Abstract
The aim of this study was to investigate the possible anxiolytic-like effects of striatal MT2 activation, and its counteraction induced by the selective blockade of this receptor. Furthermore, we analyzed this condition under the paradigm of rapid eye movement (REM) sleep deprivation (REMSD) and the animal model of Parkinson’s disease (PD) induced by rotenone. Male Wistar rats were infused with intranigral rotenone (12 μg/μL), and 7 days later were subjected to 24 h of REMSD. Afterwards the rats underwent striatal micro-infusions of selective melatonin MT2 receptor agonist, 8-M-PDOT (10 μg/μL) or selective melatonin MT2 receptor antagonist, 4-P-PDOT (5 μg/μL) or vehicle. Subsequently, the animals were tested in the open-field (OP) and elevated plus maze (EPM) tests. Results indicated that the activation of MT2 receptors produced anxiolytic-like effects. In opposite, the MT2 blockade did not show an anxiogenic-like effect. Besides, REMSD induced anxiolytic-like effects similar to 8-M-PDOT. MT2 activation generated a prevalent locomotor increase compared to MT2 blockade in the context of REMSD. Together, these results suggest a striatal MT2 modulation associated to the REMSD-induced dopaminergic supersensitivity causing a possible dopaminergic influence in the MT2 anxiolytic-like effects in the intranigral rotenone model of PD.
Keywords: Melatonin, MT2 receptor, Rotenone, Anxiety, Striatum, Parkinson’s disease
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, afflicting about 1% of people over 65 years old and 4–5% of people over 85 years old [1]. It is characterized by major cardinal motor disturbances, namely rigidity, rest tremor and bradykinesia [2], [3]. These alterations are the result of the progressive dopaminergic neuronal loss in the substantia nigra pars compacta (SNpc) and consequently reductions in the striatal levels of dopamine [4]. In addition to the motor dysfunction, PD patients usually display non-motor features of the disease [2], such as sleep disorders, autonomic dysfunctions, olfactory deficits, and neuropsychiatric symptoms particularly depression, anxiety and apathy [2], [5].
Anxiety is frequently reported in more than 50% of PD patients [6]. In general, all types of anxiety disorders found in PD are associated with generalized anxiety disorder, agoraphobia, specific phobia, and social anxiety disorder, panic disorder, and obsessive-compulsive disorder or related disorders (according to DSM-V) [7], [8]. Anxiety disorder, in some patients, is a “reactive” response to the diagnosis of PD, as a result of stress and motor disability [9]. However, PD patients have greater risks developing anxiety before the diagnosis of PD [5], [10]. In addition, loss of striatal serotonin [11], dopamine and noradrenaline [12] innervation are present in living patients with PD. Therefore, these evidence suggest that anxiety is an early non-motor symptom in Parkinson’s disease [13].
Moreover, another non-motor alteration that aggravates anxiety, in humans, and consequently PD, is rapid eye movement (REM) sleep loss [3]. Furthermore, REM sleep deprivation (REMSD) is known to increase anxiety behaviors in humans [14]. While preclinical studies, in rodents, are still inconsistent, suggesting that REMSD triggers anxiolytic-like [15], [16], [17] or anxiogenic-like effects [18], [19], [20]. These lack of consistency difficult translational applicability of preclinical data of anxiety and sleep deprivation [21].
The research of the motor and non-motor disturbances of PD frequently adopts a number of animal models based on neurotoxins injected within the brain. Rotenone is a pesticide which, freely crosses cellular membranes and accumulates in subcellular organelles such as mitochondria, where inhibits the mitochondrial complex Ι of electrical transport chain, in this way inducing apoptosis of the nigrostriatal pathway [22], [23]. This dopaminergic neuronal loss mimics several motor [23] and non-motor features of PD, such as olfactory dysfunction [24] and depression [25], [26]. However, studies reporting anxiety-like behaviors and their consequences after REMSD, in neurotoxin-based models, are sparse and somewhat inconsistent [6], [27]. It is worth mentioning, that only one study, according to our knowledge, demonstrated the effects of rotenone in anxiety-like behaviors, after intraperitoneal injections of this neurotoxin [28].
There is a growing interest in exploring novel pharmacotherapeutic strategies for anxiety in the context of PD [13], [26], [29]. Among these emerging targets, melatoninergic drugs have gained considerable attention. Several reports have demonstrated anxiolytic-like effects of melatonin, observed in the elevated plus maze test (EPM) by an increase in the number of entries and time spent into the open arms [30], [31], [32]. The main roles of this neuropeptide are related to the control of the circadian sleep-wake states, regulation of sleep and seasonal biorhythm [33], [34], [35] by activating MT1 and MT2 receptors, two G-protein-coupled membrane receptors [36].
