Abstract
Objectives/Hypothesis
To assess the comparative effectiveness of botulinum toxin and propranolol in patients with essential vocal tremor (EVT).
Study Design
Individual prospective cohort study.
Methods
Study patients were recruited at the Emory Voice Center from patients seeking treatment for EVT. Exclusion criteria included current β-blocker treatment, spasmodic dysphonia, or other disease that prevented the use of propranolol therapy. A 10-week washout period from prior botulinum toxin treatment occurred before enrollment. Patients were assessed via the Voice-Related Quality-Of-Life (VRQOL) questionnaire, Quality of life in Essential Tremor questionnaire, and blinded perceptual voice assessment. These assessments were made at baseline voice 2 weeks after propranolol therapy and 4 weeks after botulinum toxin injection.
Results
Eighteen patients were enrolled. After 2 to 4 weeks of propranolol therapy (with a maximum dosage of 60 mg to 90 mg per day), patients report an average ΔVRQOL of 9.31. Six patients report significant VRQOL improvement >10, with the rest reporting changes between −7.5 and 7.5. Fifteen patients were followed for at least 4 weeks after botulinum toxin injection, reporting an average improvement in scaled VRQOL of 22.00. Blinded perceptual voice assessment demonstrates an improvement in overall severity of tremor with botulinum toxin.
Conclusions
In some patients with EVT, propranolol led to significant vocal improvement with no major side effects. Although botulinum toxin remains the gold-standard therapy for patients with EVT, propranolol represents a possible alternative or adjuvant therapy for certain patients.
Keywords: Propranolol, botulinum, essential vocal tremor
INTRODUCTION
Essential tremor (ET) is the most common adult-onset movement disorder, and essential voice tremor (EVT) is the phonatory manifestation of essential tremor.1 Essential tremor is characterized by both postural and kinetic tremor of 4 to 12 Hz. It affects approximately 5% of adults over the age of 65, and although it was once called “benign essential tremor,” this term has fallen out of favor due to the morbidity associated with the many manifestations of ET.2 Patients may demonstrate problems with activities of daily living including eating, dressing, and speaking. Approximately 90% of tremor patients experience hand tremor, 30% manifest head tremor, and 20% may have voice tremor.2
Although EVT is typically reported to occur in 10% to 25% of patients with ET, some case series report a prevalence of up to 62%.1 EVT may be a later-onset manifestation of ET, and can lead to increased vocal effort, decreased intelligibility, and misconstrued emotional state.3 A study to characterize the signs and symptoms of EVT found kinetic tremor of the muscles of the larynx, pharynx, palate, and tongue. Women are more often affected than men, and a substantial percentage of affected patients (one-third to one-half) report a familial component.1 EVT symptoms are often caused by horizontal glottic tremor (94%) or vertical tremor (81%), with 75% having both horizontal and vertical tremor and 31% with pharyngeal tremor.3
The limited published data suggest that botulinum toxin leads to functional voice improvement,1 and it is a commonly used clinical treatment. Horizontal glottic tremor can be reduced in amplitude through injecting the thyroarytenoid/lateral cricoarytenoid (TA/LCA) muscles with botulinum toxin, leading to chemical denervation. Results peak around week 4, and in a study of 13 subjects, all noted an effect.4 Chemical denervation with botulinum toxin is only modestly effective in reducing limb tremor in ET and complicated by side effects of weakness. However, botulinum toxin seems to be more effective in treating EVT than other manifestations of ET. Although prior studies have not enlisted validated assessments of voice quality to measure the effect of botulinum toxin injections on EVT, they have shown subjective improvement in acoustic measures of tremor and ratings of videotaped speech after therapy with botulinum toxin.4–6 Botulinum injections constitute an invasive procedure that requires repeated treatments and office visits, with side effects that include transient breathiness and dysphagia.3
The United States Food and Drug Administration (FDA) has approved propranolol, an antihypertensive agent, to treat essential tremor. Anecdotal experience suggests that it is most useful for hand tremors, and less useful for axial tremors, such as those of the head and neck. A small case series found that three out of four patients with suspected parkinsonism/vocal tremor responded to treatment with propranolol.7 Propranolol has never been prospectively and objectively evaluated for EVT, and therefore is only infrequently offered. If effective, propranolol would provide an affordable and noninvasive alternative to botulinum toxin injections for patients with EVT. We propose a prospective study to further elucidate the effect of propranolol (oral medication) on EVT patients as compared to botulinum toxin injections for the same patients. This will help establish the best therapy for patients who suffer from EVT.
