Significance
The phototactic behavior of the unicellular green alga Chlamydomonas reinhardtii is thought to rely on photoreception by the eyespot apparatus. Here, we isolated an eyespot-less mutant that clearly exhibits phototaxis. Intriguingly, the phototactic sign (the direction of cell migration) in this mutant is opposite to that of the wild type after treatment with reagents that enhance the sign, a property that we also detected in previously reported eyespot-less mutants. The reversed phototactic-sign phenotype was attributed to the fact that the photoreceptors were exposed to condensed light from their rear side. This report demonstrates the importance of the eyespot, in which carotenoid layers shield the photoreceptors from light condensed by the cell body, which functions as a convex lens.
Keywords: Chlamydomonas, eyespot, lens, phototaxis, carotenoids
Abstract
The biflagellate green alga Chlamydomonas reinhardtii exhibits both positive and negative phototaxis to inhabit areas with proper light conditions. It has been shown that treatment of cells with reactive oxygen species (ROS) reagents biases the phototactic sign to positive, whereas that with ROS scavengers biases it to negative. Taking advantage of this property, we isolated a mutant, lts1-211, which displays a reduction-oxidation (redox) dependent phototactic sign opposite to that of the wild type. This mutant has a single amino acid substitution in phytoene synthase, an enzyme that functions in the carotenoid-biosynthesis pathway. The eyespot contains large amounts of carotenoids and is crucial for phototaxis. Most lts1-211 cells have no detectable eyespot and reduced carotenoid levels. Interestingly, the reversed phototactic-sign phenotype of lts1-211 is shared by other eyespot-less mutants. In addition, we directly showed that the cell body acts as a convex lens. The lens effect of the cell body condenses the light coming from the rear onto the photoreceptor in the absence of carotenoid layers, which can account for the reversed-phototactic-sign phenotype of the mutants. These results suggest that light-shielding property of the eyespot is essential for determination of phototactic sign.
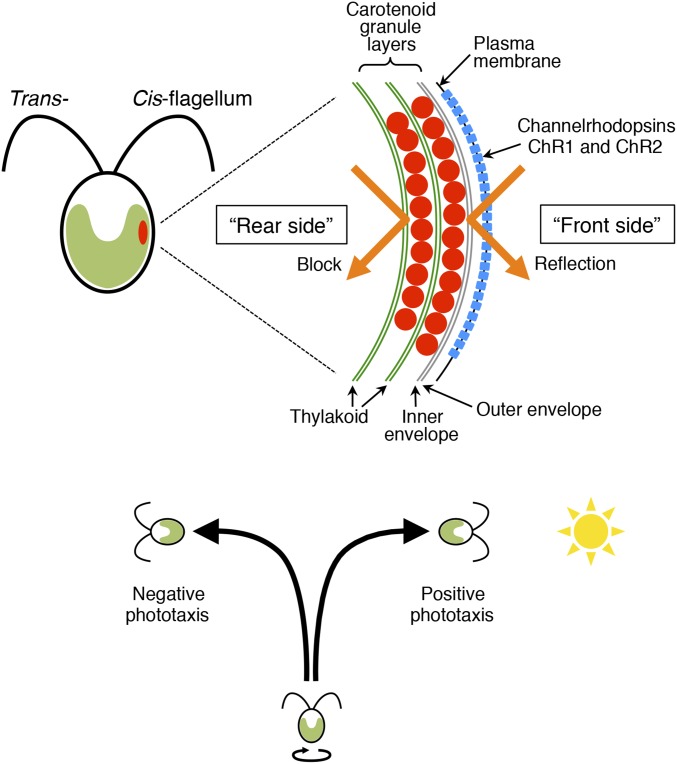
The biflagellate unicellular green alga Chlamydomonas reinhardtii exhibits both positive and negative phototaxis (i.e., swimming toward and away from the light source) to inhabit areas with the proper light conditions for photosynthesis. The phototactic response is triggered by photoreception by an elaborate subcellular organelle, the eyespot (Fig. 1). This organelle is observed as an orange spot located near the cell equator. It contains the carotenoid-rich granule layers in the chloroplast and the channelrhodopsin photoreceptor proteins ChR1 and ChR2 in the plasma membrane (1–4). The carotenoid layers of the eyespot function as a light reflector (5).
Fig. 1.
Schematic diagrams of a Chlamydomonas cell and its phototactic behavior. (Top) The eyespot is located near the cell equator and contains the carotenoid granule layers (red) and photoreceptor proteins, channelrhodopsins (ChR1 and ChR2; blue). The carotenoid layers reflect a light beam (orange arrows) and amplify the light signal from the outside of the cell on ChR (the “front side”) and block the light from the inside of the cell (the “rear side”). The flagellum closest to the eyespot is called the cis-flagellum, whereas the other one is called the trans-flagellum. Modified from refs. 24 and 41. (Bottom) As the cell swims with self-rotation, the eyespot apparatus scans the incident light around the cell’s swimming path. After photoreception by the channelrhodopsins, the cell changes the beating balance of the two flagella and exhibits either positive or negative phototaxis (swimming toward or away from the light source, respectively).
Recent studies suggested that the Chlamydomonas phototactic pathway primarily consists of four steps: (i) photoreception by ChRs; (ii) excitation of the cellular membrane; (iii) increase in intraflagellar [Ca2+]; and (iv) a change in the beating balance between the two flagella, i.e., the cis-flagellum (the one closest to the eyespot) and the trans-flagellum (the one farthest from the eyespot) (Fig. 1) (6–9). During step 1, the eyespot plays a crucial role in directional photoreception. ChRs localize to the plasma membrane over the carotenoid layers, which reflect and amplify the light signal coming from the outside of the cell (the “front side” of ChRs) while blocking the light from the inside of the cell (the “rear side” of ChRs) (Fig. 1). Rotation of the Chlamydomonas cell around its long axis during swimming, and light reflection and blocking at the carotenoid layers, produce a periodic modulation of the light intensity received by ChRs. This light signal modulation decreases in amplitude as the cell’s swimming path becomes closer to parallel to the light beam. According to the prevailing theory, phototaxis results from the cell’s response minimizing the amplitude of light signal modulation (5, 10).
There are several conflicting studies debating the importance of the reflective and absorptive properties of the eyespot in determining the direction (or “sign”) of phototaxis by the cell (11). These properties are important because positive phototaxis requires that the trans-flagellum beat more strongly than the cis-flagellum when the eyespot is facing the light source, and vice versa. Based on the hypothesis put forward originally by Foster and Smyth (5), the asymmetric layered structure of the eyespot was thought to act as a quarter wave-plate, reflecting light from the front back onto the photoreceptors in the plasma membrane and blocking light from the back coming through the cell. Data collected by Schaller and Uhl (12), with cells held on micropipettes, was used to argue that reflection does little to enhance photoreception from the front and that the pigment granule layers do not shield the photoreceptors from the backside. These authors suggested that the chlorophyll pigments in the cell were responsible for absorbing “backside” light. In another study with cells lacking both chlorophyll and pigment granule layers, active photoreceptors were reconstituted in the plasma membrane with exogenously added retinal. Interestingly, the sign of phototaxis of these rescued, clear cells was reversed relative to that of wild-type green cells (13). It was hypothesized that a “lens effect” or “focusing effect” of the transparent cell body was condensing light on the backside of the photoreceptors on the other side of the cell. However, it had been questioned earlier whether the refractive index of the cell was much different from the surrounding water, which would be required for the cell to act as a convex lens (5). Here, we sought to show whether green Chlamydomonas cells can act as lenses, because we found that several strains with missing eyespot granule layers, including newly isolated lts1-211, demonstrated a reversal in the sign of phototaxis.
Results
Isolation of a Chlamydomonas Mutant with a Reversed Sign of Phototaxis.
Several years ago, we showed that cellular reduction-oxidation (redox) poise acts as a strong signal that determines the phototactic sign: Cells show positive phototaxis after treatment with reactive oxygen species (ROS), whereas they show negative phototaxis after treatment with ROS-scavenging reagents (14). Although the molecular basis of this redox-based sign switching of phototaxis remains to be clarified, the effects of ROS/ROS scavengers (hereafter referred to as “redox reagents”) on the phototactic sign are intense. With the goal of isolating mutants defective in the signal transduction pathway affected by ROS, we screened for mutants defective in sign switching and isolated a mutant (lts1-211) exhibiting an opposite phototactic sign change (compared with the wild type) after treatment with redox reagents, i.e., positive phototaxis after treatment with ROS scavengers and negative phototaxis after treatment with ROS.