It has been hypothesized that dysregulations of these melatonin receptors may be involved in the installation of mood disorders [37]. As a matter of fact, it has been demonstrated that MT2 receptors play an important role in depression [38] as well as in anxiety [39]. Activation of MT2 receptors, by the partial agonist UCM765, elicits an anxiolytic-like effect in rats [39]. However, it is still unexplained if this effect is also observed in the intranigral rotenone model of PD. Besides, it remains to be clarified if striatal MT2 receptors could elicit anxiety-like effects, since these receptors are present within the striatum [40], [41]. Hence, we hypothesize that striatal MT2 receptors associated to dopamine depletion might be implicated in the pathogenesis of anxiety in PD [42], [43]. Therefore, in the present study we sought to investigate the existence of a purportedly anxiolytic-like effect generated by the striatal MT2 activation, achieved by the infusion of a selective agonist 8-methoxy-2-propionamidotetralin (8-M-PDOT), and counteracted by the selective antagonist 4-phenyl-2-propionamidotetralin (4-P-PDOT), after dopaminergic degeneration, induced by intranigral rotenone. This hypothesis was tested under the PD model and the REMSD protocol.
2. Material and methods
2.1. Ethics statement
The studies were carried out in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals, United States National Institutes of Health. In addition, the protocol complies with the recommendations of Federal University of Paraná and was approved by the Institutional Ethics Committee (approval ID # 695).
2.2. Animals
Male Wistar rats from our breeding colony were used, weighing 280–320 g at the beginning of the experiments. The animals were housed in groups of five, in polypropylene cages and maintained under standard conditions of temperature (22±2 °C) and illumination (12/12 h light–dark cycle). The animals had free access to water and food throughout the experiment.
2.3. Drugs
Xylazine and ketamine were purchased from Syntec (Brazil). Rotenone was dissolved in dimethylsulfoxide (DMSO) at a final concentration of 12 µg/µL, both drugs were purchased by Sigma–Aldrich (United States). This solution was administered by bilateral intranigral injections through stereotaxic surgery. The selective MT2 agonist 8-methoxy-2-propionamidotetralin (8-M-PDOT) and the selective MT2 antagonist 4-phenyl-2-propionamidotetralin (4-P-PDOT) were purchased from TOCRIS (San Diego, CA, USA), and were dissolved with DMSO. The final concentrations were 10 µg/µL and 5 µg/µL, respectively, which were injected into the striatum, through bilateral cannulas also positioned by stereotaxic surgery. The vehicle group was injected with DMSO.
2.4. Experimental design
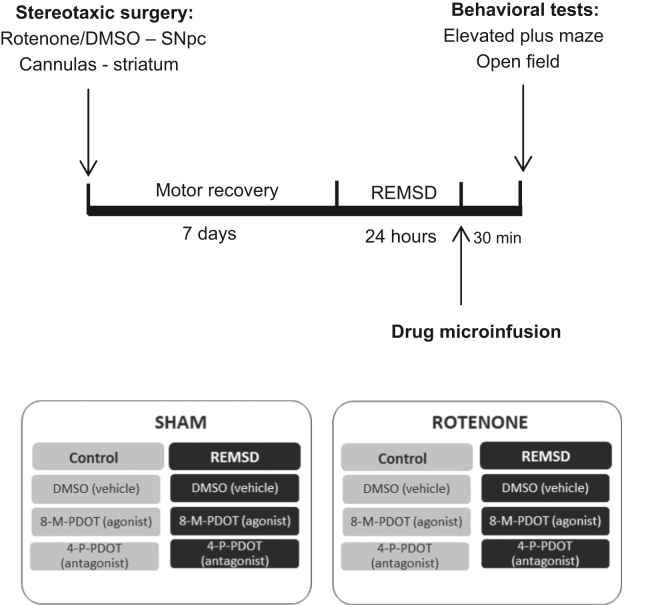
In the present study, the rats were randomly distributed into two groups named sham and rotenone. Seven days after the stereotaxic surgery (Fig. 1) the animals underwent 24 h of REMSD (REMSD group). In the control group the animals were kept in their home cages undisturbed. Under these groups, three subgroups were obtained as: vehicle, 8-M-PDOT, and 4-P-PDOT. During the experiment the rats were randomly assigned to 12 groups: sham control vehicle (n=13), sham control 8-M-PDOT (n=10), sham control 4-P-PDOT (n=10); sham REMSD vehicle (n=14), sham REMSD 8-M-PDOT (n=12), sham REMSD 4-P-PDOT (n=12); rotenone control vehicle (n=8), rotenone control 8-M-PDOT (n=10), rotenone control 4-P-PDOT (n=9); rotenone REMSD vehicle (n=10), rotenone REMSD 8-M-PDOT (n=12) and rotenone REMSD 4-P-PDOT (n=15). The drugs were administrated into the dorsal striatum by bilateral cannulas immediately after the REMSD period. All animals were tested, thirty minutes after the drugs administration, in the EPM for anxiety-like behaviors quantification and in the open-field test (OFT) for locomotion activity. For further details see Fig. 1.
