Abstract
Bupropion metabolites formed via oxidation and reduction exhibit pharmacological activity, but little is known regarding their stereoselective disposition. A novel stereoselective liquid chromatography–tandem mass spectrometry (LC-MS/MS) method was developed to separate and quantify enantiomers of bupropion, 4-hydroxybupropion, and erythro- and threo-dihydrobupropion. Liquid-liquid extraction was implemented to extract all analytes from 50µL human plasma. Acetaminophen (APAP) was used as an internal standard. The analytes were separated on a Lux 3µ Cellulose-3 250×4.6 mm column by methanol: acetonitrile: ammonium bicarbonate: ammonium hydroxide gradient elution and monitored using an ABSciex 5500 QTRAP triple-quadrupole mass spectrometer equipped with electrospray ionization probe in positive mode. Extraction efficiency for all analytes was ≥70%. The stability at a single non-extracted concentration for over 48 hours at ambient temperature resulted in less than 9.8% variability for all analytes. The limit of quantification (LOQ) for enantiomers of bupropion and 4-hydroxybupropion was 0.3ng/mL, while the LOQ for enantiomers of erythro- and threo-hydrobupropion was 0.15ng/mL. The intra-day precision and accuracy estimates for enantiomers of bupropion and its metabolites ranged from 3.4% to 15.4% and from 80.6% to 97.8%, respectively, while the inter-day precision and accuracy ranged from 6.1% to 19.9% and from 88.5% to 99.9%, respectively. The current method was successfully implemented to determine the stereoselective pharmacokinetics of bupropion and its metabolites in a healthy volunteer administered a single 100 mg oral dose of racemic bupropion. This novel, accurate, and precise HPLC-MS/MS method should enhance further research into bupropion stereoselective metabolism and drug interactions.
Keywords: Bupropion, 4-Hydroxybupropion, Threo-dihydrobupropion, Erythro-dihydrobupropion, enantiomers, Stereoselective, HPLC-MS/MS
1. Introduction
Bupropion ([(±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl) amino]-1-propanone], a dual dopamine-norepinephrine uptake inhibitor and a nicotine receptor antagonist, is widely used in the management of depression and as a smoking cessation aid. More recently, bupropion, in combination with naltrexone, was approved by the FDA for the management of weight. It is also being assessed, in clinical trials, as a candidate for the treatment of drug abuse and attention-deficit hyperactivity disorder [1– 3, 21]. Human studies conducted at the end of 1970 and beginning of 1980’s have reported that bupropion undergoes extensive hepatic metabolism, with <1% of the administered dose excreted as unchanged in urine over 48 hours after bupropion dosing, and identified three primary metabolites: 4-hydroxybupropion (a morpholinol) formed via hydroxylation of the t-butyl moiety; and two amino alcohols (erythro- and threo-dihydrobupropion) formed via reduction of the aminoketone group of bupropion [22–27]. These metabolites exhibit pharmacological and toxicological activities in different preclinical depression, behavioral and biochemical models as well as in humans [4–11, 28]. The plasma exposure of 4-hydroxybupropion and threo-dihydrobupropion at steady-state is much higher (~17- and 7-fold respectively) than that of the parent drug bupropion [15, 25, 26, 29]. Thus, it has been suggested that bupropion metabolites contribute significantly to the beneficial and/or adverse effects of bupropion.
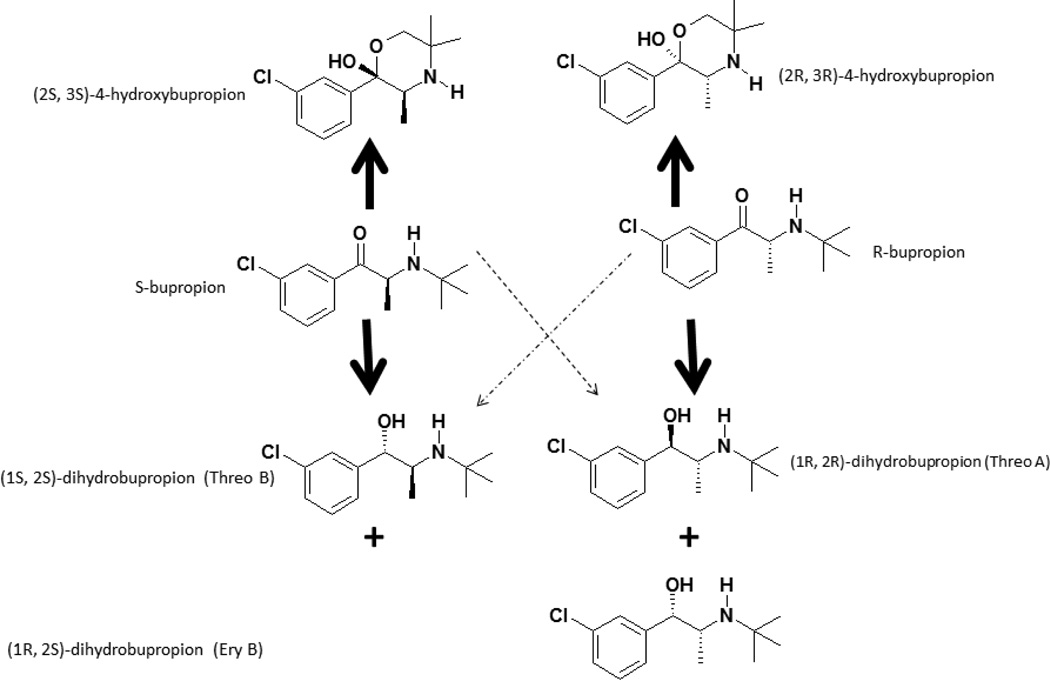
Bupropion 4-hydroxylation is exclusively catalyzed by CYP2B6 [17, 18] and this reaction has been widely used as a marker of CYP2B6 activity in vitro [30–31] and in vivo [13, 15, 29, 32]. In addition, recent studies have focused on whether reduced plasma exposure to 4-hydroxybupropion as a result of CYP2B6 genetic variations or non-genetic factors results in altered clinical outcomes following administration of bupropion [11]. As shown in Figure 1, bupropion has one chiral center and clinically it is administered as racemic mixture. The hydroxylation of bupropion creates an additional chiral center, potentially generating four diastereomers (RR-, SS-, RS- and SR-4-hydroxybupropion), but only (2R,3R)- and (2S,3S)-4-hydroxybupropion have been quantified in human plasma [12–14]. The plasma exposure of (2R,3R)-4-hydroxybupropion is over 20-fold higher than that of (2S,3S)-4-hydroxybupropion [29], but there is evidence to support that most of the pharmacological activities of bupropion reside in (2S,3S)-4-hydroxybupropion [4, 8, 10].
