Abstract
Objectives To determine whether late mortality after sepsis is driven predominantly by pre-existing comorbid disease or is the result of sepsis itself.
Deign Observational cohort study.
Setting US Health and Retirement Study.
Participants 960 patients aged ≥65 (1998-2010) with fee-for-service Medicare coverage who were admitted to hospital with sepsis. Patients were matched to 777 adults not currently in hospital, 788 patients admitted with non-sepsis infection, and 504 patients admitted with acute sterile inflammatory conditions.
Main outcome measures Late (31 days to two years) mortality and odds of death at various intervals.
Results Sepsis was associated with a 22.1% (95% confidence interval 17.5% to 26.7%) absolute increase in late mortality relative to adults not in hospital, a 10.4% (5.4% to 15.4%) absolute increase relative to patients admitted with non-sepsis infection, and a 16.2% (10.2% to 22.2%) absolute increase relative to patients admitted with sterile inflammatory conditions (P<0.001 for each comparison). Mortality remained higher for at least two years relative to adults not in hospital.
Conclusions More than one in five patients who survives sepsis has a late death not explained by health status before sepsis.
Introduction
Sepsis, an inflammatory response to infection, is a leading cause of admission to hospital in the developed world.1 2 While mortality in hospital is falling,2 3 4 5 longer term mortality after sepsis has remained high as many patients die in the subsequent months.6 Some argue that this late mortality is simply a reflection of the underlying comorbidity burden of patients who develop sepsis, whereas others argue it is the result of sepsis itself.7 8 9 10
Previous studies have provided evidence on both sides of the debate. Several studies explicitly assert that late mortality after critical illness is largely because of pre-existing comorbid disease.7 8 Quartin and colleagues, however, showed that risk of mortality remained higher for up to five years after sepsis, even compared with carefully matched controls in hospital with similar burden of comorbidity at baseline.10 Quartin and colleagues, however, assessed patients admitted to hospital in the mid-1980s—before the development of a consensus definition for sepsis11 and before widespread improvements in recognition and treatment of sepsis2 3 4 5—and were able to account for only a limited number of potential confounders. Because of the limitations of existing literature, experts have recently called for additional epidemiological studies of late mortality attributable to sepsis that use patient level data to deal with confounding.12
To guide the development of future treatments, we measured the excess late (31 days to two years) mortality directly attributable to sepsis—and not to the comorbidities and sociodemographic factors that predispose one to developing sepsis. We looked at patients with sepsis compared with three control groups: adults not currently in hospital, patients in hospital with non-sepsis infection, and patients in hospital with an acute sterile inflammatory process. These comparisons allowed us to determine the excess mortality associated with sepsis (including that associated with admission to hospital) and the incremental excess mortality associated with sepsis, beyond what is associated with admission for the two cardinal features of sepsis11 13 14: infection (by comparing sepsis with inflammatory conditions not caused by infection) and inflammation (by comparing sepsis with infections without evidence of inflammatory storm).
We examined an ongoing longitudinal prospective cohort, in which we could assess the health of a national sample of older Americans independent of whether they were admitted to hospital for sepsis. This allowed us to assess not just traditional risk factors for sepsis but also a range of potential confounders.
Methods
Study population
We studied participants in the US Health and Retirement Study (HRS), a longitudinal survey of 37 000 adults aged over 50 in 23 000 households.15 The study uses a multistage probability sample to identify participants.15 The socioeconomic and racial distribution of the cohort is broadly representative of the older US population.16 17 18 The cohort is interviewed every two years with a follow-up rate consistently over 90%.15 Survey questions focus on wealth, health, cognition, and employment. Data are also linked to federal health insurance (Medicare) claims.15 Participants provide informed consent on enrollment in HRS and again for linkage to Medicare insurance claims.
We considered Medicare beneficiaries aged ≥65 who took part in at least one survey during 1998-2008 for inclusion in the study. We excluded younger beneficiaries as Medicare is available only to select people aged <65 (for example, certain younger people with disabilities and people with end stage renal disease). We also excluded participants enrolled in Medicare Advantage Plans (managed care plans administered by private health insurance companies) as Medicare does not require claims for these beneficiaries.
Patient involvement
Patients were not involved in the study design, recruitment, or conduct of the study. Key results of studies involving HRS are disseminated back to participants through a newsletter twice a year. Patients were not involved in the development of the research question or outcome measures.