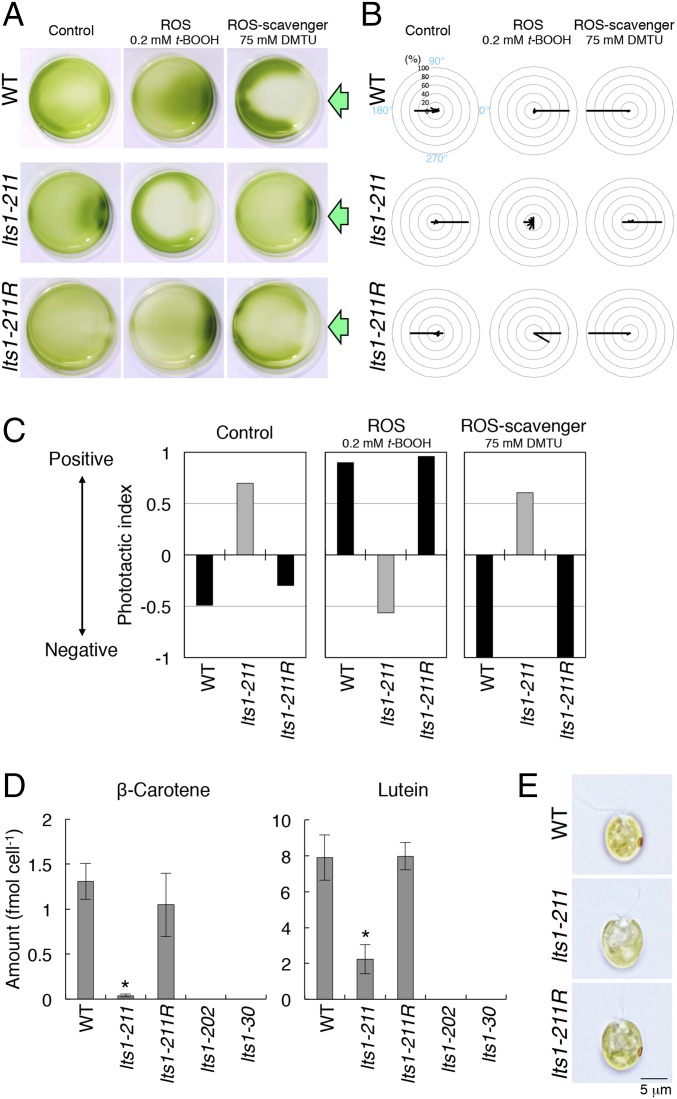
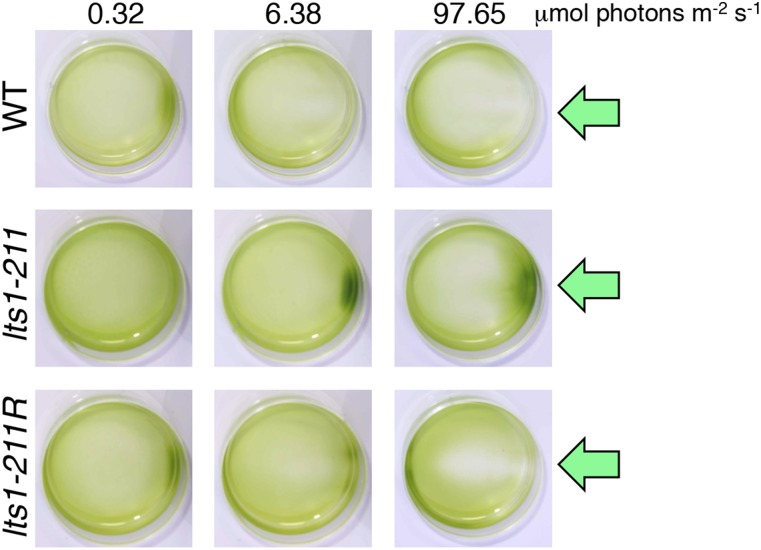
We generated a panel of mutants by UV irradiation and screened for cells showing an opposite sign of phototaxis to that of the wild type. After treatment with oxidizing reagents, wild-type cells exhibited strong positive phototaxis (Fig. 2 A–C) (14). We chose isolates showing negative phototaxis after treatment with 0.2 mM tertiary-butylhydroperoxide (t-BOOH), a ROS reagent. One clone always exhibited a reversed-phototactic sign after treatment with redox reagents: positive after treatment with ROS scavengers and negative after treatment with ROS (Fig. 2 A–C).
Fig. 2.
The lts1-211 mutant lacks eyespots and exhibits the opposite sign of phototaxis relative to the wild type. (A) Dish phototaxis assays of the wild type, lts1-211, and lts1-211R (rescued strain) with or without treatment with redox reagents. Cell suspensions in Petri dishes were photographed after illumination with a green light-emitting diode (LED) from the side for 5 min (green arrows). The areas without cells on the horizontal axis (e.g., ROS scavenger-treated lts1-211R) are likely caused by the photophobic responses of some cells. (B) Polar histograms representing the percentage of cells moving in a particular direction relative to light illumination from the right (12 bins of 30°; n = 20–30 cells per condition) for 1.5 s following 15-s illumination. (C) The sign of phototactic index in lts1-211 (gray) is opposite to that of WT or lts-211R (black) with or without treatment with redox reagents. The phototactic index was calculated as an average value of cosθ in B. When cells are not illuminated and swim in random directions, the phototactic index should be ∼0. When 100% of cells show clear positive or negative phototaxis, the phototactic index is 1 or −1, respectively. (D) lts1-211 produces less carotenoids than the wild type. β-Carotene and lutein levels in each strain (PSY null mutants lts1-202 and lts1-30 cells were grown in the dark) are shown [average values ± SEM for six (WT, lts1-211 and lts1-211R) or three (PSY null mutants) independently prepared samples]. Asterisks represent significant differences (P < 0.05, paired t test). (E) Bright-field images of the wild-type, lts1-211, and lts1-211R cells. Note that lts1-211 is eyespot-less.
