Abstract
The atypical 7-transmebrane chemokine receptor, CXCR7 transactivates the epidermal growth factor receptor (EGFR) leading to increased tumor growth in several tumor types. However, the molecular mechanism of CXCR7-ligand independent EGFR-trans-activation is unknown. We used cDNA knock-in, RNA interference (RNAi) and analysis of mitogenic signaling components in both normal prostate epithelial cells and prostate cancer cells to decipher the proliferation inducing mechanism of the CXCR7-EGFR interaction. The data demonstrate that CXCR7-induced EGFR transactivation is independent of both the release of cryptic EGFR ligands (e.g., AREG/amphiregulin) and G-protein coupled receptor (GPCR) signaling. An alternate signaling mechanism involving β-arrestin-2 (ARRB2/β-AR2) was examined by manipulating the levels of β-AR2 and analyzing changes in LNCaP cell growth and phosphorylation of EGFR, ERK1/2, Src and Akt. Depletion of β-AR2 in LNCaP cells increased proliferation/colony formation and significantly increased activation of Src, phosphorylation of EGFR at Tyr-1110 and phosphorylation/activation of ERK1/2 compared to that with control shRNA. Moreover, β-AR2 depletion downregulated the proliferation suppressor, p21. Stimulation of β-AR2 expressing cells with EGF resulted in rapid nuclear translocation of phosphorylated/activated EGFR. Downregulation of β-AR2 enhanced this nuclear translocation. These results demonstrate that β-AR2 is a negative regulator of CXCR7/Src/EGFR-mediated mitogenic signaling.
Implications
This study reveals that β-AR2 functions as a tumor suppressor, underscoring its clinical importance in regulating CXCR7/EGFR-mediated tumor cell proliferation.
Keywords: CXCR7, EGFR, β-Arrestin-2, Src, Prostate Cancer Proliferation
Introduction
Chemokine receptors are members of the seven- transmembrane guanine nucleotide-binding protein (G protein)-coupled receptor (GPCR) superfamily. The intracellular signaling of chemokine receptors is mainly dependent on binding of heterotrimeric G-proteins on intracellular loop epitopes and the carboxyl-terminal tail of these receptors(1). The CXC-chemokine receptor 7 (CXCR7) is a unique receptor that can be engaged by the chemokines CXCL11 and CXCL12/SDF-1(2). CXCR7 is overexpressed in many cancer cell types and elevated expression of CXCR7 promotes tumor cell proliferation and tumor growth (3–6). Unlike other classical chemokine receptors, CXCR7 does not mobilize intracellular calcium, does not elicit motility in normal cells and preferentially signals through the β-arrestin-2 pathway upon binding to its ligands (6, 7).
Arrestins belong to a family of proteins that consists of four members. Visual (arrestin 1) and cone arrestins (arrestin 4) are exclusively expressed in the retina, while β-arrestin-1 (arrestin 2) and β-arrestin-2 (arrestin 3) are ubiquitously expressed in most tissues (8, 9). β-arrestins are well known negative regulators of GPCR signaling. β-arrestin binding to GPCRs both uncouples receptors from heterotrimeric G proteins and targets them to clathrin coated pits for endocytosis. Recent evidence however demonstrates that β-arrestins can function as adaptor molecules that mediate G-protein independent signaling by serving as scaffolds that link signaling networks and regulate signaling molecules such as the mitogen activated protein kinases ERK, JNK, and p38 as well as Akt and Phospho-inositide 3-kinase (PI3K)(10). Importantly, while β-arrestin-1 was shown to mediate metastatic growth in breast cancer cells (11), the role of β-arrestin-2 (β-AR2) in tumor progression is more enigmatic and needs further clarification. Some reports suggest that β-AR2 may induce cancer cell progression (12), however, there is also evidence that depletion of β-AR2 promotes tumor growth in a murine model of lung cancer, indicating that β-AR2 might function as a tumor suppressor (13).
We reported previously that CXCR7 increases cell proliferation independent of its ligands (CXC11 and CXCL12/SDF-1α). Further, alteration in CXCR7 expression levels directly affects phosphorylation of EGFR at Tyrosine1110. In addition, we have reported direct coupling of EGFR and CXCR7 in PCa and breast cancer cells both in vitro and in tumors in vivo(6, 14). Although CXCR7 is an atypical GPCR, the mechanism of transactivation of EGFR by unstimulated-CXCR7 is unknown. In the present work we aimed to evaluate the role of β-AR2 in CXCR7 mediated EGFR transactivation in PCa cells. We also elucidated the signaling pathways that are regulated by β-AR2, including Src activation and downstream mitogenic signaling (e.g., ERK1/2 and the PI3K/Akt) pathway, and the role of β-AR2 in regulating prostate tumor cell proliferation.
MATERIALS AND METHODS
Materials
Smart-Pool short interfering RNA (siRNA) for various cellular targets including β-AR2 and non-target control siRNA (C-siRNA), and Dharmafect-2 transfection reagent were purchased from Dharmacon Inc. (Dharmacon-GE Life Sciences, Lafayette, CO). Recombinant chemokines and growth factors (Human SDF-1α, CXCL11, IL-8, EGF and amphiregulin) were purchased from R&D Systems (Minneapolis, MN). Full-length CXCR7 cDNA was obtained from Origene and was cloned in Ampicillin vectors for stable transfection in RWPE-1 cells. CXCR7-GFP and β-AR2 expression vectors were a gift from Dr. Kathleen Luker, University of Michigan. HuSH /shRNA Plasmid Panels for transient or stable down regulation of β-AR2 (GI326261 (1), GI326262 (2), (GI326263 (3), GI326264 (4)) and scrambled shRNA (TR30013 (13)) were purchased from Origene (Rockville, MD).
Cell Culture and Transfection
Nonmalignant prostate epithelial cells (RWPE-1) and PCa cell lines (LNCaP, C4-2B and PC-3) were obtained from American Type Culture Collection (Rockville, MD) and were used within 10 passages of cell authenticity confirmation by genetic profiling (Genetica DNA Laboratory Inc., Cincinnati, OH). Fetal bovine serum (FBS) was from Atlanta Biologicals (Atlanta, GA), antibiotics (gentamycin) and cell culture media were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO). LNCaP, C4-2B and PC-3 cells were grown in RPMI 1640 containing 10% FBS. RWPE-1 cells were grown in keratinocyte serum-free medium (SFM) supplemented with bovine pituitary extract and EGF (Invitrogen). For stable transfections, RWPE-1 cells were grown to 50–60% confluence and transfected with 0.6–1.0 μg of DNA using Lipofectamine 2000 (Life Technologies). Stable clones (RWCX7) were selected and maintained in G418 (250 μg /ml). Lipofectamine 2000 was also used for co-transient transfection of RWCX7 cells with the β-AR2 cDNA constructs. For stable down regulation of β-AR2, LNCaP cells were seeded in 12-well plates (2 × 105 cells/well) in the absence of antibiotics. 24h later, cells were transfected with HuSH/shRNA Plasmid Panels (500 ng/well for LNCaP) using Lipofectamine 2000 (2.125 μl/well for LNCaP). Stable clones were created through puromycin selection (2 μg/mL).
