Abstract
Respiratory infections are a major cause of morbidity and mortality in the elderly. Previous reports have suggested that mucociliary clearance (MCC) is impaired in older individuals, but the cause is unclear. To unravel the mechanisms responsible for the age-associated decline in MCC, we investigated the MCC system in young (3 mo) and old (2 yr) C57BL/6 mice. We found that old mice had significantly reduced MCC function in both the upper and lower airways compared with young mice. Measurement of bioelectric properties of isolated tracheal and bronchial tissue revealed a significant decrease in Cl− secretion, suggesting that the older mice may have a reduced ability to maintain a sufficiently hydrated airway surface for efficient MCC. Ciliary beat frequency was also observed to be reduced in the older animals; however, this reduction was small relative to the reduction in MCC. Interestingly, the level of the major secreted mucin, Muc5b, was found to be reduced in both bronchioalveolar lavage and isolated tracheal tissue. Our previous studies of Muc5b−/− mice have demonstrated that Muc5b is essential for normal MCC in the mouse. Furthermore, examination of Muc5b+/− and wild-type animals revealed that heterozygous animals, which secrete ∼50% of the wild-type level of Muc5b, also demonstrate a markedly reduced level of MCC, confirming the importance of Muc5b levels to MCC. These results demonstrate that aged mice exhibit a decrease in MCC and suggest that a reduced level of secretion of both Cl− and Muc5b may be responsible.
Keywords: aging, mucociliary clearance
mucociliary clearance (MCC) is the first line of defense in preventing viral and bacterial infections in both the upper and lower airways (23, 49). Efficient MCC, whereby debris and pathogens trapped in mucus are cleared from the airways by ciliary beating, depends on the proper functioning of three major systems: 1) the synthesis and secretion of mucin and other mucus proteins, 2) the proper hydration of the airway surface, and 3) the coordinated activity of the cilia. A perturbation in any of these components can result in impaired MCC. For example, patients with primary ciliary dyskinesia have mutations in genes necessary for the proper formation or function of cilia and in many cases have a complete absence of MCC (6, 24, 31). These patients suffer from recurrent/chronic rhinosinusitis, otitis media, and pulmonary infections and develop bronchiectasis in an age-dependent fashion, clearly demonstrating the importance of MCC to host defense (24). In cystic fibrosis, mutations in the CFTR gene cause a defect in Cl− and hence water secretion that results in a dehydration of mucus and impaired MCC that leads to chronic pulmonary infections, bronchiectasis, and eventually lung failure (9, 17). Similar mechanisms likely play a role in the pathogenesis of chronic bronchitis and chronic obstructive pulmonary disease (2). These and other diseases clearly demonstrate the importance of MCC to lung host defense and the prevention of pulmonary infections.
It is well known that the elderly human population exhibits an age-related increase in the incidence of pulmonary infections, resulting in substantial morbidity and mortality (8, 14, 27). In one study, the hospitalization rate for pneumonia increased 12-fold in individuals over the age of 75 compared with the general population (12). Although there are a number of possible etiologies for these age-related pulmonary diseases, including immunosenescence, a loss of muscle tone in respiratory accessory muscles, and an increased incidence of aspiration (for reviews, see Refs. 14, 28), a decrease in MCC has also been suggested as a contributing factor. Although there is good evidence in humans that MCC declines with age (19, 33, 41), no mechanisms for this age-related decline in MCC have been suggested. With the exception of one study on aging beagles (50), no studies could be located on the effect of aging on MCC in species other than humans. The paucity of data on the effect of aging on MCC reflects in part the lack of a well-characterized model system.
The purpose of our studies was to first characterize MCC in both young and old mice to determine whether the mouse is a suitable model to study the age-dependent decline in MCC. We therefore measured MCC in both the upper and lower airways of young and old mice and found a significant decrease in MCC in the older animals. To begin to investigate the mechanism responsible for this decrease, we investigated each of the major components involved in MCC (cilia, mucus, and ion transport). Our results suggest that a lower level of mucin and Cl− secretion may play a key role in the age-dependent reduction of MCC. Furthermore, our results suggest that strategies to improve/maintain adequate MCC in the elderly may reduce the incidence and severity of pulmonary infections in this susceptible population.
MATERIAL AND METHODS
All mouse studies were approved by the University of North Carolina (UNC) Institutional Animal Care and Use Committee. Male C57BL/6 mice, 3 mo or 2 yr of age, obtained from the NIA aged rodent colonies were used in this investigation. A 3-mo-old mouse is roughly equivalent to a 20- to 30-yr-old human (mature adult), and a 2-yr-old mouse is roughly equivalent to a 55- to 70-yr-old human (www.jax.org). Mean body mass of 3-mo-old males was 26.3 ± 0.5 g and of 2-yr-old males was 33.8 ± 0.6 g (means ± SE). After arrival at UNC the mice were allowed to acclimate for at least 1 wk prior to being studied. All animals were allowed food and water ad libitum until the time of study.
Mucociliary clearance measurement in the upper airways.
To determine the rate of MCC in the anterior nasopharynx (ANP) of the nasal cavity, the mice were anesthetized (2.5% isoflurane) and then euthanized by severing the abdominal aorta. The lower jaw was removed and a small incision made in the lateral wall of the ANP (hard palate) at the level of the nasopalatine duct. A 35-gauge silica cannula (WPI, Sarasota, FL) was dipped to a depth of ∼5 mm into an aliquot of dry green fluorescent 7-μm beads (Thermo Scientific, Fremont, CA). The dry beads adhered to the outside of the cannula by static electricity. The cannula with the adherent beads was then introduced into the ANP via the incision. Enough beads were deposited on the surface of the nasal tissue by contact with the cannula to allow easy quantification of MCC by tracking the beads in the ANP. The preparation was placed under a dissecting microscope with a fluorescent lamp outfitted with a video camera (MTI, Michigan City, IN). The camera was interfaced to a DVD recorder for image acquisition. The beads could be clearly seen moving caudally inside the nasal cavity toward the posterior nasopharynx. The rate of MCC was measured downstream of the site of bead introduction. Before the MCC was recorded, a slide micrometer was placed on the stage of the dissecting scope to calibrate distance measurements. Once the video was recorded, MCC was determined by playing back the video and determining the time it took fluorescent particles to traverse a calibrated distance on the screen monitor (corresponding to an in vivo distance of 0.5–1 mm). Usually 10–30 particles were tracked per mouse over a 10-min period. There was no systematic change in the rate of MCC over the 10-min time period in the majority of the mice. The rate of MCC was calculated as millimeters per minute.
