SUMMARY
Methanobactins (mbs) are low-molecular-mass (<1,200 Da) copper-binding peptides, or chalkophores, produced by many methane-oxidizing bacteria (methanotrophs). These molecules exhibit similarities to certain iron-binding siderophores but are expressed and secreted in response to copper limitation. Structurally, mbs are characterized by a pair of heterocyclic rings with associated thioamide groups that form the copper coordination site. One of the rings is always an oxazolone and the second ring an oxazolone, an imidazolone, or a pyrazinedione moiety. The mb molecule originates from a peptide precursor that undergoes a series of posttranslational modifications, including (i) ring formation, (ii) cleavage of a leader peptide sequence, and (iii) in some cases, addition of a sulfate group. Functionally, mbs represent the extracellular component of a copper acquisition system. Consistent with this role in copper acquisition, mbs have a high affinity for copper ions. Following binding, mbs rapidly reduce Cu2+ to Cu1+. In addition to binding copper, mbs will bind most transition metals and near-transition metals and protect the host methanotroph as well as other bacteria from toxic metals. Several other physiological functions have been assigned to mbs, based primarily on their redox and metal-binding properties. In this review, we examine the current state of knowledge of this novel type of metal-binding peptide. We also explore its potential applications, how mbs may alter the bioavailability of multiple metals, and the many roles mbs may play in the physiology of methanotrophs.
INTRODUCTION
Methanobactins (mbs) were first identified in aerobic methane-oxidizing bacteria (methanotrophs). This remarkable group of bacteria can grow using methane as their sole source of carbon and energy. They are ubiquitous in environments where oxygen and methane are available, and they play a major role in consuming much of the methane produced in the biosphere, thus mitigating its effects in global warming (1–4). Due to their growth on an inexpensive, readily available, and, if generated via methanogenesis (5), renewable carbon source, methanotrophs also have considerable potential for the production of bulk and fine chemicals and for bioremediation of pollutants in the environment (2, 6–8).
The first report of a bacterium growing on methane was by Söhngen, working in Beijerinck's laboratory in Delft in The Netherlands, who in 1906 reported the isolation of Bacillus methanicus from aquatic plants and pond water (9). It was not until 50 years later that this microbe was reisolated and renamed Pseudomonas methanica (10, 11). A second methanotroph, Methylococcus capsulatus (Texas strain), was isolated in 1966 (12). A landmark in methanotroph biology came in 1970, when Whittenbury and colleagues isolated, from a variety of terrestrial and freshwater environments, and described over 100 new aerobic methanotrophs growing on methane (13). They then devised a classification scheme, i.e., type I versus type II, based on the ability of these methanotrophs to grow on methane, pathways of carbon assimilation, formation of resting stages (cysts and spores), morphology, the possession of complex intracytoplasmic membrane arrangements, and the moles percent G+C content of their DNA. Subsequently, Bowman and colleagues isolated a similar number of methanotrophs from various environments and classified them according to the scheme of Whittenbury and colleagues and according to their 16S rRNA phylogeny (14, 15). It is remarkable that though there was no DNA sequencing at the time, the overall classification scheme of Whittenbury and colleagues still remains a robust and convenient way of grouping methanotrophs today.
Accordingly, there are currently 15 genera of methanotrophs within the family Methylococcaceae and 3 in the Methylothermaceae family of the Gammaproteobacteria class. Methylobacter, Methylocaldum, Methylococcus, Methylogaea, Methyloglobulus, Methylomagnum, Methylomarinum, Methylomicrobium, Methylomonas, Methyloparacoccus, Methyloprofundus, Methylosoma, Methylosphaera, Methylosarcina, and Methylovulum are the methanotrophs in the Methylococcaceae family, and Methylohalobius, Methylomarinovum, and Methylothermus are the methanotrophs in the Methylothermaceae family (16–21, 227). Within the class Alphaproteobacteria, the genera Methylosinus and Methylocystis are found in the family Methylocystaceae and the genera Methylocella, Methyloferula, and Methylocapsa in the family Beijerinkiaceae. In the last 15 years, there have been increasing reports of facultative methanotrophs within the Methylocella, Methylocapsa, and Methylocystis genera that can use multicompounds for growth in addition to methane (22–26). Also known today are filamentous methanotrophs from other genera, such as Crenothrix and Clonothrix, and nonproteobacterial (verrucomicrobial) methanotrophs of the genus Methylacidiphilum growing at high temperatures and low pH have also been discovered recently (27). Finally, it was shown that “Candidatus Methylomirabilis oxyferans,” a member of the NC10 phylum, generates dioxygen for the oxidation of methane despite being an obligate anaerobe (28, 29). Taken together, these data clearly illustrate the widespread nature of methanotrophic bacteria in most ecosystems of our planet.
Physiology and Biochemistry of Methanotrophs
Methanotrophs can use methane as an energy source and also to provide carbon for all of their cellular constituents (6, 30, 31). The initial oxidation of methane to methanol is catalyzed by the enzyme methane monooxygenase (MMO). There are two structurally and biochemically distinct forms of MMO, a membrane-associated or particulate MMO (pMMO) and a cytoplasmic or soluble MMO (sMMO), which represent evolutionarily independent solutions to the same molecular problem of methane oxidation (32–37). The sMMO is a three-component binuclear iron active-center monooxygenase that belongs to a large group of bacterial hydrocarbon oxygenases, known as soluble di-iron monooxygenases (SDIMOs) (38), which are also homologous to the R2 subunit of class I ribonucleotide reductase. Two very similar sMMO systems, from Methylococcus capsulatus (Bath) (39–43) and Methylosinus trichosporium OB3b (44–47), have been studied in detail. sMMO is encoded by a six-gene operon, mmoXYBZDC, and has three components: (i) a 250-kDa hydroxylase with an α2β2γ2 structure in which the α subunits (MmoX) contain the binuclear iron active center where substrate oxygenation occurs, (ii) a 39-kDa NAD(P)H-dependent reductase (MmoC) with flavin adenine dinucleotide (FAD) and Fe2S2 prosthetic groups, and (iii) a 16-kDa component (MmoB) known as protein B or coupling/gating protein that does not contain prosthetic groups or metal ions (39, 48). There are X-ray crystal structures for the hydroxylase component (49–52), nuclear magnetic resonance (NMR)-derived structures for protein B (39, 53, 54), and an NMR-derived structure for the flavin domain of the reductase (55). The complex formed by the three components has been studied structurally via small-angle X-ray scattering analysis and biophysically by electron paramagnetic resonance spectroscopy, ultracentrifugation, and calorimetric analysis (56, 57). The catalytic cycle of sMMO has been extensively studied, and excellent progress has been made toward understanding the mechanism of oxygen and hydrocarbon activation at the binuclear iron center (45) (45, 58–62). Detailed reviews of the structure and catalytic mechanisms of sMMO are available (6, 37, 47, 63, 64).
The pMMO, in contrast, is a copper- and possibly iron-containing, membrane-associated enzyme that is associated with unusual intracytoplasmic membranes that take the form of vesicular disks in type I methanotrophs and paired peripheral layers in type II organisms (65–75). Intracytoplasmic membranes are enriched in pMMO and can be physically separated from the cytoplasmic membrane on the basis of sedimentation velocity in sucrose density gradients (76). An understanding of the structure and mechanism of pMMO has emerged more slowly than that for sMMO because of losses of activity when the enzyme is solubilized. pMMO consists of three polypeptides of approximately 49, 27, and 22 kDa encoded by the genes pmoCAB (77). There are often multiple copies of these pmo genes in methanotrophs (78, 79). Recent studies have shown that native pMMO forms a complex with methanol dehydrogenase (MeDH), which may supply electrons to the pMMO (80, 81), similar to what has been found for the hydroxylamine oxidoreductase and ammonia monooxygenase redox couples (82–85).
Some methanotrophs, such as M. capsulatus (Bath) and M. trichosporium OB3b, can produce either form of MMO. Most known methanotrophs possess only pMMO, e.g., Methylomonas methanica, Methylomicrobium album BG8, Methylocystis parvus OBBP, and the verrucomicrobial and NC10 methanotrophs. Only a few methanotrophs within the Beijerinckiaceae family, e.g., Methylocella silvestris and Methyloferula stellata, have sMMO but do not possess pMMO (21, 86).
The methanol produced by MMO is oxidized to formaldehyde by a calcium or rare-earth-dependent pyrroloquinoline quinone (PQQ)-containing MeDH (87–91). Formaldehyde is an important branch point in the metabolism of methanotrophic metabolism and represents the point at which one-carbon (C1) intermediates can be either oxidized to CO2 to derive energy or assimilated into biomass. Since formaldehyde is toxic, methanotrophs must protect themselves against accumulation of this metabolic intermediate. Multiple pathways for metabolism of formaldehyde are found in methanotrophs (2, 26, 92–96). For example, oxidative dissimilation of formaldehyde can occur by its conjugation to tetrahydromethanopterin (H4MPT) (97, 98), via dye-linked membrane-associated (93) or via NAD+-dependent (95, 96, 99) formaldehyde dehydrogenases. Formate, resulting from the oxidation of formaldehyde by formaldehyde dehydrogenases, is further oxidized to carbon dioxide by an NAD+-dependent formate dehydrogenase which generates NADH, which is then available for oxidation of methane, biosynthetic reactions, and energy generation within the cell (100–102). Methanotrophs also possess two pathways for fixation of formaldehyde into biomass, the serine and ribulose monophosphate (RuMP) cycles, which are active in alphaproteobacterial and gammaproteobacterial methanotrophs, respectively. The pathways of carbon fixation in methanotrophs have been reviewed extensively (see, e.g., reference 6).
The “Copper Switch” in Methanotrophs
Early attempts to characterize methane oxidation were complicated by different reports on the cellular location of the MMO. MMOs were described either as soluble or as membrane associated, depending on the strain and, for some strains, on the reporting laboratory. Several groups initially reported activity in the particulate or membrane fraction (103, 104), whereas other groups detected activity in the soluble fraction (105, 106). Subsequent studies showed that the cellular location varied with cultivation conditions. Oxygen limitation was reported to induce methane oxidation in the soluble fraction in M. trichosporium OB3b (36, 107). However, it was subsequently shown that oxygen was not the regulatory factor and that the switch between the membrane-associated and soluble activities was related to biomass concentration (108). The defining moment in the discovery of this “switch” was when Dalton and colleagues attempted to grow M. parvus OBBP to high cell densities in chemostat culture. They observed that cultures of M. parvus OBBP, when supplied with methane, air, and a nitrate mineral salts (NMS) solution at relatively low cell densities, simply stopped growing. However, when additional trace element solution was added, the cultures immediately started growing again. The “secret ingredient” in the trace element solution was narrowed down to copper ions (108). It was subsequently realized that M. parvus OBBP contained only pMMO, resulting in the high requirements for copper ions, and did not contain an sMMO which would have allowed it to grow to the same high cell densities observed at the time with M. capsulatus Bath and M. trichosporium OB3b under copper limitation. The latter strains, when confronted with the same conditions, switched to expression of the sMMO and carried on growing (35, 109). Interestingly, copper had earlier been shown to enhance growth of Methanomonas margaritae, a methanotroph which does not contain sMMO, but these original observations were never investigated further (110).
Dalton and colleagues followed up their observations in detail and established the existence of this “copper switch,” i.e., the regulation of expression of the two different forms of MMO in methanotrophs in response to the copper-to-biomass ratio of cultures of methanotrophs which possess both sMMO and pMMO. This allowed them to explain many of the earlier observations on metal-dependent growth of methanotrophs. For instance, they showed that in M. capsulatus Bath, expression of the sMMO was observed only at high cell densities when copper ions in the medium were depleted, whereas additions of excess copper ions allowed this methanotroph to express active pMMO. Subsequently, Murrell and colleagues showed at the molecular level that under growth with low concentrations of copper ions, expression of sMMO was initiated at a σ54 promoter upstream of the sMMO gene cluster (mmoXYBZDC). Conversely, under high-copper growth conditions, expression of sMMO was repressed, and high levels of expression of the genes encoding pMMO (pmoCAB) allowed both M. trichosporium OB3b and M. capsulatus Bath to grow using pMMO (34, 111–113).
Further research established that copper affects methanotrophic physiology and gene expression much more broadly. For example, it was found that the intracytoplasmic membrane content in methanotrophs increased with increasing copper in the growth medium (66, 109, 114). This was not totally unexpected, however: considering that the pMMO is localized in the intracytoplasmic membranes, then greater expression and activity of pMMO would logically require more of these membranes. More surprisingly, however, it was recently discovered that pMMO and the PQQ-linked MeDH encoded by the mxa operon form a supercomplex anchored in the intracytoplasmic membranes and that electron transfer from the PQQ-linked MeDH to pMMO in vivo may drive the oxidation of methane (80, 81). In support of the latter finding, it was recently found that not only does expression of pmo genes increase with increasing copper, but that of genes in the mxa operon does so as well (91).
It has also been shown by proteomics that additional steps in the oxidation of methane to carbon dioxide are overexpressed with increasing availability of copper, such as that of proteins involved in lipid, cell wall, and membrane synthesis (66, 115). Conversely, the ability of methanotrophs to direct carbon from methane to poly-3-hydroxybutyrate increases with decreasing copper availability (66, 116), suggesting that to some extent energy metabolism of methanotrophs is controlled by copper. Such a conclusion was actually already reached earlier by Dalton and colleagues, who showed that the biomass yield and carbon conversion efficiency in methanotrophs when grown on methane increased as copper increased, i.e., when methanotrophs switch from expressing sMMO to expressing pMMO (117). The “surfaceome,” or proteins on the outer surface of the outer membrane, is also controlled by copper availability in some methanotrophs. The expression of several multi-c-type cytochromes and proteins believed to be involved in copper uptake also varies, and in most cases decreases, as copper availability increases (118–123).
Additional insight into how methanotrophs handle copper has been provided by the recent discovery of the Csps, a new family of copper storage proteins, in M. trichosporium OB3b (124). This methanotroph possesses three Csps: Csp1 and Csp2, which have predicted twin arginine translocase-targeting signal peptides and are therefore thought to be exported after folding, as well as the cytosolic Csp3. Csp1 forms a tetramer of four helix bundles that can bind up to 52 Cu1+ ions via Cys residues that point into the core of the bundle. Switchover to sMMO is accelerated in the Δcsp1 csp2 mutant compared to the wild type, suggesting that these proteins play a role in storing copper for the pMMO and provide an internal copper source when copper becomes limiting. Under such conditions, mb is produced, which can readily remove all Cu1+ from Csp1 and therefore may play a role in helping to utilize Csp1-bound copper.
Evidence Suggesting a Copper-Specific Uptake System
The first evidence for a copper-specific uptake system and for the production of an extracellular copper-binding ligand came during the phenotypic characterization of constitutive sMMO mutants (sMMOC) of M. trichosporium OB3b (125, 126). Phelps et al. (126) isolated five sMMOC mutants by culturing M. trichosporium OB3b in the presence of dichloromethane, acting as a mutagen following its cometabolic transformation by methane monooxygenase to formyl chloride. In addition to the sMMOC phenotype, sMMOC mutants were defective in copper acquisition (125, 127, 128). A drastic increase in soluble versus insoluble copper in the culture medium was also observed and fostered speculation on the production of an extracellular Cu2+-complexing agent(s) analogous to Fe3+-complexing siderophores (127). Subsequent studies revealed the presence of a low-molecular-mass copper-binding ligand, although the identity of this compound was not determined (125, 127).
Initial Identification and Isolation of Methanobactin (a Copper-Binding Compound or “Chalkophore”)
Somewhat paradoxically, the “copper-binding ligand” or “copper-binding compound” was first isolated from M. capsulatus Bath during the purification of pMMO and thus under conditions of high copper concentration (73). Separation of this copper-binding compound from the pMMO revealed a low-molecular-mass yellow-fluorescent copper-containing molecule. The color and fluorescence properties of this molecule were similar to those of the water-soluble pigment observed in cells cultured in low-copper medium (73). Subsequent characterization of this water-soluble pigment revealed that it was identical to the copper-binding compound that copurified with the pMMO (73, 128). The production of water-soluble pigments by methanotrophs had been noted during the initial isolation of many type strains over 40 years ago but was associated with cells cultured in low-iron medium (13). The copper-binding compound was eventually termed methanobactin (mb), based on the molecule's antimicrobial activity toward Gram-positive bacteria (129, 130). Once identified, this copper-binding compound was isolated or identified in a number of different methanotrophs, including Methylomicrobium album BG8 (131), Methylocystis strain SB2 (132), Methylocystis rosea (strain SV97) (133), Methylocystis hirsuta CSC1 (133), and Methylocystis strain M (133). The crystal structures of the mbs from M. trichosporium OB3b (134, 135), M. hirsuta CSC1 (133), and Methylocystis strain M (133) have been determined. Furthermore, the chemical structures of the mbs from Methylocystis strain SB2 (132) and M. rosea (133) have been deduced. This review will focus on these mbs that have allowed putative mbs to be identified from methanotrophs with sequenced genomes.
Methanobactins as Chalkophores
Functionally, mbs are similar to siderophores. Like siderophores, they are all low-molecular-mass (<1,200-Da) compounds produced by bacteria under low-copper conditions (125, 126, 128, 136–138). Following the nomenclature of siderophores, which is from the Greek for iron bearing or iron carrying, mbs are chalkophores (copper bearing or copper carrying) (134, 139). mbs are currently the only known representatives of this group. Consistent with their role as the extracellular component of a copper acquisition system, mbs have some of the highest known binding affinities for copper ions (2, 131, 135, 138, 140, 141) (Table 1).
TABLE 1.