Fig. 1.
Experimental design. SNpc – Substantia nigra pars compacta, REMSD – REM sleep deprivation.
2.5. Stereotaxic surgery
Rats were sedated with intraperitoneal xylazine (10 mg/kg) and anaesthetized with intraperitoneal ketamine (90 mg/kg). The following coordinates were used (having the bregma as a reference) to allow the bilateral nigral lesion with rotenone: SNpc (AP)=−5.0 mm, (ML)=±2.1 mm and (DV)=−8.0 mm [44]. Needles were guided to the region of interest for a bilateral infusion of 1 µL of rotenone (12 µg/µL) using an electronic infusion pump (Insight Instruments, Ribeirão Preto, Brazil) at a rate of 0.33 µL/min for 3 min [45], [46], [47]. Sham operations followed the same procedure, but 1 µL of DMSO was injected instead. Complementarily, bilateral guide cannulas were implanted in the dorsal striatum of each rat allowing a subsequent infusion 1 µL of 8-M-PDOT (10 µg/µL), 4-P-PDOT (5 µg/µL) or vehicle (DMSO) at a rate of 0.33 µL/min for 3 min, in their respective groups. Coordinates with reference to bregma for implantation of guide cannulas were: AP=−1.0 mm, ML=±3.0 mm and DV=−6.0 mm [44]. This administration protocol was performed during the light-cycle between 7:00 a.m. and 9:00 a.m.
2.6. REMSD procedure
REMSD was attained by means of the single platform method. Rats were individually placed on a circular platform (6.5 cm in diameter) in a cage (23 cm×23 cm×30 cm) filled with water up to 1 cm below the platform level. At the onset of each REM sleep episode, the animal experiences a loss of muscle tonus and falls into the water, thus being awakened. When platforms of this size are used, REM sleep is completely eliminated [48]. Throughout the study, the experimental room was maintained at controlled conditions (22±2 °C, 12/12 h light/dark cycle, lights on 7:00 a.m. and off on 7:00 p.m.). The control group was kept in the same room as the REMSD rats during the study. Food and water were provided ad libitum by placing chow pellets in a dispenser positioned inside the cage and water bottles on a grid located on top of the tank.
2.7. Elevated plus maze test
The elevated plus maze apparatus was made of wood, and consisted in two opposite open-arms (50 cm×10 cm), and two enclosed-arms (50 cm×10 cm×40 cm). The whole apparatus was elevated 50 cm above the floor. The rats were placed on the central platform (10 cm×10 cm) facing one of the enclosed arms. Animal behavior was recorded by videotape for 5 min, with the following variables evaluated: time spent in open arms and number of entries in open arms. Data were expressed as the percentage of time spent in the open arms. An increase in both the time spent on the open arms and the number of entries into them are interpreted as anxiolytic responses.
2.8. Open-field test
The apparatus consists of a circular arena (1 m of diameter) limited by a 40 cm high wall and illuminated by four 60 W lamps situated 48 cm above the arena floor, providing illumination around 300 lx. The animals were gently placed in the center of the arena and were allowed to freely explore the area for 5 min. During the experiments, the open-field was video recorded, and the measures of total distance were computed online by an image analyzer system (Smart junior, PanLab, Harvard Apparatus, Spain).
2.9. Statistical analysis
Differences between the groups in the elevated plus maze and open-field test were analyzed by one-way analysis of variance (ANOVA) followed by the Neuman-Keuls post hoc test. Values are expresses as mean±standard error of mean (SEM). The level of significance was set at P≤0.05. At this point a note of caution should be added: the analysis of the groups could also be performed by a two-way ANOVA, however, our sample size has prevented us to perform it.