Figure 1.
Proposed metabolic pathway of R-bupropion, S-bupropion. The main pathways are expressed with the bold arrows, while the minor pathways are shown with the dotted arrows.
Despite the identification of threo- and erythro-dihydrobupropion for more than three decades, the disposition and pharmacological effect of these metabolites is less investigated when compared with 4-hydroxybupropion. Reduction of the aminoketone group of bupropion by carbonyl reductases [16, 19] creates an additional chiral center adjacent to that of the bupropion chiral center (Figure 1). 11β-hydroxysteroid dehydrogenase 1 appears to be the main enzyme catalyzing the formation of threo-dihydrobupropion [16], while other carbonyl reductases seem to catalyze the formation of erythro-dihydrobupropion [19]. The steady-state plasma exposure of racemic threo-dihydrobupropion is much higher than that of racemic erythro-dihydrobupropion [15], probably due to enantioselective reduction by microsomal 11β-hydroxysteroid dehydrogenase type 1 [16]. However, threo- and erythro-dihydrobupropion have been reported only as racemic mixtures. It is possible, as with 4-hydroxybupropion, that these metabolites show marked stereoselective disposition and effect, but detailed studies on their stereoselective metabolism and disposition are particularly hampered by the lack of a chiral assay. These metabolites are of interest because: 1) they exhibit pharmacological activity [7] and threo-dihydrobupropion’ s plasma exposure is ~7-fold higher than that of bupropion [15]; and 2) in vitro and in vivo evidence suggests that reduction of bupropion to threo-dihydrobupropion is a major clearance mechanism of bupropion [15].
The aims of the present study were to: a) develop a novel chiral LC-MS/MS assay that allows simultaneous separation and quantification of enantiomers of bupropion, 4-hydroxybupropion, threo- dihydrobupropion, and erythro-dihydrobupropion in human plasma; and b) test the application of this method to enantioselective pharmacokinetics of bupropion and its metabolites in healthy volunteers administered a single 100 mg oral dose of racemic bupropion.
2. Method
2.1. Chemicals and reagents
R-Bupropion (R-BUP) (Lot# 7-DHL-47-1), S-bupropion (S-BUP) (Lot# 7-DHL-52-1), (2R,3R)-4-hydroxy bupropion (R,R-OH BUP) (Lot# 7-DHL-32-6), (2S,3S)-4-hydroxy bupropion (S,S-OH BUP) (Lot# 7-DHL-43-1), rac-erythro-dihydrobupropion (Lot# 1-SHG-40-1), rac-threo-dihydrobupropion (Lot# 1-SHG-39-2) were purchased from Toronto Research Chemicals (Toronto, Ontario). Nomenclature of the enantiomers for erythro- and threo-dihydrobupropion was based on the chromatography of the first eluting peak as “A” and the second eluting peak as “B”. Thus enantiomers of erythro-dihydrobupropion (Ery) were denoted as Ery A and Ery B and enantiomers of threo-dihydrobupropion (Threo) are denoted as Threo A and Threo B. The configurations of these enantiomers are provided in Figure 1, the lack of synthetic standards precludes accurate identification of enantiomers of Ery and Threo. The internal standard acetaminophen (APAP) (Lot# 122K0021), β-nicotinamide adenine dinucleotide 2’-phosphate reduced tetra sodium salt hydrate (NADPH) (HPLC >97%, Lot# SLBL4046V), magnesium chloride (Lot# SLBJ5509V), and ammonium bicarbonate (Bioultra, Lot# BCBL6295V) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO). Ammonium hydroxide (certified ACS, Lot# 131698), methanol (Optima LC/MS, Lot# 153322), acetonitrile (Optima LC/MS, Lot# 134035), sodium phosphate monobasic certified dehydrate (Lot# 007029), and ethyl acetate (HPLC grade, Lot# 153702) were purchased from Fisher Scientific (Fairlawn, NJ). Mixed gender pooled 20-donor human liver microsomes (HLM) S9 fractions (20mg/mL protein concentration) (Lot# 4041007) were purchased from Corning (Woburn, MA). Deionized water was purified using a Barnstead Nanopure Infinity ultrapure water system (Boston, Massachusetts). Plasma from human whole blood (tri-K EDTA, male, drug free, nonsmoker) for standard and quality control preparations was purchased from Biological Specialty Corp. (Colmar, PA)
2.2. Standard Curve and Quality Control Samples
Standard stock solutions (1mg/mL each) of R-bupropion, S-bupropion, (2R,3R)-4-hydroxybupropion, (2S,3S)-4-hydroxybupropion, and acetaminophen (the internal standard), were prepared separately in polypropylene tubes by adding methanol. Erythro- and threo-dihydrobupropion are commercially available only as 50:50 racemic mixtures. Therefore, the adjusted concentration of the stock solution was 0.5 mg/mL each for Ery A, Ery B, Threo A, and Threo B. Separate stock solutions were created for all analytes for quality control (QC) samples. All solutions were stored at −20°C. Working standard solutions were prepped from the standard stock solutions daily by performing 1:10 serial dilutions in methanol into 12×75 polypropylene tubes. An aliquot from the working solutions was added to human plasma (total volume of 200 µL) and 50 µL was transferred to clean 12×75 polypropylene tubes. The standard curves for R-BUP, S-BUP, RR-OH BUP, and SS-OH BUP had the following concentrations: 0.3, 1, 3, 10, 30, 100, and 300 ng/mL. The standard curves for Ery A, Ery B, Threo A, and Threo B had the following concentrations: 0.15, 0.5, 1.5, 5, 15, 50, and 150 ng/mL. Quality control samples were prepared in duplicate. The QC samples had the following concentrations for R-BUP, S-BUP, RR-OH BUP, SS-OH BUP: 1 ng/mL (low QC), 30 ng/mL (medium QC), and 200 ng/mL (high QC). The QC samples had the following concentrations for Ery A, Ery B, Threo A, and Threo B: 0.5 ng/mL (low QC), 15 ng/mL (medium QC), and 100 ng/mL (high QC).