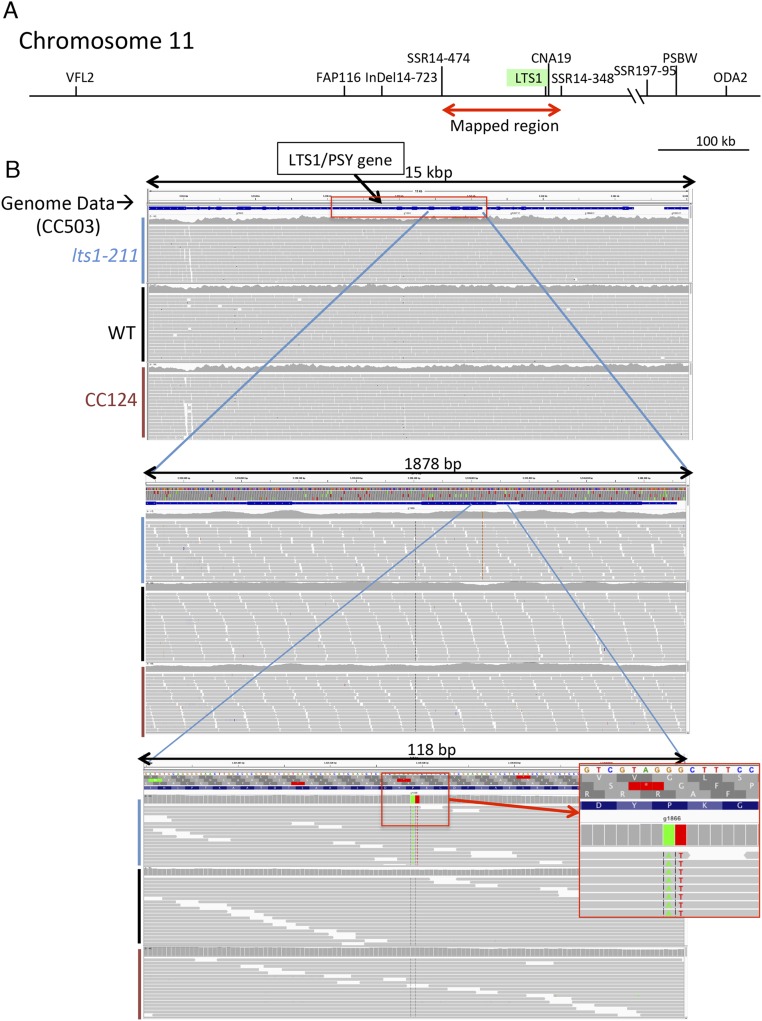
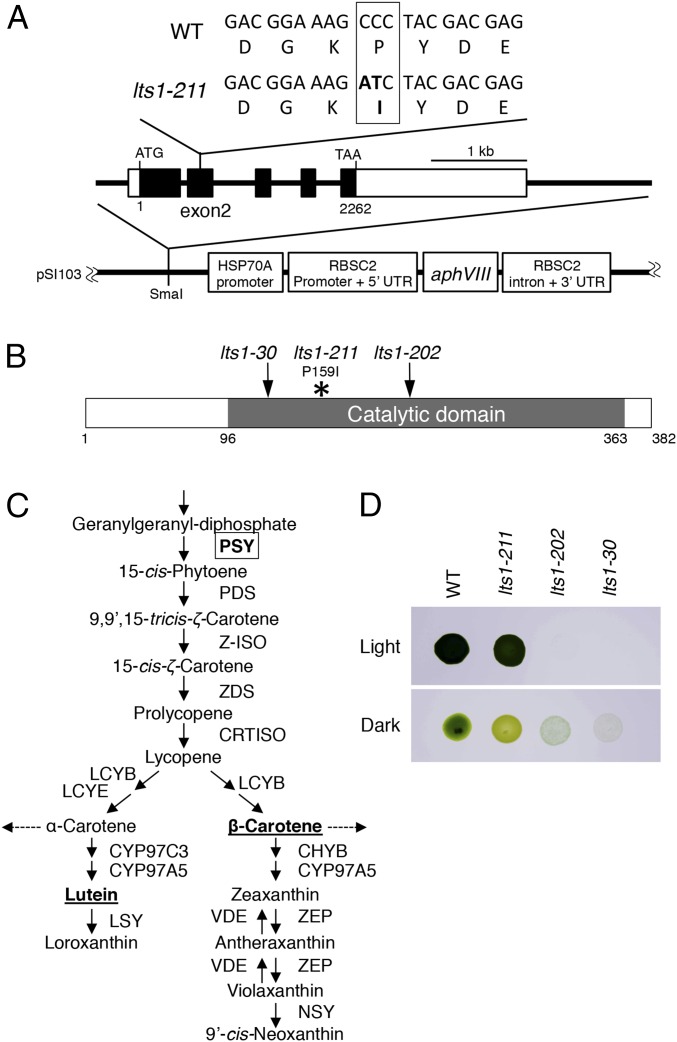
The mutation in this strain was mapped (by a PCR-based method) to a region (∼131 kb) on chromosome 11 (Fig. S1A, see SI Materials and Methods for details) (15). We also performed whole genome sequencing of this mutant as well as wild-type strains (CC124 and WT, a progeny from the cross CC124 × CC125; Table 1). Comparisons with the Chlamydomonas genome sequence database (based on the CC503/cw92 strain) (https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Creinhardtii), as well as pairwise comparisons with wild-type strains to remove CC125-specific SNPs from candidate mutations, revealed a two-base substitution in the phytoene synthase (PSY) gene that produces a single amino acid substitution (P159I) in the catalytic domain of PSY (Fig. 3 A and B and Fig. S1B).
Fig. S1.
Identification of the mutant gene in lts1-211. (A) lts1-211 was mapped to a 131-kb region on chromosome 11. (B) A series of magnified screen shots from genome viewer IGV showing the genomes of lts1-211, WT, and CC124, which were compared with the genome sequence database based on CC503/cw92 (Top; blue bars indicate genes, and thick bars indicate exons). Gray bars in each genome indicate the paired-end reads of Illumina sequencing, in which colored regions indicate mutations, as detected via comparisons to the database. Visual comparison of the data revealed only one missense mutation in PSY (CCC to ATC, leading to a substitution of isoleucine for proline) in the mapped region.
Table 1.
Chlamydomonas reinhardtii strains used in this study
| Strain | Description | Source |
| WT | A progeny from the mating of two wild-type strains, CC124 (mt-) and CC125 (mt+), devoid of the agg1 mutation | This study |
| CC124 | A wild-type strain that carries agg1-, nit1-, nit2-, shows strong negative phototaxis and cannot grow on nitrate as sole nitrogen source (mt-) | 33–36 |
| CC125 | Basic wild-type strain that carries nit1-, nit2- and cannot grow on nitrate as sole nitrogen source (mt+) | 33–36 |
| lts1-211 | Point mutation in phytoene synthase | This study |
| lts1-30 | Null mutant of phytoene synthase | 16, 37 |
| lts1-202 (a.k.a. FN68) | Null mutant of phytoene synthase | 16, 38 |
| eye1-1 | Lacks eyespots during logarithmic growth; phototactic orientation impaired | 39 |
| eye2-1 | Eyespots not formed; defect in thioredoxin-like protein | 22, 23 |
| eye3 | Eyespots not formed; defect in putative ABC1 kinase | 23, 24 |
Fig. 3.
Phyotene synthtase gene in lts1-211 and genetic/phenotypic differences from the other lts1 alleles. (A) Structure of the Chlamydomonas PSY gene and the mutation in lts1-211 (mid). DNA and amino acid sequences in the vicinity of the mutation in exon 2 in the wild-type and lts1-211 genomes (Top) are shown. For the rescue experiment, lts1-211 was transformed with a 6,000-kb DNA fragment containing the PSY gene, which was cloned into pSI103 plasmid (Bottom) (42). (B) Domain structure of PSY. The P159I mutation in lts1-211 occurs in the catalytic domain of PSY. Mutations in the previously reported PSY null mutants are also shown as follows: In lts1-30, W123 is substituted for a stop codon, whereas in lts1-202 (previously called FN68), a frameshift occurs (16). (C) Part of the carotenoid-biosynthesis pathway in Chlamydomonas modified from ref. 19. PSY (boxed) synthesizes phytoene from geranylgeranyl-diphosphate. β-Carotene and lutein, the two major carotenoids in Chlamydomonas analyzed in Fig. 2D, are underlined. (D) Growth phenotypes of the wild type, lts1-211, and two PSY null mutants. Cell suspensions from each mutant containing ∼105 cells were spotted onto TAP-agar plates and incubated in the light (∼50 µmol photons⋅m−2⋅sec−1; Top) or dark (Bottom) for 3 d.
Phytoene is an intermediate in the carotenoid-biosynthesis pathway (Fig. 3C). A series of mutants lacking PSY (named lts1 mutants) was reported (16). However, the growth phenotype of lts1-211 is different from that of previously reported PSY null mutants. These null mutants, lts1-30 and lts1-201 through lts1-210, cannot grow in the light and are white or pale green when grown in the dark (referred to as “white mutants”) (16). By contrast, lts1-211 cells grow in the light, and their green color is indistinguishable from that of the wild type (Fig. 3D). In the dark, lts1-211 cells appear pale green (Fig. 3D). As a previously unidentified lts1 allele, we thus named this mutant lts1-211.
Without application of the redox reagents, lts1-211 did not show significant phototaxis in low light (∼0.3 μmol photons⋅m−2⋅s−1), whereas WT cells showed positive phototaxis (Fig. S2). In stronger light (>5 μmol photons⋅m−2⋅s−1), WT cells showed negative phototaxis, whereas lts1-211 showed positive phototaxis (Fig. S2). Thus, as far as cells show phototaxis, lts1-211 almost always showed phototaxis with a sign opposite to that of WT. For ease of phototactic sign analyses, strong light (∼10 μmol photons⋅m−2⋅s−1 for polar histograms and ∼100 μmol photons⋅m−2⋅s−1 for dish assays) was used in the following analyses (except for Fig. S2).