Western Blotting/Immunobloting
Forty-eight hours after transfection, cells were serum starved for 16h and then stimulated with EGF (10 ng/mL, 2 or 5 min). Cell lysates were subjected to western blot analysis as previously described (6). Please see Supplementary Table 1 (Table S1) for details of primary antibodies, sources and dilutions used. In parallel experiments, cells were cultured in growth factor depleted (1% FBS) medium for 24 h, pretreated with Pertussis toxin, Amphiregulin or neu-ab (Amphiregulin neutralizing Ab) at concentrations and times indicated in the figure legends, followed by stimulation with EGF (10 ng/mL, 2 or 5 min). Pretreatment with PP2 or AG148 was used to block the activation of endogenous or stimulated EGFR as indicated in figures legends.
Co-Immunoprecipitation
Co-Immunoprecipitation assays were performed in LNCaP cells to visualize HSP90 association with CXCR7. Cells were lysed following incubation for 72 h and cell lysates were cleared through incubation with Protein A-agarose beads. Lysates were incubated with anti-HSP90 antibody overnight followed by incubation with Protein A-agarose beads (Sigma-Aldrich) and immunoprecipitated CXCR7 was detected by western blot analysis. Whole cell lysates were used as input controls. Anti-EGFR antibodies were used as positive controls for immunoprecipitation of CXCR7 (6).
Immunofluorescence
We evaluated CXCR7 and HSP90 co-localization in PCa cells by immunofluorescence staining. PC-3 cells were seeded and cultured in multi-chamber slides (Thermo Fisher Scientific Inc., Waltham, MA) until they were semi-confluent. Cells were fixed in situ with 3% paraformaldehyde and permeabilized with 0.25% Triton X-100 in PBS. Cells were incubated with anti-CXCR7 and anti-HSP90 primary antibodies and, following washing with PBS, incubated with fluorophore (Alexa Fluor 488 or Alexa Fluor 555) labeled secondary antibodies (Thermo Fisher). Nuclei were stained with DAPI. Labeled cells were observed under a confocal microscope (Zeiss LSM 700) and images were analyzed by Zeiss imaging software.
Proximity ligations assay/In-cell co-immunoprecipitation Assay
The Proximity Ligations assay (PLA) was performed in order to visualize and quantify HSP90/CXCR7 co-localization. PLA was performed using a kit and the contained instructions (Duolink In Situ, Olink Bioscience-Sigma) as described previously(14). Combination of a primary rabbit anti-CXCR7 antibody and a nonspecific mouse isotype antibody was used as a negative control. Combination of a primary rabbit anti-CXCR7 antibody and a primary mouse anti-CXCR4 antibody were used as a positive controls.
Chemotactic motility assay
Chemotactic motility of LNCaP cells and the sublines created in this study was evaluated using 8 μm-pore size Boyden chambers (Transwell plates, Corning/Costar Inc., Corning, NY)(15). Cells were harvested, re-suspended in culture medium containing 1% FBS and deposited (2 × 105 cells/filter) in the top wells of the Transwell plates. The bottom well contained 10% FBS as a chemoattractant (500 μl/well). Percent of cells in each treatment group that migrated through the filter in 48 hours was determined by MTT assay as described (15, 16). Migratory/chemotactic activity (% Migration) was defined as the ratio of optical density (OD) from the bottom wells (including the cells from the bottom side of the filters) to that of the total OD (OD of bottom plus top wells), multiplied by 100. Assays were repeated at least 3 times.
Multisite-directed mutagenesis for generation of mutant EGFR
EGFR-wt (without stop codon) was blunt-end inserted into the HindIII site of the pLPCX Vector (Clontech). The MYC epitope tag (with stop codon) was blunt-end cloned into the EcoRI site. The primers for the mutagenesis assays were designed using QuikChange Primer Design software (Stratagene) to introduce a nucleotide substitution within the EGFR hybridizing sequence to generate a Tyrosine to Phenylalanine substitution at position 1110 (Y1110 to F1110). Mutagenesis reaction was carried out using the Stratagene Multisite-Directed Mutagenesis kit (Agilent Technologies). DNA from five transformed bacterial colonies was isolated using Wizard Plus SV DNA Miniprep kit (Promega) and sequenced to verify the presence of the designed mutation. The plasmid thus generated was transfected into LNCaP cells and stable lines were selected with puromycin, as described above. Selected lines were tested for pEGFRY1110 by anti-pEGFRY1110 Rabbit monoclonal IgG.
Statistical analysis
All quantitative data shown, except the western blot quantification, were from three separate experiments; each data point represents the mean of the triplicate results. Western blots have been conducted twice. Significance of data was analyzed with the Prism graph pad software (GraphPad Inc., San Diego, CA).
RESULTS
G-protein signaling is not involved in CXCR7 mediated EGFR phosphorylation
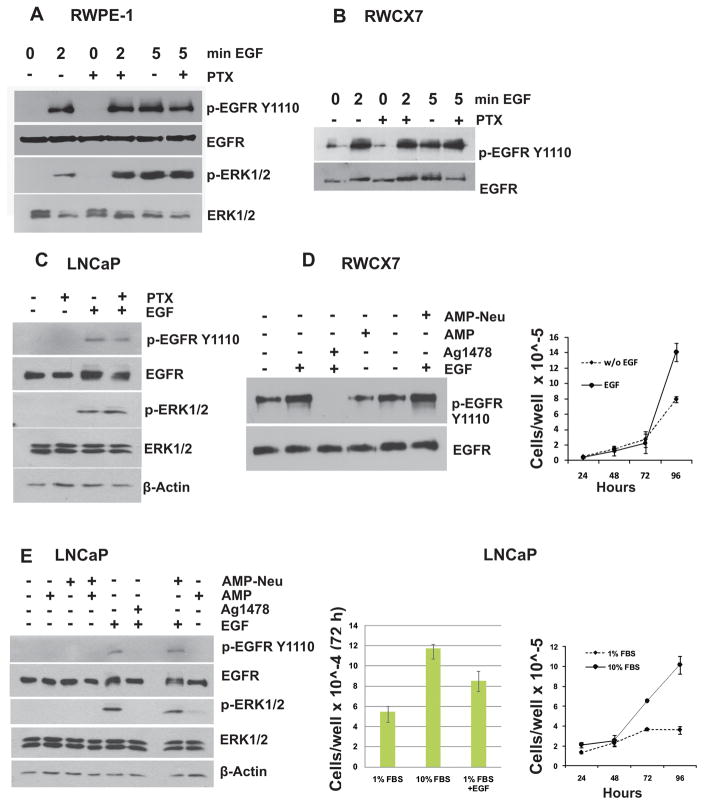
Previous studies have demonstrated the inability of CXCR7 to generate typical G protein responses such as an increase in intracellular calcium after stimulation with either SDF-1α or CXCL11 (3, 17–19). We attempted to investigate the involvement of G protein, if any, in the CXCR7 mediated increase in EGF induced EGFR phosphorylation at EGFRY1110. We treated non-tumorigenic prostate epithelial cells, RWPE-1 and CXCR7 over expressing RWPE-1 (RWCX7) cells for 2h with pertussis toxin (PTX, 0.5 μg/ml), a known inhibitor of GPCR-mediated G-protein coupling. We stimulated EGFR phosphorylation with 10 nM EGF for 2 to 5 min. We did not observe any detectable change in EGFR phosphorylation in PTX treated RWPE-1 or RWCX7 cells compared to that of cells treated with EGF alone (Fig 1A–B). Similar results were observed in LNCaP cells (Fig 1C). This observation suggested that G-protein signaling does not affect CXCR7 mediated EGFR phosphorylation.