2.3. Sample Preparation
Frozen plasma samples (stored in a −80° freezer) were thawed to ambient temperature and 50 µL were transferred to 12 × 75 polypropylene tubes. Then, 20 µL of 0.1 ng/µL of APAP (internal standard) and 2 mL of ethyl acetate were added to the tube, and the sample was vortex mixed for 30 seconds. After centrifugation at 3000rpm at ambient temperature for three minutes, the upper organic phase was transferred to a clean 12×75 polypropylene tube and evaporated to dryness. A 50 µL volume of methanol was added to each tube and the tube was mixed for 20 seconds on a vortex mixer. A 10 µL aliquot of each sample was injected into the HPLC.
2.4. Conditions for HPLC-MS/MS
Chromatographic separation was achieved using an Agilent 1290 series HPLC coupled with a PAL HTC-XT Leap autosampler using reverse phase chromatography, at 40°C, with a Lux 3µ Cellulose-3 250×4.6 mm chiral column. The buffer for mobile phase consisted of: 5mM ammonium bicarbonate and 0.1% ammonium hydroxide prepared as one solution in HPLC grade water (pH=8.5). Mobile phase A was methanol: acetonitrile: 5mM ammonium bicarbonate plus 0.1% ammonium hydroxide (25:15:60, v/v/v). Mobile phase B was methanol: acetonitrile: 5mM ammonium bicarbonate plus 0.1% ammonium hydroxide (60:30:10, v/v/v). The mobile phase was delivered as a gradient with a constant flow rate of 400 µL/min. The gradient began after 6 minutes at 100% of mobile phase A. From 6–12 min mobile phase A was decreased to 95% in a linear fashion. From 12–30 min mobile phase A was decreased from 95% to 40% in a linear fashion. Next, mobile phase A was decreased from 40% to 0% in a linear fashion from 30–37 min. At 37.1 min the gradient was stepped to 100% A until 40 min, which was the end of the run. The column effluent was monitored using an ABSciex 5500 QTRAP triple-quadrupole mass spectrometer (Foster City, CA) equipped with an electrospray ionization probe in positive mode. The mass spectrometer was controlled by Analyst software (version 1.6.2) in conjunction with Windows 7®. A flow injection analysis was performed on each analyte to maximize sensitivity. The responses of the analytes were optimized at a source temperature of 650° C, under unit resolution for quadrupole 1 and 3. In addition, the analytes were given a dwell time of 200 msec and a settling time of 10 msec. The ion spray voltage was 5500 V and the interface heater was on. Optimal gas pressures for all of the analytes were: collision gas medium, curtain gas 10, ion source gas (1) 25, ion source gas (2) 25. Multiple reaction monitoring was used to measure Q1/Q3 transitions for: R-BUP and S-BUP at 240.1/184.0; R,R-OH BUP and S,S-OH BUP at 255.9/139.0; ERY A, ERY B, Threo A, and Threo B at 241.9/116.0; and APAP at 152.0/109.9. Mass spectrometry settings for the voltages are listed in Table 1.
Table 1.
MS/MS settings (ABSciex 5500) for R-bupropion, S-bupropion, (2R,3R)-4-hydroxybupropion, (2S,3S)-4-hydroxybupropion, erythro-dihydrobupropion A and B, threo-dihydrobupropion A and B, and acetaminophen (internal standard)
| Compound | Q1 (m/z) |
Q3 (m/z) |
Declustering Potential (volts) |
Entrance Potential (volts) |
Collision Energy (volts) |
Exit Potential (volts) |
|---|---|---|---|---|---|---|
| R- and S-bupropion | 240.1 | 184.0 | 66 | 8 | 17 | 10 |
| (2R,3R)-4-hydroxybupropion and (2S,3S)- 4-hydroxybupropion |
255.9 | 139.0 | 66 | 6 | 35 | 10 |
| erythro-dihydrobupropion A and B, and threo-dihydroburpopion A and B |
241.9 | 116.0 | 51 | 10 | 45 | 8 |
| acetaminophen | 152.0 | 109.9 | 86 | 4 | 21 | 4 |
2.5. Method Validation
2.5.1. Intra-day variability
Standards (in singular) and QC samples (in duplicate) were prepared with additional samples at the following concentrations (n=6 for each): the limit of quantification (LOQ) at 0.3 ng/mL for R-BUP, S-BUP, RR-OH BUP, SS-OH BUP and 0.15ng/mL for Ery A, Ery B, Threo A, and Threo B, low QC, medium QC, and high QC concentrations. The mean and standard deviation were calculated for each concentration from the six samples. Precision was assessed using percent coefficient of variation (% C.V.) and accuracy was calculated in term of percent recovery. A single measurement was excluded from the analysis if that measurement was greater than three standard deviations from the mean of the remaining samples per published FDA Guidelines [20].
2.5.2. Inter-day variability
Standards (in singular) and QC samples (in duplicate) at the low QC, medium QC and high QC concentrations were prepared each day for 5 days. The duplicates for each QC sample of each day were averaged. The mean and standard deviation were estimated for each average of the 5 days. Accuracy and precision were assessed from the mean and standard deviation.
2.5.3. Extraction Efficiency
The efficiency of the extraction procedure was calculated by comparing the peak area response of an extracted analyte (in duplicate) from plasma to the peak area response of a non-extracted analyte dissolved in mobile phase.
2.5.4. Stability
Stability of the analytes, at ambient temperature, over 48 hours was assessed. A non-extracted standard was diluted in methanol and injected into the HPLC approximately every 2 hours for over 48 hours. Precision was then assessed. Acceptable precision was less than 10%.