Study cohorts
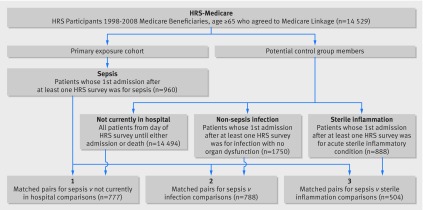
We identified four study cohorts. The primary cohort was patients admitted to hospital with sepsis. The comparison cohorts were adults not currently in hospital, patients admitted with non-sepsis infection, and patients admitted with an acute sterile inflammatory condition (“sterile inflammation”). Appendices 1 and 2 give more detailed descriptions of the matching process (using hypothetical data). In addition, we present Stata code for matching patients with sepsis to adults not in hospital in appendix 3. Fig A in appendix 4 shows a conceptual diagram and explanation of our matching approach. Details of the study cohort definitionare in table A in appendix 5.
We considered only a first admission to hospital after each HRS survey to determine a patient’s eligibility in the cohorts, so that the propensity score (using variables from the HRS survey and described below) would accurately reflect a patient’s pre-illness status.
The cohort not in hospital consisted of adults who had not yet been admitted to hospital in the interval from HRS survey to the day on which they were matched to a patient with sepsis—but no exclusions were placed on this cohort having been admitted before the HRS survey or after the date of matching. Similarly, patients in the sepsis, non-sepsis infection, and sterile inflammation cohorts could have other types of admissions either before the HRS survey or after the index admission. To prevent individual patients from being double counted within any comparison (due to having multiple first admissions, for example, after 2004 and 2006 surveys), we selected at random one admission (or time not in hospital) per patient for each comparison. We also carried out a sensitivity analysis including all first admissions after an HRS survey.
Following the procedure of Angus and colleagues, we defined admissions with sepsis by synchronous ICD-9 (international classification of diseases, ninth revision) CM codes for infection and acute organ dysfunction, or explicit coding of severe sepsis or septic shock1 19 (see table A in appendix 5). This reflects the third international consensus definition that sepsis is “life-threatening organ dysfunction caused by a dysregulated host response to infection”20 or what the previous sepsis definition conceptualized as “severe sepsis.”14 We did not require explicit sepsis codes because they were not introduced until 2002, after the start of our study.
We defined non-sepsis infection as admissions with a principal ICD-9-CM code for infection and no secondary codes for sepsis or acute organ dysfunction. We defined sterile inflammation as admissions with a principal code for traumatic injury, fracture, burn, pancreatitis, inflammatory bowel disease, or connective tissue disease, with no secondary code for infection or sepsis. This definition reflects the concept of non-infectious systemic inflammatory response described in the 1992 and 2001 consensus definitions of sepsis.11 13 14
Propensity matching
At the time of each HRS survey, we used multiple logistic regression to estimate each participant’s risk of having a hospital admission for sepsis in the next two years. We selected each predictor variable based on previous research or clinical experience suggesting it is an important risk factor for sepsis. We abstracted (or calculated) the following predictors from HRS survey and linked Medicare data: self reported race and ethnicity, partnership status (married or part of a couple), limitations of five activities and six instrumental activities of daily living (I/ADLs), self rating of health on a 5 point Likert scale, body mass index (BMI), wealth (sum of all assets and debts) standardized to 2013 $ using the annual gross domestic product price index,21 previous or current use of food stamps (a government assistance program for low income families and individuals), residence in a nursing home, 17 Charlson comorbidities,22 23 admissions to hospital in the previous year, and admissions for sepsis in the previous year. Limitations of activities and instrumental activities of daily living, self reported health, government assistance, and BMI were missing in 7.1%, 0.1%, 0.1%, 0.6%, and 1.3%, respectively, and these values were imputed with multiple imputation with chained equations and five imputations.24 The remaining covariates were present for all of the study population. We considered select interactions and non-linear forms based on a priori knowledge of the relations between the predictors and the likelihood of developing sepsis.
We matched patients in the sepsis cohort 1:1 to those in the non-sepsis infection and sterile inflammation cohorts by age at hospital admission, sex, and centile of risk for admission for sepsis in the next two years (propensity score) using coarsened exact matching.25 We examined the balance of each covariate between the sepsis and comparison cohorts using χ2 and t tests, as appropriate, without consideration of any outcome variable. We then re-matched the sepsis and comparison cohorts including any covariates that were unbalanced on the initial match. We then re-examined each covariate to ensure adequate balance between the sepsis and comparison cohorts before examining any results.
We also matched patients in the sepsis cohort 1:1 to those in the adult cohort not in hospital, again using an iterative process to ensure covariate balance. As there is no age at hospital admission for adults not in hospital, however, we instead matched on age at last HRS survey, sex, centile of risk of developing sepsis, and number of days (rounded to nearest seven) since the last HRS survey.