Mucociliary clearance measurement in the lower airways.
To measure MCC in the lower airways, the mice were anesthetized and euthanized as described above. The muscle and connective tissue covering the uppermost trachea (∼1 cm) was retracted and a very small incision (∼0.5 mm) was made through the ventral wall of the trachea. Then, via a fine-bore 34-gauge silica cannula (WPI) interfaced to a 0.5-μl SGE analytical syringe (Trajan Scientific Americas, Austin, TX), 200 nl of PBS containing fluorescent microspheres (3 μm, Molecular Probes FluoSpheres, Nile Red, Invitrogen, Carlsbad, CA) were deposited in the main stem bronchi just below the tracheal bifurcation. After deposition, the cannula was removed and the wound closed with a wound clip. After 15 min the lungs and trachea were removed, weighed, and placed in 3 M KOH for 24 h to dissolve the tissue; 10 μl of the dissolved lung preparation was placed on a hemocytometer under a ×2 objective (Nikon Eclipse scope with fluorescence) and photographed for counting with Image J software. Three aliquots from each mouse were counted and averaged. The percentage of beads cleared in the 15-min period was calculated by determining the difference between the number of beads injected and the number of beads recovered. To determine the number of beads injected, six aliquots of the bead suspension (200 nl) were counted in a similar manner prior to injection. The coefficient of variation on the six replicates was less than 5%, demonstrating that the number of beads deposited in the trachea was highly reproducible. Instilling small beads into the lower airways and quantifying the percentage of beads remaining in the airways after a defined time interval as a measure of MCC has previously been used in both rats (10) and mice (32, 35). In the modified procedure used here, a small opening is made in the trachea so that very small volumes (∼200 nl) of beads can be introduced; thus the airway surface liquid volume is minimally disturbed.
Measurement of ciliary beat frequency.
Tracheas were isolated from 3-mo-old and 2-yr-old male mice immediately after euthanasia and placed in a petri dish containing sterile F12 media. Tracheal rings (∼0.5–1.0 mm) were cut from the trachea with a razor blade and five to six rings were transferred to a 60-mm petri dish containing 1.5 ml F12 media. The dish was placed on the heated stage (25°C; Zeiss TempControl 37-2) of a Nikon Eclipse TE2000 inverted microscope with phase optics (×20). Videos (60 fps; 2 s) of active areas of ciliary activity (usually 1–3/tracheal ring) were captured with a Redlake ES-310T camera driven by SAVA software (Ammons Engineering, Clio, MI). The SAVA software was also used for ciliary beat frequency (CBF) determination with the whole field analysis option. The measurements from all tracheal rings were combined to provide an average CBF for each animal, and old and young animals were studied alternately.
Lung histology.
Lung tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Sections (4–6 μm) were prepared and stained with hematoxylin and eosin to examine lung morphology and Alcian blue/periodic acid-Schiff to visualize mucus. For quantification of lymphoid hyperplasia, one section from each lobe, sectioned to maximize visualization of the primary bronchi, was scored semiquantitatively from 0 to 3, as previously described (25). Sections with no areas of hyperplasia = 0, one area = 1, two areas = 2, and those with 3 or more = 3. Two different groups of animals were examined.
Tracheal and bronchial bioelectrics.
Mice were euthanized as described above and tracheas and bronchi were immediately excised and mounted in Ussing chambers. Ussing chamber measurements of the bioelectric properties of tracheal and bronchial epithelia were made under short-circuit current (Isc) conditions, as previously described (15, 16). Tissues were bathed bilaterally in Krebs-Ringer bicarbonate solution. The experimental protocol was; amiloride (10−4 M) added to the apical surface to block electrogenic Na+ absorption, forskolin (10−5 M) and then UTP (10−4 M) were added to the apical surface to induce anion secretion via an increase in cellular cAMP and Ca2+, respectively, and finally bumetanide (10−4) was added to the basolateral side to block Cl− secretion. All chemicals were purchased from Sigma, with the exception of UTP (Amersham Pharmacia Biotech).
Quantification of mucin.
Mice (n = 10/age group) were weighed, anesthetized with 2,2,2-tribromoethanol, and exsanguinated by resection of the inferior vena cava. The thoracic cavity was opened, the thymus removed, and the trachea exposed. An 18-gauge blunt needle was inserted just above the airway bifurcation and secured in place with suture. The upper four tracheal rings were collected as “submucosal glands” sample, and the remainder of the trachea was collected as “trachea” sample. Both were weighed, opened longitudinally by microdissection, submerged in 120 μl of 6 M urea in PBS, and incubated at 4°C for 24 h, with occasional mixing through a 200-μl pipette tip. Bronchoalveolar lavage (BAL) was performed by instilling and retrieving 1 ml of room temperature PBS containing protease inhibitors (Roche, cOmplete tablets mini, Indianapolis, IN) through the 18-gauge blunt needle. This procedure was repeated three times and the three fractions were pooled to generate a ∼3-ml BAL sample, to which powdered urea was added to reach 6 M concentration (0.4 g/ml). All samples were reduced with 10 mM dithiothreitol and alkylated with iodoacetamide to analyze mucin levels by agarose Western blot, as described in Ref. 25. Nitrocellulose membranes were probed with rabbit polyclonal antibodies against Muc5b [UNC223, 1:2,000 dilution in Odyssey blocking buffer (LI-COR Biotechnology, Lincoln, NE) (51)] and Muc5ac [UNC294, 1:1,000 dilution in Odyssey blocking buffer +0.1% Tween-20 (11)].
Statistics.
All data are shown as means ± SE. A Student's t-test was used to compare means between two groups. When more than two groups were compared, an ANOVA was used for the comparison. P ≤ 0.05 was considered statistically significant.
RESULTS
Mucociliary clearance is reduced in both the upper and lower airways of aged mice.