Metal binding affinities (K) of mbs from M. trichosporium OB3b, Methylocystis strain M, Methylocystis hirsuta CSC1, Methylocystis rosea, and Methylocystis strain SB2a
| mb | Method | pH | Reference(s) |
K (M−1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu1+ | Cu2+ | Cu2+/Cu1+ b | Au3+ | Ag1+ | Hg2+ | Pb2+ | Cd2+ | Co2+ | Fe3+ | Mn2+ | Ni2+ | Zn2+ | ||||
| mb4-OB3b | ITC | 6.8 | 141, 148 | —c | — | 3.2 × 1034 | 1.0 × 1040 | — | UTF | — | — | — | — | — | — | — |
| mb2-OB3b | 6.8 | — | — | 2.6 × 108 | 1.0 × 105 | 2.6 × 107 | 9.9 × 109 | — | — | — | — | — | — | — | ||
| mb-OB3b | 6.8 | — | — | 6.5 × 106 | 1.8 × 105 | 4.7 × 104 | 9.0 × 105 | 6.8 × 105 | — | — | — | — | — | — | ||
| mb4-OB3b | PT | 6.8 | 172 | — | — | 1.0 × 1058 | — | — | — | — | — | — | — | — | — | — |
| mb2-OB3b | 6.8 | — | — | 1.0 × 1052 | — | — | — | — | — | — | — | — | — | — | ||
| mb-OB3b | 6.8 | — | — | 1.0 × 1025 | — | — | — | — | — | — | — | — | — | — | ||
| mb-OB3b | Competition titrations with BCS | 7.5 | 135 | ∼7 × 1020 | — | — | — | — | — | — | — | — | — | — | — | — |
| mb-OB3b | 7.5 | — | ∼3 × 1012d | — | — | — | — | — | — | — | — | — | — | — | ||
| mb-M | Competition against mb-OB3b | 7.5 | 133 | ∼1 × 1021 | — | — | — | — | — | — | — | — | — | — | — | — |
| mb-M | 7.5 | — | ∼2 × 1011d | — | — | — | — | — | — | — | — | — | — | — | ||
| mb-CSC1 | Competition titrations with BCS | 7.5 | 133 | ∼8 × 1020 | — | — | — | — | — | — | — | — | — | — | — | — |
| mb-CSC1 | 7.5 | — | ∼5 × 1014d | — | — | — | — | — | — | — | — | — | — | — | ||
| mb-roseae | Competition against mb-OB3b | 7.5 | 133 | ∼5 × 1020 | — | — | — | — | — | — | — | — | — | — | — | — |
| mb-rosea | 7.5 | — | ∼7 × 1011d | — | — | — | — | — | — | — | — | — | — | — | ||
| mb4-SB2 | DITC | 6.9 | 154 | — | — | 7.6 × 1026 | — | — | — | — | — | — | — | — | — | — |
| mb2-SB2 | DITC | 6.6 | — | — | 1.2 × 109 | — | — | — | — | — | — | — | — | — | — | |
| mb-SB2 | DITC | 6.9 | — | — | 1.0 × 106 | — | — | — | — | — | — | — | — | — | — | |
| mb-SB2 | Competition titrations with TRIEN | 6.8 | — | — | 1.9 × 1027 | — | — | — | — | — | — | — | — | — | — | |
| mb-SB2 | ITC | 6.8 | — | — | — | — | 1.2 × 108 | — | — | — | — | — | — | — | — | |
| mb4-OB3b | ITC | 6.8 | 148 | — | — | — | — | — | — | — | 1.3 × 106 | — | 9.7 × 105 | — | — | 4.5 × 106 |
| mb2-OB3b | 6.8 | — | — | — | — | — | — | — | 1.1 × 107 | 1.1 × 106 | 1.7 × 105 | 7.7 × 105 | 4.9 × 105 | 1.8 × 104 | ||
Abbreviations, ITC, isothermal titration calorimetry; PT, potentiometric titration; DITC, displacement isothermal titration calorimetry; mb, methanobactin; mb2, mb dimer; mb4, mb tetramer; UTF, unable to fit; BCS, bathocuproine disulfonate; TRIEN, triethylenetetramine.
Copper was added as Cu2+, and therefore the oxidation state of the copper bound by mb is in question due to its ability to reduce Cu2+ to Cu1+.
—, not determined.
Calculated using the reduction potential of the Cu2+-mb/Cu1+-mb couple.
For the form of mb-rosea missing the C-terminal Asn and Thr residues.
Although chalkophores and siderophores share a number of properties, these two groups of metal-binding compounds can be distinguished in a number of ways. With the exception of the phytosiderophore domoic acid (142), siderophores are expressed under iron limitation, whereas chalkophores are expressed under copper limitation. Many siderophores bind copper, and chalkophores can bind iron (142–148); however, different metal binding constants characterize the two groups, and colorimetric assays have been developed to distinguish between them based on this difference (138, 149, 150). Structurally, chalkophores differ from siderophores by their typical heterocyclic rings and associated thioamide groups (Fig. 1 and 2). Different ring systems have characteristic UV-visible absorbance, circular dichroism (CD), and fluorescent spectral properties, which can be used for identification and to characterize the metal binding properties of the molecule (131, 133–136, 138, 148, 151–153). Excitation energy transfer occurs between the two rings in mb (136, 154), as observed for the chromophores of light-harvesting complexes (155–157), resulting in the fluorescent properties of mbs. For example, the emission intensity of mbs increases with selective hydrolysis of the one of the rings, and emission intensity often increases following metal addition (132, 136–138, 141, 148, 154). Again, this property can be used in both identification and characterization of mbs.
FIG 1.
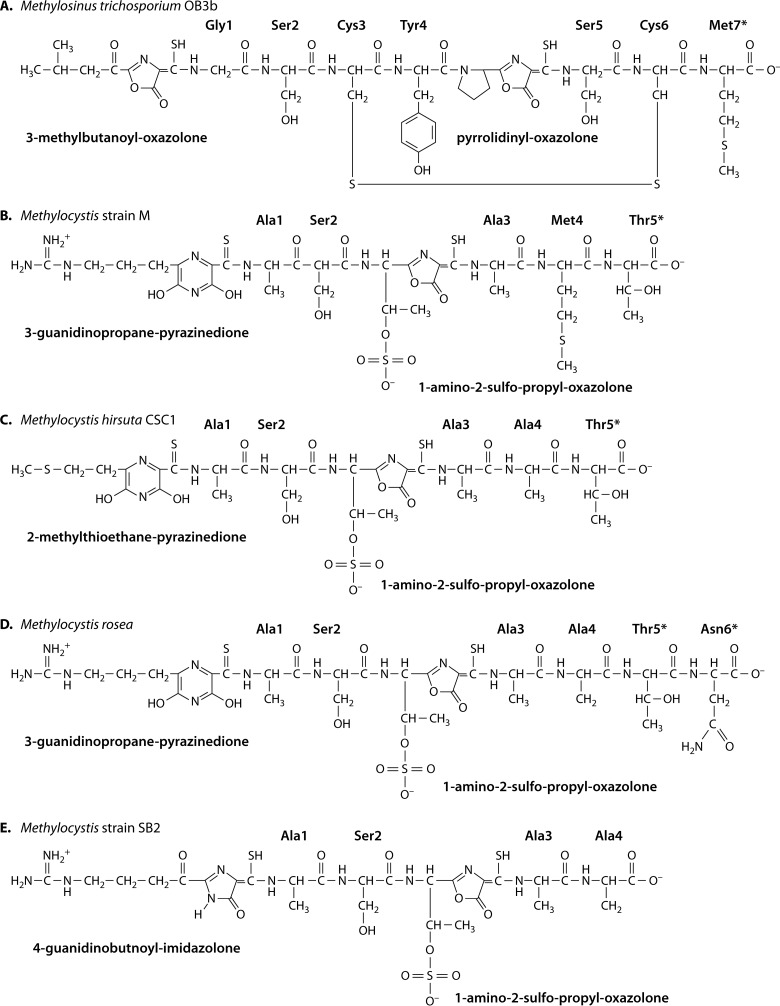
Chemical structures of full-length mb-OB3b (135, 151) (A), mb-M (133) (B), mb-CSC1 (133) (C), mb-rosea (133) (D), and mb-SB2 (132) (E). Amino acids marked with asterisks are observed in some but not all samples.
FIG 2.
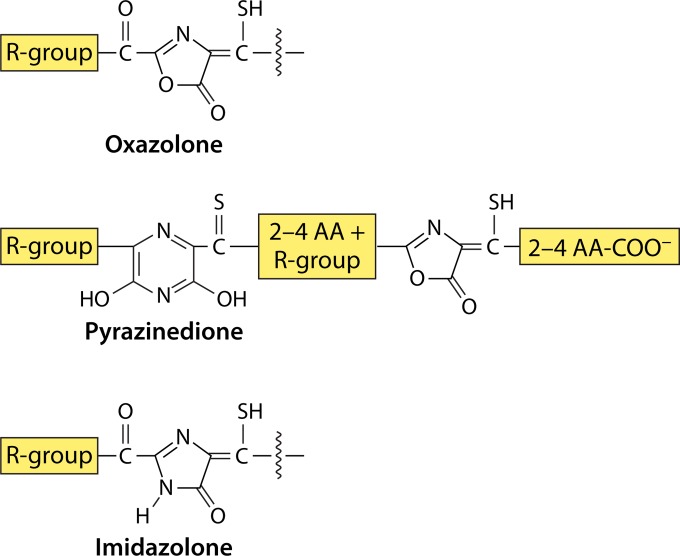
Core features of mbs. AA, amino acid(s). R-groups can be Arg, Ile, Met, or Pro.
However, not all of the properties just described are sufficient to identify mbs. For instance, Clostridium cellulolyticum produces a copper-binding secondary metabolite, closthioamide, with a molecular mass similar to that of mbs (158–160), and like mbs, closthioamide has thioamide groups, will reduce Cu2+ to Cu1+, and has a high Cu1+ binding affinity (>1015 M−1). Closthioamine will also test positive with the copper-chrome azural S (Cu-CAS) assay, a liquid or plate assay used to screen for mb production (138, 149, 161). However, closthioamide can be distinguished spectroscopically from mbs; e.g., closthioamide shows a single UV-visible absorption maximum at 270 nm arising from its two characteristic phenolic groups separated by six thioamide moieties. Furthermore, closthioamide is able to form a dinuclear Cu1+ complex. Also in contrast to that of mbs, closthioamide synthesis is not regulated by copper, and unlike for mbs, the molecule is believed to be produced by a polyketide synthase (see below).
Similarly, under low-copper conditions, Paracoccus denitrificans also produces a low-molecular-mass 716.18-Da porphyrin, coproporphyrin III, that appears to be involved in copper acquisition (162). Unfortunately, the copper binding properties were not reported in the initial publication, and we are not aware of any follow-up studies. Coproporphyrin III has a typical heme UV-visible absorption spectrum and shows different γ, α, and β maxima depending on the metal coordinated by the heme group, allowing it to be distinguished from mbs based on this property.
STRUCTURAL PROPERTIES OF METHANOBACTINS
Structural Diversity and Core Features
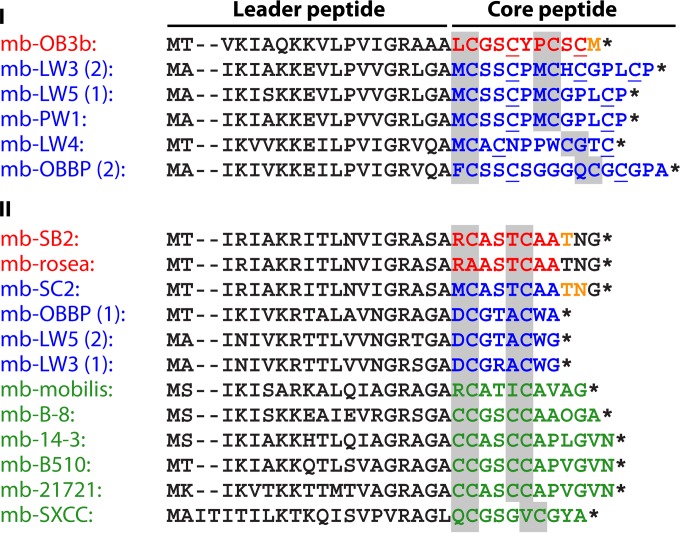
The core structural features of mbs are shown in Fig. 1 and 2. As mentioned above, mbs are modified peptides characterized by the presence of one oxazolone ring and a second oxazolone, imidazolone, or pyrazinedione ring, which are separated by 2 to 4 amino acid residues (see Biosynthesis of Methanobactin below). Each ring has an adjacent thioamide group. Structurally, mbs can be divided into two types (Fig. 3 and 4). One type (group I) is represented by mb from M. trichosporium OB3b (mb-OB3b) (Fig. 1A, 3A, and 4). In addition to these core properties, mb-OB3b also contains Cys residues in the mature peptide, which are linked by an intramolecular disulfide bond (Fig. 1A and 3A). Cu1+-mb-OB3b has a pyramid-like shape with the metal coordination site at the base (133–135). The disulfide bond is present in both apo- and Cu1+-mb-OB3b. mb-OB3b is structurally the most complex mb characterized so far and is currently the only structurally characterized representative of this mb type. However, based on sequence similarity and alignments, the putative mbs from Methylosinus sp. strain LW3 (mb-LW3), Methylosinus sp. strain LW5 (mb-LW4), and Methylosinus sp. strain PW1 (mb-PW1) and one of the two mbs from M. parvus OBBP [mb-OBBP(1)] would also fall within this group (Fig. 4). In this group, the core peptide is predicted to contain 2 or more Cys residues in the mature peptide and/or to contain an additional ring(s). If the Cys residues are not incorporated into a ring, the second Cys and either the third or fourth Cys are predicted to form an intermolecular disulfide bond.
FIG 3.
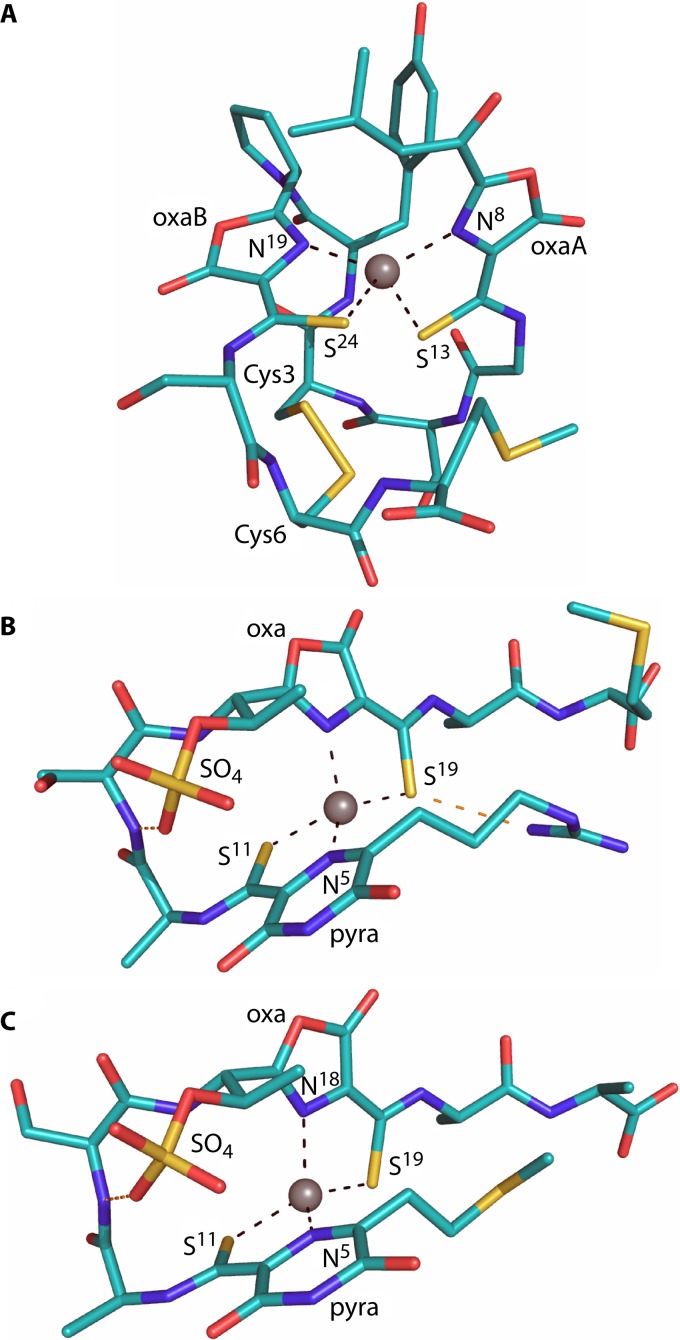
Crystal structures of Cu1+-mb-OB3b (A), Cu1+-mb-M (B), and Cu1+-mb-CSC1 (C), with a Thr residue missing at the C terminus in panels B and C compared to the largest form isolated. The copper ions are represented as gray spheres, and the oxazolone (oxa) and pyrazinedione (pyra) rings are labeled, as are the coordinating atoms. Hydrogen-bonding interactions are shown in panels B and C as dashed orange lines.
FIG 4.
mb precursor peptides. Sequences were detected in bacteria with known genome sequences, in methanotrophs with characterized mbs as well as from other methanotrophs, and from selected nonmethanotrophs, directly from the DNA sequence using Fuzztran in Mobyle (http://mobyle.pasteur.fr), with the optimized mb sequence motif (Prosite format) [ILMV]-[AIKST](1,3)-[IV]-[KNRT]-[IV]-X-[AKQ]-[KRT]-X-[ILM]-X-[IV]-X-[GV]-R-X(2)-[AL]-X-C(1,2)-[GA](0,1)-[ST](0,2)-X(0,2)-C(1,2) as a query. Only sequences also featuring downstream mb biosynthesis cassette genes mbnB and mbnC (see the text and Table 2) are shown. Known and predicted leader sequences and sequences not observed in the final product are shown in black, sequences detected in bacteria of known genome sequence from methanotrophs with mbs either whose crystal structure has been determined (mb-OB3b) or whose primary structures were predicted (mb-SB2 and mb-rosea) are shown in red, sequences detected in bacteria of known genome sequence from methanotrophs are shown in blue, and sequences detected in bacteria of known genome sequence from nonmethanotrophs are shown in green. Amino acids observed in some but not all samples are shown in tan. Amino acids with a gray background represent the amino acid pair shown or predicted to be posttranslationally modified into an oxazolone, imidazolone, or pyrazinedione group in structurally characterized mbs; underlined Cs represent Cys residues known or predicted to be present in the mature peptide. mbs were from M. trichosporium OB3b (mb-OB3b), Methylosinus sp. strains LW3 (mb-LW3), LW4 (mb-LW4), and PW1 (mb-PW1), M. parvus OBBP (mb-OBBP), M. rosea (mb-rosea), Methylocystis strains SB2 (mb-SB2), SC2 (mb-SC2), and LW5 (mb-LW5), Cupriavidus basiliensis B-8 (mb-B-8), Pseudomonas extremaustralis 14-3 (mb-14-3), Azospirillum sp. strain B510 (mb-B510), Tistrella mobilis KA081020-065 (mb-mobilis), Comamonas composti DSM 21721 (mb-21721), and Gluconoacetobacter sp. strain SXCC (mb-SXCC). Numbers in parentheses after a strain name are used for species with more than one mb gene cluster. Stop codons are indicated by an asterisk and gaps in the sequence alignment by a hyphen.