Outcomes and statistical analysis
Our principal outcome of interest was mortality. Patients were followed for two years. We generated Kaplan-Meyer survival curves for the sepsis and matched comparison cohorts. We used multiple logistic regression to calculate adjusted odds of late mortality (31 days to two years). This technique of matching patients by propensity for sepsis, then also adjusting for sepsis propensity in the regression analysis, is known as “doubly robust” estimation because it combines two methods for reducing bias and is less sensitive to mis-specification.26
Beyond measuring odds of late mortality, we also measured odds of death at multiple time intervals (0-30 days, 31-90 days, 91-180 days, 181 days-one year, and one-two years) to measure how long excess mortality persists after sepsis. We also measured rates of readmissions by 30 days, 90 days, and 365 days among patients who survived to hospital discharge.
As an exploratory analysis, we examined whether the excess late mortality of sepsis relative to adults not currently in hospital differed by age, sex, self rating of health, Charlson comorbidity index score, functional limitations, residence in a nursing home, number of organ dysfunctions, or infectious etiology of sepsis (as determined by the highest coded type of infection on the hospital claim).
As a second exploratory analysis to assess potential mechanisms of excess late mortality, we measured principal diagnoses of terminal admissions to hospital (last admission before death) among patients who survived their initial admission and died within two years. We assigned principal ICD-9-CM codes to one of 185 mutually exclusive diagnosis codes using Healthcare Cost and Utilization Project’s clinical classification software.
We performed a sensitivity analysis to examine whether results differed by early (1998-2002 surveys) versus later (2004-2008 surveys) study periods. We also did a sensitivity analysis in which we limited admissions with sterile inflammation to those for fracture or trauma.
We conducted all analyses with Stata MP version 14 (StataCorp, College Station, TX). We used two sided hypothesis testing and set significance at P<0.05.
Results
From 14 529 HRS participants with Medicare linkage and at least one survey completed in 1998-2008, we identified 960 patients (with 1012 total admissions for sepsis) for inclusion in the sepsis cohort, 1750 patients for the non-sepsis infection cohort, and 888 patients for the sterile inflammation cohort (fig B in appendix 4, fig 1). We were able to match 777 (80.9% of all patients in the sepsis cohort) to an adult not currently in hospital, 788 (82.0%) to a patient admitted for non-sepsis infection, and 504 (55.6%) to a patient admitted for acute sterile inflammation (fig 1).
Fig 1 Flow of participants and match of patients with sepsis
In the sepsis cohort the mean age was 79, 549 (54%) were women, 819 (81%) were white, and 126 (12%) were nursing home residents, with a median of one functional limitation and two medical comorbidities (table 1). There were no significant differences in demographics, socioeconomic characteristics, baseline health status, or recent healthcare use between patients within each of the matched comparisons (tables B-D in appendix 5). At two years, mortality was 25.4% (95% confidence interval 22.7% to 28.1%) at 30 days, 35.3% (32.3% to 38.2%) at 90 days, 41.3% (38.3% to 44.3%) at 180 days, 48.5% (45.4% to 51.6%) at one year, and 56.5% (53.5% to 59.6%) at two years.
Table 1.
Baseline characteristics of cohort of patients with sepsis. Figures are numbers (percentage) of patients unless stated otherwise
| Demographics | Data (n=1012) |
|---|---|
| Mean (SD) age (years) | 79.1 (8.2) |
| Men | 463 (45.8) |
| Race: | |
| White | 819 (80.9) |
| Black/African American | 173 (17.1) |
| Other | 20 (2.0) |
| Hispanic | 78 (7.7) |
| Married or with partner | 433 (42.8) |
| Economic status | |
| Total wealth (fifth of positive assets): | |
| 5 (most assets) | 133 (13.1) |
| 4 | 137 (13.5) |
| 3 | 161 (15.9) |
| 2 | 197 (19.5) |
| 1 | 271 (26.8) |
| Net negative or zero assets | 113 (11.2) |
| Government assistance | 91 (9.1) |
| Health status | |
| Median (IQR) Charlson comorbidity index | 2 (0-4) |
| Congestive heart failure | 247 (24.4) |
| Dementia | 60 (5.9) |
| Moderate or severe liver disease | 8 (0.8) |
| Cancer | 115 (11.4) |
| Renal disease | 134 (13.2) |
| Connective tissue disease | 42 (4.2) |
| Median (IQR) I/ADL limitations | 1 (0-5) |
| Self rating of health: | |
| Excellent | 28 (2.8) |
| Very good | 130 (12.9) |
| Good | 268 (26.5) |
| Fair | 325 (32.1) |
| Poor | 261 (25.8) |
| BMI: | |
| Very severely obese | 36 (3.6) |
| Severely obese | 51 (5.1) |
| Obese | 138 (13.9) |
| Overweight | 298 (30.0) |
| Normal | 395 (39.7) |
| Underweight | 77 (7.7) |
| Use of healthcare | |
| Median (IQR) No of admissions in previous year | 0 (0-1) |
| Severe sepsis in previous year | 64 (6.3) |
| Residence in nursing home | 126 (12.5) |
IQR=interquartile range; I/ADL=activities and instrumental activities of daily living.