2.5.5. Specificity
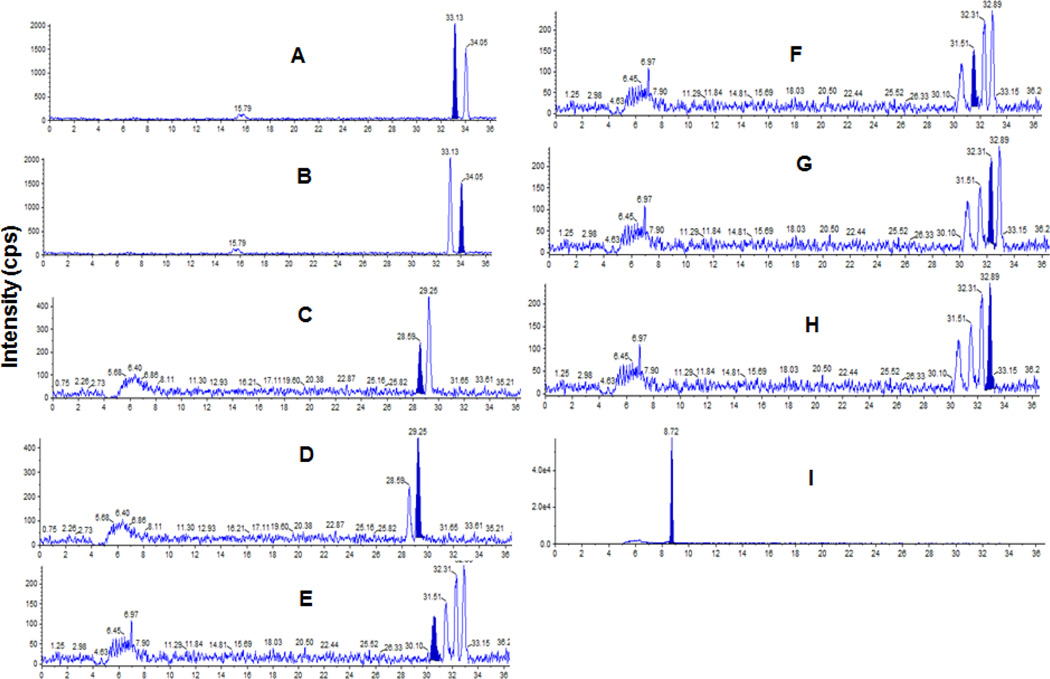
Samples, containing internal standard only, were prepared with five different non-supplemented plasma samples (Lot numbers 81784, 65406, 76480, 38331, 78030) following the sample preparation previously stated. Acceptable specificity occurred when all five samples exhibited an undetectable (below LOQ) concentration of the anaylte.
2.5.6. Identification of enantiomers of bupropion metabolites
Synthetic standards of 4-hydroxybupropion diastereomers (RR-OH BUP, SS-OH BUP) were available commercially and thus positively confirmed (Figure 1). However, no authentic standards of diastereomers of erythro- and threo-hydrobupropion were available. In addition, the formation of erythro- and threo-hydrobupropion metabolites is catalyzed by carbonyl reductases [16, 19], but no information is available regarding their stereoselective formation. To gain preliminary structural insight into the formation of these diastereomers, R- and S-bupropion (10 µM) were incubated with human liver S-9 fraction in the presence and absence of NADPH. The S-9 fraction containing cytosol and microsomes was studied because bupropion undergoes oxidation and reduction in microsomes and reduction in cytosol; thus the cellular fraction was used to capture oxidation to 4-hydroxybuproion and reduction to dihydrobupropion”. R- or S-bupropion (10 µM), in methanol, was added to polypropylene tubes and evaporated to dryness. Then, 1 mg of the S9 mixture were added to the tubes along with incubation buffer (0.1M Na2HPO4 plus 5 mM MgCl2, pH 7.4).The reaction was initiated with 1 mM NADPH and incubated at 37°C for 30 minutes. The reaction was terminated by adding 3mL of ice-cold ethyl acetate. Next, the samples were centrifuged at 3000 rpm for 5 minutes, the organic layer was transferred to clean polypropylene tubes, and evaporated to dryness. The dried samples were reconstituted with 100µL of methanol, and a 10µL aliquot was injected to the 5500 Q-TRAP. Metabolite peaks were detected using the LC-MS/MS method described above.
2.6. Pharmacokinetics studies
2.6.1. Study subjects
Healthy non-smoking male and female volunteers (18 to 49 years old), who were within 32% of their ideal body weight, and who agreed to refrain from taking any prescription medications, over-the counter medications, hormonal agents, and herbal, dietary, and alternative supplements at least 2 weeks prior to the start of the study and during the conduct of the study, were enrolled into a trial at the Indiana University School of Medicine Clinical Research Center. The Indiana University School of Medicine Institutional Review Board (IRB) approved the study protocol and all participants signed an IRB-approved informed consent prior to enrollment. The trial is registered at the ClinicalTrials.gov (Identified # NCT02401256).
2.6.2. Study design
After obtaining pre-dose blood (~10 mL) samples, subjects received a single 100 mg dose of bupropion in a tablet form (Sandoz NDC 0781-1064-01 Lot EM0855) by mouth on empty stomach together with ~250 mL water. A standard meal was served 3 hours after drug dosing. Blood samples (~10 mL) were collected from the intravenous catheter at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 16, 24, 48 and 72 hours after bupropion dosing. Plasma was separated by centrifugation at 3000 rpm for 20 minutes and immediately stored at −80°C until analysis by the LC-MS/MS.
2.6.3. Pharmacokinetic analysis
Plasma pharmacokinetic parameters of bupropion and its metabolites were estimated by standard non-compartmental pharmacokinetic analysis using PK solver software (33).
