] [PubMed] [Google Scholar]
- 22. Guan G., Dai P., and Shechter I. (2003) cDNA cloning and gene expression analysis of human myo-inositol 1-phosphate synthase. Arch. Biochem. Biophys. 417, 251–259 [DOI] [PubMed] [Google Scholar]
- 23. Draskovic P., Saiardi A., Bhandari R., Burton A., Ilc G., Kovacevic M., Snyder S. H., and Podobnik M. (2008) Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chem. Biol. 15, 274–286 [DOI] [PubMed] [Google Scholar]
- 24. Bhandari R., Juluri K. R., Resnick A. C., and Snyder S. H. (2008) Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc. Natl. Acad. Sci. U.S.A. 105, 2349–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maslanski J. A., and Busa W. B. (1990) in Methods in Inositide Research (Irvine R. F., ed) pp. 113–126, Raven Press, Ltd., New York [Google Scholar]
- 26. Ju S., and Greenberg M. L. (2003) Valproate disrupts regulation of inositol responsive genes and alters regulation of phospholipid biosynthesis. Mol. Microbiol. 49, 1595–1603 [DOI] [PubMed] [Google Scholar]
- 27. Zhu A., Romero R., and Petty H. R. (2009) An enzymatic fluorimetric assay for glucose 6-phosphate: application in an in vitro Warburg-like effect. Anal. Biochem. 388, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dowler S., Kular G., and Alessi D. R. (2002) Protein lipid overlay assay. Science's STKE 2002, pl6. [DOI] [PubMed] [Google Scholar]
- 29. Jadav R. S., Chanduri M. V., Sengupta S., and Bhandari R. (2013) Inositol pyrophosphate synthesis by inositol hexakisphosphate kinase 1 is required for homologous recombination repair. J. Biol. Chem. 288, 3312–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seelan R. S., Lakshmanan J., Casanova M. F., and Parthasarathy R. N. (2009) Identification of myo-inositol-3-phosphate synthase isoforms: characterization, expression, and putative role of a 16-kDa γ(c) isoform. J. Biol. Chem. 284, 9443–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seelan R. S., Pisano M. M., Greene R. M., Casanova M. F., and Parthasarathy R. N. (2011) Differential methylation of the gene encoding myo-inositol 3-phosphate synthase (Isyna1) in rat tissues. Epigenomics 3, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. González B., Schell M. J., Letcher A. J., Veprintsev D. B., Irvine R. F., and Williams R. L. (2004) Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol. Cell 15, 689–701 [DOI] [PubMed] [Google Scholar]
- 33. Miller G. J., and Hurley J. H. (2004) Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase. Mol. Cell 15, 703–711 [DOI] [PubMed] [Google Scholar]
- 34. Saiardi A., Nagata E., Luo H. R., Sawa A., Luo X., Snowman A. M., and Snyder S. H. (2001) Mammalian inositol polyphosphate multikinase synthesizes inositol 1,4,5-trisphosphate and an inositol pyrophosphate. Proc. Natl. Acad. Sci. U.S.A. 98, 2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Padmanabhan U., Dollins D. E., Fridy P. C., York J. D., and Downes C. P. (2009) Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J. Biol. Chem. 284, 10571–10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newell-Price J., Clark A. J., and King P. (2000) DNA methylation and silencing of gene expression. Trends Endocrinol. Metab. 11, 142–148 [DOI] [PubMed] [Google Scholar]
- 37. Chakraborty A., Koldobskiy M. A., Bello N. T., Maxwell M., Potter J. J., Juluri K. R., Maag D., Kim S., Huang A. S., Dailey M. J., Saleh M., Snowman A. M., Moran T. H., Mezey E., and Snyder S. H. (2010) Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popkie A. P., Zeidner L. C., Albrecht A. M., D'Ippolito A., Eckardt S., Newsom D. E., Groden J., Doble B. W., Aronow B., McLaughlin K. J., White P., and Phiel C. J. (2010) Phosphatidylinositol 3-kinase (PI3K) signaling via glycogen synthase kinase-3 (Gsk-3) regulates DNA methylation of imprinted loci. J. Biol. Chem. 285, 41337–41347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burton A., Azevedo C., Andreassi C., Riccio A., and Saiardi A. (2013) Inositol pyrophosphates regulate JMJD2C-dependent histone demethylation. Proc. Natl. Acad. Sci. U.S.A. 110, 18970–18975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamaru H., Zhang X., McMillen D., Singh P. B., Nakayama J., Grewal S. I., Allis C. D., Cheng X., and Selker E. U. (2003) Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 34, 75–79 [DOI] [PubMed] [Google Scholar]
- 41. Tamaru H., and Selker E. U. (2001) A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414, 277–283 [DOI] [PubMed] [Google Scholar]
- 42. Ryu K. H., Kang Y. H., Park Y. H., Hwang I., Schiefelbein J., and Lee M. M. (2005) The WEREWOLF MYB protein directly regulates CAPRICE transcription during cell fate specification in the Arabidopsis root epidermis. Development 132, 4765–4775 [DOI] [PubMed] [Google Scholar]
- 43. Yao H., Wang G., Guo L., and Wang X. (2013) Phosphatidic acid interacts with a MYB transcription factor and regulates its nuclear localization and function in Arabidopsis. Plant Cell 25, 5030–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liscovitch M., Czarny M., Fiucci G., Lavie Y., and Tang X. (1999) Localization and possible functions of phospholipase D isozymes. Biochim. Biophys. Acta 1439, 245–263 [DOI] [PubMed] [Google Scholar]
- 45. Fan L., Zheng S., Cui D., and Wang X. (1999) Subcellular distribution and tissue expression of phospholipase Dα, Dβ, and Dγ in Arabidopsis. Plant Physiol. 119, 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith C. D., and Wells W. W. (1983) Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J. Biol. Chem. 258, 9368–9373 [PubMed] [Google Scholar]
- 47. Baker R. R., and Chang H. (2001) Phosphatidic acid is the prominent product of endogenous neuronal nuclear lipid phosphorylation, an activity enhanced by sphingosine, linked to phospholipase C and associated with the nuclear envelope. Biochim. Biophys. Acta 1534, 110–120 [DOI] [PubMed] [Google Scholar]
- 48. D'Santos C. S., Clarke J. H., Irvine R. F., and Divecha N. (1999) Nuclei contain two differentially regulated pools of diacylglycerol. Curr. Biol. 9, 437–440 [DOI] [PubMed] [Google Scholar]
- 49. Shears S. B. (2009) Diphosphoinositol polyphosphates: metabolic messengers? Mol. Pharmacol. 76, 236–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Choi K., Mollapour E., Choi J. H., and Shears S. B. (2008) Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Mol. Pharmacol. 74, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szijgyarto Z., Garedew A., Azevedo C., and Saiardi A. (2011) Influence of inositol pyrophosphates on cellular energy dynamics. Science 334, 802–805 [DOI] [PubMed] [Google Scholar]