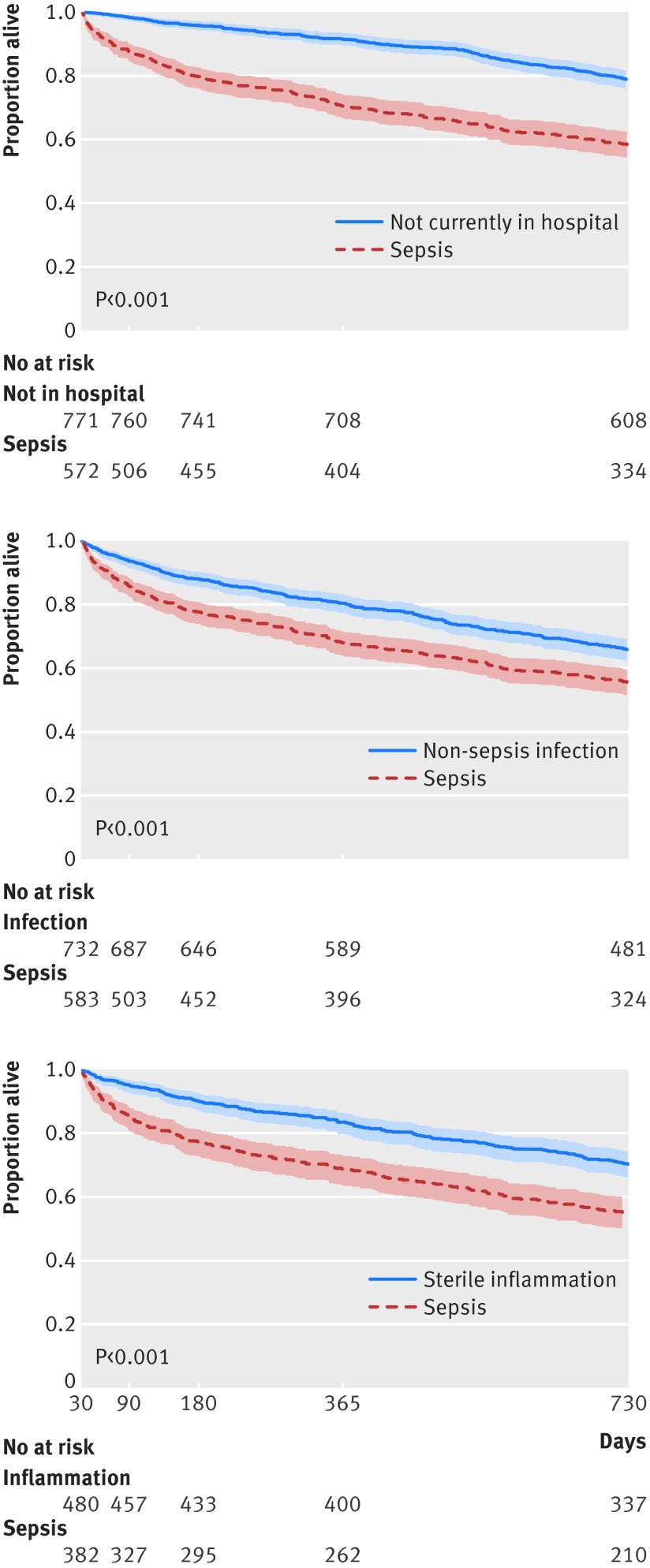
Compared with patients not in hospital matched on all baseline characteristics, those with sepsis experienced a 22.1% (95% confidence interval 17.5% to 26.7%) absolute increase (or 2.2-fold relative increase) in late mortality (adjusted 31 day-two year mortality 40.4% v 18.3%, P<0.001) (table E in appendix 5). The higher mortality in the sepsis cohort persisted for the full two year follow-up period (table 2, fig 2, table E in appendix 5). Among patients with sepsis who survived to a year, the adjusted two year mortality was 16.0% (12.5% to 19.5%) versus 10.7% (8.6% to 12.9%) in their controls who were not currently in hospital at the point of matching; the adjusted odds ratio of one to two year mortality was 1.6 (1.1 to 2.4).
Table 2.