3. Results and discussion
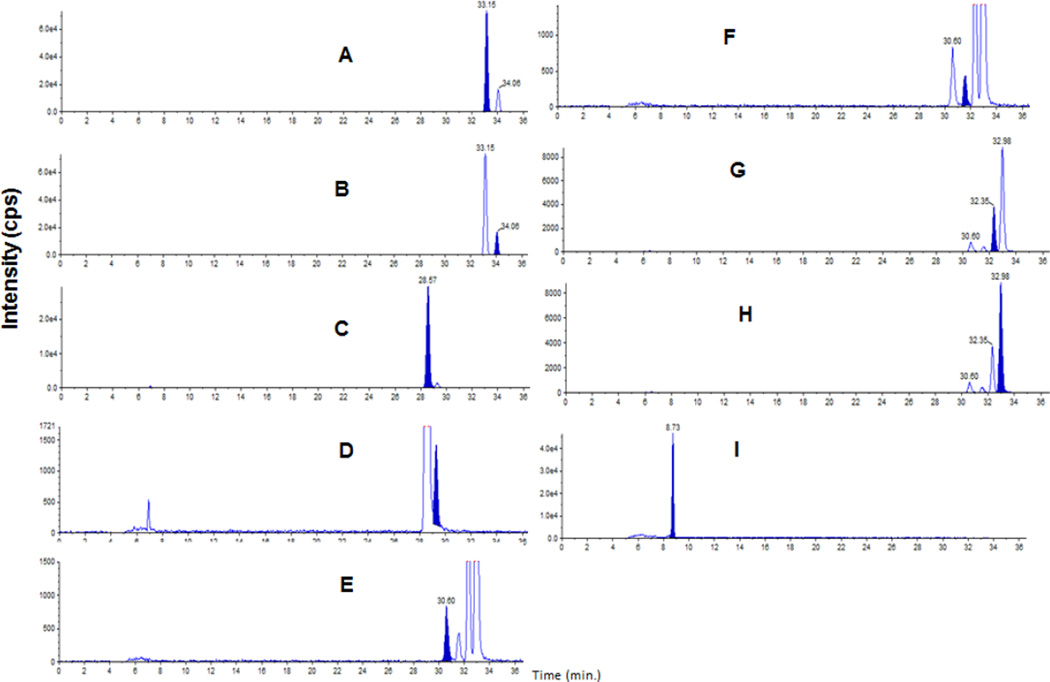
A chromatogram at the LLOQ of R-BUP, S-BUP, RR-OH BUP, SS-OH BUP Ery A, Ery B, Threo A, Threo B, and APAP (internal standard) show successful separation of enantiomers of bupropion and metabolites (Figure 2). Intra-day and inter-day accuracy and precision for BUP and metabolites are listed in Table 2. The intra-day precision ranged from 3.4% to 15.4% and the intra-day accuracy ranged from 80.6% to 97.8%. The inter-day precision ranged from 6.1% to 19.9% and the inter-day accuracy ranged from 88.5% to 99.9%. The extraction efficiency was calculated by comparing peak areas of non-extracted analyte to the peak areas of extracted analyte. The extraction efficiency of all analytes was 70% or greater. The stability at a single non-extracted concentration for over 48 hours at ambient temperature resulted in less than 9.8% variability for all analytes. The method exhibited acceptable specificity because five plasma samples had undetectable (below LOQ) concentrations of all analytes (figures 5–9 in supplementary materials).
Figure 2.
Chromatograms of bupropion and metabolites at the lowest limit of quantification: 0.3ng/mL for R-bupropion (A), S-bupropion (B), (2R,3R)-4-hydroxybupropion (C), (2S,3S)-4-hydroxybupropion (D); and 0.15ng/mL for erythrodihydrobupropion A (E), erythro-dihydrobupropion B (F), threo-dihydrobupropion A(G), threo-dihydrobupropion B (H), and the internal standard acetaminophen (I). The filled peak is the analyte of interest.
Table 2.
Intra-day and Inter-day mean, standard deviation, accuracy and precision for R-bupropion, S-bupropion, 2R,3R-4-hydroxybupropion, 2S,3S-4-hydroxybupropion, erythro-dihydrobupropion A and B, threo-dihydrobupropion A and B.
| R-bupropion and S-bupropion, respectively | ||||||||
| Intra-day | Inter-day | |||||||
|
Theoretical (ng/mL) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
| 0.3 | 0.3, 0.3 | 0.0, 0.0 | 91.6, 91.9 | 12.3, 14.5 | NA | NA | NA | NA |
| 1 | 1.2, 1.2 | 0.1, 0.1 | 80.9, 83.1 | 8.0, 7.4 | 1.0, 1.1 | 0.2, 0.2 | 96.9, 90.0 | 15.7, 16.6 |
| 30 | 33.9, 34.2 | 2.5, 2.8 | 87.1, 86.0 | 7.4, 8.2 | 30.3, 30.1 | 4.7, 4.3 | 98.9, 99.7 | 15.6, 14.4 |
| 200 | 216.2, 215.3 | 10.2, 13.1 | 91.9, 92.3 | 4.7, 6.1 | 211.6, 204.9 | 15.5, 27.9 | 94.2, 97.5 | 7.3, 13.6 |
| 2R,3R-4-hydroxybupropion and 2S,3S-4-hydroxybupropion, respectively | ||||||||
| Intra-day | Inter-day | |||||||
|
Theoretical (ng/mL) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
| 0.3 | 0.3, 0.3 | 0.0, 0.0 | 91.7, 83.6 | 11.5, 4.9 | NA | NA | NA | NA |
| 1 | 1.2, 1.1 | 0.0, 0.1 | 81.0, 86.5 | 4.0, 5.6 | 1.1, 1.0 | 0.2, 0.2 | 93.4, 99.9 | 14.2, 16.6 |
| 30 | 35.6, 35.4 | 1.7, 2.0 | 81.3, 82.0 | 4.8, 5.6 | 29.7, 30.8 | 4.7, 3.9 | 99.0, 97.2 | 15.7, 12.7 |
| 200 | 192.3, 198.5 | 15.4, 13.7 | 96.2, 99.3 | 8.0, 6.9 | 196.0, 205.0 | 23.3, 12.5 | 98.0, 97.5 | 11.9, 6.1 |
| Erythro-dihydrobupropion A and B, respectively | ||||||||
| Intra-day | Inter-day | |||||||
|
Theoretical (ng/mL) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
| 0.15 | 0.14, 0.15 | 0.0, 0.0 | 94.1, 97.8 | 7.6, 14.0 | NA | NA | NA | NA |