The second group (group II) is represented by the isolated mbs from M. rosea (mb-rosea) and Methylocystis strain SB2 (mb-SB2), which show high similarity to the structurally characterized (133) mbs from M. hirsuta CSC1 (mb-CSC1) and Methylocystis strain M (mb-M). In this group, mbs lack the Cys residues in the mature peptide and are smaller and probably less rigid due to the absence of the disulfide bond found in mb-OB3b. Heterocyclic rings are separated by two or three amino acids. In contrast to members of group II mbs from methanotrophic organisms, putative mbs from nonmethanotrophs (mb-B-8, mb-14-3, mb-B510, and mb-21721) contain four Cys residues in the apo-protein. However, based on the location of Cys residues, we predict that all 4 Cys residues are incorporated in the two heterocyclic rings. mbs from the structurally characterized members in this group contain a sulfate group, which appears to aid in the formation of a tight bend in the molecule (Fig. 3B) by making a hydrogen bond with the backbone amide of Ser2. The sulfate group also increases affinity for copper ions (133). Overall, mbs from the Methylocystis strains display a hairpin-like shape (Fig. 3B and C) (133). The conserved T/S adjacent to the putative C-terminal ring suggests that the other members of this group also contain a sulfate group (Fig. 4).
Truncated forms have been identified for all characterized mbs (133, 135, 138), which results from the loss of one or more C-terminal amino acid residues (Fig. 1 and 4). The different forms show very similar copper binding properties (133, 135) but distinct reduction potentials (133, 135) and spectral properties (138). The mechanism leading to the loss of C-terminal amino acids remains an open question but does not appear to involve N-protonation as observed for microcin B17 (163), since the C-terminal oxazolone ring of mb remains intact. In microcin B16, this reaction results in autoproteolysis of the ring and protein splicing. In Methylocystis strains, the sulfate group can also be lost, which does affect copper affinity (133).
mbs thus have a number of unusual structural features. Modifications of two residues to form oxazolone or imidazolone rings are rare but have been observed in several classes of ribosomally synthesized and posttranslationally modified peptide (RiPP) secondary metabolites (163–166). Pyrazinedione rings are even more uncommon and have been detected only in the non-amino-acid-containing molecules selerominol (167) and flutamide (168) from fungi. The presence of thioamide groups in natural products is also rare and has been identified only in closthioamide from Clostridium cellulolyticum (153, 160) and thioviridamide from Streptomyces olivoviridis (169, 170). Although rare, O-sulfonation of Ser or Thr has been reported in eukaryotic proteins (171), but to our knowledge, mb is the only bacterial peptide that contains this posttranslational modification and is ribosomally produced, as described below.
Copper Coordination Site
In the three mbs whose crystal structures have been determined, Cu1+ is coordinated in a similar manner by an N2S2 ligand set with a distorted tetrahedral geometry (Fig. 3) (133–135). The C-terminal S and N ligands in the mb-CSC1 and mb-M are switched compared to their position in mb-OB3b (133); however, the resulting coordination geometry is very similar. The coordination sites are stabilized by the two 5-membered chelate rings formed upon ligation and a number of hydrogen bonding and π interactions (Fig. 3), for example, between the backbone amide of Cys3 and a coordinating sulfur in Cu1+-mb-OB3b and a π-anion interaction between the sulfate and the pyrazinedione ring in mb-CSC1 and mb-M (133, 135).
METAL BINDING PROPERTIES
Binding and Reduction of the Primary Metal, Copper
mb binds both Cu2+ and Cu1+, and the binding by mb appears to depend on pH (135, 172) and on the ratio of Cu2+/Cu1+ to mb (136, 141, 172) (Table 1). As discussed below, mb is able to complex both soluble and insoluble forms of Cu1+ and Cu2+. Thermodynamic, spectral, and kinetic studies have been carried out on the addition of Cu2+ to mb-OB3b and mb-SB2 (136, 141). These studies (136, 141, 172) are complicated by the fact that mb rapidly reduces Cu2+ to Cu1+. Since the oxidation state of copper responsible for the high bonding constant for experiments in which Cu2+ has been added to apo-mbs is not known, we will indicate that a mixture of these oxidation states is present by using “Cu2+/Cu1+” from this point on. At low ratios of Cu2+/Cu1+ to mb-OB3b, mb-OB3b initially binds Cu2+/Cu1+ as an oligomer/tetramer (136, 141). Pre-steady-state kinetic data also suggest that the initial binding of metal is on only one of the rings and its associated thioamide. In mb-OB3b, initial Cu2+/Cu1+ coordination is to the oxazolone A, followed by a short (8- to 10-ms) lag period and then coordination to oxazolone B (141). At higher ratios of Cu2+/Cu1+ to mb-OB3b, mb-OB3b coordinates Cu2+/Cu1+ as a dimer, followed by a monomer at Cu2+/Cu1+-to-mb-OB3b ratios above 0.5 Cu2+ per mb-OB3b. mb-SB2 appears to follow a similar tetramer-dimer-monomer binding sequence depending on the copper-to-mb ratio. For mb-SB2, the initial binding of Cu2+/Cu1+ is to the imidazolone ring, followed by coordination to the oxazolone ring (154).
A number of different methods have been used to determine metal binding affinity constants for mbs (Table 1). Affinities for Cu1+ can be determined from competition studies using a well-established approach (173–175) with chromophoric ligand such as bathocuproine disulfonate. The measurement of the reduction potential of the Cu-mb then allows the Cu2+ affinity to be calculated. The Cu1+ affinity is ∼1021 M−1 for all mbs analyzed using this approach (133, 135) and is one of the highest known for biological systems. Such a high affinity raises the issue of how copper is released from mb within a methanotroph. The reduction of the disulfide, in mb-OB3b (135), and removal of the sulfate, in mb-CSC1 (133), do decrease the Cu1+ affinity by ∼2 orders of magnitude, but the values are still high. Given that the Cu2+ affinities are significantly weaker (Table 1), oxidation may be utilized for release of the metal, which may be more likely for those Cu-mbs with lower reduction potentials, such as Cu-mb-CSC1 (133).
Other copper-chelating compounds, primarily for Cu2+, have also been used to estimate affinities (91, 125, 128, 133, 135), as well as isothermal titration calorimetry (136, 141) and displacement isothermal titration calorimetry (136) (Table 1). mbs have been shown to solubilize and bind insoluble forms of Cu1+ under anaerobic conditions (141) and to extract Cu from copper minerals (176), humic material (177), and glass (178). The ability of mbs to extract copper from copper-containing minerals as well as other organic molecules should be considered in modeling bioavailable sources of copper (179–183).
Binding of Other Metals
In addition to copper, mb-OB3b and mb-SB2 have been shown to bind a number of transition and near-transition metals (137, 148, 154, 184) (Table 1). In general, the spectral properties following binding are unique for each metal and can be used in initial analysis of metal binding and in metal competition studies (137, 154). The primary function of mbs appears to be copper acquisition, and the capacity to bind other metals appears to be an inadvertent consequence of the Cu1+ coordination site. The peptide backbone of mbs appears to influence the binding of other metals. For example, the order of metal binding preference by mb-OB3b is Cu2+/Cu1+ = Hg2+ = Au3+ > Zn2+ > Cd2+ > Co2+ > Fe3+ > Mn2+ > Ni2+, whereas the metal binding preference for mb-SB2 is Au3+ > Hg2+ > Cu2+/Cu1+ > Zn2+ > Cd2+ = Co2+ = Fe3+ > Mn2+ > Ni2+ (137, 154).
Although strong binding of other metals such as mercury by mbs may seem counterproductive for methanotrophic growth, it may actually be of some benefit. Specifically, by strongly binding mercury, mbs may significantly alter its speciation and bioavailability, thereby reducing its toxicity. Indeed, growth studies showed that in the presence of mercury, added as mercuric chloride, methanotrophic growth was completely inhibited, but growth did occur when mb was also added (184). Interestingly, such a protective effect was seen in a variety of methanotrophs when exposed to mercury and mb-OB3b. These results suggest that mb secreted by any methanotroph could serve to protect the broader microbial community from mercury toxicity (184).
Further indirect evidence of the importance of mb binding to metals other than copper was afforded by the observation that in M. trichosporium OB3b sMMO is expressed and active in the presence of both gold and copper. This finding was unexpected since, as discussed above, copper strongly represses expression of sMMO. Also, the amount of copper associated with biomass significantly decreased in the presence of gold, compared to when gold was not added (91). This suggests that mb-OB3b is limited in its ability to bind copper in the presence of gold. Indeed, if mb was preloaded with copper and added exogenously to M. trichosporium OB3b, sMMO expression was not observed, and copper levels associated with biomass increased (91). These findings suggest that although methanotrophs synthesize mb for copper uptake, such uptake may be compromised if other metals such as gold are also present due to their ability to compete effectively for binding to mb. These observations suggest that in such situations, sMMO may be expressed and active even in the presence of copper, which may alter the methanotrophic community structure as well as activity.
Metal Reduction
As mentioned above, another property exhibited by all mbs examined so far is their ability to reduce Cu2+ to Cu1+ (133, 135, 136, 141, 185). In addition, mb-OB3b and mb-SB2 have also been shown to reduce Au3+ and Ag+ to Au0 and Ag0, respectively (137, 148). In the case of gold, no other oxidation states were observed. In contrast, no change in the oxidation states of Zn2+, Cd2+, Co2+, Fe3+, Mn2+, and Ni2+ was observed following binding by mb-OB3b and mb-SB2 (137, 148). Surprisingly, in the absence of an external reductant, both mb-OB3b and mb-SB2 have been shown to reduce multiple Cu2+and Au3+ ions, which raises the question of the electron donor for this reaction.
BIOSYNTHESIS OF METHANOBACTIN
Identification of the Polypeptide Precursor of Methanobactin
Although a potential peptide sequence precursor of mb-OB3b was identified following hydrolysis of the oxazolone rings (132), the question whether this peptide was assembled by a nonribosomal peptide synthetase or encoded by DNA and synthesized on the ribosome remained. Determination of the genome sequence of M. trichosporium OB3b helped resolve this question. BLAST searches using the predicted mb peptide precursor revealed a short open reading frame (ORF) with a perfect match at a location within the M. trichosporium OB3b genome sequence where no protein product had been predicted (Fig. 4). The genome region of the putative mb precursor matching sequence in M. trichosporium OB3b had a number of distinctive and striking features (Fig. 5; Table 2). These included (i) a precursor peptide composed of leader and core peptide sequences followed by a stop codon, as expected for posttranslationally modified peptide natural products (164), (ii) a potential cleavage site between the leader and core peptide, suggestive of secretion, and (iii) genes upstream and downstream of the mb precursor gene encoding protein sequences compatible with possible roles in maturation of the mb precursor sequence, transport, and regulation of mb biosynthesis (Fig. 5, Table 2).
FIG 5.
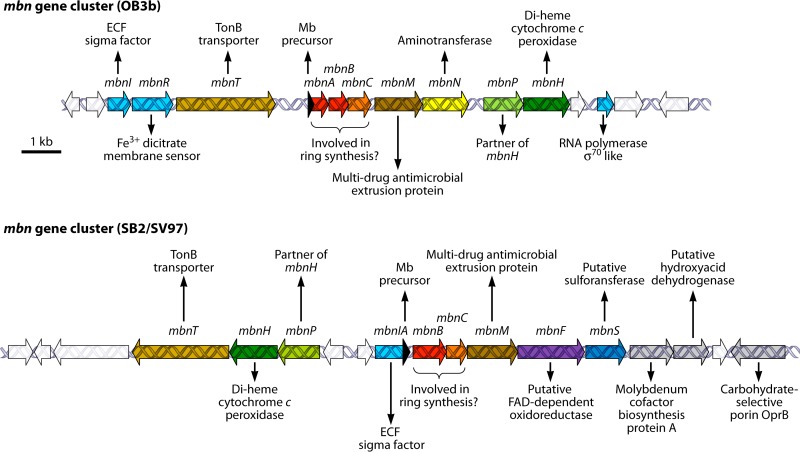
mb gene clusters. Gene clusters of complete genomes of methanotrophs M. trichosporium OB3b (OB3b) and Methylocystis sp. strain SB2 and M. rosea SV97 (SB2/SV97), which produce mbs whose chemical structures have been determined/deduced (Fig. 1). Shown are the gene for the mb peptide precursor MbnA (black) and associated genes for the mb biosynthesis cassette protein MbnB (red), mb biosynthesis cassette protein MbnC (orange), MATE efflux pump MbnM (dark brown), aminotransferase MbnN (yellow), diheme cytochrome c peroxidase MbnH (dark green) and associated MbnP of unknown function (light green), FAD-dependent oxidoreductase MbnF (violet), sulfotransferase MbnS (dark blue), TonB-dependent receptor domain MbnT (light brown), FecI-like RNA polymerase sigma-70 domain MbnI (light blue), and FecR-like membrane sensor MbnR (light purple). Other genes of unknown function are shown in white, and genes flanking the clusters with predicted functions not thought to be associated with mb maturation or transport are shown in gray.
TABLE 2.
Genes in the methanobactin gene cluster
| Gene | Proposed annotation | Characteristics/comments | InterProScan ID |
|---|---|---|---|
| mbnA | mb peptide precursor | Short ORF with N-terminal leader peptide | NA |
| mbnB | mb biosynthesis cassette protein MbnB | TIM barrel enzyme (aldolase/isomerase type) | IPR026432, IPR0078801 |
| mbnC | mb biosynthesis cassette protein MbnC | Less well defined than MbnB | IPR023973 |
| mbnE | FAD-dependent oxidoreductase | Often also found when mbnH and mbnP are missing | IPR003042 |
| mbnH | Di-heme cytochrome c peroxidase | IPR023929, IPR004852 | |
| mbnI | RNA polymerase sigma 70 domain | Putative σ70 factor; in some cases (e.g., strains SB2, SC2, SV97), methanobactin precursor peptide is the C-terminal peptide of MbnI | |
| mbnM | MATE efflux pump | Multiantimicrobial extrusion protein | IPR004839, IPR005814 |
| mbnN | Aminotransferase | Rarely found (e.g., strains OB3b, LW4), pyridoxal-phosphate dependent | IPR023977 |
| mbnP | Partner of mbnH | Conserved protein of unknown function | |
| mbnR | FecR-like, putative sigma factor activator | IPR000863 | |
| mbnS | Sulfotransferase | Rarely found (e.g., strains SB2, SC2, SV97); sulfonation of Thr? | IPR012373 |
| mbnT | TonB-dependent receptor | Plug domain, large membrane protein family | IPR000531 |
Elaboration on this initial search revealed a series of genomes containing gene clusters with characteristics matching those of the M. trichosporium OB3b mb gene cluster, e.g., in M. parvus OBBP (186, 187) and Methylosinus sp. strain LW3 (186), as well as nonmethanotrophs Azospirillum sp. strain B510 (132, 187), Azospirillum sp. strain B506 (186), Pseudomonas extremaustralis (187), Pseudomonas extremaustralis subsp. laumondii TT01 (186), Tistrella mobilis (187), Gluconacetobacter sp. strain SXCC (132, 186). Gluconacetobacter oboediens (186, 187) Methylobacterium sp. strain B34 (186), Cupriavidus basilensis B-8 (186), Photorhabdus luminescens (186), and Vibrio caribbenthicus BAA-2122 (186). Notably, this list includes not only methanotrophic strains known to produce mb for which a genome sequence was available but also genomes of other strains, suggesting that such gene clusters may encode peptide-derived products with more diverse functions than those associated with mbs.
Considering the mb precursor peptides identified so far, it is striking that the leader peptides are much more strongly conserved than core peptide sequences (Fig. 4). It is also interesting to note that sequence conservation does not follow taxonomic affiliation and that identical precursor sequences are found in gene clusters of strains from different genera (Fig. 4). This strongly suggests that horizontal gene transfer has contributed to dissemination of mb gene clusters in the environment, and indeed, mobile genetic elements have often been identified in the sequences immediately adjacent to mb gene clusters (186).
The lack of sequence conservation in mb core peptides also implies that designing sequence motifs to detect mb gene clusters is challenging and that database hits using sequence motifs based on known mb precursor sequences need to be substantiated with other evidence. In our view, for a sequence hit to be considered indicative of an mb precursor, additional evidence should include (i) the presence of the only conserved genes in the mb gene cluster (Fig. 5), i.e., the genes for mb biosynthesis cassette proteins MbnB and MbnC, in the immediate vicinity of the mb peptide precursor and (ii) the presence of 2 or more cysteines in the core mb peptide sequence (Fig. 4; Table 2). Regarding the latter, it is striking that mb peptide precursors identified so far contain either 2 or at least 4 cysteines, just as in the two types of mbs characterized so far. Nevertheless, the spacing between cysteine residues in the mb peptide precursor sequence appears to be quite variable, with 1 or 2 residues between the first and the second cysteines and, if present, 2 to 4 residues between the second and the third cysteines and 1 to 3 residues between the third and the fourth cysteines, respectively (Fig. 4). It is also striking to note that “doublets” of two cysteine residues immediately adjacent to each other have so far been found only in putative mb precursor peptides of presumably nonmethanotrophic strains (Fig. 4).
The overall structure of mb gene clusters and related gene clusters is beyond the scope of this review, but their analysis, using a combination of sequence-based, motif-based, and gene synteny-based searches, will undoubtedly yield many interesting findings in the future, as more mb gene clusters are identified in the increasing number of microbial genomes that are being sequenced. Summarizing the quite large diversity of mb gene clusters that have already been detected (see, e.g., reference 186), two major types of mb gene clusters seem to emerge with respect to the localization of the mbnA gene for the mb precursor peptide (Fig. 5). Whereas mbnA is always directly upstream of and in the same orientation as mbnB and mbnC, it may be found as a short ORF, as exemplified for strain OB3b (Fig. 5), or actually represent the 3′ end of regulatory gene mbnI, as in Methylocystis strain SB2 and M. rosea (strain SV97) (Fig. 5). The implications of the latter localization of the mbnA gene, notably in terms of regulation of mb expression in strains with this gene arrangement (also see Regulation of Gene Expression below), are completely unknown at present. Currently, the cytochrome c peroxidase MbnH (Fig. 5; Table 2), as well as the FAD-dependent oxidoreductase MbnF, present instead or sometimes in addition to MbnH in methanotroph gene clusters (186, 187), are likely candidates to be involved in the oxidation steps required for ring formation (see below) (Fig. 6). In addition, the aminotransferase MbnN, found in the mb-OB3b but not the mb-SB2/mb-rosea gene cluster (Fig. 5), may be involved in formation of the N-terminal keto-isopropyl group from Ile1 in the peptide precursor, and the sulfotransferase MbnS, found in the mb-SB2 and mb-rosea but not the mb-OB3b gene cluster, may catalyze sulfonation of the threonine (Fig. 1 and 5). Two other gene products, TonB receptor and multidrug and toxin extrusion (MATE) protein) (Fig. 5; Table 2) have been suggested (186, 187) to be involved in uptake and secretion of mature mbs, respectively. See a recent review by Kenney and Rosenzweig (186) for a detailed description and phylogenetic analysis of several mb gene clusters.
