Abstract
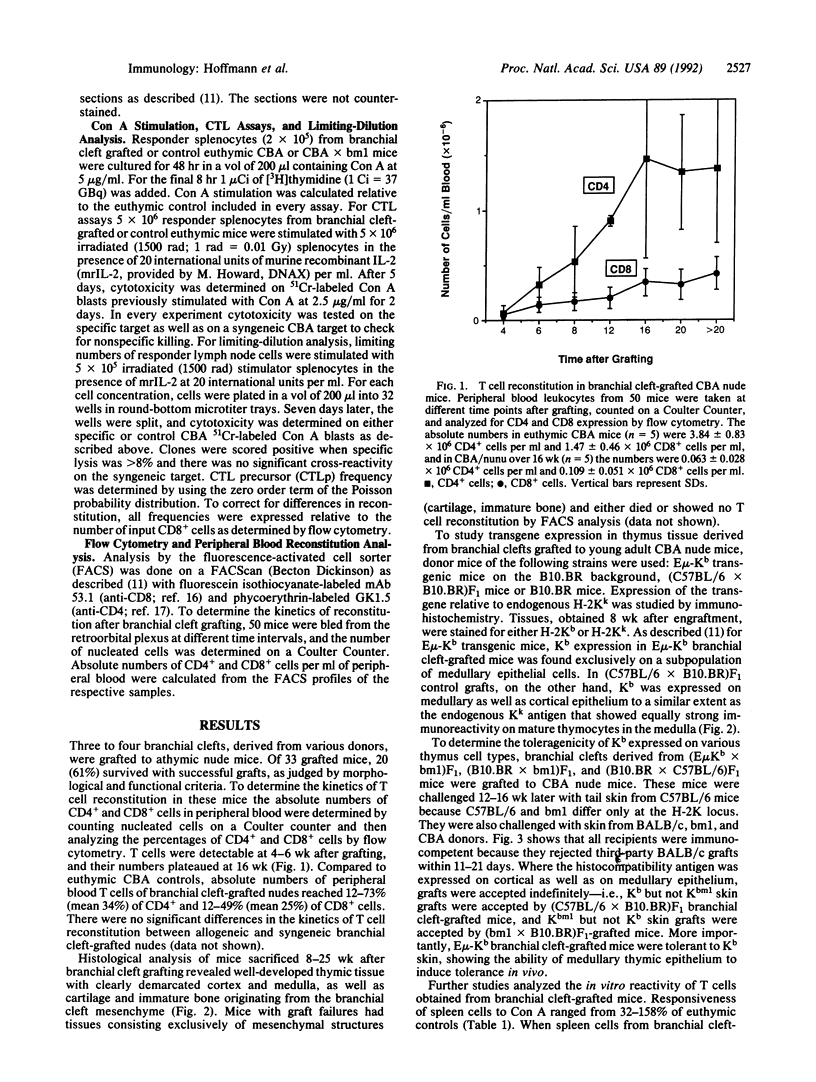
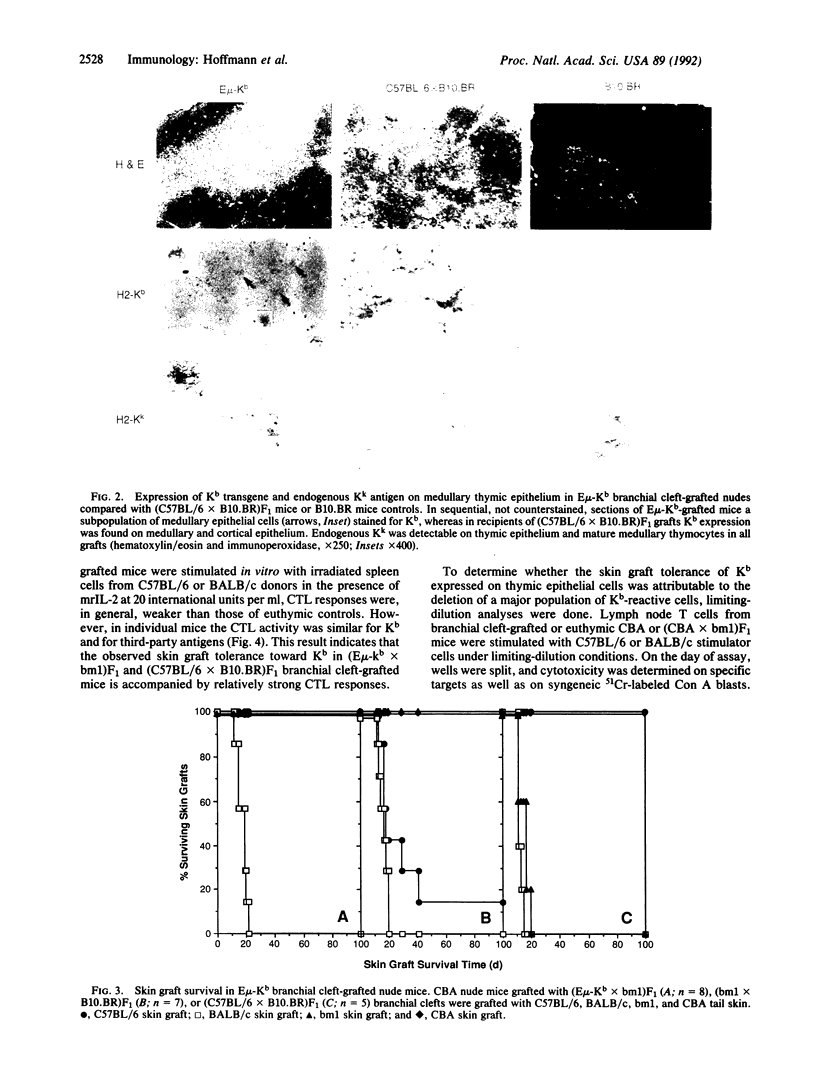
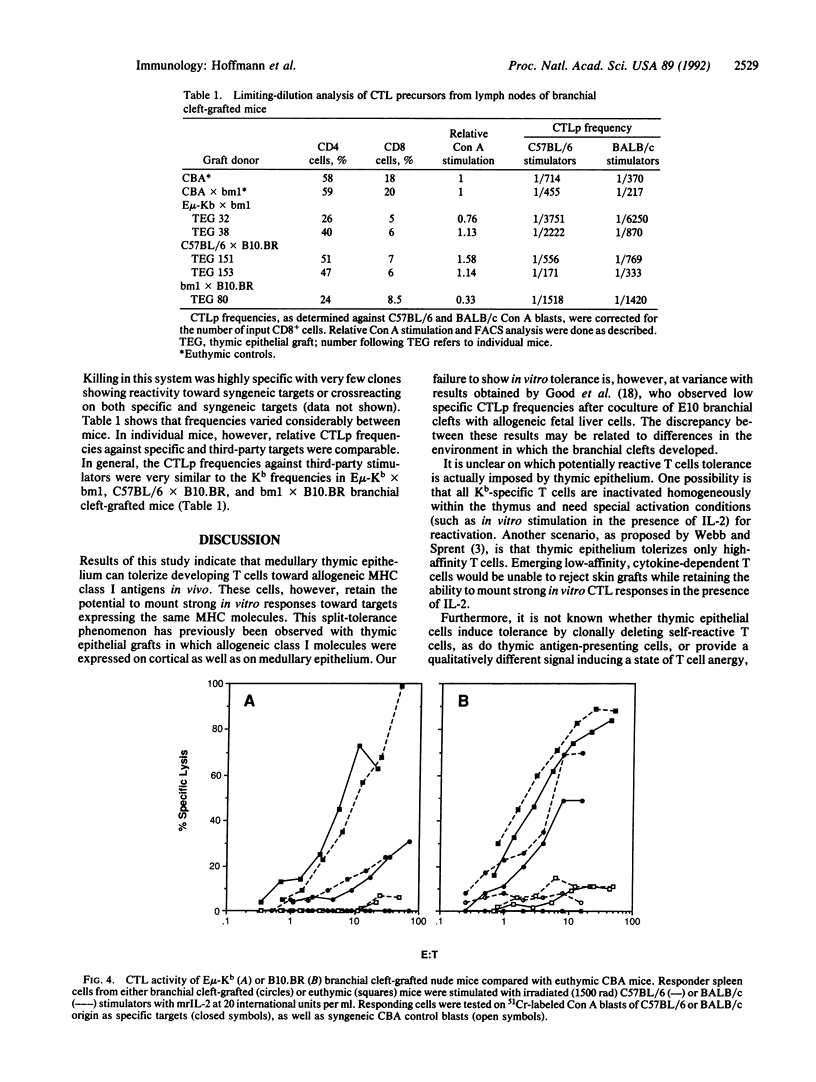
To study the role of thymic medullary epithelium in tolerance induction, the third and fourth branchial clefts of embryos from E mu-Kb transgenic mice, which express the major histocompatibility complex class I antigen H-2Kb exclusively on medullary thymic epithelium, were grafted to athymic nude mice. The grafts differentiated into tissue that morphologically resembled normal thymus. These grafts expressed the H-2Kb antigen appropriately and gave rise to a functional T cell repertoire. In vivo tolerance to H-2Kb disparate skin grafts was invariably found in mice expressing H-2Kb in the medulla or in both medulla and cortex of C57BL/6 branchial cleft-grafted controls. In marked contrast, in vitro cytotoxicity assays demonstrated reactivity toward H-2Kb in the presence of interleukin 2, and limiting-dilution analyses showed similar frequencies of cytolytic T cell precursors reactive to H-2Kb and to third-party stimulators. Medullary epithelium can, therefore, induce split tolerance, in which in vivo tolerance is accompanied by strong in vitro responses in the presence of interleukin 2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Müllbacher A., Cox K., Morahan G., Boyd R., Scollay R., Blanden R. V., Miller J. F. Selection of the T-cell repertoire in transgenic mice expressing a transplantation antigen in distinct thymus subsets. Proc Biol Sci. 1990 Sep 22;241(1302):170–178. doi: 10.1098/rspb.1990.0082. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gao E. K., Lo D., Sprent J. Strong T cell tolerance in parent----F1 bone marrow chimeras prepared with supralethal irradiation. Evidence for clonal deletion and anergy. J Exp Med. 1990 Apr 1;171(4):1101–1121. doi: 10.1084/jem.171.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Pyke K. W., Nossal G. J. Functional clonal deletion of cytotoxic T-lymphocyte precursors in chimeric thymus produced in vitro from embryonic Anlagen. Proc Natl Acad Sci U S A. 1983 May;80(10):3045–3049. doi: 10.1073/pnas.80.10.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Schönrich G., Momburg F., Auphan N., Malissen M., Malissen B., Schmitt-Verhulst A. M., Arnold B. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immunol Rev. 1991 Aug;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Jordan R. K., Robinson J. H., Hopkinson N. A., House K. C., Bentley A. L. Thymic epithelium and the induction of transplantation tolerance in nude mice. Nature. 1985 Apr 4;314(6010):454–456. doi: 10.1038/314454a0. [DOI] [PubMed] [Google Scholar]
- Khazaal I., Salaün J., Coltey M., Calman F., Le Douarin N. Restoration of T-cell function in nude mice by grafting the epitheliomesenchymal thymic rudiment from 10-day-old euthymic embryos. Cell Differ Dev. 1989 May;26(3):211–220. doi: 10.1016/0922-3371(89)90752-1. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Sharrow S. O., Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990 Mar 1;171(3):935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün J., Bandeira A., Khazaal I., Calman F., Coltey M., Coutinho A., Le Douarin N. M. Thymic epithelium tolerizes for histocompatibility antigens. Science. 1990 Mar 23;247(4949 Pt 1):1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- Sprent J., Webb S. R. Function and specificity of T cell subsets in the mouse. Adv Immunol. 1987;41:39–133. doi: 10.1016/s0065-2776(08)60030-9. [DOI] [PubMed] [Google Scholar]
- Von Boehmer H., Schubiger K. Thymocytes appear to ignore class I major histocompatibility complex antigens expressed on thymus epithelial cells. Eur J Immunol. 1984 Nov;14(11):1048–1052. doi: 10.1002/eji.1830141116. [DOI] [PubMed] [Google Scholar]
- Webb S. R., Sprent J. Tolerogenicity of thymic epithelium. Eur J Immunol. 1990 Nov;20(11):2525–2528. doi: 10.1002/eji.1830201127. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Hafen K. Minor but not major histocompatibility antigens of thymus epithelium tolerize precursors of cytolytic T cells. Nature. 1986 Apr 17;320(6063):626–628. doi: 10.1038/320626a0. [DOI] [PubMed] [Google Scholar]