3. Results
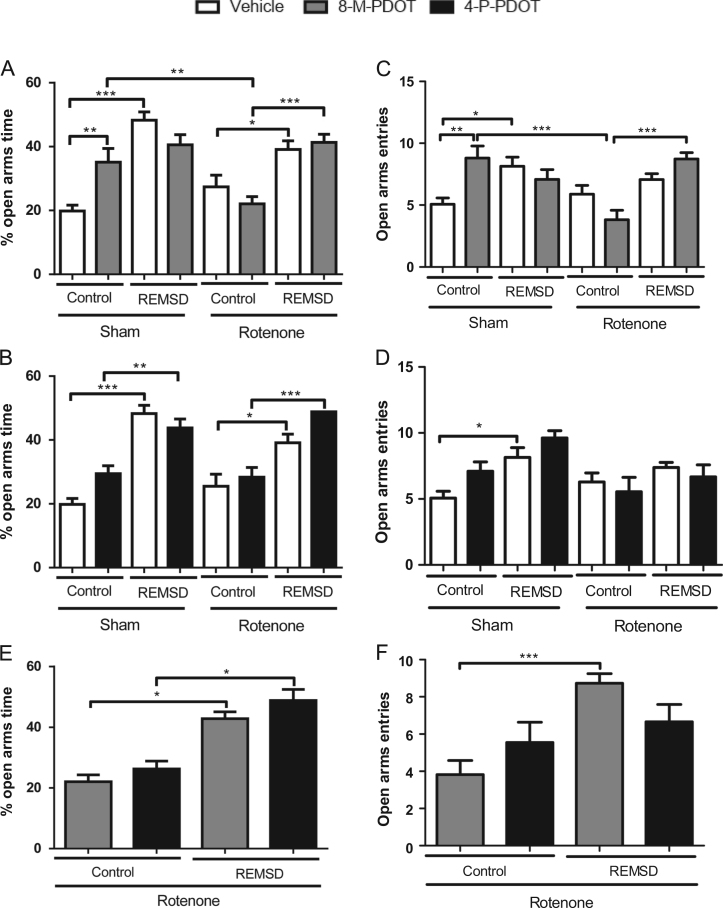
The anxiety-like effects of 8-M-PDOT and 4-P-PDOT treatment, in the EPM are shown in Fig. 2. The sham control 8-M-PDOT increased the percentage of time spent in open arms, compared with the sham control vehicle group (P≤0.01) (Fig. 2A). An increase in this parameter can also be observed after the REMSD in the sham REMSD vehicle group, compared with their respective control (P≤0.001). The rotenone control group remained unchanged. However, REMSD increased the percentage of open arms time as observed in the rotenone REMSD vehicle (P≤0.05) and rotenone REMSD 8-M-PDOT (P≤0.001) groups, compared with their respective controls [F(7,88)=13.26, P<0.001]. The treatment with 4-P-PDOT (Fig. 2B) did not elicit any change in this parameter. However, the only change in percentage of open arms time was as a result of REMSD, observed in the sham REMSD 4-P-PDOT (P≤0.05) and rotenone REMSD 4-P-PDOT (P≤0.001) groups [F(7,91)=14.71, P<0.001]. The treatment with 8-M-PDOT (Fig. 2C) increased the number of open arms entries compared with the respective vehicle group (P≤0.01). The same result is observed in the sham REMSD vehicle group, compared with their respective control group (P≤0.05). Further, the rotenone REMSD 8-M-PDOT (P≤0.001) group presented an increase in this parameter compared with the rotenone control 8-M-PDOT; however the latter group demonstrated a decrease in the number of open arms entries compared to the sham control 8-M-PDOT group (P≤0.05) [F(7,119)=7.050, P<0.0001]. The number of open arms entries was not altered by the 4-P-PDOT treatment (Fig. 2D). Nonetheless, the sham REMSD vehicle group exhibited an increase (P≤0.05) in this parameter compared to the sham control vehicle group [F(7,96)=4.334, P=0.0004]. Fig. 2E depicts the comparisons between treatments of the rotenone groups considering the percentage of time in the open arms. Accordingly, it was not observed significant differences between the 8-M-PDOT and 4-P-PDOT treated groups. In spite of that, only significant increments in the percentage of time in the open arms is observed for the rotenone REMSD 8-M-PDOT and 4-P-PDOT in comparison to their respective rotenone control group [F(3,48)=8.510, P=0.0001]. Likewise, the same type of analysis has been performed for the open arms entries (Fig. 2F), indicating a similar result, with only the increase of this parameter for the rotenone REMSD 8-M-PDOT compared to the rotenone control 8-M-PDOT group [F(3,55)=9.540, P=0.0001].
Fig. 2.
Anxiety-like parameters during the plus maze test. A. Percentage of open arms time after 8-M-PDOT treatment and REMSD, B. Percentage of open arms time after 4-P-PDOT treatment and REMSD C. Open arms entries after 8-M-PDOT treatment and REMSD, D. Open arms entries after 4-P-PDOT treatment and REMSD, E. Percentage of open arms time after 8-M-PDOT and 4-P-PDOT treatment (rotenone groups), F. Open arms entries after 8-M-PDOT and 4-P-PDOT treatment (rotenone groups). The bars represent the mean±standard error of the mean. n=15 per group, *P≤0.05, **P≤0.01, ***P≤0.001. One-way ANOVA followed by Newman Keuls post hoc test.