MATERIALS AND METHODS
We conducted a prospective study on 18 patients with EVT to determine the effect of propranolol on vocal tremor in the treatment-seeking population and to determine the effect of botulinum toxin injections on vocal tremor in the treatment-seeking population.
Subjects with EVT were identified from patients at the Emory Voice Center who have already received or were planning to receive botulinum toxin injections for the treatment of EVT. Patients were ineligible to participate if they were already receiving a β-blocker or had a contraindication to β-blocker therapy, including hypotension, bradycardia, or uncontrolled asthma or diabetes. Patients underwent three clinical and voice evaluations: one at baseline voice, one after a 2-week trial of propranolol, and one after botulinum toxin therapy. Orthostatic blood pressure and heart rate were taken for each patient at every visit.
Each evaluation consisted of patient-reported measures as well as a recorded, objective voice assessment. Voice assessment measures included patient-reported outcomes, including the Voice-Related Quality of Life (VRQOL) questionnaire8 and the Quality of Life in Essential Tremor (QUEST) questionnaire.9 The VRQOL questionnaire consists of 10 questions for patients to score from 1 to 5, consisting of statements such as “I have trouble speaking loudly or being heard in noisy situations” or “I run out of air and need to take frequent breaths.” The QUEST questionnaire, a 30-item, ET-specific quality of life scale includes such statements as “My tremor interferes with ability to communicate with others,” “My tremor interferes with my ability to write (for example, writing letters, completing forms),” “My tremor interferes with my relationships with others (for example, my family, friends, coworkers),” and “I use alcohol more frequently than I would like to because of my tremor.”
Voice recordings were made in a quiet room with the microphone held at 30 cm from the mouth at a 90° angle.10 Participants were asked to sustain the vowel /i/ for 3 to 5 seconds, read the rainbow passage, and produce 1 minute of spontaneous speech. Recordings were preserved for later analysis using a digital audio recorder and saved in WAV format.
After the first evaluation of baseline voice, the patient received a prescription for 2 weeks of propranolol therapy. This prescription consisted of a starting dose of generic immediate-release at 10 mg three times per day (TID) (30 mg each day), with an increase in dose in 5 days to 60 mg each day, and then to 90 mg each day if the patient had demonstrated no side effects. At the second evaluation, propranolol therapy ceased and the patient underwent an injection of botulinum toxin into the TA/LCA complex muscles. The third evaluation took place 4 to 6 weeks after botulinum toxin therapy.
Three trained listeners with greater than 10 years of experience were used to compare voice recordings. Listeners underwent training with anchor voices to improve reliability. For each patient visit, 3 seconds of the sustained /i/ and /a/ vowels and the second sentence of the rainbow passage were presented to the listeners. The three recording conditions were randomized for presentation. Each recording was played twice to improve listener reliability. The listening environment was free from noise. The Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V),11 a validated clinically useful perceptual voice assessment tool was used for the comparison. Per the standard CAPE-V protocol, listeners are asked to make a tick mark on a 1- to 100-mm visual analog scale to indicate overall severity of tremor.
Eighteen patients had evaluations for both visit 1 (base-line) and visit 2 (after taking propranolol for at least 2 weeks). Fifteen patients had evaluations for visit 3 (post–botulinum toxin injection therapy). One patient, new to the Voice Center, decided that she no longer wanted to receive her initial botulinum toxin injection therapy and chose to withdraw from the study. Two patients were lost to follow-up after receiving botulinum toxin injections.