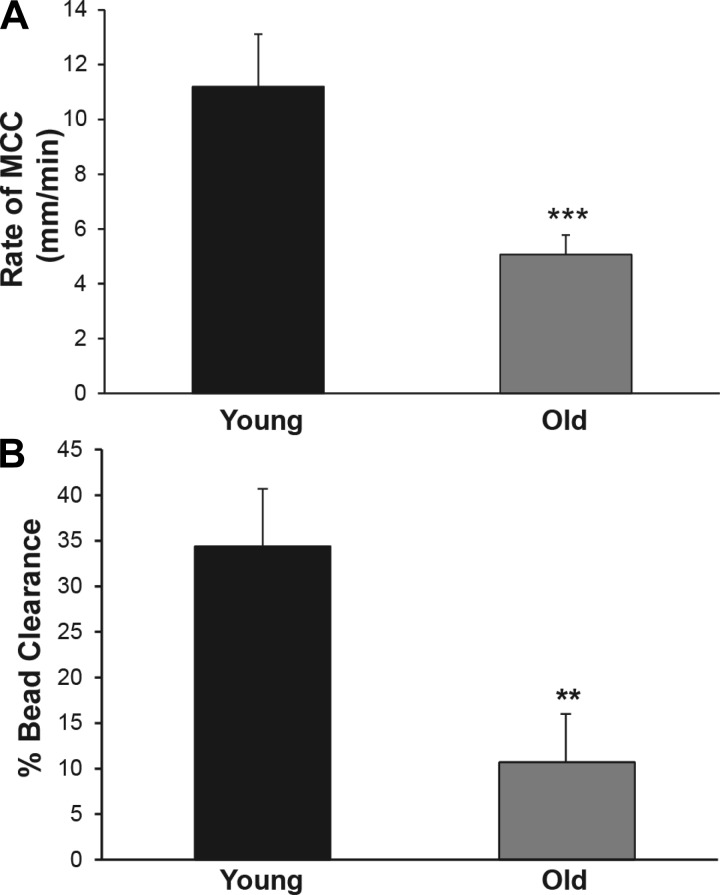
To determine whether old mice display a reduced rate of MCC and would therefore be suitable as a model to investigate the mechanisms responsible for reduced MCC in older humans, we first compared the rate of MCC in a cohort of adult (young; 3 mo old) and old (old; 2 yr old) mice. The rate of MCC in the nasal cavity (ANP) of the young animals averaged 11.2 ± 1.3 mm/min (Fig. 1A), which is in good agreement with our previous studies using this technique (32). The rate of clearance in the old mice was reduced to 5.6 ± 0.8 min/min, which was significantly decreased compared with the young animals (∼55%; P ≤ 0.001; Fig. 1A). In the lower airways, the percentage of beads cleared was again significantly decreased in the old mice (10.7 ± 5.4%) compared with the younger animals (34.4 ± 6.3%; P ≤ 0.01; Fig. 1B). These results demonstrate that MCC decreases with age in mice, similar to observations in humans, and suggest that the mouse may be a good model system to study the mechanisms responsible for the age-dependent decline in MCC.
Fig. 1.
A: effect of age on the rate of mucociliary clearance (MCC) from the nasal cavity (anterior nasopharynx) in 3-mo-old and 2-yr-old male mice. Data shown are means ± SE, n = 12 mice both groups. There was a statistically significant difference between the 2 groups, ***P ≤ 0.001. B: effect of age on the % beads cleared per 15 min from the lungs/trachea of 3-mo-old or 2-yr-old male mice; n = 10 both groups. There was a statistically significant difference between the 2 groups, **P ≤ 0.01.
Ciliary beat frequency is reduced in old mice.
To determine whether a reduction in CBF could be responsible for the reduced MCC observed in the older animals, CBF was measured in the tracheas of old and young mice. Tracheal rings were prepared from both groups of animals and CBF was measured in several rings from each animal. Average CBF in the old animals was 10.4 ± 0.2 Hz (average ± SE; n = 22) compared with 11.2 ± 0.3 Hz (n = 23) in the younger animals. This result indicates that CBF is significantly reduced in older mice (P < 0.02). However, the magnitude of the reduction (<10%) is small relative to the reduction in MCC (50–70%).
Measurement of airway bioelectric properties reveals a decrease in chloride secretion in old mice.
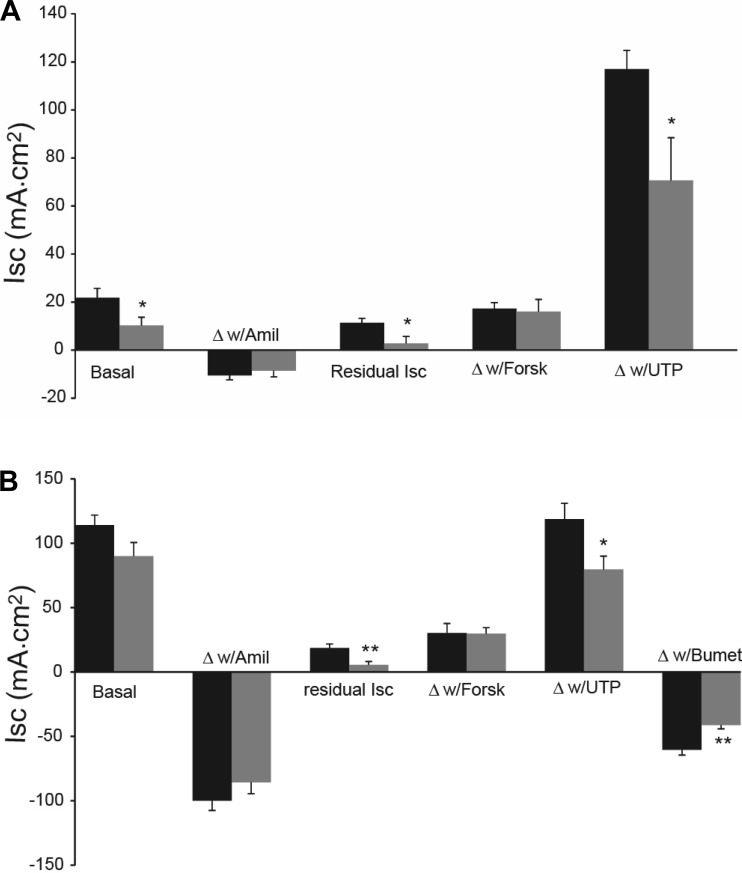
The absorption and secretion of ions (principally Na+ and Cl−) in airway epithelia is accompanied by osmotically driven water flow that is important for maintaining proper hydration (i.e., mucus concentration) of the airway surface. We measured bioelectric properties of both the trachea and bronchi from young and old mice to determine whether age affects Na+ absorption or Cl− secretion of airway epithelia. The basal Isc as well as the postamiloride residual Isc were significantly decreased in the tracheal epithelium of the old mice compared with that of the younger animals (Fig. 2A). The residual Isc was also significantly decreased in the bronchi from the old mice (Fig. 2B). Previous studies indicate that the basal Isc is a combination of ENaC-mediated Na+ absorption and Cl− secretion (34). The response to amiloride was not age dependent in either the trachea or bronchi, suggesting that the decrease in basal Isc, and particularly the reduced residual Isc, in the old mice was a result of a decrease in Cl− secretion. In both the trachea and bronchi from the old mice, there was a significant decrease in the response to UTP, a Ca++-activated Cl− secretory response (Fig. 2, A and B). As we have previously reported, the trachea of neither group responded to the Cl− channel blocker bumetanide (Ref. 16; data not shown because the responses were zero). However, both groups of bronchi responded to bumetanide, but again the old mice had a significantly attenuated response, further suggesting a reduced rate of Cl− secretion by the bronchi of the old mice.