| 0.5 | 0.59, 0.58 | 0.0, 0.0 | 81.9, 84.3 | 5.9, 5.6 | 0.5, 0.5 | 0.1, 0.1 | 97.8, 99.4 | 15.0, 11.8 |
| 15 | 17.91, 17.37 | 0.8, 1.1 | 80.6, 84.2 | 4.6, 6.2 | 15.2, 14.5 | 2.0, 2.9 | 98.5, 96.4 | 13.3, 19.9 |
| 100 | 102.43, 111.29 |
13.3, 9.9 | 97.6, 88.7 | 13.0, 8.9 | 95.0, 99.8 | 7.6, 6.1 | 95.0, 99.8 | 8.0, 6.1 |
| Threo-dihydrobupropion A and B, respectively | ||||||||
| Intra-day | Inter-day | |||||||
|
Theoretical (ng/mL) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
Mean (ng/mL) |
s.d. (ng/mL) |
Accuracy (%) |
Precision (% CV) |
| 0.15 | 0.14, 0.18 | 0.0, 0.0 | 90.9, 81.7 | 17.0, 3.7 | NA | NA | NA | NA |
| 0.5 | 0.41, 0.47 | 0.1, 0.1 | 82.3, 93.1 | 12.9, 15.4 | 0.4, 0.5 | 0.1, 0.1 | 88.5, 96.3 | 18.7, 15.6 |
| 15 | 17.72, 17.32 | 0.6, 1.2 | 81.9, 92.3 | 3.4, 6.5 | 14.4, 14.5 | 1.5, 1.9 | 96.0, 96.4 | 10.7, 12.9 |
| 100 | 110.17, 107.72 |
7.5, 7.0 | 89.8, 92.3 | 6.8, 6.5 | 102.7, 105.3 | 9.8, 9.1 | 97.3, 94.7 | 9.6, 8.6 |
The advantages of the current method include simple liquid-liquid extraction, small sample volume, and good chromatographic separation of enantiomers of bupropion, 4-hydroxybupropion, threo-dihydrobupropion, and erythro-dihydrobupropion. Chromatographic separation of the enantiomers was optimized for injection volume, column temperature, mobile phase, and buffers, as slight deviations from these conditions resulted in co-elution of the enantiomers. In addition, the HPLC column was washed for 1 hour with methanol: water; (90:10 v/v), then equilibrated with mobile phase A, overnight at a flow rate of 400µL/min. The main disadvantages of this method are the long run time and a short column life span. The HPLC column was changed after approximately 500 sample injections because of loss of baseline resolution of analytes.
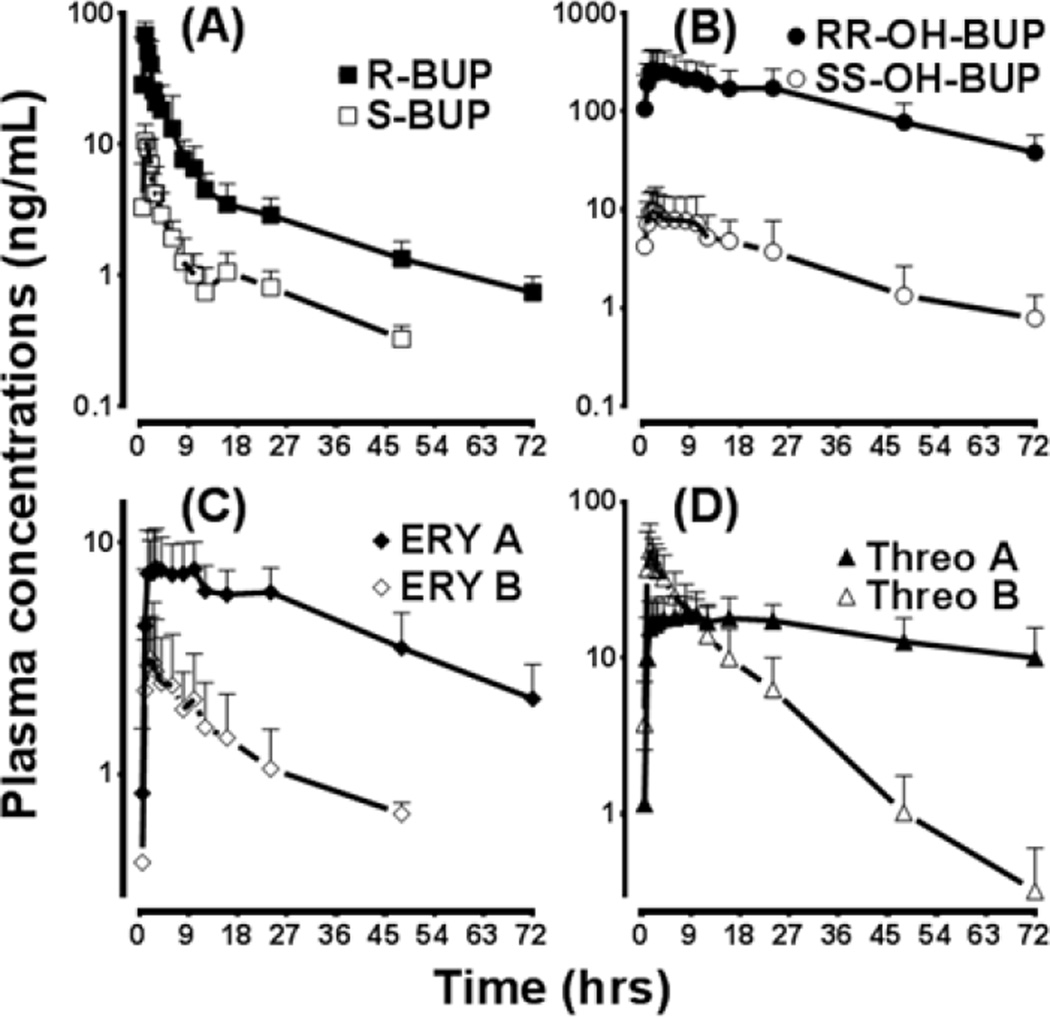
The current method was successfully implemented to determine the stereoselective pharmacokinetics of bupropion and its metabolites in a subject administered a single 100 mg oral dose of racemic bupropion. A chromatogram from a subject sample 1.5 hours after drug administration is shown in Figure 3. The pharmacokinetic profiles of BUP and metabolites of this subject are depicted in Figure 4, and the pharmacokinetic parameters are listed in Table 3. These pharmacokinetic data show highly stereoselective disposition of bupropion and its metabolites. While the patterns of stereoselectivity were reported for bupropion and 4-hydroxybupropion previously [12, 13, 14, 29], ours is the first to demonstrate: chromatographic separation of erythro- and threo-dihydrobupropion enantiomers; and demonstrate unique stereoselective disposition of erythro- and threo-dihydrobupropion in humans. The marked differences in the elimination half-life of threo-dihydrobupropion and to extent erythro-dihydrobupropion suggest differential accumulation of these metabolites during chronic dosing with racemic bupropion. For example, Ery A and Threo A, which show relatively longer half-lives, are expected to accumulate during multiple dosing compared to Ery B and Threo B.