Statistical analysis was conducted using Microsoft Excel 2003 (Microsoft Corp., Redmond, WA) and Stata 12 (StataCorp, College Station, TX).
Means, medians, and standard deviations of the quality-of-life instruments and questionnaires were tabulated. All P values were two sided and were considered statistically significant at the .05 level. To account for clustering of observers on the CAPE-V instrument, the difference in means over time was adjusted by using repeated measures analysis of variance (ANOVA). Our repeated measures ANOVA assumed a compound symmetric correlation structure. For simplicity, we averaged the CAPE-V scores for all raters for each time period.
RESULTS
Average patient age was 67.9 years. Thirteen (72%) of our patients were white. The majority tolerated a 30-mg TID dosage of propranolol, whereas four preferred a 20-mg TID dosing regimen due to dizziness or gastrointestinal distress. Sixteen (89%) of our patients received a bilateral injection to the TA/LCA complex muscles, whereas two received a unilateral injection per request (and previous experience with laryngeal botulinum toxin). The average dose was a total of 3.18 units. For botulinum-naïve patients, we used a starting dose of 1.25 U to the bilateral TA-LCA muscle complex (Table I).
TABLE I.
Patient Characteristics.
| No. (%) | |
|---|---|
| Age, mean | 67.9 |
| Race | |
| White | 13 (72.2) |
| Black | 4 (22.2) |
| Hispanic | 1 (5.6) |
| Propranolol dose | |
| 20 mg TID | 4 (22.2) |
| 30 mg TID | 14 (77.8) |
| Mean botulinum toxin dose | 3.18 |
| Botulinum toxin location | |
| Bilateral TA/LCA | 16 (88.8) |
| Unilateral TA/LCA | 2 (11.2) |
TA/LCA = thyroarytenoid/lateral cricoarytenoid; TID = three times per day.
Fifty-one unique voice evaluations were performed, consisting of 18 first visits (of baseline voice), 18 second visits (of voice after propranolol therapy), and 15 third visits (of voice after botulinum toxin injection therapy). This produced 51 VRQOL questionnaires (a subjective patient-reported measure), 51 QUEST questionnaires (a subjective patient-reported measure), and 51 audio recordings to be edited for our standardized voice listening session. The questionnaire data were entered into Excel for statistical analysis, whereas portions of the rainbow passage and sustained vowel were edited to provide approximately 12-second audio clips for the standardized voice listening session.
Orthostatic blood pressure was taken for every patient at every visit and reported via to the board of our Data Safety Monitoring Plan. No patient was required to stop the trial due to a change in blood pressure or bradycardia. No patient suffered an exacerbation of diabetes or asthma while taking propranolol.
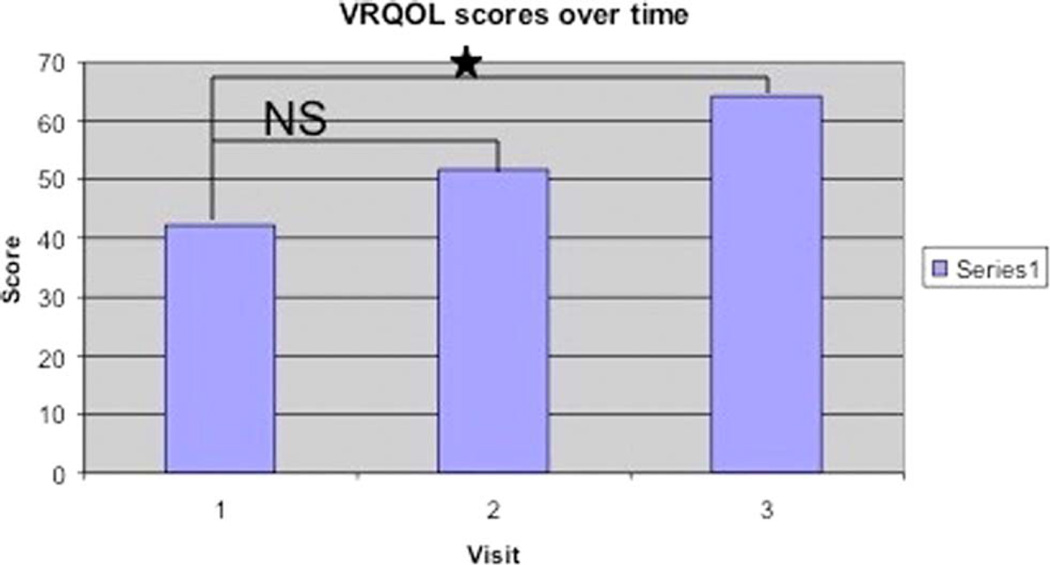
The scaled VRQOL score increased from 42.22 at baseline to 51.53 on propranolol to 64.16 on botulinum toxin (Table II), representing a significant improvement for botulinum toxin as compared to baseline voice (P<.01) (Table III, Fig. 1).
