Abstract
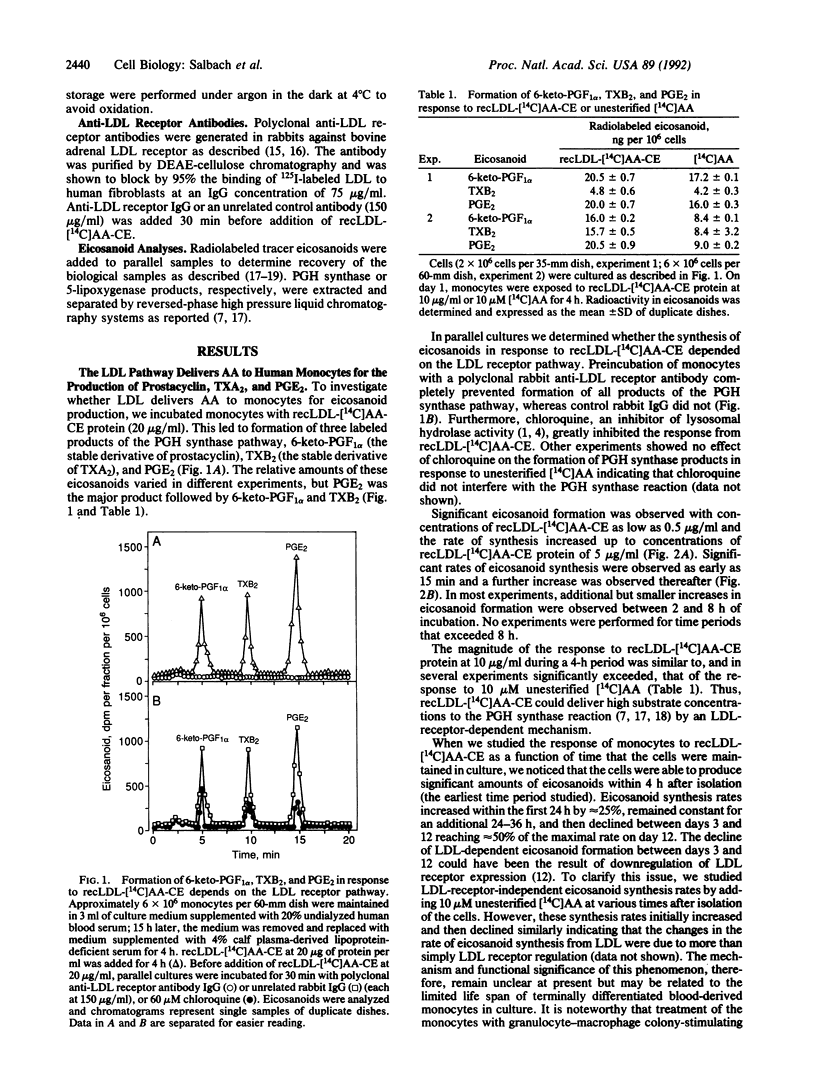
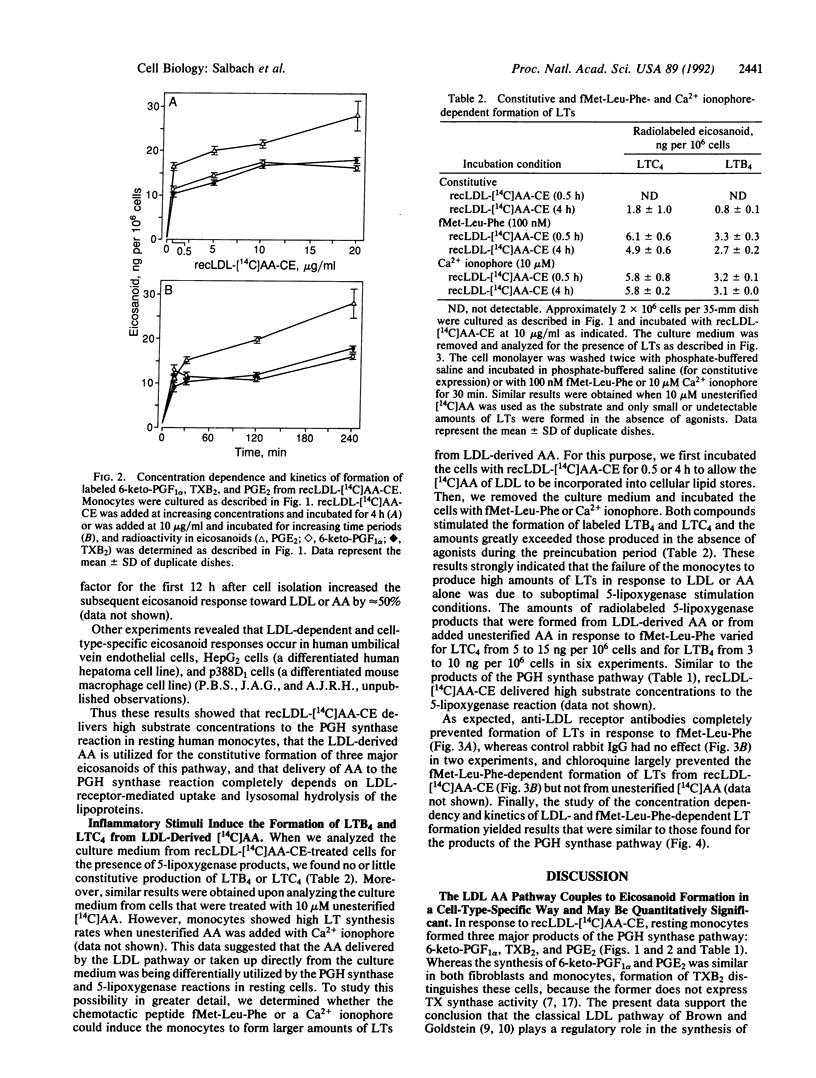
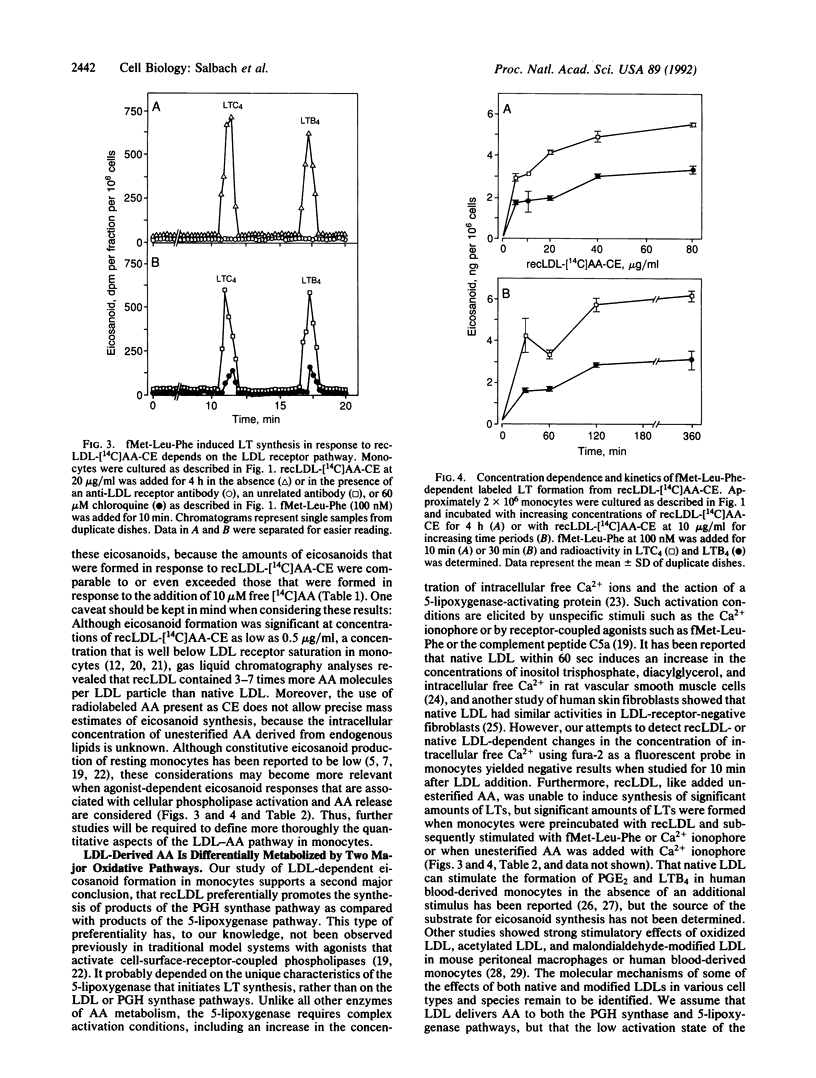
We studied the ability of low density lipoproteins (LDLs) to provide arachidonic acid (AA) for eicosanoid biosynthesis in human blood-derived monocytes. When incubated in the presence of reconstituted LDL that contained cholesteryl [1-14C]arachidonate (recLDL-[14C]AA-CE), resting monocytes formed three labeled products of the prostaglandin (PG) H synthase pathway: 6-keto-PGF1 alpha, thromboxane B2, and PGE2. The amounts of these eicosanoids in response to recLDL-[14C]AA-CE were comparable to or exceeded those that were produced in response to the addition of 10 microM unesterified [1-14C]AA. By contrast, resting monocytes formed only small amounts of products of the 5-lipoxygenase pathway, leukotriene (LT) B4 and LTC4 from either recLDL-[14C]AA-CE or [14C]AA, indicating preferential utilization of AA in the PGH synthase reaction. However, they converted LDL-derived [14C]AA efficiently into LTB4 and LTC4, when they were first incubated with recLDL-[14C]AA-CE and subsequently stimulated with the chemotactic peptide N-formylmethionylleucylphenylalanine or the Ca2+ ionophore A23187. The classical LDL receptor pathway mediated the synthesis of all of the above eicosanoids from LDL but not from unesterified AA. These results demonstrate that the LDL receptor pathway preferentially promotes the synthesis of PGH synthase products in resting human blood-derived monocytes and that an additional mechanism is required to promote effective synthesis of 5-lipoxygenase pathway products from AA that originates in LDL cholesteryl esters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisiegel U., Kita T., Anderson R. G., Schneider W. J., Brown M. S., Goldstein J. L. Immunologic cross-reactivity of the low density lipoprotein receptor from bovine adrenal cortex, human fibroblasts, canine liver and adrenal gland, and rat liver. J Biol Chem. 1981 Apr 25;256(8):4071–4078. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990 Jan 18;343(6255):282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Zeh W., Salbach P., Kommerell B., Rothe D. E., Nastainczyk W., Glomset J. A. Evidence for coordinate, selective regulation of eicosanoid synthesis in platelet-derived growth factor-stimulated 3T3 fibroblasts and in HL-60 cells induced to differentiate into macrophages or neutrophils. J Biol Chem. 1988 Dec 25;263(36):19384–19391. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Dresel H. A., Goerig M., Weber J. A., Stoehr M., Glomset J. A., Ross R., Schettler G. Low density lipoprotein receptor-dependent prostaglandin synthesis in Swiss 3T3 cells stimulated by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1344–1348. doi: 10.1073/pnas.83.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Rothe D. E., Specht E., Ziegler R., Glomset J. A., Graf T. Early reversible induction of leukotriene synthesis in chicken myelomonocytic cells transformed by a temperature-sensitive mutant of avian leukemia virus E26. Proc Natl Acad Sci U S A. 1989 Feb;86(3):921–924. doi: 10.1073/pnas.86.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Salbach P., Goerig M., Zeh W., Janssen-Timmen U., Blattner C., King W. C., Glomset J. A. The LDL receptor pathway delivers arachidonic acid for eicosanoid formation in cells stimulated by platelet-derived growth factor. Nature. 1990 Jun 14;345(6276):634–636. doi: 10.1038/345634a0. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Kladetzky R. G., Melnik B., Hennerici M. Stimulation of the scavenger receptor on monocytes-macrophages evokes release of arachidonic acid metabolites and reduced oxygen species. Lab Invest. 1986 Aug;55(2):209–216. [PubMed] [Google Scholar]
- Kelley J. L., Rozek M. M., Suenram C. A., Schwartz C. J. Activation of human peripheral blood monocytes by lipoproteins. Am J Pathol. 1988 Feb;130(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- Knight B. L., Soutar A. K. Changes in the metabolism of modified and unmodified low-density lipoproteins during the maturation of cultured blood monocyte-macrophages from normal and homozygous familial hypercholesterolaemic subjects. Eur J Biochem. 1982 Jul;125(2):407–413. doi: 10.1111/j.1432-1033.1982.tb06698.x. [DOI] [PubMed] [Google Scholar]
- Koo C., Wernette-Hammond M. E., Garcia Z., Malloy M. J., Uauy R., East C., Bilheimer D. W., Mahley R. W., Innerarity T. L. Uptake of cholesterol-rich remnant lipoproteins by human monocyte-derived macrophages is mediated by low density lipoprotein receptors. J Clin Invest. 1988 May;81(5):1332–1340. doi: 10.1172/JCI113460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. Reconstitution of the hydrophobic core of low-density lipoprotein. Methods Enzymol. 1986;128:608–613. doi: 10.1016/0076-6879(86)28094-5. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Nigon F., Rouis M., Foster S. J., Chapman M. J. Native low-density lipoproteins stimulate leukotriene B4 production by human monocyte-derived macrophages. Biochim Biophys Acta. 1991 Jun 3;1083(3):230–234. doi: 10.1016/0005-2760(91)90076-t. [DOI] [PubMed] [Google Scholar]