Adjusted odds ratios for mortality after sepsis by time period
| Time period | Adjusted* OR (95% CI) | ||
|---|---|---|---|
| Sepsis v not in hospital | Sepsis v infection | Sepsis v sterile inflammation | |
| Early mortality: | |||
| 0-30 days | 47.2† (20.7 to 107.5) | 4.8† (3.5 to 6.7) | 6.6† (3.6 to 8.4) |
| Late mortality‡: | |||
| 31 days-2 years | 3.5† (2.7 to 4.5) | 1.6† (1.3 to 2.1) | 2.3† (1.7 to 2.1) |
| Late mortality, by discrete time interval‡: | |||
| 31-90 days | 9.8† (5.1 to 18.7) | 2.5† (1.7 to 3.7) | 3.6† (2.2 to 6.0) |
| 91-180 days | 4.0† (2.4 to 6.9) | 1.8† (1.2 to 2.8) | 2.0† (1.1 to 3.4) |
| 181 days-1 year | 3.2† (2.0 to 5.2) | 1.6† (1.1 to 2.5) | 1.7 (1.0 to 2.9) |
| >1-2 years | 1.6† (1.1 to 2.4) | 1.0 (0.7 to 1.4) | 1.4 (0.9 to 2.2) |
*Adjusted for age, sex, and propensity for sepsis. All patients included in regression also matched by sepsis propensity, which included age, race, ethnicity, sex, partnership, wealth, use of food stamps, Charlson comorbidity index, I/ADL limitations, self rating of health, BMI, admission to hospital in previous year, sepsis in previous year, and residence in nursing home.
†Significant at P<0.05.
‡To be included in models for late mortality, patients had to be alive at start of time period.
Fig 2 Kaplan-Meier survival curves for sepsis cohort versus three matched comparisons showing long term survival of patients who survived at least 30 days after their match day
Compared with matched patients admitted to hospital for non-sepsis infection, patients with sepsis experienced a 10.4% (95% confidence interval 5.4% to 15.4%) absolute increase (or 1.3-fold relative increase) in late mortality. The higher mortality in the sepsis cohort persisted for a year (adjusted 181 day-one year mortality was 11.6% (7.0% to 12.1%) v 4.2% (2.8% to 5.6%); adjusted odds ratio 1.6 (1.1 to 2.5)). Among patients with sepsis who survived to a year, survival to two years was statistically indistinguishable from those who survived a non-sepsis infection.
Compared with matched patients in hospital for sterile inflammatory conditions, patients with sepsis who survived to 31 days experienced a 16.2% (95% confidence interval 10.2% to 22.2%) absolute increase (or 1.6-fold relative increase) in late mortality. The higher mortality in the sepsis cohort persisted for at least 180 days. Among patients with sepsis who survived to 90 days, adjusted 90-180 day mortality was 9.5% (95% confidence interval 6.3% to 12.8%) versus 5.3% (3.4% to 7.3%); adjusted odds ratio 2.0 (1.1 to 3.4). The point estimate, however, suggests persistent risk to one year (adjusted 181 day-one year mortality 11.4% (7.8% to 15.0%) v 7.3% (4.9% to 9.7%); adjusted odds ratio 1.7 (1.0 to 2.9)).
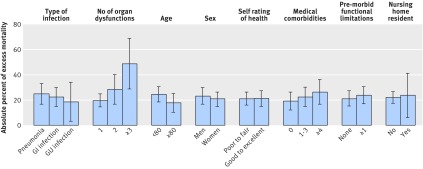
In stratified analysis, the excess late (31 day-two year) mortality with sepsis was relatively constant across patient subgroups defined by source of sepsis (pneumonia v genitourinary v gastrointestinal), age, sex, comorbidity burden, functional limitations, self rating of health, and nursing home residence (fig 3). Late mortality, however, was higher in patients with more organ dysfunctions during sepsis. In sensitivity analyses, the results were similar between earlier and later time periods (fig B in appendix 4); when admission for sterile inflammation was limited to those with fractures, injuries, and trauma; and when all first admissions after an HRS survey were included in the analysis.
Fig 3 Absolute excess late mortality of sepsis v adults not currently in hospital, stratified by subgroup. GI=gastrointestinal; GU=genitourinary
Among patients who survived their initial admission (or were in the cohort not in hospital), rates of 30 day and 90 day re-admission were higher in the sepsis cohort than in the infection and not currently admitted cohorts (table F in appendix 5). Rates, however, were similar between the sepsis and sterile inflammation cohorts. Of the patients who survived their initial admission and died within two years, most (65-75% across cohorts) were admitted at least once before death. An infection—either sepsis or pneumonia—was the most common diagnosis for the terminal admission for each of the cohorts (table G in appendix 5). Infection (sepsis, pneumonia, urinary tract infection, intestinal infection or skin/soft tissue infection) accounted for 22.1-30.4% of terminal diagnoses across cohorts and was not different between sepsis versus any matched comparison cohort (P>0.05 for each comparison).