Fig. 2.
A: bioelectrics of tracheal epithelium for 2-mo-old (solid bars) and 2-yr-old (gray bars) male mice. The “basal” Isc is the steady-state short circuit prior to drug additions. Then drugs were added in the order shown and as described in the text. “Δw/Amil” is change in Isc in response to amiloride and the “Residual Isc” is the steady-state Isc remaining following amiloride addition. Forsk, forskolin. The basal Isc, residual Isc, and the response to UTP were significantly different (*P ≤ 0.05) between the 2 groups. The response to bumetanide is not shown as the responses were ∼0 for both groups of mice. Data are from n = 6 mice, both groups. B: bioelectrics of bronchial epithelium for 2-mo-old (solid bars) and 2-yr-old (gray bars) male mice. Drug additions were as indicated above. The residual Isc (**P ≤ 0.01), the response to UTP (*P ≤ 0.05), and the response to bumetanide (Bumet; **P ≤ 0.01) differed significantly between groups; n = 6 for the 3-mo-old mice and n = 5 for the 2-yr-old animals.
Older mice have reduced levels of Muc5b.
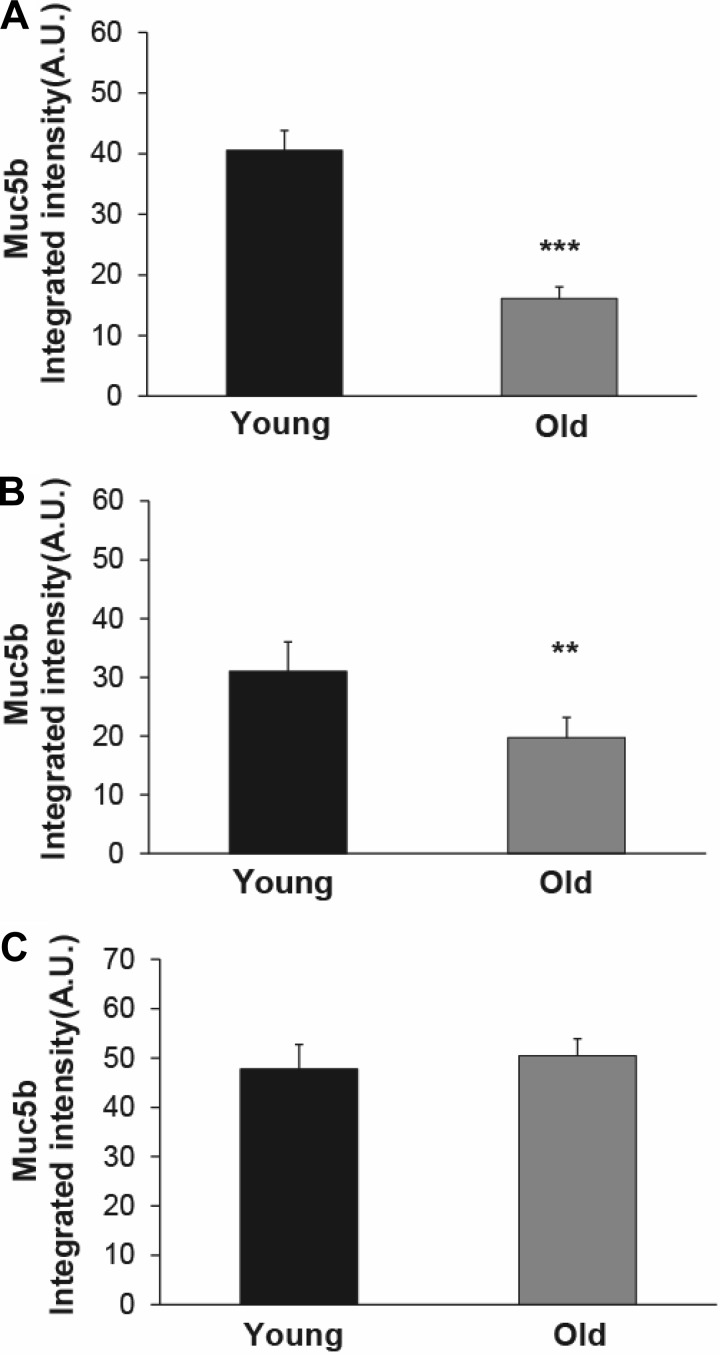
To test whether age affected the mucin content of mouse airways, we performed mucin agarose Western blots on selected tissue representative of three different lower airway compartments. Specifically, we studied tissue extracts from submucosal glands (contained in the first four tracheal rings), trachea, and BAL in young and old mice. As expected, tracheal sample weight was higher in old mice compared with young mice and correlated with the expected difference in body weight between the two groups (data not shown). In contrast, there was no significant difference in submucosal gland samples' weight between young and old mice. Notably, we detected a marked decrease in Muc5b levels in both tracheal and BAL samples from old mice compared with young mice (Fig. 3, A and B), whereas Muc5b levels were similar between the two age groups in submucosal gland preparations (Fig. 3C). Muc5ac was undetectable in all samples, regardless of origin (not shown).
Fig. 3.