TABLE II.
Descriptive Statistics of the Scoring Instruments.
| Mean | Standard Deviation | Median | |
|---|---|---|---|
| QUEST total score | |||
| Visit 1 | 39.41 | 27.89 | 29.51 |
| Visit 2 | 29.50 | 23.73 | 20.70 |
| Visit 3 | 23.60 | 24.49 | 15.56 |
| VRQOL total score | |||
| Visit 1 | 42.22 | 23.64 | 46.25 |
| Visit 2 | 51.53 | 26.21 | 48.75 |
| Visit 3 | 64.16 | 22.23 | 65 |
| CAPE-V | |||
| Visit 1 | 43.61 | 23.26 | 46 |
| Visit 2 | 45.89 | 24.57 | 44 |
| Visit 3 | 35.67 | 22.14 | 34 |
QUEST decreased (improved) with propranolol and botulinum toxin as compared to baseline. Scaled VRQOL increased (improved) with propranolol and botulinum injection toxins as compared to baseline. Our portion of the CAPE-V decreased (improved) with botulinum toxin as compared to baseline.
CAPE-V = Consensus Auditory-Perceptual Evaluation of Voice; QUEST = Quality of Life in Essential Tremor questionnaire; VRQOL = Voice-Related Quality-of-Life questionnaire.
TABLE III.
Regression Results of Subjective Questionnaires Against Time.
| β (95% Confidence Interval) | P Value | |
|---|---|---|
| QUEST total score | ||
| Visit 1 | Reference | |
| Visit 2 | −9.91 (−27.00 to 7.17) | .25 |
| Visit 3 | −15.81 (−33.74 to 2.10) | .08 |
| VRQOL total score | ||
| Visit 1 | Reference | |
| Visit 2 | 9.31 (−6.91 to 25.12) | .25 |
| Visit 3 | 21.94 (4.94 to 38.95) | .01* |
Visit 3 (botulinum toxin injections) represented a statistically significant improvement in VRQOL as compared to baseline voice.
Statistically significant at the P = .05 level.
QUEST = Quality of Life in Essential Tremor questionnaire; VRQOL = Voice-Related Quality-of-Life questionnaire.