FIG 6.
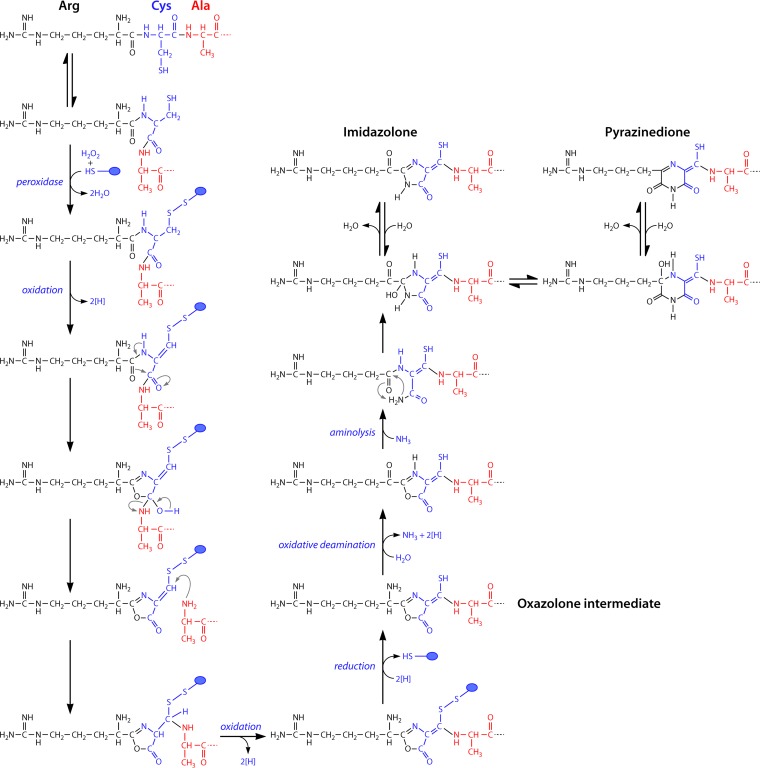
Proposed reaction schemes for biosynthesis of the oxazoline rings with associated thioamide groups via a tandem two-step sequence of peroxidation and dehydration reactions. Cysteine thiols are likely protected against oxidation, possibly as disulfides involving one of the proteins of the mb gene cluster (blue circles). For imidazolone and pyrazinedione ring formation, oxazolone rings are modified via a transamination/deamination step followed by an aminolysis step to open the oxazolone ring followed by ring formation and dehydration.
Current Hypotheses on the Nature of the Methanobactin Biosynthesis Pathway
Despite the identification of the genetic determinants of mb production, the precise mechanism involved in maturation of mbs from peptide precursors still is an open question. The presence of heterocyclic rings would suggest a pathway similar to that of other postribosomal peptide synthesis (PRPS) proteins (163–166, 188). For instance, in the PRPS protein microcin B17, McbB (cyclodehydrase), McbC (flavin mononucleotide [FMN] dehydrogenase), and McbD convert Gly-Cys and Gly-Ser dipeptide sequences into thiazole and oxazole rings, respectively (163). In this reaction sequence, the cyclodehydrase catalyzes ring formation via the amine bond with the thiol or alcohol, followed by oxidation by an FMN dehydrogenase (163–166). In mb, ring formation is initiated from an X-Cys dipeptide sequence, resulting in formation of either an oxazolone, imidazolone, or pyrazinedione ring with a neighboring thioamide group. If the catalytic sequence in ring formation in mb followed the microcin B17 example, the thiol group on Cys from mb would have to be replaced by a hydroxyl group, possibly with an amine intermediate. The thioamide group then would have to be introduced via an amide-to-thioamide replacement, as proposed for two other natural products with thioamide groups (153), closthioamide from Clostridium cellulolyticum (160) and thioviridamide from Streptomyces olivoviridis (169, 170). As an alternative, the thioamide group of mbs was proposed to originate from the Cys thiol, as hydrolysis of the oxazolone rings resulted in an X-Gly-thioamide sequence (132). Based on the precursor peptide sequences that have now been identified, the latter reaction mechanism now appears highly likely.
The absence of a cyclodehydrase-like gene in mb gene clusters also suggest that ring formation differs from that of other natural products in other aspects as well. The simplest and shortest reaction sequence in oxazolone ring formation in mbs would involve an oxidation followed by a rearrangement that changes the connectivity of the peptide backbone (Fig. 6). In such a scheme, Cys thiols would likely need to be protected against oxidation (blue circle), as protein-linked thioesters, or alternatively as disulfides (as shown in Fig. 6), possibly via glutathionylation as observed with MetE in Escherichia coli (189). This reaction may occur during the initial reaction of peroxidase, which is one of the proteins of the mb gene cluster (Table 2). The reaction sequence could operate during formation of both oxazolone rings of mb-OB3b, from Leu1 and Cys2 and from Pro7 and Cys8 of the precursor peptide, respectively. Methylocystis strain SB2 and M. rosea share the same mb gene clusters and identical precursor peptides (Fig. 4 and 5), but the mature mb-SB2 (132) as characterized is suggested to contain an imidazolone ring, whereas mb-rosea is predicted to have a pyrazinedione ring that is also present in mb-CSC1 and mb-M (133). However, imidazolone and pyrazinedione rings are actually isomeric and can be interconverted by a simple sequence of hydration, rearrangement, and dehydration reactions (190). Nevertheless, the mechanism of formation of this ring is likely to be more complex than that of oxazolone ring formation (Fig. 6). One possible sequence would replace the transamination reaction, proposed for mb-OB3b, with an oxidative deamination of the N-terminal amine, which would produce ammonia that could be used for subsequent aminolysis of the oxazolone ring (Fig. 6). A condensation reaction would then lead to cyclization and the formation of the imidazolone or, with an intervening rearrangement step, to the pyrazinedione. It should be noted that the Methylocystis species lack the aminotransferase found in the mb-OB3b gene cluster. Alternate reaction schemes would involve two changes of connectivity in the peptide backbone and not just one as proposed for oxazolone ring formation (Fig. 6). Clearly, the formation of the different types of heterocyclic ring systems in mbs now needs to be addressed experimentally. The production and characterization of mutants will hopefully help in this respect, and such studies have now been initiated (187).
REGULATION OF GENE EXPRESSION
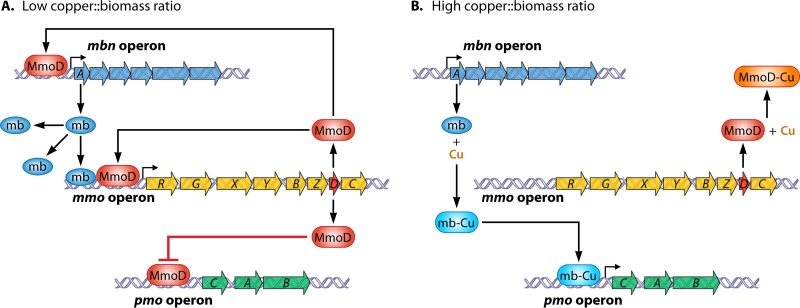
The discovery of the mb biosynthesis gene cluster raised two main questions. First, is mb biosynthesis regulated with respect to copper? Second, what is the role of mb in the “copper switch” controlling the expression of the two forms of MMO? The first question was easily answered through the use of reverse transcription-quantitative PCR (RT-qPCR) using primers specific to some gene(s) of the mbn operon. Initial studies found that in M. trichosporium OB3b, expression of mbnA, the gene encoding the polypeptide precursor of mb, did indeed vary with respect to copper, with expression dropping over three orders of magnitude when copper concentrations increased from zero (no amendment) to 1 μM copper, and expression was largely invariant at higher copper concentrations (140). Such a decrease in expression was reflected in the finding that mb in the spent medium was highest at copper concentrations less than 1 μM and dropped ∼5-fold when the copper concentration was increased to 5 μM (136, 138, 141). Given that the two forms of MMO are also regulated by copper, it was possible that mb was directly involved in this copper switch. Further genetic analyses, however, found that it is not (187). RT-qPCR of mmoX and pmoA from both wild-type M. trichosporium OB3b and an mbnA mutant where a gentamicin cassette was inserted, knocking out mbnA, showed that mmoX and pmoA expression followed similar patterns in both strains as the copper/biomass ratio varied but that the magnitudes of such changes were lower in the mbnA::Gmr mutant than in the wild-type strain (187). To answer the second question above, a mutant in which the mmo operon was deleted was constructed. In this mutant, the “copper switch” was inverted; i.e., pmoA expression was greatest in the absence of copper and dropped ∼2 orders of magnitude when copper concentrations increased (187). Further, expression of mbnA was largely invariant in this mutant with respect to copper. Since all deleted genes in the mmo operon with the exception of mmoD are known to encode polypeptides of sMMO and since MmoD is not required for sMMO activity (32, 51, 63), these expression data suggest that MmoD is a key component of the copper switch, with MmoD serving to regulate expression of mb and mb then amplifying the magnitude of the response in the copper switch.
On the basis of these findings, a model for the regulation of expression of the mmo and pmo operons by mb and MmoD was proposed (187). During growth at low copper/biomass ratios, MmoD protein represses expression of the pmo operon and also upregulates expression of the mmo operon, including that of mmoR and mmoG, which were shown previously to play key roles in regulating mmo expression (113). Further, MmoD is postulated to enhance expression of mb, which serves to amplify expression of the mmo operon. During growth with high copper concentrations, mb binds copper and can no longer enhance the expression of the mmo operon. Under these conditions, it is presumed that MmoD also binds copper and no longer represses expression of the pmo operon or induces expression of sMMO or mb.
There are, however, a number of issues with the model as proposed in Fig. 7. For such a model to be accurate, mmoD would be required to be constitutively expressed with respect to copper in order for sMMO and mb expression to occur after copper is removed. This version of the model implies, however, that as the copper/biomass ratio increases, expression of the entire mmo operon no longer occurs. Recent qRT-PCR analysis, however, shows that mmoD, unlike mmoX, is constitutively expressed with respect to copper (J. D. Semrau, unpublished data). Nevertheless, expression of mmoD, like that of mmoX, increases with increasing addition of exogenous mb, suggesting that expression of mmoD is at least partly coregulated with that of the entire mmo operon.
FIG 7.
Model for the regulation of gene expression in the mmo, pmo, and mbn gene clusters by copper, mb, and MmoD. (A) Low copper/biomass ratio; (B) high copper/biomass ratio. (Reproduced from reference 187 with permission of the publisher.)
In addition, this model, although explaining the genetic basis for the copper switch in methanotrophs, does not explicitly consider the role of two genes known to play roles in the regulation of the mmo operon, i.e., mmoR and mmoG upstream of mmoX (113, 191). Based on available data, it appears that mmoR and mmoG do not play a significant role in the copper switch; i.e., they do not appear to have any control over expression of the pmo operon. It is possible, however, that regulation of the mmo operon is the result of a concerted interaction of mb and/or MmoD with these gene products.
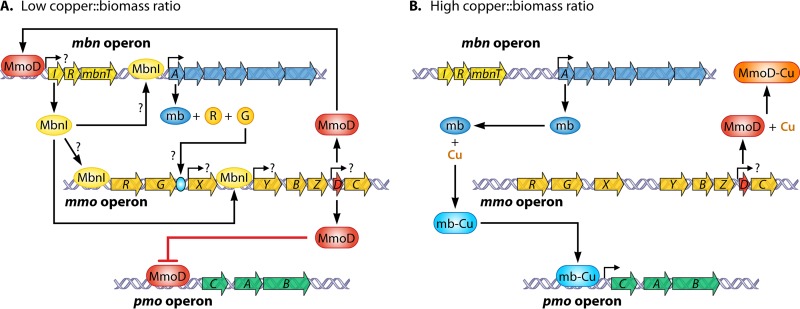
This model also ignores the possibility that the presence of putative regulatory genes (e.g., mbnI and mbnR [Fig. 5]) in mb gene clusters may play a role in regulating gene expression, particularly the mb gene cluster and/or the mmo operon. Given this, a revised model of the copper switch in methanotrophs is now proposed in Fig. 8. Here MmoD is again postulated to be a key component of the copper switch, but MbnI is proposed to be responsible for inducing expression of the mb gene cluster, as well as mmoR and mmoG. Collectively, mb, MmoR, and MmoG interact to induce expression from the σN promoter upstream of mmoX, while MbnI binds to the σ70 promoter upstream of mmoY. In this updated and revised current model, mmoD is constitutively expressed but associates with copper when it is present, preventing repression of the pmo operon or expression of the mb gene clusters. Further experiments will show whether this new model is correct, but it is already clear that the exact details of the copper switch mechanism are more complex than previously thought.
FIG 8.
Revised regulatory scheme for expression of the mmo, pmo, and mbn gene clusters as a function of copper, mb, and MbnI. (A) Low copper/biomass ratio; (B) high copper/biomass ratio.
PHYSIOLOGICAL FUNCTIONS
Copper Acquisition
Although mb has a high affinity for copper, its importance in copper acquisition by methanotrophs is still unclear. Nonnative Cu1+-mbs have been shown to be taken up by methanotrophs and facilitate switchover to pMMO (133). Copper uptake and switchover are faster if the native mb is used but still take more than 24 h. However, the role of mb in meeting the overall copper requirements of the cell is still in question. It has been found that M. trichosporium OB3b possesses at least two mechanisms for copper uptake, one clearly based on mb and involving active transport of copper-mb complexes and another, nonspecific passive transport pathway (192). It was also found that the amount of copper associated with biomass was the same regardless of whether copper was added as CuSO4 or as the Cu1+-mb complex at the same concentration. Subsequently, it was found for M. trichosporium OB3b that when the gene for the precursor polypeptide of mb (mbnA) is knocked out, copper is still associated with biomass, and it increases with increasing amounts of copper in the growth medium, as does copper found associated with wild-type cultures. The role of mb in copper uptake thus remains unclear. It should be kept in mind that these studies were performed using a well-defined growth medium with limited diversity of copper speciation and little if any copper-containing precipitate. It is tempting to speculate that mb may have a more significant role in copper uptake in situ, where copper speciation and distribution will be much more complex and will include copper associated with a wide range of organic materials (e.g., humic and fulvic acids), as well as found either sorbed onto or part of various mineral phrases. Indeed, it has been shown that expression of pmoA is strongly dependent on the form of copper present, with copper associated with metal oxides found to induce smaller changes in pmoA expression, and that concomitant addition of mb increased the magnitude of the copper switch in M. trichosporium OB3b (176, 193).
Do Methanobactins Have a Role as Signaling Molecules?
Given the small size of mbs and their secretion into the environment when copper availability is low, a role for mbs as signaling molecules may be envisaged. Indeed, addition of mb-OB3b enhances expression of mmoX in in cultures of both wild-type M. trichosporium OB3b and its mbnA::Gmr mutant (184, 187), suggesting that mb may control gene expression by acting as a signaling molecule. Further, given that all known forms of mb have significant structural similarity, mbs may allow for cross-species communication. Recent studies show that this is indeed the case. mb-SB2 increased both mmoX expression and sMMO activity in M. trichosporium OB3b in the presence of copper. However, it had no significant effect (P > 0.05) on expression of either pmoA or mbnA, nor did it increase the amount of cell-associated copper (228). The addition of mb-SB2 preloaded with copper, however, reduced both mmoX and mbnA expression when M. trichosporium OB3b was grown in the absence of any added copper, and no sMMO activity was detected. The latter results were likely due to the increased amount of cell-associated copper and may reflect the role of mb in copper uptake.
Membrane Development
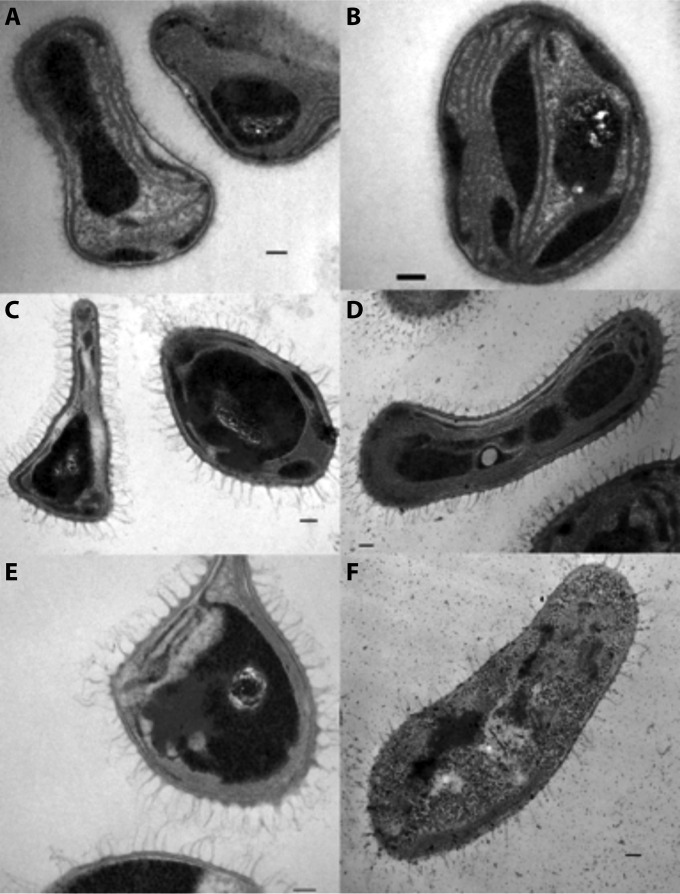
Several studies have demonstrated a direct correlation between intracytoplasmic membrane development and expression of pMMO in methanotrophs (33, 35, 66, 109, 114, 194). As stated above, mbnA plays a secondary role in the regulation of both the membrane-bound and soluble MMO (see Regulation of Gene Expression above). However, deletion of mmoD along with the rest of the sMMO operon had little effect on intracytoplasmic membrane development in M. trichosporium OB3b (Fig. 9C and D). In contrast, a deletion mutation in the mbnA structural gene for mb appears to affect intracytoplasmic membrane development well beyond its effects on pMMO expression (187) (Fig. 9E and F). Currently, knowledge of the effect(s) of mb on intracytoplasmic membrane development is observational, and additional research is required to determine whether mb is directly involved in the development of these membranes in methanotrophs.