Figure 3.
Chromatogram of a subject sample after 1.5 hours of a single 100 mg oral dose of racemic of bupropion. The filled chromatogram peak shows R-bupropion (A), S-bupropion (B), 2R,3R-4-hydroxybupropion (C), 2S,3S-4-hydroxybupropion (D), erythro-dihydrobupropion A (E), erythro-dihydrobupropion B (F), threo-dihydrobupropion A(G), threo-dihydrobupropion B (H) and acetaminophen (I).
Figure 4.
Plasma concentration vs. time plot of enantiomers of bupropion and diastreomers of its metabolites in healthy volunteers (N=3; 1 white male, 1 Asian female, and 1 white female) administered a single 100 mg oral dose of racemic bupropion.
Table 3.
Pharmacokinetic parameters of bupropion and metabolites in healthy volunteers (n=3; 1 white male, 1 Asian female and 1 white female) administered single 100 mg oral dose of racemic bupropion.
| t1/2 | tmax | Cmax | AUC0–∞ | Cl/F | |
|---|---|---|---|---|---|
| (hr) | (hr) | (ng × mL−1) | (ng × mL−1 × hr) | (L × hr−1) | |
| R-bupropion | 17.6±5.9 | 1.0 | 68.5±16.5 | 322.9±114.0 | 169.0±61.2 |
| S-bupropion | 13.9±9.2 | 1.5 | 11.6±2.3 | 56.0±26.3 | 1112±702 |
| (2R,3R)-4-hydroxybupropion | 23.3±1.9 | 2.0 | 276.0±157 | 10148±5408 | - |
| (2S,3S)-4-hydroxybupropion | 15.7±2.2 | 2.0 | 11.5±6.7 | 233±194 | - |
| Erythro-dihydrobupropion A | 31.7±5.4 | 4 | 8.7±3.0 | 433±157 | - |
| Erythro-dihydrobupropion B | 22.4±4.6 | 2.0 | 3.8±2.0 | 75.4±35.1 | - |
| Threo-dihydrobupropion A | 59.9±25.9 | 6 | 20.6±4.9 | 2038±1323 | - |
| Threo-dihydrobupropion B | 9.8±2.7 | 1.5 | 55.1±237.2 | 124.8±80.6 | - |
The metabolism of R- and S-bupropion in human liver S9-fractions and NADPH is shown in Table 4. As expected, R- and S-bupropion were metabolized predominantly to RR-OH BUP and SS-OH BUP, respectively, and served as a positive control. In addition, our data show that Ery A and Threo A are mainly formed from R-bupropion, while Ery B and Threo B are mainly formed from S-bupropion. However, our data also suggest potential chiral inversion of the substrates or the metabolites during the incubation as there was no 100 percent selectivity, even though “pure” enantiomers were used during the incubation (see the ratios in Table 4). When R- and S-bupropion were incubated with human liver S9-fractions without NADPH, no RR-OH BUP or SS-OH BUP metabolite peak was detected and this finding was expected because CYP-mediated reactions do not occur in the absence of a co-factor (data not shown). On the other hand, the reductive metabolites of bupropion were generated in similar patterns as described above for S9-fraction + NADPH. Based on these preliminary in vitro data and the possible structural configuration, we propose that R-bupropion was reduced mainly to (1S, 2R)-dihydrobupropion (Ery A) and (1R, 2R)-dihydrobupropion (Threo A), while S-bupropion was reduced mainly to (1R, 2S)-dihydrobupropion (Ery B) and (1S, 2S)-dihydrobupropion (Threo B) (Figure 1).
Table 4.
The metabolism of R- and S-bupropion with human S9 fraction.
| Peak Area (counts) | ||||||
|---|---|---|---|---|---|---|
| R,R OH BUP |
S,S OH BUP |
Ery A | Ery B | Threo A |
Threo B | |
| R-BUP + S9 | 26800 | 12900 | 6440 | 3090 | 43900 | 211000 |
| S-BUP + S9 | 4370 | 41300 | 351 | 15000 | 3800 | 562000 |
|
Metabolic ratio (R- BUP/S-BUP) |
6.1 | 0.3 | 18.3 | 0.21 | 11.6 | 0.38 |
In summary, a novel reversed phase chiral-HPLC-MS/MS assay method involving a simple liquid-liquid extraction procedure and a small plasma sample volume (50µL) was developed that allowed simultaneous separation and quantification of enantiomers of bupropion, 4-hydroxybupropion, and, for the first time, those of threo- and erythro-dihydrobupropion in human plasma. This method was successfully implemented to determine the unique stereoselective pharmacokinetics of bupropion and its metabolites in one research human volunteer administered a single 100 mg oral dose of racemic bupropion. To our knowledge, this is the first evidence showing stereoselective reduction of bupropion in vitro and in vivo. Although needs further confirmation, we also provide plausible structural features of threo- and Erythro-dihydrobupropion (Figure 1). This new quantification method should now enhance further research into bupropion stereoselective metabolism, drug interactions, and effect.
Supplementary Material
Highlights.
Analytical methods for the separation and quantification of enantiomers of bupropion and 4-hydroxybupropion are very limited. Despite the identification of threo- and erythro-dihydrobupropion for more than three decades, information on their stereoselective disposition is nonexistent.
A novel, accurate and precise HPLC-MS/MS method was developed to quantify enantiomers of bupropion and 4-hydroxybupropion and for the first time of threo- and erythro-dihydrobupropion in human plasma samples.
This method was successfully implemented to determine the unique stereoselective pharmacokinetics of bupropion and its metabolites in human.
This new quantification method should enhance further research into bupropion stereoselective metabolism.
Acknowledgments
The project described here was in part supported by an RO1 grant (Award Number R01GM078501) from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, MD). Analytical work was performed by the Clinical Pharmacology Analytical Core laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center supported by the National Cancer Institute grant P30 CA082709.