Levels of Muc5b in young and old mice. A: level of Muc5b in tracheal tissue from old mice was significantly less than the level in young mice; n = 10 mice/group. B: level of Muc5b in BAL fluid from old mice was also significantly reduced, relative to the young mice; n = 9 young mice; 10 old mice. C: Muc5b levels in submucosal gland tissues were the same in young and old mice; n = 10 mice/group. Data are means ± SE. **P < 0.01, ***P < 0.001.
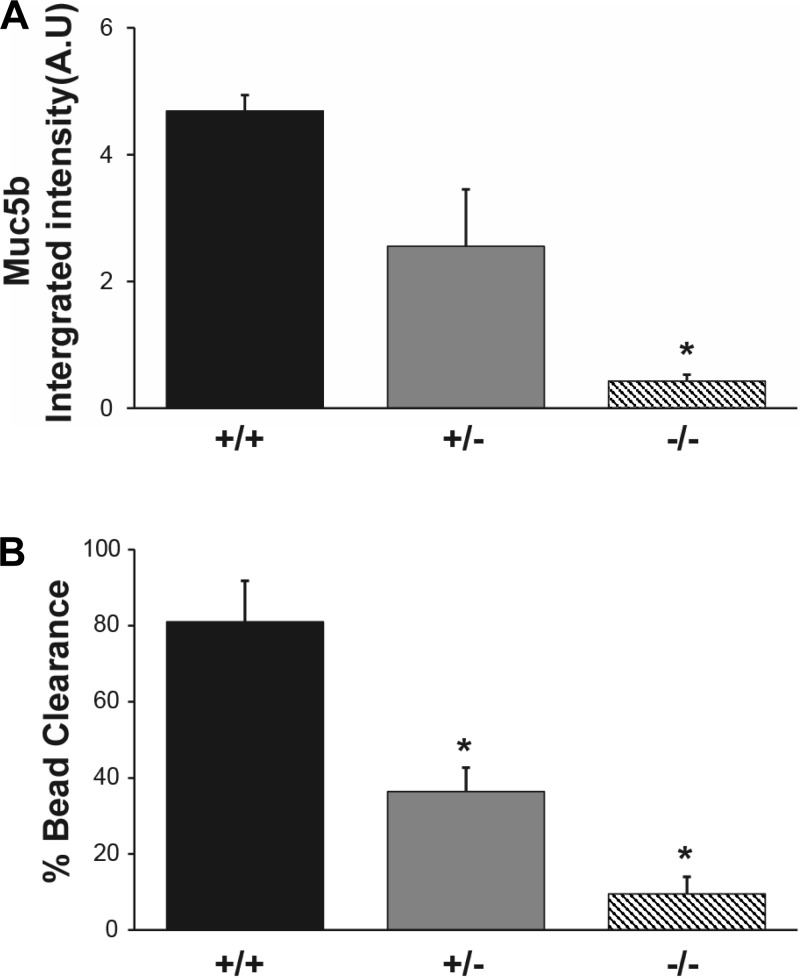
To further investigate the relationship between reduced levels of Muc5b and MCC, we utilized the Muc5b knockout mouse (Muc5b−/−). We have previously reported that the Muc5b-deficient mice have severely reduced MCC that results in the retention/accumulation of debris in the airways, leading to infection and death (35). Interestingly, whereas the Muc5b−/− mouse has no detectable Muc5b, the heterozygotes (Muc5b+/−) have ∼1/2 the level of Muc5b in BAL compared with wild-type (WT) mice (Fig. 4A). To directly examine the effect of reduced levels of Muc5b on MCC, we measured MCC in the lower airways of Muc5b−/−, Muc5b+/−, and WT mice. In agreement with our previous study, the Muc5b−/− animals had a severely reduced rate of MCC, clearing only ∼10% of the fluorescent beads compared with the WT mouse (Fig. 4B). Surprisingly, the Muc5b+/− animals had a level of clearance slightly less than half of that exhibited by the WT mice, thus demonstrating that a reduced level of Muc5b is sufficient to cause a significant inhibition of MCC (Fig. 4B). This suggests that the reduction of Muc5b levels in the old mice may be responsible for the decrease in MCC.
Fig. 4.
A: effect of Muc5b genotype on levels of Muc5b in BAL. Solid bars, WT mice; gray bars, Muc5b+/− mice; striped bars, Muc5b−/− mice (n = 6 each genotype; 5 wk old). The level of Muc5b was significantly reduced in the Muc5b−/− animals compared with WT (*P ≤ 0.05). B: effect of Muc5b genotype on MCC. Solid bars, WT mice (n = 3); gray bars, Muc5b+/− mice (n = 10); striped bars, Muc5b−/− mice (n = 6). All animals were 6 mo old. ANOVA indicated statistical difference (*P ≤ 0.05) from WT and each genotype from the other (*P ≤ 0.05).
Old mice show signs of lung disease.