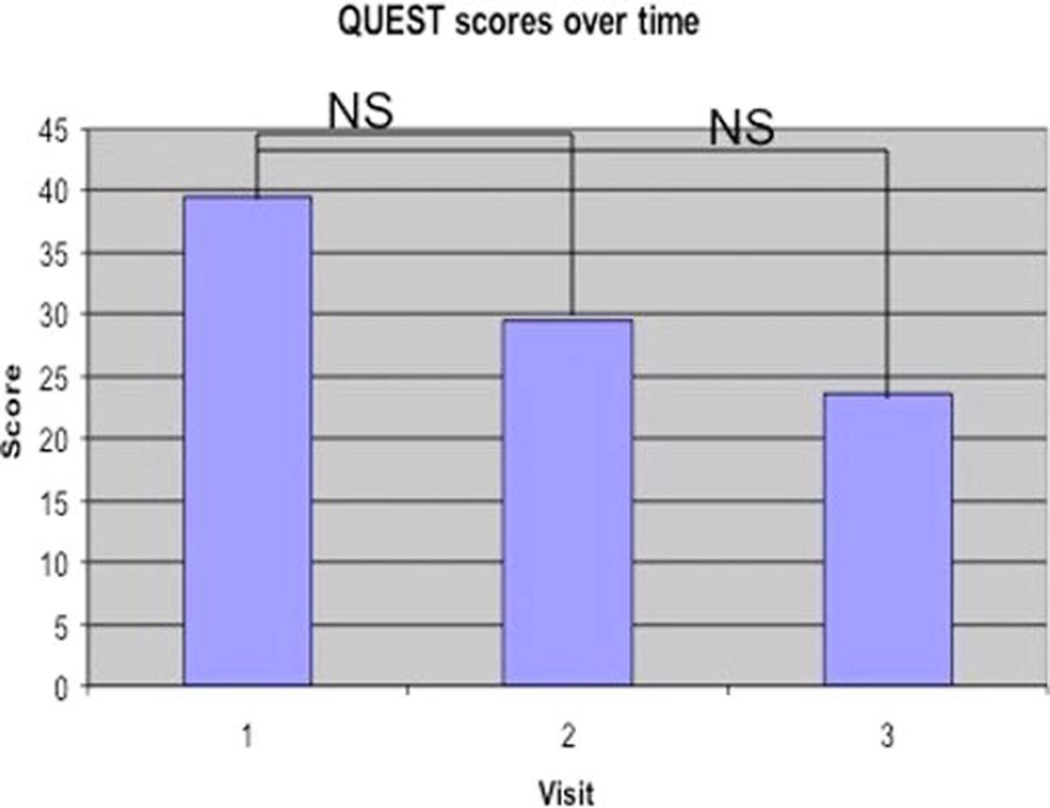
Fig. 1.
QUESTscores. NS = not significant; QUEST = Quality of Life in Essential Tremor questionnaire. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Comparing the scaled VRQOL for the difference between voice on propranolol and voice at baseline showed an average scaled VRQOL improvement on propranolol of 9.31. Six patients reported significant VRQOL improvement of 10 points or greater, with the rest reporting changes between −7.5 and 7.5. Of the 10 patients who reported any improvement in VRQOL for propranolol, four had an overall tremor severity that decreased in the standardized voice listening session.
Comparing the scaled VRQOL for the difference between voice on botulinum toxin and voice at baseline showed an average scaled VRQOL improvement on botulinum toxin of 22.00 for 15 patients. Nine patients reported VRQOL scaled improvement of 10 or greater, with the rest reporting changes between −10 and 2.5. Seven of the 11 patients who reported any improvement in the VRQOL had an overall tremor severity that decreased in the standardized voice listening session.
The mean QUEST score decreased from 39.41 at baseline to 29.50 on propranolol to 23.60 on botulinum toxin, representing improvement over the study interval. Although this demonstrates a trend, QUEST score improvement demonstrated a P value of .08 (Table III, Fig. 2).
Fig. 2.
VRQOL scores. NS = not significant; VRQOL = Voice-Related Quality-of-Life questionnaire. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
The standardized listening session provided an objective and systematic review of the portion of the CAPE-V passage. Each of the three expert listeners/speech-language pathologists marked their rating of the severity of overall tremor. These three measurements were averaged together for each patient. Baseline voice demonstrated an overall severity to tremor of 43.61, whereas voice on propranolol demonstrated an overall severity of tremor of 45.89. After botulinum toxin injections, expert voice listeners rated the overall severity due to tremor at 35.67.
Linear regression demonstrated improved quality-of-life scores on both instruments (VRQOL and QUEST), but the change from baseline voice to voice after the injection of botulinum toxin was only statistically significant for the VRQOL instrument (B = −21.94, 95% confidence interval = 4.94 to 38.95).
Results from the ANOVA analysis of the CAPE-V showed no difference in score over time; however, the rater was statistically significantly associated with perception of tremor.