Figure 1. CXCR7 mediated EGFR transactivation is independent of Gαi/o and EGF-like ligands.
Growth factor starved RWPE-1 (A), RWCX7 (B) or LNCaP cells (C) were pretreated with Pertussis toxin (B. pertussis, PTX, 0.5 μg/ml) for 2 hr at 37 °C or left untreated and then incubated in presence or absence of EGF (10 ng/ml) for 2 or 5 min at 37 °C. Total cell lysates were collected and subjected to Western blot analysis using antibodies to phosphorylated and total EGFR and ERK1/2. D, E: RWCX7 cells (RWPE-1 cells stably expressing CXCR7) (D) or LNCaP cells (E) were growth factor starved for 16 hr and then pre-incubated with Amphiregulin (Amp) for 4 hr followed by incubation in presence or absence of EGF (10 ng/ml, 5 min). Cell lysates were analyzed by western blotting using anti-phospho-EGFR and anti-ERK1/2 antibodies. Growth curves for starved and non-starved cells are shown in the right panels.
CXCR7 mediated phosphorylation of EGFR is not associated with EGF-like ligands
Several previous studies have shown a role of EGF-like ligands such as amphiregulin (Amp) and heparin-bound-EGF (HB-EGF) in chemokine receptor (e.g., CXCR4) induced EGFR trans-activation (20, 21). These cryptic EGF-like compounds sequestered in the extracellular matrix (ECM) are unmasked and released as active EGF by CXC-chemokine mediated extracellular proteolysis by Matrix Metallopeptidase 9 (MMP9). We hypothesized such a scenario with CXCR7 enhanced phosphorylation of EGFR. RWCX7 or LNCaP cells were treated with recombinant Amp, EGF, or both and phosphorylation of EGFR was analyzed by western blotting. EGF stimulation resulted in rapid phosphorylation of EGFR (pEGFRY1110) which was almost completely blocked by the EGFR kinase inhibitor, tyrphostin (AG1478) (Fig. 1D, E). In contrast, stimulation with Amp did not affect the pEGFRY1110 level in RWCX7 cells. Similarly, treatment of RWCX7 cells with neutralizing anti-Amp-antibody (Amp-Neu) prior to stimulation with EGF did not affect pEGFRY1110 levels, indicating Amp does not contribute to increased pEGFRY1110 in RWCX7 and LNCaP cells.
We also observed increased activation of the mitogenic signaling pathway (phosphorylation of ERK1/2) upon treatment with EGF which is consistent with increased cell proliferation relative to growth factor starved cells (Fig. 1D, E).
β-Arrestin-2 is a negative regulator of EGFR phosphorylation/transactivation
We have previously shown that CXCR7 mediates EGFR phosphorylation/transactivation in a CXCR7-ligand independent fashion(6, 14). Rajagopal et al.,(7) observed recruitment of β-AR2 by CXCR7 in human embryonic kidney-29 (HEK29) cells that are overexpressing CXCR7. This resulted in phosphorylation of ERK1/2. Attempts by us to stimulate LNCaP or RWPE-1 cells with SDF1α did not result in redistribution of β-AR2 or increase ERK1/2 phosphorylation (data not shown). However, stimulation with EGF alone, results in an increased pEGFRY1110/Total EGFR ratio in RWCX7 cells when compared to vector-only transfected-RWPE-1 cells (Fig. 1A, B). In addition, stimulation of LNCaP cells over-expressing β-AR2-EGFP with SDF1α did not alter the distribution of β-AR2-EGFP. Stimulating these cells with EGF triggered redistribution of β-AR2-EGFP into the cytoplasm and nucleus, indicating dynamic β-AR2-EGFP relocalization following EGFR phosphorylation (Supplementary Fig. 3).
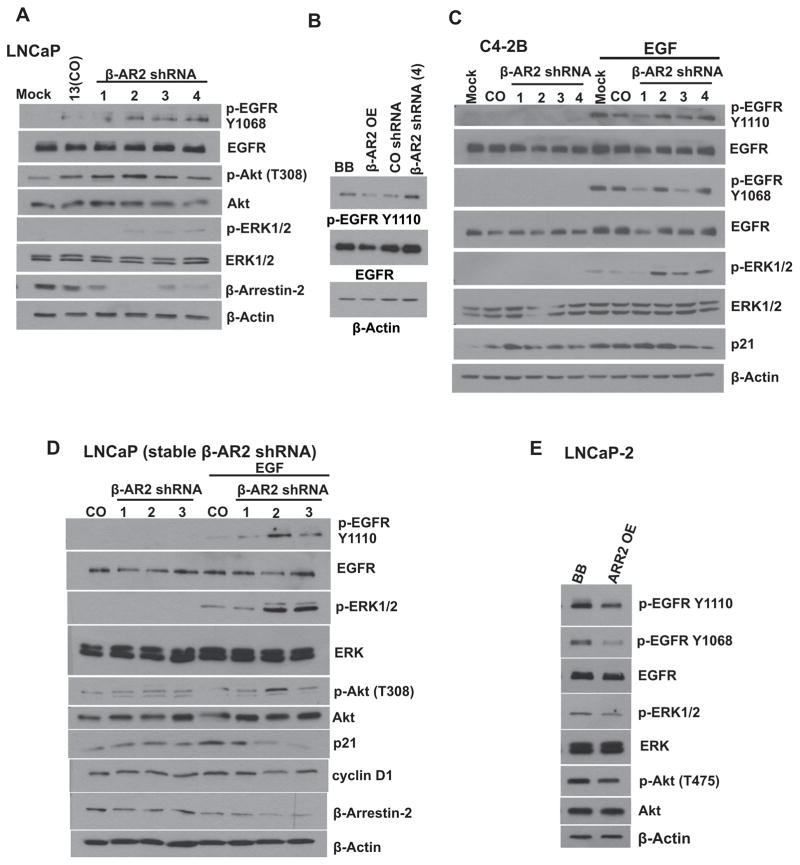
We next attempted to dissect the role of β-AR2 in CXCR7-induced EGFR trans-activation when CXCR7 is signaling independently of its ligands. First, we depleted β-AR2 in LNCaP cells through transient transfection with β-AR2 shRNA (Construct 1, 2, 3 or 4) and evaluated the phosphorylation status of EGFR by western blot analysis following stimulation with EGF. Furthermore, we examined the effects of shRNA mediated β-AR2 down regulation on ERK1/2 activation and the PI3K/Akt pathway. In parallel experiments we overexpressed β-AR2 in LNCaP cells to evaluate its effect on EGFR phosphorylation. The shRNA mediated β-AR2 depletion resulted in an increase of EGFR phosphorylation at the tyrosine1068 and 1110 (Y1068 and Y1110) residues. Transient depletion of β-AR2 in LNCaP cells increased the levels of phosphorylated ERK1/2 and Akt (Figure 2A). These results were also confirmed in C4-2B cells, a castration-resistant LNCaP subline (Figure 2C). In Contrast, overexpression of β-AR2 resulted in a decrease of phosphorylated EGFR in LNCaP cells (Figure 2B). Taken together, our results show the phosphorylation status of EGFR inversely correlates with the expression of β-AR2 in prostate cancer cells.