Fig. S2.
Dish phototaxis assay of the wild type, lts1-211 and lts1-211R without redox reagents at various light intensities. Cell suspensions in Petri dishes were photographed after illumination with a green LED from the side for 5 min (green arrows). Against weak light (∼0.3 μmol photons⋅m−2⋅s−1), WT and lts1-211R showed positive phototaxis, whereas lts1-211 did not show significant phototaxis (Left). As the light intensity gets stronger (Middle and Right), more cells of WT and lts1-211R showed negative, and those of lts1-211 showed positive phototaxis.
lts1-211 Has Low Levels of Carotenoid and Defective Eyespot Formation.
To confirm that lts1-211 impaired PSY activity, we quantified the carotenoid contents in the cells by reversed phase chromatography. β-Carotene and lutein are the two major carotenoids in wild-type cells (17, 18). Both carotenoids were absent in the two strains of the PSY null mutants, and their levels were significantly reduced in lts1-211 cells compared with wild-type cells (β-carotene, 3%; lutein, 28%; Fig. 2D).
These data prompted us to examine whether lts1-211 has a normal eyespot. As shown in Fig. 1, the Chlamydomonas eyespot contains multiple layers of carotenoid-rich granules, the main component of which is β-carotene (19). As expected, most lts1-211 cells did not have a detectable eyespot (Fig. 2E). Approximately <1% of lts1-211 cells had a faint orange spot on the cell surface, suggesting that these cells have eyespots containing only small amounts of carotenoids.
The phenotype of lts1-211 was rescued by transformation with a wild-type genome fragment containing the PSY gene (Fig. 3A). A rescued strain, lts1-211R, contained a normal level of carotenoids, normal eyespots, and showed the same sign of phototaxis as the wild type, with or without redox-reagent treatment (Fig. 2 and Fig. S2). These data suggest that PSY carrying the P159I mutation has significantly reduced activity.
All Eyeless Mutants Exhibit Redox-Dependent Reversal of Phototactic Sign.
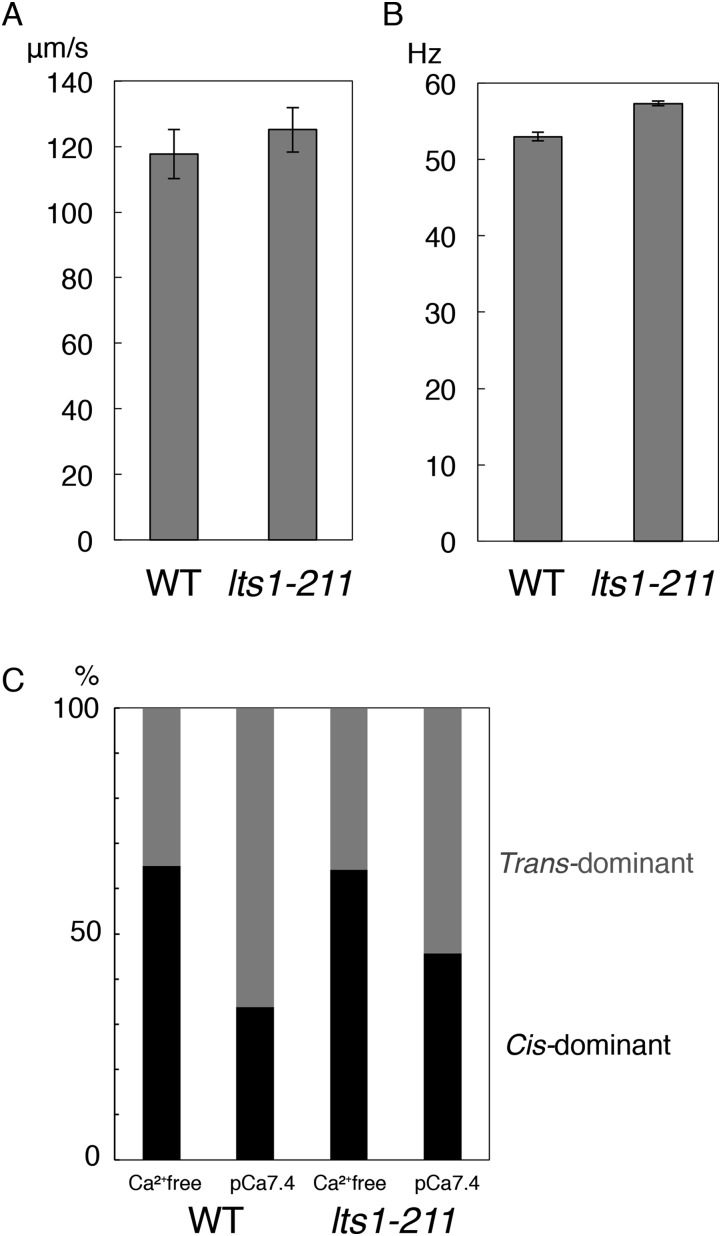
Why do lts1-211 cells show an opposite sign of phototaxis compared with that of the wild type? The swimming velocity and flagellar beat frequency of this mutant did not significantly differ from those of the wild type (Fig. S3 A and B). Similarly, Ca2+-dependent dominance switching between the two flagella, which is thought to be the basis for phototactic turning of the cell, apparently occurs normally in lts1-211, as assessed by using demembranated and reactivated “cell models” (Fig. S3C) (20). These cells also exhibited a normal photophobic response, which is characterized by transient backward swimming upon sudden light stimulation of ChRs (mainly ChR1) (6). Therefore, overall, the motility of lts1-211 cells appears to be normal.
Fig. S3.
Motility analyses of lts1-211. Swimming velocity (average ± SD: n = 47 for WT and n = 60 for lts1-211) (A) and flagellar beat frequency (average ± SEM of three independent experiments) (B). Analysis showing that lts1-211 swims at almost the same speed as, or slightly faster than, the wild type. (C) Dominance between the two flagella in demembranated cell models, reactivated with 1 mM ATP with and without submicromolar Ca2+ (pCa 7.4). No significant differences were detected between the wild-type and lts1-211 strains. Average data from three independent experiments are shown (n = 32–121 per experiment).
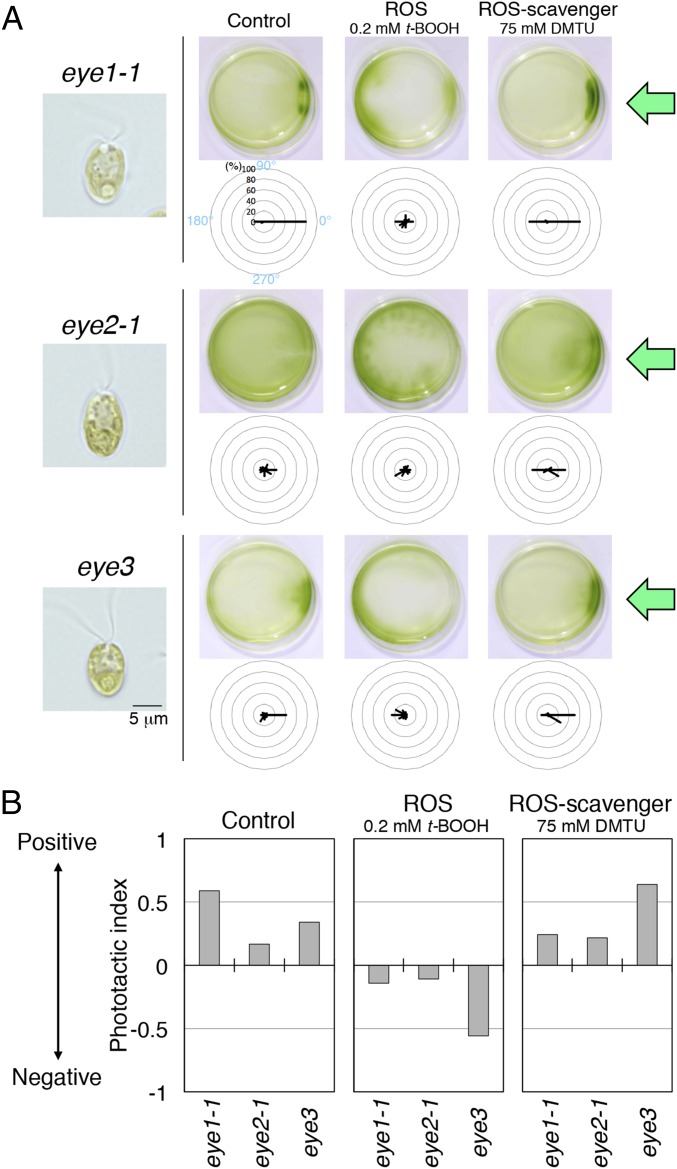
We thus surmised that the lack of eyespot pigments alone caused reversal of the phototactic sign. When the mutant displays phototaxis in a direction opposite to that of wild type, the photoreceptors may sense the light from the rear side, i.e., the light coming through the cell body, more strongly than from the front side. Such rear-side stimulation could take place if the cell body acts as a convex lens and condenses light on the photoreceptor. In fact, previous studies suggested that the cell bodies of lts1 null mutants are almost transparent and act as convex lenses, which condense light on the farthest side of the cell (13). It is possible, however, that such a lens effect is not limited to the transparent cell body of the mutant; the cell bodies of other Chlamydomonas strains with normal (green) pigmentation may also function as convex lenses, which help stimulate the photoreceptor from the rear side. If this hypothesis is the case, other eyeless mutants with green cell bodies might also exhibit a reversed sign of phototaxis. We thus examined the phototactic signs in eyeless mutants eye1, eye2, and eye3. Intriguingly, all three eye mutants exhibited an opposite sign of phototaxis compared with the wild type after treatment with redox reagents (Fig. 4).