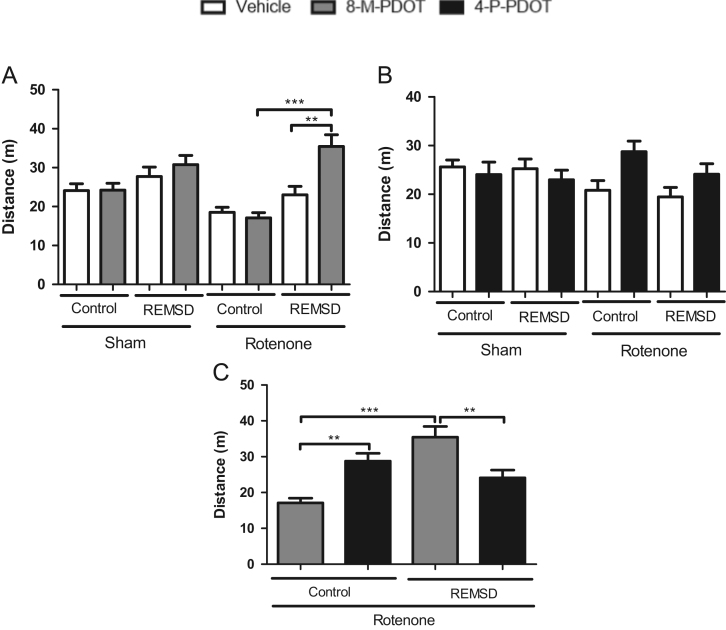
Fig. 3 shows the effects of 8-M-PDOT and 4-P-PDOT treatment in the locomotion as demonstrated by the OFT. The rotenone REMSD 8-M-PDOT group (Fig. 3A) exhibited an increase in the locomotion compared to their respective vehicle (P<0.01) and control (P<0.001) groups [F(7,76)=7.902, P<0.0001]. Furthermore, neither the treatment with 4-P-PDOT nor the REMSD exposure modified the general activity of the animals (Fig. 3B) [F(7,90)=1.607, P=0.1448]. Once more, the comparisons between treatments of the rotenone groups (Fig. 3C), considering this parameter, demonstrated an increased locomotion in the rotenone control 4-P-PDOT group (P<0.01) compared to the rotenone control 8-M-PDOT group. Additionally, the rotenone REMSD 8-M-PDOT group demonstrated a locomotion increment (P<0.01) compared to the rotenone REMSD 4-P-PDOT group [F(3,38)=12.38, P<0.001].
Fig. 3.
Locomotion parameter during the open field test. A. Locomotion after 8-M-PDOT treatment and REMSD, B. Locomotion after 4-P-PDOT treatment and REMSD, C. Locomotion after 8-M-PDOT, 4-P-PDOT treatment in the rotenone groups. The bars represent the mean±standard error of the mean. n=15 per group, **P≤0.01, ***P≤0.001. One-way ANOVA followed by Newman Keuls post hoc test.
4. Discussion
The activation of striatal MT2 receptors by the selective agonist 8-M-PDOT triggered an anxiolytic-like effect, demonstrated, consistently, by the increased percentage of time spent in the open arms and in the number of open arms entries. Conversely, the selective blockade of striatal MT2 receptors, induced by 4-P-PDOT, did not show an anxiogenic-like effect, as assumed. The anxiolytic effects of melatonin in animal models are well described by previous reports [30], [31], [32], and more recently, it has been demonstrated the involvement of MT2 receptors in the melatonin anxiolytic-like behavior [39]. The activation of MT2 receptors, by the partial agonist UCM-765, increased the time and entries in the open arms. This effect was blocked by the selective MT2 receptor antagonist, 4-P-PDOT [39]. Thus, anxiolytic-like effect is purportedly associated to MT2 activation. Notwithstanding, in our study we focused on the role of striatal MT2 receptors hence, we investigated if after a dopaminergic degeneration, induced by intranigral rotenone, the administration of MT2 agonist or antagonist would reduce the anxiogenic-like effect of rotenone. In fact, rotenone did not increase the anxiety-like behavior, however this neurotoxin was able to prevent the anxiolytic-like effect promoted by 8-M-PDOT. Another important consideration relies on the time of MT2 drugs infusion (between 7:00 a.m. and 9:00 a.m.), which is nadir of melatonergic blood levels. Conversely, it is demonstrated that melatonin receptors, in the striatum, did not suffer diurnal variations [49]. Furthermore, it is important to state that our rationale is strictly based on pharmacological activation/blockade of the receptors and not necessarily influenced by the physiological melatonin blood, or brain levels.
Another important finding in this study is that REMSD was able to induce remarkable anxiolytic-like effects similar to 8-M-PDOT. This outcome becomes evident due to the ceiling effect induced by REMSD (Fig. 2E). However, these results cannot be interpreted as a merely consequence of an alteration in locomotion inflicted by REMSD, since REMSD itself did not appear to produce motor changes. Nonetheless, locomotor adjustments occurred only in the presence of a dopaminergic lesion induced by rotenone. In fact, striatal MT2 blockade produced an increased locomotion not replicated in the context of REMSD. Also, MT2 activation appeared to generate a prevalent locomotor increase compared to MT2 blockade. This result could also be interpreted as a possible anxiogenic effect or even a mania-like effect [50], probably caused by REMSD. Indeed, REMSD is known to cause hyperactivity as a result of dopaminergic supersensitivity [51], and is also considered as a model of mania [50]. Many studies describe anxiolytic-like effects as consequence of insufficient sleep [15], [16], [17]. Meanwhile, anxiogenic-like effects are also reported in animal studies [18], [19], [20]. One possible explanation for this discrepancy is due to the variations in the REMSD protocols and different behavioral tests employed to access anxiety [52].