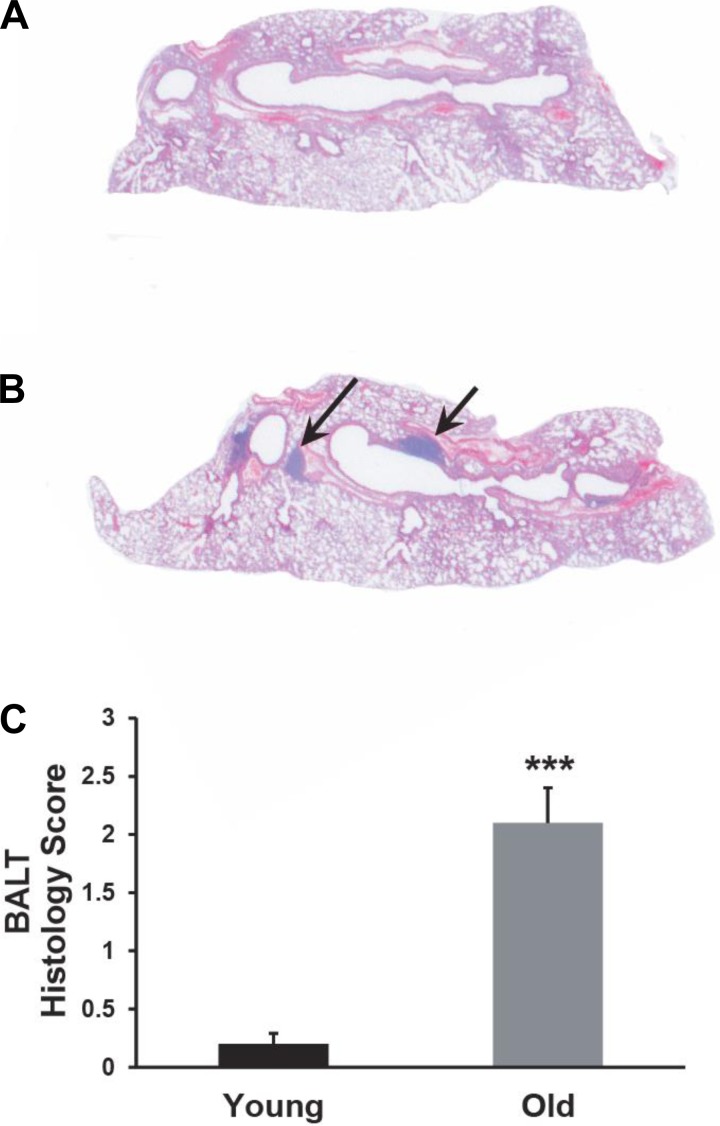
Whereas histological examination of lungs from the young animals typically displayed normal morphology, with only an occasional area of lymphoid hyperplasia, lungs from the older animals consistently showed a high incidence of bronchus-associated lymphoid tissue (BALT) (Fig. 5, A and B). A semiquantitative analysis revealed a significant increase in the average number of BALT foci in the lungs of the older animals (2.1 vs. 0.2; P ≤ 0.001, Fig. 5C). Morphologically, these lymphoid aggregates appeared loosely organized and lacking germinal centers, suggesting inducible BALT (iBALT). As iBALT formation has been associated with local infection or inflammation (13, 30), our results suggest that decreased MCC in older mice might increase the incidence of endogenous or environmental insults to the airway epithelium.
Fig. 5.
Old mice have a high incidence of BALT. A: hematoxylin and eosin (H&E) section of left lobe from a young (3 mo old) mouse. B: H&E section of left lobe from an old (2 yr) mouse showing BALT (arrows). C: semiquantitative analysis of the incidence of BALT in the lungs of young and old mice demonstrates a clear increase in the older animals. ***P ≤ 0.001; n = 9 for each age group.
DISCUSSION
The aging of the population has been associated with increased morbidity and mortality due to pulmonary diseases, while morbidity and mortality from other diseases has remained stable or declined (44). The increased incidence of lung disease in the elderly is likely due to the complex interaction of many age-associated changes (for reviews, see Refs. 14, 28). These changes can include an altered immune response, an increased risk of aspiration, a decrease in clearance mechanisms (both cough and MCC), the presence of other systemic diseases, and smoking or exposure to environmental pollutants. The importance of each of these risk factors will vary between individuals. For example, poor oral hygiene and increased aspiration alone may not be pathogenic, but when accompanied by a reduction in airway clearance mechanisms, may result in an increase in pneumonias. Although there is evidence that immune function declines with advanced age, even very old individuals can often mount robust immune responses (28). Thus, to reduce the incidence and severity of lung disease in the elderly, it is necessary to understand the effect of aging on both the innate and adaptive immune systems and to develop treatment strategies appropriate for each.
One feature of the aging lung that is thought to contribute to the susceptibility of the elderly to lung disease but has not been well studied is a decline in MCC (14, 28). MCC is known to be one of the important defense mechanisms in maintaining pulmonary health; however, there have been few investigations of the effect of aging on MCC in the lungs of older individuals (19, 29, 33, 41), with most reporting a decline in MCC with increasing age. Other investigators have studied the effect of aging on MCC in the nasal cavity with conflicting results. Ho et al. (18) reported a significant negative correlation of age with the rate of nasal MCC, whereas Kao et al. (20) did not find an effect. Presently, it is not known whether nasal MCC is representative of MCC in the lower airways.
The effect of aging on the individual components of MCC is also not known. For example, in the study by Ho et al. (18), the authors reported a significant decrease in nasal MCC, a decrease in CBF, and an increase in ciliary abnormalities in individuals over the age of 40 (18). However, it is not clear whether the ciliary defects were due to the process of aging or whether they were the result of exposure to environmental challenges, e.g., cigarette smoke (38, 40, 42). Indeed, aging of humans is accompanied by years of exposure to air pollution, environmental pathogens (viral, bacterial) and other insults to the respiratory tract, making it difficult to separate the effect of these insults from the normal response to aging. In contrast, in the mouse model utilized in this study, both the old and young animals were raised in a controlled environment with filtered air, limiting the exposure to environmental pollutants and pathogens. Thus, in our mouse model, the responses we observe will be more likely directly related to the normal aging process. Moreover, we are not aware of any published studies that attempted to determine whether the production of mucin is increased or decreased in elderly individuals. Although it is accepted that mucin hypersecretion is associated with diseases, including chronic bronchitis and asthma (47, 48), it is less clear what effect mucus hyposecretion may have on MCC. We recently reported that deletion of the secreted mucin Muc5b in a mouse model caused a severe decrease in MCC in the upper and lower airways (35). Thus either too little or too much mucin could be a cause of impaired MCC in the elderly. Finally, the effect of aging on the bioelectric properties of the airways is also unknown.
In this study, we sought to determine whether a mouse model would exhibit a decline in MCC as a function of age, and if so which of the major components of MCC (ion transport, CBF, or mucin composition/quantity) may be involved. We found that, in both the upper (nasal) and lower airways, the rate of MCC was significantly decreased (∼50 and 60% respectively) in old mice compared with the younger cohort. These data agree with earlier studies in both humans and dogs (19, 33, 41, 50) and demonstrate that mice may be useful to investigate the mechanisms responsible for the age-dependent decrease in MCC.
We then examined three major components of MCC individually to determine why MCC is decreased in old mice. As CBF has been found to be positively correlated with the rate of MCC (36), a reduction in CBF could be responsible for the reduced rate of MCC we observed in the older mice. Other studies have previously reported CBF to be reduced in older animals (3). Therefore, we measured CBF in ex vivo preparations, i.e., isolated tracheal rings, from both young and old mice. These studies revealed a small (<1 Hz) but significant reduction in the CBF of older animals. CBF is known to increase with increasing temperature, and therefore CBF in vivo is likely greater then what we observed in vitro at 25°C. However, because the increase in CBF with increasing temperature is approximately linear (36, 39), the difference between old and young mice is expected to remain small. Our result is also consistent with the study of Bailey et al. (3), who reported a significantly reduced (∼ 3 Hz) CBF in 2-yr-old mice compared with 2-mo-old mice. These investigators demonstrated that the decrease in CBF is related to an increase in the level of protein kinase C-ε. Based on studies of human ciliated cells in culture, a reduction in CBF of 1 Hz can be estimated to reduce MCC from ∼5 to ∼4.5 mm/min, a difference of only ∼10% (36). Although this likely contributes to the slowing of MCC in the older mice, it does not appear to be sufficient by itself to account for the 50% reduction of MCC observed.