Abbreviations
- R-BUP
R-Bupropion
- S-BUP
S-Bupropion
- R,R-OH BUP
(2R,3R)-4hydroxy bupropion
- S,S-OH BUP
(2S,3S)-4hydroxy bupropion
- Ery A and Ery B
rac-erythro-dihydrobupropion, nomenclature A and B is for the chromatographic first eluting peak as “A” and the second eluting peak as “B”
- Threo A and Threo B
rac-threo-dihydrobupropion, nomenclature A and B is for the chromatographic first eluting peak as “A” and the second eluting peak as “B”
- PK
pharmacokinetics
- QC
quality control
- OH
hydroxyl
- Bup
bupropion
- T1/2
terminal elimination half-life
- Tmax
time to maximum concentration (Cmax)
- Auc
area under the plasma concentration versus time up to infinity (∞)
- Cl/F
apparent oral clearance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhillon Sohita, Yang Lily PH, Curran Monique P. Bupropion: A Review of its Use in the Management of Major Depressive Disorder. Drugs. 2008;68(5):653–689. doi: 10.2165/00003495-200868050-00011. [DOI] [PubMed] [Google Scholar]
- 3.Dwoskin Linda P, Rauhut Anthony S, King-Pospisil Kelley A, Bardo Michael T. Review of the Pharmacology and Clinical Profile of Bupropion, an Antidepressant and Tobacco Use Cessation Agent. CNS Drug Reviews. 12(3–4):178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003 Aug 1;474(1):85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 5.Perumal AS, Smith TM, Suckow RF, Cooper TB. Effect of plasma from patients containing bupropion and its metabolites on the uptake of norepinephrine. Neuropharmacology. 1986 Feb;25(2):199–202. doi: 10.1016/0028-3908(86)90041-9. [DOI] [PubMed] [Google Scholar]
- 6.Golden RN, De Vane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ. Bupropion in depression. II. The role of metabolites in clinical outcome. Arch Gen Psychiatry. 1988 Feb;45(2):145–149. doi: 10.1001/archpsyc.1988.01800260055007. [DOI] [PubMed] [Google Scholar]
- 7.Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990 Jul;23(4):187–194. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- 8.Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004 Sep;66(3):675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 9.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995 Sep;56(9):395–401. [PubMed] [Google Scholar]
- 10.Hansard MJ, Jackson MJ, Smith LA, Rose S, Jenner P. A major metabolite of bupropion reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmosets. Behav Pharmacol. 2011 Jun;22(3):269–274. doi: 10.1097/FBP.0b013e328345ca37. [DOI] [PubMed] [Google Scholar]
- 11.Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. CYP2B6 and bupropion's smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther. 2012 Dec;92(6):771–777. doi: 10.1038/clpt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suckow RF1, Zhang MF, Cooper TB. Enantiomeric determination of the phenylmorpholinol metabolite of bupropion in human plasma using coupled achiralchiral liquid chromatography. Biomed Chromatogr. 1997 May-Jun;11(3):174–179. doi: 10.1002/(SICI)1099-0801(199705)11:3<174::AID-BMC681>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Loboz KK, Gross AS, McLachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007 Mar;19(3):163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 14.Coles R, Kharasch ED. Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Sep 15;857(1):67–75. doi: 10.1016/j.jchromb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., 3rd Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics. 2013 Mar;23(3):135–141. doi: 10.1097/FPC.0b013e32835d9ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Vuorinen A, Zielinska AE, Strajhar P, Lavery GG, Schuster D, Odermatt A. Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1. Drug Metab Dispos. 2013 Sep;41(9):1671–1678. doi: 10.1124/dmd.113.052936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000 Oct;28(10):1222–1230. [PubMed] [Google Scholar]
- 18.Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- 19.Skarydova L, Tomanova R, Havlikova L, Stambergova H, Solich P, Wsol V. Deeper insight into the reducing biotransformation of bupropion in the human liver. Drug Metab. Pharmacokinet. 2014;29(2):177–184. doi: 10.2133/dmpk.dmpk-13-rg-051. [DOI] [PubMed] [Google Scholar]
- 20.FDA. Guidance for Industry - Analytical Procedures and Methods Validation for Drugs and Biologics. 2014 [Google Scholar]
- 21.Aubin HJ, Luquiens A, Berlin I. Pharmacotherapy for smoking cessation: pharmacological principles and clinical practice. Br J Clin Pharmacol. 2014 Feb;77(2):324–336. doi: 10.1111/bcp.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983 May;44(5 Pt 2):79–81. Schroeder. [PubMed] [Google Scholar]
- 23.Schroeder DH. The isolation and identification of some basic urinary metabolites of bupropion—HCL in man. The Pharmacologist. 1979;21(3):191. [Google Scholar]
- 24.Lai AA, Schroeder DH. Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry. 1983 May;44(5 Pt 2):82–84. [PubMed] [Google Scholar]
- 25.Posner J, Bye A, Dean K, Peck AW, Whiteman PD. The disposition of bupropion and its metabolites in healthy male volunteers after single and multiple doses. Eur J Clin Pharmacol. 1985;29(1):97–103. doi: 10.1007/BF00547376. [DOI] [PubMed] [Google Scholar]
- 26.Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther. 1985 Nov;38(5):586–589. doi: 10.1038/clpt.1985.228. [DOI] [PubMed] [Google Scholar]
- 27.Findlay JW, Van Wyck Fleet J, Smith PG, Butz RF, Hinton ML, Blum MR, Schroeder DH. Pharmacokinetics of bupropion, a novel antidepressant agent, following oral administration to healthy subjects. Eur J Clin Pharmacol. 1981;21(2):127–135. doi: 10.1007/BF00637513. [DOI] [PubMed] [Google Scholar]
- 28.Silverstone PH, Williams R, McMahon L, Fleming R, Fogarty S. Convulsive liability of bupropion hydrochloride metabolites in Swiss albino mice. Ann Gen Psychiatry. 2008 Oct 15;7:19. doi: 10.1186/1744-859X-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008 Apr;48(4):464–474. doi: 10.1177/0091270008314254. [DOI] [PubMed] [Google Scholar]
- 30.Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- 31.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, Flockhart DA, Zanger UM. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–558. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- 32.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005 Jun;77(6):553–559. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.