FIG 9.
Transmission electron micrographs of wild-type M. trichosporium OB3b (A and B), an M. trichosporium OB3b sMMO deletion mutant (C and D), and an M. trichosporium OB3b mb deletion mutant (E and F) cultured in nitrate mineral salts (NMS) medium (A, C, and E) or NMS medium amended with 5 μM CuSO4 (B, D, and F).
Detoxification of Reactive Oxygen Species
Copper is one of the essential metals required by microorganisms for growth (179). Like iron, however, copper in oxygenated solutions can generate a variety of toxic reactive oxygen species via Fenton and Haber-Weiss reactions (195–197). The coordination of copper by mb is likely to reduce this toxicity, as observed with the iron- and copper-binding siderophore schizokinen (146). Nevertheless, both copper-bound and copper-free forms of mb-OB3b have been shown to reduce dioxygen to superoxide in the presence of a reductant (198). In general, this would represent a daunting problem for bacteria that accumulate high concentrations of Cu-mb. Perhaps not unexpectedly, the superoxide dismutase (SOD) activity of Cu-mb is approximately 30,000 times higher than the oxidase activity associated with either mb-OB3b or Cu-mb-OB3b. In addition, in the presence of a reductant, Cu-mb-OB3b also has a hydrogen peroxide reductase (HPR) activity which is approximately 50 times the oxidase activity (198). The overall result of these three activities is the reduction of O2 to H2O with concomitant loss of reductant. In addition to their protective roles in the context of mb-catalyzed oxidations, high SOD and HPR activities of mb are also likely to provide protection against oxygen radicals that are potentially formed during methane oxidation and respiration of dioxygen (196, 199, 200) and may thus explain the stabilizing effects of mb-OB3b on pMMO activity in cell extracts (66, 73, 198). Similar activities have also been observed for mb-SB2 (N. L. Bandow et al., unpublished results), and we predict that this is a property of all mbs.
MEDICAL, INDUSTRIAL, AND ENVIRONMENTAL IMPLICATIONS
Metal Mobilization or Immobilization for Remediation of Polluted Subsurface Environments
Based on the published yields of mb in laboratory cultures (136, 138), methanotrophs are predicted to export 3 to 50 copies of mb per cell per second depending on the copper concentration during growth. Given this high export rate and the fact that mb can bind most transition and near-transition metals, it is likely that mb is able to influence metal mobilization and thus bioavailability of different metals in soil systems and that this affects the structure and functioning of microbial communities. Recent studies have shown that mb will bind both inorganic and organic forms of mercury (154) and thereby protect the host bacterium as well as other bacteria from mercury toxicity (184). However, additional studies are required to determine which metals are affected in their mobility by mbs and the effects of mbs on the soil microflora.
Formation of Gold Nanoparticles
The potential application of gold nanoparticles as anti-inflammatory agents, in the targeting of cancer cells, in the detection of melamine, as biosensors, and in glucose oxidation has motivated the development of various production methods for such materials (201–206). Most approaches require either high (>100°C) or low (−18°C) temperatures in the presence of reducing and stabilizing agents. Alternatively, microbially mediated production of gold nanoparticles, both intracellularly and extracellularly, has also been extensively investigated (207). However, the size distribution of gold nanoparticles produced by microbial processes is usually quite broad, ranging from 10 to 6,000 nm (208). Further, most reported processes yield particles with a wide variety of shapes (spherical, octahedral, triangular, irregular, etc.), and rates of formation vary over a large range, from minutes to hours (209). Crucially, both mb-OB3b and mb-SB2 have been shown to efficiently reduce Au3+ ions to metallic gold (typically >5 ions/mb) and in so doing to form well-defined size distributions of spherical nanoparticles (137, 148). In a further study with mb-SB2, gold spherical nanoparticles of well-defined sizes (2.0 ± 0.7 nm) were formed, with mb-SB2 suggested to act both as a reductant and as a stabilizing agent, thereby demonstrating that the use of mb-SB2 represents a viable approach to produce well-defined gold nanoparticles in this size range (137).
Possible Therapeutic Treatment of Human Ailments
Copper has been implicated as a key factor in the development of a variety of human ailments, perhaps most notably Wilson's disease (210, 211), in which affected individuals have mutations that inactivate the Cu-transporting P-type ATPase ATP7B (212), impeding copper export to the bile and causing copper accumulation in the liver. Based on in vivo and in vitro studies, copper accumulation specifically affects liver mitochondria, via copper-induced modification of membrane protein thiols leading to multiprotein cross-linking (213). In the final stages of Wilson's disease, oxidative damage, primarily in the liver, is observed. Other symptoms of Wilson's disease include serious neurological issues that can lead to additional psychiatric disorders (210, 214, 215).
There is as yet no cure for Wilson's disease. Currently, treatment consists of one or more of the following options: (i) the use of chelating agents to remove copper via enhanced urine excretion, (ii) implementation of a low-copper diet, and (iii) zinc supplements added to the diet to stimulate production of the copper-binding protein metallothionein (210, 214). Of these options, the use of chelating agents is the most commonly prescribed treatment, with two drugs, penicillamine and trientine, currently approved by the U.S. Food and Drug Administration (FDA). Penicillamine, a breakdown product of penicillin, binds copper (216) and also induces the production of metallothionein. Although the clinical benefit of penicillamine is widely reported, it has serious side effects, including bone marrow suppression, degeneration of elastic tissue, and proteinuria. In a large fraction of cases (20 to 50%), neurological deterioration is observed (217). In such cases, the polyamine trientine, which has fewer reported side effects than penicillamine (217) and a comparable if not higher affinity for copper, is substituted. In both penicillamine and trientine treatments, copper is excreted through the urine and not the bile. Another chelating agent, tetrathiomolybdate, allows for copper to be excreted from liver primarily into the blood, but some evidence suggests that part of the copper may also be transferred to the bile (219) and that insoluble copper is found in both liver and kidneys at tetrathiomolybdate/copper ratios greater than two (219–221). The physiological impact of such an increased burden of copper in the blood, as well as the formation of insoluble copper, is unclear.
mbs may represent a superior alternative as a copper-chelating agent to treat Wilson's disease, as indicated by preliminary studies. In the animal model for Wilson's disease, the Long Evans Cinnamon (LEC) rat, intravenous application of mb-OB3b extracted copper from the liver into the bile as Cu-mb-OB3b, without measurable side effects (213, 222). It was also found that mb-OB3b reversed both the buildup of copper in liver mitochondria and protein cross-linking, allowing the liver to function normally (213), and also stripped copper from metallothionein (222). Insoluble forms of copper were not observed. Lastly, and in contrast to other chelators used for the treatment of Wilson's disease, mb-OB3b was shown to work in circumstances of advanced liver failure, further suggesting that mb may be a viable alternative in the treatment of Wilson's disease in the future. It is also tempting to speculate that the use of mbs may be of interest in other copper-associated pathologies as well, including certain forms of cancer (223–226). For example, it was recently reported that active oncogenesis of a wide variety of cancers is directly affected by the presence of copper through the role of BRAF kinase (226), since disrupting kinase copper binding through site-directed mutagenesis or preventing copper uptake through the addition of tetrathiomolybdate inhibited tumorigenesis. In our view, these observations make it clear that mbs, first discovered as an elusive factor associated with the exclusively bacterial process of methane utilization, may hold great promise as molecules useful to humankind as well.
CONCLUSIONS
The combination of a heterocyclic ring and an associated thioamide is a unique structural feature of mbs among all natural products and the primary cause of the capabilities observed for these molecules. Although rare, oxazolone and imidazolone rings have been identified in several ribosomally synthesized and posttranslationally modified natural products, and this feature appears to be responsible for the observed antimicrobial and metal binding properties of such molecules. Pyrazinediones, in contrast, have so far been observed only in two nonribosomally synthesized natural products, selerominol and flutamide, which show activity against several cancer cell lines and antiviral activity, respectively. Like for thioamide groups, they also confer antimicrobial and metal binding properties in the two natural products identified so far that contain this functional group. Crucially, combining both of these structural features in small peptide derivatives such as mbs appears to enhance various properties, including copper acquisition, metal detoxification, detoxification of reactive oxygen species, electron transfer, nanoparticle formation, and gene regulation. Production of high concentrations of mbs by a ubiquitous microbial group also raises a number of ecological questions such as its effects on metal bioavailability and mobilization, as well as on the structure and functioning of microbial communities in the environment. From an application viewpoint, mbs now have demonstrated potential for the production of nanoparticles as well as in the treatment of copper-related diseases, and we predict that a variety of other commercial and medical applications have yet to be identified.
ACKNOWLEDGMENTS
This work was supported by the Office of Science (BER), U.S. Department of Energy (DE-SC0006630) (J.D.S. and A.A.D.), National Science Foundation (CHE10112271) (A.A.D.), NERC (grant NE/F00608X) (C.D.), and BBSRC (grant BB/K008439/1) (C.D.).
Biographies

Alan A. DiSpirito received his B.S. in biology from Providence College and his M.S. and Ph.D. in microbiology from The Ohio State University with Olli H. Tuovinen and Patrick Dugan. His postdoctoral training was with Alan B. Hooper at The University of Minnesota and with Mary E. Lidstrom at The University of Washington—Seattle, Center for Great Lakes Studies, and California Institute of Technology. He was an Assistant Professor at The University of Texas at Arlington before moving to the Department of Microbiology at Iowa State University. He is currently a Professor in the Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology at Iowa State University. His basic research interest is in the basic metabolism, environmental importance, and commercial application of methanotrophic and chemoautotrophic bacteria. Current research projects include structural and functional characterization of methanobactins, the mechanism of methanobactin biosynthesis, and the influence of methanobactin on metal mobility in soil systems.

Jeremy D. Semrau, Arthur F. Thurnau Professor, has appointments in the College of Engineering and the College of Literature, Science and Arts at the University of Michigan. His research focuses primarily on methanotrophs, with a particular emphasis on the biochemistry, genetics, and regulation of methane monooxygenase and methanobactin synthesis. As part of his research, Professor Semrau has also isolated and characterized novel facultative methanotrophs, developed applications of methanotrophy for use in bioremediation and biofuel production, and developed novel applications of methanobactin for the production of gold nanoparticles and the treatment of Wilson's disease. He holds several patents in these areas and has published many papers, abstracts, and book chapters on fundamental and applied aspects of methanotrophy. Professor Semrau is currently on the editorial board of Applied and Environmental Microbiology.

J. Colin Murrell is Professor in Microbiology in the School of Environmental Sciences and Director of the Earth and Life Systems Alliance (ELSA) at the University of East Anglia, United Kingdom. He has wide-ranging research interests centered around the bacterial metabolism of methane and other one-carbon compounds, such as methanol, methylamines, methanesulfonate, dimethyl sulfide, methyl halides, and isoprene, in the terrestrial, aquatic, and marine environments. Other areas of research include the microbiology of the rhizosphere, the sea surface microlayer, caves, alkaline soda lakes, salt marshes, and cold-water corals and cultural heritage microbiology, regulation of gene expression by metals, microbial genomics, metagenomics, bioremediation, biocatalysis, and industrial biotechnology. His research over the past 35 years has resulted in around 280 publications and six edited books. Dr. Murrell is a member of the editorial boards of Environmental Microbiology, The ISME Journal, and FEMS Microbiology Letters and was the Chair of the 2015 Gordon Research Conference on Applied and Environmental Microbiology. Dr. Murrell is Vice President of The International Society for Microbial Ecology and was recently elected Member of the European Molecular Biology Organization and Member of the European Academy of Microbiology.

Warren H. Gallagher was born in Providence, RI, in 1952 and received his A.B. degree in chemistry and biology from Albion College, Albion, MI, in 1974. He went on to receive his Ph.D. in biophysics from the University of Pittsburgh in 1980 under the direction of Professor Max Lauffer. He then held postdoctoral positions with Professor Victor Bloomfield and Professor Clare Woodward at the University of Minnesota, Twin Cities, before joining the chemistry faculty at the University of Wisconsin—Eau Claire, where his current title is Professor and Chair of Chemistry. He has a long-term interest in using spectroscopic methods to determine the structures of peptide-derived molecules, and over the past several years the methanobactins have presented him and his students with some exciting challenges that demanded detective-like solutions.

Christopher Dennison received his Ph.D. in inorganic chemistry (1994) from Newcastle University with Professor A. G. Sykes and Professor W. McFarlane and then worked as a postdoctoral fellow with Professor G. W. Canters at Leiden University. He was appointed as a Lecturer at University College Dublin in 1997 and returned to Newcastle University in 1999 as a Lecturer in Inorganic Chemistry in the Department of Chemistry. In 2004 he became a Senior Lecturer in the Institute for Cell and Molecular Biosciences (Medical School) and in 2010 Professor of Biological Chemistry. He studies the role of metals, and particularly copper, in biological systems. This has included the analysis of electron transfer proteins and multicopper redox enzymes. More recently, his work has focused on copper homeostasis in a wide range of organisms, including understanding how methane-oxidizing bacteria acquire, handle, and store copper.

Stéphane Vuilleumier studied natural sciences at ETH Zurich, Switzerland, and obtained a Ph.D. in organic chemistry at the University of Basel, Switzerland. After postdoctoral work in protein engineering at the University of Cambridge, United Kingdom, he returned to ETH Zurich, where he became a group leader at the Institute of Microbiology and obtained his habilitation. He has been a Professor of Environmental Biology and Microbiology in Strasbourg, France, since 2002, heads a CNRS research team, and shares responsibility for the Chemistry and Biology Master at Université de Strasbourg. In 2005, his interest in bacterial metabolism of halogenated methanes led him to become involved in international collaborative genome sequencing projects on methylotrophic and methanotrophic bacteria at JGI (United States) and Genoscope (France) and in their subsequent analysis using the MicroScope platform at Genoscope. Dr. Vuilleumier was elected Chair of the 2016 Gordon Research Conference “Molecular Basis of Microbial One-Carbon Metabolism.”