The intranigral infusion of rotenone did not induce an anxiogenic-like effect, suggesting that this neurotoxin, or perhaps our protocol (or both), presented limitations in mimicking anxiety-like alterations such as observed in PD. Rotenone is a classical inhibitor of the mitochondrial complex I of the electron transport chain, hence, inducing apoptosis mechanisms of dopaminergic neurons very similarly to PD [2]. That is, lipid peroxidation, changes in the mitochondrial membrane potential, caspase-3 activation and DNA fragmentation [53]. In fact, 40–80% of striatal dopamine depletion can be observed after intranigral administration of rotenone [46], [54], [55], while serotoninergic neurons is not affected by this neurotoxin [54].
The use of rotenone as a PD model is gaining growing attention particularly in light of non-motor disturbances like REM sleep behavior disorder [56]. Further, this neurotoxin mimics olfactory dysfunction [24] and depression [25], [26] which are pathophysiological conditions with a strong dopaminergic influence. Meanwhile, the findings presented in the literature regarding anxiety behaviors induced by animal models of PD have been inconsistent. While some studies suggest the neurotoxin induction of anxiogenic-like behaviors [6], [57], [58], others reported absence of these effects [13], [59]. Therefore, the present study takes place with this investigation and becomes the first report, to our knowledge, to show the effects of intranigral rotenone in the context of anxiety-like behaviors in rats.
Another important point to be examined is the possible sedative effect that could be induced by the MT2 activation. In this regard we did not detect such effect, according to our protocol, as can be seen by the locomotion profile of the 8-M-PDOT treated animals. Interestingly, there is a strong relationship between anxiety disorders and glutamate receptors [60], [61]. In particular, abnormalities in the glutamatergic transmission underlie anxiety [62]. Preclinical data indicate that antagonists of the N-methyl-D-aspartate (NMDA) and group I mGlu receptors reduce anxiety [61], [63]. However, the involvement of glutamate in the neurobiology of anxiety in PD is not yet clear. Another neuropsychiatric condition relevant for PD is depression. It has been demonstrated that melatonin operates as a mediator of the antidepressant-like effects induced by NMDA receptors [64]. Although, more studies about the glutamatergic role of melatonin are needed and this constitutes a very interesting field for futures studies.
5. Conclusions
Our results demonstrated that the activation of striatal melatonin MT2 receptors elicited anxiolytic-like effects. In opposite, the selective blockade of striatal MT2 receptors, induced by 4-P-PDOT, did not show an anxiogenic-like effect. REMSD was able to induce anxiolytic-like effects similar to 8-M-PDOT. MT2 activation generated a prevalent locomotor increase compared to MT2 blockade in the context of REMSD. Together, these results suggest a striatal MT2 modulation associated to the REMSD-induced dopaminergic supersensitivity causing a possible dopaminergic influence in the MT2 anxiolytic-like effects in the intranigral rotenone model of PD.
Conflict of interests
The authors have declared that no conflict of interests exists.