Fig. 4.
All eyespot-deficient mutants show a redox-dependent sign of phototaxis opposite to that of the wild type. (A) Cell images, dish phototaxis assays, and polar histograms of eye1-1, eye2-1, and eye3, with or without treatment with redox reagents (12 bins of 30°; n = 24–56 cells per condition). (B) Phototactic index calculated as an average value of cosθ measured in A. After treatment with redox reagents, all eyeless mutants showed signs of phototaxis opposite to those of strains with eyespots (wild type and lts1-211R) and same as lts1-211 (Fig. 2C).
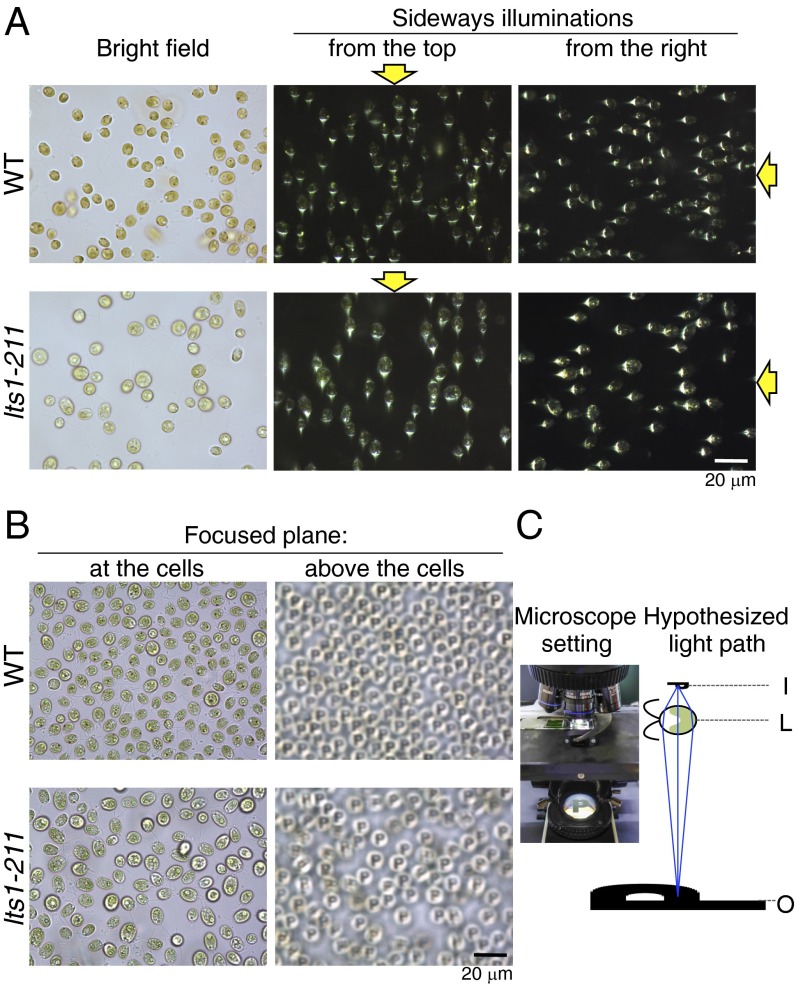
To confirm the presence of a lens effect in “green” cells, we observed the wild-type and the lts-211 cells under a microscope by using sideways illumination (Fig. 5A). Regardless of the location of the eyespot, a small, bright area appeared on the side of each cell edge opposite the light source. Furthermore, we observed images of an object in the light path of the microscope, which were formed by the cellular lens effect (Fig. 5B, Fig. S4, and Movie S1). These observations indicate that even a normally pigmented cell body acts as a convex lens. The redox-dependent reversal of phototactic sign in lts1-211 and the three eye mutants suggests that the carotenoid layers of the eyespot play a crucial role in determining the phototactic sign in Chlamydomonas (Fig. 6).
Fig. 5.
The Chlamydomonas cell body has a lens effect. (A) Wild-type and lts1-211 cells were observed with bright-field illumination (Left) or with sideways illumination (Middle and Right; yellow arrows indicate the direction of illumination). A small bright area is observed in each cell on the side opposite the light source. (B) The letter “P” (for “photo”) set on a field stop ring of the microscope was imaged through the cells of both strains by the lens effect. The letter “P” appeared on each cell as the plane of focus was moved from the cells (Left) to above the cells (Right). (C) The setting of the microscope and a hypothetical optical path are shown. I, image; L, cell as a lens; O, object.
Fig. S4.
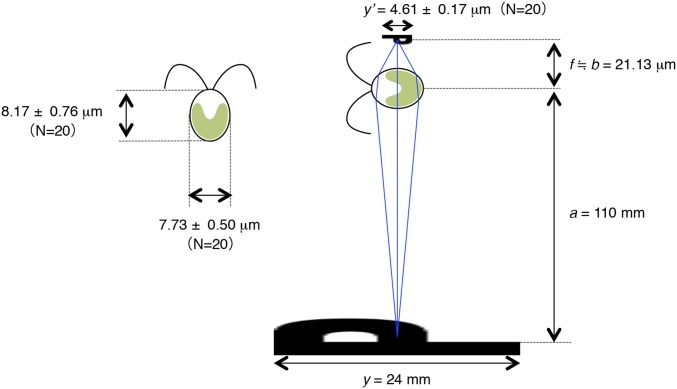
Estimation of the refractive index of Chlamydomonas cell. Approximate focal length, f = 21.13 μm, can be evaluated with the magnification (M = y′/y) and the distance from the object to the lens a. (The value for y′ was measured when “P” seemed smallest and best focused.) When the cell acts as a ball lens, radius r, relative refractive index n, and the focal length f are related by f = nr/2(n−1) from well-known lens-maker's formula. Using the value r = 3.98 μm (the average of the longest and shortest radii of WT cells) and the refractive index of water n1 = 1.333, the refractive index of the cell n2 is estimated to be 1.47.
Fig. 6.