REFERENCES
- 1.Henckel T, Roslev P, Conrad R. 2000. Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ Microbiol 2:666–679. doi: 10.1046/j.1462-2920.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 2.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol Rev 34:496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 3.Jacinthe PA. 2015. Carbon dioxide and methane fluxes in variably-flooded riparian forest. Geoderma 241:41–50. [Google Scholar]
- 4.Jacinthe PA, Vidon P, Fisher K, Lou X, Baker M. 2015. Methane and carbon dioxide fluxes from cropland and riparian buffers in different hydrogeomorphic settings. J Environ Quality 44:1080–1090. doi: 10.2134/jeq2015.01.0014. [DOI] [PubMed] [Google Scholar]
- 5.Rouviere PE, Wolfe RS. 1988. Novel biochemistry of methanogens. J Biol Chem 263:7913–7916. [PubMed] [Google Scholar]
- 6.Trotsenko YA, Murrell JC. 2008. Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63:183–229. doi: 10.1016/S0065-2164(07)00005-6. [DOI] [PubMed] [Google Scholar]
- 7.Khmelenina VN, Rozova ON, But SY, Mustakhimov II, Reshetnikov AS, Beschastnyi AP, Trotsenko YA. 2015. Biosynthesis of secondary metabolites in methanotrophs: biochemical and genetic aspects. Appl Biochem Microbiol 51:150–158. [DOI] [PubMed] [Google Scholar]
- 8.Strong PJ, Xie S, Clarke WP. 2015. Methane as a resource: can the methanotrophs add value? Environ Sci Technol 49:4001–4018. doi: 10.1021/es504242n. [DOI] [PubMed] [Google Scholar]
- 9.Söhngen NL. 1906. Ueber Bakterien, welche Methan als Kohlenstoffnahrung und Energiequelle gebrauchen. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt II 15:513–517. [Google Scholar]
- 10.Leadbetter ER, Foster JW. 1958. Studies on some methane utilizing bacteria. Archiv Mikrobiol 30:91–118. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin M, Foster JW. 1956. Studies on Pseudomonas methanica (Söhngen) nov. comb. J Bacteriol 72:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster JW, Davis RH. 1966. A methane-dependent coccus, with notes on classification and nomenclature of obligate methane-utilizing bacteria. J Bacteriol 91:1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittenbury R, Phillips KC, Wilkinson JF. 1970. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 14.Bowman JP, Sly LI, Nichols PD, Hayward AC. 1993. Revised taxonomy of the methanotrophs: description of Methylobacter gen nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes on the group I methanotrophs. Int J Syst Bacteriol 43:735–753. doi: 10.1099/00207713-43-4-735. [DOI] [Google Scholar]
- 15.Bowman JP, Sly LI, Stackebrandt E. 1995. The phylogenetic position of the family Methyloccoaceae. Int J Syst Evol Microbiol 45:182–185. [DOI] [PubMed] [Google Scholar]
- 16.Hirayama H, Fuse H, Abe M, Miyazaki M, Nakamura T, Nunoura T, Furushima Y, Yamamoto H, Takai K. 2013. Methylomarinum vadi gen. nov., sp. nov., a methanotroph isolated from two distinct marine environments. Int J System Environ Microbiol 63:1073–1082. doi: 10.1099/ijs.0.040568-0. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama H, Abe M, Miyazaki M, Nunoura T, Furushima Y, Yamamoto H, Takai K. 2014. Methylomarinovum caldicuralii gen. nov., sp. nov., a moderately thermophilic methanotroph isolated from a shallow submarine hydrothermal system, and proposal of the family Methylothermaceae fam. nov. Int J Syst Environ Microbiol 64:989–999. doi: 10.1099/ijs.0.058172-0. [DOI] [PubMed] [Google Scholar]
- 18.Tavormina PL, Hatzenpichler R, McGlynn S, Chadwick G, Dawson KS, Connon SA, Orphan VJ. 2015. Methyloprofundus sedimenti gen. nov., sp. nov., an obligate methanotroph from ocean sediment belonging to the ‘deep sea-1′ clade of marine methanotrophs. Int J Syst Environ Microbiol 65:251–259. doi: 10.1099/ijs.0.062927-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoefman S, Van der Ha D, Iguchi H, Yurimoto H, Sakai Y, Boon N, Vandamme P, Heylen K, De Vos P. 2014. Methyloparacoccus murrellii gen. nov., sp. nov., a methanotroph isolated from pond water. Int J Syst Evol Microbiol 64:2100–2107. doi: 10.1099/ijs.0.057760-0. [DOI] [PubMed] [Google Scholar]
- 20.Poehlein A, Deutzmann JS, Daniel R, Simeonova DD. 2013. Draft genome sequence of the methanotrophic Gammaproteobacterium Methyloglobulus morosus DSM 22980 strain KoM1. Genome Announc 1:e01078-13. doi: 10.1128/genomeA.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN. 2011. Methyloferula stellata gen. nov., sp nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61:2456–2463. doi: 10.1099/ijs.0.028118-0. [DOI] [PubMed] [Google Scholar]
- 22.Belova SE, Baani M, Suzina NE, Bodelier PLE, Liesack W, Dedysh SN. 2011. Acetate utilization as a survival strategy ofpeat-inhabiting Methylocystis spp. Environ Microbiol Rep 3:36–46. doi: 10.1111/j.1758-2229.2010.00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Dedysh SN, Knief C, Dunfield PF. 2005. Methylocella species are facultatively methanotrophic. J Bacterol 187:4665–4670. doi: 10.1128/JB.187.13.4665-4670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunfield PF, Belova SE, Vorob'ev AV, Cornish SL, Dedysh SN. 2010. Methylocapsa aurea sp. nov., a facultative methanotroph possessing a particulate methane monooxygenase, and emended description of the genus Methylocapsa. Int J Syst Evol Microbiol 60:2659–2664. doi: 10.1099/ijs.0.020149-0. [DOI] [PubMed] [Google Scholar]
- 25.Im J, Lee SW, Yoon S, Dispirito AA, Semrau JD. 2011. Characterization of a novel facultative Methylocystis species capable of growth on methane, acetate and ethanol. Environ Microbiol Rep 3:174–181. doi: 10.1111/j.1758-2229.2010.00204.x. [DOI] [PubMed] [Google Scholar]
- 26.Semrau JD, DiSpirito AA, Vuilleumier S. 2011. Facultative methanotrophy: false leads, true results, and suggestions for future research. FEMS Microbiol Lett 323:1–12. doi: 10.1111/j.1574-6968.2011.02315.x. [DOI] [PubMed] [Google Scholar]
- 27.Anvar SY, Frank J, Pol A, Schmitz A, Kraaijeveld K, den Dunne JT, Op den Camp HJ. 2014. The genomic landscape of verrucomicrobial methanotroph Methylacidiphilum fumariolicum SolV. BMC Genomics 15:914–926. doi: 10.1186/1471-2164-15-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJ, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJ, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MS, Strous M. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. doi: 10.1038/nature08883. [DOI] [PubMed] [Google Scholar]
- 29.Luesken FA, Wu ML, Op den Camp HJM, Keltjeb JT, Stunnenberg H, Francoijs K-J, Strous M, Jetten MSM. 2012. Effect of oxygen on the anaerobic methanotroph ‘Candidatus Methylomirabilis oxyfera’: kinetic and transcriptional analysis. Environ Microbiol 14:1024–1034. doi: 10.1111/j.1462-2920.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 30.Chistoserdova L, Lidstrom ME. 2013. Aerobic methylotrophic prokaryotes, p 267–285. In Rosenberg E, DeLong EF, Thompson F, Lory S, Stackebrandt E (ed), The prokaryotes, 4th ed Springer, New York, NY, USA. [Google Scholar]
- 31.Dalton H. 2005. The Leeuwenhoek Lecture 2000: the natural and unnatural history of methane-oxidizing bacteria. Philos Trans R Soc Lond B Biol Sci 360:1207–1222. doi: 10.1098/rstb.2005.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murrell JC. 1994. Molecular genetics of methane oxidation. Biodegradation 5:145–159. doi: 10.1007/BF00696456. [DOI] [PubMed] [Google Scholar]
- 33.Dalton H, Prior SD, Leak DJ, Stanley SH. 1984. Regulation and control of methane monooxygenase, p 75–82. In Crawford RL, Hanson RS (ed), Microbial growth on C1 compounds. American Society for Microbiology, Washington, DC. [Google Scholar]
- 34.Murrell JC, McDonald IR, Gilbert B. 2000. Regulation of expression of methane monooxygenases by copper ions. Trend Microbiol 8:221–225. doi: 10.1016/S0966-842X(00)01739-X. [DOI] [PubMed] [Google Scholar]
- 35.Prior SD, Dalton H. 1985. Copper stress underlines the fundamental change in intracellular location of the membrane monooxygenase in methane oxidizing organisms: studies in batch and ontinuous culture. J Gen Microbiol 131:155–163. [Google Scholar]
- 36.Scott D, Branna J, Higgins IL. 1981. The effect of growth conditions on intracytoplasmic membranes and methane monooxygenase activities in Methylosinus trichosporium OB3b. J Gen Microbiol 125:63–72. [Google Scholar]
- 37.Hakemian AS, Rosenzweig AC. 2007. The biochemistry of methane oxidation. Annu Rev Biochem 76:223–241. doi: 10.1146/annurev.biochem.76.061505.175355. [DOI] [PubMed] [Google Scholar]
- 38.Leahy JG, Batchelor PJ, Morcumb SM. 2003. Evolution of the soluble diiron monooxygenases. FEMS Microbiol Rev 27:449–479. doi: 10.1016/S0168-6445(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 39.Green J, Dalton H. 1985. Protein B of soluble methane monooxygenase from Methylococcus capsulatus (Bath). A novel regulatory protein of enzyme activity. J Biol Chem 260:15795–15801. [PubMed] [Google Scholar]
- 40.Colby J, Stirling DI, Dalton H. 1977. The soluble methane monooxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkane, n-alkene, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J 165:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colby J, Dalton H. 1978. Resolution of the methane monooxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J 171:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colby J, Dalton H. 1979. Characterization of the second prosthetic group of the flavoprotein NADH-acceptor reductase (component C) of the methane monooxygenase from Methylococcus capsulatus (Bath). Biochem J 177:903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodland MP, Dalton H. 1984. Purification and characterization of component A of the methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem 259:53–60. [PubMed] [Google Scholar]
- 44.Fox BG, Froland WA, Dege JE, Lipscomb JD. 1989. Methane monooxygenase from Methylosinus trichosporium Ob3b—purification and properties of a 3-component system with high specific activity from a type-II methanotroph. J Biol Chem 264:10023–10033. [PubMed] [Google Scholar]
- 45.Fox BG, Hendrich MP, Surerus KK, Andersson KK, Froland WA, Lipscomb JD, Münck E. 1993. Mössbauer, EPR, and ENDOR studies of the hydroxylase and reductase components of methane monooxygenase from Methylosinus trichosporium Ob3b. J Am Chem Soc 115:3688–3701. doi: 10.1021/ja00062a039. [DOI] [Google Scholar]
- 46.Fox BG, Surerus KK, Münck E, Lipscomb JD. 1988. Evidence for a mu-oxo-bridged binuclear iron cluster in the hydroxylase component of methane monooxygenase—Mössbauer and electron-paramagnetic-resonance studies. J Biol Chem 263:10553–10556. [PubMed] [Google Scholar]
- 47.Wallar BJ, Lipscomb JD. 1996. Dioxygen activation by enzymes containing binuclear non-heme iron clusters. Chem Rev 96:2625–2657. doi: 10.1021/cr9500489. [DOI] [PubMed] [Google Scholar]
- 48.Liu KE, Lippard SJ. 1991. Redox properties of the hydroxylase component of methane monooxygenase from Methylococcus capsulatus (Bath)—effects of protein-B, reductase, and substrate. J Biol Chem 266:12836–12839. [PubMed] [Google Scholar]
- 49.Elango N, Radhakrishnan R, Froland WA, Wallar BJ, Earhart CA, Lipscomb JD, Ohlendorf DH. 1997. Crystal structure of the hydroxylase component of methane monooxygenase from Methylosinus trichosporium OB3b. Protein Sci 6:556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenzweig AC, Frederick CA, Lippard SJ. 1992. Crystalization and preliminary X-ray analysis of the methane monooxygenase hydroxylase protein from Methylococcus capsulatus (Bath). J Mol Biol 227:583–585. doi: 10.1016/0022-2836(92)90913-5. [DOI] [PubMed] [Google Scholar]
- 51.Rosenzweig AC, Frederick CA, Lippard SJ, Nordlund P. 1993. Crystal stucture of a bacterial non-heme iron hydroxylase protein from Methylococcus capsulatus (Bath). Nature 366:357–543. [Google Scholar]
- 52.Sazinsky MH, Lippard SJ. 2005. Product bound structures of the soluble methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath): protein motion in the r-subunit. J Am Chem Soc 127:5814–5825. doi: 10.1021/ja044099b. [DOI] [PubMed] [Google Scholar]
- 53.Walters KJ, Gassner GT, Lippard SJ, Wagner G. 1999. Structure of the soluble methane monooxygenase regulatory protein B. Proc Natl Acad Sci U S A 96:7877–7882. doi: 10.1073/pnas.96.14.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang SL, Wallar BJ, Lipscomb JD, Mayo KH. 1999. Solution structure of component B from methane monooxygenase derived through heteronuclear NMR and molecular modeling. Biochemistry 38:5799–5812. doi: 10.1021/bi982992f. [DOI] [PubMed] [Google Scholar]
- 55.Chatwood LL, Müller J, Gross JD, Wagner G, Lippard SJ. 2004. NMR structure of the flavin domain from soluble methane monooxygenase reductase from Methylococcus capsulatus (Bath). Biochemistry 43:11983–11991. doi: 10.1021/bi049066n. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JZ, Wallar BJ, Popescu CV, Renner DB, Thomas DD, Lipscomb JD. 2006. Methane monooxygenase hydroxylase and B component interactions. Biochemistry 45:2913–2926. doi: 10.1021/bi052256t. [DOI] [PubMed] [Google Scholar]
- 57.Fox BG, Liu Y-N, Dege JE, Lipscomb JD. 1991. Complex formation between the protein components of the methane monooxygenase from Methylosinus trichosporium OB3b. J Biol Chem 266:540–550. [PubMed] [Google Scholar]
- 58.Lee SK, Fox BG, Froland WA, Lipscomb JD, Münck E. 1993. A transient intermediate of the methane monooxygenase catalytic cycle containing a FeIVFeIV cluster. J Am Chem Soc 115:6450–6451. doi: 10.1021/ja00067a086. [DOI] [Google Scholar]
- 59.Lee SK, Nesheim JC, Lipscomb JD. 1993. Transient intermediates of the methane monooxygenase catalytic cycle. J Biol Chem 268:21569–21577. [PubMed] [Google Scholar]
- 60.Liu KE, Valentine AM, Qiu D, Edmondson DE, Applman EH, Spiro TG, Lippard SJ. 1995. Characterization of a diiron(III) peroxo intermediate in the reaction cycle of methane monoxygenase hydroxylase from Methylococcus capsulatus (Bath). J Am Chem Soc 117:4997–4998. doi: 10.1021/ja00122a032. [DOI] [Google Scholar]
- 61.Liu KE, Wang D, Huynh BH, Edmondson DE, Salifogloi A, Lippard SJ. 1994. Spectroscopic detection of intermediates in the reaction of dioxygen with the reduced methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath). J Am Chem Soc 116:7465–7466. doi: 10.1021/ja00095a083. [DOI] [Google Scholar]
- 62.Banerjee R, Proshlyakov Y, Lipscomb JD, Proshlyakov DA. 2015. Structure of the key species in the enzymatic oxidation of methane to methanol. Nature 518:431–435. doi: 10.1038/nature14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipscomb JD. 1994. Biochemistry of the soluble methane monooxygenase. Annu Rev Microbiol 48:371–399. doi: 10.1146/annurev.mi.48.100194.002103. [DOI] [PubMed] [Google Scholar]
- 64.Guallar V, Gherman BF, Lippard SJ, Friesner RA. 2002. Quantum chemical studies of methane monooxygenase: comparison with P450. Curr Opin Chem Biol 6:236–242. doi: 10.1016/S1367-5931(02)00310-1. [DOI] [PubMed] [Google Scholar]
- 65.Basu P, Katterle B, Andersson KK, Dalton H. 2003. The membrane-associated form of methane mono-oxygenase from Methylococcus capsulatus (Bath) is a copper/iron protein. Biochem J 369:417–427. doi: 10.1042/bj20020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi DW, Kunz RC, Boyd ES, Semrau JD, Antholine WE, Han JI, Zahn JA, Boyd JM, de la Mora AM, DiSpirito AA. 2003. The membrane-associated methane monooxygenase (pMMO) and pMMO-NADH:quinone oxidoreductase complex from Methylococcus capsulatus Bath. J Bacteriol 185:5755–5764. doi: 10.1128/JB.185.19.5755-5764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieberman RL, Kondapalli KC, Shrestha DB, Hakemian AS, Smith SM, Telser J, Kuzelka J, Gupta R, Borovik AS, Lippard SJ, Hoffman BM, Rosenzweig AC, Stemmler TL. 2006. Characterization of the particulate methane monooxygenase metal centers in multiple redox states by X-ray absorption spectroscopy. Inorg Chem 45:8372–8381. doi: 10.1021/ic060739v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lieberman RL, Rosenzweig AC. 2005. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434:177–182. doi: 10.1038/nature03311. [DOI] [PubMed] [Google Scholar]
- 69.Lieberman RL, Shrestha DB, Doan PE, Hoffman BM, Stemmler TL, Rosenzweig AC. 2003. Purified particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a dimer with both mononuclear copper and a copper-containing cluster. Proc Natl Acad Sci U S A 100:3820–3825. doi: 10.1073/pnas.0536703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinho M, Choi DW, Dispirito AA, Antholine WE, Semrau JD, Münck E. 2007. Mössbauer studies of the membrane-associated methane monooxygenase from Methylococcus capsulatus Bath: evidence for a Diiron center. J Am Chem Soc 129:15783–15785. doi: 10.1021/ja077682b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen HHT, Elliott SJ, Yip JHK, Chan SI. 1998. The particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a novel copper-containing three-subunit enzyme—isolation and characterization. J Biol Chem 273:7957–7966. doi: 10.1074/jbc.273.14.7957. [DOI] [PubMed] [Google Scholar]
- 72.Takeguchi M, Miyakawa K, Okura I. 1998. Purification and properties of particulate methane monooxygenase from Methylosinus trichosporium OB3b. J Mol Catal Chem 132:145–153. doi: 10.1016/S1381-1169(97)00275-6. [DOI] [PubMed] [Google Scholar]
- 73.Zahn JA, DiSpirito AA. 1996. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath). J Bacteriol 178:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hakemian AS, Kondapalli KC, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC. 2008. The metal centers of particulate methane monooxygenase from Methylosinus trichosporium OB3b. Biochemistry 47:6793–6801. doi: 10.1021/bi800598h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Culpepper MA, Cutsail GEI, Gunderson WA, Hoffman BM, Rosenzweig AC. 2014. Identification of the valence and coordination environment of the particulate methane monooxygenase copper centers by advanced EPR characterization. J Am Chem Soc 136:11767–11775. doi: 10.1021/ja5053126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brantner CA, Buchholz LA, Remsen CC, Collins MLP. 2000. Isolation of intracytoplasmic membrane from the methanotrophic bacterium Methylomicrobium album BG8. Curr Microbiol 40:132–134. doi: 10.1007/s002849910026. [DOI] [PubMed] [Google Scholar]