Acknowledgments
This paper was supported by CAPES and the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq-Brasil Grants Casadinho/Procad # 552226/2011-4 and Universal # 473861/2012-7 to MMSL. MMSL is recipient of CNPq fellowship.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Lang A.E., Lozano A.M. Parkinson׳s disease. Second of two parts. N Engl J Med. 1998;339(16):1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 2.Lima M.M. Motor and non-motor features of Parkinson׳s disease – a review of clinical and experimental studies. CNS Neurol Disord Drug Targets. 2012;11(4):439–449. doi: 10.2174/187152712800792893. [DOI] [PubMed] [Google Scholar]
- 3.Lima M.M. Sleep disturbances in Parkinson׳s disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev. 2013;17(5):367–375. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Maloteaux J.M. [3H]GBR 12935 binding to dopamine uptake sites: subcellular localization and reduction in Parkinson׳s disease and progressive supranuclear palsy. Eur J Pharmacol. 1988;156(3):331–340. doi: 10.1016/0014-2999(88)90278-6. [DOI] [PubMed] [Google Scholar]
- 5.Shiba M. Anxiety disorders and depressive disorders preceding Parkinson׳s disease: a case-control study. Mov Disord. 2000;15(4):669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Eskow Jaunarajs K.L. Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav Pharmacol. 2010;21(7):627–637. doi: 10.1097/FBP.0b013e32833e7e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri K.R., Schapira A.H. Non-motor symptoms of Parkinson׳s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 8.Pontone G.M. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson׳s disease. Mov Disord. 2009;24(9):1333–1338. doi: 10.1002/mds.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J.J., Marsh L. Anxiety in Parkinson׳s disease: identification and management. Ther Adv Neurol Disord. 2014;7(1):52–59. doi: 10.1177/1756285613495723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menza M.A., Robertson-Hoffman D.E., Bonapace A.S. Parkinson׳s disease and anxiety: comorbidity with depression. Biol Psychiatry. 1993;34(7):465–470. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- 11.Kerenyi L. Positron emission tomography of striatal serotonin transporters in Parkinson disease. Arch Neurol. 2003;60(9):1223–1229. doi: 10.1001/archneur.60.9.1223. [DOI] [PubMed] [Google Scholar]
- 12.Remy P. Depression in Parkinson׳s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(Pt 6):1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 13.Prediger R.D. Anxiety in Parkinson׳s disease: a critical review of experimental and clinical studies. Neuropharmacology. 2012;62(1):115–124. doi: 10.1016/j.neuropharm.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Dement W. The effect of dream deprivation. Science. 1960;131(3415):1705–1707. doi: 10.1126/science.131.3415.1705. [DOI] [PubMed] [Google Scholar]
- 15.Alvarenga T.A. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem. 2008;90(4):624–632. doi: 10.1016/j.nlm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Pokk P., Zharkovsky A. Small platform stress attenuates the anxiogenic effect of diazepam withdrawal in the plus-maze test. Behav Brain Res. 1998;97(1–2):153–157. doi: 10.1016/s0166-4328(98)00036-9. [DOI] [PubMed] [Google Scholar]
- 17.Suchecki D., Tiba P.A., Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14(7):549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 18.Polesel D.N. Anxiety-like effects of meta-chlorophenylpiperazine in paradoxically sleep-deprived mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:70–77. doi: 10.1016/j.pnpbp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Silva R.H. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology (Berl) 2004;176(2):115–122. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 20.Vollert C. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224(2):233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Pires G.N., Tufik S., Andersen M.L. Sleep deprivation and anxiety in humans and rodents-translational considerations and hypotheses. Behav Neurosci. 2015 doi: 10.1037/bne0000076. [DOI] [PubMed] [Google Scholar]
- 22.Betarbet R. Intersecting pathways to neurodegeneration in Parkinson׳s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22(2):404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Betarbet R. Chronic systemic pesticide exposure reproduces features of Parkinson׳s disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues L.S. Olfactory impairment in the rotenone model of Parkinson׳s disease is associated with bulbar dopaminergic D2 activity after REM sleep deprivation. Front Cell Neurosci. 2014;8:383. doi: 10.3389/fncel.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassani T.B. Neuroprotective and antidepressant-like effects of melatonin in a rotenone-induced Parkinson׳s disease model in rats. Brain Res. 2014;1593:95–105. doi: 10.1016/j.brainres.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 26.Santiago R.M. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson׳s disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1104–1114. doi: 10.1016/j.pnpbp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Shin K.S. Effects of gypenosides on anxiety disorders in MPTP-lesioned mouse model of Parkinson׳s disease. Brain Res. 2014:57–65. doi: 10.1016/j.brainres.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Gokul K., Muralidhara Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: relevance to Parkinson׳s disease. Neurochem Res. 2014;39(7):1382–1394. doi: 10.1007/s11064-014-1323-1. [DOI] [PubMed] [Google Scholar]
- 29.Santiago R.M. Induction of depressive-like behavior by intranigral 6-OHDA is directly correlated with deficits in striatal dopamine and hippocampal serotonin. Behav Brain Res. 2014;259:70–77. doi: 10.1016/j.bbr.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Golombek D.A., Martini M., Cardinali D.P. Melatonin as an anxiolytic in rats: time dependence and interaction with the central GABAergic system. Eur J Pharmacol. 1993;237(2–3):231–236. doi: 10.1016/0014-2999(93)90273-k. [DOI] [PubMed] [Google Scholar]