Discussion
In this national cohort of older Americans, late mortality was substantially increased after sepsis. Among patients who survived for 30 days after an admission for sepsis, over 40% died in the next two years. This high rate of late mortality was not fully explained by age, sociodemographics, or health status before sepsis. Compared with adults not in hospital who were indistinguishable on various potential confounders before acute illness, patients with sepsis had a nearly 22% absolute increase and 2.2-fold relative increase in late mortality. This suggests that more than one in five patients who survives sepsis dies acutely within the next two years as a consequence of sepsis. Compared with patients admitted to hospital with non-sepsis infection or sterile inflammatory conditions, patients with sepsis experienced a 10% increase in late mortality—or roughly one in 10 had a late death related to sepsis.
The high proportion of late mortality unexplained by baseline health status suggests that health status before sepsis is insufficient to explain the poor long term mortality after sepsis. Furthermore, it suggests that late mortality after sepsis could be more amenable to intervention than previously thought, as it is not explained by pre-existing chronic health conditions. The excess late mortality experienced by patients with sepsis relative to other control patients admitted to hospital suggests that patients could benefit from intervention during the admission for sepsis (timely resuscitation, early mobility) rather than just generic post-admission care.
Findings in context
Our results are consistent with previous studies that have shown excess late mortality after sepsis relative to the general population27 28 and that the late mortality is not explained by differences in health status before sepsis.10 29 30 These past studies, however, have been inconclusive because of the substantial risk of residual confounding by incomplete capture of patients’ baseline health status. Our study advances the existing literature by examining a cohort with extensive serial data collection—allowing careful control for chronic health conditions and sociodemographic factors. Our measures of baseline health status include not only measures of comorbidity but also previous hospital admissions, BMI,31 functional status,32 self rating of health,33 and measures of poverty (wealth and government assistance)34—all important predictors of long term mortality that have not been included in previous studies of late mortality after sepsis.
Furthermore, we also compared the sepsis cohort with controls in hospital with non-sepsis infection and sterile inflammatory conditions. Through these analyses, we found that about 50-60% of the excess late mortality after sepsis is explained by admission for non-sepsis infection or sterile inflammation, and about 40-50% is incremental excess mortality above and beyond that associated with these other types of admissions. Taken together, our findings do not refute the importance of baseline burden of comorbidity to patients’ long term outcomes after sepsis. They do, however, indicate that sepsis confers an additional risk of late mortality above and beyond that predicted by status before sepsis alone.
If our results are interpreted strictly as a prognostic association, they might have value to patients, families, and health systems seeking information about life after an admission for sepsis. The high rate of late mortality suggests that physicians should perhaps discuss advanced directives and end of life planning in patients who survive sepsis.
Biological plausibility
While our study cannot offer definitive proof that sepsis causes this increased mortality, there are several credible biological mechanisms and observations that support a potentially mechanistic interpretation of the impact of sepsis on subsequent mortality. Mice that recover from experimental sepsis have accelerated development of atherosclerosis,35 they die with subsequent bacterial or fungal challenge,36 37 38 and they show increased tumor growth.39 High rates of infection, cancer, and deaths related to cardiovascular disease have also been seen in studies of humans who survive sepsis.40 Recent studies suggest that epigenetic regulation is a driving mechanism of post-sepsis immunosuppression and atherosclerosis,41 which could confer persistent immunosuppression and pro-atherosclerotic conditions.41
Of the patients with late mortality after sepsis in our study, the most common terminal admission diagnoses were infection, respiratory failure, and aspiration pneumonitis. With the relatively small number of terminal admissions, we were not able to detect a difference in the proportion for infection between the matched comparison cohorts. Nonetheless, the high number of terminal admissions for infection suggests that heightened risk of infection, perhaps as a result of a sustained immunosuppression or microbiome perturbation,42 could be a potential target for reducing late mortality from sepsis.
Limitations and strengths of study
There are some potential limitations to our study. First, we used claims based algorithms to identify our cohorts. The method for identifying sepsis is commonly used and has similar specificity and greater sensitivity to other claims based methods.19 For the non-sepsis infection and sterile inflammation cohorts, we required a principal diagnosis of infection and sterile inflammatory condition, respectively, to increase the specificity of these cohorts. While some misclassification might have occurred compared with individually adjudicated diagnoses, this allows us to represent a broad range of patients and diagnoses commonly used in clinical practice. Second, we had no measure for the severity of systemic inflammatory response so cannot rule out the possibility that our sterile inflammation cohort was less “inflamed” than our sepsis cohort. Third, we excluded patients aged under 65 because our study relied on Medicare claims, and only limited groups aged under 65 qualify for Medicare coverage. Nonetheless, in our stratified analysis we found no evidence that the excess mortality associated with sepsis attenuates with younger age. Fourth, as with any observational study, we cannot rule out the possibility of unmeasured confounding, such as genetically based differences in immune function.