- 77.Semrau JD, Chistoserdov A, Lebron J, Costello AM, Davagnino J, Kenna E, Holmes AJ, Finch R, Murrell JC, Lidstrom ME. 1995. Particulate methane monooxygenase genes in methanotrophs. J Bacteriol 177:3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stolyar S, Costello AM, Peeples TL, Lidstrom ME. 1999. Role of multiple gene copies in particulate methane monooxygenase activity in the methane-oxidizing bacterium Methylococcus capsulatus Bath. Microbiology 145:1235–1244. doi: 10.1099/13500872-145-5-1235. [DOI] [PubMed] [Google Scholar]
- 79.Stolyar S, Franke M, Lidstrom ME. 2001. Expression of individual copies of Methylococcus capsulatus Bath particulate methane monooxygenase genes. J Bacteriol 183:1810–1812. doi: 10.1128/JB.183.5.1810-1812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myronova N, Kitmitto A, Collins RF, Miyaji A, Dalton H. 2006. Three-dimensional structure determination of a protein supercomplex that oxidizes methane to formaldehyde in Methylococcus capsulatus (Bath). Biochemistry 45:11905–11914. doi: 10.1021/bi061294p. [DOI] [PubMed] [Google Scholar]
- 81.Culpepper MA, Rosenzweig AC. 2014. Structure and protein-protein interactions of methanol dehydrogenase from Methylococcus capsulatus (Bath). Biochemistry 53:6211–6219. doi: 10.1021/bi500850j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hooper AB, Arciero DM, Bergman B, Hendrich MP. 2004. The oxidation of ammonia as an energy source in bacteria, p 121–147. In Zannoni D. (ed), Respiration in archaea and bacteria, vol 16 Springer, Norwell, MA, USA. [Google Scholar]
- 83.Hooper AB, Vannelli T, Bergman DJ, Arciero DM. 1997. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Van Leeuwenhoek 71:59–67. doi: 10.1023/A:1000133919203. [DOI] [PubMed] [Google Scholar]
- 84.Hooper AB, DiSpirito AA. 1985. In bacteria which grow on simple reductants, generation of a proton gradient involves extracytoplasmic oxidation of substrate. Microbiol Rev 49:140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whittaker MR, Bergmann DJ, Arciero DM, Hooper AB. 2000. Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim Biophys Acta 1459:346–355. doi: 10.1016/S0005-2728(00)00171-7. [DOI] [PubMed] [Google Scholar]
- 86.Dunfield PF, Khmelenina VN, Suzina NE, Trotsenko YA, Dedysh SN. 2003. Methylocella silvestris sp. nov., a novel methanotroph isolated from an acidic forest cambisol. Int J Syst Evol Microbiol 53:1231–1239. doi: 10.1099/ijs.0.02481-0. [DOI] [PubMed] [Google Scholar]
- 87.Waechter-Brulla D, DiSpirito AA, Chistoserdova LV, Lidstrom ME. 1993. Methanol oxidation genes in the marine methanotroph Methylomonas sp. strain A4. J Bacteriol 175:3767–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anthony C. 1992. The structure of bacterial quinoprotein dehydrogenases. Int J Biochem 24:29–30. doi: 10.1016/0020-711X(92)90226-Q. [DOI] [PubMed] [Google Scholar]
- 89.Al-Taho NM, Cornish A, Warner PJ. 1990. Molecular cloning of the methanol dehydrogenase structural gene from Methylosinus trichosporium OB3B. Curr Microbiol 20:153–157. doi: 10.1007/BF02091990. [DOI] [Google Scholar]
- 90.Pol A, Barends TRM, Dietl A, Khadem AF, Eygensteyn J, Jetten MMS, Op den Camp HJ. 2014. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 16:255–264. doi: 10.1111/1462-2920.12249. [DOI] [PubMed] [Google Scholar]
- 91.Farhan U-H M, Kalidass B, Bandow NL, Turpin E, DiSpirito AA, Semrau JD. 2015. Cerium regulates expression of alternative methanol dehydrogenases in Methylosinus trichosporium OB3b. Appl Environ Microbiol 81:7446–7554. doi: 10.1128/AEM.02542-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vorholt JA, Chistoserdova L, Stolvar SM, Thauer RK, Lidstrom ME. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J Bacteriol 181:5750–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zahn JA, Bergmann DJ, Boyd JM, Kunz RC, DiSpirito AA. 2001. Membrane-associated quinoprotein formaldehyde dehydrogenase from Methylococcus capsulatus Bath. J Bacteriol 183:6832–6840. doi: 10.1128/JB.183.23.6832-6840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vorholt JA. 2002. Cofactor-dependent pathways of formaldehyde oxidation in methylotrophic bacteria. Arch Microbiol 178:239–249. doi: 10.1007/s00203-002-0450-2. [DOI] [PubMed] [Google Scholar]
- 95.Stirling DI, Dalton H. 1978. Purification and properties of an NAD(P)+-linked formaldehyde dehydrogenase from Methylococcus capsulatus (Bath). J Gen Microbiol 107:19–29. doi: 10.1099/00221287-107-1-19. [DOI] [PubMed] [Google Scholar]
- 96.Tate S, Dalton H. 1999. A low-molecular mass protein from Methylococcus capsulatus (Bath) is responsible for the regulation of formaldehyde dehydrogenase activity in vitro. Microbiology 145:159–167. doi: 10.1099/13500872-145-1-159. [DOI] [PubMed] [Google Scholar]
- 97.Chistoserdova L, Vorholt JA, Thauer RK, Lidstrom ME. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria with methanogenic Archaea. Science 281:99–102. doi: 10.1126/science.281.5373.99. [DOI] [PubMed] [Google Scholar]
- 98.Vorholt JA, Chistoserdova L, Lidstrom ME, Thauer RK. 1998. The NADP-dependent methylene tetrahydromethanopterin dehydrogenase in Methylobacterium extorquens AM1. J Bacteriol 180:5351–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Speer B, Chistoserdova L, Lidstrom ME. 1994. Sequence of the gene for a NAD(P)+-dependent formaldehyde dehydrogenase (class III alcohol dehydrogenase) from a marine methanotroph Methylobacter marinus A45. FEMS Microbiol Lett 121:349–355. doi: 10.1111/j.1574-6968.1994.tb07125.x. [DOI] [PubMed] [Google Scholar]
- 100.Yoch DC, Chen CL, Hardt MG. 1990. Formate dehydrogenase from the methane oxidizer Methylosinus trichosporium OB3b. J Bacteriol 172:4456–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jollie DR, Lipscomb JD. 1990. Formate dehyrogenase from Methylosinus trichosporium OB3b. Methods Enzymol 188:331–334. doi: 10.1016/0076-6879(90)88051-B. [DOI] [PubMed] [Google Scholar]
- 102.Jollie DR, Lipscomb JD. 1991. Formate dehydrogenase from Methylosinus trichosporium OB3b. J Biol Chem 266:21853–21863. [PubMed] [Google Scholar]
- 103.Tonge GM, Harrison DEF, Knowles CJ, Higgins IL. 1975. Properties and partial purification of the methane-oxidizing enzyme systems from Methylosinus trichosporium OB3b. FEBS Lett 58:293–299. doi: 10.1016/0014-5793(75)80282-1. [DOI] [PubMed] [Google Scholar]
- 104.Ribbons DW, Michalover JL. 1970. Methane oxidation by cell-free extracts of Methylococcus capsulatus. FEBS Lett 11:41–44. doi: 10.1016/0014-5793(70)80487-2. [DOI] [PubMed] [Google Scholar]
- 105.Stirling DI, Dalton H. 1979. Properties of the methane monooxygenae from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to Methylococcus capsulatus (Bath). Eur J Biochem 96:205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- 106.Stirling DI, Colby J, Dalton H. 1979. Comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from 3 strains of methane-oxidizing bacteria. Biochem J 177:361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott D, Best DJ, Higgins IL. 1981. Intracytoplasmic membranes in oxygen-limited chemostat cultures of Methylosinus trichosporium OB3b; biocatalytic implications of physiological balanced growth. Biotech Lett 3:641–644. doi: 10.1007/BF00158693. [DOI] [Google Scholar]
- 108.Stanley SH, Prior SD, Leak DJ, Dalton H. 1983. Copper stress underlies the fundamental change in intracellular location of methane mono-oxygenase in methane-oxidizing oganisms—studies in batch and continuous cultures. Biotechnol Lett 5:487–492. doi: 10.1007/BF00132233. [DOI] [Google Scholar]
- 109.Green J, Prior SD, Dalton H. 1985. Copper ions as inhibitors of protein C of the soluble methane monooxygenase of Methylococcus capsulatus (Bath). Eur J Biochem 153:137–144. doi: 10.1111/j.1432-1033.1985.tb09279.x. [DOI] [PubMed] [Google Scholar]
- 110.Takeda K, Takahara Y. 1980. Ultrastructure of intracytoplasmic membranes of Methanomonas margaritae cells grown under different conditions. Antonie Van Leeuwenhoek 46:15–25. doi: 10.1007/BF00422225. [DOI] [PubMed] [Google Scholar]
- 111.Nielsen AK, Gerdes K, Degn H, Murrell JC. 1996. Regulation of bacterial methane oxidation: transcription of the soluble methane monooxygenase operon of Methylococcus capsulatus is regulated by copper ions. Microbiology 142:1289–1296. doi: 10.1099/13500872-142-5-1289. [DOI] [PubMed] [Google Scholar]
- 112.Nielsen AK, Gerdes K, Murrell JC. 1997. Copper-dependent reciprocal transcriptional regulation of methane monooxygenase genes in Methylococcus capsulatus and Methylosinus trichosporium OB3b. Mol Microbiol 25:399–409. doi: 10.1046/j.1365-2958.1997.4801846.x. [DOI] [PubMed] [Google Scholar]
- 113.Stafford G, Scanlan J, McDonald IR, Murrell JC. 2003. rpoN, mmoR and mmoG genes involved in the expression of soluble methane monooxygenase in Methylosinus trichosporium OB3b. Microbiology 149:1771–1784. doi: 10.1099/mic.0.26060-0. [DOI] [PubMed] [Google Scholar]
- 114.Collins MLP, Buchholz LA, Remsen CC. 1991. Effect of copper on Methylomonas albus BG8. Appl Environ Microbiol 57:1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kao W-C, Chen Y-R, Yi EC, Lee H, Tian Q, Wu K-M, Tsai S-F, Yu SSF, Chen Y-J, Aebersold R, Chan SI. 2004. Quantitative proteomic analysis of metabolic regulation by copper ions in Methylococcus capsulatus (Bath). J Biol Chem 279:51554–51560. doi: 10.1074/jbc.M408013200. [DOI] [PubMed] [Google Scholar]
- 116.Peija AT, Rostkowski KH, Criddle CS. 2011. Distribution and selection of poly-3-hydroxybutyrate production capacity in methanotrophic. Microbiol Ecol 62:564–473. doi: 10.1007/s00248-011-9873-0. [DOI] [PubMed] [Google Scholar]
- 117.Leak DJ, Dalton H. 1986. Growth yields of methanotrophs. 1. Effect of copper on the energetics of methane oxidation. Appl Microbiol Biotechnol 23:470–476. [Google Scholar]
- 118.Berson O, Lidstrom ME. 1997. Cloning and characterization of corA, a gene encoding a copper-repressible polypeptide in the type I methanotroph, Methylomicrobium album BG8. FEMS Microbiol Lett 148:169–174. doi: 10.1111/j.1574-6968.1997.tb10284.x. [DOI] [PubMed] [Google Scholar]
- 119.Karlsen OA, Lillehaug JR, Jensen HB. 2008. The presence of multiple c-type cytochromes at the surface of the methanotrophic bacterium Methylococcus capsulatus (Bath) is regulated by copper. J Biol Chem 279:51544. doi: 10.1111/j.1365-2958.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 120.Karlsen OA, Lillehaug JR, Jensen HB. 2011. The copper responding surfaceome of Methylococcus capsulatus Bath. FEMS Microbiol Lett 23:97–104. doi: 10.1111/j.1574-6968.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- 121.Johnson KA, Ve T, Larsen Ø, Pedersen RB, Lellehaug JR, Jensen HB, Helland R, Karlsen OA. 2014. CorA is a copper repressible surface-associated copper(I)-binding protein produced in Methylomicrobium album BG8. PLoS One 9:e87750. doi: 10.1371/journal.pone.0087750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bergmann DJ, Zahn JA, DiSpirito AA. 1999. High-molecular-mass multi-c-heme cytochromes from Methylococcus capsulatus Bath. J Bacteriol 181:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shchukin VN, Khmelenina VN, Eshinimayev BT, Suzina NE, Trotsenko YA. 2011. Primary characterization of dominant cell surface proteins of halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Microbiology (Russ) 80:595–605. [PubMed] [Google Scholar]
- 124.Vita N, Platsaki S, Basle A, Allen SJ, Paterson NG, Crombie AT, Murrell JC, Waldron KJ, Dennison C. 2015. A four-helix bundle stores copper for methane oxidation. Nature 525:140–143. doi: 10.1038/nature14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fitch MW, Graham DW, Arnold RG, Agarwal SK, Phelps P, Speitel GE, Georgiou G. 1993. Phenotypic characterization of copper-resistant mutants of Methylosinus trichosporium Ob3b. Appl Environ Microbiol 59:2771–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phelps PA, Agarwal SK, Speitel GE, Georgiou G. 1992. Methylosinus trichosporium OB3b mutants having constitutive epression of soluble methane monooxygenase in the presence of high-levels of copper. Appl Environ Microbiol 58:3701–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tellez CM, Gaus KP, Graham DW, Arnold RG, Guzman RZ. 1998. Isolation of copper biochelates from Methylosinus trichosporium OB3b and soluble methane monooxygenase mutants. Appl Environ Microbiol 64:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DiSpirito AA, Zahn JA, Graham DW, Kim HJ, Larive CK, Derrick TS, Cox CD, Taylor A. 1998. Copper-binding compounds from Methylosinus trichosporium OB3b. J Bacteriol 180:3606–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DiSpirito AA, Zahn JA, Graham DW, Kim HJ, Alterman M, Larive C. September 2004. Methanobactin: a copper binding compound having antibiotic and antioxidant activity. US patent 7,199,099.
- 130.Johnson CL. 2006. Methanobactin: a potential novel biopreservative used against the foodborne pathogen Listeria monocytogenes. Ph.D. thesis. Iowa State University, Ames, IA. [Google Scholar]
- 131.Choi DW, Bandow NL, McEllistrem MT, Semrau JD, Antholine WE, Hartsel SC, Gallagher W, Zea CJ, Pohl NL, Zahn JA, DiSpirito AA. 2010. Spectral and thermodynamic properties of methanobactin from gamma-proteobacterial methane oxidizing bacteria: a case for copper competition on a molecular level. J Inorg Biochem 104:1240–1247. doi: 10.1016/j.jinorgbio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Krentz BD, Mulheron HJ, Semrau JD, DiSpirito AA, Bandow NL, Haft DH, Vuilleumier S, Murrell JC, McEllistrem MT, Hartsel SC, Gallagher WH. 2010. A comparison of methanobactins from Methylosinus trichosporium OB3b and Methylocystis strain SB2 predicts methanobactins are synthesized from diverse peptide precursors modified to create a common core for binding and reducing copper ions. Biochemistry 49:10117–10130. doi: 10.1021/bi1014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El Ghazouani A, Basle A, Gray J, Graham DW, Firbank SJ, Dennison C. 2012. Variations in methanobactin structure influences copper utilization by methane-oxidizing bacteria. Proc Natl Acad Sci U S A 109:8400–8404. doi: 10.1073/pnas.1112921109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim HJ, Graham DW, DiSpirito AA, Alterman MA, Galeva N, Larive CK, Asunskis D, Sherwood PM. 2004. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305:1612–1615. doi: 10.1126/science.1098322. [DOI] [PubMed] [Google Scholar]
- 135.El Ghazouani A, Basle A, Firbank SJ, Knapp CW, Gray J, Graham DW, Dennison C. 2011. Copper-binding properties and structures of methanobactins from Methylosinus trichosporium OB3b. Inorg Chem 50:1378–1391. doi: 10.1021/ic101965j. [DOI] [PubMed] [Google Scholar]
- 136.Bandow N, Gilles VS, Freesmeier B, Semrau JD, Krentz B, Gallaghe W, McEllistrem MT, Hartse SC, Cho DW, Hargrove MS, Heard TM, Chesner LM, Braunreiter KM, Cao BV, Gavitt MM, Hoopes JZ, Johnson JM, Polster EM, Schoenick BD, Umlauf AM, DiSpirito AA. 2012. Spectral and copper binding properties of methanobactin from the facultative methanotroph Methylocystis strain SB2. J Inorg Biochem 110:72–82. doi: 10.1016/j.jinorgbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Bandow NL. 2014. Isolation and binding properties of methanobactin from the facultative methanotroph Methylocystis strain SB2. Ph.D. thesis. Iowa State University, Ames, IA. [DOI] [PubMed] [Google Scholar]
- 138.Bandow NL, Gallagher WH, Behling L, Choi DW, Semrau JD, Hartsel SC, Gilles VS, Dispirito AA. 2011. Isolation of methanobactin from the spent media of methane-oxidizing bacteria. Methods Enzymol 495:259–269. doi: 10.1016/B978-0-12-386905-0.00017-6. [DOI] [PubMed] [Google Scholar]
- 139.Neilands JB. 1995. Siderophores—structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 140.Khmelenina VN, Beck DA, Munk C, Davenport K, Daligault H, Erkkila T, Goodwin L, Gu W, Lo CC, Scholz M, Teshima H, Xu Y, Chain P, Bringel F, Vuilleumier S, Dispirito A, Dunfield P, Jetten MS, Klotz MG, Knief C, Murrell JC, Op den Camp HJ, Sakai Y, Semrau J, Svenning M, Stein LY, Trotsenko YA, Kalyuzhnaya MG. 2013. Draft genome sequence of Methylomicrobium buryatense strain 5G, a haloalkaline-tolerant methanotrophic bacterium. Genome Announc 1:e00053-13. doi: 10.1128/genomeA.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choi DW, Zea CJ, Do YS, Semrau JD, Antholine WE, Hargrove MS, Pohl NL, Boyd ES, Geesey GG, Hartsel SC, Shafe PH, McEllistrem MT, Kisting CJ, Campbell D, Rao V, de la Mora AM, Dispirito AA. 2006. Spectral, kinetic, and thermodynamic properties of Cu(I) and Cu(II) binding by methanobactin from Methylosinus trichosporium OB3b. Biochemistry 45:1442–1453. doi: 10.1021/bi051815t. [DOI] [PubMed] [Google Scholar]
- 142.Rue E, Bruland K. 2001. Domoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Marine Chem 76:127–134. doi: 10.1016/S0304-4203(01)00053-6. [DOI] [Google Scholar]
- 143.Pelludat C, Rakin A, Jacobi CA, Schubert S, Heesemann J. 1998. The Yersiniabactin biosynthetic gene cluster of Yersinia enterocolitia: Organization and siderophore-dependent regulation. J Bacteriol 180:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rehmani FS, Siddiqa T. 2008. Comparative studies of copper catechol siderophore complexes with copper hydroxamate complexes. J Chem Soc Pak 30:85–89. [Google Scholar]
- 145.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. 2012. The siderophore yersiniabactin binding copper to protect pathogens during infections. Nat Chem Biol 8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clarke SE, Stuart J, Sanders-Loehr J. 1987. Induction of siderophore activity in Anabaena spp. and its moderation of copper toxicity. Appl Environ Microbiol 53:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grass G, Thakali K, Klebba PE, Thieme D, Muller A, Wilder GF, Rensing CJ. 2004. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J Bacterol 186:5826–5833. doi: 10.1128/JB.186.17.5826-5833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi DW, Do YS, Zea CJ, McEllistrem MT, Lee SW, Semrau JD, Pohl NL, Kisting CJ, Scardino LL, Hartsel SC, Boyd ES, Geesey GG, Riedel TP, Shafe PH, Kranski KA, Tritsch JR, Antholine WE, DiSpirito AA. 2006. Spectral and thermodynamic properties of Ag(I), Au(III), Cd(II), Co(II), Fe(III), Hg(II), Mn(II), Ni(II), Pb(II), U(IV), and Zn(II) binding by methanobactin from Methylosinus trichosporium OB3b. J Inorg Biochem 100:2150–2161. doi: 10.1016/j.jinorgbio.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 149.Yoon S, Kraemer SM, DiSpirito AA, Semrau JD. 2010. An assay for screening microbial cultures for chalkophore production. Environ Microbiol Rep 2:295–303. doi: 10.1111/j.1758-2229.2009.00125.x. [DOI] [PubMed] [Google Scholar]