DISCUSSION
EVT is the phonatory manifestation of ET, which demonstrates multiple sites of involvement within the larynx. Tremor of the voice can greatly affect overall quality of life, ranging from communication to activities of daily living to psychosocial components.
EVT affects women more often than men, and all 18 of the patients consented for this study were women. None were of child-bearing age. Although it is known that EVT symptoms are caused by horizontal glottic tremor or vertical tremor (or both) or pharyngeal tremor, there is no simple way to distinguish the pathophysiology for each particular patient.
The limited published data suggest that botulinum toxin has been shown to lead to functional voice improvement, and it is a commonly used clinical treatment in our Voice Center. Voice tremor can be reduced in amplitude through injecting the TA/LCA complex muscles with botulinum toxin, leading to chemical denervation. In the small body of literature, botulinum toxin has emerged as the standard of care, as it seems to be more effective in treating EVT than medical therapies.
Vocal tremor involves the glottis, and when severe may manifest in phonatory offsets and breaks and effortful phonation. Botulinum toxin represents a focused treatment that directly targets involved muscles of the TA/LCA complex muscles and alleviates these breaks. If tremor exists in other locations, botulinum toxin does not treat these other symptoms as it is not a systemic therapy. Botulinum toxin is usually well-tolerated, but represents a procedure that can lead to dysphagia and weakness.
The FDA approved propranolol, an antihypertensive agent, to treat ET as a whole. We aimed to study the effect of propranolol on EVT, which has never been objectively prospectively studied. Oral medications, such as propranolol, are generally not considered to be particularly effective for voice tremor.
Some patients perceived or reported an improvement in EVT while on propranolol. This did not closely align with the speech-language pathologists’ interpretation of better voice or decreased tremor severity as measured on the CAPE-V. Because some patients improved and desired to continue the propranolol therapy, and the risks associated with propranolol are small, it is reasonable to attempt a trial or propranolol in all patients with EVT. Some of our patients preferred propranolol because they found it to be a noninvasive alternative. Others preferred that they no longer had to commute to the Voice Center in Atlanta. Propranolol may serve an additional therapeutic benefit for patients with concomitant conditions such as hypertension, stable angina, and migraine headache. The side effects of orthostatic hypotension and bradycardia must be monitored, especially after initially beginning propranolol therapy. None of our patients reported difficulty in controlling their type II diabetes or asthma while taking propranolol. Some did not like the TID dosing regimen, but propranolol is available in a sustained-release formulation. Overall, the benign side-effect profile and extensive knowledge base on propranolol therapy make it an appropriate initial medical therapy for patients who seek treatment for EVT.
This study of comparative effectiveness lacks a placebo or control group, with patients representing their own internal controls. In this study of treatment-seeking patients with EVT, they identified botulinum toxin injections as a preferable treatment for tremor symptoms overall. However, for patients who experience improvement in voice while on propranolol, dual therapy with botulinum toxin makes logical sense and would provide an excellent area of further study.
There exists a number of possibilities why an improvement in voice tremor was not perceived as significant on audio-perceptual ratings by our panel of expert voice listeners. First, voice quality of tremor on propranolol may not have actually improved. Second, limitations in audio-perceptual ratings may mean that our current methods are not sensitive enough to detect changes in voice that may actually be present between voice on propranolol and voice at baseline. Interestingly, even the standard of care, botulinum toxin therapy, was not perceived as statistically significant to the panel of experts.
Furthermore, the lack of a significance on the CAPE-V analysis could be due to a number of other statistical factors. The agreement between the raters on the level of tremor based on exploratory analysis was poor. This would reduce our ability to detect any difference if one is in fact present. Although we attempted to use control samples to detect differences between our raters, the kappa statistic for agreement among raters on the control sample was 0.25, which falls just barely into the fair category. Furthermore, there was a wide amount of variation in scoring as noted by our variance being equal to our mean. Given the significant improvement in VRQOL scores seen in this study raises the question about whether an objective assessment is important compared to improvement in tremor based on patient report. It would also be of interest to explore whether the patient’s family members and coworkers find the patients voice to be improved.