Our study has several strengths. We examined a national cohort with detailed survey data collected every two years and linked claims data. These granular data allow for robust adjustment for confounding, not possible in prior studies of late mortality after sepsis. Second, we compared outcomes after sepsis with several relevant comparators, allowing us to disentangle the proportion of excess mortality related to hospital admissions and the incremental mortality associated specifically with sepsis. These carefully chosen comparisons add to the robustness of the findings because they explore potential mechanisms and show similar associations in expected strengths.
Conclusions
Using a national cohort of older Americans, we have shown that sepsis is associated with a high rate of late mortality that is not explained by health status before sepsis. More than one in five patients who survives sepsis experiences a late death related to sepsis. The degree of late mortality unexplained by baseline health status suggests that long term mortality after sepsis could be more amenable to intervention than previously thought. The mechanisms of excess late mortality remain unproved and warrant future study.
What is already known on this topic
Numerous observational studies have shown a high rate of later mortality in people who survive an episode of sepsis
It is unclear whether late mortality after sepsis is driven predominantly by pre-existing comorbid disease or is also the result of sepsis itself
What this study adds
More than one in five older patients who survives sepsis has a late death not explained by pre-sepsis health status
Sepsis was associated with a 22% absolute increase in late mortality relative to adults not in hospital, a 10% absolute increase relative to patients admitted to hospital with non-sepsis infection, and a 16% absolute increase relative to patients admitted with sterile inflammatory conditions
Web Extra.
Extra material supplied by the author
Appendix 1: Process for matching admissions for sepsis to admission for non-sepsis infection
Appendix 2: Process for matching admissions for sepsis to adults not currently in hospital
Appendix 3: Stata code for matching patients with sepsis to adult cohort not in hospital
Appendix 4: Supplemental figures A-C
Appendix 5: Supplemental tables A-G
We thank the participants in the Health and Retirement Study whose data were included in this study.
Contributors: HCP designed the study, analyzed the data, interpreted the data, and drafted the manuscript. JJO, DCA, and TJI interpreted the data and revised the manuscript critically for intellectual content. KML acquired the data, interpreted the data, and revised the manuscript critically for intellectual content. HCP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by grants T32 HL007749 (HCP), L30 GM116118 (HCP), and K08 GM115859 (HCP) from the National Institutes of Health and IIR 11-109 (TJI) from the US Department of Veterans Affairs Health Services Research and Development Service. The Health and Retirement Study is sponsored by the National Institute on Aging (U01 AG009740) and performed at the Institute for Social Research, University of Michigan. The funders were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: University of Michigan IRB approved this study. Informed consent was obtained on enrollment into the Health and Retirement Study and again for Medicare linkage
Data sharing: Important components of the statistical code are included in appendix 3. Additional code can be obtained from the corresponding author on request. HRS survey data are available through the HRS website.
Transparency: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. 10.1097/00003246-200107000-00002 pmid:11445675. [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. 10.1001/jama.2014.2637. pmid:24638143. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med 2014;42:625-31. 10.1097/CCM.0000000000000026. pmid:24201173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RR 3rd, , Dong L, Nelson NC, et al. Intermountain Healthcare Intensive Medicine Clinical Program. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. 10.1164/rccm.201212-2199OC. pmid:23631750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott HC, Kepreos KM, Wiitala WL, Iwashyna TJ. Temporal Changes in the Influence of Hospitals and Regional Healthcare Networks on Severe Sepsis Mortality. Crit Care Med 2015;43:1368-74. 10.1097/CCM.0000000000000970. pmid:25803652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012;60:1070-7. 10.1111/j.1532-5415.2012.03989.x. pmid:22642542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland A, Olafson K, Ramsey CD, Yogendran M, Fransoo R. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med 2014;40:1097-105. 10.1007/s00134-014-3348-y. pmid:25011575. [DOI] [PubMed] [Google Scholar]
- 8.Clermont G, Angus DC, Linde-Zwirble WT, Griffin MF, Fine MJ, Pinsky MR. Does acute organ dysfunction predict patient-centered outcomes?Chest 2002;121:1963-71. 10.1378/chest.121.6.1963 pmid:12065364. [DOI] [PubMed] [Google Scholar]
- 9.Leibovici L. Long-term consequences of severe infections. Clin Microbiol Infect 2013;19:510-2. 10.1111/1469-0691.12160. pmid:23397980. [DOI] [PubMed] [Google Scholar]
- 10.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Magnitude and duration of the effect of sepsis on survival. JAMA 1997;277:1058-63. 10.1001/jama.1997.03540370048035 pmid:9091694. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992;101:1481-3. 10.1378/chest.101.6.1481 pmid:1600757. [DOI] [PubMed] [Google Scholar]
- 12.Shankar-Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld GD. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Crit Care 2016;20:101 10.1186/s13054-016-1276-7. pmid:27075205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. 10.1097/01.CCM.0000050454.01978.3B. pmid:12682500. [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, et al. International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. 10.1007/s00134-003-1662-x. pmid:12664219. [DOI] [PubMed] [Google Scholar]
- 15.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014;43:576-85. 10.1093/ije/dyu067 pmid:24671021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer E, Karoly LA. Representativeness of the Low-Income Population in the Health and Retirement Study. 2013.