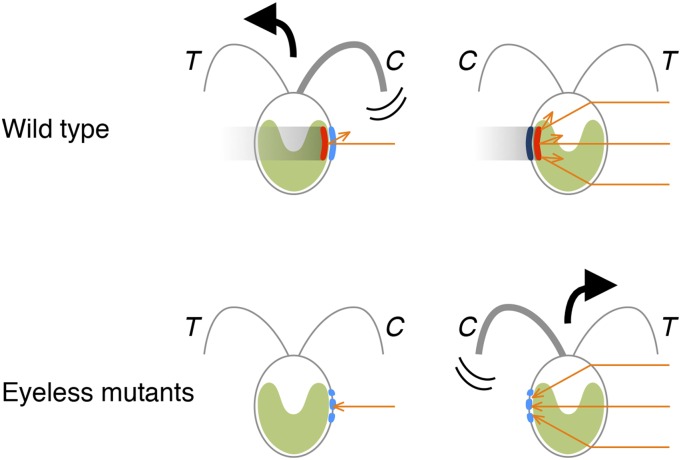
Model illustrating the effect of light illumination on the photoreceptors and the phototactic sign of the wild type (Top) and eyeless mutants (Bottom). Carotenoid layers (red) reflect and amplify the light signal (orange arrows) onto the photoreceptors (blue) when the eyespot faces the light source. These layers shield the photoreceptors from the light condensed by the lens effect of the cell when the eyespot faces the side opposite the light source. The photoreceptors in an eyeless mutant cell localize to several patches around the “correct” position but function normally (24). The photoreceptors receive stronger light stimulation when facing away from the light source, i.e., in an opposite manner to that of wild-type photoreception. When the wild type cells are illuminated by strong light, they show negative phototaxis by beating the cis-flagellum (C) stronger than the trans-flagellum (T) when the eyespot faces the light source (Top Left). In contrast, the eyeless mutant cells show positive phototaxis by beating the cis-flagellum stronger than the trans-flagellum when the eyespot faces the side opposite the light source (Bottom Right).
SI Materials and Methods
Isolation and Rescue of the lts1-211 Mutant.
CC125 wild-type cells were mutagenized by UV illumination for 2 min. After being kept in the dark for 12 h, 0.2 mM t-BOOH (see below) was added to the culture medium, and the side of the plastic dish was illuminated with green light. Cells showing negative phototaxis were collected and spread onto a TAP-agar plate. Single colonies were inoculated into a 96-well plate, and each clone’s phototactic phenotype was assessed by visual inspection. Clones that always exhibited a reversed sign of phototaxis were backcrossed twice with the wild type [a strain derived from a cross between two wild-type strains, CC124 (minus mating type) and CC125 (plus mating type); the agg1 mutation, a mutation responsible for negative phototaxis in CC124 (35), had been removed].
To rescue lts1-211 mutant phenotype, a 6.0-kb genome DNA fragment containing wild-type PSY gene was cloned into SmaI site of pSI103 plasmid (42) that contains paromomycin resistant aphVIII gene using primers 5′- CCTTAATGCAGCGAATCCTT-3′ and 5′- CTGCATTGCTACAGCTGCA-3′ by Hot Fusion method (43) (Fig. 3A). The plasmid linearized by KpnI digestion was introduced to lts1-211 cells by the method of Yamano et al. (44). A rescued strain lts1-211R was isolated by paromomycin selection and eyespot observation.
Phototaxis Assay.
The phototaxis assay was performed by using cells in the light phase (6∼8 h after the light on) as described with modifications (14). Cells were washed with an experimental solution [5 mM Hepes (pH 7.4), 0.2 mM EGTA, 1 mM KCl, and 0.3 mM CaCl2] and kept under red light for more than 50 min before the assays. For the dish assays, 2-mL cell suspensions (∼107 cells/mL) were placed into Petri dishes (35 mm in diameter, 10 mm thick), illuminated with a green LED (λ = 525 nm, ∼100 μmol photons⋅m−2⋅s−1; for weaker light, neutral-density filters were set in front of the LED) from one side for 5 min, and photographed (DSC-RX100M2; Sony). Photon flux density was measured by LI-250 Light Meter (Meiwafosis). The cell suspensions were then used for single-cell analysis; they were gently agitated, placed on the stage of an inverted microscope (IX70; Olympus), and illuminated with a green LED from one side (λ = 525 nm, ∼10 μmol photons⋅m−2⋅s−1). The behavior of the cells was observed under dim red light (λ, > 600 nm) and video recorded by using a CCD camera (1129HMN1/3; Wraymer). The angle (θ) between the light direction and the swimming direction was measured for 1.5 s following illumination with a green LED for 15 s. Images of swimming cells were autotracked by using Image Hyper software (Science Eye). The angles were measured from the cell trajectories. t-BOOH (final concentration of 0.2 mM; Wako Pure Chemical Industries) was used as a ROS reagent, and dimethylthiourea (final concentration of 75 mM; Sigma-Aldrich) was used as a ROS-scavenging reagent (45).
Microscopy.
Cells were observed under a BX-53 microscope (Olympus) equipped with either a bright-field condenser and were photographed by using a CMOS camera (STC-5MUSB3; Sentech). Cells were stuck onto a glass surface coated with 0.1% polyethyleneimine. The side of the microscope stage was illuminated by using optical fiber illuminators (FLH-150A; Shimadzu and PCS-UMX250; Nippon PI). To directly observe the cellular lens effect, the letter “P” was printed on a transparent sheet and placed onto a field stop ring (P stands for photo and is a vertically and horizontally asymmetrical letter; see Fig. 5B). The cells were observed under a BX-53 microscope without a condenser (27).
Detection of Carotenoids.
β-Carotene and lutein were measured by HPLC as described with minor modifications (46). Approximately 1 × 107 cells were suspended in 1 mL of extraction solution (equal amounts of acetone and methanol) and centrifuged at 20,000 × g for 5 min at 4 °C. The pigments were separated on a Symmetry C18 reversed phase column (250 × 4.6 mm; Senshu) by using the Nexera X2 HPLC system (Shimadzu) by elution with solvent A [80% (vol/vol) acetonitrile and 20% (vol/vol) ethanol]. The flow rate was 2 mL/min at 40 °C. β-Carotene and lutein were detected at 450 nm. The concentrations were calculated based on comparisons with known concentrations of pigment standard (β-carotene and lutein; Wako Chemical Industries).
Whole Genome Sequencing.
Cell walls were removed from lts1-211, CC124, and WT (Table 1) cells by treatment with autolysin, which was prepared as described (47). DNA was prepared from each sample by using a DNeasy Plant Mini kit (QIAGEN) following the manufacturer’s instructions. Two micrograms of each DNA sample was fragmented by using a Covaris sonicator. Next, ∼300-bp DNA fragments were selectively purified with a Pippin Prep system (Sage Science) and subjected to sequencing library construction by using an Illumina TruSeq library prep kit (Illumina) following the manufacturer’s instruction with one modification: The number of PCR cycles in the amplification enrichment step was reduced to six to minimize the PCR amplification bias. The libraries were sequenced by using Illumina HiSeq 2000 to produce 2 × 101-bp paired-end reads. In total, 40.4 M (8.2 Gbp), 48.2 M (9.7 Gbp), and 71.6 M paired-end reads (14.5 Gbp) were obtained for lts1-211, CC124, and WT, respectively.
Sequence reads were aligned onto the Joint Genome Institute (JGI) version 5.3.1 (Creinhardtii_236.fa.gz) Chlamydomonas genome sequence (downloaded from https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Creinhardtii) using bowtie2 (bowtie-bio.sourceforge.net/bowtie2/index.shtml). The resulting Sequence Alignment/Map files were converted to Binary Sequence Alignment/Map and sorted by SAMtools version 0.1.18 (samtools.sourceforge.net/). The alignment data were visualized by using IGV software (version 2.3.39; https://www.broadinstitute.org/igv/). The causal mutation of lts1-211 was identified by comparing the genome sequence of lts1-211 with that of WT, CC124, or CC503 (JGI database) focusing on the 131-kb region on chromosome 11, the candidate locus determined by genetic mapping (see next section).
Linkage Mapping of the lts1-211 Mutation.
The lts1-211 mutant was crossed to polymorphic strain S1C5 (mt-, CC-1952; ref. 48), and the tetrad progeny were dissected as described (47). Recombination frequencies between lts1-211 and genetic markers were determined by detecting polymorphic PCR products in 112 progeny that displayed phototaxis defects (15). The lts1-211 mutation was mapped to a 131-kb region between two genetic markers on chromosome 11: SSR14-348 (5′-TGAGAGTATTTGCGGCTTTGAACA-3′ and 5′-TCAGCTTATTCCTCATTGGCATCA-3′) and SSR14-474 (5′-TGGTACCAGCATGTACTCAGCGAT-3′ and 5′-ATGTGCTCACACAGATGCCGACT-3′).