The amount of the airway surface liquid (ASL) is also critical to maintaining normal MCC. The volume of this layer is regulated primarily by the rate of ion transport across the airway epithelia. An increase in ion absorption, primarily Na+, from the lumen will result in a decrease in ASL volume as water will passively follow the ions to maintain osmolality. The decreased volume of ASL will dehydrate the mucus, collapse the cilia (7) and result in a markedly decreased rate of MCC, as demonstrated by the overexpression of β-ENaC in a mouse model (26). Similarly, an imbalance in ion transport, e.g., reduced Cl− secretion with persistent Na+ absorption, will decrease water flow onto the epithelial surface and result in a compromised ASL volume and reduced MCC, as observed in cystic fibrosis (4, 5). Therefore, we measured airway bioelectric properties in both tracheal and bronchial tissue from old and young mice as an indicator of the ion transport capacity and thus ASL regulation. Similar age-related changes were observed in both tissues. The basal (unstimulated) Isc reflects components of both electrogenic Na+ absorption and Cl− secretion (1, 34). In both the trachea and bronchi of the aged mice, there was a significant reduction in Cl− secretory component of the basal Isc, reflected as a reduced postamiloride residual Isc, with no significant age-related change in amiloride-sensitive Na+ absorption in either tissue. In the tracheas of the aged mice, because constitutive Cl− secretion was reduced, it is likely that there was a decrease in coupled water secretion. From our data, it is not possible to determine whether this defect in basal Cl− secretion caused a decrease in ASL volume and a decrease in MCC.
In addition, in both the aged tracheas and bronchi there was a significant decrease (∼40%) in the response to UTP. We have previously found that a significant fraction of the UTP response in murine airway tissue is due to TMEM 16A-mediated Cl− secretion (34), thus it is likely that secretion through this channel was reduced in both airway tissues as a result of aging. Because nucleotide release rates can regulate MCC, reduced UTP sensitive Cl− secretion could contribute to reduced ASL volume and reduced MCC.
In both humans and mice, MUC5B and MUC5AC are the major secreted mucins comprising the primary component of airway mucus and are mainly responsible for its rheological properties (22, 45, 46). We have recently shown that, in mice, Muc5b, but not Muc5ac, is necessary for normal airway MCC (35). In our aged mice, we found a significant decrease in the levels of Muc5b in BAL fluid, which sampled the lower airways, and in tracheal extracts, compared with the Muc5b levels detected in the same samples from young mice. The Muc5b present in both the BAL and tracheal samples likely reflects the baseline contribution from the club cells lining the trachea and lower airways (51), as opposed to the Muc5b pool found in the tracheal glands, which is mainly secreted following neural stimulation. The constitutively secreted mucin from the superficial epithelium is likely responsible for lining the healthy airways and allowing normal MCC (51). In support of this hypothesis, we have found that the levels of Muc5b correlated with the rate of MCC in mouse airways, since the Muc5b−/− mice exhibited very low MCC and Muc5b heterozygotes exhibited an ∼50% decrease in the levels of BAL Muc5b (Fig. 4A) and MCC (Fig. 4B). Because we observed a similar decrease in both Muc5b and MCC in the old mice, it is likely that the lower level of Muc5b contributes to the reduced MCC in the old animals.
Old mice also showed an increase in the incidence of BALT (43). Similarly, we have observed an increased incidence of BALT over time in a mouse model of primary ciliary dyskinesia in which MCC is also reduced (Ostrowski LE and Livraghi-Butrico A, unpublished observation). Thus we further hypothesize that the development of BALT in older mice may arise in response to progressively poorer MCC, through stimulation of mucosal innate immunity.
A major advantage of using the mouse to study the age-related decline in MCC is that, because of its short life span, these changes can be studied in a much shorter time frame compared with humans or other animal models. In addition, because mice are raised in a controlled environment, the changes observed are less likely to be the result of exposure to air pollutants, infectious agents, or other environmental factors. However, there are differences between murine and human airways that may impact on the usefulness of the mouse as a model of human aging. For example, Muc5b is the predominant secreted mucin under baseline conditions in the mouse, whereas human sputum contains both MUC5B and MUC5AC (21, 22). In the mouse, only the most proximal trachea contains submucosal glands, whereas in the human, submucosal glands are abundant throughout the cartilaginous airways. Thus the mouse trachea may be a good model for human small airways that also lack glands. Additionally, in mouse airways, CFTR does not play the major role in Cl− secretion that it does in human airways; instead Ca++ mediated Cl− secretion predominates (16). The impact of these differences between mouse and human airways needs to be taken into account when interpreting the data. In the mouse model, we observed a decrease in MCC in the nasal cavity as well as the lower airways of the old mice. This finding suggests that the nasal cavity may be a good model for studying the effects of aging on the lower airways. Furthermore, if these data are applicable to the human, the human nasal cavity may be a good model for studying the effect of aging on MCC, as nasal epithelia are clearly much easier to study than the lower airways.
We could not locate any published studies regarding the levels of MUC5B protein in human airways as a function of aging. However, in humans, many muco-obstructive lung diseases are characterized by an increase in mucin secretion, including MUC5B [e.g., chronic bronchitis, cystic fibrosis (17, 22)]. With these diseases, it is likely that a marked increase in the total concentration of mucus contributes to the reduced MCC. Current and developing therapies for these diseases are designed to clear mucus from the airways by improving mucus transportability (DNase, hypertonic saline, mucolytics), or decreasing mucus secretion. Ironically, at least in the aged mouse, therapies aimed at increasing the level of Muc5b production may be beneficial in normalizing the age-related compromise in MCC.