Figure 2. Down regulation of β-arrestin-2 leads to increased mitogenic signaling.
A: Specific shRNAs (1, 2, 3, 4) were used to transiently downregulate β-AR2 in LNCaP cells. Culture medium was replaced 48 hr following transfection and cells were then serum-starved (1% FBS) for additional 16 hr. Cell lysates were collected following stimulation with EGF (10 ng/ml, 5 min) and were subjected to western blot analysis for detection of pEGFR, EGFR, pAkt, Akt, pERK1/2, ERK1/2 and β-AR2. β-Actin served as a loading control. B: β-AR2 was overexpressed or downregulated in LNCaP cells. Cell lysates were collected following stimulation with EGF (10 ng/ml, 5 min) and were subjected to western blot analysis for detection of pEGFR and EGFR. β-Actin served as a loading control. C: Specific shRNAs (1, 2, 3, 4) were used to transiently downregulate β-AR2 in C4-2B cells. Experiment was performed as described in A. D: β-AR2 specific shRNAs (1, 2, 3) were introduced in LNCaP cells via transfection and cells were cultured under puromycin selection pressure to create stable cell lines. E: Following generation of stable cells, β-AR2 was overexpressed in the stable LNCaP-2 β-AR2 shRNA cell line to see whether the effects of β-AR2 depletion can be rescued. Cell lysates were collected following stimulation with EGF (10 ng/ml, 5 min) and were subjected to western blot analysis for detection of pEGFR, EGFR, pAkt, Akt, pERK1/2, ERK1/2, p21, CyclinD1 and β-AR2. β-Actin served as a loading control. 13= stable control-shRNA LNCaP cells; 1, 2, 3= stable β-AR2-shRNA LNCaP cells; BB= Plasmid backbone, OE= Over expression.
We next generated LNCaP cells with stably downregulated β-AR2 (β-AR2shRNA clones 1, 2, 3) and evaluated the effects on phosphorylation of EGFR, ERK1/2, and Akt, as well as the expression of the cell cycle regulator p21. Stable downregulation of β-AR2 (LNCaP- 1, 2, 3) resulted in an increase of phosphorylated EGFR, Akt and ERK1/2 in EGF stimulated cells, whereas the level of p21 was dramatically decreased (Figure 2D). Importantly, when β-AR2 was transiently overexpressed in the stable β-AR2 shRNA expressing clone, LNCaP-2, the above described effects were reversed (Figure 2E).
Src mediates CXCR7 induced EGFR transactivation
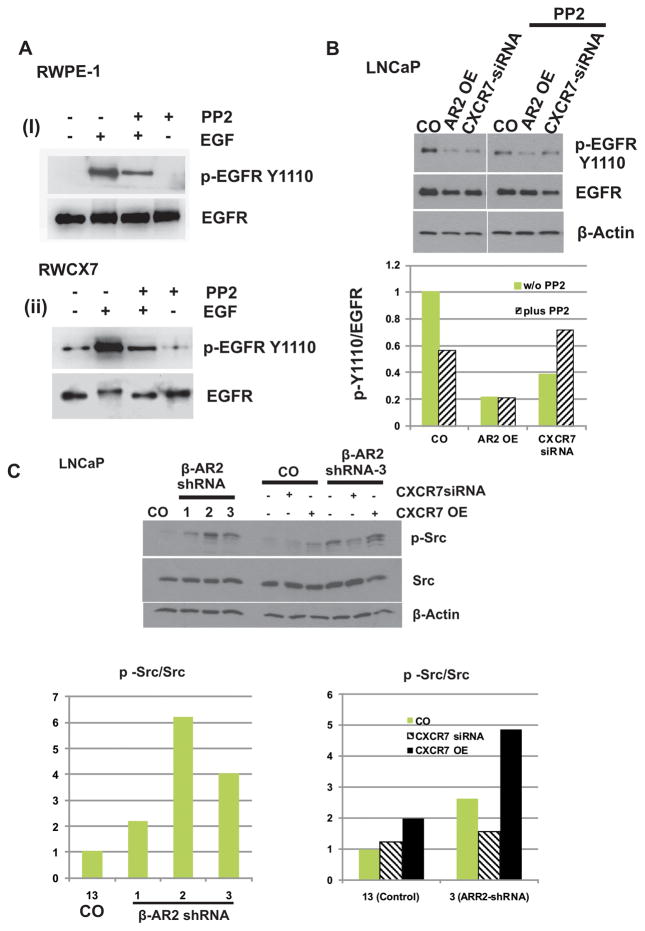
The inability of externally added CXCR7 cognate ligands to enhance EGFR phosphorylation, and the absence of activation of other cryptic EGFR ligands in CXCR7 over-expressing cells, made us inquire about the role of Src-kinase in CXCR7-mediated- EGFR activation. RWPE-1 and RWCX7 cells were pre-treated with the Src kinase inhibitor PP2 with or without EGF and the relative increase in p-EGFR was determined by western blot analysis. In RWPE-1 cells (Fig. 3A(i)), constitutive p-EGFR was undetectable even when we loaded twice the amount of total cell lysates as that of RWCX7 cells (Fig. 3A(ii), lane 1). Following addition of EGF, we observed a rapid increase in p-EGFR in both the cell lines; this increase was significantly inhibited in the presence of PP2. PP2 alone was also able to inhibit basal level of pEGFRY1110 in RWCX7 cells. In a parallel approach we investigated the effect of PP2 on EGFR phosphorylation in LNCaP cells (Fig 3B). Pretreatment with PP2 remarkably down regulated EGF mediated EGFRY1110 phosphorylation. These results indicate that Src is upstream of EGFR in the CXCR7-EGFR axis, and is required for EGFR phosphorylation at Y1110. Furthermore, in the absence of PP2, overexpression of β-AR2, similar to the down regulation of CXCR7, resulted in a prominent decrease of pEGFRY1110. Addition of PP2 to these cells did not further reduce the level of pEGFRY1110.