- Pomerantz K. B., Tall A. R., Feinmark S. J., Cannon P. J. Stimulation of vascular smooth muscle cell prostacyclin and prostaglandin E2 synthesis by plasma high and low density lipoproteins. Circ Res. 1984 May;54(5):554–565. doi: 10.1161/01.res.54.5.554. [DOI] [PubMed] [Google Scholar]
- Sachinidis A., Locher R., Mengden T., Vetter W. Low-density lipoprotein elevates intracellular calcium and pH in vascular smooth muscle cells and fibroblasts without mediation of LDL receptor. Biochem Biophys Res Commun. 1990 Feb 28;167(1):353–359. doi: 10.1016/0006-291x(90)91772-k. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Funk C. D. Enzymes involved in the biosynthesis of leukotriene B4. J Biol Chem. 1989 Nov 25;264(33):19469–19472. [PubMed] [Google Scholar]
- Schneider W. J., Goldstein J. L., Brown M. S. Purification of the LDL receptor. Methods Enzymol. 1985;109:405–417. doi: 10.1016/0076-6879(85)09106-6. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Baur U., Box R. J., Bühler F. R. Induction of growth-related metabolism in human vascular smooth muscle cells by low density lipoprotein. J Biol Chem. 1989 Jul 25;264(21):12582–12589. [PubMed] [Google Scholar]
- Spector A. A., Scanu A. M., Kaduce T. L., Figard P. H., Fless G. M., Czervionke R. L. Effect of human plasma lipoproteins on prostacyclin production by cultured endothelial cells. J Lipid Res. 1985 Mar;26(3):288–297. [PubMed] [Google Scholar]
- Yokode M., Kita T., Kikawa Y., Ogorochi T., Narumiya S., Kawai C. Stimulated arachidonate metabolism during foam cell transformation of mouse peritoneal macrophages with oxidized low density lipoprotein. J Clin Invest. 1988 Mar;81(3):720–729. doi: 10.1172/JCI113377. [DOI] [PMC free article] [PubMed] [Google Scholar]