- 31.Karakas A. The effects of the intraamygdalar melatonin injections on the anxiety like behavior and the spatial memory performance in male Wistar rats. Behav Brain Res. 2011;222(1):141–150. doi: 10.1016/j.bbr.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Nava F., Carta G. Melatonin reduces anxiety induced by lipopolysaccharide in the rat. Neurosci Lett. 2001;307(1):57–60. doi: 10.1016/s0304-3940(01)01930-9. [DOI] [PubMed] [Google Scholar]
- 33.Carpentieri A. New perspectives in melatonin uses. Pharmacol Res. 2012;65(4):437–444. doi: 10.1016/j.phrs.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3(2):194–225. [PMC free article] [PubMed] [Google Scholar]
- 35.Hardeland R. Melatonin – a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Nosjean O. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275(40):31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 37.Jockers R. Melatonin receptors, heterodimerization, signal transduction and binding sites: what׳s new? Br J Pharmacol. 2008;154(6):1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noseda A.C. Putative role of monoamines in the antidepressant-like mechanism induced by striatal MT2 blockade. Behav Brain Res. 2014:136–145. doi: 10.1016/j.bbr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa-Sanchez R. Anxiolytic effects of the melatonin MT(2) receptor partial agonist UCM765: comparison with melatonin and diazepam. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):318–325. doi: 10.1016/j.pnpbp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Imbesi M. Drug- and region-specific effects of protracted antidepressant and cocaioce treatment on the content of melatonin MT(1) and MT(2) receptor mRNA in the mouse brain. Int J Neuroprot Neuroregener. 2006;2:185–189. [PMC free article] [PubMed] [Google Scholar]
- 41.Niles L.P. Melatonin receptors in brain. Eur J Pharmacol. 1979;55(2):219–220. doi: 10.1016/0014-2999(79)90397-2. [DOI] [PubMed] [Google Scholar]
- 42.Erro R. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson׳s disease patients. Parkinsonism Relat Disord. 2012;18(9):1034–1038. doi: 10.1016/j.parkreldis.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Eskow Jaunarajs K.L., George J.A., Bishop C. L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson׳s disease. Neuroscience. 2012;218:243–256. doi: 10.1016/j.neuroscience.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G., Watson C. 5th ed. vol. 1. Academic Press; Amsterdam: 2005. The rat brain in stereotaxic coordinates. [Google Scholar]
- 45.Dos Santos A.C. REM sleep deprivation generates cognitive and neurochemical disruptions in the intranigral rotenone model of Parkinson׳s disease. J Neurosci Res. 2013;91(11):1508–1516. doi: 10.1002/jnr.23258. [DOI] [PubMed] [Google Scholar]
- 46.Moreira C.G. Behavioral, neurochemical and histological alterations promoted by bilateral intranigral rotenone administration: a new approach for an old neurotoxin. Neurotox Res. 2012;21(3):291–301. doi: 10.1007/s12640-011-9278-3. [DOI] [PubMed] [Google Scholar]
- 47.Saravanan K.S., Sindhu K.M., Mohanakumar K.P. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res. 2007;42(3):247–253. doi: 10.1111/j.1600-079X.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 48.Machado R.B. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004(1–2):45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Laudon M., Nir I., Zisapel N. Melatonin receptors in discrete brain areas of the male rat. Impact of aging on density and on circadian rhythmicity. Neuroendocrinology. 1988;48(6):577–583. doi: 10.1159/000125066. [DOI] [PubMed] [Google Scholar]
- 50.Gessa G.L. Animal models of mania. Adv Biochem Psychopharmacol. 1995;49:43–66. [PubMed] [Google Scholar]
- 51.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology (Berl) 1981;72(3):257–260. doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- 52.Alkadhi K. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11(3):231–249. doi: 10.2174/1570159X11311030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tada-Oikawa S. Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 2003;73(25):3277–3288. doi: 10.1016/j.lfs.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Saravanan K.S., Sindhu K.M., Mohanakumar K.P. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson׳s disease. Brain Res. 2005;1049(2):147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 55.Sindhu K.M., Saravanan K.S., Mohanakumar K.P. Behavioral differences in a rotenone-induced hemiparkinsonian rat model developed following intranigral or median forebrain bundle infusion. Brain Res. 2005;1051(1–2):25–34. doi: 10.1016/j.brainres.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 56.Verhave P.S. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. Sleep. 2011;34(8):1119–1125. doi: 10.5665/SLEEP.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L. Alterations of emotion, cognition and firing activity of the basolateral nucleus of the amygdala after partial bilateral lesions of the nigrostriatal pathway in rats. Brain Res Bull. 2011;85(6):329–338. doi: 10.1016/j.brainresbull.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Sy H.N. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacol Biochem Behav. 2010;95(2):158–165. doi: 10.1016/j.pbb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Vuckovic M.G. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32(2):319–327. doi: 10.1016/j.nbd.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Filippis B. The role of group II metabotropic glutamate receptors in cognition and anxiety: comparative studies in GRM2(−/−), GRM3(−/−) and GRM2/3(−/−) knockout mice. Neuropharmacology. 2015;89:19–32. doi: 10.1016/j.neuropharm.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitsikas N. The metabotropic glutamate receptors: potential drug targets for the treatment of anxiety disorders? Eur J Pharmacol. 2014;723:181–184. doi: 10.1016/j.ejphar.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 62.Bergink V., van Megen H.J., Westenberg H.G. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14(3):175–183. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 63.Palucha A., Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Mantovani M. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine-nitric oxide pathway. Neurosci Lett. 2003;343(1):1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]