Figure 3. Src-kinase is associated with CXCR7-mediated EGFR transactivation.
A : Growth factor starved RWPE-1 and RWCX7 cells were incubated with the Src inhibitor PP2 or vehicle control (ethanol) followed by stimulation with EGF (10 ng/ml, 5 min). Cell lysates were subjected to Western blot analysis using anti-pEGFR and anti-ERK1/2 antibodies. B: β-AR2 was overexpressed or CXCR7 was downregulated in LNCaP cells. Then, cells were incubated with the Src inhibitor PP2 or vehicle control (ethanol) and subjected to western blot analysis following stimulation with EGF (10 ng/ml, 5 min). OE= Over expression. C: LNCaP cells with stable β-AR2 down regulation (1, 2, and 3) and control (13, Control-shRNA) were subjected to western blot analysis for evaluation of Src phosphorylation/activation. In parallel experiments, CXCR7 was downregulated with siRNA or overexpressed (right panel).
The results presented above suggest that β-AR2 is a negative regulator of Src. Therefore, we evaluated the activation of Src in LNCaP cells with stable β-AR2 depletion (Figure 3C). Consistently, depletion of β-AR2 resulted in an induction of phosphorylated/activated Src. Moreover, overexpression of CXCR7, further induced Src activation (Figure 3C, right panel). Taken together, these data demonstrate that a Src-kinase mediated mechanism is responsible for CXCR7-induced EGFR phosphorylation at Y1110 and that this mechanism is negatively regulated by β-AR2.
β-Arrestin-2 regulates nuclear translocation of EGFR in prostate cancer cells
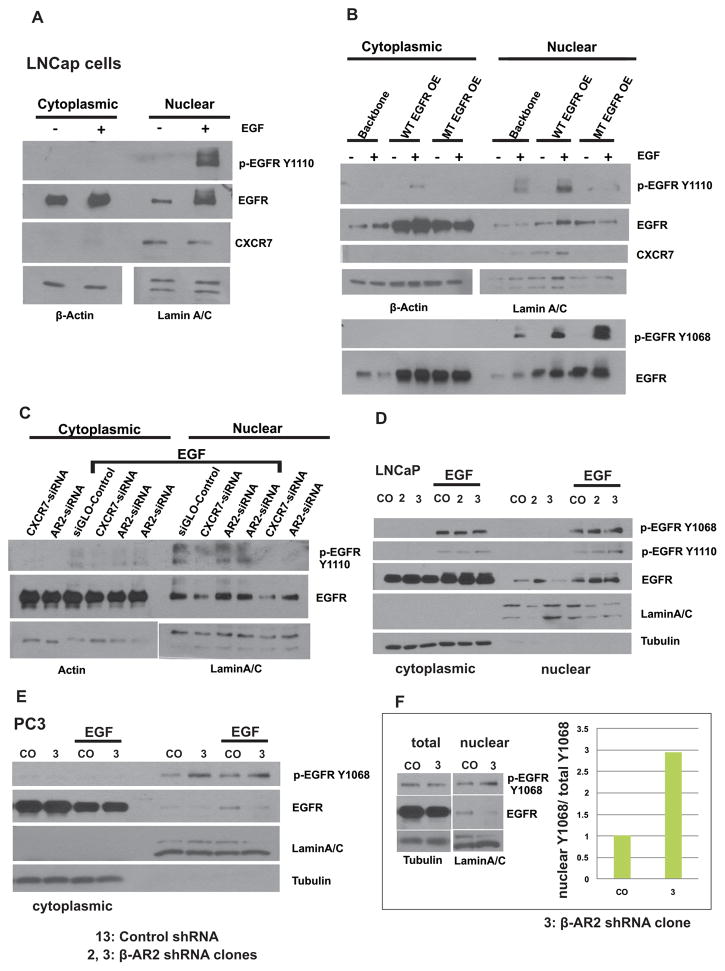
Accumulating evidence suggests that there is a nuclear translocation of EGFR in cancer cells(22–26). In the nucleus, EGFR may drive expression of genes required for proliferation, such as Cyclin D1 and Myc (24). We aimed to evaluate the regulatory roles of both CXCR7 and β-AR2 in the nuclear translocation of EGFR in PCa cells. To first elucidate how phosphorylation regulates the translocation of EGFR to the nucleus, LNCaP cells were transfected with either wild type (WT) EGFR, mutant (MT) EGFR (with a non-phosphorylatable amino acid substitution: tyrosyl (Y) to phenylalanine (F) (Y1110 to F1110), or backbone plasmid. Cells were stimulated with EGF and nuclear and cytoplasmic protein fractions were collected and subjected to western blot analysis for expression of pEGFR. Stimulation of LNCaP cells with EGF resulted in a rapid nuclear translocation of pEGFR (Figure 4A). Translocation of pEGFR to the nucleus occurred independent of phosphorylation at Y1110 in LNCaP cells as shown by strongly increased pEGFRY1068 in the nuclear fraction of both EGF stimulated WT-EGFR and MT-EGFR transfected LNCaP cells (Figure 4B). Down regulation of CXCR7 significantly inhibited nuclear translocation of EGFR (Figure 4C). Furthermore, we evaluated nuclear and cytoplasmic extracts in LNCaP cells with stable β-AR2 depletion (LNCaP-2 and LNCaP-3) compared to control shRNA transfected LNCaP (LNCaP-13). LNCaP-3 cells show a higher level of nuclear pEGFRY1110 compared to LNCaP-13 cells (CO) (Figure 4D). Similar results were observed for PC-3 cells (Fig. 4E). In summary, depletion of β-AR2 via shRNA results in an increase of nuclear phosphorylated EGFR, suggesting that β-AR2 negatively regulates nuclear translocation of EGFR and nuclear-EGFR signaling.
Figure 4. Downregulation of β-arrestin-2 interferes with nuclear translocation of phosphorylated EGFR.
A, B: Untransfected LNCaP cells (A) or LNCaP transfected with Backbone Plasmid, wild type EGFR (WT EGFR OE), or mutant EGFR (MT EGFR OE, Y1110F) (B) were serum starved for 16 hr, then nuclear and cytoplasmic extracts were collected following stimulation with EGF (10 ng/mL, 5 min) and subjected to Western blot analysis for detection of phospho-EGFR Y1110. C: LNCaP cells were transfected with specific siRNA against β-AR2 or CXCR7. Culture medium was replaced 48 hr following transfection and cells were then starved (1% FBS) for additional 16 hr. Nuclear and cytoplasmic extracts were collected following stimulation with EGF (10 ng/ml, 5 min) and were subjected to western blot analysis. β-Actin and Lamin A/C served as a loading controls for cytoplasmic and nuclear extracts, respectively. D: β-AR2 specific shRNAs (2, 3) were introduced in LNCaP cells via transfection and cells were cultured under puromycin selection pressure to create stable cell lines (LNCaP-2, LNCaP-3). Nuclear and cytoplasmic extracts were collected following stimulation with EGF (10 ng/ml, 5 min) and were subjected to western blot analysis for detection of pEGFR. α-Tubulin and Lamin A/C served as a loading controls for cytoplasmic and nuclear extracts, respectively. E: β-AR2 specific shRNA (3) was introduced in PC3 cells via transfection and nuclear and cytoplasmic extracts were subjected to western blot analysis as described above. F: Quantification of nuclear pEGFR in 10 ng/ml EGF treated PC3 cells relative to total cell lysate.