Further analysis could also be directed at the pattern of CAPE-V scoring by the raters. There may be systematic decreases or in increases in the amount of tremor detected as the raters become more astute or become fatigued.
In summary, these data are consistent with some of the literature that has been published previously that propranolol is less effective for voice tremor than for axial tremors. The reason why some patients improve and others do not improve while on propranolol is unclear, and represents an area of potential future research. There may exist a subset of patients who improve on propranolol more than others. Several of our 18 patients have decided to continue on propranolol therapy at the cessation of their study involvement, perceiving enough of an increase in voice quality that they desired to continue the medical therapy.
CONCLUSION
Before this study, propranolol had never been prospectively and objectively evaluated for essential voice tremor, and therefore is only infrequently offered. For some patients, propranolol provides an affordable, convenient, and noninvasive alternative to botulinum toxin injections. A subset of our patients perceived improvement in voice quality, and we therefore recommend a trial of propranolol for medical therapy for patients with essential voice tremor.
Acknowledgments
This study was internally funded by the Department of Otolaryngology–Head & Neck Surgery, Emory University School of Medicine, Atlanta, Georgia, U.S.A.
Dr. Jinnah is a Site PI for a study grant for Merz, an Organizing PI for an education grant for Merz, Ipsen, and Allergen, and a consultant for Medtronics.
Footnotes
This study was completed at the Emory Voice Center under the direction of Dr. Johns.
Presented orally at the 136th Annual Meeting of the American Laryngological Association at the Combined Otolaryngological Spring Meetings (COSM), Boston, Massachusetts, U.S.A., April 22–23, 2015.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Sulica L, Louis ED. Clinical characteristics of essential voice tremor: a study of 34 cases. Laryngoscope. 2010;120:516–528. doi: 10.1002/lary.20702. [DOI] [PubMed] [Google Scholar]
- 2.Zesiewicz TA, Shaw JD, Allison KG, Staffetti JS, Okun MS, Sullivan KL. Update on treatment of essential tremor. Curr Treat Options Neurol. 2013;15:410–423. doi: 10.1007/s11940-013-0239-4. [DOI] [PubMed] [Google Scholar]
- 3.Gurey LE, Sinclair CF, Blitzer A. A new paradigm for the management of essential vocal tremor with botulinum toxin. Laryngoscope. 2013;123:2497–2501. doi: 10.1002/lary.24073. [DOI] [PubMed] [Google Scholar]
- 4.Adler CH, Bansberg SF, Hentz JG, et al. Botulinum toxin type A for treating voice tremor. Arch Neurol. 2004;61:1416–1420. doi: 10.1001/archneur.61.9.1416. [DOI] [PubMed] [Google Scholar]
- 5.Warrick P, Dromey C, Irish J, Durkin L. The treatment of essential voice tremor with botulinum toxin A: a longitudinal case report. J Voice. 2000;14:410–421. doi: 10.1016/s0892-1997(00)80086-7. [DOI] [PubMed] [Google Scholar]
- 6.Koller W, Graner D, Mlcoch A. Essential voice tremor: treatment with propranolol. Neurology. 1985;35:106–108. doi: 10.1212/wnl.35.1.106. [DOI] [PubMed] [Google Scholar]
- 7.Massey EW, Paulson GW. Essential vocal tremor: clinical characteristics and response to therapy. South Med J. 1985;78:316–317. [PubMed] [Google Scholar]
- 8.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13:557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 9.Tröster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in Essential Tremor Questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord. 2005;11:367–373. doi: 10.1016/j.parkreldis.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Titze IR, Winholtz WS. Effect of microphone type and placement on voice perturbation measurements. J Speech Hear Res. 1993;36:1177–1190. doi: 10.1044/jshr.3606.1177. [DOI] [PubMed] [Google Scholar]
- 11.Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Pathol. 2009;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]