The genomic sequence of this region is found on chromosome 11 in the JGI database, version 4, although it is found on chromosome 2 in the same database, version 5.5. This discrepancy is likely due to gene misassembly in the version 5.5 genome because two genetic markers of linkage group XI (chromosome 11), CNA19 and ODA2, are linked to the lts1-211 mutation (15), which is consistent with the results from version 4.
Motility Analysis.
Flagellar beat frequency was determined from the power spectra of cell body vibration signals in microscope images of a population of cells (49). Median frequency was obtained from the power spectra averaged for ∼20 s. For swimming velocity analysis, video-recorded images of swimming cells were autotracked for 1.5 s by using Image Hyper software (Science Eye). The swimming velocities were measured based on the cell trajectories.
Reactivation of Demembranated Cell Models.
Cell models were prepared and analyzed by using the method of Kamiya and Witman (20) with modifications. Calcium buffers (Ca2+-free and pCa 7.4) were prepared according to Wakabayashi et al. (50) with modifications (14). Cell models reactivated with 1 mM ATP frequently rotated within small areas by predominantly beating one of the two flagella. The dominance of the cis- or trans-flagellum over the other in these cells was determined by visual inspection.
Discussion
Screening for Chlamydomonas mutants defective in phototactic sign switching resulted in the isolation of lts1-211, a weak-allele mutant of the PSY gene. The mutant cells contained low amounts of carotenoids, and most lacked detectable eyespots. These cells displayed phototaxis against light stronger than ∼5 μmol photons⋅m−2⋅s−1, but its sign was opposite to that of wild-type cells with or without the application of redox reagents, which strongly biases the sign of phototaxis. Interestingly, all previously known eye mutants also exhibited the same phenotype after redox-reagent treatment.
Previously isolated eye mutants were reported to exhibit weak or no phototaxis. The eye1 mutant exhibits weak phototaxis because of the less precise orientation of the cells’ swimming direction (21). The eye2 mutant also exhibits weak phototaxis because it is ∼100-fold less sensitive to light than the wild type (22). The eye3 mutant does not exhibit phototaxis unless it is under special conditions (e.g., nitrogen starvation or a prolonged incubation at the stationary phase) (23). In the eye2 and eye3 mutants, ChR1 localizes to several patches around the “correct” position where the eyespot would normally occur, suggesting that the focused localization of channelrhodopsins, but not their approximate localization, requires the presence of the carotenoid layers (24, 25). Individual ChR1 molecules present in the membrane of a cell without a detectable eyespot appear to function normally, because eye1 exhibits a normal photophobic response (6, 21). In contrast to previous studies, in the present study, the use of redox reagents produced rather strong (but oppositely directed) phototaxis in all eyeless mutants examined, including the newly isolated mutant lts1-211. Strong phototaxis in eyeless mutants was detected in this study, most likely because redox reagents fixed the phototactic sign and, thereby, stabilized this behavior (14). In the dish phototaxis assay without redox reagent, only eye2 did not exhibit obvious positive phototaxis among eyespot-less mutants (Fig. 2A and Fig. 4A). A previous study showed that eye2 shows weak negative phototaxis at approximately 120∼150 μmol photons⋅m−2⋅s−1 (22). Because Eye2p is a thioredoxin family protein, its absence may change the intracellular redox poise (22).
The reversed-phototactic sign in the eyeless mutants after treatment with redox reagents can be explained by the lens effect of the cell body (Figs. 5 and 6), which was previously found in several organisms including cyanobacteria, fungi, dinoflagellates, colonial Volvocine algae, and colorless Chlamydomonas mutants (12, 13, 26–28). The present study directly demonstrates that a normally pigmented Chlamydomonas cell can also function as a convex lens such that light illuminated sideways on the cell is condensed on the farther side, forming a small, bright patch, and that the images of an object are formed through the cells. In an apparent contradiction to our observations, a previous study (12) concluded that chlorophylls or other pigments in the cell body, rather than the eyespot, act as shields against light from the rear. However, our results indicate that shielding by chlorophylls or other pigments dispersed throughout chloroplast is insufficient to cancel the cellular lens effect on the ChRs, and that the carotenoid layers underneath the ChRs, where the incident light most strongly concentrates in the cell, are necessary.
Because we directly observed the lens effect of the cell bodies (Fig. 5B), we then were able to estimate that the refractive index of Chlamydomonas cells is 1.47, which is closed to the refractive index of most of the cells of green algae Dunaliella salina (1.46) and Chlorella sp. (1.40–1.45), as well as plants (1.48) (Fig. S4) (29–31). This value is higher than the previously reported value for Chlamydomonas estimated by a laser scanning flow cytometer (1.39∼1.43) (32). Our method can be applied to evaluation of refractive indices of other spheroidal organisms without special equipment.
In conclusion, a new screening method using redox reagents allowed us to isolate a previously unidentified Chlamydomonas mutant and to detect a previously unknown aspect of eyespot function affecting phototaxis. The isolation of the mutant, lts1-211, revealed that the cellular lens effect affects cellular behavior in the absence of carotenoid layers. The carotenoid pigment granules therefore have a crucial role in determining the sign of phototaxis, by shielding the ChRs in the plasma membrane from light condensed by the cellular lens onto the back of the eyespot.
Materials and Methods
The strains used in this study are listed in Table 1. All cells were grown in Tris-acetate-phosphate (TAP) medium (40) with aeration at 22 °C under a 12 h/12 h light/dark cycle, except for lts1-202 and lts1-30, which were grown in the dark for pigment and growth-phenotype analyses.
See SI Materials and Methods for more information.
Supplementary Material
Acknowledgments
We thank Drs. Masakatsu Watanabe (Graduate School for the Creation of New Photonics Industries), Tetsuo Takahashi, Mineo Iseki (Toho University), Oleg A. Sineshchekov (University of Texas Med School), Kenjiro Yoshimura (Shibaura Institute of Technology), Takako Kato-Minoura (Chuo University), and Takeyuki Wakabayashi (Teikyo University) for fruitful discussions about phototaxis; Dr. Tatsuya Kitazume, Ms. Hiroyo Asao (National Institute for Basic Biology, NIBB), and Ms. Mishio Toh (University of Tokyo) for Illumina sequencing; Ms. Yuka Misawa (University of Tokyo) for mutant isolation; Ms. Naomi Miyamoto (Hosei University) for linkage mapping; and Dr. Ritsu Kamiya (Gakushuin University) for critical reading of this manuscript. This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 25291058, 26650093, 15H01206, 15H01314 (to K.W.), 26251033 (to J.M.), and 15K20985 (to Y.K.); NIBB Collaborative Research Program 14-733 (to K.W.); Network Joint Research Center for Materials and Devices Grant 2015298 (to M.H.); and the New Energy and Industrial Technology Development Organization (P07015 to J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525538113/-/DCSupplemental.
References
- 1.Nagel G, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296(5577):2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 2.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2002;99(13):8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki T, et al. Archaeal-type rhodopsins in Chlamydomonas: Model structure and intracellular localization. Biochem Biophys Res Commun. 2003;301(3):711–717. doi: 10.1016/s0006-291x(02)03079-6. [DOI] [PubMed] [Google Scholar]
- 5.Foster KW, Smyth RD. Light Antennas in phototactic algae. Microbiol Rev. 1980;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthold P, et al. Channelrhodopsin-1 initiates phototaxis and photophobic responses in chlamydomonas by immediate light-induced depolarization. Plant Cell. 2008;20(6):1665–1677. doi: 10.1105/tpc.108.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegemann P, Berthold P. 2009. Sensory photoreceptors and light control of flagellar activity. The Chlamydomonas Sourcebook, ed Witman G-B (Academic, Oxford), 2nd Ed, Vol 3, pp 395–430.