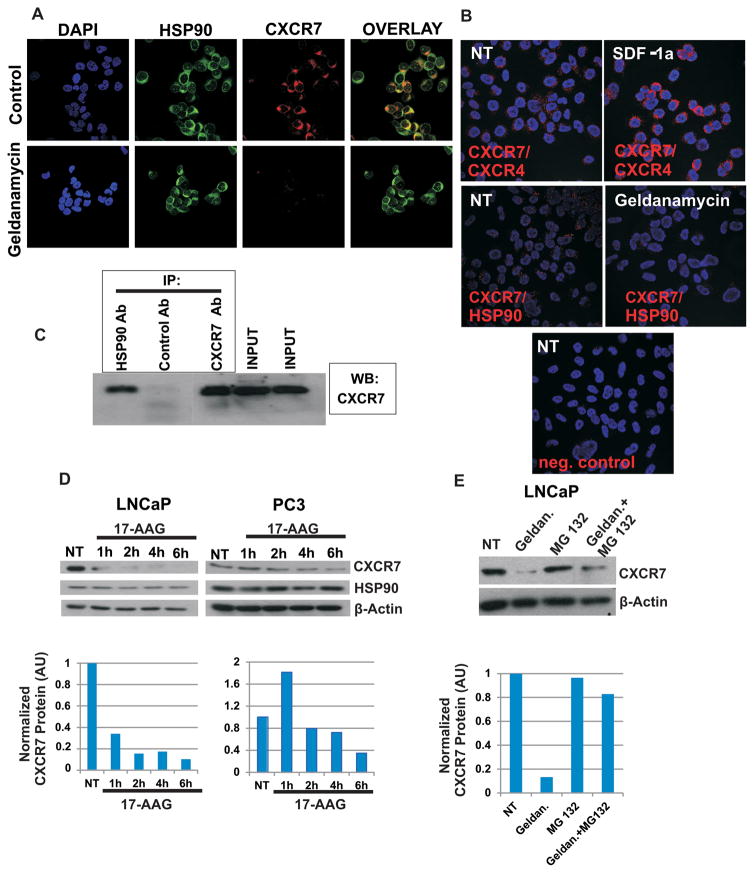
Hsp90 interacts with the CXCR7/EGFR/Arrestin axis and protects it from proteasome-mediated degradation
Hsp90 is a molecular chaperone that is constitutively expressed in eukaryotic cells and can be further stimulated in its expression level by physiological stress. It participates in stabilizing over 200 proteins, many of which are essential for constitutive cell signaling (and adaptive responses to stress). As such, we were interested in evaluating whether Hsp90 stabilizes CXCR7.
Immunofluorescence staining and proximity ligation assay (Duolink) were performed in order to evaluate co-localization of CXCR7 and Hsp90 in LNCaP cells. Co-Immunoprecipitation assays were performed in LNCaP cells for examination of the Hsp90-CXCR7 interaction. Our results show that Hsp90 associates with CXCR7 in LNCaP and PC-3 cells (Figure 5A, B, C). Hsp90 and CXCR7 show a predominantly cytoplasmic and peri-nuclear co-localization (Figure 5A, B). Treating cells with inhibitors of Hsp90 (e.g., Geldanamycin/17-AAG) resulted in decreased CXCR7/Hsp90 colocalization.
Figure 5. Hsp90 interacts with CXCR7/EGFR/β-Arrestin-2 axis and protects it from proteasome mediated degradation.
A: PC-3 cells were seeded in 8 well chamber slides (20,000 cells per well). Forty-eight hours later, cells were treated with Hsp90 inhibitor Geldanamycin (3 μM, 2 hr) or were left untreated. Cells were fixed with 3% paraformaldehyde, permeabilized with 0.25% Triton X-100 and subjected to double immunofluorescence staining. Expression of Hsp90 was detected by mouse anti-Hsp90 antibody (Enzo Life Sciences, ADI-SPA-830) and Alexa 488 conjugated goat anti-mouse IgG (green). Expression of CXCR7 was analyzed using rabbit anti-CXCR7 antibody (Genetex, GTX100027) and visualized by Alexa 555 conjugated goat anti-rabbit IgG (red). B: PC-3 cells were seeded in 8 well chamber slides (20,000 cells per well). Seventy-two hours later cells were treated with Hsp90 inhibitor Geldanamycin (3 μM, 2 hr) or were left untreated. Cells were fixed with 3% paraformaldehyde and proximity ligation assay (DUOLINK) was performed to detect CXCR7/Hsp90 and CXCR7/CXCR4 co-localization. Mouse anti-Hsp90 antibody (Enzo Life Sciences, ADI-SPA-830) and rabbit anti-CXCR7 antibody (Genetex, GTX100027) were used for detection of Hsp90/CXCR7 co-localization. NT= non-treated cells. C: Co-Immunoprecipitation of CXCR7 and Hsp90: Cell lysates of LNCaP were immunoprecipitated with an anti-Hsp90 IgG followed by western blotting against CXCR7. D: Prostate cancer cells were seeded in 6 well plates. Twenty-four hours later cells were treated with 17-AAG (2 μM) for 1–6 hr, or were left untreated (NT). Protein extracts were collected and subjected to western blotting. Actin served as a loading control. E: LNCaP cells were seeded in 6 well plates. Twenty-four hours later, cells were treated with Geldanamycin (3 μM, 2 hr), proteasome inhibitor MG132 (10 μM, 2 hr) or were left untreated (NT). Protein extracts were subjected to western blotting. Actin served as a loading control. AU: Arbitrary Units
Furthermore, we performed Western blot analysis of nuclear, cytoplasmic and total protein extracts in the presence or absence of Geldanamycin/17-AAG to evaluate the role of Hsp90 in the stabilization of CXCR7. Kinetic analysis showed that treatment with Geldanamycin/17-AAG resulted in a decrease of CXCR7 total protein level (Figure 5D and Additional file 2A).
Moreover, we show that in the presence of the proteasome inhibitor MG132, Geldanamycin/17-AAG mediated CXCR7 degradation was rescued in LNCaP and PC-3 cells, suggesting Hsp90 interacts with CXCR7 and protects it from proteasome-mediated degradation (Figure 5E and Supplemental Fig. 2B). Furthermore, treatment with Geldanamycin resulted in down regulation of β-AR2 and EGFR (Supplemental Figure 2C, D).