- 17.Ofstedal MB, Weir DR. Recruitment and retention of minority participants in the health and retirement study. Gerontologist 2011;51(Suppl 1):S8-20. 10.1093/geront/gnq100. pmid:21565822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy H, Gutierrez I. Documentation and Benchmarking of Health Insurance Measures in the Health And Retirement Study. 2009.
- 19.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care 2014;52:e39-43. 10.1097/MLR.0b013e318268ac86. pmid:23001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. 10.1001/jama.2016.0287. pmid:26903338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Commerce. Bureau of Economic Analysis Website. 2014. http://bea.gov/iTable/iTable.cfm?ReqID=9&step=1#reqid=9&step=1&isuri=1. Updated: September 29, 2014.
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. 10.1016/0895-4356(92)90133-8 pmid:1607900. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67. 10.1016/S0895-4356(00)00256-0 pmid:11146273. [DOI] [PubMed] [Google Scholar]
- 24.Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41. [Google Scholar]
- 25.Iacus SM, King G, Porro G. CEM: Software for Coarsened Exact Matching. J Stat Softw 2009;30:1-27. http://j.mp/Te8KP5 10.18637/jss.v030.i09.21666874 [DOI] [Google Scholar]
- 26.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011;173:761-7. 10.1093/aje/kwq439. pmid:21385832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghelani D, Moran JL, Sloggett A, Leeson RJ, Peake SL. Long-term survival of intensive care and hospital patient cohorts compared with the general Australian population: a relative survival approach. J Eval Clin Pract 2009;15:425-35. 10.1111/j.1365-2753.2008.01030.x. pmid:19366395. [DOI] [PubMed] [Google Scholar]
- 28.Davis JS, He V, Anstey NM, Condon JR. Long term outcomes following hospital admission for sepsis using relative survival analysis: a prospective cohort study of 1,092 patients with 5 year follow up. PLoS One 2014;9:e112224 10.1371/journal.pone.0112224. pmid:25486241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder A, Guh D, Boyd JH, Walley KR, Anis AH, Russell JA. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Crit Care Med 2014;42:2211-8. 10.1097/CCM.0000000000000503. pmid:25054672. [DOI] [PubMed] [Google Scholar]
- 30.Wang HE, Szychowski JM, Griffin R, Safford MM, Shapiro NI, Howard G. Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 2014;4:e004283 10.1136/bmjopen-2013-004283. pmid:24441058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211-9. 10.1056/NEJMoa1000367. pmid:21121834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 1998;279:1187-93. 10.1001/jama.279.15.1187 pmid:9555758. [DOI] [PubMed] [Google Scholar]
- 33.Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol 1997;50:517-28. 10.1016/S0895-4356(97)00045-0 pmid:9180644. [DOI] [PubMed] [Google Scholar]
- 34.Fiscella K, Franks P. Poverty or income inequality as predictor of mortality: longitudinal cohort study. BMJ 1997;314:1724-7. 10.1136/bmj.314.7096.1724 pmid:9185498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaynar AM, Yende S, Zhu L, et al. Effects of intra-abdominal sepsis on atherosclerosis in mice. Crit Care 2014;18:469 10.1186/s13054-014-0469-1. pmid:25182529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol 2003;163:2605-17. 10.1016/S0002-9440(10)63615-2. pmid:14633632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol 2004;75:408-12. 10.1189/jlb.0503214. pmid:14557384. [DOI] [PubMed] [Google Scholar]
- 38.Deng JC, Cheng G, Newstead MW, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 2006;116:2532-42. 10.1172/JCI28054. pmid:16917541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavassani KA, Carson WF 4th, , Moreira AP, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 2010;115:4403-11. 10.1182/blood-2009-09-241083. pmid:20130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yende S, D’Angelo G, Kellum JA, et al. GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008;177:1242-7. 10.1164/rccm.200712-1777OC. pmid:18369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics 2011;6:273-83. 10.4161/epi.6.3.14017 pmid:21048427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization Type and Subsequent Severe Sepsis. Am J Respir Crit Care Med 2015;192:581-8. 10.1164/rccm.201503-0483OC. pmid:26016947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Process for matching admissions for sepsis to admission for non-sepsis infection
Appendix 2: Process for matching admissions for sepsis to adults not currently in hospital
Appendix 3: Stata code for matching patients with sepsis to adult cohort not in hospital
Appendix 4: Supplemental figures A-C
Appendix 5: Supplemental tables A-G