- 8.Rüffer U, Nultsch W. Flagellar photoresponses of Chlamydomonas cells held on micropipettes: II. Change in flagellar beat pattern. Cell Motil Cytoskeleton. 1991;18(4):269–278. [Google Scholar]
- 9.Rüffer U, Nultsch W. Flagellar photoresponses of ptx1, a nonphototactic mutant of Chlamydomonas. Cell Motil Cytoskeleton. 1997;37(2):111–119. doi: 10.1002/(SICI)1097-0169(1997)37:2<111::AID-CM3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Schaller K, David R, Uhl R. How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys J. 1997;73(3):1562–1572. doi: 10.1016/S0006-3495(97)78188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreimer G. The green algal eyespot apparatus: A primordial visual system and more? Curr Genet. 2009;55(1):19–43. doi: 10.1007/s00294-008-0224-8. [DOI] [PubMed] [Google Scholar]
- 12.Schaller K, Uhl R. A microspectrophotometric study of the shielding properties of eyespot and cell body in Chlamydomonas. Biophys J. 1997;73(3):1573–1578. doi: 10.1016/S0006-3495(97)78189-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sineshchekov OA, Govorunova EG, Dér A, Keszthelyi L, Nultsch W. Photoinduced electric currents in carotenoid-deficient Chlamydomonas mutants reconstituted with retinal and its analogs. Biophys J. 1994;66(6):2073–2084. doi: 10.1016/S0006-3495(94)81002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakabayashi K, Misawa Y, Mochiji S, Kamiya R. Reduction-oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2011;108(27):11280–11284. doi: 10.1073/pnas.1100592108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kathir P, et al. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryot Cell. 2003;2(2):362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy SS, Kobayashi MC, Niyogi KK. White mutants of Chlamydomonas reinhardtii are defective in phytoene synthase. Genetics. 2004;168(3):1249–1257. doi: 10.1534/genetics.104.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichenberger W, Boschetti A, Michel HP. Lipid and pigment composition of a chlorophyll beta-deficient mutant of Chlamydomonas-reinhardii. Physiol Plant. 1986;66(4):589–594. [Google Scholar]
- 18.Niyogi KK, Björkman O, Grossman AR. The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA. 1997;94(25):14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohr M. 2009. Carotenoids. The Chlamydomonas Sourcebook (Academic, Oxford), 2nd Ed, Vol 2, pp 799–817.
- 20.Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel-Laurens NML, Feinleib MEH. Photomovement in an “eyeless” mutant of Chlamydomonas. Photochem Photobiol. 1983;37(2):189–194. [Google Scholar]
- 22.Roberts DG, Lamb MR, Dieckmann CL. Characterization of the EYE2 gene required for eyespot assembly in Chlamydomonas reinhardtii. Genetics. 2001;158(3):1037–1049. doi: 10.1093/genetics/158.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb MR, Dutcher SK, Worley CK, Dieckmann CL. Eyespot-assembly mutants in Chlamydomonas reinhardtii. Genetics. 1999;153(2):721–729. doi: 10.1093/genetics/153.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd JS, Mittelmeier TM, Lamb MR, Dieckmann CL. Thioredoxin-family protein EYE2 and Ser/Thr kinase EYE3 play interdependent roles in eyespot assembly. Mol Biol Cell. 2011;22(9):1421–1429. doi: 10.1091/mbc.E10-11-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittelmeier TM, Boyd JS, Lamb MR, Dieckmann CL. Asymmetric properties of the Chlamydomonas reinhardtii cytoskeleton direct rhodopsin photoreceptor localization. J Cell Biol. 2011;193(4):741–753. doi: 10.1083/jcb.201009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shropshire W. The lens effect and phototropism of phycomyces. J Gen Physiol. 1962;45(5):949–958. doi: 10.1085/jgp.45.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler JO, Nedelcu AM, Solari CA, Shelton DE. 2015. Cells acting as lenses: A possible role for light in the evolution of morphological asymmetry in multicellular Volvocine algae. Evolutionary Transitions to Multicellular Life, eds Ruiz-Trillo I, Nedelcu AM (Springer, Dordrecht, The Netherlands), pp 225–243.
- 28.Schuergers N, et al. Cyanobacteria use micro-optics to sense light direction. eLife. 2016;5:e14169. doi: 10.7554/eLife.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bricaud A, Bédhomme AL, Morel A. Optical properties of diverse phytoplanktonic species: Experimental results and theoretical interpretation. J Plankton Res. 1998;10(5):851–873. [Google Scholar]
- 30.Spinrad RW, Brown JF. Relative real refractive index of marine microorganisms: A technique for flow cytometric estimation. Appl Opt. 1986;25(12):1930–1934. doi: 10.1364/ao.25.001930. [DOI] [PubMed] [Google Scholar]
- 31.Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009;50(4):684–697. doi: 10.1093/pcp/pcp034. [DOI] [PubMed] [Google Scholar]
- 32.Spizzichino V, et al. First studies of pico- and nanoplankton populations by a laser scanning flow cytometer. J Quant Spectrosc Radiat Transf. 2011;112(5):876–882. [Google Scholar]
- 33.Nichols GL, Syrett PJ. Nitrate reductase deficient mutants of Chlamydomonas reinhardii. Isolation and genetics. J Gen Microbiol. 1978;108:71–77. [Google Scholar]
- 34.Fernández E, Matagne RF. Genetic analysis of nitrate reductase-deficient mutants in Chlamydomonas reinhardii. Curr Genet. 1984;8(8):635–640. doi: 10.1007/BF00395710. [DOI] [PubMed] [Google Scholar]
- 35.Smyth RD, Ebersold WT. Genetic investigation of a negatively phototactic strain of Chlamydomonas reinhardtii. Genet Res. 1985;46(2):133–148. doi: 10.1017/s001667230002262x. [DOI] [PubMed] [Google Scholar]
- 36.Pröschold T, Harris EH, Coleman AW. Portrait of a species: Chlamydomonas reinhardtii. Genetics. 2005;170(4):1601–1610. doi: 10.1534/genetics.105.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemerilova VI. Investigation of pigmentation modifying mutations in Chlamydomonas-reinhardii strains of different ploidy. 2. Lts1 mutation compounds and their use for obtaining triploid cultures. Genetika. 1978;14(1):154–162. [Google Scholar]
- 38.Foster KW, et al. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- 39.Hartshorne JN. The function of the eyespot in Chlamydomonas. New Phytol. 1953;52(3):292–297. [Google Scholar]
- 40.Gorman DS, Levine RP. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1965;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieckmann CL. Eyespot placement and assembly in the green alga Chlamydomonas. BioEssays. 2003;25(4):410–416. doi: 10.1002/bies.10259. [DOI] [PubMed] [Google Scholar]
- 42.Sizova I, Fuhrmann M, Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277(1–):221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 43.Fu C, Donovan WP, Shikapwashya-Hasser O, Ye X, Cole RH. Hot Fusion: An efficient method to clone multiple DNA fragments as well as inverted repeats without ligase. PLoS One. 2014;9(12):e115318. doi: 10.1371/journal.pone.0115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamano T, Iguchi H, Fukuzawa H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J Biosci Bioeng. 2013;115(6):691–694. doi: 10.1016/j.jbiosc.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Wakabayashi K, King SM. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J Cell Biol. 2006;173(5):743–754. doi: 10.1083/jcb.200603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homma K, Nishitani M, Mita Y, Mawatari M, Nakashima H. A simultaneous determination method of carotenes by reversed phase high performance liquid chromatography. J Tsuruma Health Sci Soc Kanazawa Univ. 2012;36:27–31. [Google Scholar]
- 47.Harris EH. Chlamydomonas in the Laboratory. In: Harris E-H, editor. The Chlamydomonas Sourcebook. 2nd Ed. Vol 1. Academic; Oxford: 2009. pp. 241–302. [Google Scholar]
- 48.Gross CH, Ranum LPW, Lefebvre PA. Extensive restriction fragment length polymorphisms in a new isolate of Chlamydomonas reinhardtii. Curr Genet. 1988;13(6):503–508. doi: 10.1007/BF02427756. [DOI] [PubMed] [Google Scholar]
- 49.Kamiya R. Analysis of cell vibration for assessing axonemal motility in Chlamydomonas. Methods. 2000;22(4):383–387. doi: 10.1006/meth.2000.1090. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi K, Yagi T, Kamiya R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motil Cytoskeleton. 1997;38(1):22–28. doi: 10.1002/(SICI)1097-0169(1997)38:1<22::AID-CM3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.