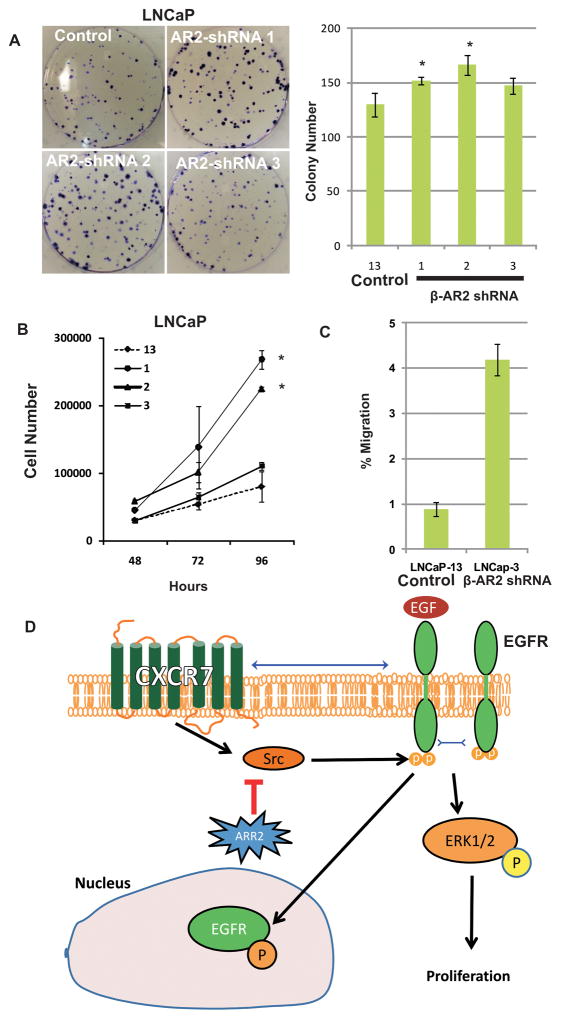
β-Arrestin-2 negatively regulates proliferation and migration of prostate cancer cells
Since our data suggest activation mitogenic signaling (e.g. activation of Akt and ERK1/2) in cells with stable shRNA-mediated β-AR2 depletion, we aimed to evaluate proliferation in those cell lines compared to control shRNA transfected cells. We performed colony assays to investigate the replicative potential of β-AR2-shRNA LNCaP compared to control-shRNA LNCaP cells. All three β-AR2 shRNA LNCaP cell lines (LNCaP-1, -2 and -3) showed increased proliferation and colony formation potential compared to control cells (LNCaP-13) (Figure 6A, B). Importantly, LNCaP-3 cells had significantly increased motility compared to LNCaP-13 (Figure 6C). A model is presented (Fig 6D) to simplify the complex signaling mechanism involved in CXCR7, EGFR, HSP90 and β-AR2 mediated regulation of PCa cell proliferation.
Figure 6. LNCaP cells with stable β-arrestin-2 downregulation show increased proliferation and clonogenic potential.
Control shRNA (13) or β-AR2 specific shRNAs (1, 2, 3) were introduced in LNCaP cells via transfection and cells were cultured under puromycin selection pressure to create stable cell lines. A: Stable control shRNA (13) or β-AR2 specific shRNA (1, 2, 3) expressing LNCaP cell lines were seeded at low density in 6-well plates and colonies were stained and counted 12 days later. B: In parallel experiments, stable cell lines were seeded in 12-plates and cell numbers were counted daily for generation of growth curves. C: Chemotactic motility of LNCaP-3 vs LNCaP-13 cells. Asterisks indicate statistical significance compared to control (13) (p<0.05).
D: A suggested pathway diagram of β-AR2 regulation of proliferation: β-AR2 inhibits CXCR7 mediated transactivation of EGFR which occurs through activation/phosphorylation of Src, and thus inhibits downstream ERK mediated signaling and proliferation and nuclear translocation of phosphorylated EGFR.
DISCUSSION
The data presented above demonstrate the unique mechanism of CXCR7 and β-AR2 mediated growth signaling in PCa cells. A novel finding in this study is the regulation of Src-mediated phosphorylation of tyrosyl1110 in EGFR and its effects on mitogenic signaling. Although Src-kinase phosphorylates EGFR at tyrosyl1173, its ability to phosphorylate EGFRY1110 is seldom reported. Another interesting finding in this study is the tumor suppressor function of β-AR2 in PCa that was not reported before. Our findings strongly support the hypothesis that β-AR2 negatively regulates tumor cell proliferation and, more importantly, their chemotactic motility. LNCaP cells have poor motility relative to more aggressive PCa tumor cells, such as PC-3 which express a lower level of β-AR2 (unpublished).
Previously, we reported that the chemokine receptor CXCR7 mediates EGFR phosphorylation/activation in a CXCR7 ligand-independent fashion and plays a crucial role in proliferation of prostate and breast cancer cells (6, 14). In the present work we show for the first time, that β-AR2 negatively regulates CXCR7 mediated phosphorylation/activation of EGFR in PCa cells. Depletion of β-AR2 in LNCaP cells resulted in a significant increase of phosphorylated/activated Src, EGFR and ERK1/2 and a dramatic decrease of p21 protein level along with an induction of proliferation and clonogenic potential. Although CXCR7 heterodimerizes with a related GPCR, CXCR4, our data show that the two molecules independently interact with other molecules in the cell, such as EGFR and Hsp90. CXCR7 associates with HSP90 as shown by the PLA to demonstrate colocalization.
Since previous work has shown that GPCR-mediated activation of EGFR may require activation of MMPs and subsequent cleavage of membrane bound RTK/EGFR ligands (20), we investigated this pathway in prostate cancer cells. Interestingly, phosphorylation of EGFR in PCa cells seems to be independent of ECM bound EGFR ligands. In a further attempt we investigated whether the above mentioned pathways can be bypassed by GPCR-induced Src activation of EGFR. Indeed, treatment of LNCaP cells with the Src inhibitor PP2 resulted in a significant decrease of pEGFRY1110 compared to that of untreated cells. Similar effects were observed when CXCR7 was depleted via siRNA; however, combined treatment with CXCR7 siRNA and PP2 did not further decrease levels of pEGFRY1110 compared to cells treated with CXCR7 siRNA or PP2 alone. These results strongly suggest that Src plays a key role in CXCR7-mediated phosphorylation/activation of EGFR and downstream proliferation signaling.
Since CXCR7 is shown to signal through β-AR2 in its ligand-dependent manner (7), we investigated the role of β-AR2 in CXCR7-ligand independent transactivation of EGFR. Surprisingly, down regulation of β-AR2 resulted in a significant increase of pSrcY416 and pEGFR as well as activation of mitogenic signaling (ERK, Akt), and an increase in proliferation and clonogenic potential. Consistently, β-AR2 overexpression resulted in a decrease of pEGFR and cell proliferation. These results demonstrate that β-AR2 is a negative regulator of CXCR7-mediated EGFR activation and subsequent cell proliferation. It is to be noted that β-arrestin has two isoforms (β-arrestin-1 and β-AR2). While β-arrestin-1 promotes GPCR mediated Src activation and Erk1/2 phosphorylation, the opposing effects have been observed for β-AR2 in HEK 293 cells (27). Our results in prostate cancer cells show a similar mechanism of β-AR2.