In conclusion, we have shown that the aged C5BL/6 mouse exhibits significantly reduced rates of MCC in both the upper and lower airways. This reduction is likely the result of a combination of a decrease in CBF and a decrease in Cl− and mucin secretion. Given the magnitude of the changes, we hypothesize that the age-dependent decline in MCC is likely the result of the significantly decreased levels of Muc5b and/or Cl− secretion. Further studies will be needed to determine the mechanism(s) for the decline in the Muc5b levels and Cl− secretion in the aging murine airways and whether therapies to increase Muc5b and/or Cl− secretion in the aged mouse will return MCC levels to those of the younger animals.
GRANTS
Funding for this research was provided by the National Institutes of Health (R03AG042706, R01HL117836, P30 DK065988, P01HL108808, P01HL110873) and the Cystic Fibrosis Foundation (R0-C11, BOUCHE15R0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.R.G., A.L.-B., and L.E.O. conception and design of research; B.R.G., A.L.-B., T.D.R., W.Y., and L.E.O. performed experiments; B.B. designed and built imaging system; B.R.G., A.L.-B., T.D.R., and L.E.O. analyzed data; B.R.G., A.L.-B., and L.E.O. interpreted results of experiments; B.R.G., A.L.-B., T.D.R., and L.E.O. prepared figures; B.R.G., A.L.-B., and L.E.O. drafted manuscript; B.R.G., A.L.-B., and L.E.O. edited and revised manuscript; B.R.G., A.L.-B., T.D.R., W.Y., B.B., and L.E.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the excellent histological support of K. Burns. We also thank Dr. R. C. Boucher for reading the manuscript and for helpful discussions and suggestions and Dr. Hong Dang for statistical consulting.
REFERENCES
- 1.Anagnostopoulou P, Riederer B, Duerr J, Michel S, Binia A, Agrawal R, Liu X, Kalitzki K, Xiao F, Chen M, Schatterny J, Hartmann D, Thum T, Kabesch M, Soleimani M, Seidler U, Mall MA. SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation. J Clin Invest 122: 3629–3634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 192: 182–190, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey KL, Bonasera SJ, Wilderdyke M, Hanisch BW, Pavlik JA, DeVasure J, Robinson JE, Sisson JH, Wyatt TA. Aging causes a slowing in ciliary beat frequency, mediated by PKCε. Am J Physiol Lung Cell Mol Physiol 306: L584–L589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 58: 157–170, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 261: 5–16, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bush A, Hogg C. Primary ciliary dyskinesia: recent advances in epidemiology, diagnosis, management and relationship with the expanding spectrum of ciliopathy. Expert Rev Respir Med 6: 663–682, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337: 937–941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong CP, Street PR. Pneumonia in the elderly: a review of severity assessment, prognosis, mortality, prevention, and treatment. South Med J 101: 1134–1140; quiz 1132, 1179, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Clunes MT, Boucher RC. Cystic fibrosis: the mechanisms of pathogenesis of an inherited lung disorder. Drug Discov Today Dis Mech 4: 63–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coote K, Nicholls A, Atherton HC, Sugar R, Danahay H. Mucociliary clearance is enhanced in rat models of cigarette smoke and lipopolysaccharide-induced lung disease. Exp Lung Res 30: 59–71, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O'Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci USA 109: 16528–16533, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis 28: 726–729, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol 3: 537–544, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother 8: 47–62, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Grubb BR, Pace AJ, Lee E, Koller BH, Boucher RC. Alterations in airway ion transport in NKCC1-deficient mice. Am J Physiol Cell Physiol 281: C615–C623, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. Am J Physiol Cell Physiol 267: C293–C300, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, Kesimer M. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 124: 3047–3060, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Sun J, Leung R, Tsang KW. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 163: 983–988, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Incalzi RA, Maini CL, Fuso L, Giordano A, Carbonin PU, Galli G. Effects of aging on mucociliary clearance. Compr Gerontol A 3 Suppl: 65–68, 1989. [PubMed] [Google Scholar]
- 20.Kao CH, Jiang RS, Wang SJ, Yeh SH. Influence of age, gender, and ethnicity on nasal mucociliary clearance function. Clin Nucl Med 19: 813–816, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 361: 537–546, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109: 571–577, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188: 913–922, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O'Neal WK, Boucher RC, Randell SH. Airway and lung pathology due to mucosal surface dehydration in β-epithelial Na+ channel-overexpressing mice: role of TNF-α and IL-4Rα signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol 182: 4357–4367, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Meyer KC. Lung infections and aging. Ageing Res Rev 3: 55–67, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer KC. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Semin Respir Crit Care Med 31: 561–574, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen J, Lange P, Nyboe J, Groth S. Lung mucociliary clearance. Eur J Nucl Med 21: 953–961, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10: 927–934, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, Boucher RC, Knowles MR. Effect of aerosolized uridine-5′-triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. Am J Respir Crit Care Med 160: 144–149, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O'Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol 43: 55–63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puchelle E, Zahm JM, Bertrand A. Influence of age on bronchial mucociliary transport. Scand J Respir Dis 60: 307–313, 1979. [PubMed] [Google Scholar]
- 34.Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O'Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 309: L99–L108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisson JH, Papi A, Beckmann JD, Leise KL, Wisecarver J, Brodersen BW, Kelling CL, Spurzem JR, Rennard SI. Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am J Respir Crit Care Med 149: 205–213, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Smith CM, Hirst RA, Bankart MJ, Jones DW, Easton AJ, Andrew PW, O'Callaghan C. Cooling of cilia allows functional analysis of the beat pattern for diagnostic testing. Chest 140: 186–190, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Stanley PJ, Wilson R, Greenstone MA, MacWilliam L, Cole PJ. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax 41: 519–523, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J 26: 609–615, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy 23: 117–122, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Tango M, Suzuki E, Gejyo F, Ushiki T. The presence of specialized epithelial cells on the bronchus-associated lymphoid tissue (BALT) in the mouse. Arch Histol Cytol 63: 81–89, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, Busse PJ, Tuder RM, Antony VB, Sznajder JI, Budinger GR. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med 191: 261–269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70: 459–486, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc 1: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans 37: 877–881, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 135: 505–512, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med 154: 1868–1902, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Whaley SL, Muggenburg BA, Seiler FA, Wolff RK. Effect of aging on tracheal mucociliary clearance in beagle dogs. J Appl Physiol 62: 1331–1334, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 586: 1977–1992, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]