- 150.Yoon S, Dispirito AA, Kraemer SM, Semrau JD. 2011. A simple assay for screening microorganisms for chalkophore production. Methods Enzymol 495:247–258. doi: 10.1016/B978-0-12-386905-0.00016-4. [DOI] [PubMed] [Google Scholar]
- 151.Behling LA, Hartsel SC, Lewis DE, DiSpirito AA, Choi DW, Masterson LR, Veglia G, Gallagher WH. 2008. NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings. J Am Chem Soc 130:12604–12605. doi: 10.1021/ja804747d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim HJ, Galeva N, Larive CK, Alterman M, Graham DW. 2005. Purification and physical-chemical properties of methanobactin: a chalkophore from Methylosinus trichosporium OB3b. Biochemistry 44:5140–5148. doi: 10.1021/bi047367r. [DOI] [PubMed] [Google Scholar]
- 153.Banala S, Sussmuth RD. 2010. Thioamides in nature: in search of secondary metabolites in anaerobic microorganisms. Chembiochem 11:1335–1337. doi: 10.1002/cbic.201000266. [DOI] [PubMed] [Google Scholar]
- 154.Baral BS, Bandow NL, Vorobev A, Freemeier BC, Bergman BH, Herdendorf T, Fuentes N, Ellias L, Turpin E, Semrau JD, Di Spirito AA. 2014. Mercury binding by methanobactin from Methylocystis strain SB2. J Inorg Biochem 141:161–169. doi: 10.1016/j.jinorgbio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 155.Stirbet A. 2013. Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynth Res 116:189–214. doi: 10.1007/s11120-013-9863-9. [DOI] [PubMed] [Google Scholar]
- 156.Georgakopoulou S, van Grondelle R, van der Zwan G. 2004. Circular dichroism of carotenoids in bacterial light-harvesting complexes: experiments and modeling. Biophys J 87:3010–3022. doi: 10.1529/biophysj.104.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Georgakopoulou S, Frese RN, Johnson E, Koolhaas C, Cogdell RJ, van Grondelle R, van der Zwan G. 2002. Absorption and CD spectroscopy and modeling of various LH2 complexes from purple bacteria. Biophyl J 82:2184–2197. doi: 10.1016/S0006-3495(02)75565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kloss F, Pidot S, Goerls H, Freidrich T, Hertweck C. 2013. Formation of a dinuclear copper(I) complex from the Clostridium-derived antibiotic closthioamide. Angewandte Chemie Int Ed 52:10745–10748. doi: 10.1002/anie.201304714. [DOI] [PubMed] [Google Scholar]
- 159.Letzel A-C, Pidott SJ, Hertweck C. 2013. A genomic approach to the cryptic secondary metabolome of the anaerobic world. Nat Prod Rep 30:392–428. doi: 10.1039/C2NP20103H. [DOI] [PubMed] [Google Scholar]
- 160.Lincke T, Behnken S, Ishida K, Roth M, Hertweck C. 2010. Closthioamide: an unprecedented polythioamide antibiotic from the strictly anaerobic bacterium Clostridium cellulolyticum. Angewandte Chemie Int ed 49:2011–2013. doi: 10.1002/anie.200906114. [DOI] [PubMed] [Google Scholar]
- 161.Yoon S, Im J, Bandow N, Dispirito AA, Semrau JD. 2011. Constitutive expression of pMMO by Methylocystis strain SB2 when grown on multi-carbon substrates: implications for biodegradation of chlorinated ethenes. Environ Microbiol Rep 3:182–188. doi: 10.1111/j.1758-2229.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 162.Anttila J, Petri Heinonen P, Nenonen T, Pino A, Iwaï H, Kauppi E, Soliymani R, Baumann M, Saksi J, Suni N, Haltia T. 2011. Is coproporphyrin III a copper-acquisition compound in Paracoccus denitrificans? Biochim Biophys Acta 1807:311–318. doi: 10.1016/j.bbabio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 163.Li Y-M, Mine JC, Madison LL, Kolter T, Walsh CT. 1996. From peptide precursors to oxazole and thiazole-containing peptide antibiotics: microbin B17 synthease. Science 274:1188–1193. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- 164.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian K-D, Fischbach MA, Gravelli JS, Goransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kiuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore B, Muller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rubuffat S, Ross RP, Sahl H-G, Schmidt EW, Selsted ME, et al. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for universal nomenclature. Nat Prod Rep 30:108–160. doi: 10.1039/C2NP20085F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Melby JO, Nard NJ, Mitchell DA. 2011. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr Opin Chem Biol 15:369–387. doi: 10.1016/j.cbpa.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmidt EW. 2010. The hidden diversity of ribosomal peptide natural products. MBC Biol 8:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Savard ME, Melzer MS, Boland GJ, Bensimon C, Blackwell BA. 2003. A New 1-hydroxy-2,6-pyrazinedione associated with hypovirulent isolates of Sclerotinia minor. J Nat Prod 66:306–309. doi: 10.1021/np020445w. [DOI] [PubMed] [Google Scholar]
- 168.Tomassini JE, Davies ME, Hastings JC, Lingham R, Mojena M, Raghoobar SL, Sing SB, Tkacz JS, Goetz MA. 1996. A novel antiviral agent which inhibits the endonuclease of influenza viruses. Antimicrob Agents Chemother 40:1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hayakawa Y, Sasaki K, Adachi H, Furihata K, Nagai K, Shin-ya K. 2006. Thioviridamide, a novel apoptosis inducer in transformed cell from Steptomyces olivoviridis. J Antibiot 59:1–5. doi: 10.1038/ja.2006.1. [DOI] [PubMed] [Google Scholar]
- 170.Hayakawa Y, Sasaki K, Nagai K, Shin-ya K, Furihata K. 2006. Structure of thioviridamide, a novel apoptosis inducer from Streptomyces olivovirdis. J Antibiot 59:6–10. doi: 10.1038/ja.2006.2. [DOI] [PubMed] [Google Scholar]
- 171.Medzihradszky KF, Darula Z, Perlson E, Faubzuber M, Chalkley RJ, Ball H, Greenbaum D, Bogyo M, Tyson DR, Bradshaw RA, Burlingame AL. 2004. O-sulfonation of serine and threoninie: mass spectrometric detection and characterization of a new posttranslational modification in diverse proteins throughout the eukaryotes. Mol Cell Proteomics 3:429–440. doi: 10.1074/mcp.M300140-MCP200. [DOI] [PubMed] [Google Scholar]
- 172.Pesch M-L, Christl I, Hoffmann M, Kraemer SM, Kretzschmar R. 2012. Copper complexation of methanobactin isolated from Methylosinus trichosporium: OB3b: pH-dependent speciation and modeling. J Inorg Biochem 116:55–62. doi: 10.1016/j.jinorgbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 173.Bagchi P, Morgan MT, Bacsa J, Fahrni CJ. 2013. Robust affinity standards for Cu(I) biochemistry. J Am Chem Soc 135:18549–18559. doi: 10.1021/ja408827d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Badarau A, Dennison C. 2011. Thermodynamics of copper and zinc distribution in the cyanobacterium Synechocystis PCC 6803. Proc Natl Acad Sci U S A 108:13007–13012. doi: 10.1073/pnas.1101448108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Badarau A, Dennison C. 2011. Copper trafficking mechanism of CXXC-containing domains: insight from the pH-dependence of their Cu(I) affinites. J Am Chem Scc 133:2983–2988. doi: 10.1021/ja1091547. [DOI] [PubMed] [Google Scholar]
- 176.Fru EC, Gray ND, McCann C, Baptista JdC, Christgen B, Talbot HM, El Ghazouani A, Dennison C, Graham DW. 2011. Effects of copper mineralogy and methanobactin on cell growth and sMMO activity in Methylosinus trichosporium OB3b. Biosciences 8:2887–2894. [Google Scholar]
- 177.Pesch ML, Hoffmann M, Christl I, Kreamer M, Kretzschmar R. 2013. Competitve ligand exchange between Cu-humic acid complexes and methanobactin. Biobiology 11:44–54. [DOI] [PubMed] [Google Scholar]
- 178.Kulczycki E, Fowle DA, Kenward PA, Leslie K, Graham DW, Roberts JA. 2011. Stimulation of methanotroph activity by Cu-substituted borosilicate glass. Geomicrobiol J 28:1–10. doi: 10.1080/01490451003614971. [DOI] [Google Scholar]
- 179.Hughes MN, Poole RK. 1991. Metal speciation and microbial growth-the hard (and soft) facts. J Gen Microbiol 137:725–734. doi: 10.1099/00221287-137-4-725. [DOI] [Google Scholar]
- 180.Zhang H, Zhao F-J, S B, Davison W, McGrath SP. 2001. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607. doi: 10.1021/es000268q. [DOI] [PubMed] [Google Scholar]
- 181.Sutherland RA, Tack FMG. 2003. Fractionation of Cu, Pb and Zn in certified reference soils SRM 2710 and SRM 2711 using optimized BCR sequential extraction procedure. Adv Environ Research 8:37–50. doi: 10.1016/S1093-0191(02)00144-2. [DOI] [Google Scholar]
- 182.Mayor DJ, Gray NB, Elver-Evans J, Midwook AJ, Thornton B. 2013. Metal-macrofauna interactions determine microbial community structure and function in copper contaminated sediments. PLoS One 8:e64940. doi: 10.1371/journal.pone.0064940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suave S, Hendershot W, Allen HE. 2000. Solid-solution partioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131. doi: 10.1021/es9907764. [DOI] [Google Scholar]
- 184.Vorobev A, Jagadevan S, Baral BS, Dispirito AA, Freemeier BC, Bergman BH, Bandow NL, Semrau JD. 2013. Detoxification of mercury by methanobactin from Methylosinus trichosporium OB3b. Appl Environ Microbiol 79:5918–5926. doi: 10.1128/AEM.01673-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hakemian AS, Tinberg CE, Kondapalli KC, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC. 2005. The copper chelator methanobactin from Methylosinus trichosporium OB3b binds copper(I). J Am Chem Soc 127:17142–17143. doi: 10.1021/ja0558140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kenney GE, Rosenzweig AC. 2013. Genome mining for methanobactin. BMC Biol 11:17. doi: 10.1186/1741-7007-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Semrau JD, Jagadevan S, DiSpirito AA, Khalifa A, Scanlan J, Bergman B, Freemeir BC, Baral BS, Bandow NL, Vorobev A, Haft DH, Vuilleumier S, Murrell JC. 2013. Methanobactin and MmoD work in concert to act as the “copper switch” in methanotrophs. Environ Microbiol 15:3077–3086. doi: 10.1111/1462-2920.12150. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Q, van der Dork WA. 2012. Catalytic promiscuity of a bacterial alpha-N-methyltrasferase. FEBS Lett 586:3391–3397. doi: 10.1016/j.febslet.2012.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hondrop ER, Matthews RG. 2004. Oxidative stress inactivates cobalamin-independent methione synthease (MetE) in Escherichia coli. PLoS Biol 2:1738–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yampolsky IV, Balashova TA, Lukyanov M-M. 2009. Synthesis and spectral and chemical properties of the yellow flourescent protein zFP38 chromophore. Biochemisty 48:8077–8082. doi: 10.1021/bi900719x. [DOI] [PubMed] [Google Scholar]
- 191.Csaki R, Bodrossy L, Klem J, Murrell JC, Kovacs KL. 2003. Genes involved in the copper-dependent regulation of soluble methane monooxygenase of Methylococcus capsulatus (Bath): cloning, sequencing and mutational analysis. Microbiology 149:1785–1795. doi: 10.1099/mic.0.26061-0. [DOI] [PubMed] [Google Scholar]
- 192.Balasubramanian R, Kenney GE, Rosenzweig AC. 2011. Dual pathways for copper uptake by methanotrophic bacteria. J Biol Chem 286:37313–37319. doi: 10.1074/jbc.M111.284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Knapp CW, Fowle DA, Kulczycki E, Roberts JA, Graham DW. 2007. Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources. Proc Natl Acad Sci U S A 104:12040–12045. doi: 10.1073/pnas.0702879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peltola P, Priha P, Laakso S. 1993. Effect of copper on membrane-lipids and on methane monooxygenase activity of Methylococcus capsulatus (Bath). Arch Microbiol 159:521–525. doi: 10.1007/BF00249029. [DOI] [Google Scholar]
- 195.Fentona R, Chemizmu KD. 2009. Fenton reaction—controversy concerning the chemistry. Ecol Chem Eng 16:347–358. [Google Scholar]
- 196.Fridovich I. 1978. The biology of oxygen radicals. Science 201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 197.Kehrer JP. 2000. The Harber-Weiss reaction mechanisms of toxicity. Toxicology 149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 198.Choi DW, Semrau JD, Antholine WE, Hartsel SC, Anderson RC, Carey JN, Dreis AM, Kenseth EM, Renstrom JM, Scardino LL, Van Gorden GS, Volkert AA, Wingad AD, Yanzer PJ, McEllistrem MT, de la Mora AM, DiSpirito AA. 2008. Oxidase, superoxide dismutase, and hydrogen peroxide reductase activities of methanobactin from types I and II methanotrophs. J Inorg Biochem 102:1571–1580. doi: 10.1016/j.jinorgbio.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 199.Tegoni M, Valensin D, Toso L, Remelli M. 2014. Copper chelators: chemical properties and bio-medical applications. Curr Med Chem 21:3785–3818. doi: 10.2174/0929867321666140601161939. [DOI] [PubMed] [Google Scholar]
- 200.Turrens JF. 2003. Mitochondrial formation of reactive oxygen species. J Physiol 522.2:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Saha K, Agasti S, Kim C, Li X, Rotello V. 2012. Gold nanoparticles in chemical and bological sensing. Chem Rev 112:2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hostetler MJ, Wingate JE, Zhong C-J, Harris JE, Vachet RW, Clark MR, Londono JD, Green SJ, Stokes JJ, Wignall GD, Glish GL, Porter MD, Evans ND, Murray RW. 1998. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 14:17–30. doi: 10.1021/la970588w. [DOI] [Google Scholar]
- 203.Chen S. 1999. 4-Hydroxythiophenol-protected gold nanoclusters in aqueous media. Langmuir 15:7551–7556. doi: 10.1021/la990398g. [DOI] [Google Scholar]
- 204.Chen S, Murray RW. 1999. Arenethiolate monolayer-protected gold clusters. Langmuir 15:682–689. doi: 10.1021/la980817u. [DOI] [Google Scholar]
- 205.Xin J-Y, Ahang L-X, Chen D-D, Lin K, Fan H-C, Wang Y, Xia C-G. 2015. Colorimetric detection of melamine based on methanobactin-mediated synthesis of gold nanoparticles. Food Chem 174:473–479. doi: 10.1016/j.foodchem.2014.11.098. [DOI] [PubMed] [Google Scholar]
- 206.Xin J-Y, Lin K, Wang Y, Xia C-G. 2014. Methanobactin-mediated synthesis of gold nanoparticles supported over Al2O3 towaard and efficient catalyst for glucose oxidation. Int J Mol Sci 15:21603–21620. doi: 10.3390/ijms151221603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Korbekandi H, Iravani S, Abbasi S. 2009. Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306. doi: 10.3109/07388550903062462. [DOI] [PubMed] [Google Scholar]
- 208.Iravani S. 2014. Bacteria in nanoparticle synthesis: current status and future prospects. Intern Sch Res Notices 2014:359361–325984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lar RH, Vikesland PL. 2014. Surface-enhanced raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sust Chem Eng 2:1599–1608. doi: 10.1021/sc500105n. [DOI] [Google Scholar]
- 210.Ala A, Walker AP, Ashkan A, Dooley JS, Schilsky M. 2007. Wilson's disease. Lancet 369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 211.Roberts EA. 2011. Wilson's disease. Medicine 39:602–604. doi: 10.1016/j.mpmed.2011.08.006. [DOI] [Google Scholar]
- 212.de Bie P, Muller P, Wijmenga C, Klomp LWJ. 2007. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet 44:678–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zischka H, Lichtmannegger J, Schmitt S, Jagemann N, Schulz S, Wartini D, Jennen L, Rust C, Larochette N, Galluzzi L, Chajes V, Bandow N, Gilles VS, DiSpirito AA, Esposito I, Goettlicher M, Summer KH, Kroemer G. 2011. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest 121:1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dalyl A, Padmanaban M. 2014. Wilson's disease: etiology, diagnosis, and treatment. Disease-a-Month 60:450–459. doi: 10.1016/j.disamonth.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 215.Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J. 2014. The neurotoxicity of iron, copper and manganese in Parkinson's and Wilson's diseases. J Trace Elem Med Biol doi: 10.1016/j.jtemb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 216.Martell AE, Smith RM. 1984. Critial stability constants, vol 6 Plenum Press, New York, NY. [Google Scholar]
- 217.Brewer GJ, Hedera P, Kluin KK, Carlson M, Askari F, Dick RB, Sitterly J, Fink JK. 2003. Treatment of Wilson disease with ammonium tetrathiomolybdate. III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch Neurol 60:379–385. [DOI] [PubMed] [Google Scholar]
- 218.Reference deleted.
- 219.Ogra Y, Ohmichi M, Suzuki KT. 2000. Metabolic fate of the insoluble copper/tetrathiomolybdate complex formed in the liver of EC rats with excess tetrathiolmolybdate. J Inorg Biochem 78:123–128. doi: 10.1016/S0162-0134(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 220.Ogra Y, Ohmichi M, Suzuki KT. 1996. Mechanisms of selective copper removal by tetrathiomolybdate from metallothionein in LEC rats. Toxicology 106:75–83. doi: 10.1016/0300-483X(95)03171-B. [DOI] [PubMed] [Google Scholar]
- 221.Suzuki KT, Yamamoto K, Kanno S, Aoki Y, Takeichi N. 1993. Selective removal of copper bound to metallothionein in the liver of LEC rats by tetrathiolmolybdate. Toxicology 83:123–128. [DOI] [PubMed] [Google Scholar]
- 222.Summer KH, Lichtmannegger J, Bandow N, Choi DW, DiSpirito AA, Michalke B. 2011. The biogenic methanobactin is an effective chelator for copper in a rat model for Wilson disease. J Trace Elem Med Biol 25:36–41. doi: 10.1016/j.jtemb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 223.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. 2002. Copper deficiency induced by tetrathiolmolybdate suppresses tumor growth and angiogenesis. Cancer Res 62:4854–4859. [PubMed] [Google Scholar]
- 224.Garber K. 2015. Targeting copper to treat breast cancer. Science 349:128–129. doi: 10.1126/science.349.6244.128. [DOI] [PubMed] [Google Scholar]
- 225.Folkman J, Shing Y. 1992. Angiogenesis. J Biol Chem 267:10931–10934. [PubMed] [Google Scholar]
- 226.Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xio K, Cambell SL, Thiele DJ, Counter CM. 2014. Copper is required for oncogenic BRAF signalling and turmorigenesis. Nature 509:492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khalifa A, Lee CG, Ogiso T, Ueno C, Dianou D, Demachi T, Katayama A, Asakawa S. 2015. Methylomagnum ishizawai gen. nov., sp. nov., a mesophilic type I methanotroph isolated from rice rhizosphere. Int J Syst Evol Microbiol 65:3527–3534. doi: 10.1099/ijsem.0.000451. [DOI] [PubMed] [Google Scholar]
- 228.Farhan Ul-Haque M, Kalidass B, Vorobev A, Baral BS, DiSpirito AA, Semrau JD. 2015. Methanobactin from Methylocystis strain SB2 affects gene expression and methane monooxygenase activity in Methylosinus trichosporium OB3b. Appl Environ Microbiol 81:2466–2473. doi: 10.1128/AEM.03981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]