A novel finding in our work is that depletion of β-AR2 resulted in an increase of nuclear EGFR. Multisite-directed mutagenesis experiments indicated that this nuclear translocation is independent of EGFR phosphorylation at Y1110. Nuclear EGFR is associated with disease progression and increased resistance to chemotherapy and anti-EGFR therapies (25). Our results show that β-AR2 negatively regulates the nuclear translocation of EGFR, underlining the potential role of β-AR2 as a tumor suppressor and its potential as a therapeutic target.
Conclusions
In conclusion, we show that CXCR7 mediates EGFR transactivation through activation of Src, and that this pathway is negatively regulated by β-AR2 which inhibits activation of Src in prostate cancer cells (Fig. 6D). Moreover, we show that heat shock protein Hsp90 stabilizes the CXCR7/EGFR/β-AR2 axis in prostate cancer cells and protects it from proteasome mediated degradation. Although β-arrestins have been known to mediate chemokine receptor induced mitogenic signaling in cancer cells, the present work demonstrates that β-AR2 functions as a tumor suppressor and negatively regulates mitogenic signaling/proliferation and migration in prostate cancer cells and thus has relevant clinical significance.
Supplementary Material
Acknowledgments
Financial support: This work was supported in parts by the Veterans Health Administration Merit Award: BX 001517-01 (BLL); the United States’ Public Health Services awards: NIH-1R01CA156776-01 (BLL); and F31 Fellowship (NS).
The authors are grateful to Mr. Gabriel Gaidosh for confocal image acquisition.
Abbreviations
- Amp
Amphiregulin
- CXCR
CXC-chemokine receptor
- EGFR
Epidermal growth factor receptor
- CXCL
CXC-chemokine receptor ligand
- SDF-1α
Stromal derived factor alpha
- EGF
Epidermal growth factor
- PLA
Proximity ligation assay
- β-AR2
Beta-arrestin-2
- PP2
Src-Kinase Inhibitor
Footnotes
Some aspects of this work was conducted while the authors were at Department of Urology University of Miami Miller School of Medicine, Miami FL 33101 and Research Service Laboratory, Miami VA Medical Center Miami FL 33136.
Authors’ contributions
All authors participated in the design and execution of this study. Specifically, GK and RKS participated in experimental design, carried out experiments, analyzed data, and wrote the manuscript; DM and JJH carried out western blotting and migration experiments and critically reviewed the manuscript. RKS participated in initial experimental design, carried out experiments in figure 1 and 3A, and helped write the manuscript. NS carried out migration experiments. BLL conceived and supervised the study, analyzed data and finalized the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Salazar N, Castellan M, Shirodkar SS, Lokeshwar BL. Chemokines and chemokine receptors as promoters of prostate cancer growth and progression. Critical reviews in eukaryotic gene expression. 2013;23(1):77–91. doi: 10.1615/critreveukaryotgeneexpr.2013006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Martin L, Sanchez-Mateos P, Cabanas C. CXCR7 impact on CXCL12 biology and disease. Trends in molecular medicine. 2013;19(1):12–22. doi: 10.1016/j.molmed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. The Journal of experimental medicine. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. The Journal of biological chemistry. 2008;283(7):4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 5.Hattermann K, Held-Feindt J, Lucius R, Muerkoster SS, Penfold ME, Schall TJ, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer research. 2010;70(8):3299–308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 6.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer research. 2011;71(9):3268–77. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(2):628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. Journal of cell science. 2007;120(Pt 2):213–8. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan FG, DuBois RN. Emerging roles of beta-arrestins. Cell cycle. 2006;5(18):2060–3. doi: 10.4161/cc.5.18.3212. [DOI] [PubMed] [Google Scholar]
- 10.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annual review of physiology. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy SK, Han S, Zhao YL, Hara MR, Oliver T, Cao Y, et al. beta-arrestin1 mediates metastatic growth of breast cancer cells by facilitating HIF-1-dependent VEGF expression. Oncogene. 2012;31(3):282–92. doi: 10.1038/onc.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, He X, Tan J, Zhou X, Zou L. beta-arrestin2 mediates beta-2 adrenergic receptor signaling inducing prostate cancer cell progression. Oncology reports. 2011;26(6):1471–7. doi: 10.3892/or.2011.1417. [DOI] [PubMed] [Google Scholar]
- 13.Raghuwanshi SK, Nasser MW, Chen X, Strieter RM, Richardson RM. Depletion of beta-arrestin-2 promotes tumor growth and angiogenesis in a murine model of lung cancer. Journal of immunology. 2008;180(8):5699–706. doi: 10.4049/jimmunol.180.8.5699. [DOI] [PubMed] [Google Scholar]
- 14.Salazar N, Munoz D, Kallifatidis G, Singh RK, Jorda M, Lokeshwar BL. The chemokine receptor CXCR7 interacts with EGFR to promote breast cancer cell proliferation. Molecular cancer. 2014;13:198. doi: 10.1186/1476-4598-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokeshwar BL, Selzer MG, Zhu BQ, Block NL, Golub LM. Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. International journal of cancer Journal international du cancer. 2002;98(2):297–309. doi: 10.1002/ijc.10168. [DOI] [PubMed] [Google Scholar]
- 16.Lokeshwar BL, Lokeshwar VB, Block NL. Expression of CD44 in prostate cancer cells: association with cell proliferation and invasive potential. Anticancer research. 1995;15(4):1191–8. [PubMed] [Google Scholar]
- 17.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–93. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 18.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132(3):463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 21.Pierce KL, Tohgo A, Ahn S, Field ME, Luttrell LM, Lefkowitz RJ. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. The Journal of biological chemistry. 2001;276(25):23155–60. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- 22.Traynor AM, Weigel TL, Oettel KR, Yang DT, Zhang C, Kim K, et al. Nuclear EGFR protein expression predicts poor survival in early stage non-small cell lung cancer. Lung cancer. 2013;81(1):138–41. doi: 10.1016/j.lungcan.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast cancer research and treatment. 2006;95(3):211–8. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nature cell biology. 2001;3(9):802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 25.Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;108(3):370–7. doi: 10.1016/j.radonc.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Brand TM, Iida M, Li C, Wheeler DL. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discovery medicine. 2011;12(66):419–32. [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo FT, Lu TL, Fu HW. Opposing effects of beta-arrestin1 and beta-arrestin2 on activation and degradation of Src induced by protease-activated receptor 1. Cellular signalling. 2006;18(11):1914–23. doi: 10.1016/j.cellsig.2006.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.